Abstract
Aims
Rhythm control therapy has shown great benefits for patients with atrial fibrillation (AF) and heart failure (HF). However, few studies have evaluated the effects of rhythm control on left ventricular ejection fraction (LVEF) trajectory across the whole HF spectrum. Our study explored the prevalence and predictors of LVEF trajectory changes and their prognostic implications following rhythm control.
Methods and results
Depending on the treatment strategy, the cohort was classified into rhythm and rate control groups. Alterations in HF types and LVEF trajectory were recorded. The observational endpoints were all‐cause mortality and HF‐related admission. Predictors of LVEF trajectory improvement in the rhythm control group were evaluated. After matching, the two groups had similar age [mean age (years): rhythm/rate control: 63.96/65.13] and gender [male: rhythm/rate control: n = 228 (55.6%)/233 (56.8%)]. Based on baseline LVEF measurement, the post‐matched cohort had 490 HF with preserved ejection fraction (rhythm/rate control: n = 260/230; median LVEF: 58.00%/57.00%), 99 HF with mildly reduced ejection fraction (rhythm/rate control: n = 50/49; median LVEF: 45.00%/46.00%), and 231 HF with reduced ejection fraction (rhythm/rate control: n = 100/131; median LVEF: 32.50%/33.00%). Trajectory analysis found that the rhythm control group had a greater percentage of LVEF trajectory improvement than the rate control group [80 (53.3%) vs. 71 (39.4%), P = 0.012]. Cox regression analysis also showed that the rhythm control group was more likely to have improved LVEF trajectory compared with the rate control group {hazard ratio [HR] 1.671 [95% confidence interval (CI) 1.196–2.335], P = 0.003}. In the survival analysis, the rhythm control group experienced significant lower risks of all‐cause mortality [HR 0.600 (95% CI 0.366–0.983), P = 0.043] and HF‐related admission [HR 0.611 (95% CI 0.496–0.753), P < 0.001]. In the rhythm control subgroup, E/e′ [odds ratio (OR) 0.878 (95% CI 0.792–0.974), P = 0.014], left ventricular end‐diastolic diameter [OR 0.874 (95% CI 0.777–0.983), P = 0.024], and CHA2DS2‐VASc score (congestive HF, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, age 65–74 years, and sex category) [OR 0.647 (95% CI 0.438–0.955), P = 0.028] were identified as three independent predictors of LVEF trajectory improvement.
Conclusions
Rhythm control is associated with improved LVEF trajectory and clinical outcomes and may thus be considered the optimal therapeutic strategy for patients with both HF and AF.
Keywords: Left ventricular ejection fraction, Trajectory, Rhythm control, Heart failure, Atrial fibrillation
Introduction
Heart failure (HF) is a multifaceted syndrome and may present with different levels of left ventricular ejection fraction (LVEF). The European Society of Cardiology (ESC) Heart Failure guidelines have classified HF into three different phenotypes according to LVEF measurement: HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%), HF with mildly reduced ejection fraction (HFmrEF, LVEF 40–49%), and HF with reduced ejection fraction (HFrEF, LVEF < 40%). 1 The primary pathophysiological mechanism of HF is adverse cardiac remodelling, which is manifested as alternations in the size, mass, and biological properties of cardiomyocytes and/or changes in the quantity and composition of the extracellular matrix. 2 Besides, previous studies have reported that the main molecular changes associated with adverse cardiac remodelling, including cardiac fibrosis, hypertrophy, inflammation, and apoptosis, were regulated by multiple miRNAs (miRs) pathways such as miR‐18, miR‐145, and miR‐181. 3 , 4 Intriguingly, anti‐HF therapies could promote sustained functional and clinical improvement via the modulation of these adaptive cardiac processes in HF patients. 5 , 6 For example, left ventricular reverse remodelling, LVEF recovery, New York Heart Association (NYHA) functional grade improvement, and B‐type natriuretic peptide (BNP) level reduction were commonly observed in HF patients treated with cardiac resynchronization therapy (CRT). 5
HF and atrial fibrillation (AF) often co‐exist through share common risk factors. 7 Mechanistically, atrial remodelling plays an important role in the pathogenesis of AF, and it might cause AF onset, recurrence, and persistence. The key pathogenic mechanisms of atrial remodelling are alteration of calcium channel currents and calcium handling mediated by sarcoplasmic endoplasmic reticulum Ca2+ ATPase (SERCA). 8 Over‐inflammation and altered expression of few miRs implied (epigenetic regulators) in cardiac fibrosis, apoptosis, and ionic channel currents have been proposed as potential player in the processes leading to atrial remodelling. 9 , 10 Catheter ablation (CA) may increase the expression of some biomarkers of AF fibrotic and electrical alterations, suggesting that this approach may relieve atrial fibrosis through positive changes in inflammatory markers, collagen turnover, and natriuretic peptide expression. 10
Clinically, previous randomized controlled trials have shown the superiority of CA for the treatment of AF in maintaining sinus rhythm (SR) and improving quality of life and prognosis in HF patients. 11 , 12 , 13 , 14 , 15 , 16 Therefore, in the 2022 American College of Cardiology HF guideline, CA is recommended as an optimal therapy for relieving symptoms and improving life quality in patients with AF and HF. 17 Currently, most clinical studies have focused on AF and HFrEF. 12 , 13 , 14 , 16 , 18 , 19 However, HF is a heterogeneous syndrome, in which disease progression is associated with the dynamic evolution of cardiac structural and functional alterations that determine the trajectories of this disease. 20 The new guideline points out that dynamic trajectory changes in LVEF are present in HF. A comprehensive analysis of LVEF trajectory over time should be conducted, focusing on LVEF‐based first classification, multiple assessments, and reclassifications. 17 To date, there has been no any research exploring the effect of rhythm control on the dynamic changes in LVEF trajectory (improvement or deterioration). This study examined the prevalence and predictors of LVEF trajectory changes and their prognostic implications after rhythm control treatment across the entire HF spectrum.
Methods
This study was a retrospective, single‐centre, observational, real‐world one and was approved by the institutional review board of Dalian Medical University. All the procedures were carried out in accordance with the Declaration of Helsinki and its amendments.
Study population
Patients diagnosed with HF and AF admitted to The First Affiliated Hospital of Dalian Medical University between 1 January 2012 and 30 December 2020 were enrolled in this study. The exclusion criteria were missing electrocardiogram (ECG) or echocardiogram results, loss of follow‐up, end‐stage renal failure, an estimated life expectancy <1 year, and a <18 or >85 years old. Baseline demographics, laboratory data, echocardiogram parameters, and medications were obtained and recorded.
Grouping
Rhythm control group
There were three methods of rhythm control used in this study: CA, electrical cardioversion (ECV), and surgical ablation. CA procedure was performed under general anaesthesia. Patients were heparinized to maintain the activated clotting time of 250–350 s during radiofrequency ablation. Left atrial (LA) anatomy was reconstructed using the PentaRay mapping electrode with the assistance of the Carto 3D cardiac mapping system. Circumferential pulmonary vein (PV) isolation was performed with an ablation catheter guided by ablation index after LA structural reconstruction. For paroxysmal AF, the endpoint of radiofrequency ablation was complete electrical PV isolation, which was characterized by the absence of PV potentials or PV–left atrial conduction. For persistent AF, the primary CA procedure was PV isolation, and additional ablation (ablation of complex fractionated atrial electrograms, creation of linear lesions, or combinations thereof) was performed at the discretion of the investigator to achieve a bidirectional block or restore SR. An initial energy of 100 or 150 J was used for ECV. Surgical ablation was achieved using the Cox–Maze procedure. All patients in rhythm control group were anticoagulated with warfarin or direct oral anticoagulants for at least 3 weeks prior to their procedures. For patients taking warfarin, an international normalized ratio (INR) was obtained weekly prior to the procedure to ensure that INR was >2.0. Amiodarone was continued until ablation. Before the procedures, intracardiac echocardiography was applied to ensure that the LA appendage was free of thrombus.
Rate control group
The target for rate control was <80 b.p.m. at rest and <110 b.p.m. during moderate exercise. Beta‐blocker was the most commonly used rate control agent. In HFrEF or HFmrEF, digoxin was also available when the ventricular rate remained faster after beta‐blocker administration or when beta‐blocker was not tolerated or contraindicated. Diltiazem was also an option for controlling heart rate in HFpEF. Notably, all enrolled patients received guideline‐directed medical therapy (GDMT) during hospitalization.
Propensity score matching
To reduce the impact of confounders, propensity score matching at 1:1 ratio between rhythm and rate control groups was performed based on age, gender, LVEF, and CHA2DS2‐VASc score (congestive HF, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, age 65–74 years, and sex category).
Clinical definitions
HF was diagnosed according to the 2022 American College of Cardiology HF guidelines. 17 In addition to typical HF symptoms and/or signs, LVEF was also an important echocardiographic indicator for the diagnosis and classification of HF (HFrEF, LVEF ≤ 40%; HFmrEF, LVEF 41–49%; and HFpEF, LVEF ≥ 50%). Additional criterion for HFmrEF and HFpEF was objective evidence of spontaneous or provokable raised left ventricular filling pressures, including increased natriuretic peptide and invasive/non‐invasive haemodynamic measurement suggesting elevated left ventricular filling pressures. HF with improved ejection fraction (HFimpEF) was defined as previous LVEF ≤ 40% and a follow‐up measurement of LVEF > 40%. As defined in this study, an improvement in LVEF trajectory is referring to HFimpEF or the transition from HFmrEF to HFpEF, while deterioration in LVEF trajectory is defined as the transition from HFpEF to HFmrEF/HFrEF or from HFmrEF to HFrEF.
AF was determined by ECG documentation, presenting with irregular R–R intervals (when atrioventricular conduction is not impaired), absence of discernible repeating P waves, and irregular atrial activations. Persistent AF was defined as persisting for at least 1 week to a maximum of 1 year, while paroxysmal AF was identified to last up to 1 week and was capable of spontaneous termination. 21
Study endpoints and follow‐up
All participants underwent at least twice echocardiographic examinations with interval exceeding 6 months. Two echocardiography findings were both obtained during hospitalization. Alterations in HF types and LVEF trajectory were recorded. The observational events were all‐cause mortality and HF‐related admission. For the subgroup analysis of rhythm control, predictors of LVEF trajectory improvement were also evaluated. All patients were required to return to the clinic regularly (1, 3, 6, and 12 months after discharge), and if they did not participate in scheduled clinic appointments, a telephone interview would be conducted. The follow‐up period was calculated from the second echocardiography to the study deadline. AF recurrence was monitored via 12‐lead ECG or Holter examination at each follow‐up visit (3, 6, and 12 months, post‐operatively) or when symptoms relapsed. Success in rhythm control referred to freedom from any atrial arrhythmia recurrence, including AF, atrial flutter, and atrial tachycardia within 12 months after rhythm intervention. All patients undergoing CA or surgical ablation were required to assess after the 90 day blanking. The deadline for follow‐up was 30 November 2021, or the occurrence of death, whichever was earlier.
Statistical analysis
All statistical analyses were performed with Statistical Package for Social Sciences, Version 24.0 (SPSS Inc., Chicago, IL, USA). Categorical data were presented as percentages (%), and χ 2 test was utilized to compare the differences between the two groups. Continuous variables with non‐normal distribution were expressed as median (inter‐quartile range) and were analysed using Kruskal–Wallis test. Continuous variables with normal distribution were presented as means ± standard deviations, and independent‐sample t‐test was applied to assess the differences. Kaplan–Meier analysis was performed to calculate the incidence of pre‐specified outcomes, with differences compared by log‐rank test. Univariate and multivariate Cox regression analyses were conducted to compare the risk of adverse endpoints, with hazard ratios (HRs) and 95% confidence intervals (CIs) presented. Covariates selected for multivariate Cox analysis were those with significantly statistical differences in the univariate analysis. Logistic regression analysis was used to detect independent predictors of improved LVEF trajectory. Odds ratio (ORs) and their relative 95% CIs were obtained. Multivariate logistic regression model was constructed by including variables with P < 0.05 in univariate analysis and entering into models stepwise. A two‐sided P value <0.05 was considered statistically different.
Results
The flowchart for the identification, inclusion, and exclusion of study participants was shown in Figure 1 . A total of 1810 consecutive HF and AF patients were initially identified. Of these, 793 patients were excluded due to missing ECG or echocardiogram results (n = 210), loss to follow‐up (n = 410), end‐stage renal failure (n = 58), estimated life expectancy <1 year (n = 91), and under 18 years or over 85 years of age (n = 24). Consequently, 1017 patients remained, with 607 cases in rate control group and 410 in rhythm control group. To reduce the imbalance, rate control group was matched to rhythm control group in a 1:1 ratio by age, gender, LVEF, and CHA2DS2‐VASc score using propensity score matching, yielding 410 cases in each group.
Figure 1.
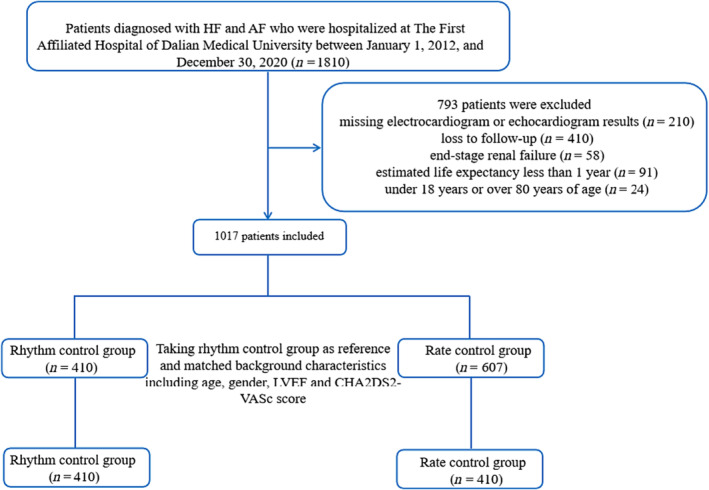
Flowchart of the study protocol. AF, atrial fibrillation; HF, heart failure; LVEF, left ventricular ejection fraction.
Baseline characteristics
After matching, the two groups had similar age [mean age (years): rhythm/rate control: 63.96/65.13] and gender [male: rhythm/rate control: n = 228 (55.6%)/233 (56.8%)]. Based on baseline LVEF measurement, the post‐matched cohort had 490 HFpEF (rhythm/rate control: n = 260/230; median LVEF: 58.00%/57.00%), 99 HFmrEF (rhythm/rate control: n = 50/49; median LVEF: 45.00%/46.00%), and 231 HFrEF (rhythm/rate control: n = 100/131; median LVEF: 32.50%/33.00%). Compared with the rhythm control group, patients in the rate control group had a higher prevalence of NYHA Class IV, higher systolic blood pressure, higher frequency of diabetes mellitus, ischaemic heart disease, and persistent AF, but lower rate of valvular heart disease. Regarding their medications, the rate control group had a higher proportion of patients who were prescribed with digoxin, beta‐blockers, angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB)/angiotensin receptor–neprilysin inhibitor (ARNI), diuretic, nitrate, and antiplatelet agents, but lower proportion on anticoagulant therapy. In terms of laboratory tests and echocardiographic findings, the rate control group had higher levels of BNP and high‐sensitivity troponin I (hs‐TnI), greater value of LA diameter (LAD) and left ventricular end‐diastolic diameter (LVEDD), but lower haemoglobin and sodium (Table 1 ).
Table 1.
Baseline characteristics
| Rhythm control group (pre‐matched, n = 410) | Rate control group (pre‐matched, n = 607) | P value | Rhythm control group (post‐matched, n = 410) | Rate control group (post‐matched, n = 410) | P value | |
|---|---|---|---|---|---|---|
| Age (years) | 63.96 ± 10.08 | 70.17 ± 10.99 | <0.001 | 63.96 ± 10.08 | 65.13 ± 9.63 | 0.089 |
| Male, n (%) | 228 (55.6) | 321 (52.9) | 0.392 | 228 (55.6) | 233 (56.8) | 0.725 |
| NYHA Class I–II, n (%) | 118 (28.8) | 138 (22.7) | 0.029 | 118 (28.8) | 89 (21.7) | 0.020 |
| NYHA Class III, n (%) | 247 (60.2) | 366 (60.3) | 0.987 | 247 (60.2) | 237 (57.8) | 0.478 |
| NYHA Class IV, n (%) | 45 (11.0) | 103 (17.0) | 0.008 | 45 (11.0) | 84 (20.5) | <0.001 |
| Left ventricular ejection fraction at baseline categories | ||||||
| HFrEF, n (%) | 100 (24.4) | 163 (26.9) | 0.379 | 100 (24.4) | 131 (32.0) | 0.016 |
| HFmrEF, n (%) | 50 (12.2) | 75 (12.4) | 0.939 | 50 (12.2) | 49 (12.0) | 0.915 |
| HFpEF, n (%) | 260 (63.4) | 369 (60.8) | 0.398 | 260 (63.4) | 230 (56.1) | 0.033 |
| Left ventricular ejection fraction at baseline | ||||||
| HFrEF (%) | 32.50 (30.00, 36.00) | 34.00 (28.00, 38.00) | 0.816 | 32.50 (30.00, 36.00) | 33.00 (26.25, 38.00) | 0.674 |
| HFmrEF (%) | 45.00 (44.00, 47.00) | 45.00 (43.00, 46.00) | 0.133 | 45.00 (43.25, 47.00) | 46.00 (44.00, 47.00) | 0.838 |
| HFpEF (%) | 58.00 (55.00, 59.00) | 57.00 (55.00, 58.00) | 0.032 | 58.00 (55.00, 59.00) | 57.00 (55.00, 58.00) | 0.098 |
| Heart rate (b.p.m.) | 96.65 ± 29.29 | 91.92 + 26.81 | 0.008 | 96.65 ± 29.29 | 92.89 + 27.84 | 0.06 |
| Systolic blood pressure (mmHg) | 127.75 ± 18.76 | 135.97 ± 22.11 | <0.001 | 127.75 ± 18.76 | 134.52 ± 23.46 | <0.001 |
| Diastolic blood pressure (mmHg) | 81.29 ± 14.68 | 81.20 ± 14.48 | 0.922 | 81.29 ± 14.68 | 82.22 ± 15.24 | 0.374 |
| Paroxysmal atrial fibrillation, n (%) | 100 (24.4) | 108 (17.8) | 0.021 | 100 (24.4) | 73 (17.8) | 0.021 |
| Persistent atrial fibrillation, n (%) | 310 (75.6) | 499 (82.2) | 0.021 | 310 (75.6) | 337 (82.2) | 0.021 |
| Ischaemic heart disease, n (%) | 55 (13.4) | 164 (27.0) | <0.001 | 55 (13.4) | 84 (20.5) | 0.007 |
| Valvular disease, n (%) | 158 (38.5) | 156 (25.7) | <0.001 | 158 (38.5) | 119 (29.0) | 0.004 |
| Dilated cardiomyopathy, n (%) | 27 (6.6) | 44 (7.2) | 0.684 | 27 (6.6) | 40 (9.8) | 0.097 |
| Hypertrophic cardiomyopathy, n (%) | 23 (5.6) | 32 (5.3) | 0.815 | 23 (5.6) | 26 (6.3) | 0.659 |
| Hypertension, n (%) | 209 (51.0) | 386 (63.6) | <0.001 | 209 (51) | 231 (56.3) | 0.123 |
| Diabetes mellitus, n (%) | 80 (19.5) | 188 (31.0) | <0.001 | 80 (19.5) | 106 (25.9) | 0.030 |
| Previous stroke or TIA, n (%) | 39 (9.5) | 95 (15.7) | 0.005 | 39 (9.5) | 45 (11) | 0.490 |
| CHA2DS2‐VASc score, n (%) | 3.13 ± 1.57 | 3.98 ± 1.67 | <0.001 | 3.13 ± 1.57 | 3.31 ± 1.36 | 0.079 |
| Pharmacotherapies | ||||||
| Digoxin, n (%) | 27 (6.6) | 216 (35.6) | <0.001 | 27 (6.6) | 161 (39.3) | <0.001 |
| Beta‐blocker, n (%) | 215 (52.4) | 540 (89.0) | <0.001 | 215 (52.4) | 366 (89.3) | <0.001 |
| ACEI or ARB or ARNI, n (%) | 163 (39.8) | 293 (48.3) | 0.007 | 163 (39.8) | 203 (49.5) | 0.005 |
| Spironolactone, n (%) | 243 (59.3) | 371 (61.1) | 0.554 | 243 (59.3) | 256 (62.4) | 0.352 |
| Diuretic, n (%) | 241 (58.8) | 404 (66.6) | 0.012 | 241 (58.8) | 274 (66.8) | 0.017 |
| Nitrate, n (%) | 42 (10.2) | 149 (24.5) | <0.001 | 42 (10.2) | 91 (22.2) | <0.001 |
| Novel oral anticoagulants, n (%) | 130 (31.7) | 157 (25.9) | 0.042 | 130 (31.7) | 100 (24.4) | 0.02 |
| Warfarin, n (%) | 239 (58.3) | 218 (35.9) | <0.001 | 239 (58.3) | 174 (42.4) | <0.001 |
| Antiplatelet, n (%) | 26 (6.3) | 202 (33.3) | <0.001 | 26 (6.3) | 115 (28) | <0.001 |
| Lipid‐lowering drug, n (%) | 148 (36.1) | 299 (49.3) | <0.001 | 148 (36.1) | 170 (41.5) | 0.115 |
| Laboratory test | ||||||
| Haemoglobin (g/L) | 140.18 ± 18.20 | 134.40 ± 20.99 | <0.001 | 140.18 ± 18.20 | 137.39 ± 20.83 | 0.042 |
| BNP level (pg/mL) | 297.54 (134.33, 562.61) | 393.66 (195.00, 826.53) | <0.001 | 297.54 (134.33, 562.61) | 399.08 (184.63, 817.72) | <0.001 |
| hs‐TnI (μg/L) | 0.02 (0.01, 0.05) | 0.02 (0.01, 0.07) | <0.001 | 0.02 (0.01, 0.05) | 0.02 (0.01, 0.06) | 0.005 |
| Creatinine (mmol/L) | 74.00 (62.00, 89.00) | 76 (63.00, 95.00) | 0.068 | 74.00 (62.00, 89.00) | 75.00 (63.00, 95.00) | 0.216 |
| Uric acid (mmol/L) | 417.25 ± 123.26 | 414.95 ± 135.55 | 0.791 | 417.25 ± 123.26 | 424.93 ± 135.03 | 0.412 |
| Potassium (mmol/L) | 4.07 ± 0.41 | 4.03 ± 0.48 | 0.132 | 4.07 ± 0.41 | 4.02 ± 0.48 | 0.097 |
| Sodium (mmol/L) | 142 (140, 144) | 141 (139, 143) | <0.001 | 142 (140, 144) | 141 (139, 143) | 0.001 |
| Echocardiographic parameters | ||||||
| LAD (mm) | 44 (40, 48) | 46 (41, 50) | 0.001 | 44 (40, 48) | 46 (42, 50) | <0.001 |
| LVEDD (mm) | 50.00 (45.00, 55.00) | 50 (45, 57) | 0.175 | 50.56 ± 7.68 | 52.25 ± 8.97 | 0.004 |
| E/e′ | 11.00 (8.20, 15.00) | 11.80 (9.00, 15.00) | 0.135 | 11.00 (8.20, 15.00) | 12 (9, 15) | 0.152 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BNP, B‐type natriuretic peptide; CHA2DS2‐VASc score, congestive HF, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, age 65–74 years, and sex category; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hs‐TnI, high‐sensitivity troponin I; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; NYHA, New York Heart Association; TIA, transient ischaemic attack.
Left ventricular ejection fraction trajectory changes between the two groups
The rhythm control group experienced a greater percentage of LVEF trajectory improvement than the rate control group [80 (53.3%) vs. 71 (39.4%), P = 0.012], including transitions from HFrEF to HFimpEF [51 (51.0%) vs. 48 (36.6%), P = 0.029] and HFmrEF to HFpEF [29 (58.0%) vs. 23 (46.9%), P = 0.270] (Figure 2 A,B ). Cox regression analysis also showed that rhythm control group was more likely to have improvements in LVEF trajectory [HR 1.671 (95% CI 1.196–2.335), P = 0.003] (Table 2 ). Additionally, the proportion of cases with LVEF improvement >10% was higher with rhythm therapies for HFrEF [47 (47.0%) vs. 34 (26.0%), P = 0.001] and HFmrEF [13 (26.0%) vs. 3 (6.1%), P = 0.007], respectively.
Figure 2.
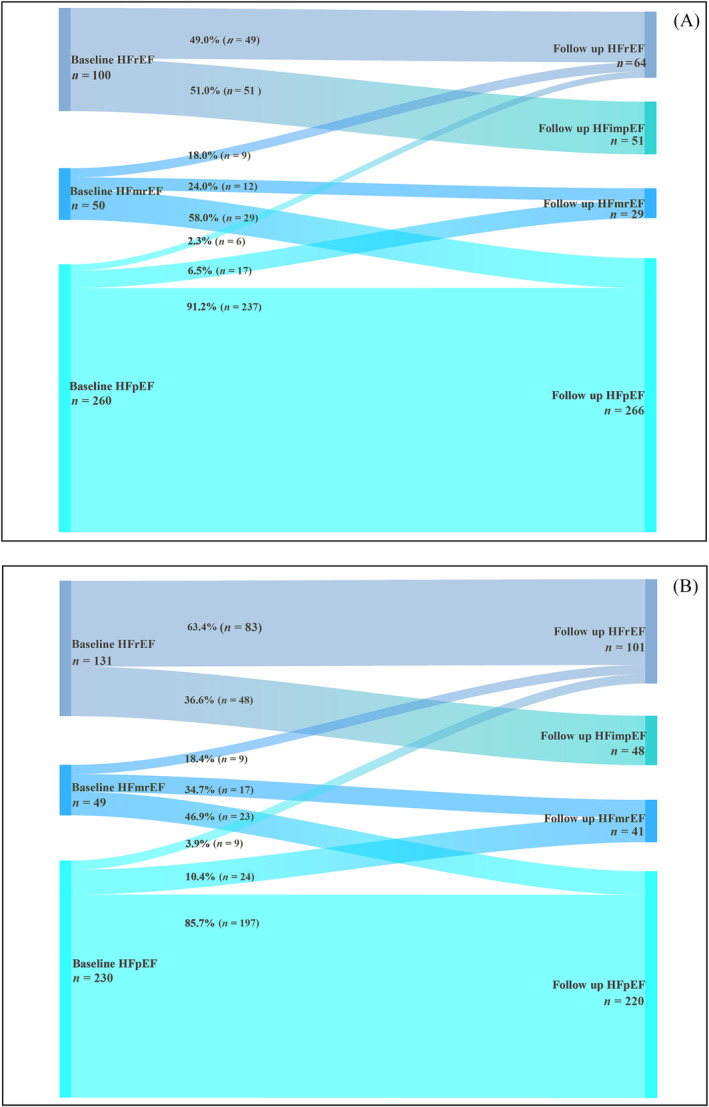
(A) Sankey diagram displaying the longitudinal evolution of heart failure (HF) types from baseline to follow‐up following rhythm control. (B) Sankey diagram displaying the longitudinal evolution of HF types from baseline to follow‐up following rate control. HFimpEF, heart failure with improved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 2.
Cox regression analysis for improved LVEF trajectory between two groups
| Unadjusted | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Rate control group | 1.000 | Reference | NA |
| Rhythm control group | 1.671 | 1.196–2.335 | 0.003 |
CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
On the other hand, the proportion of deteriorated LVEF trajectory in the rhythm control group was lower than that in the rate control group [32 (10.3%) vs. 42 (15.1%)], although there was no significant statistical difference (P = 0.084). The percentages of patients who transitioned from HFpEF to HFmrEF/HFrEF [17 (6.5%) vs. 24 (10.4%), P = 0.120 or 6 (2.3%) vs. 9 (3.9%), P = 0.303] and HFmrEF to HFrEF [9 (18.0%) vs. 9 (18.4%), P = 0.962] were lower in the rhythm control group compared with the rate control group (Figure 2 A,B ).
Adverse outcomes on follow‐up
The median follow‐up was 3.8 years. The number of patients with mortality was 26 (6.3%) and 40 (9.8%) for rhythm and rate control groups, respectively. A total of 156 (38.0%) and 208 (50.7%) cases for rhythm and rate control groups were re‐hospitalized due to worsening HF, respectively. Kaplan–Meier showed that patients in the rhythm control group experienced a lower incidence of the primary endpoints than the rate control group (Figure 3 ). Cox regression analysis also demonstrated that the rhythm control group had significant lower risks of all‐cause mortality [HR 0.600 (95% CI 0.366–0.983), P = 0.043] and HF‐related admission [HR 0.611 (95% CI 0.496–0.753), P < 0.001]. Similar results were found after full adjustment with related factors [all‐cause mortality: HR 0.569 (95% CI 0.325–0.996), P = 0.048; HF‐related admission: HR 0.654 (95% CI 0.518–0.826), P < 0.001] (Table 3 ).
Figure 3.
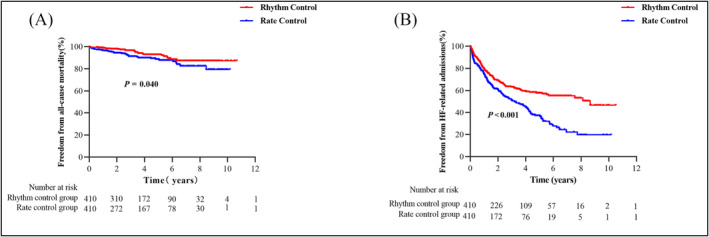
Adverse outcomes stratified by rhythm and rate control.
Table 3.
Risks of adverse outcomes between rhythm and rate control groups
| Adverse outcome | Unadjusted | Adjusted a | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| All‐cause mortality | 0.600 | 0.366–0.983 | 0.043 | 0.569 | 0.325–0.996 | 0.048 |
| HF re‐hospitalization | 0.611 | 0.496–0.753 | <0.001 | 0.654 | 0.518–0.826 | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CI, confidence interval; HR, hazard ratio; NYHA, New York Heart Association.
Adjusted for the NYHA Class I–II, NYHA Class IV, coronary artery disease, hypertension, diabetes mellitus, systolic blood pressure, ACEI or ARB or ARNI, beta‐blocker, spironolactone, and potassium.
Rhythm control subgroup analysis
In those treated with rhythm control, 150 patients were found to have HFrEF or HFmrEF. After a second echocardiographic assessment, 80 cases were identified as having improved LVEF trajectory. The univariate logistic regression analysis for predictors of LVEF trajectory improvement after rhythm control were shown in Table S1. Multivariate logistic regression analysis revealed that greater values of early transmitral flow velocity‐to‐early diastolic mitral annulus velocity (E/e′) [OR 0.878 (95% CI 0.792–0.974), P = 0.014], LVEDD [OR 0.874 (95% CI 0.777–0.983), P = 0.024], and CHA2DS2‐VASc score [OR 0.647 (95% CI 0.438–0.955), P = 0.028] were three negative predictors of LVEF trajectory improvement following rhythm control treatment (Table 4 ).
Table 4.
Multivariate logistic regression analysis for detecting predictors of improvement in LVEF trajectory after rhythm control
| Multivariate analysis | |||
|---|---|---|---|
| Exp(B) | 95% CI | P | |
| Heart rate | 1.005 | 0.985–1.024 | 0.641 |
| Diastolic blood pressure | 1.021 | 0.983–1.061 | 0.280 |
| Ischaemic heart disease | 0.259 | 0.054–1.251 | 0.093 |
| Dilated cardiomyopathy | 0.758 | 0.147–3.909 | 0.741 |
| CHA2DS2‐VASc score | 0.647 | 0.438–0.955 | 0.028 |
| ACEI or ARB or ARNI | 0.497 | 0.141–1.753 | 0.277 |
| Spironolactone | 0.185 | 0.031–1.105 | 0.064 |
| Diuretic | 2.300 | 0.441–11.997 | 0.323 |
| Nitrate | 1.857 | 0.330–10.449 | 0.483 |
| Lipid‐lowering drug | 0.767 | 0.242–2.429 | 0.652 |
| LAD | 0.971 | 0.860–1.097 | 0.640 |
| LVEDD | 0.874 | 0.777–0.983 | 0.024 |
| LVEF | 1.028 | 0.950–1.112 | 0.493 |
| E/e′ | 0.878 | 0.792–0.974 | 0.014 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CHA2DS2‐VASc score, congestive HF, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, age 65–74 years, and sex category; CI, confidence interval; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction.
In a follow‐up analysis, 153 (71.2%), 62 (47.0%), and 26 (41.3%) subjects remained SR at 12th month after CA, ECV, and surgical ablation, respectively (data not shown).
Discussion
The main finding of this study was that rhythm control treatment for AF may shift LVEF trajectory in a favourable direction and was generally associated with improved clinical outcomes across the whole HF spectrum.
Dynamic changes in left ventricular ejection fraction trajectory
Several randomized controlled trials have demonstrated that CA for AF could improve LVEF in patients with HF. 14 , 22 Despite this, the extent of improvement after rhythm control among the HF population remains inadequately studied, and most published studies thus far focus solely on LVEF improvement in HFrEF population. In our study, dynamic changes in LVEF trajectory across the entire HF spectrum were evaluated. The results showed that after rhythm intervention, approximately half of the patients with HFrEF and HFmrEF transitioned to a higher category, which was significantly higher than that on rate control therapy. In spite of this, 39.4% of HFrEF and HFmrEF patients in the rate control group also showed improvements in LVEF trajectory, possibly due to the high proportion of GDMT use in this population. Accordingly, active rhythm control and standardized medications may be considered for all types of HF patients to improve LVEF and prevent its deterioration.
Rhythm control on prognosis in atrial fibrillation and HF
In clinical practice, GDMT could also positively condition clinical outcomes and reduce AF recurrence in patients with HF and cardiac resynchronization therapy with defibrillator (CRTd) and particularly in those CRTd non‐responders via the modulation of few miRs. 6 However, a growing body of evidence has further confirmed the clinical benefits of rhythm therapy, such as raising LVEF, increasing 6 min walk distance, and improving the quality of life in patients with HF and AF. 11 , 12 , 13 , 14 , 15 , 16 The famous CASTLE‐AF trial showed that CA treatment had a marked improvement in LVEF and a significant reduction in the composite endpoint of all‐cause mortality or HF‐related admission compared with medical therapy. 12 In CABANA trial, the subgroup analysis demonstrated that CA treatment produced considerable clinical benefits in freedom from AF recurrence, survival, and quality of life compared with drug therapy. 11 A similar study, EAST‐AFNET4 trial, also revealed that early rhythm control reduced a composite primary endpoint of acute coronary syndrome or HF admission compared with conventional treatment. 15 Due to these excellent findings, the recent HF guideline recommends the use of CA in the treatment of deteriorating HF and symptomatic AF (IIA class). 17 Our study also found a favourable effect of rhythm control on survival and HF‐related admission, which was consistent with previously published studies.
Predictors of improvements in left ventricular ejection fraction trajectory
It takes into account several factors when determining who are likely to benefit from rhythm control. For instance, NYHA Class I/II and non‐ischaemic aetiologies may benefit the most; in contrast, those with enlarged atria or advanced atrial fibrosis on cardiac magnetic resonance (CMR) are less likely to benefit from rhythm control treatment. 18 , 22 , 23 In this study, we observed that a smaller baseline LVEDD, E/e′, and CHA2DS2‐VASc score were associated with a higher likelihood of improvement in LVEF trajectory following rhythm control. Smaller baseline LVEDD was an independent predictor of improved LVEF after CA in patients with persistent AF, which had been previously reported. 24 E/e′ correlates with invasive left ventricular filling pressures and acts as an important echocardiographic parameter to evaluate left ventricular diastolic function. 25 Higher E/e′ ratio is associated with myocardial fibrosis and diastolic dysfunction, as well as an increased risk of short‐ and long‐term mortality. 26 , 27 A study conducted by Yang et al. showed that E/e′ was an independent predictor of recovered ejection fraction in systolic HF patients who received CA for AF. 28 These findings suggest that patients with a lower E/e′ ratio appear to have an improved LVEF and are more likely to benefit from rhythm control therapy.
Limitations
We must recognize that there were several limitations in this study. First, it was a single‐centre, retrospective, observational study, and selection and recall bias were inevitable with this study design. Second, due to a relatively small sample size involved in this study, the findings may need to be further confirmed in a larger multi‐centre clinical study. Third, monitoring recurrence of arrhythmia after rhythm control relied on 12‐lead ECG measurement, lacking more accurate detection strategies, such as implantable loop recorder (determination of AF pattern, number of episodes, AF burden, and AF density). 29 At last, metabolic syndrome (MS) could negatively increase arrhythmic burden and reduce the effects of anti‐HF and anti‐arrhythmic therapies and may affect the clinical outcomes in patients with HF. 30 , 31 Nevertheless, waist circumference data are only available for a small percentage of patients in this study, making it difficult to explore its role.
Conclusions
Our findings supported that rhythm control therapy was associated with improved LVEF trajectory and better clinical outcomes and thus be considered as the optimal therapeutic strategy for patients with HF and AF.
Conflict of interest
None declared.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Numbers U1908209 and 82170385).
Supporting information
Table S1. Univariate logistic regression analysis to detect predictors of improved LVEF trajectory after rhythm control.
Si, J. , Sun, Y. , Bai, L. , Tse, G. , Ding, Z. , Zhang, X. , Zhang, Y. , Chen, X. , Xia, Y. , and Liu, Y. (2024) Trajectory change of left ventricular ejection fraction after rhythm control for atrial fibrillation in heart failure. ESC Heart Failure, 11: 681–691. 10.1002/ehf2.14590.
Contributor Information
Yunlong Xia, Email: yunlong_xia@126.com.
Ying Liu, Email: yingliu.med@gmail.com.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: Myth, magic, or molecular target? J Am Coll Cardiol 2012;60:2465‐2472. doi: 10.1016/j.jacc.2012.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sardu C, Barbieri M, Rizzo MR, Paolisso P, Paolisso G, Marfella R. Cardiac resynchronization therapy outcomes in type 2 diabetic patients: Role of microRNA changes. J Diabetes Res 2016;2016:7292564. doi: 10.1155/2016/7292564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marfella R, Di Filippo C, Potenza N, Sardu C, Rizzo MR, Siniscalchi M, et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non‐responders. Eur J Heart Fail 2013;15:1277‐1288. doi: 10.1093/eurjhf/hft088 [DOI] [PubMed] [Google Scholar]
- 5. Sardu C, Marfella R, Santulli G, Paolisso G. Functional role of miRNA in cardiac resynchronization therapy. Pharmacogenomics 2014;15:1159‐1168. doi: 10.2217/pgs.14.76 [DOI] [PubMed] [Google Scholar]
- 6. Sardu C, Massetti M, Scisciola L, Trotta MC, Santamaria M, Volpicelli M, et al. Angiotensin receptor/neprilysin inhibitor effects in CRTd non‐responders: From epigenetic to clinical beside. Pharmacol Res 2022;182:106303. doi: 10.1016/j.phrs.2022.106303 [DOI] [PubMed] [Google Scholar]
- 7. Mesubi OO, Anderson ME. Heart failure and atrial fibrillation—Chicken or egg? Circ Res 2022;130:1011‐1013. doi: 10.1161/CIRCRESAHA.122.320930 [DOI] [PubMed] [Google Scholar]
- 8. Sardu C, Santulli G, Guerra G, Trotta MC, Santamaria M, Sacra C, et al. Modulation of SERCA in patients with persistent atrial fibrillation treated by epicardial thoracoscopic ablation: The CAMAF study. J Clin Med 2020;9:544. doi: 10.3390/jcm9020544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sardu C, Santulli G, Santamaria M, Barbieri M, Sacra C, Paolisso P, et al. Effects of alpha lipoic acid on multiple cytokines and biomarkers and recurrence of atrial fibrillation within 1 year of catheter ablation. Am J Cardiol 2017;119:1382‐1386. doi: 10.1016/j.amjcard.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sardu C, Santamaria M, Paolisso G, Marfella R. microRNA expression changes after atrial fibrillation catheter ablation. Pharmacogenomics 2015;16:1863‐1877. doi: 10.2217/pgs.15.117 [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Piccini JP, Monahan KH, al‐Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: Results from the CABANA trial. Circulation 2021;143:1377‐1390. doi: 10.1161/CIRCULATIONAHA.120.050991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417‐427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 13. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31‐38. doi: 10.1161/CIRCEP.113.000806 [DOI] [PubMed] [Google Scholar]
- 14. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: The CAMERA‐MRI study. J Am Coll Cardiol 2017;70:1949‐1961. [DOI] [PubMed] [Google Scholar]
- 15. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021;144:845‐858. doi: 10.1161/CIRCULATIONAHA.121.056323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Circulation 2016;133:1637‐1644. doi: 10.1161/CIRCULATIONAHA.115.019406 [DOI] [PubMed] [Google Scholar]
- 17. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263‐e421. [DOI] [PubMed] [Google Scholar]
- 18. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: The randomized AMICA trial. Circ Arrhythm Electrophysiol 2019;12:e007731. doi: 10.1161/CIRCEP.119.007731 [DOI] [PubMed] [Google Scholar]
- 19. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman‐Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894‐1903. [DOI] [PubMed] [Google Scholar]
- 20. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur Heart J 2019;40:2155‐2163. doi: 10.1093/eurheartj/ehz158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373‐498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 22. Sohns C, Zintl K, Zhao Y, Dagher L, Andresen D, Siebels J, et al. Impact of left ventricular function and heart failure symptoms on outcomes post ablation of atrial fibrillation in heart failure: CASTLE‐AF trial. Circ Arrhythm Electrophysiol 2020;13:e008461. doi: 10.1161/CIRCEP.120.008461 [DOI] [PubMed] [Google Scholar]
- 23. Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5‐year follow‐up data. J Am Heart Assoc 2018;7:e006313. doi: 10.1161/JAHA.117.006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ukita K, Egami Y, Nakamura H, Matsuhiro Y, Yasumoto K, Tsuda M, et al. Predictors of improvement of left ventricular systolic function after catheter ablation of persistent atrial fibrillation in patients with heart failure with reduced ejection fraction. Heart Vessels 2021;36:1212‐1218. doi: 10.1007/s00380-021-01795-1 [DOI] [PubMed] [Google Scholar]
- 25. Daubert MA. Diastolic function in heart failure with reduced ejection fraction: The overlooked prognosticator? JACC Heart Fail 2019;7:818‐820. doi: 10.1016/j.jchf.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 26. Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK, et al. Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail 2019;7:808‐817. doi: 10.1016/j.jchf.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 27. Maniu CV, Redfield MM. Diastolic dysfunction: Insights into pathophysiology and pharmacotherapy. Expert Opin Pharmacother 2001;2:997‐1008. doi: 10.1517/14656566.2.6.997 [DOI] [PubMed] [Google Scholar]
- 28. Yang M, Zhang R, Tang H, Li G, Guan X, Yang Y, et al. E/E′ is a new independent predictor of recovered ejection fraction in patients with systolic heart failure undergoing ablation for atrial fibrillation. Front Cardiovasc Med 2021;8:707996. doi: 10.3389/fcvm.2021.707996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee R, Mittal S. Utility and limitations of long‐term monitoring of atrial fibrillation using an implantable loop recorder. Heart Rhythm 2018;15:287‐295. doi: 10.1016/j.hrthm.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 30. Sardu C, Santamaria M, Funaro S, Sacra C, Barbieri M, Paolisso P, et al. Cardiac electrophysiological alterations and clinical response in cardiac resynchronization therapy with a defibrillator treated patients affected by metabolic syndrome. Medicine (Baltimore) 2017;96:e6558. doi: 10.1097/MD.0000000000006558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sardu C, Marfella R, Santamaria M, Papini S, Parisi Q, Sacra C, et al. Stretch, injury and inflammation markers evaluation to predict clinical outcomes after implantable cardioverter defibrillator therapy in heart failure patients with metabolic syndrome. Front Physiol 2018;9:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate logistic regression analysis to detect predictors of improved LVEF trajectory after rhythm control.


