Key Points
Question
To what extent is shared decision-making (SDM) used in interventions aimed at improving cardiovascular risk factor management, and how does SDM affect decisional outcomes, cardiovascular risk factors, and health behaviors?
Findings
In this systematic review and meta-analysis of 57 randomized clinical trials that included 88 578 patients on SDM interventions for cardiovascular risk management and 1341 clinicians, SDM interventions were associated with a slight decrease in decisional conflict and an improvement in hemoglobin A1c levels with substantial heterogeneity.
Meaning
These findings may help advance the field of SDM interventions for cardiovascular risk management.
This systematic review and meta-analysis examines the use and outcomes of shared decision-making in interventions to enhance management of cardiovascular risk factors.
Abstract
Importance
The effect of shared decision-making (SDM) and the extent of its use in interventions to improve cardiovascular risk remain unclear.
Objective
To assess the extent to which SDM is used in interventions aimed to enhance the management of cardiovascular risk factors and to explore the association of SDM with decisional outcomes, cardiovascular risk factors, and health behaviors.
Data Sources
For this systematic review and meta-analysis, a literature search was conducted in the Medline, CINAHL, Embase, Cochrane, Web of Science, Scopus, and ClinicalTrials.gov databases for articles published from inception to June 24, 2022, without language restrictions.
Study Selection
Randomized clinical trials (RCTs) comparing SDM-based interventions with standard of care for cardiovascular risk factor management were included.
Data Extraction and Synthesis
The systematic search resulted in 9365 references. Duplicates were removed, and 2 independent reviewers screened the trials (title, abstract, and full text) and extracted data. Data were pooled using a random-effects model. The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
Decisional outcomes, cardiovascular risk factor outcomes, and health behavioral outcomes.
Results
This review included 57 RCTs with 88 578 patients and 1341 clinicians. A total of 59 articles were included, as 2 RCTs were reported twice. Nearly half of the studies (29 [49.2%]) tested interventions that targeted both patients and clinicians, and an equal number (29 [49.2%]) exclusively focused on patients. More than half (32 [54.2%]) focused on diabetes management, and one-quarter focused on multiple cardiovascular risk factors (14 [23.7%]). Most studies (35 [59.3%]) assessed cardiovascular risk factors and health behaviors as well as decisional outcomes. The quality of studies reviewed was low to fair. The SDM intervention was associated with a decrease of 4.21 points (95% CI, −8.21 to −0.21) in Decisional Conflict Scale scores (9 trials; I2 = 85.6%) and a decrease of 0.20% (95% CI, −0.39% to −0.01%) in hemoglobin A1c (HbA1c) levels (18 trials; I2 = 84.2%).
Conclusions and Relevance
In this systematic review and meta-analysis of the current state of research on SDM interventions for cardiovascular risk management, there was a slight reduction in decisional conflict and an improvement in HbA1c levels with substantial heterogeneity. High-quality studies are needed to inform the use of SDM to improve cardiovascular risk management.
Introduction
Cardiovascular risk factors such as hypertension, diabetes, obesity, and current smoking are modifiable, and the prevention and management of these conditions is important in decreasing cardiovascular disease (CVD)–related morbidity and mortality.1,2,3 Shared decision-making (SDM) is a collaborative approach that incorporates the active involvement of patients in decisions concerning their care.4 This approach enables patients, in concert with their clinicians, to make informed decisions about their health care that incorporate their goals, values, and preferences.5
Previous systematic reviews showed that the implementation of SDM in primary care may be effective in reducing decisional conflict and improving knowledge of diseases and treatment options, awareness of risk, and satisfaction with the decisions made.6,7,8,9 The incorporation of SDM in cardiovascular risk prevention and management has the potential to increase patient engagement in lifestyle changes, medication adherence, and glycemic and blood pressure control and lead to improved health outcomes.5 Shared decision-making may promote equity by involving clinicians and patients, sharing the best available evidence, and recognizing the needs, values, and experiences of individuals and their families when faced with the task of making decisions.5
Although SDM is increasingly embraced in health care and recommended in cardiovascular guidelines,1,2 the extent to which SDM is used in research assessing interventions for managing cardiovascular risk factors remains unclear. In addition, the effect of SDM-based interventions that focus on managing cardiovascular risk factors on decisional outcomes, cardiovascular risk factors, and health behaviors remains uncertain.
To address this gap, we performed a systematic review of trials to assess the extent to which SDM is used in interventions aimed at enhancing the management of cardiovascular risk factors (diabetes, hypertension, dyslipidemia, overweight and obesity, and tobacco use) in clinical practice. To achieve this, we developed an evidence map by thoroughly exploring the extensive literature to identify existing interventions (for patients and clinicians) and outcomes (including decisional outcomes, cardiovascular risk factor outcomes, and health behavioral outcomes) studied in trials that used SDM interventions to manage cardiovascular risk factors. Furthermore, we performed meta-analyses to evaluate the association of SDM interventions with the main outcomes of interest, including decisional conflict, hemoglobin A1c (HbA1c), and systolic blood pressure (SBP) levels.
Methods
Protocol and Guidance
The protocol for this systematic review and meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and the Cochrane Handbook for Systematic Reviews of Interventions.10 The protocol was registered with PROSPERO (CRD42021261433).
Information Sources and Search Strategy
A public health informationist supported the team in the development of the search strategy. The Medline (via PubMed), CINAHL (Cumulative Index of Nursing and Allied Health Literature), Embase, Cochrane, Web of Science, Scopus, and ClinicalTrials.gov databases were searched for all primary studies published through June 24, 2022, without language restrictions. The search strategy included MeSH (Medical Subject Headings) terms, CINAHL headings, and Emtree terms. In addition, we used various combinations of search terms related to the concepts of shared decision-making, decision-making, patient participation, communication, and professional-patient relations and terms related to cardiovascular health. The full search strategy is presented in eMethods 1 in Supplement 1.
Eligibility Criteria
Studies were included in this review if they met the following criteria: (1) the population included adults (aged ≥18 years) with cardiovascular risk factors (diabetes, hypertension, dyslipidemia, overweight and obesity, and tobacco use); (2) an SDM-based intervention type was used; (3) outcomes examined included decisional outcomes (decisional conflict, decisional quality, or SDM scores), cardiovascular risk factor outcomes (HbA1c, SBP levels, low-density lipoprotein, total cholesterol, smoking cessation, or CVD risk), and health behavioral outcomes (physical activity, healthy diet, or medication management); and (4) a randomized clinical trial (RCT) study design was used. In our systematic review, we identified interventions as SDM based if they met the following criteria based on the published scientific statement5: clinicians and patients made decisions together, the best available evidence was used, and decisions were based on patients’ informed values or preferences.
We excluded letters, editorials, and protocols. We also excluded nonrandomized trials, interrupted time-series studies, controlled before-and-after studies, prospective and retrospective comparative cohort studies, case-control or nested case-control studies, cross-sectional studies, reviews, case series, and case reports.
Study Selection and Data Collection Process
All studies identified via database searches were imported into Covidence, a web-based collaboration software platform, for screening and data extraction.11 For the screening, data extraction, and quality appraisal, each article was assigned to 2 investigators from the team (S.E., Y.C., X.L., S.S., R.-A.T.-O., B.O., S.T., S.B., B.K., R.A., D.-L.B., N.L.M., and N.N.) and to a third author also from the team who was not one of the first 2 authors who initially evaluated studies. Titles and abstracts, and later full-text articles, were independently screened for relevance by 2 investigators, and discrepancies in study selection were discussed via consensus in Covidence by a third author. All articles from primary studies that met the eligibility criteria were included (1) to fully appraise the evolution of SDM in research evaluating interventions for managing cardiovascular risk factors and (2) to evaluate the association of SDM with the decision-making process and the management of cardiovascular risk factors. Full-text articles were obtained for all citations that met the inclusion criteria during the screening phase.
Two independent investigators extracted data from the studies included in the systematic review using a standardized data extraction form that was incorporated into Covidence. Disagreements were resolved via consensus in Covidence and by a third author.11
Quality Appraisal
We used the Quality Assessment Tool for Controlled Intervention Studies Criteria (eMethods 2 and eTable 1 in Supplement 1) developed by the National Heart, Lung, and Blood Institute.12 This tool addresses 14 elements of quality and risk assessment, which provides an overall rating of good, fair, or poor based on critical appraisal of characteristics relevant to high-quality research studies.13 Two researchers independently assessed each article, and a third researcher evaluated any discrepancies in the cross-check for consensus.
Data Synthesis
Meta-analyses were conducted to assess the pooled effect size of SDM interventions on decisional conflict, HbA1c (percentages), and SBP levels (in millimeters of mercury). We chose these 3 outcomes for meta-analysis due to the consistent measurement methods or tools used across studies, and because they represent the main outcomes commonly reported in trials. Decisional conflict is defined as the individual’s personal uncertainty regarding the choice of options,14 which is one of the frequently reported outcomes in studies examining SDM-based interventions. Decisional conflict was evaluated using the Decisional Conflict Scale, a validated measure assessing patient perception of uncertainty in choosing between options, with summary scores ranging from 0 (no decisional conflict) to 100 (extremely high decisional conflict).14
Statistical Analysis
We used means (SDs) of the outcomes measured at the follow-up time points to perform meta-analyses. The effect size is presented as the mean difference between the experimental intervention group and the control group accompanied by the 95% CI (data transformation method presented in eMethods 3 in Supplement 1). Heterogeneity was assessed using the Cochran Q test and the Higgins I2 statistic.15 The interpretation of I2 values followed Cochrane guidelines.10 The Hedges test was used to evaluate publication bias, and funnel plots were examined visually. Subgroup meta-analyses were carried out for different study durations as reported across the studies. All meta-analyses were conducted using Stata/SE, version 17.0 (StataCorp LLC).
Results
Search Results
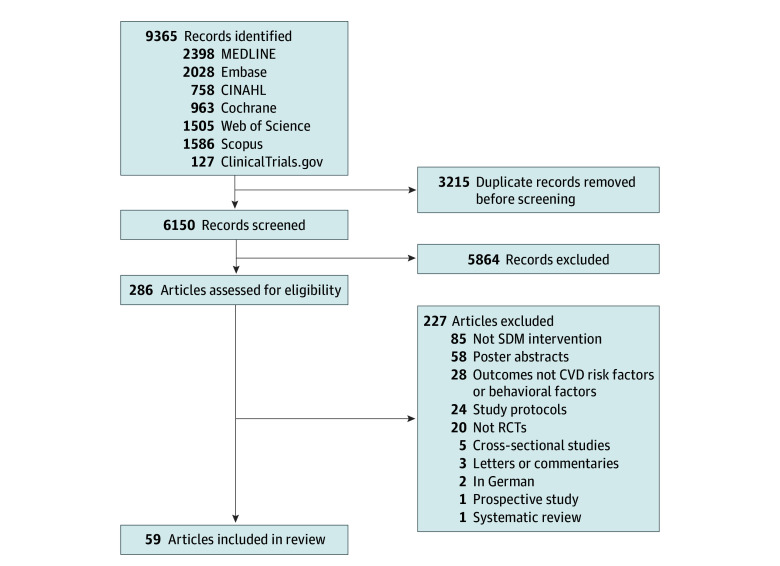
Our systematic search resulted in 9365 references, 3215 of which were duplicates. After the titles and abstracts of the identified references were read, 5863 articles were excluded for not fulfilling the inclusion criteria. Full-text screening was performed on the remaining 286 articles, and 227 were excluded (Figure 1). Thus, 57 RCTs with 88 578 patients and 1341 clinicians were included in this review.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74 A total of 59 articles were included, as 2 RCTs were reported twice.44,45,70,71
Figure 1. Study Flow Diagram.
CINAHL indicates Cumulative Index of Nursing and Allied Health Literature; CVD, cardiovascular disease; SDM, shared decision-making; and RCT, randomized clinical trial.
Study and Participant Characteristics
The 59 included articles were published between 198854 and 202234,42 (Table 1 and eTable 2 in Supplement 1). Of these studies, 26 (44.1%) were conducted in the US,20,21,27,28,29,31,34,35,36,37,41,42,46,49,52,53,54,55,58,59,64,68,69,70,71,73 9 (15.3%) in Germany,17,19,25,26,43,60,66,67,72 6 (10.2%) in the Netherlands,23,44,45,50,61,63 4 (6.8%) in the United Kingdom,16,38,56,57 3 (5.1%) in China,22,51,65 3 (5.1%) in Spain,30,32,62 and the remaining 8 (13.6%) in Australia,33 Canada,18 Denmark,39 Estonia,47 Finland,24 Greece,48 India,74 or South Korea.40 Sample sizes ranged from 40 participants52 to 10 815 participants60 across the included studies. The mean age of patients in the included studies ranged from 24.5 years56 to 78.962 years. Among the 59 articles, 20 also reported health care clinician characteristics,18,19,20,23,28,29,30,32,34,36,42,43,44,45,55,59,62,64,69,72 with sample sizes ranging from 11 participants34 to 230 participants.55 The mean age of clinicians ranged from 38 years45 to 49 years.23
Table 1. Summary of Study Characteristics.
| Study | Location | Target and main topic | Purpose and population | Design and setting | Sample size | |
|---|---|---|---|---|---|---|
| No. of patients | No. of clinicians | |||||
| Adarkwah et al,72 2016 | Germany | Patients and clinicians; multiple CVD risk factors | To compare the new TTE illustration with the established emoticons looking at the degree of SDM in the consultation process and various secondary outcomes of decisional conflict and accessibility | Cluster RCT: practice, clinic, or office | 304 | 32 |
| Applegate et al,73 2021 | US | Patients only; multiple CVD risk factors | To determine whether Project ACTIVE improved utilization of preventive care, estimated life expectancy compared with usual care, or both | RCT: practice, clinic, or office; hospital | 132 | NR |
| Bailey et al,71 2016 | US | Patients only; diabetes | To test the effectiveness of the intervention in clinical practice and assess whether an EHR-linked clinical decision support system slowed increases in modifiable cardiovascular risk among adults with serious mental illness | Pragmatic RCT: practice, clinic, or office | 225 | NR |
| Bailey et al,70 2018 | US | Patients only; diabetes | To assess the effectiveness of different interventions on knowledge transfer and behavior modification to improve PROMs for T2D | Pragmatic RCT: practice, clinic, or office | 225 | NR |
| Boulware et al,68 2020 | US | Patients only; hypertension | To assess whether an intervention to help patients prioritize goals for their visit would improve patient-clinician communication and clinical outcomes | Pragmatic, comparative effectiveness RCT: practice, clinic, or office; home | 159 | NR |
| Branda et al,69 2013 | US | Patients and clinicians; diabetes | To evaluate the effect of PDAs vs usual care on decision-making measures, metabolic control, and medication adherence among patients with T2D | Cluster RCT: practice, clinic, or office; other (nonacademic and rural primary care practice) | 103 | 41 |
| Buhse et al,66 2015 | Germany | Patients and clinicians; diabetes | To investigate the cardiovascular risk factor profile among young men (aged 18-50 y) with hypertension in family practices and analyze the effectiveness of a computer-based decision aid promoting SDM in modifying cardiovascular risk factors | RCT: practice, clinic, or office | 154 | NR |
| Buhse et al,67 2018 | Germany | Patients and clinicians; diabetes | To determine whether communicating personalized statin therapy effects obtained by a prognostic algorithm leads to lower decisional conflict associated with statin use in patients with stable CVD compared with standard (nonpersonalized) therapy effects | Cluster RCT: practice, clinic, or office | 279 | NR |
| Cheng et al,65 2021 | China | Patients only; diabetes | To compare the new TTE illustration with the established emoticons looking at the degree of SDM in the consultation process and various secondary outcomes such as decisional conflict and accessibility | RCT: practice, clinic, or office; hospital | 242 | NR |
| Cooper et al,64 2011 | US | Patients and clinicians; hypertension | To compare the effectiveness of patient and physician interventions (separately and in combination with one another) with the effectiveness of minimal interventions, by evaluating the effect of the intervention on (1) patient and physician communication behaviors, (2) patient ratings of the interpersonal process of care, (3) patient adherence to medications, and (4) BP levels and control over 12 mo | RCT: practice, clinic, or office | 279 | 41 |
| Coronado-Vázquez et al,62 2019 | Spain | Patients and clinicians; multiple CVD risk factors and medication | To determine the effectiveness of an SDM intervention for medication appropriateness in patients with chronic diseases and polypharmacy | Randomized, multicenter quasi-experimental study: practice, clinic, or office | 122 | 22 |
| Den Ouden et al,63 2017 | Netherlands | Patients and clinicians; multiple CVD risk factors | To evaluate in a cluster-randomized practical trial the effect of PDAs vs usual care on decision-making measures, metabolic control, and medication adherence in nonacademic and rural primary care practices and their patients with T2D | Cluster RCT: practice, clinic, or office | 153 | NR |
| Denig et al,61 2014 | Netherlands | Patients only; diabetes | To evaluate an informed SDM program for individuals with T2D under high-fidelity conditions | Pragmatic RCT: practice, clinic, or office | 344 | NR |
| Dwinger et al,60 2020 | Germany | Patients only; multiple CVD risk factors | To determine the effectiveness of a PDA for patients with T2D receiving metformin who require treatment intensification | Prospective, pragmatic RCT: community | 10 815 | NR |
| Eaton et al,59 2011 | US | Patients and clinicians; multiple CVD risk factors | To determine whether an intervention based on patient activation and a physician decision support tool is more effective than usual care for improving adherence to National Cholesterol Education Program guidelines | Cluster RCT: practice, clinic, or office | 4105 | 55 |
| Eckman et al,58 2012 | US | Patients only; multiple CVD risk factors | To evaluate the effectiveness of an empowerment self-management intervention on psychological distress and quality of life among patients with poorly controlled T2D | RCT: ambulatory setting | 170 | NR |
| Edwards et al,57 2006 | UK | Patients only; diabetes | To compare the effectiveness of patient and physician interventions (separately and in combination with one another) on (1) patient and physician communication behaviors, (2) patient ratings of the interpersonal process of care, (3) patient adherence to medications, and (4) BP levels and control over 12 mo | RCT: online | 508 | NR |
| Farmer et al,56 2005 | UK | Patients only; diabetes | To determine whether real-time telemedicine support can improve glycemic control in T1D | RCT: practice, clinic, or office | 93 | NR |
| Grant et al,55 2008 | US | Patients only; diabetes | To examine whether the use of diabetes-specific personal health records improves diabetes care management by increasing patient knowledge and engagement in their own care and by facilitating patient-physician communication | RCT: practice, clinic, or office; hospital; community | 244 | 230 |
| Greenfield et al,54 1988 | US | Patients only; diabetes | To examine the effects of an intervention designed to increase the involvement of patients with diabetes in medical decision-making on blood glucose control and quality of life | RCT: practice, clinic, or office | 59 | NR |
| Heisler et al,53 2014 | US | Patients only; diabetes | To evaluate the effectiveness of iDecide in improving key diabetes outcomes compared to delivery by CHWs of the same evidence-based information without tailoring using print consumer booklets developed by the AHRQ | RCT: practice, clinic, or office; home; or other agreed-upon place | 188 | NR |
| Hsu et al,52 2016 | US | Patients only; diabetes | To test the efficacy of a cloud-based diabetes management program in helping individuals starting basal insulin achieve better glycemic control | RCT: practice, clinic, or office | 40 | NR |
| Hu et al,51 2021 | China | Patients only; diabetes and medication | To evaluate a combined fasting blood glucose–based dosage self-titration and decision-supported telephone coaching intervention on glycemic control and diabetes self-management skills | RCT: practice, clinic, or office; via telephone calls | 869 | NR |
| Jaspers et al,50 2021 | Netherlands | Patients only; CVD risk | To determine whether communicating personalized statin therapy effects obtained by a prognostic algorithm leads to lower decisional conflict associated with statin use in patients with stable CVD | RCT: practice, clinic, or office | 303 | NR |
| Jouni et al,49 2017 | US | Patients only; multiple CVD risk factors | To assess the effect of disclosing CHD genetic risk on LDL-C levels | RCT: practice, clinic, or office | 207 | NR |
| Karagiannis et al,48 2016 | Greece | Patients only; diabetes | To assess the efficacy of the Diabetes Medication Choice decision aid among patients with T2D in Greece in primary and secondary care practice | Cluster RCT: practice, clinic, or office | 204 | NR |
| Kask-Flight et al,47 2021 | Estonia | Patients and clinicians; multiple CVD risk factors | To analyze the effectiveness of a computer-based decision aid promoting SDM in changing cardiovascular risk factors among young men (aged 18-50 y) with hypertension in family practices | Cluster RCT: practice, clinic, or office | 130 | NR |
| Keyserling et al,46 2014 | US | Patients only; smoking | To assess the effectiveness, acceptability, and cost-effectiveness of a combined lifestyle and medication intervention to reduce CHD risk offered in counselor-delivered and web-based formats | RCT: practice, clinic, or office | 385 | NR |
| Koelewijn-van Loon et al,44 2009 | Netherlands | Patients and clinicians; multiple CVD risk factors | To investigate whether a nurse-led intervention in primary care had a positive effect on lifestyle and 10-y cardiovascular risk | Cluster RCT: practice, clinic, or office | 615 | 24 |
| Koelewijn-van Loon et al,45 2010 | Netherlands | Patients and clinicians; multiple CVD risk factors | To determine whether lifestyle and risk perception improved with an intervention to involve patients in cardiovascular risk management by the practice nurse | Cluster RCT: practice, clinic, or office | 615 | 24 |
| Krones et al,43 2008 | Germany | Patients and clinicians; CVD risk | To determine the effect of promoting the effective communication of absolute CVD risk and SDM through dissemination of a simple decision aid for use in family practice consultations | Pragmatic, cluster RCT: practice, clinic, or office | 1132 | 91 |
| Kulzer et al,17 2018 | Germany | Patients and clinicians; diabetes and medication | To investigate whether taking care of patients with insulin-treated T2D using integrated personalized diabetes management improves glycemic control, PROs, and physician treatment satisfaction and intensifies therapy adjustments | Cluster RCT: practice, clinic, or office | 907 | NR |
| Kunneman et al,42 2022 | US | Patients and clinicians; diabetes and medication | To determine the effectiveness of an SDM tool vs guideline-informed usual care in translating evidence into primary care, and to explore how the tool changed patient perspectives about diabetes medication decision-making | Mixed methods cluster RCT: practice, clinic, or office | 350 | 99 |
| Lauffenburger et al,41 2019 | US | Patients only; diabetes and medication | To evaluate the effect of a telephone-based patient-centered intervention on glycated HbA1c control for individuals with poorly controlled diabetes | Pragmatic RCT: insurance community | 1400 | NR |
| Lee et al,40 2016 | South Korea | Patients only; smoking | To develop a culturally tailored decision aid for smoking cessation and evaluate its effect on the use of smoking cessation medication | Pragmatic cluster RCT: practice, clinic, or office | 414 | NR |
| Maindal et al,39 2014 | Denmark | Patients and clinicians; diabetes | To assess whether a 12-wk participant-driven health education program offered to individuals with screening-detected hyperglycemia in Danish primary care would lead to improvements in cardiovascular risk factors, health behavior, and PROs after 3 y | RCT: practice, clinic, or office | 509 | NR |
| Mathers et al,38 2012 | UK | Patients and clinicians; diabetes | To determine the effectiveness of a PDA on improving decision quality and glycemic control in individuals with diabetes making treatment decisions | Pragmatic cluster RCT: practice, clinic, or office | 175 | NR |
| Moin et al,37 2019 | US | Patients only; diabetes and medication | To test the effectiveness of a prediabetes SDM intervention | Cluster RCT: practice, clinic, or office | 1379 | NR |
| Montgomery et al,16 2003 | UK | Patients only; hypertension and medication | To evaluate 2 interventions for assisting patients with newly diagnosed hypertension in the decision whether to start drug therapy for reducing BP | Factorial RCT: practice, clinic, or office; community | 217 | NR |
| Mullan et al,36 2009 | US | Patients only; diabetes and medication | To determine the ability of a decision aid to promote patient involvement in choosing antihyperglycemic agents and to evaluate the effects of this strategy on medication adherence and patient outcomes | Cluster RCT: practice, clinic, or office | 85 | 40 |
| Naik et al,35 2011 | US | Patients only; diabetes | To evaluate the comparative effectiveness of 2 diabetes group clinic interventions on glycated HbA1c levels in primary care | RCT: practice, clinic, or office | 87 | NR |
| O’Malley et al,34 2022 | US | Patients and clinicians; multiple CVD risk factors and medication | To assess whether an intervention to help patients prioritize goals for their visit would improve patient-clinician communication and clinical outcomes | RCT: practice, clinic, or office | 120 | 11 |
| Peiris et al,33 2015 | Australia | Patients and clinicians; CVD risk | To test whether a multifaceted quality improvement intervention comprising computerized decision support, audit/feedback tools, and staff training improved (1) guideline-indicated risk factor measurements and (2) guideline-indicated medications for those at high CVD risk | Cluster RCT: practice, clinic, or office | 38 725 | NR |
| Perestelo-Pérez et al,32 2016 | Spain | Patients and clinicians; CVD risk and medication | To test whether a multifaceted quality improvement intervention comprising computerized decision support, audit/feedback tools, and staff training improved (1) guideline-indicated risk factor measurements and (2) guideline-indicated medications for those at high CVD risk | Cluster RCT: practice, clinic, or office | 168 | 29 |
| Prabhakaran et al,74 2019 | India | Patients and clinicians; multiple CVD risk factors | To evaluate the effectiveness of a nurse-facilitated, mHealth-based EDS for the integrated management of 5 chronic conditions in primary care settings in India as part of the mWellcare trial | Pragmatic RCT: community health centers | 3324 | NR |
| Ramallo-Fariña et al,30 2021 | Spain | Patients and clinicians; diabetes | To assess the effectiveness of different interventions of knowledge transfer and behavior modification to improve PROMs in patients with T2D | Open, community-based pragmatic, multicenter, controlled trial with random allocation by cluster: practice, clinic, or office | 2334 | 211 |
| Rost et al,31 1991 | US | Patients only; diabetes | To determine whether a short intervention that enhanced patient information seeking and decision-making during hospitalization improved metabolic control and functional status in patients with diabetes | RCT: clinical research center | 61 | NR |
| Smith et al,29 2008 | US | Patients and clinicians; diabetes | To assess the effect of a specialist telemedicine intervention for improving diabetes care using the CCM | RCT: primary care clinic | 639 | 97 |
| Sperl-Hillen et al,28 2018 | US | Patients and clinicians; cardiovascular risk | To evaluate whether a clinical decision support intervention can improve 10-y CVD risk trajectory in patients in primary care settings | RCT: primary care clinics | 7914 | 102 |
| Swoboda et al,27 2017 | US | Patients; diabetes | To evaluate a telephone-based goal setting and decision support coaching intervention among adults with T2D and to evaluate the effect of these approaches for diet and physical activity behavior changes in relation to an attention control group | RCT: community setting | 54 | NR |
| Tinsel et al,26 2013 | Germany | Clinicians; hypertension | To implement an evaluated SDM training program for GPs within the context of hypertension treatment, and to examine whether the SDM training enhanced patients’ perceived participation and lowered BP | Cluster RCT: general practices | 1120 | NR |
| Tinsel et al,25 2018 | Germany | Patients; CVD risk | To investigate the applicability of the DECADE intervention and the potential effects of the intervention on patients with cardiovascular risk factors | RCT: primary care | 78 | NR |
| Tusa et al,24 2021 | Finland | Patients; CVD risk | To examine the influence of a participatory patient care plan on health-related quality of life and disease-specific outcomes related to diabetes, ischemic heart disease, and hypertension | RCT: primary care | 605 | NR |
| Tutino et al,22 2017 | China | Patients; diabetes | To test whether the delivery of integrated care augmented by a web-based disease management program and nurse coordinator could improve treatment target attainment and health-related behavior | RCT: hospital | 3586 | NR |
| van Steenkiste et al,23 2007 | Netherlands | Clinicians and patients; multiple CVD risk factors | To test whether a decision support tool can improve primary prevention of CVD in primary care | Cluster RCT: hospital | 490 | 34 |
| Warner et al,21 2015 | US | Clinicians and patients; smoking | To develop and pilot test a decision aid to increase patient involvement in decisions regarding smoking behavior of cigarette smokers scheduled for elective surgery | RCT: academic medical center | 130 | NR |
| Weymiller et al,20 2007 | US | Clinicians and patients; diabetes | To examine a decision aid tool’s acceptability to patients and its effect on patient knowledge of the information about the potential merits and demerits of the options and decisional conflict | Cluster RCT: metabolic clinic | 98 | 21 |
| Wollny et al,19 2019 | Germany | Clinicians and patients; diabetes | To investigate whether an educational intervention facilitated by GPs increased patient-centeredness and perceived SDM in the treatment of patients with poorly controlled T2D | Cluster RCT: primary care | 833 | 108 |
| Yu et al,18 2020 | Canada | Clinicians and patients; diabetes | To assess the effect of MyDiabetesPlan on decisional conflict, diabetes distress, health-related quality of life, and patient assessment of chronic illness care at the individual patient level | Cluster RCT: primary care | 213 | 53 |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; BP, blood pressure; CCM, chronic care model; CHD, coronary heart disease; CHW, community health worker; CVD, cardiovascular disease; DECADE, decision aid, action planning, and follow-up support for patients to reduce the 10-year risk of cardiovascular diseases; EDS, electronic decision support; EHR, electronic health record; GP, general practitioner; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; NR, not reported; PDA, patient decision aid; PRO, patient-reported outcome; PROM, patient-reported outcome measure; RCT, randomized clinical trial; SDM, shared decision-making; T1D, type 1 diabetes; T2D, type 2 diabetes; TTE, time-to-event.
Quality Appraisal
Among the included studies, 30 (50.8%) were rated fair,16,19,20,24,25,28,29,30,33,34,35,37,38,39,41,42,44,45,46,48,49,50,59,62,63,65,66,67,73,74 29 (49.2%) were rated poor,17,18,21,22,23,26,27,31,32,36,40,43,47,51,52,53,54,55,56,57,58,60,61,64,68,69,70,71,72 and none were rated good. These ratings were primarily driven by inadequate blinding, unclear assessment of adherence to protocols, and high study attrition rates. Details of the quality assessment can be found in eTable 1 in Supplement 1.
Interventions for Patients and Clinicians
Interventions for patients and clinicians are summarized in Table 2 and eTable 3 in Supplement 1. The SDM interventions were directed toward both patients and clinicians in 29 studies (49.2%),17,18,19,21,23,30,32,33,34,36,37,38,39,41,42,43,45,52,53,59,61,62,63,64,66,67,72,73,74 29 studies (49.2%) exclusively targeted patients,16,20,22,24,25,27,28,29,31,35,40,44,46,47,48,49,50,51,54,55,56,57,58,60,65,68,69,70,71 and only 1 study (1.6%) targeted clinicians.26 Regarding the frequency of interventions, 18 studies (30.5%) reported using a one-time intervention,19,20,21,26,32,34,36,40,42,50,57,61,66,68,69,70,71,72 whereas others included interventions that were performed between 2 and 7 times during the study period.
Table 2. Intervention Characteristics of Included Articles.
| Study | Location | Delivery mode | Frequency; duration | Patient intervention description (technology, decision aid, and messaging) | Clinician intervention description (training, tools, EHR best practice alerts, and PDA use) |
|---|---|---|---|---|---|
| Adarkwah et al,72 2016 | Germany | In-person consultation and computer | 1-Time intervention, follow-up in 3 mo; NR | Decision aid used to show 10-y CVD risk | Training on use of showing risk in ARRIBA-Herz |
| In-person consultation focused on specific medications, dose adjustments, and behavioral measures | Advice on how to communicate risk with patients | ||||
| Applegate et al,73 2021 | US | In-person consultation, telephone consultation, and printed material | 4 Monthly visits, followed by 2 quarterly visits; 40 min | SDM intervention focused on identifying the highest-priority unfulfilled clinical goals and monitoring action steps to reach them | Formal training on SDM not provided to intervention staff |
| Behavior change techniques | Formal training in using behavior change techniques provided | ||||
| Bailey et al,71 2016 | US | Web-based and recorded video | Once before follow-up; 30 min | Decision aid to show recommendations for diabetes management | NR |
| Emails and daily messages to send reminders | |||||
| Bailey et al,70 2018 | US | In-person consultation and online | Once; NR | Interactive online diabetes decision aid for T2D to address decisions about adding therapy to metformin due to poor glycemic control | NR |
| Boulware et al,68 2020 | US | In-person consultation and telephone consultation | Once; NR | Training in SDM skills provided by a CHW | NR |
| Workbook and reminder card provided by a CHW | |||||
| Branda et al,69 2013 | US | In-person consultation | Once; NR | Decision aids during the clinical encounter | NR |
| Buhse et al,66 2015 | Germany | In-person consultation and web-based or recorded video | Once; 90 min | Evidence-based decision aid for patients on the prevention of heart attack | Training DVD, including basic principles of SDM |
| Structured patient teaching by diabetes educators | Training focused on evidence-based practice within the decision aid and patient teaching | ||||
| Magnet board to visualize the quantity risk | |||||
| Buhse et al,67 2018 | Germany | In-person consultation | NR | Evidence-based PDA about primary prevention of myocardial infarction and other diabetes-related complications | 6-h Training offered to prepare GPs for consultations in terms of SDM |
| Patient-held documentation sheet with patient-defined treatment goals | |||||
| Cheng et al,65 2021 | China | In-person consultation and telephone consultation | Weekly intervention; 6 wk | Brief intake session, 2 structured face-to-face small group discussion sessions, and 4 telephone-based individualized consultation and maintenance sessions | NR |
| Cooper et al,64 2011 | US | In-person consultation; telephone consultation; printed material; photographic novels; newsletter | Bimonthly and monthly; NR | Pocket-sized diaries provided by CHWs to patients to record appointments, medications, and questions | Physician review of videotape of personal interviews with the simulated patient and completion of CD-ROM or workbook exercises |
| Bimonthly photographic novels that reinforce the coaching messages; monthly health education newsletter | |||||
| Coronado-Vázquez et al,62 2019 | Spain | In-person consultation; telephone consultation; web-based or recorded video | 1-Time visit to physician’s office; NR | Decision support tool in paper format aimed at helping patients with decision-making by providing information about the secondary risks associated with inappropriate medications in their treatments | Information on the designed decision support tool and a link to a video about the web-based SDM process provided to physicians |
| Den Ouden et al,63 2017 | Netherlands | In-person consultation | 2 Times 12 mo apart; NR | OPTIMAL decision support aid provided on how to (1) consider the pros and cons of 2 almost equally effective evidence-based multifactorial treatments; (2) prioritize treatment targets according to the chosen treatment protocol; and (3) select treatment (medication, lifestyle change, or both) | Training on the SDM approach provided to GPs from the intervention group during just one 2-h training session |
| Role-plays used to train GPs on the SDM process | |||||
| Denig et al,61 2014 | Netherlands | In-person consultation and printed material | Once; NR | Decision aid presented, which contained several graphs with individually tailored information on risks and treatment options for multiple risk factors; patients received a printed version | Training course in motivational interviewing offered to all practices |
| Dwinger et al,60 2020 | Germany | Telephone consultation | Every 6 wk; 1 y | SDM sharing information on advantages and disadvantages of health behaviors and a joint decision | NR |
| Motivational interviewing | |||||
| Eaton et al,59 2011 | US | In-person consultation; printed material; web-based or recorded video | NR; 1 h | Patient education toolkit consisting of smoking cessation, weight loss, healthy diets, exercise, and lipid-lowering medication adherence materials | 1-h Academic session provided to all practices, which detailed using (1) a patient education toolkit and (2) a personal digital assistant–based decision support tool for each physician |
| Eckman et al,58 2012 | US | In-person consultation; printed material | NR; 70 min | Booklet for the foundation for informed medical decision-making | NR |
| DVD/VCR video (about 30 min) with similar information to that in the booklet | |||||
| Edwards et al,57 2006 | UK | Web-based or recorded video | Once; NR | Web-based video with detailed numeric information (absolute or relative risk, numbers needed to treat) and graphics (bar charts, thermometer scales, crowd figure formats) | NR |
| Farmer et al,56 2005 | UK | In-person consultation; telephone consultation; printed material; web-based or recorded video | 3 Times over 9 mo; NR | In-person clinical advice and structured counseling provided by a diabetes specialist nurse in response to real-time blood glucose test results | NR |
| Nurse telephone call to patients to identify concerns and problems and possible solutions | |||||
| Grant et al,55 2008 | US | Web-based or recorded video | NR | SDM module for (1) providing patients with their own clinical information linked to tailored decision support and (2) generating a diabetes care plan | NR |
| Greenfield et al,54 1988 | US | In-person consultation | Twice; 20 min | A 20-min session in which the assistant and the patient reviewed standardized educational materials in the diabetes treatment clinic | NR |
| Heisler et al,53 2014 | US | In-person consultation; telephone consultation; printed material; web-based or recorded video | 4 Times during 6 wk; 1.5-2 h | Initial one-on-one, face-to-face session with a CHW and a copy of the printed materials to take home | 80 h of Initial training in motivational interviewing–based communication approaches and diabetes self-management support provided to CHWs, with 4-8 h of booster training annually |
| CHWs contacted participants twice (at 3 and 6 wk after the session after the initial face-to-face session) by telephone to address any additional questions | |||||
| Hsu et al,52 2016 | US | In-person consultation; live video conference or telehealth; other (secure text messages) | Weekly; 12 wk | Meeting between patients and their health care clinicians during initial visit; for self-management of diabetes, patients received (1) a tablet computer preloaded with the diabetes management program (including the medication regimen and the initial insulin dose) and (2) a glucose meter that was wirelessly connected to the tablet | Tablet to model expert decision-making |
| Combination of virtual visits (real-time video and voice communication along with shared screen control), asynchronous text messages, or custom features in the software for making collaborative decisions and communicating dosage recommendations | |||||
| Hu et al,51 2021 | China | In-person consultation; telephone consultation | 5 Times over 12 wk; NR | Basal insulin self-titration decision support program that included 1 baseline in-person dosage setting and decision coaching session to empower adjustment, followed by 5 coaching calls delivered by the same nurse | NR |
| Jaspers et al,50 2021 | Netherlands | In-person consultation; telephone consultation; printed material | Once; NR | A personalized health profile in leaflet form | NR |
| USB device containing educational videos | |||||
| Structured telephone consultation enforcing uptake of the information | |||||
| Jouni et al,49 2017 | US | In-person consultation | NR | Modified version of the Statin Choice decision aid to emphasize that CHD risk assessment was probabilistic and not deterministic | NR |
| Visit with a genetic counselor and a physician in the cardiovascular health clinic | |||||
| Karagiannis et al,48 2016 | Greece | In-person consultation | Initial visit, 12 wk, and 24 wk; 6 mo | Greek version of the Diabetes Medication Choice decision aid used by clinicians and patients during the initial clinical encounter | NR |
| Physicians presented patients with 7 cards and asked which cards they preferred to discuss | |||||
| Kask-Flight et al,47 2021 | Estonia | In-person consultation | NR; 3 mo | Computer-based decision aid program, ARRIBA-Herz, with interactive visual prompts used to present 10-y morbidity risk for heart attack and stroke based on the Framingham algorithm | NR |
| Keyserling et al,46 2014 | US | In-person consultation; web-based or recorded video | 7 Times; 45-60 min, followed by 15-30 min | Decision aid used to calculate 10-y CVD risk factors, educate participants about their CHD risk factors and the pros and cons of risk-reducing strategies, and show participants the potential CHD risk reduction by changing ≥1 CVD risk factor | NR |
| Counseling tailored to the choice of risk-reducing strategy | |||||
| Koelewijn-Van Loon et al,44 2009 | Netherlands | In-person consultation; telephone consultation; printed material | Twice; 15-20 min | Risk communication tool used by nurses in an in-person meeting to present 10-y risk of CVD mortality, in which they (1) explained the options for risk reduction and gave patients a decision aid to review at home and (2) guided patients in formulating their main personal goals for lifestyle change or medication in telephone consultation using the motivational interviewing method | NR |
| Koelewijn-Van Loon et al,45 2010 | Netherlands | In-person consultation; telephone consultation | Twice; 20 min | Patients in the intervention group received two 20-min face-to-face consultations regarding SDM and a 10-min telephone consultation | 2-d Training course involving risk assessment, risk communication, distribution of a decision support tool, and adapted motivational interviewing |
| Krones et al,43 2008 | Germany | In-person consultation | NR | Decision aid to calculate CVD risk for stroke and myocardial infarction and compare age- and sex-adjusted population risk and the effects of their choice | 2 CME sessions emphasizing practical communication strategies and materials to be applied during consultation |
| In-person counseling with 6 steps | Practice using a script-like decision aid through role-playing | ||||
| Kulzer et al,17 2018 | Germany | In-person consultation | 6 times; NR | SDM intervention involving 6 recurring steps: (1) structured assessment and patient education, (2) structured and therapy-adapted SMBG, (3) structured documentation, (4) systematic analysis, (5) personalized treatment, and (6) treatment effectiveness assessment | Training based on a structured curriculum during four 1-h sessions that included video instruction programs and role-play exercises |
| Kunneman et al,42 2022 | US | In-person consultation | Once; NR | Diabetes Medication Choice conversation aid used by patients and clinicians during the clinical encounter; the aid presents general considerations and adverse effects of diabetes medication, and each topic occupies a card | Training on how to use the conversation aid provided during a 10-min group session, with access to an online demonstration and a 1-page storyboard and option to request ad hoc, one-on-one training during the study |
| A 1-page handout version of the conversation aid | |||||
| Lauffenburger et al,41 2019 | US | Telephone consultation | 4 Times; 30 min | Consultation conducted by trained clinical pharmacists using a semistructured call guide | Pharmacist training included script development and role-play exercises |
| Based on the call, patients identified 1 of 3 strategies to improve diabetes control: (1) treatment intensification, (2) adherence improvement, or (3) lifestyle improvement | |||||
| Simple pillbox and postcard-sized SDM tool used to prime patients for the telephone consultations | |||||
| Lee et al,40 2016 | South Korea | In-person consultation; web-based or recorded video | Once; 7-min video and 5-15 min of routine medical care and 5-10 min of smoking cessation counseling and prescription | A 7-min video on smoking cessation information and options and a decision aid | NR |
| Maindal et al,39 2014 | Denmark | Other (in-person individual counseling and group session) | 2 Interviews and 8 group sessions; 18 h over 3 mo | 2 Individual counseling interviews provided by a nurse | Training on target-driven intensive behavioral and pharmacological treatment provided to GPs |
| 8 Participant-centered group sessions led by nurses, dieticians, physiotherapists, and GPs, covering lifestyles according to participants’ own goals and requests | |||||
| Final individual session with a nurse | |||||
| Mathers et al,38 2012 | UK | In-person consultation | NR | PDA reviewed by patients before the consultation | Short training session (between 1 and 2 h) for physicians and nurses on how to use the PANDAs decision aid, the principles of SDM, the evidence for various treatment options for poorly controlled T2D, and essential skills in risk communication |
| PDA presented in a consultation with the GP or the practice nurse | |||||
| Moin et al,37 2019 | US | In-person consultation | NR; 35-45 min | Face-to-face SDM visit with a pharmacist who used a decision aid to describe prediabetes and 4 possible options for diabetes prevention | Pharmacist training in SDM and decision aid use, with quarterly refresher training sessions throughout the trial |
| Printed summary report with decision and plan provided to patients at the end of the SDM visit | |||||
| Pharmacist prescription given to patients choosing metformin | |||||
| Montgomery et al,16 2003 | UK | In-person consultation; printed material; web-based or recorded video | NR; 60 min | Simple decision tree to include likely outcomes of treatment options | NR |
| Individual absolute CVD risk calculated and combined with utilities using decision analysis software | |||||
| Printed sheet detailing participants’ CVD risk factors and summarizing the decision analysis | |||||
| Mullan et al,36 2009 | US | In-person consultation | Once; 3 min | Diabetes Medication Choice decision aid tool (6 cards describing the possible effects of the medications on 6 outcomes) used to enable clinicians to discuss with patients the potential advantages and disadvantages of adding an agent from 1 antihyperglycemic classes to their regimen | Brief demonstration from the study coordinator on how to use the decision aid (lasting <3 min; as seen in the video) |
| Copy of the cards in the form of a take-home pamphlet provided to patients | |||||
| Naik et al,35 2011 | US | In-person consultation; other (group session) | Every 3 wk; 3 mo, 1 h each session | A 4-h group sessions focused on the diabetes ABCs, personalized decision-making goals and action plans on lifestyles and diabetes, proactive patient behavior, effective physician-patient communication, and how to develop and obtain feedback on goals and action plans | NR |
| 10 min of individual interaction with the study clinician after group sessions | |||||
| O’Malley et al,34 2022 | US | Printed material | Once; NR | Card to prompt patients to reflect on their specific goals for the medical encounter, prioritize those goals, and engage in a discussion with their physician on their concerns and expectations | 1 h of Training on the importance of addressing patient concerns and expectations provided to physicians |
| Peiris et al,33 2015 | Australia | Other (software) | Monthly; 48 min | Point-of-care decision support; risk communication interface; clinical audit tool to assess performance on CVD-related indicators; and quality improvement component comprising peer-ranked data feedback and support to develop strategies to improve performance | Intervention practices received an average of 48 min of support per month comprising on-site training, remote clinical webinars, and help desk services |
| Clinical staff trained in use of the tools and received access to a technical support desk | |||||
| Bimonthly webinars offered with a focus on the practical demonstrations of the tools | |||||
| Perestelo-Pérez et al,32 2016 | Spain | In-person consultation | Once; 1 h | Decision aid applied by physicians in the intervention group | Group sessions of 1 h, in which physicians were trained to apply the decision aid by a member of the research team |
| Take-home copy of decision aid provided to patients | |||||
| Prabhakaran et al,74 2019 | India | Software and text message | NR; 12 mo | mWellcare system (Android application built on the CommCare platform) to generate a longitudinal trend or summary | Centralized training on the current clinical management guidelines and on-site training for orientation to the mWellcare system provided to physicians |
| Pamphlets provided by a nurse to each participant | Nurses trained on the management of hypertension, diabetes, depression, and tobacco/alcohol use, and received a 3-d training on using the mWellcare system and another 2 d of on-site supervision and support | ||||
| Short message service reminders for scheduled follow-up visits and medication adherence sent to participants | |||||
| Ramallo-Fariña et al,30 2021 | Spain | In-person consultation, a paper workbook, a website platform, and text message | Every 3 mo; 2 y | An 8-session conventional, group educational program given by a nurse specializing in diabetes | Complex intervention of knowledge transfer and decision support |
| Paper workbook used to monitor lifestyle daily | Intervention included (1) an educational and interactive group program of 2 sessions to update clinical management information and promote patient-centered care, (2) an automated decision aid tool, and (3) monthly computerized graphic feedback | ||||
| Website platform to upload paper workbook data weekly | |||||
| Text message use to send personalized feedback according to website information | |||||
| Rost et al,31 1991 | US | In-person consultation | 2 Intervention sessions and 4-mo follow-up; 45 min (first session) and 1 h (second session) | Component 1: 45-min patient activation session facilitated by a nurse before the patient’s discharge to introduce a decision tree about treatment choices in managing diabetes-related problems and to discuss facilitators and barriers of active patient participation and potential strategies | NR |
| Component 2: 1-h instructional package, which included self-assessment and 3 modules of question-asking skills, completed by patient at home before next outpatient visit | |||||
| Smith et al,29 2008 | US | Telemedicine consultation | NR | Specialty advice and evidence-based messages regarding medication management for CVD risk provided to patients and clinicians | NR |
| Sperl-Hillen et al,28 2018 | US | Web-based intervention | NR | Evidence-based treatment options for lipid, BP, weight, tobacco, or aspirin management identified and prioritized by the clinical decision support system based on potential benefit to patients | NR |
| Swoboda et al,27 2017 | US | In-person consultation and telephone consultation | 1 In-person session and biweekly telephone call until week 16; NR | 1 Motivational interviewing and decision support session and 7 biweekly telephone coaching calls | NR |
| Multiple-goal intervention: 1 diet goal and 1 physical activity goal established during the first session, and goals in both domains subsequently set during every coaching call | |||||
| Single-goal intervention: a goal for either a diet-related or physical activity–related behavior set during the first session based on individual preference | |||||
| Tinsel et al,26 2013 | Germany | NR | Once; NR | NR | SDM training program for GPs included information on arterial hypertension, physician-patient communication and risk communication, the process steps of SDM, motivational interviewing, introduction of a decision table listing options to lower cardiovascular risk score, and use of case vignettes for role-plays simulating physician-patient consultations |
| Tinsel et al,25 2018 | Germany | NR | 4 Consultation sessions; NR | DECADE brochures that contained evidence-based decision aids and action plans, and 4 structured follow-up consultations | NR |
| Tusa et al,24 2021 | Finland | In-person consultation | 1 Health care visit and 12-mo follow-up; 30-60 min with nurse and 30-40 min with GP | Participatory patient care plan formulated in collaboration with the patient, nurse, and physician during the first health care visit | NR |
| Tutino et al,22 2017 | China | In-person consultation and telephone consultation | 1 Intervention session and at least 2 follow-up sessions facilitated by a nurse coordinator; 2-4 h of diabetes education | Web-based JADE portal: assessment module including templates for periodic assessment, risk stratification, personalized reporting, and automated decision support plus nurse-coordinated structured follow-up module including templates for documentation of modifiable risk factors, hypoglycemia, and key events to track clinical progress and reinforce adherence | NR |
| van Steenkiste et al,23 2007 | Netherlands | In-person consultation | 1 Training and 2 consultations; 8 mo | A 4-h interactive small group training session for clinicians | 4-h Interactive small group training session instructed physicians about the risk table and the key recommendations for treatment of patients at high cardiovascular risk |
| Decision support tool for patients consisting of a booklet providing education on absolute 10-y CVD risk consultations with clinicians | Role-play used to allow physicians to practice how to use the decision support tool | ||||
| Warner et al,21 2015 | US | In-person consultation | Once; NR | Decision aid consisting of 3 laminated cards with pros and cons of continuing smoking, attempting temporary abstinence, or attempting to quit | Clinicians delivering the decision aid watched an 8-min video demonstrating the use of the decision aid |
| Weymiller et al,20 2007 | US | In-person consultation | Once and 3-mo follow-up; NR | Statin Choice tailored decision aid presenting estimated 10-y cardiovascular risk, absolute risk reduction with use of statin drugs, and disadvantages of using statin drugs | NR |
| Wollny et al,19 2019 | Germany | In-person consultation | Once; NR | Patients in the intervention group received SDM intervention (including patient-centered communication, decision aid) from the trained GPs | Outreach educational peer visit conducted to sensitize patients’ concepts of disease and their views, attitudes, and behaviors by using patient-centered communication |
| Additional training on patient communication skills | |||||
| Decision aid used HbA1c levels and associated risk factors to visualize the probability of experiencing macrovascular events and present the effect of antidiabetic medication and lifestyle changes on CVD | |||||
| Yu et al,18 2020 | Canada | In-person consultation | NR | Individualized diabetes-specific goals and strategies for patients generated using the MyDiabetesPlan decision aid, which later resulted in an action plan. At 6 mo, patients were provided with a patient-directed how-to guide and video and directed to update MyDiabetesPlan according to their progress before the appointment | One-on-one 60-min tutorial with access to a 1-page how-to guide and 2-min video |
| Subsequent review of the resultant action plan with the patient |
Abbreviations: BP, blood pressure; CHD, coronary heart disease; CHW, community health worker; CME, continuing medical education; CVD, cardiovascular disease; DECADE, decision aid, action planning, and follow-up support for patients to reduce the 10-year risk of cardiovascular diseases; EHR, electronic health record; GP, general practitioner; HbA1c, hemoglobin A1c; JADE, Joint Asia Diabetes Evaluation; NR, not reported; PANDAs, Patient and Decision Aids; PDA, patient decision aid; SDM, shared decision-making; SMBG, self-monitoring of blood glucose; T2D, type 2 diabetes.
Regarding interventions for patients, 47 studies (79.7%) conducted individual consultations16,17,18,19,20,21,22,23,24,25,27,30,31,32,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50,51,52,53,54,56,58,59,61,62,63,65,66,67,68,69,70,72,73 and 4 studies (6.8%) utilized group sessions.30,35,39,65 Almost half the 59 studies (26 [44.1%]) incorporated workbooks, newsletters, cards, diaries, and other printed materials.16,20,21,23,25,30,31,32,34,36,37,41,42,44,48,50,53,56,58,59,61,64,67,68,73,74 In terms of digital intervention formats, 19 (32.2%) studies used websites,16,22,28,30,40,44,46,47,48,49,53,55,56,59,62,66,70,71,72 16 (27.1%) used videos,16,18,29,40,46,50,52,53,55,56,57,58,59,62,66,71 11 (18.6%) used telephone-based interventions,27,41,44,45,50,53,60,62,65,68,73 5 (8.5%) used software applications,16,33,52,72,74 4 (4.8%) used text messages,30,52,71,74 and 1 (1.7%) used email.71 Among the included studies, 3 (5.1%) integrated behavior change techniques (eg, motivational interviewing) into the SDM interventions.27,60,73
In terms of interventions for clinicians, 36 studies (61.0%) provided training on using decision aids.16,18,19,20,21,22,23,25,28,29,30,31,32,36,37,38,40,41,42,43,44,46,47,48,49,59,61,62,63,66,67,69,70,71,72,74 In addition, 13 studies (22.0%) provided training to enhance SDM skills17,18,19,26,30,34,37,38,41,63,64,66,67; 11 (18.6%) focused on improving communication, educational, and motivational interviewing skills18,19,26,38,43,45,53,59,61,66,72; and 3 (5.1%) targeted behavior change techniques.23,39,73
Decision Aid Use
A detailed description of the decision aids included in this systematic review is presented in eTable 4 in Supplement 1. Of the 59 articles, 36 (61.0%) used decision aids to enhance SDM.16,18,19,20,21,22,23,25,28,29,30,31,32,36,37,38,40,41,42,43,44,46,47,48,49,59,61,62,63,66,67,69,70,71,72,74 In terms of the formats of the decision aids used among the 36 studies, 19 (52.8%) were online based,18,19,20,22,28,29,30,32,37,42,43,46,48,49,66,67,69,70,71 9 (25.0%) were paper based (including postcards, booklet, and brochures),21,23,25,36,38,41,44,62,63 2 (5.6%) were software applications,72,73 2 (5.6%) utilized used computer screens,47,61 2 (5.6%) used decision-making trees,16,31 1 (2.8%) involved a personal digital assistant,59 and 1 (2.8%) utilized used video.40 Among the 36 included articles with decision aids, 19 (52.8%) were focused on diabetes.18,19,20,22,29,30,31,36,37,38,41,42,48,61,66,67,69,70,71
Risk Factors Targeted and Outcomes
The majority of included studies (32 [54.2%]) concentrated on diabetes only,17,18,19,20,22,27,28,29,30,31,35,36,37,38,39,41,42,48,51,52,53,54,55,56,57,61,65,66,67,69,70,71 followed by multiple cardiovascular risk factors (14 [23.7%]),23,34,44,45,47,49,58,59,60,62,63,72,73,74 CVD risk (6 [10.2%]),24,25,32,33,43,50 hypertension (4 [6.8%]),16,26,64,68 and smoking cessation (3 [5.1%])27,34,54 (Table 1 and eTable 5 in Supplement 1). In terms of outcomes reported, 8 studies (13.6%) focused solely on decisional outcomes,18,19,21,49,65,70,71,72 35 (59.3%) measured both cardiovascular risk factors and health behaviors as well as decisional outcomes,16,17,20,23,25,26,27,30,31,32,34,35,36,38,39,42,43,45,46,48,50,51,52,53,54,55,57,58,60,61,64,66,67,68,69 and 16 (27.1%) evaluated only cardiovascular risk factors and health behaviors.22,24,28,29,33,37,40,41,44,47,56,59,62,63,73,74
Multiple studies reported improvements in decisional outcomes after SDM intervention. Improvements following SDM intervention were reported for decisional conflict (7 of 14 [50.0%]) and patient satisfaction with the decisions or treatment (6 of 14 [42.9%]). Other decisional outcomes reported among the articles that demonstrated improvement following SDM intervention included knowledge (13 of 20 [65.0%]), patient-centeredness (6 of 8 [75.0%]), decision quality (5 of 9 [55.6%]), risk perception (4 of 5 [80.0%]), empowerment (4 of 5 [80.0%]), patient activation (4 of 5 [80.0%]), and diabetes-related distress (4 of 7 [57.1%]).
The effect of SDM interventions on cardiovascular risk factors and CVD risk was inconsistent. Among studies measuring the specific outcomes, improvements were reported for the following: tobacco use (5 of 10 [50.0%]), CVD risk (3 of 9 [33.3%]), diabetes (6 of 23 [26.1%]), dyslipidemia (2 of 14 [14.3%]), hypertension (1 of 18 [5.6%]), and overweight and obesity (2 of 13 [15.4%]). The most frequently reported outcomes were HbA1c and SBP levels (as described in the next section).
Improvements following SDM interventions were reported for the following cardiovascular health behaviors: self-management (3 of 5 [60.0%]), medication management (7 of 12 [58.3%]), physical activity (3 of 8 [37.5%]), nutrition and diet (1 of 4 [25.0%]), and adherence (3 of 18 [16.7%]).
Meta-Analysis for Decisional Conflict, HbA1c, and SBP Levels
The SDM interventions were associated with a decrease of 4.21 points (95% CI, −8.21 to −0.21) in Decisional Conflict Scale scores, with substantial heterogeneity among 9 trials16,18,32,38,48,50,53,71,72 (I2 = 85.6%; P < .001; Figure 2). The SDM intervention was associated with a decrease of 0.20% (95% CI, −0.39% to −0.01%) in HbA1c levels, with substantial heterogeneity (I2 = 84.2%; P < .001; eFigure 1 in Supplement 1) observed across 18 trials.17,24,31,35,36,38,41,42,46,48,51,52,53,54,56,63,66,74 However, no statistically significant changes were observed in SBP levels for SDM interventions among 10 trials22,24,25,26,29,34,46,47,63,64 (mean difference, 0.02 [95% CI, −0.58 to −0.63]; I2 = 37.0%; eFigure 2 in Supplement 1). Publication bias of these outcomes is presented in eFigures 3 to 5 in Supplement 1.
Figure 2. Forest Plot of Mean Differences in Decisional Conflict Between the Shared Decision-Making (SDM)–Based Intervention Group and the Control Group.
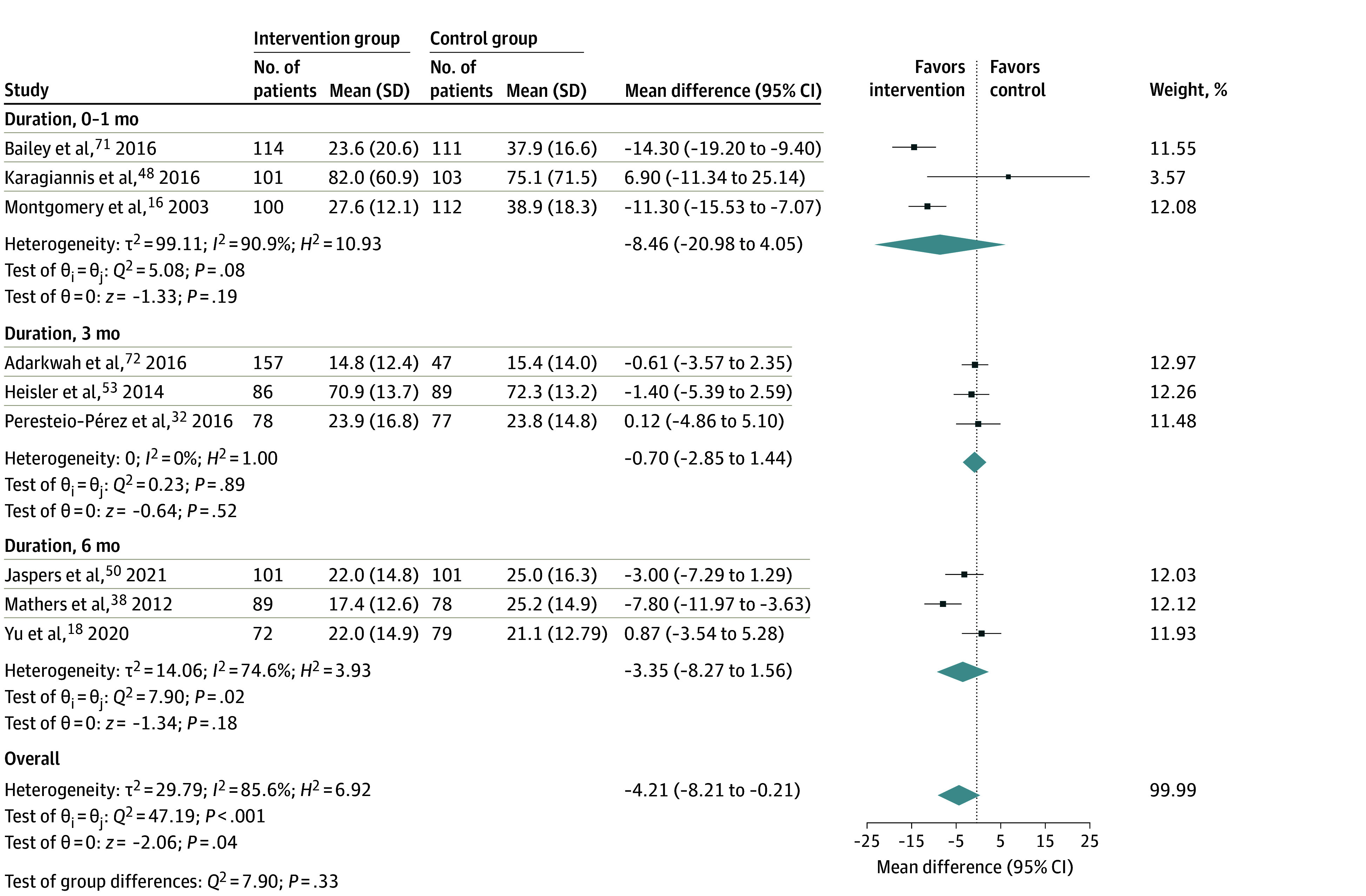
Results of the random-effects Hedges model are presented. The means (SDs) are the outcomes measured at the follow-up time points to perform meta-analyses. The size of the squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study, diamonds are the pooled estimate with 95% CI (weight, 100%), and the vertical dotted line is the line of no effect. Heterogeneity was assessed using the Cochran Q test (H2) and the Higgins I2 statistic. Interpretation of I2 values followed Cochrane guidelines (0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; and 75% to 100%, considerable heterogeneity).10
Discussion
In this protocol-driven systematic review, we conducted a comprehensive search for RCTs to ensure that only the highest level of evidence regarding the efficacy of SDM interventions was included. This review provides a comprehensive overview of the current state of research on SDM interventions for cardiovascular risk management. The meta-analyses suggested that the SDM intervention was associated with a slight reduction in decisional conflict and HbA1c levels, with substantial heterogeneity.
A limited number of systematic reviews specifically addressing SDM in cardiovascular management have been published.6,7,8,9 Some of these reviews focused on multiple cardiac conditions and reported solely on SDM outcomes,6 whereas others examined online decision aids for primary CVD prevention7 or focused on specific cardiovascular risk factors such as diabetes.9 Additionally, some reviews have investigated the effect of computerized decision support systems on cardiovascular risk factors.8 To our knowledge, this review is the first comprehensive exploration of SDM in cardiovascular risk factor management. Furthermore, we identified a wide range of SDM interventions that have been tested for managing cardiovascular risk factors. Approximately half of the included studies assessed both SDM process outcomes and cardiovascular risk factors and health behaviors.
A previous systematic review and meta-analysis conducted by Mitropoulou et al6 evaluated the effectiveness of interventions to improve SDM in cardiology with a particular focus on SDM process-related outcomes. Mitropoulou et al6 found that interventions to increase SDM had a significant effect on reducing decisional conflict and increasing patient knowledge compared with standard of care. Similar to the review by Mitropoulou et al,6 we also observed that implementing SDM interventions in cardiovascular risk management has potential to improve various decisional outcomes, including reducing decisional conflict and improving risk perception, empowerment, patient activation, patient-centeredness, disease-related knowledge, distress, decision quality, and satisfaction. In addition, the findings from our meta-analysis of SDM interventions suggest a positive effect on reducing decisional conflict. However, it is important to note that substantial heterogeneity was observed, suggesting variability in the decisional conflict.
In addition to aligning with the findings of Mitropoulou et al,6 our findings suggest that use of SDM interventions may also positively affect cardiovascular risk factors such as tobacco use, diabetes, dyslipidemia, hypertension, overweight and obesity, and overall CVD risk. Regarding the association of SDM intervention with HbA1c levels, our meta-analysis suggested a statistically significant reduction of −0.2% across 18 trials. Similar to decisional conflict, substantial heterogeneity was observed, suggesting diversity in the association of SDM interventions with HbA1c levels. However, no statistically significant changes in SBP levels were observed among 10 trials. Although the SDM interventions seemed to be associated with reduced decisional conflict and improved HbA1c levels, the substantial heterogeneity observed warrants further investigation and consideration of potential biases in the reported outcomes. Nevertheless, among trials in our review measuring other cardiovascular risk factors and health behaviors, the findings were inconsistent. This may be explained by the substantial variation across the SDM interventions tested and the methods and time points used to measure those outcomes.
Nearly half of the included studies (29 [49.2%]) implemented interventions targeting both clinicians and patients, whereas the remaining studies focused solely on patients. Despite the protracted nature of cardiovascular risk management efforts, approximately one-third of studies (18 [30.5%]) included one-time SDM interventions without incorporating ongoing interventions to enhance the SDM process. The lack of sustained interventions may hinder the long-term effectiveness of SDM interventions on decisional outcomes and cardiovascular health outcomes.5
Limitations
One limitation of this review is the predominance of studies rated as fair or poor in terms of their methodological quality among the included literature. The absence of studies rated as good suggests potential limitations in the overall quality and rigor of the available evidence. As a result, the reliability and generalizability of these findings may be compromised, as low-quality studies can introduce bias or have methodological flaws that affect the validity of the results. Additionally, the heterogeneity of some outcome measures, such as satisfaction with the decision process and ultimate decisions, across the included studies (eTable 6 and eFigures 6 and 7 in Supplement 1) posed a challenge in synthesizing the findings.
Conclusions
This systematic review and meta-analysis of 57 RCTs provides a comprehensive overview of the current state of research on SDM interventions for cardiovascular risk management. These findings suggest that the SDM intervention shows promise in alleviating decisional conflict and enhancing HbA1c levels. However, the notable heterogeneity observed underscores the need for additional scrutiny and the careful examination of potential biases inherent in the reported outcomes. High-quality studies are needed to inform the use of SDM to improve cardiovascular risk management in clinical practice.
eMethods 1. Search Summary
eMethods 2. Quality Appraisal
eMethods 3. Data Synthesis and Data Transformation
eTable 1. Quality Assessment Using the Quality Assessment of Controlled Intervention Studies Criteria
eTable 2. Summary of Patient and Clinician Sociodemographic Characteristics and Outcomes
eTable 3. Summary of Interventions
eTable 4. Studies Incorporating Decision Aids in the Intervention
eTable 5. Summary of Outcomes
eTable 6. Studies Reporting Satisfaction Outcome
eFigure 1. Forest Plot of HbA1c
eFigure 2. Forest Plot of Systolic Blood Pressure
eFigure 3. Funnel Plot of Decisional Conflict
eFigure 4. Funnel Plot of HbA1c
eFigure 5. Funnel Plot of Systolic Blood Pressure
eFigure 6. Forest Plot of Satisfaction About the Decision or Treatment
eFigure 7. Funnel Plot of Satisfaction About the Decision or Treatment
eReferences
Data Sharing Statement
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177-e232. doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 4.Magnani JW, Mujahid MS, Aronow HD, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; Stroke Council . Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation. 2018;138(2):e48-e74. doi: 10.1161/CIR.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himmelfarb CRD, Beckie TM, Allen LA, et al. ; American Heart Association Council on Cardiovascular and Stroke Nursing; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Stroke Council . Shared decision-making and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2023;148(11):912-931. doi: 10.1161/CIR.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 6.Mitropoulou P, Grüner-Hegge N, Reinhold J, Papadopoulou C. Shared decision making in cardiology: a systematic review and meta-analysis. Heart. 2022;109(1):34-39. doi: 10.1136/heartjnl-2022-321050 [DOI] [PubMed] [Google Scholar]
- 7.Bonner C, Patel P, Fajardo MA, Zhuang R, Trevena L. Online decision aids for primary cardiovascular disease prevention: systematic search, evaluation of quality and suitability for low health literacy patients. BMJ Open. 2019;9(3):e025173. doi: 10.1136/bmjopen-2018-025173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenhof TKJ, Asselbergs FW, Groenwold RHH, Grobbee DE, Visseren FLJ, Bots ML; UCC-SMART study group . The effect of computerized decision support systems on cardiovascular risk factors: a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2019;19(1):108. doi: 10.1186/s12911-019-0824-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Jin Y, Lu C, Luo R, Wang J, Liu Y. Effects of patient decision aids in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Nurs Pract. 2021;27(6):e12914. doi: 10.1111/ijn.12914 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.4. Cochrane Collaboration; 2023. [Google Scholar]
- 11.Covidence systematic review software. Veritas Health Innovation. 2022. https://www.covidence.org
- 12.Risk Assessment Working Group . Assessing cardiovascular risk: systematic evidence review from the risk assessment work group. US Dept of Health and Human Services. 2013. Accessed January 4, 2024. https://www.nhlbi.nih.gov/health-topics/assessing-cardiovascular-risk
- 13.Uffelman CN, Chan NI, Davis EM, Wang Y, McGowan BS, Campbell WW. An assessment of mushroom consumption on cardiometabolic disease risk factors and morbidities in humans: a systematic review. Nutrients. 2023;15(5):1079. doi: 10.3390/nu15051079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25-30. doi: 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br J Gen Pract. 2003;53(491):446-453. [PMC free article] [PubMed] [Google Scholar]
- 17.Kulzer B, Daenschel W, Daenschel I, et al. Integrated personalized diabetes management improves glycemic control in patients with insulin-treated type 2 diabetes: results of the PDM-ProValue study program. Diabetes Res Clin Pract. 2018;144:200-212. doi: 10.1016/j.diabres.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Yu C, Choi D, Bruno BA, et al. Impact of MyDiabetesPlan, a web-based patient decision aid on decisional conflict, diabetes distress, quality of life, and chronic illness care in patients with diabetes: cluster randomized controlled trial. J Med Internet Res. 2020;22(9):e16984. doi: 10.2196/16984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wollny A, Altiner A, Daubmann A, et al. Patient-centered communication and shared decision making to reduce HbA1c levels of patients with poorly controlled type 2 diabetes mellitus—results of the cluster-randomized controlled DEBATE trial. BMC Fam Pract. 2019;20(1):87. doi: 10.1186/s12875-019-0977-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167(10):1076-1082. doi: 10.1001/archinte.167.10.1076 [DOI] [PubMed] [Google Scholar]
- 21.Warner DO, LeBlanc A, Kadimpati S, Vickers KS, Shi Y, Montori VM. Decision aid for cigarette smokers scheduled for elective surgery. Anesthesiology. 2015;123(1):18-28. doi: 10.1097/ALN.0000000000000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tutino GE, Yang WY, Li X, et al. ; China JADE Study Group . A multicentre demonstration project to evaluate the effectiveness and acceptability of the web-based Joint Asia Diabetes Evaluation (JADE) programme with or without nurse support in Chinese patients with type 2 diabetes. Diabet Med. 2017;34(3):440-450. doi: 10.1111/dme.13164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Steenkiste B, van der Weijden T, Stoffers HE, Kester AD, Timmermans DR, Grol R. Improving cardiovascular risk management: a randomized, controlled trial on the effect of a decision support tool for patients and physicians. Eur J Cardiovasc Prev Rehabil. 2007;14(1):44-50. doi: 10.1097/01.hjr.0000239475.71805.1e [DOI] [PubMed] [Google Scholar]
- 24.Tusa N, Kautiainen H, Elfving P, Sinikallio S, Mäntyselkä P. Randomized controlled study of the impact of a participatory patient care plan among primary care patients with common chronic diseases: a one-year follow-up study. BMC Health Serv Res. 2021;21(1):715. doi: 10.1186/s12913-021-06716-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinsel I, Siegel A, Schmoor C, Poguntke I, Maun A, Niebling W. Encouraging self-management in cardiovascular disease prevention: a randomized controlled study of a structured advice and patient activation intervention in primary care. Dtsch Arztebl Int. 2018;115(27-28):469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinsel I, Buchholz A, Vach W, et al. Shared decision-making in antihypertensive therapy: a cluster randomised controlled trial. BMC Fam Pract. 2013;14(1):135-147. doi: 10.1186/1471-2296-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swoboda CM, Miller CK, Wills CE. Impact of a goal setting and decision support telephone coaching intervention on diet, psychosocial, and decision outcomes among people with type 2 diabetes. Patient Educ Couns. 2017;100(7):1367-1373. doi: 10.1016/j.pec.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Sperl-Hillen JM, Crain AL, Margolis KL, et al. Clinical decision support directed to primary care patients and providers reduces cardiovascular risk: a randomized trial. J Am Med Inform Assoc. 2018;25(9):1137-1146. doi: 10.1093/jamia/ocy085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SA, Shah ND, Bryant SC, et al. ; Evidens Research Group . Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clin Proc. 2008;83(7):747-757. doi: 10.4065/83.7.747 [DOI] [PubMed] [Google Scholar]
- 30.Ramallo-Fariña Y, Rivero-Santana A, García-Pérez L, et al. ; INDICA Team . Patient-reported outcome measures for knowledge transfer and behaviour modification interventions in type 2 diabetes—the INDICA study: a multiarm cluster randomised controlled trial. BMJ Open. 2021;11(12):e050804. doi: 10.1136/bmjopen-2021-050804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rost KM, Flavin KS, Cole K, McGill JB. Change in metabolic control and functional status after hospitalization. Impact of patient activation intervention in diabetic patients. Diabetes Care. 1991;14(10):881-889. doi: 10.2337/diacare.14.10.881 [DOI] [PubMed] [Google Scholar]
- 32.Perestelo-Pérez L, Rivero-Santana A, Boronat M, et al. Effect of the statin choice encounter decision aid in Spanish patients with type 2 diabetes: a randomized trial. Patient Educ Couns. 2016;99(2):295-299. doi: 10.1016/j.pec.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 33.Peiris D, Usherwood T, Panaretto K, et al. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8(1):87-95. doi: 10.1161/CIRCOUTCOMES.114.001235 [DOI] [PubMed] [Google Scholar]
- 34.O’Malley PG, Jackson JL, Becher D, Hanson J, Lee JK, Grace KA. Tool to improve patient-provider interactions in adult primary care: randomized controlled pilot study. Can Fam Physician. 2022;68(2):e49-e58. doi: 10.46747/cfp.6802e49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453-459. doi: 10.1001/archinternmed.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568. doi: 10.1001/archinternmed.2009.293 [DOI] [PubMed] [Google Scholar]
- 37.Moin T, Duru OK, Turk N, et al. Effectiveness of shared decision-making for diabetes prevention: 12-month results from the Prediabetes Informed Decision and Education (PRIDE) trial. J Gen Intern Med. 2019;34(11):2652-2659. doi: 10.1007/s11606-019-05238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open. 2012;2(6):e001469. doi: 10.1136/bmjopen-2012-001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maindal HT, Carlsen AH, Lauritzen T, Sandbaek A, Simmons RK. Effect of a participant-driven health education programme in primary care for people with hyperglycaemia detected by screening: 3-year results from the Ready to Act randomized controlled trial (nested within the ADDITION-Denmark study). Diabet Med. 2014;31(8):976-986. doi: 10.1111/dme.12440 [DOI] [PubMed] [Google Scholar]
- 40.Lee JE, Shin DW, Suh B, Chun S, Nam YS, Cho B. Development and application of culturally appropriate decision aids for smoking cessation in Korea: a pragmatic clustered randomization crossover trial. Patient Prefer Adherence. 2016;10:1929-1936. doi: 10.2147/PPA.S114387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauffenburger JC, Ghazinouri R, Jan S, et al. Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: the ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial. PLoS One. 2019;14(4):e0214754. doi: 10.1371/journal.pone.0214754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunneman M, Branda ME, Ridgeway JL, et al. Making sense of diabetes medication decisions: a mixed methods cluster randomized trial using a conversation aid intervention. Endocrine. 2022;75(2):377-391. doi: 10.1007/s12020-021-02861-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krones T, Keller H, Sönnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6(3):218-227. doi: 10.1370/afm.854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koelewijn-van Loon MS, van der Weijden T, van Steenkiste B, et al. Involving patients in cardiovascular risk management with nurse-led clinics: a cluster randomized controlled trial. CMAJ. 2009;181(12):E267-E274. doi: 10.1503/cmaj.081591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koelewijn-van Loon MS, van der Weijden T, Ronda G, et al. Improving lifestyle and risk perception through patient involvement in nurse-led cardiovascular risk management: a cluster-randomized controlled trial in primary care. Prev Med. 2010;50(1-2):35-44. doi: 10.1016/j.ypmed.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 46.Keyserling TC, Sheridan SL, Draeger LB, et al. A comparison of live counseling with a web-based lifestyle and medication intervention to reduce coronary heart disease risk: a randomized clinical trial. JAMA Intern Med. 2014;174(7):1144-1157. doi: 10.1001/jamainternmed.2014.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kask-Flight L, Durak K, Suija K, Rätsep A, Kalda R. Reduction of cardiovascular risk factors among young men with hypertension using an interactive decision aid: cluster-randomized control trial. BMC Cardiovasc Disord. 2021;21(1):543. doi: 10.1186/s12872-021-02339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karagiannis T, Liakos A, Branda ME, et al. Use of the Diabetes Medication Choice decision aid in patients with type 2 diabetes in Greece: a cluster randomised trial. BMJ Open. 2016;6(11):e012185. doi: 10.1136/bmjopen-2016-012185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jouni H, Haddad RA, Marroush TS, et al. Shared decision-making following disclosure of coronary heart disease genetic risk: results from a randomized clinical trial. J Investig Med. 2017;65(3):681-688. doi: 10.1136/jim-2016-000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaspers NEM, Visseren FLJ, van der Graaf Y, et al. Communicating personalised statin therapy-effects as 10-year CVD-risk or CVD-free life-expectancy: does it improve decisional conflict? three-armed, blinded, randomised controlled trial. BMJ Open. 2021;11(7):e041673. doi: 10.1136/bmjopen-2020-041673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X, Deng H, Zhang Y, et al. Efficacy and safety of a decision support intervention for basal insulin self-titration assisted by the nurse in outpatients with T2DM: a randomized controlled trial. Diabetes Metab Syndr Obes. 2021;14:1315-1327. doi: 10.2147/DMSO.S297913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67. doi: 10.1089/dia.2015.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heisler M, Choi H, Palmisano G, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Ann Intern Med. 2014;161(suppl 10):S13-S22. doi: 10.7326/M13-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenfield S, Kaplan SH, Ware JE Jr, Yano EM, Frank HJ. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448-457. doi: 10.1007/BF02595921 [DOI] [PubMed] [Google Scholar]
- 55.Grant RW, Wald JS, Schnipper JL, et al. Practice-linked online personal health records for type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2008;168(16):1776-1782. doi: 10.1001/archinte.168.16.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farmer AJ, Gibson OJ, Dudley C, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446). Diabetes Care. 2005;28(11):2697-2702. doi: 10.2337/diacare.28.11.2697 [DOI] [PubMed] [Google Scholar]
- 57.Edwards A, Thomas R, Williams R, Ellner AL, Brown P, Elwyn G. Presenting risk information to people with diabetes: evaluating effects and preferences for different formats by a web-based randomised controlled trial. Patient Educ Couns. 2006;63(3):336-349. doi: 10.1016/j.pec.2005.12.016 [DOI] [PubMed] [Google Scholar]
- 58.Eckman MH, Wise R, Leonard AC, et al. Impact of health literacy on outcomes and effectiveness of an educational intervention in patients with chronic diseases. Patient Educ Couns. 2012;87(2):143-151. doi: 10.1016/j.pec.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 59.Eaton CB, Parker DR, Borkan J, et al. Translating cholesterol guidelines into primary care practice: a multimodal cluster randomized trial. Ann Fam Med. 2011;9(6):528-537. doi: 10.1370/afm.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dwinger S, Rezvani F, Kriston L, Herbarth L, Härter M, Dirmaier J. Effects of telephone-based health coaching on patient-reported outcomes and health behavior change: a randomized controlled trial. PLoS One. 2020;15(9):e0236861. doi: 10.1371/journal.pone.0236861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denig P, Schuling J, Haaijer-Ruskamp F, Voorham J. Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial. BMJ. 2014;349:g5651. doi: 10.1136/bmj.g5651 [DOI] [PubMed] [Google Scholar]
- 62.Coronado-Vázquez V, Gómez-Salgado J, Cerezo-Espinosa de Los Monteros J, Ayuso-Murillo D, Ruiz-Frutos C. Shared decision-making in chronic patients with polypharmacy: an interventional study for assessing medication appropriateness. J Clin Med. 2019;8(6):904. doi: 10.3390/jcm8060904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Den Ouden H, Vos RC, Rutten GEHM. Effectiveness of shared goal setting and decision making to achieve treatment targets in type 2 diabetes patients: a cluster-randomized trial (OPTIMAL). Health Expect. 2017;20(5):1172-1180. doi: 10.1111/hex.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011;26(11):1297-1304. doi: 10.1007/s11606-011-1794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng L, Sit JWH, Choi KC, et al. The effects of an empowerment-based self-management intervention on empowerment level, psychological distress, and quality of life in patients with poorly controlled type 2 diabetes: a randomized controlled trial. Int J Nurs Stud. 2021;116:103407. doi: 10.1016/j.ijnurstu.2019.103407 [DOI] [PubMed] [Google Scholar]
- 66.Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116. doi: 10.1136/bmjopen-2015-009116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buhse S, Kuniss N, Liethmann K, Müller UA, Lehmann T, Mühlhauser I. Informed shared decision-making programme for patients with type 2 diabetes in primary care: cluster randomised controlled trial. BMJ Open. 2018;8(12):e024004. doi: 10.1136/bmjopen-2018-024004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulware LE, Ephraim PL, Hill-Briggs F, et al. Hypertension self-management in socially disadvantaged African Americans: the Achieving Blood Pressure Control Together (ACT) randomized comparative effectiveness trial. J Gen Intern Med. 2020;35(1):142-152. doi: 10.1007/s11606-019-05396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301. doi: 10.1186/1472-6963-13-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey RA, Shillington AC, Harshaw Q, Funnell MM, VanWingen J, Col N. Changing patients’ treatment preferences and values with a decision aid for type 2 diabetes mellitus: results from the treatment arm of a randomized controlled trial. Diabetes Ther. 2018;9(2):803-814. doi: 10.1007/s13300-018-0391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey RA, Pfeifer M, Shillington AC, et al. Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Serv Res. 2016;16:10. doi: 10.1186/s12913-016-1262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adarkwah CC, Jegan N, Heinzel-Gutenbrunner M, et al. Time-to-event versus ten-year-absolute-risk in cardiovascular risk prevention—does it make a difference? results from the Optimizing-Risk-Communication (OptRisk) randomized-controlled trial. BMC Med Inform Decis Mak. 2016;16(1):152. doi: 10.1186/s12911-016-0393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Applegate M, Scott E, Taksler GB, et al. Project ACTIVE: a randomized controlled trial of personalized and patient-centered preventive care in an urban safety-net setting. J Gen Intern Med. 2021;36(3):606-613. doi: 10.1007/s11606-020-06359-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prabhakaran D, Jha D, Prieto-Merino D, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation. 2019;139(3):380-391. doi: 10.1161/CIRCULATIONAHA.118.038192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search Summary
eMethods 2. Quality Appraisal
eMethods 3. Data Synthesis and Data Transformation
eTable 1. Quality Assessment Using the Quality Assessment of Controlled Intervention Studies Criteria
eTable 2. Summary of Patient and Clinician Sociodemographic Characteristics and Outcomes
eTable 3. Summary of Interventions
eTable 4. Studies Incorporating Decision Aids in the Intervention
eTable 5. Summary of Outcomes
eTable 6. Studies Reporting Satisfaction Outcome
eFigure 1. Forest Plot of HbA1c
eFigure 2. Forest Plot of Systolic Blood Pressure
eFigure 3. Funnel Plot of Decisional Conflict
eFigure 4. Funnel Plot of HbA1c
eFigure 5. Funnel Plot of Systolic Blood Pressure
eFigure 6. Forest Plot of Satisfaction About the Decision or Treatment
eFigure 7. Funnel Plot of Satisfaction About the Decision or Treatment
eReferences
Data Sharing Statement