Key Points
-
•
Allo-HCT can be performed with tolerable side-effects, and successfully restores ARSA levels in adult patients with MLD.
-
•
Patients who achieved complete chimerism showed a clinically stable condition within the first year.
Visual Abstract
Abstract
Metachromatic leukodystrophy (MLD) is a rare genetic disorder caused by pathogenic variants of the ARSA gene, leading to a deficiency of the arylsulfatase A enzyme (ARSA) and consecutive accumulation of galactosylceramide-3-0-sulfate in the nervous system. The condition leads to severe neurological deficits and subsequently results in profound intellectual and motoric disability. Especially, the adult form of MLD, which occurs in individuals aged >16 years, poses significant challenges for treating physicians because of the rarity of cases, limited therapeutic options, and different allogeneic hematopoietic cell transplantation (allo-HCT) protocols worldwide. Here, we report the results of allo-HCT treatment in 4 patients with a confirmed adult MLD diagnosis. Bone marrow or mobilized peripheral progenitor cells were infused after a reduced intensity conditioning regime consisting of fludarabine and treosulfan. In 3 patients, allo-HCT was followed by an infusion of mesenchymal cells to further consolidate ARSA production. We observed a good tolerability and an increase in ARSA levels up to normal range values in all patients. A full donor chimerism was detected in 3 patients within the first 12 months. In a 1-year follow-up, patients with complete donor chimerism showed a neurological stable condition. Only 1 patient with an increasing autologous chimerism showed neurological deterioration and a decline in ARSA levels in the first year. In summary, allo-HCT offers a therapeutic option for reconstituting ARSA enzyme levels in adult patients with MLD, with tolerable side effects.
Introduction
Metachromatic leukodystrophy (MLD) is a rare neurological disorder (incidence, 1 per 100 000 births).1 The disease belongs to the lysosomal storage disorders and is caused by mutations of the arylsulfatase A enzyme (ARSA) gene, resulting in an ARSA deficiency2 and accumulation of galactosylceramide-3-0-sulfate in the nervous systems.1,2 MLD is classified based on the age of onset into late-infantile (<30 months), juvenile (<16 years), and adult (≥16 years) forms.3,4 MLD commonly leads to devastating and progressive cognitive and motor impairment, with ataxia, spasticity, and neuropathy, as well as incontinence.5 The natural course of the disease consequently causes severe disabilities and a reduced life expectancy.6 In contrast to the infantile and juvenile forms, which primarily manifest clinically through motor symptoms, the adult form is characterized initially by psychiatric and cognitive symptoms. Motor impairments often develop later in the course of the disease.
The diagnosis of MLD is made based on the measurement of reduced ARSA activity in leukocytes and the identification of biallelic pathogenic variants in the ARSA gene. Furthermore, enhanced excreted sulfatide levels can be detected in urine samples across all MLD types.4 Magnetic resonance imaging (MRI) examinations of patients with MLD typically show white matter lesions,7 whereas nerve conduction studies show demyelinating polyneuropathy in most cases.8
Potential therapies in infantile and juvenile MLD aim to enhance ARSA levels, for example, by allogeneic hematopoietic cell transplantation (allo-HCT),3,9,10 ex vivo gene therapy,11,12 or (intrathecal) enzyme replacement.13 Although the exact mechanism is not fully understood,14 allo-HCT is used to restore ARSA activity through the migration of donor-derived macrophages into the central nervous system (CNS) releasing ARSA, which is taken up by cells of the recipient. This procedure may slow down MLD progression, when done early during the disease course.3,9,10 Although allo-HCT has gained significant importance in the juvenile form of the disease, its role in the adult form is currently unclear, with only few cases reported for this condition.10,15, 16, 17, 18
Methods
Here, we report the results obtained from 4 patients with a confirmed diagnosis of adult MLD treated with allo-HCT at our center. All patients had evidence of white matter lesions on MRI, demyelinating neuropathy in nerve conduction studies, and documented ARSA deficiency in peripheral blood cells (ARSA activity range, 0.00-0.06 E514nm/10−6 leukocytes), as well as corresponding genetic mutation (Table 1). At diagnosis, patients 1, 2, and 4 were symptomatic, exhibiting behavioral abnormalities and memory loss, with additional signs of ataxia in patients 2 and 4. In contrast, patient 3 was clinically asymptomatic at diagnosis and diagnosed with MLD through genetic screening triggered by positive family history. As part of the neurological assessment before allo-HCT, neurofunctional tests such as the scale for the assessment and rating of ataxia (SARA), Montreal cognitive assessment test (MoCA), or the mini-mental status test were conducted to evaluate the extent of impairments. Further nerve conduction studies and MRI examinations were assessed. MoCA tests were performed with patients 1 through 3, revealing scores ranging from 21 to 28 points (Table 1). The patient who was initially asymptomatic scored 28 points. Patient 4 underwent a mini-mental status test, achieving a score of 30 points. As expected in adult MLD, the conducted SARA test in 2 patients revealed only mild motor impairments at the time of diagnosis (Table 1).
Table 1.
Patient characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Sex | Male | Male | Female | Female |
| Age (y) at diagnosis | 30 | 17 | 17 | 37 |
| Age (y) at HCT | 30 | 18 | 18 | 37 |
| ARSA activity, E514nm/ 10−6 leukocytes |
0.04 | 0.06 | 0 | 0.03 |
| Blood type | Ad | AD | AD | 0D |
|
ARSA variants (NM_000487.6) |
c.465+1G>A, p? and c.542T>G, p.IIe181Ser | c.465+1>G>A, p? and c.542T>G, p.Ile181Ser | c.1283C>T, p.Pro428Leu homozygous | c.979G>A, p.Gly325Ser homozygous |
| EMG before HCT | Demyelinating polyneuropathy | Demyelinating polyneuropathy | Demyelinating polyneuropathy | Demyelinating polyneuropathy |
| MRI before HCT | White matter lesions | White matter lesions | White matter lesions | White matter lesions |
| SARA at diagnosis (1-y follow-up) | 2.5 (2.5) | - | - | 1.1 (1.0) |
| MoCA at diagnosis (1-y follow-up) | 24 (23) | 21 (19) | 28 (pending) | 30 MMS (-) |
| Karnofsky Index at diagnosis (1-y follow-up) | 70 (90) | 100 (90) | 100 (100) | 100 (-) |
| Initial symptoms | Behavioral change and memory loss | Behavioral change, memory loss, and ataxia | Asymptomatic | Behavioral change, memory loss, and ataxia |
| Related donor | No | No | Yes | No |
| Graft type | Peripheral blood | Bone marrow | Bone marrow | Peripheral blood |
| HLA typing | Identical (MUD 10 of 10) | Identical (MUD 12 of 12) | Identical (MSD 12 of 12) | Identical (MUD 10 of 10) |
| Conditioning regime | Fludarabin/treosulfan | Fludarabin/treosulfan | Fludarabin/treosulfan | Fludarabin/treosulfan |
| Composition CD34+ cells per kg | 7.82 × 106 | 2.2 × 106 | 5.16 × 106 | 3.82 × 108 |
| MSCs | No | Yes | Yes | Yes |
| DLI | No | Yes | No | No |
| Reg. thrombocytes (d) | 14 | 21 | 15 | 14 |
| Reg. ANC (d) | 17 | 21 | 20 | 16 |
| Adverse events | Allergic reaction on CsA, hyperkalemia, and loss of appetite | Hepatitis and loss of appetite | Diarrhea and exanthema | None |
ANC, absolute neutrophile count; CsA, cyclosporine A; DLI, donor lymphocyte infusion; EMG, electromyography; MUD, matched unrelated donor; Reg., regeneration.
Allo-HCT was performed between 18 and 37 years of age (mean, 25.75 years) and within 1 year after diagnosis (Table 1). Graft sources were bone marrow or mobilized peripheral blood hematopoietic stem cells in 2 patients each, respectively. The related donor of patient 3 was tested on pathogeneic ARSA gene variants; an MLD-associated mutation was ruled out. Hematopoietic cells were infused at a dose of 2.2 × 106 to 7.82 × 106 CD34+ cells per kg after a reduced intensity conditioning regime with fludarabine (30 mg/m2 from day −6 to −2) and treosulfan (10 or 14 g/m2 from day −4 to −2; Table 1). Donor mesenchymal stromal cells (MSCs) in a dose of 0.98 × 106 to 1.44 × 106 kg/KG were used to consolidate enzyme production at approximately day 30 after transplantation (Table 1). One patient received a second MSC infusion 6 months after transplantation. MSCs are able to release high amounts of ARSA, which are taken up by the deficient tissue.14,19,20 Donor lymphocytes were given in cases of transplant failure. For posttransplant immunosuppression tacrolimus, methotrexate, mycophenolate mofetil, or antithymocyte globulin (Grafalon, Neovii, 10 mg/kg) were used.
This retrospective analysis was performed in accordance with the Declaration of Helsinki and approved by the ethical committee of the University of Tuebingen (reference no. 567/2023BO2). Because person-specific data in our study are anonymized and cannot be traced by third parties, informed consent has been waived.
Results
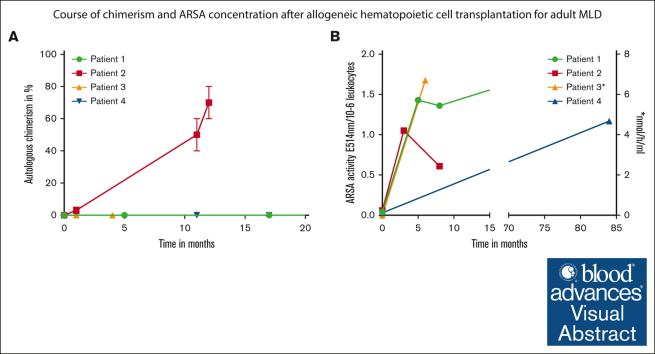
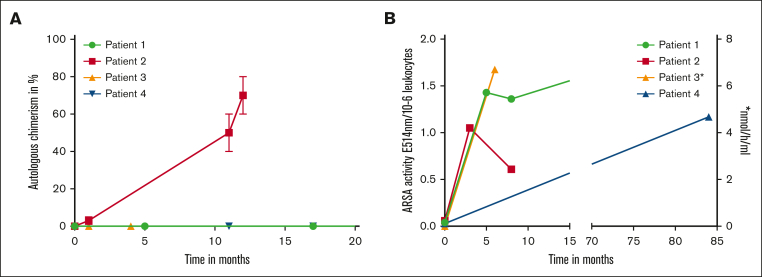
The tolerability of the intervention was good in all 4 patients. Transient adverse events, such as diarrhea and loss of appetite occurred in 3 patients. In 2 patients, hepatitis and exanthema associated with antithymocyte globulin administration were observed. Patient 1 had a mild allergic reaction to tacrolimus. A mild graft-versus-host disease (GVHD) of the skin occurred only in patient 4, 1 year after transplantation; a full remission of the GVHD was achieved using tacrolimus and steroids. All patients were engrafted within 16 to 21 days after HCT (platelet, 14-21 days [median, 14.5 days] and neutrophils, 16 to 21 days [median, 18.5 days]). A full donor chimerism was detected in 3 of 4 patients within the first 11 months after HCT (Figure 1A). In patient 2, an 18-year-old male, autologous chimerism increased to between 60% and 80% within 12 months after HCT (Figure 1A). An increase in ARSA levels reaching normal values after treatment was documented in all patients (Figure 1B). However, in patient 2, the ARSA activity decreased from 1.05 E514nm/10−6 leukocytes to 0.65 E514nm/10−6 leukocytes in month 8 after transplantation (Figure 1B), corresponding to the reduction in donor-derived hematopoietic chimerism. To improve donor-derived engraftment, donor lymphocyte infusions were administrated twice (1.0 × 106 CD3+ cells per kg and 10 × 106 CD3+ cells per kg). Although donor lymphocyte infusions were well tolerated, an improvement in chimerism has unfortunately not yet been achieved.
Figure 1.
Course of chimerism and enzyme concentration after HCT. (A) Percentage of autologous chimerism in whole peripheral blood mononuclear cells over time after HCT. (B) ARSA activity over time after HCT (∗ARSA activity in patient 3 was measured in nmol/h per mL). ARSA activity in E514nm/10−6 leukocytes. Lower cutoff for normal values is 0.4 E514nm/10−6 leukocytes, respectively 3.3 nmol/h per mL.
In addition to assessing ARSA activity and chimerism, presentations were conducted with the treating neurologist. Here, we report on the neurological course of the patients within the first year after allo-HCT. Patients with a full donor chimerism (patients 1, 3, and 4) did not report new symptoms within the first year. Moreover, preexisting symptoms seemed to remain stable during this period in these patients. The SARA scores for patients 1 and 4 remained stable within the first year, and the MoCA test also yielded an unchanged result for patient 1. At present, corresponding test procedures are still pending for patient 3. Unfortunately, in patient 2, a deterioration of neurological symptoms was observed, corresponding to declining ARSA activity and the increasing autologous fraction in chimerism. The patient’s relatives reported an increasingly impaired short-term memory. This was also confirmed by a lower MoCA test result of 19 points. However, no new symptoms seem to have emerged.
Furthermore, values for the development of nerve conduction velocity under therapy after 1 year are available for 2 patients. Patient 4 demonstrated a slightly improved nerve conduction velocity of motor nerves (the ulnaris nerve increased from 47-52 m/s, and the tibialis nerve increased from 33-46 m/s), whereas sensitive nerves showed a stable conduction velocity. Patient 1 exhibited an overall stable, to slightly improved, nerve conduction velocity (ulnaris nerve increased from 44.4-51 m/s, and tibialis nerve decreased from 35-34 m/s).
Discussion
The potential benefits and risks of allo-HCT in adult MLD are currently not sufficiently studied. So far, there are only a few case reports or small case series of allo-HCT in adult MLD.10,15,16,18 A case series by de Hosson et al reported poor transplantation outcomes, with 2 of 5 patients failing to achieve engraftment and the fifth patient dying shortly after allo-HCT because of GVHD.16 However, reliable data from larger cohorts are currently only available for the infantile and juvenile forms of the disease.3 In this case series, we analyzed the feasibility and safety of allo-HCT in our unicentric cohort. Currently, it remains unclear at which stage of the disease allo-HCT should be performed. Treatment of presymptomatic patients showed improved outcome in late-juvenile patients compared with treatment in more advanced disease stages. Therefore, this procedure might also prevent disease progression in the adult form.3,10,18 A longer clinical follow-up, higher patient numbers, and further prospective trials are needed to evaluate the therapeutic benefit of allo-HCT in adult MLD. Another issue are potential differences in conditioning regimes for patients with MLD. For instance, Boucher et al and Videbæk et al used busulfan and cyclophosphamide as conditioning regimens, whereas Groeschel et al used busulfan and cylclophosphomide as well as treosulfan and fludarabin.3,10,15 Currently, clear recommendations for potential conditioning regimens are lacking. However, it should be noted that busulfan demonstrated better donor-derived CNS engraftment in in vivo mouse studies with inherited metabolic disorders.21 This could potentially be attributed to a better ablative effect on functionally defined brain-resident myeloid precursors, thereby facilitating improved turnover with donor-derived microglia in the CNS.22 Furthermore, alternative treatment approaches such as enzyme replacement or ex vivo gene therapy need to be tested on adult patients.13 Potential advantages of these strategies include more favorable adverse event profiles. In this context, head-to-head studies are needed to ascertain the value of each treatment. Because of the low incidence of adult MLD, conducting such studies proves to be exceedingly challenging.
The clinical significance of administrating MSCs to patients with MLD remains unclear. So far, only sporadic significant clinical improvements have been demonstrated, such as improvements in nerve conduction velocity in 4 patients with juvenile MLD.23 Furthermore, as reported by Cabanilla Stanchi et al in a retrospective study, ARSA levels, as well as leukocyte and platelet counts, appear to be significantly higher after 1 year, after MSC transfusions.24 However, no significant differences in terms of clinical outcomes could be identified.
In summary, our case series document that allo-HCT can be performed with tolerable side effects and successfully restores ARSA levels in adult MLD. Based on this experience and previously reported cohorts in juvenile MLD, our data reinforce the notion that allo-HCT represents a relevant therapeutic option for adult MLD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The work of L.S. has been supported by the German Ministry of Health to the LeukoExpert Network (grant ZMVI1-2520DAT94E). H.H. was supported by the Deutsche Forschungsgemeinschaft (HE 8803/1–1).
Authorship
Contribution: A.R. contributed to data collection and analysis, and manuscript writing and editing; C.F. and K.R. performed data collection; J.C.S., L.S., and P.J.L. contributed to manuscript writing and editing; C.L. contributed to project development, data interpretation, and manuscript writing and editing; N.W. and L.S. contributed to data collection; H.H. contributed to data interpretation, and manuscript writing and editing; S.G. contributed to data interpretation, and manuscript writing and editing; and W.A.B. contributed to project development, data analysis, and manuscript writing/editing.
Footnotes
Data are available on request from the corresponding author, Andreas Riedel (andreas.riedel@med.uni-tuebingen.de).
References
- 1.Heim P, Claussen M, Hoffmann B, et al. Leukodystrophy incidence in Germany. Am J Med Genet. 1997;71(4):475–478. [PubMed] [Google Scholar]
- 2.Gieselmann V, Krägeloh-Mann I. Metachromatic leukodystrophy--an update. Neuropediatrics. 2010;41(1):1–6. doi: 10.1055/s-0030-1253412. [DOI] [PubMed] [Google Scholar]
- 3.Groeschel S, Kühl JS, Bley AE, et al. Long-term outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 2016;73(9):1133–1140. doi: 10.1001/jamaneurol.2016.2067. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Ospina N. In: GeneReviews. Adam MP, Mirzaa GM, Pagon RA, et al., editors. University of Washington; 1993. Arylsulfatase A deficiency. [PubMed] [Google Scholar]
- 5.Shaimardanova AA, Chulpanova DS, Solovyeva VV, et al. Metachromatic leukodystrophy: diagnosis, modeling, and treatment approaches. Front Med. 2020;7 doi: 10.3389/fmed.2020.576221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehrer C, Elgün S, Raabe C, et al. Association of age at onset and first symptoms with disease progression in patients with metachromatic leukodystrophy. Neurology. 2021;96(2):e255–e266. doi: 10.1212/WNL.0000000000011047. [DOI] [PubMed] [Google Scholar]
- 7.Schoenmakers DH, Beerepoot S, Krägeloh-Mann I, et al. Recognizing early MRI signs (or their absence) is crucial in diagnosing metachromatic leukodystrophy. Ann Clin Transl Neurol. 2022;9(12):1999–2009. doi: 10.1002/acn3.51692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beerepoot S, Nierkens S, Boelens JJ, Lindemans C, Bugiani M, Wolf NI. Peripheral neuropathy in metachromatic leukodystrophy: current status and future perspective. Orphanet J Rare Dis. 2019;14(1):240. doi: 10.1186/s13023-019-1220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krägeloh-Mann I, Groeschel S, Kehrer C, et al. Juvenile metachromatic leukodystrophy 10 years post transplant compared with a non-transplanted cohort. Bone Marrow Transplant. 2013;48(3):369–375. doi: 10.1038/bmt.2012.155. [DOI] [PubMed] [Google Scholar]
- 10.Boucher AA, Miller W, Shanley R, et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J Rare Dis. 2015;10:94. doi: 10.1186/s13023-015-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fumagalli F, Calbi V, Natali Sora MG, et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet. 2022;399(10322):372–383. doi: 10.1016/S0140-6736(21)02017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148) doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 13.í Dali C, Sevin C, Krägeloh-Mann I, et al. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: results from a phase 1/2 clinical trial. Mol Genet Metabol. 2020;131(1-2):235–244. doi: 10.1016/j.ymgme.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Wolf NI, Breur M, Plug B, et al. Metachromatic leukodystrophy and transplantation: remyelination, no cross-correction. Ann Clin Transl Neurol. 2020;7(2):169–180. doi: 10.1002/acn3.50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Videbæk C, Stokholm J, Sengeløv H, et al. Allogenic hematopoietic stem cell transplantation in two siblings with adult metachromatic leukodystrophy and a systematic literature review. JIMD Rep. 2021;60(1):96–104. doi: 10.1002/jmd2.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Hosson LD, van de Warrenburg BP, Preijers FW, et al. Adult metachromatic leukodystrophy treated by allo-SCT and a review of the literature. Bone Marrow Transplant. 2011;46(8):1071–1076. doi: 10.1038/bmt.2010.252. [DOI] [PubMed] [Google Scholar]
- 17.Solders M, Martin DA, Andersson C, et al. Hematopoietic SCT: a useful treatment for late metachromatic leukodystrophy. Bone Marrow Transplant. 2014;49(8):1046–1051. doi: 10.1038/bmt.2014.93. [DOI] [PubMed] [Google Scholar]
- 18.van Rappard DF, Boelens JJ, van Egmond ME, et al. Efficacy of hematopoietic cell transplantation in metachromatic leukodystrophy: the Dutch experience. Blood. 2016;127(24):3098–3101. doi: 10.1182/blood-2016-03-708479. [DOI] [PubMed] [Google Scholar]
- 19.Meuleman N, Vanhaelen G, Tondreau T, et al. Reduced intensity conditioning haematopoietic stem cell transplantation with mesenchymal stromal cells infusion for the treatment of metachromatic leukodystrophy: a case report. Haematologica. 2008;93(1):e11–13. doi: 10.3324/haematol.11802. [DOI] [PubMed] [Google Scholar]
- 20.Müller I, Kustermann-Kuhn B, Holzwarth C, et al. In vitro analysis of multipotent mesenchymal stromal cells as potential cellular therapeutics in neurometabolic diseases in pediatric patients. Exp Hematol. 2006;34(10):1413–1419. doi: 10.1016/j.exphem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves KA, Hyzy SL, Brooks ML, Hertzler HJ, Boitano AE, Cooke MP. High dose hematopoietic stem cell transplantation leads to rapid neural and peripheral disease cross-correction via robust hematopoietic and microglia recovery. Biol Blood Marrow Transplant. 2020;26(3):S207–S208. [Google Scholar]
- 22.Capotondo A, Milazzo R, Politi LS, et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc Natl Acad Sci U S A. 2012;109(37):15018–15023. doi: 10.1073/pnas.1205858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30(4):215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 24.Cabanillas Stanchi KM, Böhringer J, Strölin M, et al. Hematopoietic stem cell transplantation with mesenchymal stromal cells in children with metachromatic leukodystrophy. Stem Cell Dev. 2022;31(7-8):163–175. doi: 10.1089/scd.2021.0352. [DOI] [PubMed] [Google Scholar]