1. Introduction
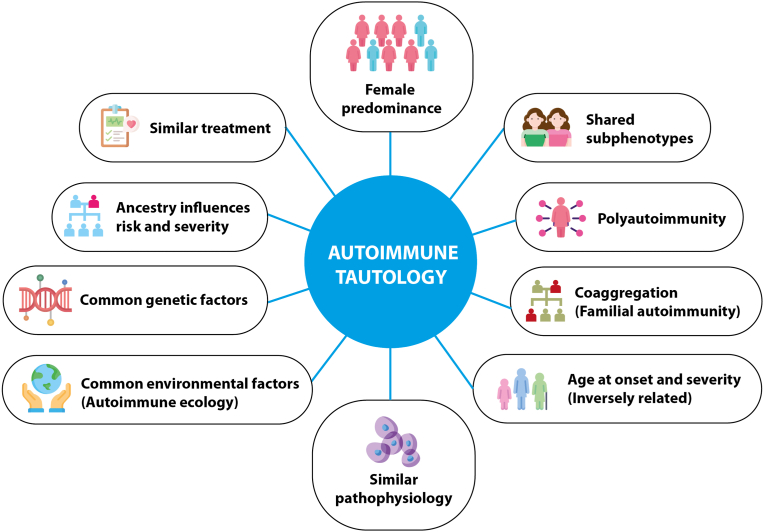
Autoimmune diseases (ADs) have a common pathophysiological mechanism as a consequence of sharing genetic and environmental factors, as well as similar clinical manifestations and therapeutic approaches, which led to the coining of the term autoimmune tautology in 2010 [1]. The arguments supporting this theory (Fig. 1) have been confirmed by several works since then.
Fig. 1.
Shared features of autoimmune diseases.
Tautology (from Greek tauto, “the same” and logos, “word/idea”) is an obvious statement. In logic, tautology is a formula, which is true in every possible interpretation. Thus, autoimmune tautology means that one AD is similar to a second one, to a third one, and so on. They cannot be equal because the target cell and organ are different in each case, and influencing factors although similar may vary from one patient to another as well as from one population to another.
Since the last summary of evidence about the topic [2] several publications sustaining the theory have been published [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]] including this special issue [[3], [4], [5], [6], [7], [8], [9], [10]], which are all discussed here.
2. Female preponderance
It is estimated that around 5%-10% of the world population lives with AD, both men and women are affected but around 70–80% are women [11]. The female immune response is stronger than in men, which is believed generates a greater risk of developing ADs, due to female sex hormones, X chromosome genes, and the microbiome, among others [11]. Although female sex hormones play an important role, female predominance has been described in both prepubertal and postmenopausal women, suggesting that there are other associated mechanisms [11]. When there are supernumerary X chromosomes, such as in men with Klinefelter syndrome (47,XXY), the susceptibility for developing systemic lupus erythematosus (SLE) and other ADs is significantly higher than in healthy men. A similar scenario occurs in women with normal karyotype and women with triple X syndrome (47,XXX) who have 25 times more risk of developing SLE [11]. Women with Turner Syndrome (45X0) have a lower risk [11,12].
The X chromosome inactivation (XCI) process occurs in the embryological development [13]. The X-inactive-specific transcript (XIST) gene induces epigenetic mechanisms resulting in a change of euchromatin into heterochromatin, which is known to be the inactive form of the genetic material. Consequently, it acts as a “gene silencing” [13]. Despite the inactivation of the X chromosome, about 15% of the genes escape from XCI, resulting in the dual expression ratio of the given gene in females [13]. Many escaped genes do not have a functional homologous gene on the Y chromosome, giving them a specific characteristic [13]. Within the escaped genes are CD40L, CD99, LAMP2, IRAK1, TLR7, USP27X, DDX3X, CXORF21 and XIAP, most of them are involved into the innate and adaptive immune system, and their proteins are produced twice in women than in men, due to the expression of two X chromosomes, contributing to the greater susceptibility of ADs in women [13].
Regarding the hormonal imbalance, progesterone, which has high levels in pregnancy as well as in the menstrual cycle at the luteal phase, generates immunosuppression, decreasing inflammatory mediators and inhibiting cellular immune responses such as decreased dendritic cell activation, natural-killer cells (NK), macrophages, and inhibition of the nuclear factor kappa B (NK-kB) [12]. For this reason, progesterone has been associated with a better clinical course of Th-1-mediated diseases such as multiple sclerosis (MS) and rheumatoid arthritis (RA) during pregnancy [12].
Estrogens are also elevated during pregnancy and decreased at menopause. There are shock receptors such as the pulsed estrogen receptor alfa (ERα) on T cells and the estrogen receptor beta (ERβ) on B cells, through which the upregulation of interferon (IFN) regulatory factor 5 (IRF5) and IFN-γ occurs, and at high concentrations they promote the Th2 response [12,14]. On the other hand, androgens induce downregulation of the immune response. In males they promote the AIRE gene in the thymus after binding to the androgen receptor, causing an important decrease of the inflammatory response [12].
3. Shared subphenotypes
Multiple subphenotypes are common among systemic ADs, like the age groups predominantly affected, arthralgia, arthritis, photosensitivity, Raynaud's phenomenon, and cardiovascular involvement [2]. The common presence of non-specific antibodies, including antinuclear antibodies (ANA), rheumatoid factor, anti-Ro antibodies [2] all of which disclose a low specificity and are common among several autoimmune conditions. Particular patters of cytokines such as TNF, IL-1, IL-6, IL-10 and IL-17 have also been described in diverse ADs as part of similarities shared by these conditions [2].
4. Polyautoimmunity
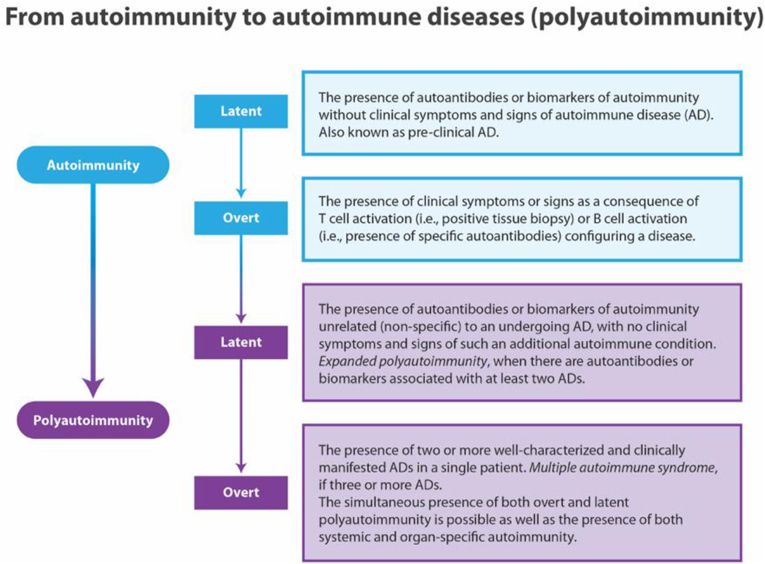
Overt polyautoimmunity (PolyA), defined as the presence of two or more well defined ADs in the same patient (Fig. 2), is present in approximately 40% of patients with SLE, Sjögren's syndrome (SS), autoimmune thyroid disease (AITD) and in 20% of RA [2]. Among the risk factors described for developing PolyA, ancestry, gender, education, familial autoimmunity (i.e., coaggregation of ADs) and economic status have been described. Latent polyautoimmunity is more frequent that overt polyautoimmunity [15]. The significance of PolyA lies in the possibility of a new classification of autoimmune illnesses based on it [15].
Fig. 2.
From Autoimmunity to autoimmune diseases [42].
In a systematic review and meta-analysis [16], of the prevalence of latent and overt PolyA on AITD, Graves' disease (GD) was the most prevalent autoimmune thyroid pathology. Overt PolyA was found in 13.46% of patients with AITD and latent PolyA in 17.45%. Within the PolyA types, type 1 diabetes (T1D) and autoimmune gastritis were the most representative, indicating that both latent and overt PolyA are common in AITD [16]. The study by Santos-Moreno et al. [17] found that 34.8% of their patients with SLE had PolyA, with antiphospholipid syndrome (APS) being the most frequent coexistent disease. PolyA in these patients was associated with sicca symptoms, thromboembolic phenomena, and fewer renal complications [17]. Unfortunately, this study did not evaluate the presence of AITD.
PolyA is a condition that may occur throughout the lifetime of an AD patient, as shown by the research of Bao et al. [18] in T1D. Even though PolyA has been identified in almost all ADs, the cross-sectional design of the majority of the studies is a factor explaining the varied PolyA prevalence rates among them.
One of the animal models of PolyA is the Non-Obese Diabetic (NOD) mice with autoimmune diabetes, in which PolyA is observed in relation with AITD and SS [19]. This phenomenon is promoted by targeting AIRE gen, IL-2 or performing thymectomy [19].
5. Familial autoimmunity
There are two types of familial clustering of ADs. The first and more common is coaggregation (i.e., familial autoimmunity), defined as the presence of diverse ADs in multiple members of a nuclear family. Second, familial autoimmune disease, where the same AD is observed in the nuclear family (i.e., aggregation) [20].
A Taiwanese study indicated that the risks of RA and other ADs increased in individuals with an RA family history, and approximately two-thirds of RA phenotypic variation was explained by familial factors [21]. Similarly, a Swedish study [22], reported that significant familial risks for discordant rheumatoid autoimmune diseases (i.e., familial autoimmunity) was observed for several ADs among patients with Polymyositis/Dermatomyositis (PM/DM), RA, SS, SLE and SSc. Another study estimated the heritability and genetic overlap in seven organ-specific ADs using a cohort of 11,814 twins [23]. Coaggregation was more pronounced in monozygotic twins (median hazard ratios (HR): 3.2, range: 2.2–9.2) than in dizygotic twins (median HR: 2.4, range: 1.1–10.0) [23]. Avalos-Díaz et al. [6], studied a set of monozygotic male twin patients who develop AITD and vitiligo associated with the HLA-DRB1*04-DQB1*03:02 and HLA-DRB1*03-DQB1*0201 haplotypes, exhibiting clinical data of multiple autoimmunity in relation to different epitopes that may be handled by MHC II molecules.
PolyA and familial autoimmunity represent extreme phenotypes that are ideal for identifying major genomic variants contributing to autoimmunity [2].
6. Age of onset
The age at onset of disease impacts the prognosis of ADs which tend to be more serious at a younger age of presentation. For instance, late-onset SLE (age at onset >50 years) is associated with less renal and neurological manifestations as compared to early-onset SLE (age at onset <50 years) [24]. Nevertheless, multimorbidity, where the co-occurrence of two conditions co-existing without any implicit ordering, can be a severity bias [25]. For this reason, the comparative analysis of ADs with respect to their prognosis based on age of onset, should be taken with caution, since older patients will have a greater burden of the same disease associated with other pathologies that may exacerbate or aggravate the underlying condition [26].
In autoimmune neurological diseases age at onset does not always influence the prognosis. A Spanish study classified myasthenia gravis (MG) based on early-onset (age at onset <50 years), late-onset (onset ≥50 and < 65 years), and very-late-onset MG (onset ≥65 years) and observed that patients with very-late-onset group presented with more life-threatening events. Late-onset MG and very-late-onset MG groups had more frequently ocular MG and a maximal worsening [27]. Neurological plasticity, especially in MS, reduce the effect of age at onset on severity. Cognitive impairment may be more accentuated in adults as compared to children, and not necessarily related. Butler Pagnotti et al. [28], compared cognition disease characteristics between adult onset (between the ages 20 and 40 years) versus late onset MS (onset after the age of 50 years), and showed that individuals with late onset disease had more impairment on tasks of visual learning and memory and working memory than the other group.
7. Similar pathophysiology
Loss of tolerance of the immune system and increased reactivity of T and B lymphocytes that end up causing tissue damage are characteristics of ADs. These disorders' similar heritability and genetic overlap point to a shared pathogenesis. In tissue damaged by autoimmunity, the predominant infiltrating cells include macrophages, neutrophils, autoreactive CD8+ cytolytic T cells, and autoreactive CD4+ T cells [2]. Increased number of memory CD4+ T cells that promote autoimmunity has been observed in animal models of autoimmunity [29,30]. Among effector T cells, Th1, Th17, and Th9 cells contribute to the pathogenesis of ADs [31]. Defective regulatory function in T and B cells and activation of the type I interferon system are other important common mechanisms in ADs [2,32].
Kourilovitch et al. [4], studied neutrophil-to-lymphocyte ratio (NLR) as a biomarker who reflex complex processes orchestrated by neutrophils. An interval of 1–2 is considered as a normal value, 2–3 as a grey area indicating subclinical inflammation and values above 3 as inflammation. Authors indicated that NLR could be also interpreted as a predictor of relapses [4]. Duarte-Delgado et al. [10], report in this issue of the Journal similar metabolites and metabolic pathways associated with RA and SLE. In both diseases, there is a decrease in several amino acids and oxidative stress-related metabolites like glutathione [10].
In the NOD mouse models, as in people with AITD, the thyroid immune cell infiltration is predominantly composed of CD4+, CD8+, T cells, B cells and macrophages, demonstrating the similar pathophysiology with humans [19].
Tolerance defects are also an important mechanism leading to ADs. Alterations that lead to ineffective clearance of immune complexes and accumulation of apoptotic cells expose the immune system to various autoantigens; inappropriate cell death or survival is also believed to be involved in the pathogenesis of various ADs [33]. Autoantibodies appear before clinical symptoms, for example, ANA can appear 2.7–3.0 years before the diagnosis of SLE [2,34]. The risk of acquiring an AD (i.e., overt autoimmunity) rises in direct proportion to the quantity of autoantibodies present before the clinical symptoms occur [35].
8. Autoimmune ecology
Ecology (Greek: οἶκος, “house”; -λογία, “study of”) studies the interactions between organisms and their environment [2,36]. Autoimmune ecology refers to the effect and relation between environmental factors and the risk and course of ADs [2,36]. Among them, tobacco, vitamin D, silicone, air pollution, and infectious agents have been the most studied.
Different infectious agents can trigger ADs, and a single one can affect the development of multiple ADs. Campylobacter jejuni and Zika virus have been cleared linked to the development of Guillain-Barré syndrome (GBS) [37,38]. Yersinia enterocolitica infection is associated with Hashimoto's thyroiditis (HT) [37]. Garcia-Carrasco's group [8], reviewed H. pylori infection and its association with ADs, like SLE, RA, and SS. The role of H. pylori infection in this process remains unclear, but eradication should be recommended in patients with ADs or when a high risk of developing them exists. Posso-Osorio et al. [9], reviewed the human endogenous retroviruses (HERV) and non-HERV viruses incorporated into the human genome, supporting they are part of many research processes to understand their biological role in normal cell physiology and pathological processes such as ADs.
Vitamin D deficiency is a risk factor for developing ADs due to its impact on the microbiota, in addition to the immunomodulatory role through its receptor in dendritic cells, monocytes and T cells activation, mainly in inflammatory bowel disease (IBD), coeliac disease (CD), autoimmune hepatitis, T1D and AITD [39,40]. Vitamin D supplementation (2000 IU/day) for five years, with or without omega 3 fatty acids, reduced ADs by 22% [41].
Another recent aspect of autoimmune ecology is Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and Coronavirus disease (COVID-19) vaccines. SARS-CoV-2 is a new incriminating pathogen in the etiology of autoimmunity. There is a close relationship between SARS-CoV-2 infection and autoimmunity, first suggested by the presence of multiple autoantibodies that were associated with acute disease severity and mortality [42]. Patients with post-Covid syndrome present, several months after the acute illness, persistent inflammation and autoantibodies remain positive [43]. In addition, some patients develop overt ADs [44]. All of the above observations anticipated that “it would be possible to observe an increase incidence of ADs in the coming years and that, therefore, health and social care services will require a new framework to treat such patients” [45]. The large propensity score-matched cohort study by Chang et al. [46] confirmed the association between of COVID-19 and ADs.
Autoimmunity has been also described after vaccination against COVID-19. Rodríguez et al. [47], carried out an updated bibliographic review, where the most frequent diseases were immune thrombocytopenia, myocarditis and GBS, being widely prevalent among women.
Sasmaca et al. [3], review in this issue of the Journal the impact of the COVID-19 pandemic on patients with CD, finding an increased incidence during the COVID-19 pandemic. Multiple environmental factors may contribute to ADs, thus these interactions warrant further studies. For example, adherence to the diet is crucial for the patient's health and quality of life. Celiac disease is not uncommon among patients with ADs, therefore gluten-free diet have been advocated as a therapeutic tool in these patients [48].
Both latent and overt autoimmunity are dramatically increasing in many parts of the world, likely due to variations in exposures to environmental factors [49]. Therefore, more study on the autoimmune ecology is required. The understanding of how environmental variables affect gene activity is far more difficult to grasp, despite the fact that a sizable number of genetic variants associated with ADs have been uncovered.
9. Common genetic factors
The genetic influence on polygenic ADs are of two types: those which are common to several ADs (e.g., PTPN22, HLADRB1) and those that are specific to a certain condition (INS in T1D) [50]. There is a significant genetic overlap amongst ADs, as shown by the occurrence of pleiotropic variations that are common to several diseases and occasionally have opposite effects. The understanding of the genetics of ADs has much advanced in recent years [[51], [52], [53], [54], [55], [56], [57]], including the discovery of monogenic ADs as part of inborn errors of immunity [58,59].
Recent and elegant original works and reviews have pointed out the genetic commonalities among ADs [51,52,54,[60], [61], [62], [63], [64]], which include hundreds of loci, of which the major histocompatibility complex (MHC) region harbors the strongest susceptibility genes for ADs.
It is anticipated that new research examining how genetic variations affect gene expression (e.g., expression QTLs, splicing, methylation, and chromatin phenotypes) in particular tissues and cell types, will clarify the biological effects of genetic variations on disease [65]. It is also expected that additional approaches such as single cell analyses, 3D chromatin structure analysis or genome editing will allow to increase the knowledge of the genetic influence of genes on ADs. As a corollary, understanding the basic processes underlying genetic variations will offer the potential to enhance disease prevention and treatment options.
10. Ancestry
Ancestry refers to the common origin of a specific population, other terms like continental ancestry and genetic ancestry, are described [66]. In ADs, genetic ancestry strongly contributes to the clinical heterogeneity, variation, and treatment response [2]. For example, African-Americans (AA) have shown 3 to 5 times more risk to develop SLE, compared with individuals with European ancestry (EA) [67]. Lupus nephritis is more severe on AA and Hispanic population, than in Europeans [67]. Ancestry influences the susceptibility of several others ADs including SS [66] T1D [68], RA [53], among others.
Ancestral differences in immune cells may contribute to the different disease course and incidence between populations [69]. Ancestry influences gene expression signatures. Much of the gene expression in patients with SLE is related to patient ancestry resulting in alterations in the proportions of hematopoietic cells, cellular processes, and signaling pathways detected [70]. There is a significant association between autoantibody profiles and differences in gene expression between ancestries (i.e., AA vs EA vs Native American ancestry) [70].
Guavita-Navarro et al. [7], compare the sensitivity of the European league against rheumatism/American College of rheumatology (EULAR/ACR) 2019 and Systemic Lupus International Collaborating Clinics (SLICC) 2012 classification criteria in a group of SLE patients of Latin American population of Amerindian ancestry. They observed no differences between the EULAR/ACR and SLICC 2012 criteria in the population studied.
To date, studies of the genetics of ADs, including ancestry, have largely focused on EA populations. In the near future, ancestry will be the understanding basis of several clinical and treatment variations between individuals with the same AD.
11. Similar treatment
The final premise of the autoimmune tautology theory follows from the earlier ones and corresponds to the fact that ADs receive identical therapies. Conventional synthetic disease-modifying antirheumatic drugs are widely used for ADs, being the first line of treatment in many ADs [71].
There are biological and synthesis inhibitors therapies such as Janus kinase (JAK) inhibitors (e.g., Tofacitinib). Within biological therapies, B cell depletion, by direct depletion with monoclonal antibodies (e.g., rituximab) and indirect depletion via survival cytokine blockade (e.g., belimumab) [72] are used for several ADs. JAK inhibitors are currently used to treat RA, psoriatic arthritis, alopecia areata, vitiligo, discoid lupus erythematosus, dermatomyositis, SLE, SS and IBD [73,74]. Finally, drug repurposing based on the physiopathology of ADs will also improve the treatment of ADs [75].
12. Conclusion
The heterogeneity of ADs may be attributable to a collection of diverse disorders based on epidemiology, pathology, or diagnostic findings, but the underlying physiopathological mechanisms are common. Identification of such common mechanisms will improve our comprehension of these complex, common, and sometimes devastating diseases and allow us to develop a new taxonomy, predict their occurrence, and discover new therapeutic interventions.
Note added in proof. A recent work by Nathalie Conrad and colleagues showed that the prevalence of ADs in the UK was 10·2%, and that the number of new AD diagnoses increased by 22% during their study period (2017–19 vs 2000–02 IRR 1·22 [1·18–1·28]), largely due to polyautoimmunity [76].
Credit author statement
Juan-Manuel Anaya: Conceptualization, writing and editing. Santiago Beltrán: writing and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the patients for teaching us the commonalities of autoimmune diseases, as well as the colleagues and students who have contributed to our research throughout the years. We apologize for our inability to reference several additional outstanding articles on this topic due to space limitations.
References
- 1.Anaya J.M. The autoimmune tautology. Arthritis Res. Ther. 2010;12 doi: 10.1186/ar3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaya J.M. The autoimmune tautology. A summary of evidence. Joint Bone Spine. 2017;84:251–253. doi: 10.1016/j.jbspin.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Samasca G., Lerner A. Celiac disease in the COVID-19 pandemic. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kourilovitch M., Galarza–Maldonado C. Could a simple biomarker as neutrophil-to-lymphocyte ratio reflect complex processes orchestrated by neutrophils? J. Transl. Autoimmun. 2022 doi: 10.1016/j.jtauto.2022.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales-Tisnés T., Quintero-Ortiz L., Quintero-Muñoz E., Sierra-Matamoros F., Arias-Aponte J., Rojas-Villarraga A. Prevalence of hospital readmissions and related factors in patients with autoimmune diseases. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avalos-Díaz E., Pérez-Pérez E., Granados J., Pacheco-Tovar D., Bollain-y-Goytia-de-la-Rosa J.J., Herrera-Esparza R. Multiple autoimmunity and epitope spreading in monozygotic twins. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guavita-Navarro D., Gallego-Cardona L., Arredondo A.M., Cubides H., Cajamarca-Barón J., Ibáñez C., Escobar A., Rojas-Villarraga A. Comparison of the sensitivity of the EULAR/ACR 2019 and SLICC 2012 classification criteria in a Colombian population with systemic lupus erythematosus. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etchegaray-Morales I., Jiménez-Herrera E.A., Mendoza-Pinto C., Rojas-Villarraga A., Macías-Díaz S., Osorio-Peña Á.D., Munguía-Realpozo P., García-Carrasco M. Helicobacter pylori and its association with autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis and Sjögren syndrome. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posso-Osorio I., Tobón G.J., Cañas C.A. Human endogenous retroviruses (HERV) and non-HERV viruses incorporated into the human genome and their role in the development of autoimmune diseases. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte-Delgado N.P., Cala M.P., Barreto A., Rodríguez C L.S. Metabolites and metabolic pathways associated with rheumatoid arthritis and systemic lupus erythematosus. J. Transl. Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miquel C.H., Faz-Lopez B., Guéry J.C. Influence of X chromosome in sex-biased autoimmune diseases. J. Autoimmun. 2023 doi: 10.1016/j.jaut.2023.102992. [DOI] [PubMed] [Google Scholar]
- 12.Billi A.C., Kahlenberg J.M., Gudjonsson J.E. Sex bias in autoimmunity. Curr. Opin. Rheumatol. 2019;31:53–61. doi: 10.1097/BOR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousavi M.J., Mahmoudi M., Ghotloo S. Escape from X chromosome inactivation and female bias of autoimmune diseases. Mol. Med. 2020;26 doi: 10.1186/s10020-020-00256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Florio D.N., Sin J., Coronado M.J., Atwal P.S., Fairweather D.L. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31 doi: 10.1016/j.redox.2020.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas M., Ramírez-Santana C., Acosta-Ampudia Y., Monsalve D.M., Rodriguez-Jimenez M., Zapata E., Naranjo-Pulido A., Suárez-Avellaneda A., Ríos-Serna L.J., Prieto C., Zambrano-Romero W., Valero M.A., Rodríguez Y., Mantilla R.D., Zhu C., Li Q.Z., Toro-Gutiérrez C.E., Tobón G.J., Anaya J.M. New insights into the taxonomy of autoimmune diseases based on polyautoimmunity. J. Autoimmun. 2022;126 doi: 10.1016/j.jaut.2021.102780. [DOI] [PubMed] [Google Scholar]
- 16.Botello A., Herrán M., Salcedo V., Rodríguez Y., Anaya J.M., Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin. Endocrinol. 2020;93:375–389. doi: 10.1111/cen.14304. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Moreno P., Arias-Aponte J., Rodríguez-Vargas G.S., Nieto-Zambrano P.D., Villarreal L., Ibatá L., Martinez S., Rubio-Rubio J.A., Rodríguez P., Rojas-Villarraga A. Polyautoimmunity in systemic lupus erythematosus patients: new insights from a cross-sectional study. J. Transl. Autoimmun. 2023;6 doi: 10.1016/j.jtauto.2022.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Y.K. Bao, L.G. Weide, V.C. Ganesan, I. Jakhar, J.B. Mcgill, S. Sahil, A.-L. Cheng, M. Gaddis, B.M. Drees, Y. Bao, High prevalence of comorbid autoimmune diseases in adults with type 1 diabetes from the HealthFacts database Running title: Comorbid Autoimmune Disease in Type 1 Diabetes, (n.d.). 10.1111/jdb.12856. [DOI] [PubMed]
- 19.Aubin A.M., Lombard-Vadnais F., Collin R., Aliesky H.A., McLachlan S.M., Lesage S. The NOD mouse beyond autoimmune diabetes. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.874769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cárdenas-Roldán J., Rojas-Villarraga A., Anaya J.M. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med. 2013;11 doi: 10.1186/1741-7015-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo C.F., Grainge M.J., Valdes A.M., See L.C., Yu K.H., Steven Shaw S.W., Luo S.F., Zhang W., Doherty M. Familial aggregation of rheumatoid arthritis and co-aggregation of autoimmune diseases in affected families: a nationwide population-based study. Rheumatology (United Kingdom). 2017;56:928–933. doi: 10.1093/rheumatology/kew500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomsen H., Li X., Sundquist K., Sundquist J., Försti A., Hemminki K. Familial associations for rheumatoid autoimmune diseases. Rheumatol. Adv. Pract. 2020;4 doi: 10.1093/rap/rkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skov J., Eriksson D., Kuja-Halkola R., Höijer J., Gudbjörnsdottir S., Svensson A.M., Magnusson P.K.E., Ludvigsson J.F., Kämpe O., Bensing S. Co-aggregation and heritability of organ-specific autoimmunity: a population-based twin study. Eur. J. Endocrinol. 2020;182:473–480. doi: 10.1530/EJE-20-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aljohani R., Gladman D.D., Su J., Urowitz M.B. Disease evolution in late-onset and early-onset systemic lupus erythematosus. Lupus. 2017;26:1190–1196. doi: 10.1177/0961203317696593. [DOI] [PubMed] [Google Scholar]
- 25.Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining comorbidity: implications for understanding health and health services. Ann. Fam. Med. 2009;7:357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu C.Y., Kuo C.F., Chou I.J., Chen J.S., Lu H.Y., Wu C.Y., Chen L.C., Huang J.L., Yeh K.W. Comorbidities of systemic lupus erythematosus prior to and following diagnosis in different age-at-onset groups. Lupus. 2022;31:963–973. doi: 10.1177/09612033221100908. [DOI] [PubMed] [Google Scholar]
- 27.Cortés-Vicente E., Álvarez-Velasco R., Segovia S., Paradas C., Casasnovas C., Guerrero-Sola A., Pardo J., Ramos-Fransi A., Sevilla T., López De Munain A., Gómez M.T., Jericó I., Gutiérrez-Gutiérrez G., Pelayo-Negro A.L., Martín M.A., Mendoza M.D., Morís G., Rojas-Garcia R., Díaz-Manera J., Querol L., Gallardo E., Vélez B., Albertí M.A., Galán L., García-Sobrino T., Martínez-Piñeiro A., Lozano-Veintimilla A., Fernández-Torrón R., Cano-Abascal Á., Illa I. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. 2020;94:e1171–e1180. doi: 10.1212/WNL.0000000000008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler Pagnotti R., Hua L.H., Miller J.B. Cognition and disease characteristics in adult onset versus late onset multiple sclerosis. Mult. Scler. J. 2022;28:933–941. doi: 10.1177/13524585211039112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raphael I., Joern R.R., Forsthuber T.G. Memory CD4+ T cells in immunity and autoimmune diseases. Cells. 2020;9 doi: 10.3390/cells9030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez-Villar M., Hafler D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018;19:665–673. doi: 10.1038/s41590-018-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda K., Takeuchi Y., Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019;41:283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 32.Marx A., Yamada Y., Simon-Keller K., Schalke B., Willcox N., Ströbel P., Weis C.-A. Thymus and autoimmunity. Semin. Immunopathol. 2021;43:45–64. doi: 10.1007/s00281-021-00842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe H., Son M. The immune tolerance role of the hmgb1-rage axis. Cells. 2021;10:1–14. doi: 10.3390/cells10030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Z.X., Miller J.S., Zheng S.G. An updated advance of autoantibodies in autoimmune diseases. Autoimmun. Rev. 2021;20 doi: 10.1016/j.autrev.2020.102743. [DOI] [PubMed] [Google Scholar]
- 35.Bieber K., Hundt J.E., Yu X., Ehlers M., Petersen F., Karsten C.M., Köhl J., Kridin K., Kalies K., Kasprick A., Goletz S., Humrich J.Y., Manz R.A., Künstner A., Hammers C.M., Akbarzadeh R., Busch H., Sadik C.D., Lange T., Grasshoff H., Hackel A.M., Erdmann J., König I., Raasch W., Becker M., Kerstein-Stähle A., Lamprecht P., Riemekasten G., Schmidt E., Ludwig R.J. Autoimmune pre-disease. Autoimmun. Rev. 2023;22 doi: 10.1016/j.autrev.2022.103236. [DOI] [PubMed] [Google Scholar]
- 36.Anaya J.M., Restrepo-Jiménez P., Ramírez-Santana C. The autoimmune ecology: an update. Curr. Opin. Rheumatol. 2018;30:350–360. doi: 10.1097/BOR.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 37.Rojas M., Restrepo-Jiménez P., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Leung P.S.C., Ansari A.A., Gershwin M.E., Anaya J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018;95:100–123. doi: 10.1016/j.jaut.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Pinto-Díaz C.A., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Molano-González N., Anaya J.M., Ramírez-Santana C. Autoimmunity in Guillain-Barré syndrome associated with Zika virus infection and beyond. Autoimmun. Rev. 2017;16:327–334. doi: 10.1016/j.autrev.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Yang C.Y., Leung P.S.C., Adamopoulos I.E., Gershwin M.E. The implication of vitamin D and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 2013;45:217–226. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dipasquale V., Lo Presti G., Milani G.P., Corsello A., Agostoni C., Romano C. Vitamin D in prevention of autoimmune diseases. Front. Biosci. - Landmark. 2022;27 doi: 10.31083/j.fbl2710288. [DOI] [PubMed] [Google Scholar]
- 41.Hahn J., Cook N.R., Alexander E.K., Friedman S., Walter J., Bubes V., Kotler G., Lee I.M., Manson J.A.E., Costenbader K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376 doi: 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anaya J.M., Monsalve D.M., Rojas M., Rodríguez Y., Montoya-García N., Mancera-Navarro L.M., Villadiego-Santana A.M., Rodríguez-Leguizamón G., Acosta-Ampudia Y., Ramírez-Santana C. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Zapata E., Ramírez-Santana C., Anaya J.M. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis. 2022;12:2155–2162. doi: 10.1093/infdis/jiac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas M., Rodríguez Y., Acosta-Ampudia Y., Monsalve D.M., Zhu C., Li Q.Z., Ramírez-Santana C., Anaya J.M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022;20 doi: 10.1186/s12967-022-03328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anaya J.M., Herrán M., Beltrán S., Rojas M. Is post-COVID syndrome an autoimmune disease? Expet Rev. Clin. Immunol. 2022;18 doi: 10.1080/1744666X.2022.2085561. [DOI] [PubMed] [Google Scholar]
- 46.Chang R., Yen-Ting Chen T., Wang S.I., Hung Y.M., Chen H.Y., Wei C.C.J. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56 doi: 10.1016/j.eclinm.2022.101783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez Y., Rojas M., Beltrán S., Polo F., Camacho-Domínguez L., Morales S.D., Gershwin M.E., Anaya J.M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022;132 doi: 10.1016/j.jaut.2022.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denham J.M., Hill I.D. Celiac disease and autoimmunity: review and controversies. Curr. Allergy Asthma Rep. 2013;13:347–353. doi: 10.1007/s11882-013-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller F.W. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023;80 doi: 10.1016/j.coi.2022.102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anaya J.M., Gómez L., Castiblanco J. Is there a common genetic basis for autoimmune diseases? Clin. Dev. Immunol. 2006;13 doi: 10.1080/17402520600876762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saurabh R., Fouodo C.J.K., König I.R., Busch H., Wohlers I. A survey of genome-wide association studies, polygenic scores and UK Biobank highlights resources for autoimmune disease genetics. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.972107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki A., Guerrini M.M., Yamamoto K. Functional genomics of autoimmune diseases. Ann. Rheum. Dis. 2021;80:689–697. doi: 10.1136/annrheumdis-2019-216794. [DOI] [PubMed] [Google Scholar]
- 53.Ishigaki K., Sakaue S., Terao C., Luo Y., Sonehara K., Yamaguchi K., Amariuta T., Too C.L., Laufer V.A., Scott I.C., Viatte S., Takahashi M., Ohmura K., Murasawa A., Hashimoto M., Ito H., Hammoudeh M., Al Emadi S., Masri B.K., Halabi H., Badsha H., Uthman I.W., Wu X., Lin L., Li T., Plant D., Barton A., Orozco G., Verstappen S.M.M., Bowes J., MacGregor A.J., Honda S., Koido M., Tomizuka K., Kamatani Y., Tanaka H., Tanaka E., Suzuki A., Maeda Y., Yamamoto K., Miyawaki S., Xie G., Zhang J., Amos C.I., Keystone E., Wolbink G., van der Horst-Bruinsma I., Cui J., Liao K.P., Carroll R.J., Lee H.S., Bang S.Y., Siminovitch K.A., de Vries N., Alfredsson L., Rantapää-Dahlqvist S., Karlson E.W., Bae S.C., Kimberly R.P., Edberg J.C., Mariette X., Huizinga T., Dieudé P., Schneider M., Kerick M., Denny J.C., Matsuda K., Matsuo K., Mimori T., Matsuda F., Fujio K., Tanaka Y., Kumanogoh A., Traylor M., Lewis C.M., Eyre S., Xu H., Saxena R., Arayssi T., Kochi Y., Ikari K., Harigai M., Gregersen P.K., Yamamoto K., Louis Bridges S., Padyukov L., Martin J., Klareskog L., Okada Y., Raychaudhuri S. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat. Genet. 2022;54:1640–1651. doi: 10.1038/s41588-022-01213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H.G., McDermott G., Seyok T., Huang S., Dahal K., L’Yi S., Lea-Bonzel C., Stratton J., Weisenfeld D., Monach P., Raychaudhuri S., Yu K.H., Cai T., Cui J., Hong C., Cai T., Liao K.P. Identifying shared genetic architecture between rheumatoid arthritis and other conditions: a phenome-wide association study with genetic risk scores. EBioMedicine. 2023;92 doi: 10.1016/j.ebiom.2023.104581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.M. Fazel-Najafabadi, L.L. Looger, H. Reddy-Rallabandi, S.K. Nath, A multilayered post-GWAS analysis pipeline defines functional variants and target genes for systemic lupus erythematosus (SLE), (n.d.). 10.1101/2023.04.07.23288295. [DOI] [PMC free article] [PubMed]
- 56.Khatri B., Tessneer K.L., Rasmussen A., Aghakhanian F., Reksten T.R., Adler A., Alevizos I., Anaya J.M., Aqrawi L.A., Baecklund E., Brun J.G., Bucher S.M., Eloranta M.L., Engelke F., Forsblad-d’Elia H., Glenn S.B., Hammenfors D., Imgenberg-Kreuz J., Jensen J.L., Johnsen S.J.A., Jonsson M.V., Kvarnström M., Kelly J.A., Li H., Mandl T., Martín J., Nocturne G., Norheim K.B., Palm Ø., Skarstein K., Stolarczyk A.M., Taylor K.E., Teruel M., Theander E., Venuturupalli S., Wallace D.J., Grundahl K.M., Hefner K.S., Radfar L., Lewis D.M., Stone D.U., Kaufman C.E., Brennan M.T., Guthridge J.M., James J.A., Scofield R.H., Gaffney P.M., Criswell L.A., Jonsson R., Eriksson P., Bowman S.J., Omdal R., Rönnblom L., Warner B., Rischmueller M., Witte T., Farris A.D., Mariette X., Alarcon-Riquelme M.E., Shiboski C.H., Wahren-Herlenius M., Ng W.F., Sivils K.L., Adrianto I., Nordmark G., Lessard C.J. Genome-wide association study identifies Sjögren’s risk loci with functional implications in immune and glandular cells. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-30773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorlacius G.E., Björk A., Wahren-Herlenius M. Genetics and epigenetics of primary Sjögren syndrome: implications for future therapies. Nat. Rev. Rheumatol. 2023 doi: 10.1038/s41584-023-00932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amaya-Uribe L., Rojas M., Azizi G., Anaya J.M., Gershwin M.E. Primary immunodeficiency and autoimmunity: a comprehensive review. J. Autoimmun. 2019;99 doi: 10.1016/j.jaut.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Gray P.E., David C. Inborn errors of immunity and autoimmune disease. J. Allergy Clin. Immunol. Pract. 2023 doi: 10.1016/j.jaip.2023.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Ortíz-Fernández L., Martín J., Alarcón-Riquelme M.E. A summary on the genetics of systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and Sjögren’s syndrome. Clin. Rev. Allergy Immunol. 2022 doi: 10.1007/s12016-022-08951-z. [DOI] [PubMed] [Google Scholar]
- 61.Shirai Y., Nakanishi Y., Suzuki A., Konaka H., Nishikawa R., Sonehara K., Namba S., Tanaka H., Masuda T., Yaga M., Satoh S., Izumi M., Mizuno Y., Jo T., Maeda Y., Nii T., Oguro-Igashira E., Morisaki T., Kamatani Y., Nakayamada S., Nishigori C., Tanaka Y., Takeda Y., Yamamoto K., Kumanogoh A., Okada Y. Multi-Trait and cross-population genome-wide association studies across autoimmune and allergic diseases identify shared and distinct genetic component. Ann. Rheum. Dis. 2022;81 doi: 10.1136/annrheumdis-2022-222460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding Y., Cui M., Qian J., Wang C., Shen Q., Ren H., Li L., Zhang F., Zhang R. Calculation of similarity between 26 autoimmune diseases based on three measurements including network, function, and semantics. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.758041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González-Serna D., Villanueva-Martin G., Acosta-Herrera M., Márquez A., Martín J. Approaching shared pathophysiology in immune-mediated diseases through functional genomics. Genes (Basel) 2020;11 doi: 10.3390/genes11121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acosta-Herrera M., Kerick M., González-Serna D., Wijmenga C., Franke A., Gregersen P.K., Padyukov L., Worthington J., Vyse T.J., Alarcón-Riquelme M.E., Mayes M.D., Martin J. Genome-wide meta-analysis reveals shared new loci in systemic seropositive rheumatic diseases. Ann. Rheum. Dis. 2019;78 doi: 10.1136/annrheumdis-2018-214127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckner J.H. Translational immunology: applying fundamental discoveries to human health and autoimmune diseases. Eur. J. Immunol. 2023 doi: 10.1002/eji.202250197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Restrepo-Jiménez P., Molano-González N., Anaya J.M. Geoepidemiology of Sjögren’s syndrome in Latin America. Joint Bone Spine. 2019;86:620–626. doi: 10.1016/j.jbspin.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Goulielmos G.N., Zervou M.I., Vazgiourakis V.M., Ghodke-Puranik Y., Garyfallos A., Niewold T.B. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene. 2018;668:59–72. doi: 10.1016/j.gene.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 68.Onengut-Gumuscu S., Chen W.M., Robertson C.C., Bonnie J.K., Farber E., Zhu Z., Oksenberg J.R., Brant S.R., Louis Bridges S., Edberg J.C., Kimberly R.P., Gregersen P.K., Rewers M.J., Steck A.K., Black M.H., Dabelea D., Pihoker C., Atkinson M.A., Wagenknecht L.E., Divers J., Bell R.A., Erlich H.A., Concannon P., Rich S.S. Type 1 diabetes risk in African-ancestry participants and utility of an ancestry-specific genetic risk score. Diabetes Care. 2019;42:406–415. doi: 10.2337/dc18-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nédélec Y., Sanz J., Baharian G., Szpiech Z.A., Pacis A., Dumaine A., Grenier J.C., Freiman A., Sams A.J., Hebert S., Pagé Sabourin A., Luca F., Blekhman R., Hernandez R.D., Pique-Regi R., Tung J., Yotova V., Barreiro L.B. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. 2016;167:657–669.e21. doi: 10.1016/j.cell.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 70.Catalina M.D., Bachali P., Yeo A.E., Geraci N.S., Petri M.A., Grammer A.C., Lipsky P.E. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kerrigan S.A., McInnes I.B. Reflections on ‘older’ drugs: learning new lessons in rheumatology. Nat. Rev. Rheumatol. 2020;16:179–183. doi: 10.1038/s41584-020-0375-7. [DOI] [PubMed] [Google Scholar]
- 72.Barnas J.L., Looney R.J., Anolik J.H. B cell targeted therapies in autoimmune disease. Curr. Opin. Immunol. 2019;61:92–99. doi: 10.1016/j.coi.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamilloux Y., El Jammal T., Vuitton L., Gerfaud-Valentin M., Kerever S., Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019;18 doi: 10.1016/j.autrev.2019.102390. [DOI] [PubMed] [Google Scholar]
- 75.Kingsmore K.M., Grammer A.C., Lipsky P.E. Drug repurposing to improve treatment of rheumatic autoimmune inflammatory diseases. Nat. Rev. Rheumatol. 2020;16 doi: 10.1038/s41584-019-0337-0. [DOI] [PubMed] [Google Scholar]
- 76.Conrad N., Misra S., Verbakel J.Y., Verbeke G., Molenberghs G., Taylor P.N., Mason J., Sattar N., McMurray J.J.V., McInnes I.B., Khunti K., Cambridge G. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–1890. doi: 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]