Abstract
Heart failure with preserved ejection fraction (HFpEF) has a high prevalence, affecting more than 50% of patients with heart failure. HFpEF is associated with multiple comorbidities, and obesity is one of the most common. A distinct phenotype has been proposed for obese patients with HFpEF. Recent data show the beneficial role of glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) for weight loss in diabetic and non‐diabetic patients with obesity or overweight when given as adjunctive therapy to diet and exercise. The mechanisms of action are related to paracrine and endocrine signalling pathways within the gastrointestinal tract, pancreas, and central nervous system that delay gastric emptying, decrease appetite, augment pancreatic beta‐cell insulin secretion, and suppress pancreatic glucagon release. These drugs are therefore potentially indicated for treatment of patients with HFpEF and obesity or overweight. Efficacy and safety need to be shown by clinical trials with a first one, Semaglutide Treatment Effect in People with obesity and heart failure with preserved ejection fraction (STEP HFpEF), recently concluded. The aim of the present review is to provide the pathophysiological and pharmacological rationale for GLP‐1 RA administration to obese patients with HFpEF.
Keywords: Heart failure with preserved ejection fraction, Glucagon‐like peptide‐1 receptor agonists, Obesity
Background: role of obesity in heart failure with preserved ejection fraction
The prevalence of heart failure (HF) with preserved ejection fraction (HFpEF) is around 4.9% in the general population aged over 60 years, and HFpEF affects more than 50% of the patients admitted for HF. 1 , 2 , 3 , 4 Thus, several millions of people are affected by HFpEF in Europe and the United States. The prevalence of obesity is growing in many developed countries. In the United States, more than 40% of the general population is obese, and it is projected that at least half of the population will be obese in 2030. 5 , 6 A specific and independent relationship exists between obesity and HFpEF so that these patients have peculiar clinical and haemodynamic features and obesity may be considered not a mere comorbidity but rather a direct cause of HFpEF itself. 7 , 8 , 9 , 10 Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) have recently been shown to be an effective treatment of obesity and diabetes. They are therefore potentially useful, if not of choice, for the patients with HFpEF and obesity. 9 , 11 This article will review the rationale for this treatment.
The obesity heart failure with preserved ejection fraction phenotype
Mechanisms
Obesity leads to a biological transformation of the adipose tissue towards an inflammatory state, and this may have adverse effects on the structure and function of the vasculature and most visceral organs. 12 , 13 Expansion of visceral adipose tissue causes oxidative stress, release of pro‐inflammatory adipokines, activation of renin–angiotensin–aldosterone system, adipocyte apoptosis, autophagy, and gut microbiota dysbiosis: these mechanisms lead to insulin resistance with type 2 diabetes mellitus (T2DM), dyslipidaemia, increased vascular stiffness and hypertension, coronary artery disease, and eventually HF, namely, HFpEF. 14 , 15 , 16
Inflammation can also cause microvascular impairment and fibrosis in the heart and also in the lungs, kidneys, liver, pancreas, and skeletal muscle, leading to the characteristic comorbidities of HFpEF. 17 , 18 , 19 , 20 , 21 Coronary microvascular endothelial dysfunction is observed with increased expression of endothelial adhesion molecules in myocardial biopsy samples of HFpEF patients, including vascular cell adhesion molecule and E‐selectin. 22 , 23 Pro‐inflammatory cytokines are also known to elicit endothelial production of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidases. 24 This can cause the high nitrosative/oxidative stress, which has been observed in HFpEF myocardium 22 , 25 and which is also exacerbated in typical comorbidities of HFpEF patients, such as T2DM, and physiological processes, such as ageing. 26 , 27
Moreover, it has been shown that overall obesity and higher amount of visceral adipose tissue are associated with greater abnormalities in cardiac structure and function, with higher left ventricular (LV) mass, greater LV concentric hypertrophy, and higher degree of LV diastolic dysfunction. 28 A higher amount of adipose tissue is also associated with plasma volume expansion and impairment in LV relaxation potentially through systemic inflammation. This may contribute to limited ventricular distensibility, higher LV filling pressures, and signs and symptoms of HF. 29 , 30 , 31 , 32
Obesity also affects both resting and exercise‐related respiratory physiology. Severe obesity classically produces a restrictive ventilatory abnormality. 33 A peculiarity in these subjects is that decreased peak work rates are usually seen in a setting of normal or decreased ventilatory reserve and normal cardiovascular (CV) response to exercise. 34 , 35 , 36
On the other hand, even asymptomatic severely obese subjects may develop abnormal echocardiographic indices of LV diastolic filling during exercise, as compared with matched lean controls. 37 This may represent a subclinical form of cardiomyopathy in obese subjects. Considering the poor prognosis of HFpEF in obese patients, we believe that the early identification of these patients and their relatively targeted treatment could represent the turning point in the natural history of the pathology.
Clinical characteristics
There is a high prevalence of T2DM in patients with HFpEF, and the presence of T2DM has been shown to increase mortality of patients with HFpEF by 30–50% even after adjustment for age, gender, hospital factors, and other patient characteristics. Unlike HF with reduced ejection fraction (HFrEF), HFpEF has distinct clinical phenotypes, and the obese–diabetic phenotype is the most often encountered phenotype in clinical practice. 38 , 39
In the Phosphodiesterase‐5 inhibition to improve clinical status and Exercise capacity in Diastolic HF (RELAX) trial, 40 body mass index (BMI) was 37.1 vs. 30.7 kg/m2 in patients with and without T2DM. In this category of patients, LV remodelling was more relevant and associated with reduced ventricular compliance with increased systemic and pulmonary venous pressures and congestion despite preserved systolic function. 41
In addition to the systemic inflammatory state, obesity is also associated with peculiar abnormalities in patients with HFpEF (Figure 1 ). Specific circulating biomarkers patterns have been identified in obese HFpEF patients, supporting the clinical definition of a distinct obese HFpEF phenotype. 42 Obese HFpEF patients exhibit higher circulating biomarkers of volume expansion [adrenomedullin (ADM)], myocardial fibrosis (thrombospondin‐2), and systemic inflammation (galectin‐9 and glycoprotein CD4) compared with obese non‐HFpEF or lean HFpEF patients. 42 With the only exception of CD4, these proteins were linearly related with increased left atrial (LA) pressure. Importantly, ADM and CD4 were associated with increased mortality in obese HFpEF patients. 42
Figure 1.
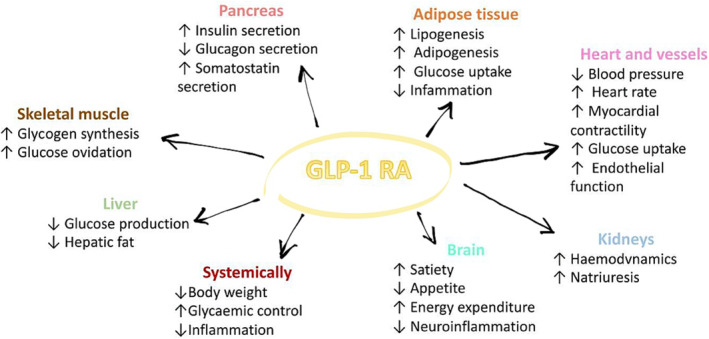
Direct and indirect effects of glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) in heart failure with preserved ejection fraction (HFpEF). The figure shows currently known or suggested direct and indirect effects of GLP‐1 RA in HFpEF in all human organs.
The characteristics of the obese patients with HFpEF were compared with those of the normal subjects and with those of the non‐obese patients with HFpEF, thus showing the peculiarity of the obese HFpEF phenotype. Obese patients with HFpEF had an increased plasma volume, epicardial fat thickness, and total heart volume. LV mass was increased with concentric LV hypertrophy, and right ventricular (RV) volume was larger with more severe RV dysfunction. The increase in heart volume and in ventricular interdependence was attended by an increased ratio of right‐ to left‐sided heart filling pressures, higher pulmonary venous pressure, relative to LV transmural pressure, and greater LV eccentricity index, defined as the ratio of the anterior–inferior and septal–posterolateral cavity dimensions at the mid‐ventricular level. Pulmonary capillary wedge pressure was slightly but significantly correlated with body mass and plasma volume in obese HFpEF (r = 0.22 and 0.27, both P < 0.05) but not in non‐obese HFpEF (P ≥ 0.3). 10 Venous compliance is decreased, thus contributing, in addition to the increased blood volume, to increased filling pressure and peripheral congestion. 15
Compared with the non‐obese HFpEF patients and control subjects, obese patients with HFpEF displayed worse exercise capacity (peak oxygen consumption, 7.7 ± 2.3 vs. 10.0 ± 3.4 and 12.9 ± 4.0 mL/kg/min; P < 0.0001), higher biventricular filling pressures with exercise, and impaired pulmonary artery vasodilator reserve. 10
Along with abnormalities related to obesity, increased epicardial adipose tissue (EAT) has been shown to be associated with cardiac abnormalities and represents a pathological feature of obese HF patients. 43 , 44 , 45 Among obese patients with HFpEF, the presence of increased EAT is associated with greater haemodynamic impairment at rest and exercise, with a greater elevation in cardiac filling pressures, more severe pulmonary hypertension, and greater pericardial restraint. 46 The greater external restraint on the heart may alter the relationship between intravascular pressures and stress markers among obese patients complaining dyspnoea. In this context, standard biomarkers, namely, natriuretic peptides, may underestimate circulatory congestion leading to under‐recognition of its clinical signs in patients with obesity. 47
EAT is greater in obese HFpEF patients compared with the HFrEF ones and is associated with worse LA and LV function as shown by echocardiographic strain analysis. 48 Among HFpEF patients, increased EAT was also associated with worse haemodynamic and metabolic profile expressed by proteomic markers of inflammation, insulin resistance, and endothelial dysfunction, 45 expressed by effort intolerance, and impaired left atrioventricular and right ventriculo‐arterial coupling. 49
Glucagon‐like peptide‐1
Glucose homeostasis is dependent upon a complex interplay of multiple hormones: (i) insulin and amylin, produced by pancreatic beta cells; (ii) glucagon, produced by pancreatic alpha cells; and (iii) gastrointestinal peptides, including glucagon‐like peptide‐1 (GLP‐1), and gastric inhibitory polypeptide, a glucose‐dependent insulinotropic polypeptide. 50 The role of GLP‐1 in glucose homeostasis is related to its incretin effect and is shown by the greater stimulatory effect on insulin secretion of oral glucose compared with intravenous glucose, as GLP‐1 is released from intestinal L cells in response to nutrients. 51
GLP‐1 is produced from the proglucagon gene in L cells of the small intestine and is secreted in response to nutrients and binds to a specific GLP‐1 receptor, which is expressed in various tissues, including pancreatic beta cells, kidney, lung, heart, brain, gastric mucosa, and other organs. 52 GLP‐1 exerts its main effect by stimulating glucose‐dependent insulin release from the pancreatic islets, but it also slows gastric emptying and inhibits inappropriate post‐meal glucagon release, thus also reducing food intake. The satiety effect of GLP‐1 may involve both meal entero‐enteric reflexes and central signalling mechanisms that mediate changes in appetite and promote satiety. 53 , 54 Given its effects on slowed gastric emptying and on appetite centres in the hypothalamus, therapy with GLP‐1 and its receptor agonists is associated with weight loss (Figure 1 ). 55
Glucagon‐like peptide‐1 receptor agonists
Synthetic GLP‐1 RAs are variably resistant to degradation by the enzyme dipeptidyl peptidase 4 and therefore have a longer half‐life, with consequent favourable pharmacological effects. They bind to the GLP‐1 receptor and stimulate glucose‐dependent insulin release from the pancreatic islets, as described above. They do not usually cause hypoglycaemia in the absence of therapies that otherwise can cause it. 56
Results in patients with type 2 diabetes
GLP‐1 receptor agonists reduce the risk of myocardial infarction (MI), stroke, and CV death in patients with T2DM. 11 , 57 Randomized controlled trials (RCTs) have demonstrated a reduction in CV events with liraglutide, 58 once‐weekly semaglutide, 59 dulaglutide, 60 and albiglutide, 61 whereas lixisenatide, extended‐release exenatide, and oral semaglutide showed a neutral effect (Table 1 ). 65 , 66 , 67
Table 1.
Randomized controlled trials of GLP‐1 RA for body weight reduction in patients with and without T2DM
| Trial | Design | Patients | Active drug | Patients | Main results |
|---|---|---|---|---|---|
| NCT00422058 62 | Double‐blind, placebo‐controlled trial | 564 | Liraglutide (1.2, 1.8, 2.4, or 3.0 mg) or placebo administered once a day subcutaneously, or orlistat three times a day orally | BMI of 30–40 kg/m2 and fasting plasma glucose ≤7 mmol/L at run‐in | Weight loss was proportional to liraglutide dose (mean 4.8–7.2 kg). With the highest doses of liraglutide (2.4 and 3.0 mg), there was higher weight lost compared with orlistat |
| SCALE Maintenance 63 | Randomized, double‐blind, placebo‐controlled trial | 422 | Liraglutide 3.0 mg/day or placebo (subcutaneous administration) for 56 weeks | BMI ≥ 30 or ≥27 kg/m2 with dyslipidaemia and/or hypertension | Estimated weight loss difference of 6.1% in the liraglutide group (P < 0.001) |
| SCALE Obesity and Prediabetes 64 | Randomized, double‐blind, placebo‐controlled trial | 731 | Liraglutide 3 mg once daily vs. placebo injection | BMI ≥ 30 or ≥27 kg/m2 with dyslipidaemia or hypertension | Estimated weight loss difference of 5.6% in the liraglutide group (P < 0.001) |
| LEADER 58 | Multicentre, double‐blind, placebo‐controlled trial | 9340 | 1.8 mg of liraglutide or placebo | ≥50 years with at least one CV coexisting condition or an age of ≥60 years with at least one CV risk factor | Weight loss was 2.3 kg higher in the liraglutide group |
| SUSTAIN‐6 59 | Randomized, double‐blind, placebo‐controlled, parallel‐group trial | 3297 | 0.5 or 1.0 mg of once‐weekly subcutaneous semaglutide or placebo | ≥50 years with CV disease, chronic HF (NYHA II or III) or CKD of stage ≥3, or ≥60 years with at least one CV risk factor | Weight loss was greater in patients taking higher doses of semaglutide (P < 0.001) |
| PIONEER 6 65 | Randomized, placebo‐controlled, Phase 3a trial | 3183 | Once‐daily oral semaglutide (14 mg) or placebo | Established CV disease or CKD if ≥50 years or with at least one cardiovascular risk factor if ≥60 years | Weight loss was 3.4 kg higher in the semaglutide group |
| REWIND 60 | Randomized, double‐blind, placebo‐controlled trial | 9901 | Weekly subcutaneous dulaglutide (1.5 mg) or placebo |
|
Lower least squares mean body weight of 1.46 kg in the dulaglutide group (P < 0.001) |
| ELIXA 66 | Multicentre, randomized, double‐blind, placebo‐controlled trial | 6068 | 10–20 μg of subcutaneous lixisenatide or placebo in addition to other diabetes medications | T2DM and either an MI or hospitalization for unstable angina in the past 180 days | Weight loss was 0.6 kg higher in the lixisenatide group (P < 0.001) |
| EXSCEL 67 | Randomized, double‐blind, placebo‐controlled, event‐driven trial | 14 752 | Subcutaneous injections of extended‐release exenatide at a dose of 2 mg or placebo once weekly | T2DM with or without previous CV events | Lower least squares mean of 1.27 kg in the exenatide group (P < 0.001) |
| FREEDOM 68 | Non‐inferiority, randomized controlled trial | 4156 | Subcutaneous exenatide (ITCA 650) or placebo | T2DM with or at risk for atherosclerotic CV disease | Weight loss was higher in the exenatide group (−4.24 kg; P < 0.001) |
| AMPLITUDE‐O 69 | Randomized, placebo‐controlled trial | 4076 | Weekly doses of efpeglenatide or placebo | T2DM and an HbA1c ≥ 7%, with history of CV disease (defined as CAD, stroke, or PAD) or if they had CKD and at least one additional CV risk factor | Weight loss was higher in the efpeglenatide group (2.6 kg, P < 0.001) |
| Harmony Outcomes 61 | Randomized, double‐blind, placebo‐controlled trial | 9463 | Subcutaneous albiglutide (30–50 mg) or of a matched volume of placebo once a week | ≥40 years old with T2DM and established CAD, cerebrovascular disease, or PAD with an HbA1c ≥ 7% | Weight loss was higher in the albiglutide group (−0.66 kg at 8 months and −0.83 kg at 16 months) |
| STEP 1 70 | Randomized, double‐blind, placebo‐controlled trial | 1961 | Once‐weekly subcutaneous 2.4 mg semaglutide or placebo, plus lifestyle intervention | Adults without T2DM and a BMI of ≥30 kg/m2 (or ≥27 with ≥1 weight‐related comorbidity) | Estimated weight loss difference of 12.4% in the semaglutide group (P < 0.001) |
| STEP 2 71 | Randomized, double‐blind, double‐dummy, placebo‐controlled, Phase 3 trial | 1210 | Semaglutide 2.4 mg or semaglutide 1.0 mg or placebo once a week for 68 weeks, plus a lifestyle intervention | BMI ≥ 27 kg/m2 and HbA1c 7–10% and diagnosis of T2DM at least 180 days before screening | Estimated weight loss difference of 6.2% in the semaglutide group (P < 0.001) |
| STEP 3 72 | Randomized, double‐blind, parallel‐group, 68 week, Phase 3a study | 611 | Once‐weekly subcutaneous semaglutide 2.4 mg vs. placebo as an adjunct to intensive behavioural therapy with initial low‐calorie diet | Adults without T2DM and with either overweight (BMI ≥ 27 kg/m2) plus at least one comorbidity or obesity (BMI ≥ 30 kg/m2) | Estimated weight loss difference of 10.3% in the semaglutide group (P < 0.001) |
| STEP 4 73 | Randomized, double‐blind, 68 week, Phase 3a withdrawal study | 902 | Once‐weekly treatment with subcutaneous semaglutide, 2.4 mg, compared with switching to placebo | Adults with overweight or obesity after a 20 week run‐in with subcutaneous semaglutide titrated to 2.4 mg weekly | Estimated weight loss difference of 14.8% in the semaglutide group (P < 0.001) |
| NCT05111912 74 (recruiting) | Phase 2, open‐label, randomized, interventional, dose‐finding study | 200 | Once‐weekly human GLP‐1 analogue, compared with once‐daily liraglutide 3 mg | BMI ≥ 30.0 and ≤40.0 kg/m2 at screening, in the absence of type 2 or any other type of diabetes | Percentage change in participants' body weight (%) from the baseline to Week 26 |
|
(recruiting) |
Randomized, interventional open‐label, Phase 3 study | 150 | Liraglutide vs. exenatide vs. exenatide microspheres for injection | BMI ≥ 28 kg/m2 or with abdominal obesity and with weight stable for ≥3 months | Weight change at 3 months measured in kilograms |
|
(recruiting) |
Multicentre, open‐label, randomized controlled, Phase 4 trial | 120 | Three times a day of subcutaneous beinaglutide or once weekly of 1.5 mg subcutaneous dulaglutide for 16 weeks | Adults with T2DM and overweight or obesity (BMI from 24 to 35 kg/m2) or waistline longer than 90 cm (male)/85 cm (female) | Change from baseline to Week 16 in HbA1c. The secondary endpoint is the change from baseline to Week 16 in weight |
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CV, cardiovascular; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; HF, heart failure; LV, left ventricular; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus.
In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, the primary composite outcome of CV death, non‐fatal MI, or non‐fatal stroke was significantly lower in the liraglutide group as compared with the placebo group (13% vs. 15%, P < 0.001 for non‐inferiority. 58 These beneficial effects observed in patients with no history of HF were, however, not replicated in patients with HF at baseline.
Results in patients with heart failure
Given the results derived from RCTs, international scientific societies currently recommend the use of GLP‐1 RAs as part of a comprehensive strategy to reduce the risk of CV events in patients with T2DM, 11 , 57 though, not yet, for the prevention of HF in patients with diabetes. 2
Overall, although hospitalization for HF did not represent the primary endpoint of the main RCTs, GLP‐1 RAs slightly reduced the risk of hospitalization for HF by 11% (Figure 2 ). 77 , 78 However, their effects on HF‐related events were different depending on the patients treated. HF events were reduced in diabetic patients with no HF at baseline whereas they were generally not changed in the RCTs enrolling patients with HF. A further distinction is possible depending on the HF phenotype with a possible increased risk of HF events in the patients with HFrEF at baseline and, on the opposite, beneficial effects in the patients with HFpEF above all with concomitant obesity. 79 , 80 , 81
Figure 2.
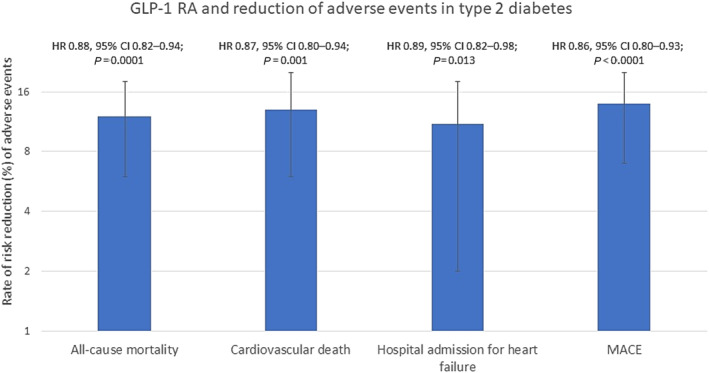
Reduction of adverse events in type 2 diabetes patients treated with glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs). The figure is adapted from Sattar et al., 77 showing the beneficial effects on mortality, hospital admission for heart failure (HF), and MACE meta‐analysed from GLP‐1 RA clinical trials (ELIXA, LEADER, SUSTAIN‐6, EXSCEL, Harmony Outcomes, REWIND, PIONEER 6, and AMPLITUDE‐O). MACE included cardiovascular death, myocardial infarction, and stroke. X axis represents the % reduction of the analysed endpoints. CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular events.
With respect of the results in patients with HFrEF, liraglutide had no effect on LV ejection fraction (LVEF), increased heart rate, and increased serious cardiac events in a randomized placebo‐controlled trial in 241 patients with HFrEF with and without diabetes. 82 A significant increase in serious cardiac events, although with small numbers, 12 (10%) with liraglutide vs. 3 (3%) with placebo (P = 0.04), occurred in another small randomized trial in patients with HFrEF. 83 Results could be ascribed to the increase in heart rate with liraglutide. Similar trends were observed also with other GLP‐1 RAs. 60 , 61
In a post hoc analysis of the Harmony Outcomes trial, 61 albiglutide, compared with placebo, reduced the composite of CV death or HF hospitalization as well as HF hospitalizations alone in patients without HF history but not in those with a history of HF (interaction P = 0.062 and 0.025, respectively). In a post hoc analysis from REWIND, dulaglutide was not associated with a reduction in HF events in patients with T2DM regardless of baseline HF status over 5.4 years of follow‐up. 60 , 84
Different results are likely in patients with HFpEF, above all if the obesity phenotype (see below).
Results in patients with obesity
Along with the benefits on CV outcome, it has been shown that the administration of GLP‐1 RA is associated with weight loss regardless of the diabetic status, although this may be less in patients with diabetes. 85 , 86 , 87 A systematic review comparing GLP‐1 RA with placebo in patients with T2DM and suboptimal control on oral agents showed that all GLP‐1 RAs except albiglutide reduce body weight.
Liraglutide has been shown to be effective for weight loss in non‐diabetic patients with obesity or overweight when given as adjunctive therapy to diet and exercise (Table 1 ). 62 , 63 , 64 , 88 Semaglutide is also highly effective in both patients with and without T2DM (Table 1 ). 70 , 71 , 73 , 89 , 90 , 91 Its efficacy was greater compared with other agents. In the SUSTAIN trials, a slightly greater weight loss has been observed with subcutaneous once‐weekly semaglutide, compared with exenatide, dulaglutide, or liraglutide. 91 , 92 , 93 Similarly, a secondary analysis of PIONEER 4 showed a greater weight loss with once‐daily oral semaglutide compared with subcutaneous liraglutide. 71 , 90
The ‘STEP Program’ trials have been designed to test the efficacy of semaglutide, at the higher dose of 2.4 mg/week, for weight loss in patients with and without type 2 diabetes. STEP 1 showed an average 14.9% reduction in body weight with semaglutide 2.4 mg plus a lifestyle intervention, compared with a 2.4% reduction in the placebo plus lifestyle intervention group (treatment difference of −12.4%, P < 0.001), among obese or overweight participants with related comorbidities, but not T2DM. 70 The STEP 2 trial showed an average body weight reduction of 9.6% and 6.9% with semaglutide 2.4 and 1.0 mg vs. 3.4% with placebo (P < 0.001) among participants with T2DM and overweight or obesity. The higher dose also achieved slightly better glycaemic control, reductions in cardiometabolic risk, and improved physical function relative to the standard dose. 71 In STEP 3, a weight reduction treatment difference of 10.3% was observed when treating overweight or obese people with related comorbidities, but not T2DM, with semaglutide 2.4 mg compared with placebo. 72 Patients who continued to take semaglutide after the first 20 weeks lost an additional 7.9% of their body weight in the STEP 4 trial. 73 Consistent results have been observed in other STEP trials. 94 , 95 , 96
Recently, the Food and Drug Administration approved semaglutide injection 2.4 mg once weekly for chronic weight management in adults with obesity or overweight with at least one weight‐related condition (such as high blood pressure, T2DM, or high cholesterol), for use in addition to a reduced calorie diet and increased physical activity. Semaglutide is the first drug approved for chronic weight management in adults with general obesity or overweight since 2014. Although tirzepatide is at an earlier stage of development, it has shown a similar, if not greater, efficacy for weight loss. 97
Treatment of patients with heart failure with preserved ejection fraction
With the recent exception of the sodium–glucose cotransporter‐2 inhibitor (SGLT2i), 98 , 99 trials in patients with HFpEF have failed to show significant results so that no specific treatment was recommended in the 2021 European Society of Cardiology (ESC) guidelines for HF. 2 It can be, however, hypothesized that, similarly to the beneficial effects of caloric restriction and physical activity leading to weight loss, also treatment with weight reducing GLP‐1 RA may be an effective for the patients with the obese HFpEF phenotype (Figure 3 ) (see below).
Figure 3.
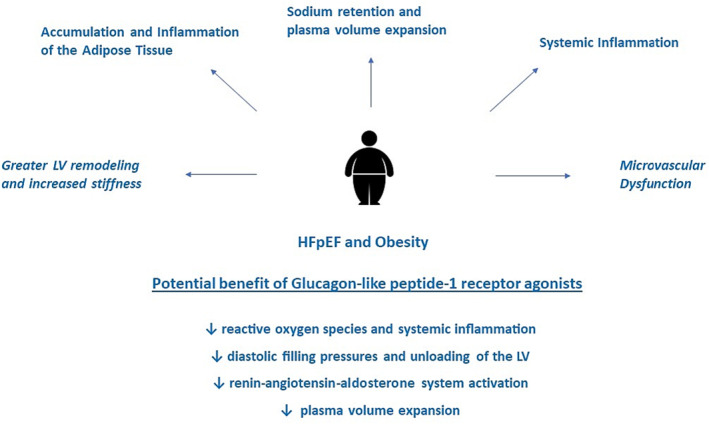
Obesity and heart failure with preserved ejection fraction (HFpEF). The figure shows the clinical feature and pathophysiology of obesity and HFpEF and the potential benefit deriving from glucagon‐like peptide‐1 receptor agonists. LV, left ventricular.
Treatment of the obese heart failure with preserved ejection fraction phenotype
Treatment of obesity includes a variety of modalities including lifestyle intervention, medications, and bariatric surgery. 100 , 101 The effect of weight loss in HF patients is still partially unsettled. The significance of obesity and, more specifically, increased epicardial fat is likely different in patients with HFrEF or HFpEF. It is associated with a reduced risk of events in HFrEF, whereas it is associated with worse symptoms and likely outcomes in HFpEF patients. 44 , 45 , 48 , 49 , 102 , 103
Weight loss should be a target of treatment only in obese patients with HFpEF. In the FLAGSHIP study, non‐obese HFpEF patients with weight loss had higher all‐cause mortality and re‐hospitalization rates than their pairs without weight loss. 104 Furthermore, at 6 months of hospital discharge, a high proportion of patients in the weight loss group in the non‐obesity group presented with functional limitations and anorexia, suggesting that their physical function and nutritional status were deteriorating.
Conversely, weight loss had beneficial effects in obese patients with HFpEF. Kitzman et al showed that among obese older patients with clinically stable HFpEF, caloric restriction and/or aerobic exercise training increased peak oxygen consumption, and their effects were additive. 101 Similarly, dietary treatment/prevention programmes among obese HFpEF patients showed that a loss of ≈7% body weight was associated with a 37% decrease in Minnesota Living With Heart Failure (MLWHF) score and a 29% increase in 6 min walking distance (6MWD) test at completion of the 15 week programme, compared with baseline. 105
Also, bariatric surgery in obese patients with HFpEF has been shown to improve symptoms and New York Heart Association (NYHA) class, as well as reduce HF readmissions and reverse LV remodelling, and improve LV distensibility. 106 , 107 , 108 In a nationwide analysis, mortality was lower among obese HFpEF patients with bariatric surgery compared with obese HFpEF patients without bariatric surgery. Obese HFpEF patients with bariatric surgery also had lower total hospitalization charges and lower total hospitalization costs compared with obese HFpEF patients without bariatric surgery. These results suggest that bariatric surgery in morbidly obese HFpEF patients may reduce mortality and improve resource utilization.
Whereas cardiac rehabilitation and intentional weight loss through caloric restriction, physical activity, and/or bariatric surgery are promising strategies to improve exercise capacity in these patients, future large studies are needed to test whether such interventions may modify the risk of long‐term adverse clinical outcomes. 109
Effects of glucagon‐like peptide‐1 receptor agonist
Agents that lead to a weight loss may be effective in patients with obesity and HFpEF. Also, the efficacy of GLP‐1 RA to reduce the generation of reactive oxygen species and reduce systemic inflammation 110 could represent a key factor to promote their use in HFpEF. Preliminary data show that GLP‐1 RA may improve diastolic function by reducing diastolic filling pressures and unloading the ventricle. 111 Beneficial effects of GLP‐1 RA also exert on the kidney by the protection from oxidative injury and by reducing the renin–angiotensin–aldosterone system activation and thereby contributing to blood pressure lowering. 112 This may be particularly important in HFpEF. A recent meta‐analysis of the main GLP‐1 RA CV outcome trials (CVOTs) has shown a reduction of the composite kidney outcome (development of macroalbuminuria, doubling of serum creatinine, end‐stage renal disease, and renal‐related deaths) by 21%. 77 , 78
RCTs are needed to test the efficacy of these drugs in this population. The STEP HFpEF trial has recently shown a significant improvement in symptoms, quality of life, and exercise tolerance, assessed by the 6MWD test, along with body weight reduction, in patients with obesity and HFpEF treated with semaglutide (2.4 mg) compared with placebo. 81 Similarly, the STEP HFpEF DM trial (NCT04916470) will test the effect of semaglutide in subjects with obesity‐related HFpEF and with T2DM. 113
SUMMIT is another ongoing RCT that will assess the efficacy and safety of tirzepatide (LY3298176), a combined gastric inhibitory peptide and GLP‐1 RA, in participants with HFpEF and obesity, compared with placebo. 97 Finally, SELECT has tested the superiority of semaglutide, compared with placebo, when added to standard of care for preventing major adverse CV events in patients with established CV disease and overweight or obese but without T2DM. Given the potential inclusion of HFpEF patients and the assessment of hospitalization for HF as a secondary outcome, SELECT will have potential for exploring new approaches to reduce CV events and HF events while targeting obesity (Table 2 ). 114 , 115
Table 2.
GLP‐1 RA and clinical outcomes in patients with heart failure and cardiovascular disease: ongoing trials
| Study or trial | Type of study | Estimate enrolment | Drug vs. comparator | Inclusion criteria | Primary endpoints |
|---|---|---|---|---|---|
| STEP HFpEF 81 | Randomized, double‐blind, placebo‐controlled Phase 3 trial | 529 | Once‐weekly subcutaneous semaglutide 2.4 mg add‐on to standard of care vs. placebo | BMI > 30 kg/m2, NYHA Class II–IV, and LVEF > 45% |
|
|
STEP HFpEF DM 113 (recruiting) |
Randomized, quadruple‐blind, placebo‐controlled Phase 3 trial | 610 | Once‐weekly subcutaneous semaglutide 2.4 mg add‐on to standard of care vs. placebo | Adults (>18 years old), with BMI > 30.0 kg/m2, NYHA Class II–IV, LVEF > 45% at screening, T2DM diagnosed ≥90 days prior to the screening, and HbA1c ≤ 10% |
|
|
SUMMIT 97 (recruiting) |
Randomized, double‐blind, placebo‐controlled Phase 3 trial | 700 | Tirzepatide administered subcutaneously vs. placebo | NYHA Class II–IV and elevated NT‐proBNP, structural heart disease, or HF decompensation within 12 months |
|
|
SELECT 114 (recruiting) |
Randomized, quadruple‐blind, placebo‐controlled Phase 3 trial | 17 500 | Once‐weekly subcutaneous semaglutide from 0.24 up to 2.4 mg add‐on to standard of care vs. placebo | BMI ≥ 27 kg/m2: prior myocardial infarction or prior stroke or PAD | Time to first occurrence of CV death, non‐fatal myocardial infarction, or non‐fatal stroke from 0 to 59 months |
6MWD, 6 min walking distance; BMI, body mass index; CV, cardiovascular; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus.
With worsening epidemiological trends for both the incidence and prevalence of HF worldwide, it is critical to implement optimal prevention and treatment strategies for patients with or without comorbidities as T2DM. 116 Consensus statements and guidelines have recommended GLP‐1 RA and SGLT2i as additions to lifestyle interventions with or without metformin in those at high atherosclerotic CV disease risk. 57 , 117 , 118 , 119 , 120
However, these recommendations fail to differentiate between the prevention and treatment of patients with HF and do not differentiate among those with different HF phenotypes.
From this perspective, GLP‐1 RA could represent a cornerstone treatment to modify the natural history of HFpEF. This could potentially lead to a breakthrough in the treatment of HF, which is constantly evolving, especially if we consider the high prevalence and adverse prognosis of patients affected by HFpEF. 121 , 122
Conclusions
The number of patients with HFpEF is expected to grow, given the increased life expectancy and the increasing prevalence of risk factors predisposing to HFpEF. It is well known that obesity is one of the most common and clinically relevant phenotypes of HFpEF with specific pathophysiological mechanisms. Therapies targeting body weight reduction are therefore promising. Trials with GLP‐1 RA in obese patients, with or without T2DM, have shown their efficacy for weight loss. Future studies are ongoing to assess whether GLP‐1 RA can prevent and treat patients with HFpEF and obesity.
Cimino, G. , Vaduganathan, M. , Lombardi, C. M. , Pagnesi, M. , Vizzardi, E. , Tomasoni, D. , Adamo, M. , Metra, M. , and Inciardi, R. M. (2024) Obesity, heart failure with preserved ejection fraction, and the role of glucagon‐like peptide‐1 receptor agonists. ESC Heart Failure, 11: 649–661. 10.1002/ehf2.14560.
References
- 1. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18:242–252. [DOI] [PubMed] [Google Scholar]
- 2. Authors/Task Force Members , McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 3. Seferovic PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinkovic I, et al. The Heart Failure Association Atlas: Heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906–914. [DOI] [PubMed] [Google Scholar]
- 4. Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: A review. JAMA 2023;329:827–838. [DOI] [PubMed] [Google Scholar]
- 5. Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol 2021;18:291–304. [DOI] [PubMed] [Google Scholar]
- 6. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. state‐level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: New insights and pathophysiological targets. Cardiovasc Res 2023;118:3434–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: Meta‐analysis of randomized control trials. Circ Heart Fail 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anker SD, Usman MS, Anker MS, Butler J, Bohm M, Abraham WT, et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur J Heart Fail 2023;25:936–955. [DOI] [PubMed] [Google Scholar]
- 10. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marx N, Federici M, Schutt K, Muller‐Wieland D, Ajjan RA, Antunes MJ, et al. ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 2023;2023. [DOI] [PubMed] [Google Scholar]
- 12. Harada T, Obokata M. Obesity‐related heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and potential therapies. Heart Fail Clin 2020;16:357–368. [DOI] [PubMed] [Google Scholar]
- 13. Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity‐induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 2016;118:1786–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia G, Jia Y, Sowers JR. Contribution of maladaptive adipose tissue expansion to development of cardiovascular disease. Compr Physiol 2016;7:253–262. [DOI] [PubMed] [Google Scholar]
- 15. Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, et al. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:1648–1658. [DOI] [PubMed] [Google Scholar]
- 16. Sorimachi H, Omote K, Omar M, Popovic D, Verbrugge FH, Reddy YNV, et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail 2022;24:1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 18. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur J Heart Fail 2016;18:588–598. [DOI] [PubMed] [Google Scholar]
- 19. Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity‐associated liver disease. J Clin Endocrinol Metab 2008;93:S74–S80. [DOI] [PubMed] [Google Scholar]
- 20. Chiyanika C, Chan DFY, Hui SCN, So HK, Deng M, Yeung DKW, et al. The relationship between pancreas steatosis and the risk of metabolic syndrome and insulin resistance in Chinese adolescents with concurrent obesity and non‐alcoholic fatty liver disease. Pediatr Obes 2020;15:e12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol 2018;221. [DOI] [PubMed] [Google Scholar]
- 22. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 23. van Heerebeek L, Hamdani N, Handoko ML, Falcao‐Pires I, Musters RJ, Kupreishvili K, et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51. [DOI] [PubMed] [Google Scholar]
- 24. D'Oria R, Schipani R, Leonardini A, Natalicchio A, Perrini S, Cignarelli A, et al. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxid Med Cell Longev 2020;2020:5732956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Heerebeek L, Hamdani N, Falcao‐Pires I, Leite‐Moreira AF, Begieneman MP, Bronzwaer JG, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 26. Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, et al. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: Inhibition by resveratrol. PLoS ONE 2011;6:e19237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanders‐van Wijk S, Tromp J, Beussink‐Nelson L, Hage C, Svedlund S, Saraste A, et al. Proteomic evaluation of the comorbidity‐inflammation paradigm in heart failure with preserved ejection fraction: Results from the PROMIS‐HFpEF study. Circulation 2020;142:2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013;6:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Packer M. Derangements in adrenergic–adipokine signalling establish a neurohormonal basis for obesity‐related heart failure with a preserved ejection fraction. Eur J Heart Fail 2018;20:873–878. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, Kitzman DW. Obesity‐related heart failure with a preserved ejection fraction: The mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium‐glucose cotransporter‐2. JACC Heart Fail 2018;6:633–639. [DOI] [PubMed] [Google Scholar]
- 31. Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail Rev 2017;22:889–902. [DOI] [PubMed] [Google Scholar]
- 32. Fontes‐Carvalho R, Fontes‐Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, et al. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol 2014;114:1663–1669. [DOI] [PubMed] [Google Scholar]
- 33. Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med 2009;30:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129:S49–S55. [DOI] [PubMed] [Google Scholar]
- 35. Buskirk E, Taylor HL. Maximal oxygen intake and its relation to body composition, with special reference to chronic physical activity and obesity. J Appl Physiol 1957;11:72–78. [DOI] [PubMed] [Google Scholar]
- 36. American Thoracic Society , American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 37. Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol 1991;68:377–381. [DOI] [PubMed] [Google Scholar]
- 38. Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin 2014;10:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mgbemena O, Zhang Y, Velarde G. Role of diabetes mellitus in heart failure with preserved ejection fraction: A review article. Cureus 2021;13:e19398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reddy YNV, Lewis GD, Shah SJ, Obokata M, Abou‐Ezzedine OF, Fudim M, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: A RELAX trial ancillary study. Mayo Clin Proc 2019;94:1199–1209. [DOI] [PubMed] [Google Scholar]
- 41. Dhore‐Patil A, Thannoun T, Samson R, Le Jemtel TH. Diabetes mellitus and heart failure with preserved ejection fraction: Role of obesity. Front Physiol 2021;12:785879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kresoja KP, Rommel KP, Wachter R, Henger S, Besler C, Kloting N, et al. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:1633–1644. [DOI] [PubMed] [Google Scholar]
- 43. Inciardi RM, Chandra A. Epicardial adipose tissue in heart failure: Risk factor or mediator? Eur J Heart Fail 2022;24:1357–1358. [DOI] [PubMed] [Google Scholar]
- 44. van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: Mechanisms, management and modern perspectives. Eur J Heart Fail 2022;24:2238–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venkateshvaran A, Faxen UL, Hage C, Michaelsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: Insights from the PROMIS‐HFpEF study. Eur J Heart Fail 2022;24:2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Obokata M, Reddy YNV, Melenovsky V, Sorimachi H, Jarolim P, Borlaug BA. Uncoupling between intravascular and distending pressures leads to underestimation of circulatory congestion in obesity. Eur J Heart Fail 2022;24:353–361. [DOI] [PubMed] [Google Scholar]
- 48. Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail 2022;24:1346–1356. [DOI] [PubMed] [Google Scholar]
- 49. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–1871. [DOI] [PubMed] [Google Scholar]
- 50. Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med 2016;48:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee YS, Jun HS. Anti‐diabetic actions of glucagon‐like peptide‐1 on pancreatic beta‐cells. Metabolism 2014;63:9–19. [DOI] [PubMed] [Google Scholar]
- 52. Muller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab 2019;30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP‐1 and analogs on cell signaling, metabolism, and function. Front Endocrinol (Lausanne) 2018;9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dailey MJ, Moran TH. Glucagon‐like peptide 1 and appetite. Trends Endocrinol Metab 2013;24:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shah M, Vella A. Effects of GLP‐1 on appetite and weight. Rev Endocr Metab Disord 2014;15:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deacon CF. Physiology and pharmacology of DPP‐4 in glucose homeostasis and the treatment of type 2 diabetes. Front Endocrinol (Lausanne) 2019;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for Diabetes, Pre‐diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 58. Alvarez‐Villalobos NA, Trevino‐Alvarez AM, Gonzalez‐Gonzalez JG. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:1797–1798. [DOI] [PubMed] [Google Scholar]
- 59. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 60. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double‐blind, randomised placebo‐controlled trial. Lancet 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 61. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double‐blind, randomised placebo‐controlled trial. Lancet 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 62. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: A randomised, double‐blind, placebo‐controlled study. Lancet 2009;374:1606–1616. [DOI] [PubMed] [Google Scholar]
- 63. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: The SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–1451. [DOI] [PubMed] [Google Scholar]
- 64. Pi‐Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 65. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 66. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 67. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ruff CT, Baron M, Im K, O'Donoghue ML, Fiedorek FT, Sabatine MS. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: A non‐inferiority randomized controlled trial. Nat Med 2022;28:89–95. [DOI] [PubMed] [Google Scholar]
- 69. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907. [DOI] [PubMed] [Google Scholar]
- 70. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002. [DOI] [PubMed] [Google Scholar]
- 71. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet 2021;13:971–984. [DOI] [PubMed] [Google Scholar]
- 72. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA 2021;325:1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA 2021;325:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. ClinicalTrials.gov . Effects of XW003 versus liraglutide on body weight of adult participants with obesity. Available from: https://clinicaltrials.gov/ct2/show/NCT05111912?term=GLP1&recrs=ab&cond=Obesity&draw=2&rank=1
- 75. ClinicalTrials.gov . Effects of GLP‐1 RAs on weight and metabolic indicators in obese patients. Available from: https://clinicaltrials.gov/ct2/show/NCT03671733?term=GLP1&recrs=ab&cond=Obesity&draw=2&rank=5
- 76. ClinicalTrials.gov . The effects of glucose control and weight loss between beinaglutide and dulaglutide in type 2 diabetes with overweight or obesity. Available from: https://clinicaltrials.gov/ct2/show/NCT05005741?term=GLP1&recrs=ab&cond=Obesity&draw=2&rank=10
- 77. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: A systematic review and meta‐analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662. [DOI] [PubMed] [Google Scholar]
- 78. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: A systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 79. Ferreira JP, Neves JS. Glucagon‐like peptide 1 receptor agonists in heart failure: The need for a rewind. Eur J Heart Fail 2022;24:1813–1815. [DOI] [PubMed] [Google Scholar]
- 80. Ferreira JP, Sharma A, Butler J, Packer M, Zannad F, Vasques‐Novoa F, et al. Glucagon‐like peptide‐1 receptor agonists across the spectrum of heart failure. J Clin Endocrinol Metab 2023. [DOI] [PubMed] [Google Scholar]
- 81. Kosiborod MN, Abildstrom SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023. [DOI] [PubMed] [Google Scholar]
- 82. Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA 2016;316:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, et al. Effect of liraglutide, a glucagon‐like peptide‐1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—A multicentre, double‐blind, randomised, placebo‐controlled trial. Eur J Heart Fail 2017;19:69–77. [DOI] [PubMed] [Google Scholar]
- 84. Branch KRHDG, Avezum A, Basile J, Conget I, Cushman WC, Jansky P, et al. Dulaglutide and cardiovascular and heart failure outcomes in patients with and without heart failure: A post‐hoc analysis from the REWIND randomized trial. Eur J Heart Fail 2022t;8:1805–1812. [DOI] [PubMed] [Google Scholar]
- 85. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA 2015;314:687–699. [DOI] [PubMed] [Google Scholar]
- 86. Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, et al. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: The SCALE insulin randomized controlled trial. Diabetes Care 2020;43:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon‐like peptide‐1 receptor agonists on weight loss: Systematic review and meta‐analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: The PIONEER 3 randomized clinical trial. JAMA 2019;321:1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double‐blind, phase 3a trial. Lancet 2019;394:39–50. [DOI] [PubMed] [Google Scholar]
- 91. Pratley RE, Aroda VR, Lingvay I, Ludemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol 2018;6:275–286. [DOI] [PubMed] [Google Scholar]
- 92. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56‐week, open‐label, randomized clinical trial. Diabetes Care 2018;41:258–266. [DOI] [PubMed] [Google Scholar]
- 93. Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020;46:100–109. [DOI] [PubMed] [Google Scholar]
- 94. Kadowaki TIJ, Khalid U, Lee SY, Nishida T, Ogawa W, Tobe K, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): A randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3a trial. Lancet Diabetes Endocrinol 2022;10:193–206. [DOI] [PubMed] [Google Scholar]
- 95. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sorrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 randomized clinical trial. JAMA 2022;327:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two‐year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat Med 2022;28:2083–2091. doi: 10.1038/s41591-022-02026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. NCT04847557 . A study of tirzepatide (LY3298176) in participants with heart failure with preserved ejection fraction and obesity (SUMMIT). 2021.
- 98. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. [DOI] [PubMed] [Google Scholar]
- 99. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 100. Tabucanon T, Wilcox J, Tang WHW. Does weight loss improve clinical outcomes in overweight and obese patients with heart failure? Curr Diab Rep 2020;20:75. [DOI] [PubMed] [Google Scholar]
- 101. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rao VN, Fudim M, Mentz RJ, Michos ED, Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I‐PRESERVE) trial. Circ Heart Fail 2011;4:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kamisaka K, Kamiya K, Iwatsu K, Iritani N, Imoto S, Adachi T, et al. Impact of weight loss in patients with heart failure with preserved ejection fraction: Results from the FLAGSHIP study. ESC Heart Fail 2021;8:5293–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. El Hajj EC, El Hajj MC, Sykes B, Lamicq M, Zile MR, Malcolm R, et al. Pragmatic weight management program for patients with obesity and heart failure with preserved ejection fraction. J Am Heart Assoc 2021;10:e022930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Miranda WR, Batsis JA, Sarr MG, Collazo‐Clavell ML, Clark MM, Somers VK, et al. Impact of bariatric surgery on quality of life, functional capacity, and symptoms in patients with heart failure. Obes Surg 2013;23:1011–1015. [DOI] [PubMed] [Google Scholar]
- 107. Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: Population‐based, self‐controlled series. J Am Coll Cardiol 2016;67:895–903. [DOI] [PubMed] [Google Scholar]
- 108. Mikhalkova D, Holman SR, Jiang H, Saghir M, Novak E, Coggan AR, et al. Bariatric surgery‐induced cardiac and lipidomic changes in obesity‐related heart failure with preserved ejection fraction. Obesity (Silver Spring) 2018;26:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, et al. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 110. Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012;97:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nguyen TD, Shingu Y, Amorim PA, Schenkl C, Schwarzer M, Doenst T. GLP‐1 improves diastolic function and survival in heart failure with preserved ejection fraction. J Cardiovasc Transl Res 2018;11:259–267. [DOI] [PubMed] [Google Scholar]
- 112. Wang C, Li L, Liu S, Liao G, Li L, Chen Y, et al. GLP‐1 receptor agonist ameliorates obesity‐induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS ONE 2018;13:e0193473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Christensen L, Davies M, et al. Design and baseline characteristics of STEP‐HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail 2023;11:1000–1010. [DOI] [PubMed] [Google Scholar]
- 114. Novo Nordisk A/S . Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT).
- 115. Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson SS, Kahn SE, et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J 2020;229:61–69. [DOI] [PubMed] [Google Scholar]
- 116. McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, et al. Heart failure with preserved ejection fraction and diabetes: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019;73:602–611. [DOI] [PubMed] [Google Scholar]
- 117. Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: A report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure, a scientific statement from the American Heart Association and Heart Failure Society of America. J Card Fail 2019;25:584–619. [DOI] [PubMed] [Google Scholar]
- 119. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 120. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kaplon‐Cieslicka A, Benson L, Chioncel O, Crespo‐Leiro MG, Coats AJS, Anker SD, et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction—Insights from the ESC‐HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2022;24:335–350. [DOI] [PubMed] [Google Scholar]
- 122. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]


