ABSTRACT
Background
The aim of this study was to provide an overview of age, sex and primary renal disease (PRD) distribution among first kidney transplant recipients across Europe.
Method
The European Renal Association (ERA) Registry database was used to obtain data on patients aged 20 years or older receiving their first kidney transplant between 2010 and 2019 from 12 European countries. The numbers and percentages of recipients in each age, sex and PRD group were calculated by country, donor type and year.
Results
In total, 99 543 adults received a first kidney transplant. Overall, 23% of the recipients were 65 years or older, 36% were female, and 21% had glomerulonephritis and 15% diabetes mellitus as PRD. Compared with deceased donor kidney transplant recipients, living donor kidney transplant recipients were less often 65 years or older (13% versus 26%), more often had glomerulonephritis (25% versus 20%) and less often diabetes mellitus (8% versus 17%) as PRD. We found large international differences, which were most prominent for age and PRD and less prominent for sex. Over time, the largest change in recipient characteristics was observed for the percentage of recipients aged 65 years or older, increasing from 18% in 2010 to 28% in 2019 for all countries combined with a similar trend in most countries.
Conclusion
We observed large differences for age and PRD distribution between recipients of living and deceased donor kidneys and between European countries. Over time, the percentage of older first kidney transplant recipients increased.
Keywords: age, kidney transplantation, patient characteristics, primary renal disease, sex
KEY LEARNING POINTS.
What was known:
Large international differences exist in the kidney transplantation rate across Europe.
The composition of the group of end-stage kidney disease patients receiving a transplant likely differs across countries.
Differences in transplant recipient populations could contribute to the explanation of existing international differences in patient survival after kidney transplantation.
This study adds:
Compared with recipients of a deceased donor kidney transplant, recipients of a first kidney transplant from a living donor were younger (proportion >65 years: 13% versus 26%), more often had glomerulonephritis (25% versus 20%) and less often diabetes mellitus (8% versus 17%) as primary renal disease.
Large international differences were found, which were most prominent for recipient age and primary renal disease and less prominent for sex.
Over time, the largest change in recipient characteristics was observed for the percentage of recipients aged 65 years or older, increasing from 18% in 2010 to 28% in 2019 for all countries combined, and with a similar trend in most countries.
Potential impact:
This study may inform clinicians and policy makers on the existence of differences in the composition of the recipient population, and may assist in the explanation of international differences in patient survival after kidney transplantation.
INTRODUCTION
Kidney transplantation is the preferred kidney replacement therapy (KRT) in terms of survival and quality of life for end-stage kidney disease (ESKD) patients compared with dialysis [1]. Large differences exist in the kidney transplantation rate across Europe [2]. For most countries the kidney transplantation rate has increased over the last decade [2].
Renal registries around the world have shown large international differences in age, sex and primary renal disease (PRD) of patients receiving a kidney transplant [3–5]. Such differences in transplant recipients may affect international differences in patient survival after kidney transplantation. Only a few studies have investigated changes over time in recipient characteristics for different countries [6–8]. An international overview of the time trends of PRDs in kidney transplant recipients is lacking.
An international comparison as well as time trends in kidney transplant recipient characteristics could elucidate disparities in ESKD patients receiving a kidney transplant. Therefore, the aim of this study was to provide an overview of age, sex and PRD distribution in adults receiving a first kidney transplant across Europe between 2010 and 2019.
MATERIALS AND METHODS
Patient data
The European Renal Association (ERA) Registry collects data on ESKD patients receiving KRT in Europe and countries bordering the Mediterranean Sea [5]. Individual patient data are collected by national or regional renal registries and sent to the ERA Registry annually. Data include country, patient identifier, month and year of birth, sex, PRD, date and type of treatment at start and during follow-up (dialysis or kidney transplantation), and date and cause of death. The ERA Registry database is nearly complete regarding age, sex and PRD.
The study population consisted of patients who were 20 years of age or older and received their first kidney transplant between 2010 and 2019 from the following countries: Austria (AT), Belgium (BE), Bosnia and Herzegovina (BA; 2011–19), Denmark (DK), Finland (FI), France (FR; 98% coverage), Greece (GR), Norway (NO), Spain (ES; 88% coverage), Sweden (SE), the Netherlands (NL), and the United Kingdom (UK).
Definition of variables
Recipient characteristics are reported by country or year as absolute numbers and percentages for age group, sex and PRD, and as median for age as a continuous variable. The age groups consisted of 20–44 years, 45–64 years, 65–74 years and 75 years or older. The PRD groups consisted of autosomal dominant polycystic kidney disease (ADPKD), congenital anomalies of the kidney and the urinary tract (CAKUT), diabetes mellitus type I and II (DM), hypertension/renal vascular disease (HT/RVD), glomerulonephritis (GN), other cause, unknown and missing. The recipient characteristics are presented for all first kidney transplants (total) and separately for deceased donor (DD) and living donor (LD) kidneys. The first kidney transplantation rate was calculated by dividing the number of first kidney transplantations by the general adult population count and multiplied by one million.
Time trends
The annual percentage change (APC) with 95% confidence intervals (95% CI) of the time trends in recipient characteristics was computed using Poisson regression provided by the Joinpoint regression program [9]. Joinpoint identifies points in time (e.g. years) where the trend of, in this case, recipient characteristics (e.g. age, sex and PRD) changes statistically significantly [10, 11]. Corresponding to the availability of 10 data points (i.e. 10 years in our study period), a maximum of one joinpoint (two trends) was used [10]. In addition, analyses were performed using zero joinpoints to obtain one trend for the entire study period. The year of kidney transplantation was added to the joinpoint model as independent variable and the country as by-variable. Separate models were made per age, sex and PRD group, adding the percentage of each age, sex and PRD group per year as dependent variable. The analyses were performed for total first kidney transplantations as well as for those from LDs and DDs separately, and for all participating countries together (ALL) and for each country separately. If the number of recipients was zero in a country for a particular age, sex or PRD group, the percentage was set on 0.1% in order to be able to calculate the APC.
For the time trends by country we present the average percentage of recipients by age, sex and PRD group for two time periods: 2010–14 and 2015–19. The corresponding APC represents the trend over the entire study period, including percentages by year, as previously described.
Software and statistical tests
The analyses were performed using SAS 9.4 (2016; SAS Institute Inc., Cary, NC, USA) [12] and Joinpoint 4.2.0.4 (2015; National Cancer Institute, Calverton, MD, USA) [9]. P-values <.05 were considered statistically significant. Pearson square (R2), with weighting for the general population counts of the participating countries, was used for the correlation between kidney transplantation rate and recipient characteristics.
RESULTS
In total, 99 543 patients received a first kidney transplant in the 12 participating European countries between 2010 and 2019. Supplementary data, Table S1 presents the number of total, DD and LD first kidney transplantations for each country by age, sex and PRD group.
Age
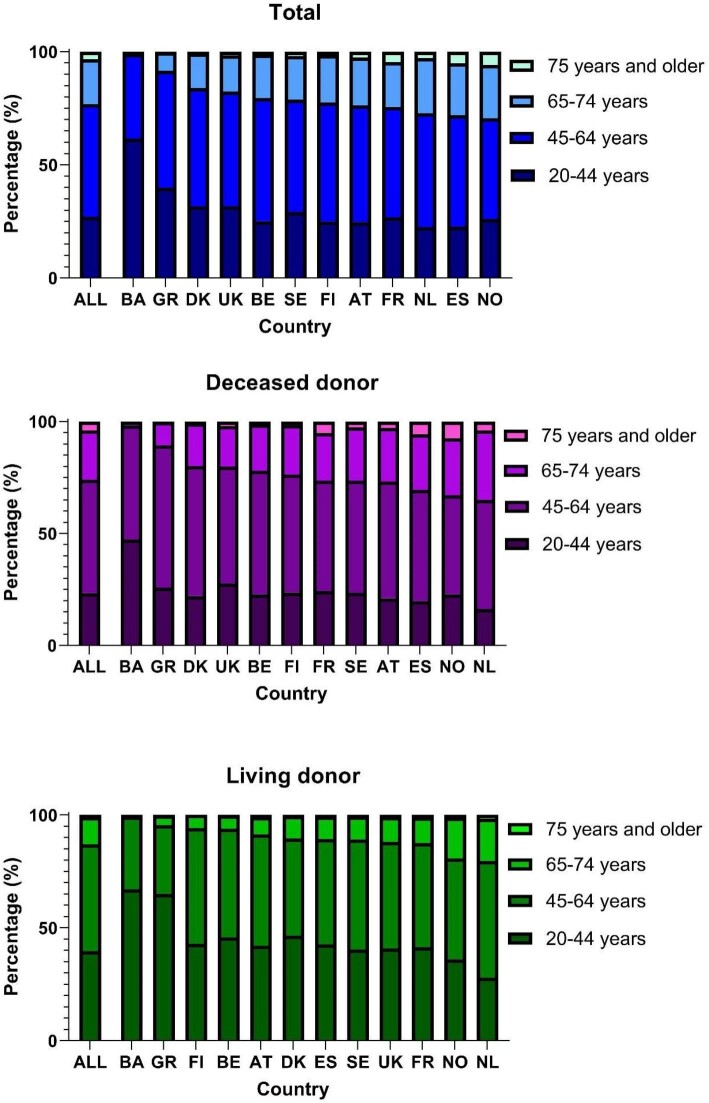
Overall, 23% of the recipients were aged 65 years or older (Fig. 1). Large international differences were observed, with Bosnia and Herzegovina and Greece having the lowest percentage of recipients aged 65 years or older (1% and 8%, respectively) and the Netherlands, Spain and Norway the highest percentages (>25%; Fig. 1). In all countries the percentage of older recipients was higher for DD (ranging between 2% in Bosnia and Herzegovina and 35% in the Netherlands) than for LD (ranging between 1% in Bosnia and Herzegovina and 20% in the Netherlands; Fig. 1).
Figure 1:
Age distribution of first kidney transplant recipients between 2010 and 2019 for total, DD and LD kidney transplantation by country. The order of countries is based on the percentage of recipients aged 65 years or older.
The number of first kidney transplantations increased between 2010 and 2019 for all age groups (Supplementary data, Table S2); however, the percentage of recipients aged 65 years or older increased (APC 4.5; 95% CI 3.8 to 5.2), while the percentage of recipients aged 20 to 64 years decreased (Supplementary data, Table S3). The size of the increase in patients aged 65 years or older differed across countries, with the largest increase in Spain and the Netherlands, both from approximately 24% in 2010–14 to 31% in 2015–19 (APC 5.3%; Table 1). For DD transplants, the percentage of older recipients increased over time (APC 4.1, 95% CI 3.2 to 4.9), with the Netherlands showing the largest increase (from 31% in 2010–14 to 39% in 2015–19, APC 6.4, 95% CI 2.4 to 10.7), followed by Spain (from 26% to 34%, APC 4.8, 95% CI 3.6 to 6.0) and Denmark (from 16% to 23%, APC 7.7, 95% CI 2.0 to 13.7; Table 1). For LD transplants, Norway and the Netherlands had the highest percentage of older recipients throughout the study period. The percentage of older recipients of a first LD kidney transplant increased over time (APC 6.4, 95% CI 5.2 to 7.6), with France, Norway and the Netherlands showing the most prominent increase (France: from 8% in 2010–14 to 15% in 2015–19, APC 15.4, 95% CI 11.2 to 19.9; Norway: 17% to 22%, APC 6.8, 95% CI 1.4 to 12.6; the Netherlands: 18% to 23%, APC 5.4, 95% CI 3.0 to 7.9; Table 1).
Table 1:
Percentage of recipients aged 65 years or older, percentage female and percentage with DM as PRD with corresponding APC between 2010 and 2019 for total, DD and LD first kidney transplantation, by country.
| Percentage aged 65 years or older | Percentage female | Percentage with DM as PRD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Cohort | APC 2010–19 | Cohort | Cohort | APC 2010–19 | Cohort | Cohort | APC 2010–19 | ||||
| 2010–14 | 2015–19 | (95% CI) | 2010–14 | 2015–19 | (95% CI) | 2010–14 | 2015–19 | (95% CI) | ||||
| Total | ||||||||||||
| ALL | 20.7 | 25.2 | 4.5 (3.8 to 5.2) | ↑ | 36.7 | 36.1 | –0.3 (–0.6 to 0.0) | ↓ | 15.0 | 15.7 | 1.0 (0.3 to 1.7) | ↑ |
| AT | 23.9 | 23.6 | –1.0 (–3.2 to 1.3) | 33.9 | 31.6 | –1.3 (–3.9 to 1.4) | 16.6 | 14.2 | –5.2 (–9.6 to –0.6) | ↓ | ||
| BA | 0.0 | 1.8 | NA | 35.6 | 33.3 | 0.3 (–11.6 to 13.8) | 7.8 | 6.3 | NA | |||
| BE | 19.4 | 21.6 | 1.7 (0.1 to 3.4) | ↑ | 37.1 | 34.3 | –0.5 (–2.8 to 1.8) | 12.1 | 13.8 | 0.6 (–3.4 to 4.7) | ||
| DK | 13.2 | 18.7 | 8.0 (3.6 to 12.6) | ↑ | 38.7 | 34.6 | –1.3 (–4.9 to 2.5) | 15.1 | 15.9 | –0.6 (–4.4 to 3.4) | ||
| ES | 23.9 | 31.5 | 5.3 (4.1 to 6.5) | ↑ | 35.5 | 34.9 | –0.5 (–1.0 to 0.0) | ↓ | 15.4 | 16.0 | 0.9 (–0.6 to 2.4) | |
| FI | 21.8 | 23.0 | 1.4 (–3.2 to 6.3) | 32.6 | 34.9 | 1.5 (–1.8 to 5.0) | 31.1 | 27.2 | –1.6 (–3.3 to 0.1) | |||
| FR | 22.0 | 26.4 | 5.3 (3.2 to 7.5) | ↑ | 37.0 | 36.4 | –0.6 (–1.4 to 0.3) | 13.5 | 14.0 | 0.8 (–0.4 to 2.2) | ||
| GR | 8.8 | 8.0 | 0.5 (–8.9 to 11.0) | 34.3 | 32.7 | –0.1 (–3.9 to 3.8) | 5.9 | 6.0 | –0.9 (–10.6 to 9.8) | |||
| NL | 23.7 | 30.5 | 5.3 (3.9 to 6.7) | ↑ | 38.4 | 38.2 | –0.1 (–1.7 to 1.6) | 13.9 | 14.3 | 1.7 (–0.5 to 4.0) | ||
| NO | 29.4 | 29.5 | 0.8 (–1.0 to 2.7) | 30.0 | 36.0 | 2.6 (–1.6 to 6.9) | 18.1 | 15.8 | –2.0 (–5.2 to 1.2) | |||
| SE | 19.6 | 22.6 | 3.9 (0.4 to 7.5) | ↑ | 36.3 | 33.0 | –1.2 (–3.6 to 1.3) | 17.4 | 16.3 | –1.6 (–4.1 to 0.9) | ||
| UK | 16.0 | 19.1 | 4.2 (3.1 to 5.4) | ↑ | 38.0 | 37.9 | 0.0 (–0.6 to 0.6) | 15.8 | 17.7 | 2.7 (1.3 to 4.1) | ↑ | |
| Deceased donor | ||||||||||||
| ALL | 23.6 | 28.1 | 4.1 (3.2 to 4.9) | ↑ | 36.4 | 36.1 | –0.3 (–0.7 to 0.2) | 17.1 | 17.8 | 1.0 (0.3 to 1.7) | ↑ | |
| AT | 27.0 | 26.7 | –0.8 (–3.0 to 1.4) | 34.7 | 32.1 | –1.5 (–4.3 to 1.5) | 18.9 | 16.2 | –5.1 (–9.6 to –0.3) | ↓ | ||
| BA | 0.0 | 3.3 | N.A. | 25.9 | 43.3 | –26.7 (–59.8 to 33.4) | 14.8 | 6.7 | N.A. | |||
| BE | 20.8 | 23.3 | 1.7 (–0.2 to 3.7) | 36.9 | 34.2 | –0.5 (–2.8 to 1.9) | 13.1 | 14.6 | 0.3 (–3.4 to 4.1) | |||
| DK | 15.7 | 23.2 | 7.7 (2.0 to 13.7) | ↑ | 40.2 | 36.4 | –0.5 (–3.5 to 2.5) | 15.5 | 17.2 | –0.7 (–7.0 to 6.1) | ||
| ES | 26.3 | 33.8 | 4.8 (3.6 to 6.0) | ↑ | 35.0 | 34.7 | –0.4 (–1.0 to 0.3) | 16.7 | 17.3 | 0.8 (–0.6 to 2.3) | ||
| FI | 22.4 | 24.7 | 2.0 (–2.4 to 6.6) | 33.0 | 34.4 | 1.2 (–2.2 to 4.7) | 31.8 | 28.9 | –0.9 (–2.8 to 1.0) | |||
| FR | 23.9 | 28.5 | 5.1 (2.8 to 7.5) | ↑ | 37.4 | 36.8 | –0.6 (–1.4 to 0.3) | 14.5 | 15.2 | 1.1 (–0.1 to 2.4) | ||
| GR | 10.8 | 10.5 | 1.6 (–9.8 to 14.4) | 35.1 | 35.5 | 0.4 (–4.0 to 5.0) | 5.6 | 6.3 | –4.7 (–24.5 to 20.2) | |||
| NL | 30.7 | 39.4 | 6.4 (2.4 to 10.7) | ↑ | 36.9 | 38.0 | –0.1 (–2.1 to 2.0) | 19.5 | 22.1 | 3.0 (1.2 to 4.9) | ↑ | |
| NO | 33.8 | 31.9 | –0.4 (–2.0 to 1.2) | 29.7 | 38.5 | 4.3 (–0.1 to 8.9) | 21.5 | 18.4 | –2.4 (–5.4 to 0.8) | |||
| SE | 24.4 | 28.0 | 3.4 (–0.1 to 7.0) | 37.9 | 33.7 | –1.9 (–4.6 to 0.8) | 22.0 | 19.4 | –1.9 (–3.5 to –0.2) | ↓ | ||
| UK | 18.9 | 21.3 | 3.2 (1.8 to 4.6) | ↑ | 37.2 | 37.3 | 0.2 (–0.6 to 1.0) | 19.4 | 21.1 | 2.2 (0.6 to 3.7) | ↑ | |
| Living donor | ||||||||||||
| ALL | 11.2 | 14.9 | 6.4 (5.2 to 7.6) | ↑ | 37.6 | 36.2 | –0.5 (–1.2 to 0.2) | 8.3 | 8.2 | –0.7 (–3.2 to 1.9) | ||
| AT | 8.0 | 9.0 | 11.3 (–23.8 to 62.7) | 29.9 | 29.2 | –0.1 (–5.4 to 5.6) | 4.8 | 4.7 | –4.5 (–14.1 to 6.2) | |||
| BA | 0.0 | 1.2 | >NA | 38.7 | 29.6 | 1.7 (–12.6 to 18.3) | 4.8 | 6.2 | NA | |||
| BE | 5.3 | 6.9 | 23.4 (–22.1 to 95.6) | 38.9 | 36.5 | –0.7 (–3.9 to 2.7) | 2.6 | 6.9 | 11.2 (–4.3 to 29.3) | |||
| DK | 9.9 | 10.9 | 6.0 (–4.9 to 18.1) | 36.7 | 31.3 | –3.7 (–6.4 to –1.0) | ↓ | 14.4 | 13.5 | –1.2 (–6.4 to 4.4) | ||
| ES | 8.8 | 12.5 | 9.3 (5.3 to 13.4) | ↑ | 39.1 | 36.2 | –0.6 (–2.8 to 1.6) | 7.3 | 6.2 | –3.5 (–10.4 to 4.0) | ||
| FI | 5.4 | 6.1 | >NA | 24.3 | 39.0 | 10.1 (–0.6 to 22.1) | 16.2 | 6.1 | >NA | |||
| FR | 8.3 | 15.1 | 15.4 (11.2 to 19.9) | ↑ | 33.7 | 34.0 | –0.1 (–1.3 to 1.2) | 6.0 | 8.0 | 2.5 (–3.7 to 9.1) | ||
| GR | 4.2 | 4.7 | –0.4 (–36.8 to 57.1) | 32.5 | 28.8 | –0.9 (–5.1 to 3.5) | 6.6 | 5.5 | –2.7 (–19.3 to 17.4) | |||
| NL | 17.5 | 23.1 | 5.4 (3.0 to 7.9) | ↑ | 39.8 | 38.4 | –0.1 (–2.1 to 1.9) | 8.9 | 7.8 | –0.5 (–4.8 to 4.1) | ||
| NO | 16.6 | 22.3 | 6.8 (1.4 to 12.6) | ↑ | 31.1 | 28.5 | –2.4 (–7.7 to 3.1) | 8.1 | 8.1 | 1.4 (–12.5 to 17.5) | ||
| SE | 12.1 | 9.4 | –1.6 (–9.1 to 6.5) | 34.0 | 31.2 | –0.3 (–3.6 to 3.2) | 10.2 | 8.8 | –4.9 (–15.1 to 6.5) | |||
| UK | 10.5 | 13.6 | 5.4 (3.6 to 7.2) | ↑ | 39.4 | 39.2 | –0.3 (–1.8 to 1.3) | 8.9 | 9.1 | 0.6 (–2.0 to 3.2) | ||
APCs were calculated using the percentages from each year. NA, not available. Arrows indicate statistically significant trends.
Overall, the median age of recipients at time of the first kidney transplantation increased by 2.9 years from 53.3 years in 2010 to 56.2 years in 2019 (Supplementary data, Table S4). For first DD transplant recipients the median age increased by 2.9 years from 54.7 to 57.6 years and for first LD transplant recipients by 2.4 years from 48.0 to 50.4 years. In all countries LD transplant recipients were on average younger than DD recipients.
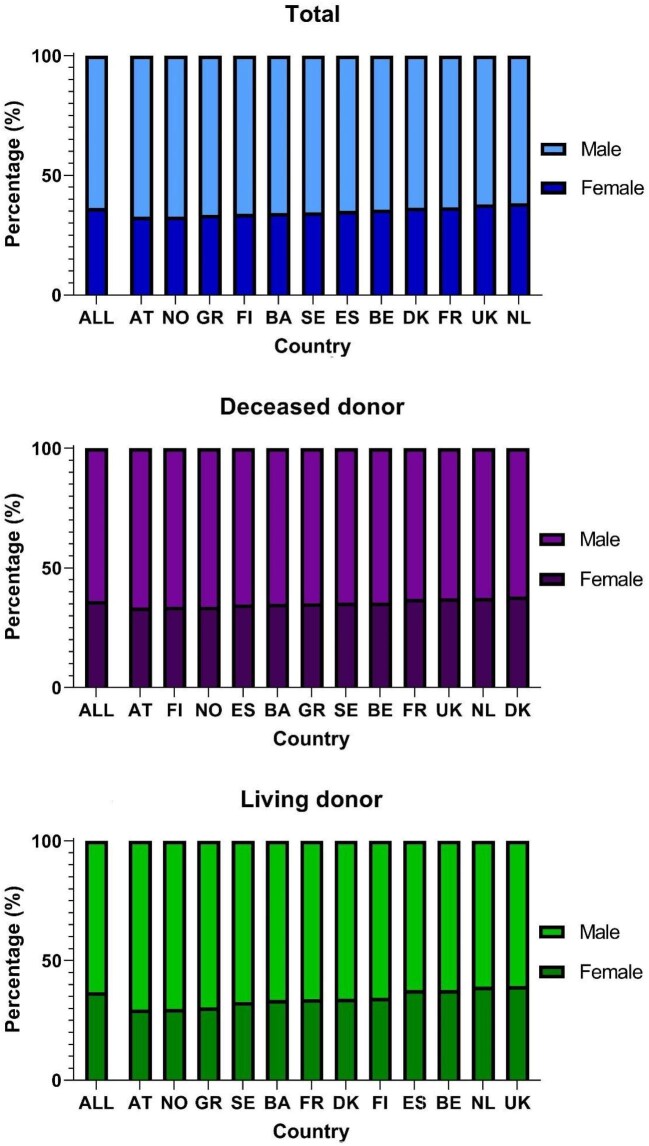
Sex
Overall, around 36% of recipients of a first kidney transplant were female, ranging between 33% in Austria and 38% in the Netherlands (Fig. 2). Among recipients of a first DD transplant this ranged between 33% in Austria and 38% in Denmark, and for LD recipients between 30% in Austria and 39% in the UK. Although the number of females receiving a first transplant increased over time (Supplementary data, Table S2), the percentage of female recipients slightly decreased from 36.9% to 36.3% (APC –0.3%, 95% CI –0.6 to 0.0; Supplementary data, Table S3). However, this decrease was not statistically significant when examining DD and LD recipients separately (Supplementary data, Table S3). For most countries, the percentage of female recipients was constant over time (Table 1).
Figure 2:
Sex distribution of first kidney transplant recipients between 2010 and 2019 for total, DD and LD kidney transplantation by country. The order of countries is based on the percentage female.
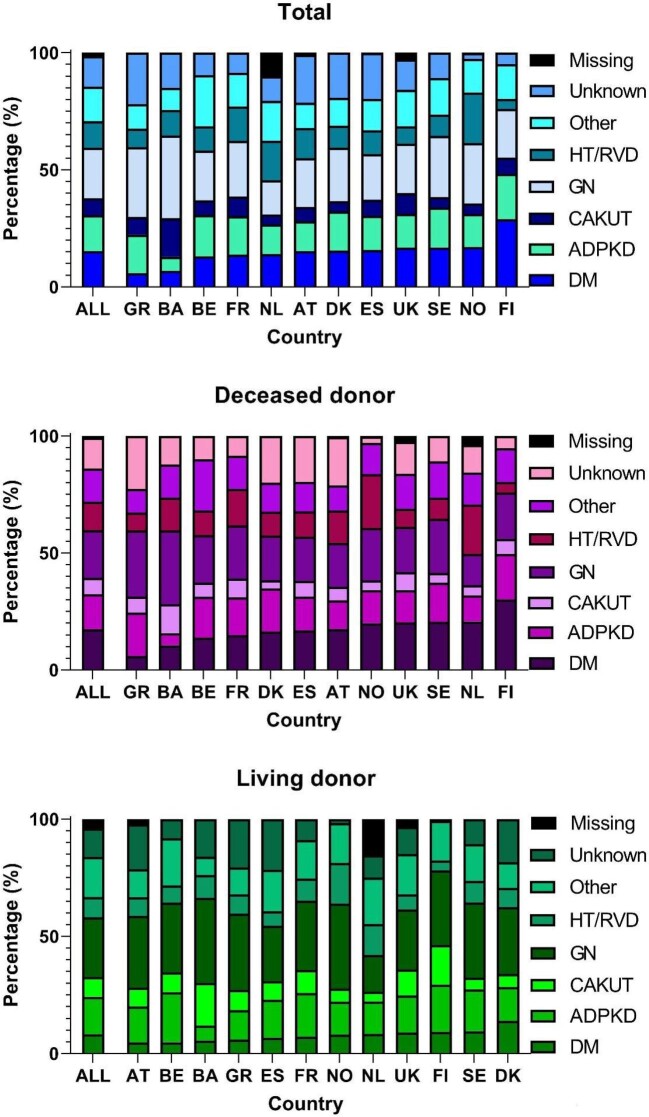
Primary renal disease
Overall, the highest percentage (21%) of recipients of a first kidney transplant had glomerulonephritis as PRD (Fig. 3). Large international differences were observed, with Bosnia and Herzegovina having a relatively high percentage of recipients with glomerulonephritis (35%) and CAKUT (16%), Finland a high percentage of recipients with DM (29%), and the Netherlands and Norway a high percentage of recipients with HT/RVD (17% and 22% respectively; Fig. 3). Compared with DD recipients, LD recipients more frequently had glomerulonephritis as PRD and less frequently DM (Fig. 3).
Figure 3:
PRD distribution of first kidney transplant recipients between 2010 and 2019 for total, DD and LD kidney transplantation by country. The order of countries is based on the percentage of recipients with DM as PRD. DM includes both DM type I and type II.
Overall, the percentage of recipients of a first kidney transplant with ADPKD or CAKUT as PRD decreased between 2010 and 2019 from 16.1% to 14.0% (APC –1.4%, 95% CI –2.0 to –0.8) and from 8.4% to 5.9% (APC –3.5%, 95% CI –4.7 to –2.4), respectively, whereas the percentage of recipients with DM increased from 15.2% to 16.0% (APC 1.0%, 95% CI 0.3 to 1.7; Supplementary data, Table S3). Similar trends were observed for recipients of a DD kidney transplant, whereas for LD kidney transplants the proportion of recipients with CAKUT decreased and the proportion of recipients with other causes as PRD increased (Supplementary data, Table S3). The increase in the percentage of recipients with DM over time was only statistically significant in the UK for total kidney transplantations and in the Netherlands and the UK for DD kidney transplantations (Table 1). Interestingly, a statistically significant decrease in the percentage of recipients with DM was observed for Austria for total kidney transplantations and for Austria and Sweden for DD kidney transplantations (Table 1).
Recipient characteristics and kidney transplantation rate
Countries with a higher first kidney transplantation rate also had a higher percentage of recipients aged 65 years or older (R2 = 0.90, P < .01; Fig. 4). This correlation was also statistically significant for first transplants from a DD (R2 = 0.65, P = .02) and first transplants from a LD (R2 = 0.76, P < .01; Supplementary data, Fig. S1). The first kidney transplantation rate in a country was correlated with neither the percentage of female recipients (data not shown), nor the percentage of recipients with DM as PRD (Fig. 4 and Supplementary data, Fig. S1).
Figure 4:
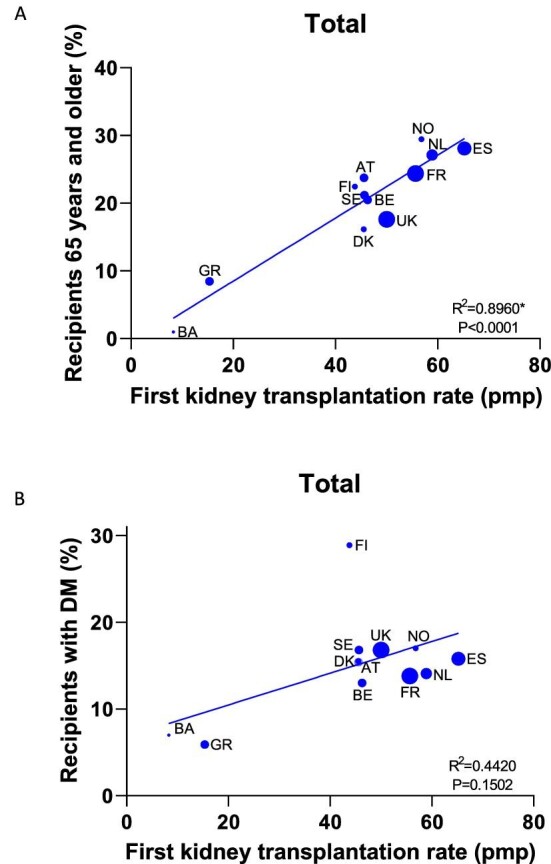
Correlation between first kidney transplantation rate (pmp) and percentage of recipients aged 65 years or older (A) and percentage of recipients with DM as PRD (B) between 2010 and 2019, by country. Asterisk indicates statistically significant R2.
DISCUSSION
This is the first study on characteristics of adults receiving a first kidney transplant, using data from 12 European countries for a decade. Overall, 23% of the recipients were 65 years or older, 36% were female whereas 21% had glomerulonephritis and 15% DM as PRD. Compared with recipients of a first DD kidney transplant, recipients of a first LD kidney transplant were less often 65 years or older (13% versus 26%), more often had glomerulonephritis (25% versus 20%) and less often diabetes mellitus (8% versus 17%) as PRD. We found large international differences, which were most prominent for age and PRD and less prominent for sex. Over time, the largest change in recipient characteristics was observed for age with the percentage of those aged 65 years or older increasing from 18% in 2010 to 28% in 2019 for all countries combined, and with a similar trend observed in most countries.
Factors influencing the distribution of recipient characteristics
Several factors may play a role in the explanation of differences in first kidney transplant recipient characteristics between countries and over time. First of all, in countries with a low number of transplants available, nephrologists tend to select the youngest and healthiest patients for kidney transplantation, since they are expected to benefit the most in terms of life expectancy [5]. In line with this, differences in the ratio of available DD and LD may play a role. Other factors may include differences in the allocation systems and organ exchange organizations across countries, such as Eurotransplant [the Euro-transplant kidney allocation system (ETKAS)], Scandiatransplant, ONT (Spain), UK transplant and AdB (France). Furthermore, older patients might have fewer potential living donors in their social network willing and able to donate a kidney. In addition, as kidney transplant recipients are a subgroup of the KRT patient population [5], the composition of the kidney transplant population may reflect that of the KRT patient population. Differences in the composition of the KRT patient population may depend on the availability of KRT or choices for conservative care, but also on the characteristics of the chronic kidney disease (CKD) patients progressing to ESKD, as well as on the predisposition for CKD in the general population due to genetic factors or risk factors such as DM and HT [13].
Age
Overall in Europe, around 23% of the recipients were aged 65 years or older, while this was 16% in Australia, 23% in New Zealand and 19% in the USA during the same time period [14, 15]. The observed differences within Europe may be partly explained by differences in the availability of kidney transplants. Indeed a higher first kidney transplantation rate was correlated with a higher recipient age, indicating an liberal acceptance for elderly patients in countries with high transplantation rates. On the other hand, for example in Greece, the transplantation rate was among the lowest in Europe [2] and only 8% of the kidney transplant recipients were aged 65 years or older. Also, access to KRT seemed to have played a role in the age distribution. For example, in Bosnia and Herzegovina the incidence of KRT was relatively low and the KRT patient population was young [5], suggesting limited access to KRT, resulting in a young KRT and transplant recipient population. Finally, the age distribution in the general population might have played a role, as Norway and Spain are among the countries with the highest life expectancy (83 and 82 years, respectively), while in Bosnia and Herzegovina the life expectancy is notably lower (76 years) [16].
The proportion of older recipients increased between 2010 and 2019, which was similar to trends observed in Australia, Canada and the USA [14, 15, 17]. This could be explained by an increased availability of kidney transplants over time resulting in an increased transplantation rate in Europe over the last decade among older patients [2]. In addition, countries have been taking initiatives aimed at increasing their kidney transplantation rate, some of which are aimed specifically at increasing the access to transplantation for older recipients. Examples are an old-for-old program for DD transplants and a higher acceptance of older recipients for LD transplants [18]. Also, some countries have increased their DD kidney transplantation rate in older recipients by facilitating the use of organs from expanded criteria and non-standard risk donors, and deceased donation after controlled cardiac death [19–21]. Finally, in Europe between 2008 and 2017 the incidence of KRT in elderly patients increased [22], which may have resulted in more elderly ESKD patients receiving a transplant over time. In line with this, elderly patients who initiated KRT more recently might have had better cardiovascular health and may have been more suitable for transplantation [23].
Sex
In Europe around 36% of the recipients were female. This percentage was similar in Canada and slightly higher at 39% in Australia, New Zealand and the USA [14, 15, 17]. During our study period, the percentage of female recipients did not change over time in most countries, implying that the sex distribution was not affected by the increased number of first kidney transplantations. The percentage of female recipients in the kidney transplantation population reflects the percentage of females initiating KRT (36%) [5]. The latter may be due to more males than females commencing KRT due to faster CKD progression in males and because elderly females more frequently choose conservative care [24].
Primary renal disease
Overall in Europe, 21% of the first kidney transplant recipients had glomerulonephritis and 15% DM as PRD, which was lower compared with percentages observed in Canada (27% and 23%, respectively) and the USA (24% and 26%, respectively) [14, 17]. Within Europe, we showed large international differences for the PRD distribution. For Bosnia and Herzegovina we found a relative large proportion of recipients with glomerulonephritis and CAKUT, while the Netherlands and Norway had a large proportion of recipients with HT/RVD. Also, in the KRT patient population these differences in PRD distribution across countries were observed, however they were less prominent than in the recipient population [5]. Therefore, we believe that both the differences in patients selected for kidney transplantation as well as the distribution of PRD within the KRT population may play a role. Countries with fewer kidney transplants available have transplanted younger patients, who more often suffer from ESKD due to genetic or congenital kidney diseases, such as CAKUT, whereas countries with a high transplantation rate have also transplanted older patients with diseases such as HT and RVD [25]. The large proportion of recipients with DM in Finland is likely due to the high incidence of KRT of patients with DM type 1 [5]. This could be explained by the high proportion of DM type 1 in the Finnish general population probably due to predisposing genes, lifestyle and environmental factors [26].
During our study period, the proportion of kidney transplant recipients with ADPKD or CAKUT decreased, whereas the proportion of recipients with DM increased. Also, in Canada the percentage of recipients with DM as PRD increased over time [17], while in the USA it remained constant at around 26% [14]. The change in PRD distribution in the recipient population could be explained by the increased access to kidney transplantation among older patients who more frequently had DM as PRD and less frequently genetic or congenital kidney diseases [6, 25]. Furthermore, Huijben et al. reported an increase in the incidence of KRT in ESKD patients with DM as PRD between 2011 and 2017 in Europe [22], following the epidemic of DM in the ageing general population [22, 27]. Notably, Austria had a decrease in the proportion of recipients with DM as PRD over time. Prischl et al. showed that around 2006 the incidence of KRT patients with DM started to decrease in Austria [28]. The authors speculate that this might be explained by the implementation of a multifactorial treatment regimen for patients with DM including intensive glucose lowering, hypertension treatment (enalapril), lipid lowering and lifestyle changes [28].
Strengths and limitations
The main strength of this study is that it presents data on recipient characteristics for total, DD and LD first kidney transplantations from 12 European countries over a decade.
This study also has some limitations. We predominantly included data from Western European countries, and therefore, the results may not be generalizable to the whole of Europe. In addition, we were unable to investigate other recipient characteristics such as comorbidities and laboratory measurements since these data are not collected by the ERA Registry for all countries. Finally, countries might differ in practices to diagnose the PRD and this could explain part of the observed differences in PRD distribution between countries [29].
CONCLUSION
Overall in Europe, 23% of the first kidney transplant recipients were aged 65 years or older, 36% were female and 21% had glomerulonephritis and 15% DM as PRD. Compared with recipients of a first DD kidney transplant, recipients of a first LD kidney transplant were younger, more often had glomerulonephritis and less often DM as PRD. We observed large international differences, especially for recipient age and PRD. These differences may be explained by differences in the availability of kidney transplants and to whom these transplants are allocated, and differences in the characteristics of the KRT population and the general population. Between 2010 and 2019, the recipient age increased in most countries and the distribution of PRDs changed, which may be the consequence of an increased transplantation rate and an aging KRT population. Our results may inform clinicians and policy makers on the existence of differences in the composition of the recipient population, and may assist in the explanation of international differences in patient survival after kidney transplantation.
Supplementary Material
Acknowledgements
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry (OEDTR) (F. Engler, R. Kramar, G. Mayer and the Austrian Society of Nephrology); Dutch-speaking Belgian Society of Nephrology (NBVN) (M. Couttenye, F. Schroven and J. De Meester); French-speaking Belgian Society of Nephrology (GNFB) (J.M. des Grottes and F. Collart); Renal Registry Bosnia and Herzegovina (H. Resić); Danish Nephrology Registry (DNS) (K. Hommel); Finnish Registry for Kidney Diseases (P. Finne and H. Niemelä); France: The Epidemiology and Information Network in Nephrology (REIN) (M. Lassalle and C. Couchoud); Hellenic Renal Registry (G. Moustakas); Norwegian Renal Registry (A. Åsberg); Spain Renal Registry (B. Mahillo Durán and M.O. Valentín Muñoz); Swedish Renal Registry (SRR) (K.G. Prütz, M. Stendahl, M. Evans, S. Schön and H. Rydell); Dutch Renal Registry (RENINE) (L. Heuveling, S. Vogelaar and M. ten Dam); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (SRR) (all of the Scottish renal units); and the regional registries of Andalusia (SICATA) (P. Castro de la Nuez, on behalf of all users of SICATA), Aragon (F. Arribas Monzón), Asturias (P. Escalada Rodríguez, P. Beltrán, M. Rodríguez, J.R. Quirós and the RERCA Working Group), Basque country (UNIPAR) (Á. Magaz, J. Aranzabal, M. Rodrigo and I. Moina), Cantabria (J.C. Ruiz San Millán), Castile and León (M.A. Palencia García and P. Ucio Mingo), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia (RMRC) (J. Comas and J. Tort), Community of Madrid (M.I. Aparicio de Madre and F Tornero Molina), Extremadura [all the renal units (Nephrology and Dialysis)], Galicia (E. Bouzas-Caamaño) and Valencian region (O. Zurriaga); and the other ERA Registry committee members not mentioned above for their advice in the analysis and the drafting of this paper: C. Wanner, P. Ambühl; M. Arici, J.E. Sánchez-Alvarez and E. Vidal; and M. Astley in the AMC Registry office for data collection and management. The ERA Registry is funded by the European Renal Association (ERA). This article was written by R. Boenink et al. on behalf of the ERA Registry, which is an official body of the ERA.
Contributor Information
Rianne Boenink, ERA Registry, Department of Medical Informatics, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Anneke Kramer, ERA Registry, Department of Medical Informatics, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Sherry Masoud, UK Renal Registry, UK Kidney Association, Bristol, UK; DPMCN, School of Medicine, Cardiff University, Cardiff, UK; Renal Medicine & Transplantation, North Bristol NHS Trust, Bristol, UK.
Alberto Rodríguez-Benot, Kidney and Pancreas Transplantation Unit, Nephrology Dept University Hospital Reina Sofia, Cordoba, Spain; Sistema de Información de la Coordinación Autonómica de Trasplantes de Andalucía (SICATA), Spain.
Jaakko Helve, Finnish Registry for Kidney Diseases, Helsinki, Finland; Abdominal Center Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Claus Bistrup, Department of Nephrology, Odense University Hospital, Odense, Denmark.
Mårten M Segelmark, Department of Clinical Sciences, Lund University, Lund, Sweden; Department of Endocrinology, Nephrology and Rheumatology, Skane University Hospital, Lund, Sweden.
Olga L Rodríguez Arévalo, Registry of Renal Patients of the Valencian Community, General Directorate of Public Health and Addictions, Ministry of Universal Health and Public Health, Valencia, , Spain; Health and Well-being Technologies Program, Polytechnic University of Valencia, Valencia, Spain.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV – Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria.
Aiko P J de Vries, Department of Medicine, Division of Nephrology, Leiden Transplant Center, Leiden University Medical Center and Leiden University, Leiden, The Netherlands.
Torbjörn Lundgren, Division of Transplantation Surgery, CLINTEC, Karolinska Institute, Stockholm, Sweden.
Samira Bell, Scottish Renal Registry, Meridian Court, Glasgow, UK; Division of Population Health and Genomics, University of Dundee, Dundee, UK.
Marta Crespo, Department of Nephrology, Hospital del Mar, Barcelona, Spain; Institut Mar d'Investigacions Médiques, Barcelona, Spain.
Søren S Sørensen, Department of Nephrology P, Rigshospitalet, University Hospital of Copenhagen, Copenhagen, Denmark.
Pietro Manuel Ferraro, U.O.S. Terapia Conservativa della Malattia Renale Cronica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Università Cattolica del Sacro Cuore, Rome, Italy.
Miha Arnol, Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia.
Sevcan A Bakkaloglu, Department of Pediatric Nephrology, Gazi University, Ankara, Turkey.
Laurent Weekers, French-Belgian ESRD Registry (GNFB), Brussels, Belgium.
Anna Varberg Reisæter, Department of Transplantation Medicine, Oslo University hospital, Rikshospitalet, Oslo, Norway.
Damir Rebić, Clinic for Nephrology, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina.
Alberto Ortiz, Fundación Jiménez Díaz, Universidad Autónoma de Madrid, Fundación Renal Iñigo Alvarez de Toledo, Madrid, Spain.
Kitty J Jager, ERA Registry, Department of Medical Informatics, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Vianda S Stel, ERA Registry, Department of Medical Informatics, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
FUNDING
K.J.J. and V.S.S. report grants from the European Renal Association.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Tonelli M, Wiebe N, Knoll G et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011;11:2093–109. [DOI] [PubMed] [Google Scholar]
- 2. Boenink R, Kramer A, Tuinhout RE et al. Trends in kidney transplantation rate across Europe: study from the ERA Registry. Nephrol Dial Transplant 2023;38:1528–39. 10.1093/ndt/gfac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Renal Data System . 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Chapter 11 International Comparisons. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. [Google Scholar]
- 4. ANZDATA Registry . 43rd Report, Chapter 7: Kidney Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2020. Available at: http://www.anzdata.org.au [Google Scholar]
- 5. Boenink R, Astley ME, Huijben JA et al. The ERA Registry Annual Report 2019: summary and age comparisons. Clin Kidney J 2022;15:452–72. 10.1093/ckj/sfab273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pippias M, Stel VS, Kramer A et al. Access to kidney transplantation in European adults aged 75–84 years and related outcomes: an analysis of the European Renal Association-European Dialysis and Transplant Association Registry. Transpl Int 2018;31:540–53. 10.1111/tri.13125 [DOI] [PubMed] [Google Scholar]
- 7. Sorensen SS. Rates of renal transplantations in the elderly-data from Europe and the US. Transplant Rev (Orlando) 2015;29:193–6. 10.1016/j.trre.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 8. Hödlmoser S, Gehrig T, Antlanger M et al. Sex differences in kidney transplantation: Austria and the United States, 1978–2018. Front Med (Lausanne) 2021;8:800933. 10.3389/fmed.2021.800933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joinpoint Regression Program, Version 4.2.0.2. June 2015. Statistical Research and Applications Branch. USA: National Cancer Institute. [Google Scholar]
- 10. National Cancer Institute . Joinpoint Help Manual 4.7.0.0. Bethesda, MD: Division of Cancer Control and Population Sciences, 2019. [Google Scholar]
- 11. Kim HJ, Fay MP, Feuer EJ et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 12. SAS software, Version 9.4. Copyright ©. Cary, USA, NC: SAS Institute Inc. [Google Scholar]
- 13. Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011) 2013;3:368–71. 10.1038/kisup.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. United States Renal Data System . 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Chapter 7 Transplantation. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. [Google Scholar]
- 15. ANZDATA Registry . Annual Reports, Chapter: Kidney Transplantation. Adelaide, Australia: Australia and New Zealand Dialysis and Transplant Registry. 2023. Available at: https://www.anzdata.org.au/anzdata/publications/reports/ [Google Scholar]
- 16. Life expectancy at birth, total. . The World Bank Group. Available at: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?end=2020&name_desc=false&view=map. [Google Scholar]
- 17. Canadian Institute for Health Information . Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2009 To 2018: End-Stage Kidney Disease and Kidney Transplants—Data Tables. Ottawa, ON: CIHI, 2019. [Google Scholar]
- 18. Boenink R, Kramer A, Vanholder RC et al. Factors influencing kidney transplantation rates: a study from the ERA Registry. Nephrol Dial Transplant 2023;38:1540–51. 10.1093/ndt/gfad001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matesanz R, Domínguez-Gil B, Coll E et al. How Spain reached 40 deceased organ donors per million population. Am J Transplant 2017;17:1447–54. 10.1111/ajt.14104 [DOI] [PubMed] [Google Scholar]
- 20. Pippias M, Stel VS, Arnol M et al. Temporal trends in the quality of deceased donor kidneys and kidney transplant outcomes in Europe: an analysis by the ERA-EDTA Registry. Nephrol Dial Transplant 2021;37:175–86. 10.1093/ndt/gfab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pérez-Sáez MJ, Arcos E, Comas J et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transplant 2016;16:2724–33. 10.1111/ajt.13800 [DOI] [PubMed] [Google Scholar]
- 22. Huijben JA, Kramer A, Kerschbaum J et al. Increasing numbers and improved overall survival of patients on kidney replacement therapy over the last decade in Europe: an ERA Registry study. Nephrol Dial Transplant 2023;38:1027–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceretta ML, Noordzij M, Luxardo R et al. Changes in co-morbidity pattern in patients starting renal replacement therapy in Europe-data from the ERA-EDTA Registry. Nephrol Dial Transplant 2018;33:1794–804. 10.1093/ndt/gfx355 [DOI] [PubMed] [Google Scholar]
- 24. Carrero JJ, Hecking M, Chesnaye NC et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 25. Mahmood U, Healy HG, Kark A et al. Spectrum (characteristics) of patients with chronic kidney disease (CKD) with increasing age in a major metropolitan renal service. BMC Nephrol 2017;18:372. 10.1186/s12882-017-0781-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knip M. Type 1 diabetes in Finland: past, present, and future. Lancet Diabetes Endocrinol 2021;9:259–60. 10.1016/S2213-8587(21)00074-7 [DOI] [PubMed] [Google Scholar]
- 27. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prischl FC, Auinger M, Säemann M et al. Diabetes-related end-stage renal disease in Austria 1965-2013. Nephrol Dial Transplant 2015;30:1920–7. 10.1093/ndt/gfv113 [DOI] [PubMed] [Google Scholar]
- 29. Fiorentino M, Bolignano D, Tesar V et al. Renal biopsy in 2015—from epidemiology to evidence-based indications. Am J Nephrol 2016;43:1–19. 10.1159/000444026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.