Key Points
-
•
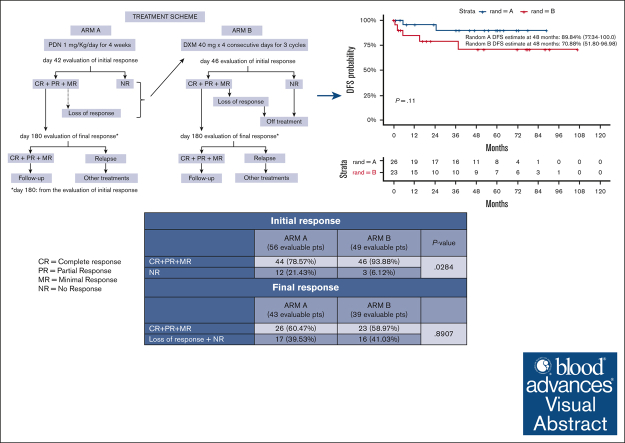
HD-DXM allows a better initial response than PDN, but less long-lasting over time, useful when a rapid platelet increase is required.
-
•
PDN seems to increase long-lasting responses but exhibits a less safe profile than HD-DXM.
Visual Abstract
Abstract
A debate exists regarding which type of corticosteroids (standard-dose prednisone [PDN] or high-dose dexamethasone [HD-DXM]) is the best first-line treatment for adult patients with newly diagnosed untreated primary immune thrombocytopenia (pITP). An ad hoc study compared PDN with HD-DXM in newly diagnosed untreated patients with pITP (aged ≥18 but ≤80 years, platelet count of ≤20 or >20 but <50 × 109/L, and bleeding score of ≥8). Patients were randomised to receive PDN 1 mg/kg per day from days 0 to 28 (Arm A) or HD-DXM 40 mg per day for 4 days, every 14 days, for 3 consecutive courses (Arm B). Fifty-nine of 113 patients (52.2%) were randomized to Arm A and 54 of 113 (47.8%) to Arm B. In evaluable patients, total initial responses (complete response [CR], partial response [PR], minimal response [MR]) were 44 of 56 (78.57%) in Arm A and 46 of 49 (93.88%) in Arm B at days 42 and 46, respectively (P = 0.0284). Total final responses (at day 180 from initial response) were 26 of 43 (60.47%) in Arm A and 23 of 39 (58.97%) in Arm B (P = 0.8907). Total persistent responses (at 12 months from initial response) were 25 of 31 (80.65%) in Arm A and 20 of 36 (55.56%) in Arm B (P = 0.0292). Seven relapses occurred. Median follow-up was 44.4 months. Overall survival was 100% at 48 months, overall disease-free survival was 81.11% at 48 months from day 180. PDN and pulsed HD-DXM were well tolerated; HD-DXM allows effective initial responses but less long lasting than PDN. This trial was registered at www.clinicaltrials.gov as #NCT00657410.
Introduction
Primary immune thrombocytopenia (pITP) is an autoimmune disorder characterized by isolated thrombocytopenia in the absence of underlying causes.1 In adults, pITP has an incidence of 2 to 4 cases per 100 000 person-years and shows 2 peaks: 1 between 20 and 30 years of age, with a slight prevalence for female sex; and another at age >60 years, with equal distribution between the 2 sexes.2,3 Physiopathology of pITP is characterized by an immune-mediated platelet destruction induced by autoantibodies directed against specific glycoproteins of platelet surface and by an impaired platelet production, leading to an increased risk of bleeding.4 Since the 1950s, corticosteroids have been recognized as the most widely used first-line treatment in adult pITP. Indeed, according to the international guidelines,4, 5, 6, 7, 8 corticosteroids are currently administered as frontline therapy to patients who need to be treated. However, the best initial approach is still a matter of debate. Usually, prednisone or prednisolone (PDN) is administered at a starting dose of 0.5 to 2 mg/kg per day for 2 to 4 weeks. This approach leads to an initial response rate of 50% to 80% or more, according to various publications4,9, 10, 11, 12, 13 whereas the long-term response rate after treatment discontinuation is ∼30%.14 A comparison between 2 different initial dosages of PDN was made in 2 randomized trials involving adults and children with newly diagnosed pITP.9,10 In both studies no statistically significant differences in response rate between arms were found. Subsequently, in the mid-1990s, for the first time, pulsed high-dose dexamethasone (HD-DXM) (40 mg per day) was administered every 28 days for a 4-day course, 6 consecutive times in a small cohort of adult patients with pITP, refractory to several therapy lines, with the aim of achieving a better outcome. Satisfactory results were obtained (response rate, 100%).15 In an uncontrolled study, HD-DXM was given in a single 4-day course (40 mg per day) in adult patients with previously untreated pITP, with very encouraging results: initial response rate was 85%, and sustained response (SR), 50%; however, a relapse rate of 50% was recorded.16 A Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) multicenter pilot study, investigating the use of pulsed HD-DXM in adults and children with newly diagnosed pITP (40 mg per day or 20 mg/m2 per day in children, for 4 consecutive days, every 14 days, for 4 therapy courses) was published in 2007 with the following results: in 90 evaluable patients (42 aged <18 years) initial response rate, at day 60, was 85.6%, without any statistically significant difference in patients aged <18 years and those aged ≥18 years. Long-term responses, lasting for a median time of 8 months, were 97% and 78% in patients aged <18 years and those aged ≥18 years, respectively.17 Although these experiences showed the high efficacy of HD-DXM, some questions remained unanswered, such as whether HD-DXM compared with conventional PDN doses could lead to a better response, reduce relapses, and prolong SRs, in newly diagnosed adults with pITP. To answer the above questions, randomized studies were considered necessary. For this reason, a GIMEMA ad hoc Study Group launched a randomized study comparing standard-dose PDN vs HD-DXM in adult patients with newly diagnosed untreated pITP.
Objectives of the trial
The primary objective was to evaluate the role of therapy intensification by HD-DXM in adult patients with newly diagnosed previously untreated pITP, in terms of response improvement at 6 months after initial response, compared with standard PDN doses. Secondary objectives were rate of initial, final, and persistent responses. The estimated sample size of the study was 150 patients, 75 for PDN treatment, and 75 for HD-DXM treatment.
Methods
Study design
The GIMEMA Protocol ITP 0207-EudraCT number 2008-000417-30, was registered at www.clinicaltrials.gov as #NCT00657410. It is a multicenter, prospective, randomized, controlled, open-label, phase 3 clinical trial for the treatment of newly diagnosed previously untreated pITP in adult patients, comparing standard-dose PDN with HD-DXM. This trial was compliant with the Declaration of Helsinki and Italian laws and conducted in accordance with International Council for Harmonization Guideline for Good Clinical Practice.18 An informed consent form was signed by the patient or by the patient’s legal representative before enrollment. The coordinating center was the Hematology Institute of Sapienza University of Rome. The study was approved by the ethical committee of each participating GIMEMA center. Data were analyzed by the GIMEMA statistician, and all authors had access to clinical trial data. The trial was started on 23 April 2008 and ended on 2 February 2016.
Procedures at diagnosis and patient selection
Clinical and laboratory evaluations are summarized in Table 1. Diagnostic criteria are shown in Table 2. Assessment of bleeding symptoms was made according to the grading scale by Khellaf et al19 (Table 3). Inclusion criteria were: signed informed consent and new diagnosis of pITP, previously untreated, in adult patients aged ≥18 years but ≤80 years with platelet count of ≤20 × 109/L or >20 × 109/L but <50 × 109/L, and bleeding score of ≥8 (Table 4). Exclusion criteria are listed in Table 5. Random arms were, Arm A: PDN, and Arm B: HD-DXM.
Table 1.
Summary of clinical and laboratory evaluations
| Baseline (before treatment initiation) | During treatment Arm A | During treatment Arm B | During treatment Arm B | During treatment Arm B | During treatment Arm B | During treatment Arm B | Initial response evaluation (Arm A and B) | Between initial and final evaluation (Arm A and B) | Final response evaluation (Arm A and B) | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 0 | 7-35 | 4 | 14 | 18 | 28 | 32 | 42-46 | 180∗ | ||
| Eligibility (inclusion/exclusion) criteria | † | ||||||||||
| Demographics | † | ||||||||||
| General medical history/present medical conditions | † | Weekly | † | † | † | † | † | † | † | † | †,‡ |
| Physical examination/vital signs | † | Weekly | † | † | † | † | † | † | † | † | †,‡ |
| Complete blood cell count | † | Weekly | † | † | † | † | † | † | Every 15 of 21 d | † | †,‡ |
| Serum biochemistry (glucose, urea, creatinine, uric acid, albumin, total protein and electrophoresis, bilirubin, alkaline phosphatase, AST, ALT, LDH, sodium, potassium, and calcium) | † | Weekly | † | † | † | † | † | † | † | † | †,‡ |
| BM and PB cytomorphology | † | †,§ | |||||||||
| Autoimmunity markers | † | ||||||||||
| Coagulation: PT, aPTT, fibrinogen, D-dimer, and AT | † | ||||||||||
| Cardiac evaluation: visit. If necessary: electrocardiogram and echocardiogram |
† | ||||||||||
| Chest radiograph | † | ||||||||||
| Abdomen ultrasound | † | ||||||||||
| Evaluation of bleeding symptoms (indication of the score) | † | Weekly | † | † | † | † | † | † | † | † | †,‡ |
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AT, antithrombin; BM, bone marrow; LDH, lactate dehydrogenase; PB, peripheral blood; PT, prothrombin time; PTT, activated partial thromboplastin time.
From initial response evaluation.
Time when an evaluation should be made.
Every month until 12th month; thereafter, almost every 2 months until 24th month, and every 3 months until 36th month.
In patients aged >60 years who were unresponsive to treatment or who had lost the response, bone marrow aspirate, bone marrow biopsy, and evaluation of karyotype are indicated.
Table 2.
Diagnostic criteria
| Normal physical examination |
| No history of congenital thrombocytopenia |
| No alterations at the blood smear apart from thrombocytopenia |
| Bone marrow aspirate morphology: compatible with isolated thrombocytopenia |
| Absence of autoimmunity markers (anti-nucleus, anti-thyroglobulin, and anti-thyroid peroxidase antibodies; anti-cardiolipin and anti–β2-glycoprotein antibodies; and Lupus anticoagulant) and direct anti-globulin test |
| Normal coagulation parameters |
| Normal serum biochemistry: glucose, urea, creatinine, uric acid, bilirubin, AST, ALT, alkaline phosphatase, LDH, sodium, potassium, calcium, total proteins and electrophoresis, and albumin |
| Absence of lymphadenopathies; liver and spleen enlargement |
| Absence of neoplasms |
| Absence of acute or chronic infectious diseases |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
Table 3.
Bleeding score
| Score | |
|---|---|
| Age, y∗ | |
| >65 | 2 |
| >75 | 5 |
| Cutaneous bleeding∗ | |
| Localized petechial purpura (legs) | 1 |
| Localized ecchymotic purpura | 2 |
| Two locations of petechial purpura (eg, legs + chest) | 2 |
| Generalized petechial purpura | 3 |
| Generalized ecchymotic purpura | 4 |
| Mucosal bleeding | |
| Unilateral epistaxis | 2 |
| Bilateral epistaxis | 3 |
| Hemorrhagic oral bullae, spontaneous gingival bleeding, or both | 5 |
| Gastrointestinal bleeding∗ | |
| Gastrointestinal hemorrhage without anemia | 4 |
| Gastrointestinal hemorrhage with acute anemia (>2 g Hb decrease in 24 h) and/or shock | 15 |
| Urinary bleeding∗ | |
| Macroscopic hematuria without anemia | 4 |
| Macroscopic hematuria with acute anemia | 10 |
| Genitourinary tract bleeding∗ | |
| Major menometrorrhagia without anemia | 4 |
| Major menometrorrhagia with acute anemia | 10 |
| Central nervous system bleeding | |
| Central nervous system bleeding and/or life-threatening hemorrhage | 15 |
According to criteria by Khellaf et al.19
Hb, hemoglobin.
For these items, only the highest values were considered.
Table 4.
Inclusion criteria
| Signed written informed consent according to IGH/EU/GCP and national local laws |
| Adult patients with newly diagnosed, previously untreated primary ITP |
| Age of >18 y but <80 y |
| Platelet count of <20 × 109/L |
| Platelet count of >20 × 109/L but ≤50 × 109/L plus bleeding score of >8 (according to grading scale reported in Table 3) |
EU, European Union; GCP, good clinical practice; ICH, International Council for Harmonization (of Technical Requirements for Pharmaceuticals for Human Use).
Table 5.
Exclusion criteria
| Exclusion criteria |
| Active malignancy at time of study entry |
| Steroid administration for >5 d before randomization∗ |
| Concomitant treatment with antiplatelet and/or anticoagulant drugs |
| Concomitant severe psychiatric disorders |
|
Unconfirmed diagnosis of primary ITP for Positivity† of autoimmunity markers: anti-nucleus (≥1:80), anti-thyroglobulin, anti-thyroperoxidase, anti-cardiolipin (≥40 GPL U/mL), anti–β2-glycoprotein (≥40 IgG U/mL) antibodies, Lupus anticoagulant (KCT ratio, dRVVT ratio ≥1.5 times the upper normal limit), direct antiglobulin test. Presence of autoimmune hemolytic anemia Presence of connective tissue disease |
| Women who are pregnant or breastfeeding |
| Cardiovascular diseases requiring treatment |
| Severe, noncontrolled despite therapy hypertension and diabetes |
| Liver and kidney function impairment (creatinine level, ALT, AST >2-times the upper normal limit) |
| HCV Ab, HIV Ab, HBsAg, HBc Ab seropositive status |
| Chronic liver disease |
| Documented viral illness by the positivity of IgM, or vaccination, both occurred 1 mo before diagnosis |
| Intake of drugs not previously taken within 1 wk before diagnosis |
| Bleeding score of 15 because of ICH or to GI bleeding (according to grading scale in Table 3) |
| Active gastric ulcer |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; dRVVT, diluted Russell viper venom time; GI, gastrointestinal; GPL, IgG phospholipid unit; HBc Ab, Hepatitis B core antibodies; HBsAg, Hepatitis B surface antigen; HCV Ab, Hepatitis C virus antibodies; HIV Ab, human immunodeficiency virus antibodies; ICH, intracranial hemorrhage; IgG, immunoglobulin G; IgM, immunoglobulin M; KCT, Kaolin clotting time.
If steroid administration lasted <5 days before randomization, only equivalent doses of PDN of <1 mg/kg per day were allowed.
The results of autoimmunity markers were mandatory for patient eligibility; however, to avoid delay in the treatment start, patients could be randomized before the results of these tests were available. If evidence of positivity, the patient was no longer eligible even if the treatment had been started.
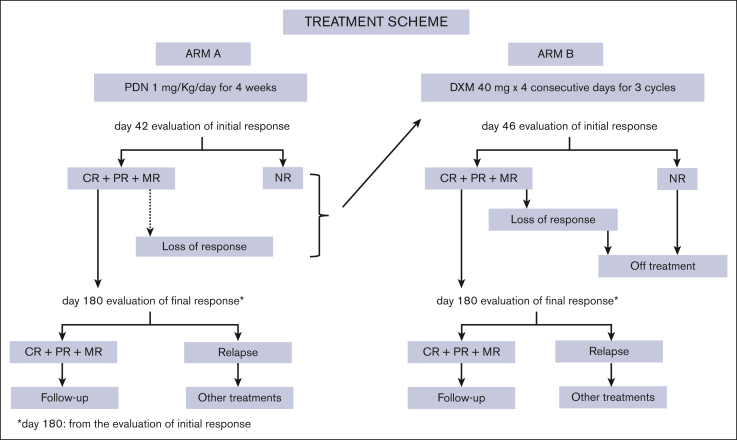
Treatment scheme is reported in Figure 1. In Arm A, PDN was administered orally at a dose of 1 mg/kg per day for 4 consecutive weeks (from day 0-day 28), then, after day 28 PDN was tapered off in 14 days according to the following scheme: days 1 to 3, 0.8 mg/kg per day; days 4 to 6, 0.6 mg/kg per day; days 7 to 9, 0.4 mg/kg per day; days 10 to 11, 0.2 mg per day; days 12 to 13, 0.1 mg per day; and day 14, stop. Patients, unresponsive at day 42 or who had lost the response before the final response assessment, could receive a nonmandatory rescue treatment with HD-DXM (no other drugs were permitted). In Arm B, DXM was administered orally at single fixed daily dose of 40 mg for 4 consecutive days, every 14 days, for 3 consecutive courses. This regimen was chosen because in the previously reported pilot study.17 There was no statistically significant difference in the overall response rate between the first and second cycle, whereas a statistically significant increase was observed between the first and third cycle (P = .001) and between the second and third cycle (P = .018) but not after the fourth cycle. Patients from both arms unresponsive at day 42 or 46 or who had lost the response before the assessment of the final response, were considered off-treatment (Figure 1).
Figure 1.
Treatment scheme, therapeutic approach scheme, and steps in both study arms are reported.
Assessment of response to therapy
Response criteria were complete response (CR), platelet count of ≥100 × 109/L; partial response (PR), platelet count ≥50 × 109/L but <100 × 109/L ; minimal response (MR), platelet count of >20 × 109/L but <50 × 109/L; no response (NR), platelet count of ≤20 × 109/L or platelet count >20 × 109/L but <50 × 109/L and bleeding score of ≥8. Definition of response required concomitant absence of bleeding symptoms. The evaluation of initial response was done in Arm A at day 42 from the start of PDN, and in Arm B at day 46 from the start of HD-DXM. Initial response had to last at least 30 days from day 42 and from day 46 for Arm A and Arm B, respectively. Final response was evaluated at day 180 from the assessment of initial response (Figure 1). Persistent response (no relapses, no therapies) was defined as a response persisting at 12 months from the assessment of the initial response. Loss of response (ie, failure) was defined as a platelet count of ≤20 × 109/L or >20 × 109/L but <50 × 109/L and bleeding score ≥8 within 180 days from the initial response. Relapse was defined as a platelet count decrease to levels of ≤20 × 109/L or >20 × 109/L but <50 × 109/L and bleeding score ≥8 after the assessment of the final response. Follow-up was defined as the time elapsing between diagnosis and the last available assessment. During the follow-up, after the final response assessment, complete blood cell count had to be repeated every month until the 12th month. Thereafter, every 2 months until the 24th month, and every 3 months until the 36th month. Adverse events and/or adverse drug reactions were recorded according to the National Cancer Institute: Common Toxicity Criteria Adverse Events, version 3.0.20
Statistics
Differences between categorical variables or response rates in subgroups were assessed by the χ2 or Fisher's exact tests. Overall survival of enrolled patients (time elapsed from study entry to death or last follow-up) and disease-free survival of responders (DFS, time from response to relapse or death in response status or last follow-up) were calculated using the Kaplan-Meier product limit estimator with subgroup comparisons by means of log-rank test. All patients not evaluable for initial, final, and persistent response were not included in the statistical analysis. All analyses were intention to treat and were performed using the SAS system software (version 9.4) and R (R Foundation for Statistical Computing, Vienna, Austria). All tests were 2-sided, accepting P ≤ .05 as indicative of a statistically significant difference. Study data were collected and managed using the Research Electronic Data Capture (REDCap) tools hosted at the GIMEMA Foundation REDCap, which is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.21,22
Results
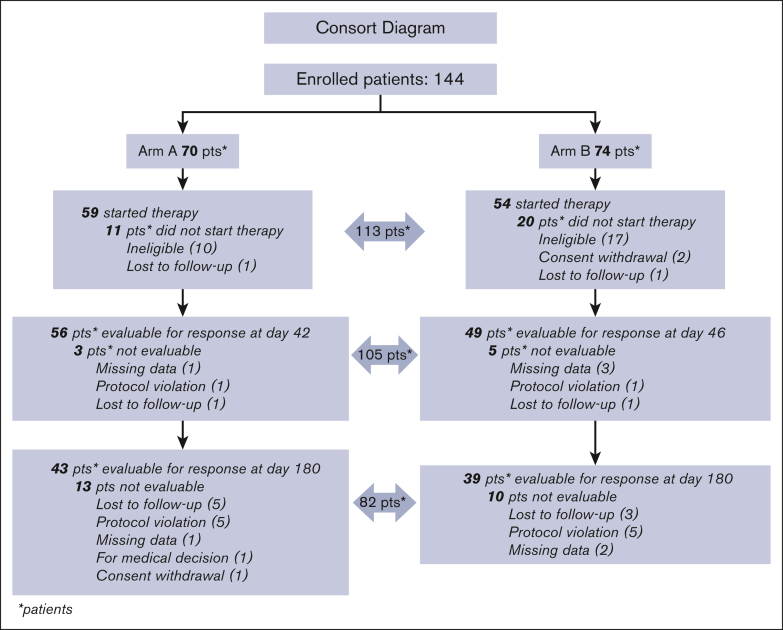
In total, 144 patients with newly diagnosed previously untreated pITP (median time in days elapsed between diagnosis and randomization: 1 day, [range, 0-85 days]) were enrolled in 29 GIMEMA Italian centers: 27 patients were considered ineligible, 2 withdrew informed consent before starting treatment, 2 were lost to follow-up immediately after randomization. Overall, 113 randomized patients were considered evaluable: 43 males (38%) and 70 females (62%); median age of 45 years (range, 18.2-79.6 years); 59 (52.2%) were randomized to Arm A, and 54 (47.8%) to Arm B (Figure 2). At randomization, median platelet count was 8.50 × 109/L (1.00 × 109/L to 48 × 109/L). Bleeding score assessment at diagnosis was available in 95 patients and ranged from 0 to 4 in 76 patients (80%), from 5 to 9 in 14 (14.7%) patients, and from 10 to 15 in 5 (5.3%) patients; 1 patient showed a bleeding score of 15, due to macroscopic hematuria with acute anemia (score 10), and hemorrhagic oral bullae (score 5). Main patient characteristics and type, site, and frequency of bleeding events are described in Table 6.
Figure 2.
CONSORT diagram description of patient flow in both study arms since enrollment to day 180 from the initial response. Causes for exit from the study are also reported.
Table 6.
Evaluable patients’ characteristics
| All | Random |
P value | |||
|---|---|---|---|---|---|
| A | B | ||||
| Patients, n | 113 | 59 | 54 | ||
| Sex | Male | 43 (38%) | 22 (37.3%) | 21 (38.9%) | .8610 |
| Female | 70 (62%) | 37 (62.7%) | 33 (61.1%) | ||
| Age, y | Median (range) | 45 (18.2-79.6) | 42.5 (18.2-79.6) | 48.2 (21.5-78.7) | .0944 |
| Platelet count | Median (range) | 8.5 × 109/L (1-48) | 9.0 × 109/L (1-48) | 8.0 × 109/L (1-27) | .9119 |
| Bleeding score (available in 95 patients) | 0-4 | 76 (80.0%) | 40 (80.0%) | 36 (80.0%) | 1.0000 |
| 5-9 | 14 (14.7%) | 7 (14.0%) | 7 (15.6%) | ||
| 10-15 | 5 (5.3%) | 3 (6.0%) | 2 (4.4%) | ||
| Bleeding site (available in 84 patients) | Cutaneous bleeding | 64 (76.2) | 33 (75.0%) | 31 (77.5%) | .8681 |
| Mucosal bleeding | 15 (17.8) | 9 (20.4%) | 6 (15.0%) | ||
| Gastrointestinal∗ | 2 (2.4) | 1 (2.3%) | 1 (2.5%) | ||
| Genitourinary tract bleeding | 2 (2.4) | 1 (2.3%) | 1 (2.5%) | ||
| Urinary bleeding | 1 (1.2) | 0 (0.0%) | 1 (2.5%) | ||
Not due to active gastric ulcer.
Therapy response
Initial response was assessed at day 42 for Arm A and at day 46 for Arm B. In total, 8 of 113 patients were not evaluable for response; 4 because of missing data, 2 because of protocol violation, and the remaining 2 were lost to follow-up. Therefore, 105 patients (56 in Arm A, and 49 in Arm B) were evaluable (Figure 2). In Arm A, CRs were 35 (62.50%); PRs, 7 (12.50%); MRs, 2 (3.57%); and NRs, 12 (21.43%). In Arm B, CRs were 31 (63.27%); PRs, 11 (22.45%); MRs, 4 (8.16%); and NRs, 3 (6.12%). Total initial responses (CR + PR + MR) were 44 (78.57%) in Arm A, and 46 (93.88%) in Arm B. The difference in initial response rate between the 2 arms was statistically significant (P = .0284), in favor of Arm B (Table 7). In Arm A, 7 of 12 patients who were unresponsive to treatment at day 42 had rescue treatment with HD-DXM: 1 obtained a CR, 3 a PR, 1 did not respond, and 2 were not evaluable. At day 180 from the initial response, 82 patients were evaluable for the final response, 43 in Arm A, and 39 in Arm B. Ten were nonassessable because of protocol violation, 3 because of missing data, 1 because of being out of study for medical decision, 1 for consent withdrawal, and 8 were lost to follow-up (Figure 2). In Arm A, CR was achieved by 22 patients (51.16%), PR by 3 (6.98%), MR by 1 (2.33%), and NR occurred in 12 patients (27.91%); 5 (11.64%) had lost response before day 180. In Arm B, CR was reached by 18 (46.15%) patients, PR by 4 (10.26%), MR by 1 (2.56%), and NR was recorded in 3 (7.69%); 13 (33.33%) had lost response before day 180. Total final responses (CR + PR + MR) were recorded in 26 of 43 (60.47%) patients in Arm A, and 23 of 39 (58.97%) patients in Arm B. The difference in final response rate between the 2 study arms was not statistically significant (P = .8907; Table 7). Overall, 15 patients were unresponsive at day 180; 15 (12 in Arm A and 3 in Arm B): during the following observation, 4 underwent splenectomy, 1 received HD-DXM plus rituximab, 9 received unspecified other treatments, and 1 was lost to follow-up. As far as persistent responses at 12 months from the initial response, 67 patients were evaluable, 31 in Arm A and 36 in Arm B. In Arm A, CRs were recorded in 22 (70.97%) patients, PRs in 1 (3.23%), MRs in 2 (6.45%), and NR in 6 of 31 (19.35%); in Arm B, CRs were recorded in 18 (50.00%) patients, PRs in 2 (5.56%), MR in 0, and NRs in 16 (44.44%). Total persistent responses (CRs + PRs + MRs) were observed in 25 of 31 (80.65%) in Arm A, and in 20 of 36 (55.56%) in Arm B. The difference between the 2 arms was statistically significant (P = .0292) in favor of Arm A (Table 7).
Table 7.
Response to therapy
| Arm A Day 42 (56 evaluable patients) |
Arm B Day 46 (49 evaluable patients) |
P value | ||
|---|---|---|---|---|
| Initial response | CR + PR + MR | 44 (78.57%) | 46 (93.88%) | .0284 |
| NR | 12 (21.43%) | 3 (6.12%) | ||
| Arm A Day 180∗ (43 evaluable patients) |
Arm B Day 180† (39 evaluable patients) |
|||
| Final response | CR + PR + MR | 26 (60.47%) | 23 (58.97%) | .8907 |
| Loss of response + NR | 17 (39.53%) | 16 (41.03%) | ||
| Arm A 12 mo∗ (31 evaluable patients) |
Arm B 12 mo† (36 evaluable patients) |
|||
| Persistent response | CR + PR + MR | 25 (80.65%) | 20 (55.56%) | .0292 |
| NR | 6 (19.35%) | 16 (44.40%) |
From day 42.
From day 46.
The sample size of the study was 150, but we did not reach it. We reassessed power of real sample size: among 144 enrolled patients, 82 were evaluable for final response. We considered the smaller number of evaluable patients in the 2 study arms (39) to recalculate power, and a group sample size of 39 per arm achieved a power of 70.3%.
We performed a post-hoc reevaluation of the initial, final, and persistent responses according to the criteria established by the International Working Group (IWG).1 The definition of CR was the same (platelet count of ≥100 × 109/L, without bleeding), but the IWG defined as response (R) a platelet count of ≥30 × 109/L and doubling of basal count, in absence of bleeding symptoms, whereas neither PR nor MR were considered. With regard the initial response, in both study arms all CRs were confirmed, and all PRs were considered responses (Rs); in Arm A the 2 MRs were reassessed as Rs, whereas in Arm B, 3 of 4 MRs were reassessed as Rs and 1 of 4 as NR. In this latter case, the patient, according to our protocol criteria, had been considered to have had a MR because he had a platelet count of 26 × 109/L, no bleeding symptoms, and showed platelet levels higher than twice that at baseline (6 × 109/L). Regarding the initial response, the difference between the combined data of CRs + Rs and of NRs in the 2 study arms, is no longer statistically significant (P = .0592). With regard the final response, all CRs were confirmed in both arms, PRs and the 2 MRs (1 for each Arm) were reassessed as Rs; therefore, the overall result remained unchanged (P = .8907). With regard persistent responses in both study arms, all CRs were confirmed, PRs and MRs were all evaluated as Rs. Difference between study arms was statistically significant (P = .0292; Table 8). In total, 7 relapses occurred: 2 in Arm A and 5 in Arm B at a median time of 15.13 (range, 5.69-24.57 months) and of 5.13 months (range, 0.72-37.70 months) from the final response, respectively. Median follow-up was 44.4 months (interquartile range, 15.7-79.9); range, 0.9-114.0 months; overall survival was estimated to be 100% (95% confidence interval [CI], 100-100) at 48 months. One death occurred 64.5 months after diagnosis due to sepsis in a 78.6-year-old woman in Arm B who had relapsed. In patients who achieved final response, overall DFS was estimated to be 81.11% (95% CI, 69.20-95.06) at 48 months from day 180, with 89.84% (95% CI, 77.34-100.00) in Arm A and 70.88% (95% CI, 51. 80-96.79) in Arm B.
Table 8.
Response to therapy according to IWG criteria
| Arm A Day 42 (56 evaluable patients) |
Arm B Day 46 (49 evaluable patients) |
P value | ||
|---|---|---|---|---|
| Initial response | CR + R | 44 (78.57%) | 45 (91.84%) | .0592 |
| NR | 12 (21.43%) | 4 (8.16%) | ||
| Arm A Arm B Day 180∗ Day 180† (43 evaluable patients) (39 evaluable patients) | ||||
| Final response | CR + R | 26 (60.47%) | 23 (58.97%) | .8907 |
| Loss of response + NR | 17 (39.53%) | 16 (41.03%) | ||
| Arm A Arm B Day 180∗ Day 180† (31 evaluable patients) (36 evaluable patients) | ||||
| Persistent response | CR + R | 25 (80.65%) | 20 (55.56%) | .0292 |
| NR | 6 (19.35%) | 16 (44.44%) | ||
Adverse drug reactions and adverse events
Nine patients experienced ≥1 adverse drug reactions (7 in Arm A, and 2 in Arm B); in 7 of 9 patients adverse drug reactions occurred before day 180, whereas in the other 2 (1 in Arm A, and 1 in Arm B) it occurred successively. In most cases, toxicities appeared to be attributable to the expected effects of corticosteroids (Table 9). Three patients each experienced a severe adverse event: in Arm A, 1 patient experienced depression (26 days after the start of therapy) probably related to treatment, and 1 patient had bronchopneumonia (13 days after the start of therapy) possibly related to PDN; in Arm B, 1 patient experienced appendicitis (34 days after therapy start), considered unrelated to HD-DXM. No major bleeding occurred during follow-up.
Table 9.
Adverse drug reactions
| Grade | Arm |
|||
|---|---|---|---|---|
| A | B | Total events | ||
| Retinal toxicity | 3 | 1 | 0 | 1 |
| Edemas | 1 | 0 | 1 | 1 |
| 2 | 3 | 0 | 3 | |
| Insomnia | 2 | 4 | 1 | 5 |
| 3 | 1 | 0 | 1 | |
| Hypertension | 3 | 1 | 0 | 1 |
| Weight gain | 2 | 5 | 0 | 5 |
| 3 | 2 | 0 | 2 | |
| Fluid retention | - | 1 | 0 | 1 |
| Cushing's syndrome | - | 1 | 0 | 1 |
Grading according to the National Cancer Institute: Common Toxicity Criteria Adverse Events, version 3.0.20
Discussion
The aim of our study was to compare the efficacy of pulsed HD-DXM vs standard-dose PDN in adult patients with newly diagnosed, previously untreated pITP. The rationale of this protocol was based on the favorable results of a nonrandomized multicenter GIMEMA pilot study.17
Over a period of ∼8 years, we reached the high number of 144 enrolled patients. However, we did not reach the estimated sample size of 150 patients and the power of study was estimated to be 70.3% at final response. In 2016, the last patients were enrolled, and, according to the protocol, every patient should have been followed-up for at least 36 months. Soon after the last controls of the last patients in 2019, there has been the COVID-19 pandemic, therefore, there was a substantial delay in data analysis and, consequently, in the writing of this report. We emphasize that enrollment criteria were extremely strict because the platelet count had to be ≤20 × 109/L or >20 × 109/L but ≤50 × 109/L if the bleeding score was ≥8. Establishing the platelet count cutoff of ≤20 × 109/L spared patients without bleeding symptoms and with a platelet count of >20 × 109/L but ≤30 × 109/L from starting treatment too early and allowed a more complete diagnosis and the possibility of a spontaneous increase in platelet count. All other causes of thrombocytopenia had to be excluded and this can explain the reason for some screening failures. We underline that bleeding score at diagnosis ranged from 0 to 4 in 80% of patients, and from 10 to 15 in only 5.3% of patients (Table 6). Assessment of bleeding score was performed following the grading scale of Khellaf et al19 because we considered this the most suitable tool for describing sites and severity of bleeding in pITP (Table 3). In contrast, when our study was launched, the IWG had not yet published its proposal for standardization of bleeding assessment in ITP.23 We point out that most patients had mild or no hemorrhagic symptoms at diagnosis and that no severe bleeding events were recorded during follow-up. Regarding the assessment of the initial response rate, total responses were higher in Arm B (93.88%) than in Arm A (78.57%; P = .0284), showing a better initial response with HD-DXM. Final responses were similar (60.47% in Arm A and 58.97% in Arm B) without a statistically significant difference (P = .8907; Table 7). These results seem to indicate that HD-DXM allows an effective initial response but a response less long lasting over time than that obtained with PDN. Of note, several patients were not evaluable for initial and especially for final response (Figure 2). Overall persistent responses were higher in Arm A (80.65%) than in Arm B (55.56%; P = .0292), but it must be considered that the patients evaluable for persistent response were few, 31 and 36 in Arm A and B, respectively (Table 7). Do these findings suggest an advantage of PDN therapy in achieving long-lasting responses? The HD-DXM therapeutic regimen chosen by us derives from the promising results of the GIMEMA pilot study, in which the best result was obtained with the administration of 3 HD-DXM courses.17 With regard pharmacological considerations, DXM has a longer half-life and considerably less mineralocorticoid activity than PDN. Repeated HD-DXM pulses might be able to counteract the autoimmune response in ITP in the early disease phase, with fewer side effects.24
The response criteria we used were the same as in the pilot study,17 but new criteria were established by the IWG1 in 2009, after our study had already begun. Of note, our definition of “final response” (sustained without interruption or rescue therapy at the 180th day after the initial response), is comparable with the “sustained response,” more recently defined as a platelet count of ≥30 × 109/L, maintained for 6 consecutive months from the achievement of the initial response, without any treatment and without bleeding symptoms, according to recent publications.25, 26, 27 The reassessment of the initial response according to the IWG criteria1 showed that the difference between study arms was no longer statistically significant in favor of Arm B (Table 8), because 1 patient with MR was considered unresponsive because his platelet count was <30 × 109/L, although he was asymptomatic and his platelet count had doubled from the baseline level. However, it is rather difficult to consider that this patient truly unresponsive to therapy. With regard final and persistent responses, the results were unchanged (Table 8).
Whether standard-dose PDN or HD-DXM should be chosen as initial treatment of ITP remains an open and relevant question, especially considering that corticosteroids are widely believed to be the most suitable first-line approach to ITP.4, 5, 6, 7, 8 Mithoowani et al13 performed a large review and a meta-analysis concerning patients with newly diagnosed pITP, to determine long-term response to HD-DXM compared with standard-dose PDN.25,28, 29, 30, 31 At the 14th day, overall platelet response was higher in the HD-DXM group, whereas long-term responses were similar in both groups. They concluded that HD-DXM might be preferred when a rapid increase of platelet count is required. Notably, this review includes the randomized study by Wei et al,25 regarding 192 patients whose initial response was better and faster in the arm treated with HD-DXM. In another prospective randomized study, HD-DXM induced significantly higher long-term remissions than standard-dose PDN.32 A more recent randomized study found that HD-DXM led to significantly higher initial response and SR rates than standard-dose PDN.33 There are also nonrandomized studies. In 1, PDN appeared to be superior to pulsed HD-DXM in achieving a SR.34 In another, HD-DXM vs standard-dose PDN provided an effective and faster response to initial treatment, SR at 6 months was better in the PDN-treated cohort but similar in both groups at 12 months.26 A retrospective study aimed to assess whether the long-term outcome of newly diagnosed ITP would be improved by using HD-DXM with sequential PDN maintenance therapy. Response was obtained in most cases and the authors considered this approach a suitable first-line treatment.27
Strengths of our study are the very long follow-up (median, 44.4 months; range, 0.9-114.0 months), the low number of relapses (more in Arm B than in Arm A; 5 vs 2), and the DFS of 81.11% at 48 months.
Both standard-dose PDN and pulsed HD-DXM were well tolerated: in fact, only 9 patients experienced ≥1 adverse drug reactions, most in Arm A (7 patients; Table 9). Overall, 2 of 3 severe adverse events were considered related to PDN therapy. The low prevalence of adverse drug reactions demonstrates that a proper management of corticosteroids allows a good therapeutic efficacy and excellent safety, both in short-term and long-term follow-up. However, we emphasize that HD-DXM courses were better tolerated than standard-dose PDN, as also reported in the literature.13,25,26
From our experience and literature data, it appears that corticosteroids, in particular HD-DXM, can currently be considered an appropriate first-line therapy for adult pITP. Is it desirable to improve the initial treatment by combining other drugs with DXM? Early inclusion of other drugs has been considered immediately after ≥1 HD-DXM courses. Firstly, rituximab was proposed. In 2 randomized studies, HD-DXM plus standard-dose rituximab vs HD-DXM alone, resulted in higher SR rates in both studies.35,36 A meta-analysis of 11 randomized studies, showed similar results.37 In another meta-analysis, HD-DXM plus rituximab was considered a suitable alternative to traditional therapy in improving patients’ long-term outcome; however, an increase in adverse events was noted.38
The combination of the thrombopoietin-receptor agonist eltrombopag with HD-DXM was considered for the first time in a pilot study as a valid frontline alternative for adult ITP: this combination was safe and effective in achieving a lasting response in a small group of patients.39 Regimens containing recombinant human thrombopoietin agonist, appeared to be beneficial up-front therapy in addition to the conventional corticosteroid monotherapies.40 Comparable results were achieved with eltrombopag plus pulsed HD-DXM.41 In a randomized study, HD-DXM plus recombinant human thrombopoietin vs HD-DXM alone significantly improved initial response and yielded a favorable SR.42
In conclusion, from our experience and literature data, corticosteroids are confirmed as efficacious and safe first-line therapy for adults with newly diagnosed pITP; HD-DXM exhibits a better initial response, whereas PDN seems to increase long-lasting responses. Limits of our study are a long period of patient enrollment, response criteria different from those from IWG, and the progressive reduction of evaluable patients for response throughout the follow-up period. For future studies, a combination of HD-DXM with other effective agents such as thrombopoietic agents seems worth of further investigation.
Conflict-of-interest disclosure: C.S. participated in advisory boards for Amgen, Novartis, and Sobi, and was a member of the speakers' bureaus for Amgen, Novartis, and Sobi. E.B. participated in advisory boards for Amgen and Novartis. F.P. received honoraria from Novartis and Sobi. E. Rivolti participated in advisory boards for Novartis and Sobi, and reports presentations for Novartis and Amgen. A.P. was a consultant for Sanofi, Alexion, and Takeda; reports presentations for Sobi, Novartis, Pfizer, and Sanofi; and received congress fees from Alexion and Takeda. M.D. participated in advisory boards for Amgen and Novartis. S. Siragus collaborated with Sobi, Bayer, Amgen, Novartis, and Novo Nordisk. V.D.S. participated in advisory boards for Argenx, Grifols, Novartis, and Sobi, and was a member of the speakers' bureaus for Amgen, Grifols, and Novartis. E. Rossi participated in advisory boards for Argenx, Grifols, Novartis, Amgen, and Sobi, and was a member of the speakers' bureaus for Amgen, Novartis, and Sobi. F.Z. received personal fees from Novartis, Amgen, Roche, AbbVie, Janssen Cilag, Takeda, Grifols, Argenx, Sobi, and BeiGene; received funding for biologic studies or clinical trials from Novartis, Amgen, AbbVie, and Janssen Cilag; and received fundings for educational projects from Janssen Cilag, AbbVie, Kyowa Kirin, Takeda, BeiGene, and AstraZeneca. M.B. received conference fees from Incyte, AstraZeneca, Novartis, and Janssen. M.C. was a member of the speakers' bureaus for Novartis and Bristol Myers Squibb. A.T. received congress fees from Novartis and research funds from Janssen. N.V. participated in advisory boards and was speaker for Novartis, Amgen, Grifols, and Sobi. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank the Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) Data Centre for the collection of data relating to the study and their analysis, and Pharmacovigilance and Regulatory Affairs units. All members of the writing committee wish to remember Franco Mandell, full professor and emeritus professor of Hematology at the Sapienza University of Rome and director of the Hematology Institute of the Policlinico Umberto I in Rome, who passed away in 2018. He was the founder and president of GIMEMA. He approved this study and followed its first stages.
Authorship
Contribution: M.G.M. wrote the manuscript; M.G.M., F.R., G.A., V.D.S., L.G., M.R., N.V., and P.F. were members of the study's writing committee and created the concept and the design of the study, all of whom reviewed the manuscript and agreed to publish it; C.S. contributed to the drafting of the manuscript and approved it for publication; F. Paoloni performed the statistical design and analysis; V.S. collected and assembled the data and performed project management activities; and E.B., A.F., B.M., I.D.V., G.C., S.F., M.D.I., P.R., F. Palandri, N.P., E.L., E. Rivolti, A.P., A.R., M.D., M.G., S. Siragusa, S. Sibilla, A.M.C., E. Rossi, R.B., F.Z., M.B., N.D.R., P.M., M.C., A.C.G., M.K., and A.T. provided study material and recruited patients, reviewed the manuscript, and approved the final version and its publication.
Footnotes
For original study data, deidentified individual participant data, and full study protocol, contact the GIMEMA Foundation (www.gimema.it).
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an International Working Group. Blood. 2009;113(11):2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235–244. doi: 10.1111/j.1365-2141.2009.07615.x. [DOI] [PubMed] [Google Scholar]
- 3.Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94(3):909–913. [PubMed] [Google Scholar]
- 4.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 5.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA, American Society of Hematology The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 6.Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia – current diagnostics and therapy: recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(suppl 5):1–30. doi: 10.1159/000492187. [DOI] [PubMed] [Google Scholar]
- 7.Provan D, Arnold DM, Bussel JB, et al. Updated International Consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzucconi MG, Francesconi M, Fidani P, et al. Treatment of idiopathic thrombocytopenic purpura (ITP): results of a multicentric protocol. Haematologica. 1985;70(4):329–336. [PubMed] [Google Scholar]
- 10.Bellucci S, Charpak Y, Chastang C, Tobelem G. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71(4):1165–1169. [PubMed] [Google Scholar]
- 11.George JN, el-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1994;331(18):1207–1211. doi: 10.1056/NEJM199411033311807. [DOI] [PubMed] [Google Scholar]
- 12.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura. Blood. 2005;106(7):2244–2251. doi: 10.1182/blood-2004-12-4598. [DOI] [PubMed] [Google Scholar]
- 13.Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3(10):e489–e496. doi: 10.1016/S2352-3026(16)30109-0. [DOI] [PubMed] [Google Scholar]
- 14.Umakanthan JM, Dhakal P, Gundabolu K, Kallam A, Almquist DR, Bhatt VR. Initial management of immune thrombocytopenia in adults based on risk stratification. Postgrad Med J. 2019;95(1128):558–562. doi: 10.1136/postgradmedj-2019-136636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen JC. Response of resistant idiopathic thrombocytopenic purpura to pulsed high-dose dexamethasone therapy. N Engl J Med. 1994;330(22):1560–1564. doi: 10.1056/NEJM199406023302203. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Wong RS, Soo YO, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349(9):831–836. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 17.Mazzucconi MG, Fazi P, Bernasconi S, et al. Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) Thrombocytopenia Working Party Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109(4):1401–1407. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 18.IFPMA ICH harmonized tripartite guideline for good clinical practice. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf [PubMed]
- 19.Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90(6):829–832. [PubMed] [Google Scholar]
- 20.National Cancer Institute Common toxicity criteria version 3.0.(CTCAEv3.0) https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf [PubMed]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Minor BL, et al. REDCap Consortium The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121(14):2596–2606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 24.Cuker A, Prak ET, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost. 2015;41(4):395–404. doi: 10.1055/s-0034-1544001. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–370. doi: 10.1182/blood-2015-07-659656. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Xu L, Hao H, et al. First line treatment of adult patients with primary immune thrombocytopenia: a real-world study. Platelets. 2020;31(1):55–61. doi: 10.1080/09537104.2019.1572875. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Zhang X, Feng S, et al. Clinical efficacy of high-dose dexamethasone with sequential prednisone maintenance therapy for newly diagnosed adult immune thrombocytopenia in a real-world setting. J Int Med Res. 2021;49(4) doi: 10.1177/03000605211007322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Praituan W, Rojnuckarin P. Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 2009;7(6):1036–1038. doi: 10.1111/j.1538-7836.2009.03359.x. [DOI] [PubMed] [Google Scholar]
- 29.Bae SH, Ryoo HM, Lee WS, et al. High dose dexamethasone vs. conventional dose prednisolone for adults with immune thrombocytopenia: a prospective multicenter phase III trial. Blood. 2010;116(21):3687. [Google Scholar]
- 30.Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 2012;20(1):7. doi: 10.1186/2008-2231-20-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Din B, Wang X, Shi Y, Li Y. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015;133(1):124–128. doi: 10.1159/000362529. [DOI] [PubMed] [Google Scholar]
- 32.Matschke J, Müller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 Study. Acta Haematol. 2016;136(2):101–107. doi: 10.1159/000445420. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghi A, Hosseini SF, Jouzdani SR. Evaluation of treatment plan by three-period pulses of high-dose dexamethasone among patients with primary immune thrombocytopenia on platelet count response and adverse events: a randomized Clinical trial. J Res Med Sci. 2020;25:88. doi: 10.4103/jrms.JRMS_257_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakazaki K, Hosoi M, Hangaishi A, Ichikawa M, Nannya Y, Kurokawa M. Comparison between pulsed high-dose dexamethasone and daily corticosteroid therapy for adult primary immune thrombocytopenia: a retrospective study. Intern Med. 2012;51(8):859–863. doi: 10.2169/internalmedicine.51.7005. [DOI] [PubMed] [Google Scholar]
- 35.Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755–2762. doi: 10.1182/blood-2009-07-229815. [DOI] [PubMed] [Google Scholar]
- 36.Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11):1976–1981. doi: 10.1182/blood-2012-09-455691. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Li Y, Wang C, et al. Efficacy and safety of the combination treatment of rituximab and dexamethasone for adults with primary immune thrombocytopenia (ITP): a meta-analysis. BioMed Res Int. 2018;2018 doi: 10.1155/2018/1316096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Q, Lin B, Wang H, Zhan W, Chen P. The efficacy of high-dose dexamethasone vs. other treatments for newly diagnosed immune thrombocytopenia: a meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.656792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Almaguer D, Herrera-Rojas MA, Jaime-Pérez JC, et al. Eltrombopag and high-dose dexamethasone as frontline treatment of newly diagnosed immune thrombocytopenia in adults. Blood. 2014;123(25):3906–3908. doi: 10.1182/blood-2014-01-549360. [DOI] [PubMed] [Google Scholar]
- 40.Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Comparison of up-front treatments for newly diagnosed immune thrombocytopenia - a systematic review and network meta-analysis. Haematologica. 2018;103(1):163–171. doi: 10.3324/haematol.2017.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Zhang M, Du X, Cheng Y, Cheng G. Safety and efficacy of eltrombopag plus pulsed dexamethasone as first-line therapy for immune thrombocytopenia. Br J Haematol. 2020;189(2):369–378. doi: 10.1111/bjh.16327. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Wang M, Hou Y, et al. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: a prospective, multicenter, randomized trial. Am J Hematol. 2020;95(12):1542–1552. doi: 10.1002/ajh.25989. [DOI] [PubMed] [Google Scholar]