Abstract
The North Atlantic and North Pacific commercial fisheries report problematic occurrences of ‘jellied’ or ‘mushy’ fish. These fish exhibit an abnormally soft and jelly-like musculature that attains a mushy consistency when cooked. The condition affects several economically important species, and is commonly termed ‘jellied condition’ or, specifically for halibut, ‘mushy halibut syndrome’. The inferior quality of ‘jellied’ or 'mushy' products reduces the market value considerably, leading to wastage and losses in the fisheries. The syndrome is associated with an abnormally high moisture content and lowered protein of the white skeletal musculature, particularly the fillets. Alterations in lipid content varies depending on species investigated. In some fish species, myxozoan infections can induce similar fillet alterations, but studies on ‘jellied’ or ‘mushy’ meat indicate a non-infectious myopathy. Several hypotheses have been launched to explain the jelly-like syndrome, including dietary deficiencies, spawning exhaustion, environmental circumstances, genetics and adaptive physiology. This review provides a comprehensive overview of the characteristics of the ‘jellied’ or ‘mushy’ syndrome including a discussion of plausible etiologies and applicable mitigation strategies. The main conclusion is that the syndrome may involve two different etiologies dependent on species and location, but new studies are needed to verify past findings and rule out alternative explanations. A growing human population and an increasing demand for food requires efficient utilization of all harvested fish products. Expanded and updated knowledge is vital to reduce food waste and losses related to ’jellied’ or ‘mushy’ fish in catches, and promote sustainable quota usage. We advocate for further research into the syndrome, including prevalence in commercial fish stocks and correlational studies with focus on association with biological parameters, chemical composition, infectious agents, geographic distribution and seasonal variation.
Keywords: Jellied condition, Mushy halibut syndrome, Histology, Myopathy, Kudoa, Fish quality
Highlights
-
•
'Jellied condition' and 'mushy halibut syndrome' appears in several commercially important species.
-
•
Affected fish present with jelly-like fillets characterized by an increased water and decreased protein content.
-
•
The etiology is possibly linked to nutritional status or spawning exhaustion.
-
•
There are currently no available technologies for detecting affected fish products.
-
•
Renewed focus is needed to minimize food wastage and economic losses.
1. Introduction
Texture is an important quality parameter of fish products. The skeletal musculature of fish is influenced by different anatomical and physiological processes, and multiple factors before, during and after harvest affect muscle firmness (see Fig. 1) [[1], [2], [3], [4]]. A continuously occurring texture problem is the jelly-like consistency of some commercial fish products, referred to as ‘jellied’, ‘soft’ or ‘mushy’ flesh. These names are often used interchangeably, but describe two different conditions seen in farmed and wild-caught commercial fish species, in which the somatic skeletal muscle tissue, commonly sold as fillets or cuts, attains an abnormally soft texture (see Table 1). Different forms of jelly-like quality deterioration exist: some fillets appear gelatinous and glistening, while others slowly decompose after capture and obtain a creamy texture. The latter form is associated with myxozoan parasite infections of the Kudoa genus, and the parasitic effect on fillet quality is recognized as cyst or pseudocyst formation and extreme softening [5,6]. This softening is known as post-mortem myoliquefaction, caused by proteolytic activity of parasitic proteases, mainly cathepsin L-enzymes. After the death of the fish host, the proteases diffuse out of the pseudocyst and into the surrounding muscle tissue, where they break-down the muscle protein [[7], [8], [9]]. In contrast, the cause of the watery, gelatinous form of fillet deterioration is unclear. In peer-reviewed studies and grey literature, affected fish products exhibit myodegenerative changes without signs of parasitic infection. This includes reports of ‘gelatinous’ or ‘mushy’ Greenland halibut (Reinhardtius hippoglossoides) [[10], [11], [12], [13]], ‘mushy halibut syndrome’ in Pacific halibut (Hippoglossus stenolepis) [14,15], ‘soft flesh’ in sablefish (Anoplopoma fimbria) [[16], [17], [18]] and the ‘jellied condition’ in American Plaice (Hippoglossoides platessoides) and Dover sole (Microstomus pacificus) [[19], [20], [21], [22], [23]]. Fish appear to be affected at capture, and may be recognized by experienced fishermen [16,21]. While some commercial vessels sort abnormally soft fish at sea, ‘jellied’ or ‘mushy’ fish often go undiscovered until the land-based processing stage or later in the supply chain. The late detection is likely due to varying degrees of deterioration, highlighting the need for effective and sensitive methods for early detection [21,[24], [25], [26]]. The unappealing nature of affected fish products reduces the market value and severely affected fillets are considered unsaleable. Despite this, the etiology of the syndrome remains unknown, although several causes have been suggested. These include dietary deficiencies, spawning exhaustion, environmental circumstances, genetics, and adaptive physiological mechanisms [14,16,20,21,23,27].
Fig. 1.
Overview of factors influencing the chemical composition and texture of fish musculature.
Table 1.
Reports of ‘jellied’, ‘mushy’ or ‘soft’ musculature and myoliquefaction in selected commercial species in the North Atlantic and North Pacific fisheries, including identified or suspected causesa.
| Aquaculture | |
|---|---|
| Affected fish species | Etiology |
| Atlantic salmon (Salmo salar) [[28], [29], [30], [31], [32]] (North America and Western Europe) | Parasitic (Kudoa thyrsites) |
| Atlantic salmon (Salmo salar) [33,34] (Northern Europe) | No causative agent identified – possibly glycogen accumulation |
| Coho salmon (Oncorhyncus kisutch) [35] | Parasitic (Kudoa thyrsites) |
| Mahi mahi (Coryphaena hippurus) [36] | Parasitic (Kudoa thyrsites) |
| Olive flounder (Paralichthys olivaceus) [37] | Parasitic (Kudoa thyrsites) |
| Capture fishery | |
|
Affected fish species |
Etiology |
| Atka mackerel (Pleurogrammus monopterygius) [38] | Parasitic (Kudoa pleurogrammi n. sp.) |
| American plaice (Hippoglossoides platessoides) [21,22] | No causative agent identified – possibly reproductive strategy, environmental factors or nutritional deficiency |
| Arrowtooth flounder (Atheresthes stomias) [39,40] | Parasitic (Kudoa thyrsites) |
| Atlantic lumpfish (Cyclopterus lumpus) [41] | Parasitic (Kudoa islandica) |
| Atlantic mackerel (Scomber scombrus) [[42], [43], [44], [45]] | Parasitic (Kudoa thyrsites) |
| Atlantic wolffish (Anarhichas lupus) [41] | Parasitic (Kudoa islandica) |
| Broad flounder (Paralichthys squamilentus) [46] | No causative agent identified |
| Dover sole (Microstomus pacificus) [19,20,23,47] | No causative agent identified – possibly reproductive strategy or environmental factors |
| Greenland halibut (Reinhardtius hippoglossoides) [[10], [11], [12], [13]] | No causative agent identified – possibly nutritional deficiency or myodegenerative disease |
| Hoki (Macruronus novaezelandaie) [48] | Nutritional deficiency |
| Mahi mahi (Coryphaena hippurus) [36] | Parasitic (Kudoa thyrsites) |
| Olive flounder (Paralichthys olivaceus) [24] | No causative agent identified – elevated endogenic proteolytic activity possibly due to abnormal regulation |
| Pacific bluefin tuna (Thunnus orientalis) [49] | Parasitic (Kudoa hexapunctata, Kudoa neothunni) |
| Pacific bluefin tuna (Thunnus orientalis) [50] | Post mortem endogenous proteolytic activity, stress during capture and insufficient cooling post-harvest |
| Pacific hake (Merluccius productus) [9,51,52] | Parasitic (Kudoa paniformis, Kudoa thyrsitis) |
| Pacific halibut (Hippoglossus stenolepis) [14,15,53] | No causative agent identified – possibly nutritional deficiency or myodegenerative disease Older reports suggestive of Kudoa sp [54,55]. Kudoa sp. reported by Davis (1924) is now named Kudoa aburakarei [56]. |
| Red barracuda (Sphyraena pinguis) [57] | Parasitic (Kudoa megacapsula) |
| Sablefish (Anoplopoma fimbria) [[16], [17], [18]] | No causative agent identified – possibly genetic variability or environmental factors and reproductive strategy |
| Skipjack tuna (Katsuwonus pelamis) [58] | Post mortem endogenous proteolytic activity, stress during capture and insufficient cooling post-harvest |
| Spotted wolffish (Ana hichas minor) [41] | Parasitic (Kudoa islandica) |
| Swordfish (Xiphsias gladius) [59,[60], [61], [62], [63]] | Parasitic (Kudoa musculoliquefaciens) |
| Yellowfin tuna (Thunnus albacares) [64] | Parasitic (Kudoa neothunni) |
| Yellowfin tuna (Thunnus albacares) [65,66] | Post mortem endogenous proteolytic activity, stress during capture and insufficient cooling post-harvest |
Reports on Kudoa infections have only been included if jelly-like quality deterioration or myoliquefaction is documented as a consequence.
The ‘jellied’ or ‘mushy’ syndrome has been known for more than a century. Mentions can be traced back to the early and mid-1900s reports, and anecdotes circulate among fishermen and people processing fish [26,59,67]. Several reviews address the problem with myoliquefaction due to Kudoa infections in commercial catches and its economic consequences for the industry [[68], [69], [70]]; yet the exact impact of the ‘jellied’ or ‘mushy’ syndrome is unclear, and most studies were conducted more than 30 years ago. Due to unnecessary processing, yield loss and unmarketability, new focus should be placed on this condition. The objective of this review is to examine characteristics and possible causes of the ‘jellied’ or ‘mushy’ syndrome seen in five commercial North Atlantic and North Pacific fish species, as well as the incentives for further investigations. The review addresses four questions.
-
I.
What are the histological and biochemical characteristics of affected fish products?
-
II.
How does the occurrence of the syndrome relate to fish size, sex, maturity, season and depth of capture in different species?
-
III.
Which etiology or etiologies are supported by past and current study findings?
-
IV.
How can research and commercial efforts mitigate the effects of ‘jellied’ or 'mushy' fish products?
In the results section, we answer question one and two, while the discussion addresses question three and four. The first part of the discussion summarizes characteristics of the syndrome with perspective to the quality deterioration caused by Kudoa spp. The second part addresses the validity of current hypotheses concerning the cause of the syndrome, while the final part concentrates on limitations in previous work, gaps in knowledge, and implications for research and commercial initiatives. It is our hope that this paper will aid both the fisheries science community and the commercial fishing industries, and encourage continued research into the syndrome.
2. Methods
A broad literature search was conducted on selected terms in several databases (see Appendix A for the full list of key terms, inclusion criteria, restrictions, date of first and last search and databases searched). Manual cross-referencing with bibliographies was carried out to identify more relevant articles, and most of the articles used in the review were identified this way. A grey literature search was conducted to minimize the risk of omitting other relevant sources. Similar criteria as with the peer-reviewed search were applied. The grey sources were identified through multiple Google scholar searches, targeted fisheries websites and by consulting professionals. For each publication, the full-text, authority, stakeholders and credibility, year published, document objectives, accuracy of resources cited, objectivity and significance was evaluated. Out-reach to North Atlantic and North Pacific fisheries and authorities was carried out to inquire about new research or unpublished data.
3. Results
The database search strategy resulted in 18 search hits. Most concerned quality deterioration due to infection with Kudoa spp. Cross-referencing and grey literature screening revealed more relevant journal articles, government publications, fisheries reports and proceedings. Ten documents (key sources) provided the foundation for this review, of which four are non-peer-reviewed. Study details are summarized in Table 2. After the initial search, articles covering concepts relevant to the discussion were obtained. Some studies conducted outside the geographic focus are cited based on relevance. Personal communication with Royal Greenland and The Fish Pathology Section at Alaska Department of Food and Game provided valuable insight and perspective.
Table 2.
Overview of selected articles concerning ‘jellied’, ‘mushy’ or ‘soft’ meat in five commercial North Atlantic and North Pacific fish species. Sorted alphabetically according to species investigated.
| Author and year | Fish species | Sample size | Sampling location and depth (m) | Study focus | Study design | Methodology |
|---|---|---|---|---|---|---|
| Templeman and Andrews [21] | American plaiceWC | 273a to 2.406b | Grand Banks, Canada 155–227 m. |
Chemical composition of jellied plaice and association of jellied plaice to life-history of the plaice (sex, size and sexual maturity) and conditions of the fishery (season, depth and temperature) | Correlational study | Sensory classification Biological analysis Chemical analysis Yield analysis Drip loss Parasitological analysis Histological analysis Statistical analysis |
| Haard [22] | American plaiceWC | 12 | Newfoundland, Canada (place of capture not disclosed) Depth N/A |
Amino acid, non-nitrogenous compounds and proteins in jellied and normal flounder fillets | Case-control study | Chemical analysis |
| Puckett and Hendrickson [20]* | Dover soleWC | N/A | Eureka, U.S.A. (place of capture not disclosed) <180–1020 m. |
Association of jellied sole to sex, maturity, length, weight, age, season, geographic location and depth of capture | Correlational study | Biological analysis Moisture content Histological analysis Bacteriological analysis parasitological analysis |
| Fisher et al. [19]* | Dover soleWC | N/A | Eureka, U.S.A. (place of capture not disclosed) 550–915 m. |
Characterization of jellied sole musculature by histology | Case-control study | Histological analysis |
| Ortega, Ofstad et al. [11] | Greenland halibutWC | 16 | Vengsøya, Norway (place of capture not disclosed) Depth N/A |
Characterization of jellied or mushy Greenland halibut by histology, hyperspectral imaging and diffusion tensor imaging | Case-control study | Histological analysis Image analysis Statistical analysis |
| Ortega, Lindberg et al. [10] | Greenland halibutWC | 23c to 62d | NAFO Divisions 1C/1D (Davis Strait), Southwest Greenland 400–1500 m. |
Detection of jellied or mushy Greenland halibut by use of hyperspectral imaging | Case-control study | Chemical analysis Image analysis Statistical analysis |
| Widera [12] | Greenland halibutWC | 6 | Various waters off Labrador and Newfoundland, Canada Depth N/A |
Fatty acid composition in lipids of gelatinous Greenland halibut meat | Case-control study | Chemical analysis |
| Widera and Madler [13] | Greenland halibutWC | 6 | Various waters off Labrador and Newfoundland, Canada Depth N/A |
Amino acid composition in proteins of gelatinous Greenland halibut meat | Case-control study | Chemical analysis |
| Norris et al. [18]** | SablefishWC | 740 | Coast of Vancouver, Canada, and Coast of Washington, Oregon and Northern California, U.S.A. 109–1235 m. |
Association of soft sablefish to four factors: fish size, depth of capture, season and fishing gear type | Correlational study | Biological analysis Yield analysis Drip loss Chemical analysis Sensory analysis Statistical analysis |
| Karinen et al. [16]*** | SablefishWC | 179 | Chatham, Icy Straits and off Cape Cross, Southeastern Alaska, U.S.A. 259–988 m. |
Association of soft sablefish to depth of capture, season, spawning condition and type of fishing gear | Correlational study | Biological analysis Yield analysis Drip loss Chemical analysis Statistical analysis |
WC = Wild-caught * = Conference Proceedings ** = Government document or report *** = Non-peer reviewed publication N/A = Data not available.
The sample sizes varied for each experiment conducted. This sample size reflects the one reported in Tables I–III in the publication.
The sample sizes varied for each experiment conducted. This sample size reflects the one reported in Table IX in the publication.
The sample size varied for each experiment conducted. This sample size reflects the one reported in Section 2.1: Sample description and Table 1 in the publication.
The sample size varied for each experiment conducted. This sample size reflects the one reported in Section 2.3: Hyperspectral image processing in the publication.
It should be noted that soft-textured Atlantic salmon are occasionally seen in Norwegian farming and processing industries [71]. However, rather than gelatinous and translucent, affected fillets appear abnormally soft with a patty-like texture, accompanied by gaping and a jelly-like band along the fillet [33]. Assumed due to intracellular glycogen accumulation, it is considered a separate phenomenon not covered in this review [34]. The same concerns the ‘burnt’ and ‘mushy’ syndromes seen in tunas, characterized by pale or brown meat color and soft texture, with surface exudate and a sour taste, related to post mortem proteolysis and lactic acidosis [58,72].
3.1. Characteristics of the ‘jellied’ or ‘mushy’ condition
3.1.1. Gross appearance
Fillets of affected fish are unanimously described as odorless, glossy and gelatinous, at times quivering to the touch. The meat is flaccid, watery and fragile, with an abnormally soft texture, notable shrinkage and a mushy cooked texture (see Fig. 2). In American plaice, Dover sole, sablefish and Greenland halibut, affected musculature is more white or opalescent compared to normal [10,11,18,19,21], while that of affected Pacific halibut has a more translucent appearance [14]. Apart from a small number of Kudoa clupeidae cysts found in Dover sole [20], no studies report visible cysts or pseudocysts. Observations on the condition of affected fish differ – some describe fish in poor condition [14,18] while others report good condition [47]. Some ‘gelatinous’ Greenland halibut have exhibited signs of kidney- and liver degeneration [12,13]. ‘Soft’ sablefish from deep waters appear darker in color [16,18].
Fig. 2.
Severely ‘jellied’ or ‘mushy’ Greenland halibut fillet (lower left) next to a normal high-quality fillet (upper right). Photo source: NLS, first and corresponding author.
3.1.2. Histological hallmarks
Histology findings point to myofiber degeneration or atrophy in the absence of parasitic infection. The syndrome is characterized by wide spacing between myofibers, which appear fragmented and coiled with a lack of orientation [11,19,21]. In American plaice and Dover sole, the enlarged intracellular space appears water-filled, presumably due to protein or fat depletion in the skeletal muscle tissue [19,21]. Irregularly shaped and bloated muscle cells with hypertrophied or pyknotic nuclei are occasionally reported. Some studies describe myofiber-necrosis, proliferation of non-supportive, loose connective tissue, and occasional inflammatory cell infiltration in the enlarged intracellular space [14,19]. In Greenland halibut, ‘mushy’ muscle sections contain less fat and connective tissue between muscle segments [11].
3.1.3. Biochemical composition
Altered biochemistry of fish exhibiting signs of the ‘jellied’ or ‘mushy’ syndrome is evident in most key sources. The white skeletal muscle is characterized by an increased water content and decreased protein, compared to unaffected fish. Studies report an average increase in water content from 1 to 10%, while protein is lower by 2–52%. The alteration in composition seems to progress with the severity of ‘jellification’; in one study, the fillet of one extremely ‘jellied’ plaice was found to have a water content of 96.18% and a protein content of 2.83%, compared to app. 82% and 16% in normal plaice fillets. Drip loss averaged 14.4% for ‘jellied’ fillets and 2.1% for normal, but ranged as high as 29% in the ‘jellied’ category depending on the level of ‘jellification’ [21].
Findings are not homogenous when it comes to fat content, as illustrated in Table 3. For plaice and sablefish, ‘jellied’ fillets contain 0.2%–12% more fat, while in Greenland halibut the fat content is 3–9% lower compared to normal fillets. Decreased yield is evident in two studies on sablefish and one on American plaice [16,18,21]. One study on Greenland halibut found affected fish to contain overall 4.24% less essential amino acids, with the exception of histidine and arginine [13]. Similar findings exist for ‘jellied’ plaice, which also exhibit lower myosin and actin levels [22].
Table 3.
Average chemical constitution of ‘jellied’, ‘mushy’, ‘soft’ and ‘gelatinous’ fish. Units depicted as in original studies.
| Author and year | Fish species | Sample size ‘Jellied’/normal |
Water content ‘Jellied’/normal |
Fat content ‘Jellied’/normal |
Protein content ‘Jellied’/normal |
Ash content ‘Jellied’/normal |
|---|---|---|---|---|---|---|
| Ortega, Ofstad et al. [11] | Greenland halibutL | 8/8 | 0.996/0.993a | 4.3e−3/6.9e−3a | – | – |
| Ortega, Lindberg et al. [10] | Greenland halibutL | 12/11 | 78.63%/74.99% | 7.6%/10.88% | 12.54%/14.10% | 1.14%/1.03% |
| Widera and Madler [13] | Greenland halibutF | 3/3 | – | – | 34.9%/39.14% | – |
| Widera [12] | Greenland halibutF | 3/3 | – | 2.43%/11.62% | – | – |
| Karinen et al. [16] | SablefishN | 115/64 | 726.1 mg/g/720.7 mg/g | 148.1 mg/g/132.6 mg/g | 118.2 mg/g/136.8 mg/g | 10.3 mg/g/10.6 mg/g |
| Templeman and Andrews [21] | American plaiceF | 108/165b | 79.01–93.0%/77.01–86% | 0.9%/0.55% | 10.87%/15.31% | – |
| Haard [22] | American plaiceF | 6/6 | 88.7%/78.5% | – | 9.2%/18.8% | – |
| Puckett and Hendrickson [20] | Dover sole | N/A | 88.6–91.0%/85.1% | – | – | – |
L = Loin N = Nape F = Fillet.
Average spectral abundance of chemical constituents in sample signals measured by hyperspectral imaging.
The sample sizes varied for each experiment conducted. This sample size reflects the one reported in Table I in the publication.
3.2. Associations of occurrence
3.2.1. Fish size, sex and maturity
Three of the ten key sources report on the effect of fish size. Two of these also investigated sexual variation. In Dover sole, only reproductively mature individuals exhibited the syndrome, especially large, spawning females. No significant correlation between length, weight and occurrence of the syndrome was found [20]. However, in America plaice, the amount of ‘jellied’ plaice did increase with length: 20% of males were reported ‘jellied’ at 48–51 cm, compared to 6% at 44–47 cm. Mature females showed no signs of ‘jellification’ below 44–47 cm. At 48–51 cm, 7% of females were ‘jellied’, going up to 23% at 52–55 cm and 58% at 60–63 cm. The percentage of ‘jellied’ females increased until 68–77 cm, at which stage 80% were ‘jellied’ and only 7% normal, the remainder characterized as ‘intermediate’. No significant differences were evident between sexes in weight-matched categories. The prevalence reported for each sex and size group is depicted in Table 4 [21]. The studies on Dover sole and American plaice both found the ‘jellied condition’ to be negligible in immature fish. In sablefish, the size range of affected fish extended from small, recently-maturing fish, to very large multiparous females, with no significant correlation with sex or size [16]. Although most of the sampled sablefish were immature or maturing juveniles, a greater percentage of mature, spawning and spent individuals occurred in the ‘soft’-category. Spent females appeared only in this category, especially in trawl catches. However, regression analysis showed that while gonad stage was related to capture depth, it was only marginally related to ‘soft’ flesh texture.
Table 4.
Prevalence of ‘jellied’ and ‘soft’ fish in two commercial North Pacific species.
| Author and year | Fish species | Sample size | Overall prevalence | Prevalence in study categories | |||
|---|---|---|---|---|---|---|---|
| Karinen et al. [16] | SablefishWC | Summer242 |
Winter 439 |
Summer60% |
Winter 33% |
Summer ♂: 61% ♀: 59% |
Winter ♂: 26% ♀: 39% |
| Templeman andAndrews [21] | American plaiceWC | 2406a | 18% | ♂ Overall: 6% ♂Mature, SG48–51 cm: 20% ♀Overall: 25% ♀Mature, SG60–63 cm: 58% ♀Mature, SG68–77 cm: 80% |
|||
WC = Wild-caught SG = Size Group.
The sample sizes varied for each experiment conducted. This sample size reflects the one reported in Table IX in the publication.
3.2.2. Season and habitat
Four of the key sources investigated seasonal variation and took into account depth of capture. The two studies on sablefish from different locations found the abundance of ‘soft’ sablefish to increase with depth. The studies found no statistical evidence of seasonal interaction [18], although one did note ‘soft’ sablefish to peak in June–August compared to January–February (see Table 4) [16]. Depth was the factor most significantly correlated with ‘soft’ texture: for each 182 m. of depth, total moisture and drip loss increased, while smoked yield and raw fillet weight declined [18]. Similarly, the percentage of ‘jellied’ American plaice increased with depth of capture and bottom temperatures of −1 °C–0 °C. At water temperatures above 0 °C, the percentage of both larger and ‘jellied’ plaice decreased. ‘Jellied’ plaice appeared throughout the year in the eastern Grand Banks area, with a decrease in January-March [21].
In addition to reproductive state, depth of capture was the factor most significantly correlated with the ‘jellied condition’ in Dover sole. A seasonal variation was reported only for deep-water samples (650–1020 m), with a peak in ‘jellied’ sole in August–September and again in December–February. A significant proportion of ‘jellied’ sole was caught in deep water spawning grounds, compared to shallower waters [20].
4. Discussion
4.1. Characteristics of the ‘jellied’ or ‘mushy’ condition: ‘jelly meat’ vs. ‘soft flesh’
One purpose of this review was to evaluate characteristics of the ‘jellied’ or ‘mushy’ syndrome in different species, with perspective to post mortem myoliquefaction caused by Kudoa spp. Several common denominators exist for the ‘jellied condition’ and ‘mushy halibut syndrome’ reported in the North Atlantic and North Pacific fisheries. Unlike the creamy disintegrated consistency associated with Kudoa-induced post mortem myoliquefaction, ‘jellied’ or ‘mushy’ fillets appear watery and gelatinous without signs of Kudoa pseudocysts. Histology reveals myofiber degeneration, and although the alterations exhibit some similarity to those inflicted by Kudoa infection [42,62,73], no parasitic plasmodia or myxospores are evident in affected fillets. The ‘jellied’ or ‘mushy’ flesh has an increased water content, with a concurrent decrease in protein. Alterations in muscle lipid content appear to depend on species physiology and mode of energy storage. This coincides well with what we know of fat content and fatty acid composition of fish, which is subject to great variability [74,75]. It would indicate that fat content is less reliable as a common hallmark, but if further studies can verify the observed trends it might be useful as an intra-species specific indicator. In contrast, Kudoa infections generally reduce the fat content of the infected tissue, while protein, ash and pH does not vary significantly [7,63] - or do not alter the chemical composition at all [76,77]. Recent studies and aquaculture initiatives have focused on inhibiting high Kudoa infection rates or stalling the proteolytic effects of parasitic proteases. To our knowledge, the enzymatic activity in ‘jellied’ or ‘mushy’ meat has only been scarcely studied [24]. Unlike Kudoa-induced post mortem myoliquefaction, neither the ‘jellied condition’ or ‘mushy halibut syndrome’ appear to progress after harvest or to be caused by parasitic infection. Rather, it seems a static state evident at capture. It is clear that we are dealing with more than myoliquefaction, and solutions therefore need to address factors other than parasite control and inhibiting proteolysis post-harvest.
4.2. Associations of occurrence
A second goal of the review was to assess evidence of associations with occurrence. In American plaice, Dover sole and sablefish, the ‘jellied condition’ is unassociated with fish sex, but there appears to be an association with sexual maturity [20,21]. At first glance, the problem with ‘jellied’ plaice and sole seems to primarily concern larger female fish. However, male fish of both species mature at much smaller sizes and do not attain the same size as their female counterparts. In age-matched categories, no sexual variation was observed in plaice and a gradual decline in protein content with increasing size was seen in both sexes [21]. This supports the notion of increased occurrence in older, larger fish, regardless of the sex. Although no correlation with length and weight was found for mature Dover sole, it has been observed that mainly larger, older fish exhibit the phenomenon, compared to smaller, younger ones [20]. Investigations of the bathymetric patterns in size, age, sexual maturity, water content, and caloric density of Dover sole similarly imply that the water content (synonymous with ‘jellied flesh’) of mature fish is a function of fish age and length, in addition to depth of capture [23].
While these findings suggest that the syndrome is most frequent in large, mature plaice and sole, with a notable occurrence in deep waters and spawning grounds, the same trend is not evident in halibut. It should be noted that none of the three studies offer details on the classification of maturity stages, including criteria of immaturity. Furthermore, the representation of spawning females was sparse [16,20], barely exceeding 2% of the sampling total in one study [20]. Inconsistency in sampling strategy and seasonal and diurnal variation in movement patterns of fish can greatly affect sample representation [78,79]. Some studies included in this review did not clearly state sampling size, depth, area or period, which complicates reproducibility and validation of results.
Seasonal trends, investigated only in American plaice and sablefish, are biased by sampling method. Although no statistically significant effect of season was found, Karinen et al. reported that 80% of fish caught during winter at depths between 365 and 730 m. had firm flesh; this number declined to 48% in the summer. Method of capture likely influenced this variation, as trawl catches contained a higher proportion of ‘soft’ fish compared to longlines and pots, and no trawling was conducted during the summer [16]. Additionally, fish were sampled down to a depth of 988 m. Sablefish spawn from winter to spring, at depths of 800 m. and deeper [80,81]. Seasonal migration of adult fish to deeper spawning grounds could add to the observed effect. For American plaice, the ‘jellied condition’ was recorded throughout the year with a decrease in January-March [21]. However, sampling strategy and size varied during the study period: sampling took place in the South during winter and in the North during summer, with the amount of plaice examined in January–March being much lower than all other months [21]. For Dover sole, a seasonal variation was found only in deep-water samples (650–1020 m), the amount of ‘jellied’ sole peaking in August-September and December–February [20]. These results are difficult to evaluate due to lacking sampling details such as depth of capture at specific locations and months. Nevertheless, spawning seasons for Dover sole are known to vary by location, but peaks from November–March in northern California and Oregon [82,83]. This may reflect in the results.
Studies on plaice, sole and sablefish indicate that the ‘jellied condition’ is associated with depth of capture. To our knowledge, no published studies on the association of ‘mushy halibut syndrome’ with fish size, sex, maturity, season and habitat exist, but illuminating these trends would be of great value (see 4.3.8). Initial investigations conducted by the Alaska Department of Fish and Game indicated that the syndrome affected mainly young immature Pacific halibut in the Cook Inlet and Seward nursing grounds [14]. However, personal communication revealed that even larger fish are afflicted, and the geographic distribution wider.1 In Greenland halibut, the syndrome also appears to affect fish of different size categories.2
4.3. Proposed etiologies: valid explanations and speculations
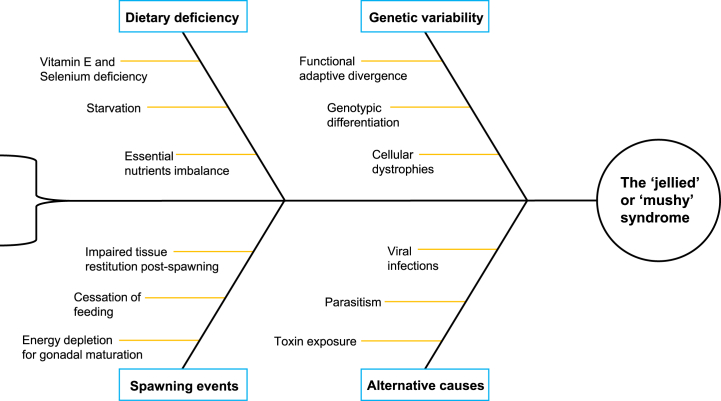
Different circumstances have been proposed to explain the ‘jellied’ or ‘mushy’ syndrome (see Fig. 3). For Greenland and Pacific halibut, the prevailing suspicion is a deficiency in essential nutrients [11,14]. Depletion of energy reserves due to gonadal maturation is the most prominent hypothesis behind the syndrome in American plaice and Dover sole [20,21]. In addition, exposure to environmental factors associated with depth, such as low temperatures, are suspected of either exacerbating or initiating the syndrome in both plaice, sole and sablefish [16,18,20,23]. Lastly, genetic variability has been proposed for sablefish [16,18]. The musculature of 'jellied' or 'mushy' fish is characterized by high water and low protein content. The chemical composition of fish and muscle texture differs between species, and is influenced by sex [84], season [84,85], feeding and movement habits [[86], [87], [88], [89]], and habitat [[90], [91], [92], [93]]. In the light of associations with occurrence, we address plausible hypotheses on the etiology and the possibility of species-specific syndromes.
Fig. 3.
Proposed causes of the ‘jellied’ or ‘mushy’ syndrome seen in the North Atlantic and North Pacific fisheries.
4.3.1. Dietary deficiency
4.3.1.1. Starvation
Starvation is known to cause myofiber-degeneration and deplete energy reserves [94]. Fat and protein content reflects food availability and nutritional status, and low levels indicate malnutrition [95,96]. While strategic food deprivation is used to improve fillet texture [[97], [98], [99]], prolonged starvation results in soft fish musculature and increased water content of white muscle tissue [100,101]. This can mask the loss of lipids and proteins during food deprivation, as reviewed by McCue [102]. Other parameters for assessing feeding and nutritional status of fish include gallbladder bile color [103,104] and mineral levels, high sodium and low potassium levels in the white muscle tissue indicating starvation [105]. Periods of starvation occur naturally in most wild fish habitats and reflects in fish condition; however, observations on the condition of ‘jellied’ or ‘mushy’ fish are either lacking or conflicting.
The one study that did investigate feeding status of affected fish did not indicate starvation [16]. Although analysis of sodium and potassium levels in ‘soft’ sablefish resembled effects of starvation, similar changes have been described in spawning sockeye salmon (Oncorhynchus nerka) [106]. Bile samples indicated well or recently fed fish, and the lipid content of ‘soft’ fish was 12% higher compared to firm. This is contrasting to the low lipid levels seen in experimentally starved sablefish [107]. The results indicate that variations in electrolyte and water content of sablefish may occur naturally or due factors other than poor feeding state. Excessive feeding on single prey items can also alter muscle texture. This is seen in post-spawning Atlantic cod, which attain a soft muscle texture with increased drip loss after feeding intensely on capelin [89]. A similar phenomenon could occur in sablefish.
4.3.1.2. Essential nutrient imbalance
Another possibility is a deficiency or imbalance in essential nutrients, as suggested for Greenland and Pacific halibut.Recent studies indicate a change in ecosystems in both the North Atlantic and Pacific [53,[108], [109], [110]]. It is natural to wonder if this change could predispose fish to nutritional deficiencies through a shift in primary food sources, due to a decrease in preferred prey items or increased predation pressure. Initial reports from Alaska indicated that the ‘mushy’ syndrome affects mainly young Pacific halibut in poor condition, with a stomach content consisting mostly of crabs. Vitamin E and selenium deficiency was suspected [14]. Vitamin E and selenium are important for growth and metabolism. Deficiency signs include muscular dystrophy, as reviewed by Hamre and El-Sayed [111,112], although some studies have reported no gross pathologies [113,114]. In Atlantic salmon, vitamin E deficiency with or without selenium results in increased carcass fat and water, and the histology resembles that of ‘jellied’ or 'mushy' tissue [115]. However, other deficiency symptoms such as anemia or alterations in organ size have not been described in conjunction with the syndrome. Other dietary imbalances may be relevant to look into, such as essential fatty acid deficiency, which can cause symptoms similar to vitamin E and selenium deficiency [116]. Hyperspectral and chemical analysis point to a decreased fat and protein content of ‘mushy’ Greenland halibut [10,11]. Previous studies have found the level of both fatty acids and essential amino acids to be lower in ‘gelatinous’ Greenland halibut compared to unaffected fish, and the composition to differ considerably [12,13]. Similar findings exist for American plaice [22]; a dietary imbalance was suspected due to unusually low concentrations of urea, β-alanine, taurine and peptides containing β-alanine in ‘jellied’ meat of plaice. It was argued that while protein emaciation does occur during gonad maturation, the subsequent increase in muscle water content is not as dramatic as seen with the ‘jellied condition’.
The amino acid composition of fish varies between species, and fluctuations are seen during and prior to spawning [[117], [118], [119]]. This is especially true for white muscle histidine levels, which are utilized rapidly both during spawning migration and food deprivation [[120], [121], [122]]. Widera (1978) [12] found ‘jellied’ muscle samples of both spawning and non-spawning Greenland halibut to contain higher levels of histidine and arginine. Could this indicate that the syndrome reflects diet composition, and that a diet high in certain amino acids is a predisposing factor?
4.3.2. Effects of depth
The effect of depth and reproductive cycle are difficult to study individually. In American plaice, Dover sole and sablefish, the ‘jellied condition’ is most prevalent in deep waters. These species all have ontogenic shifts in distribution pattern, with adult fish inhabiting the ocean floor at depths of 200–2000 m. The same is true for halibut [[123], [124], [125], [126], [127]].
The physiology and biochemistry of fish living in deeper waters below the epipelagic zone differs significantly from that of shallower-living ones [128,129]. Several authors have found the muscle water content of meso- and bathypelagic fish to increase and protein content to decrease, with increasing depth. This trend is prominent in species lacking a swim bladder [90,130,131]. The high water content is thought to reflect low locomotory needs and declining prey density, and allows for attaining and maintaining neutral buoyancy and larger body size at a lower metabolic cost [132,133]. Some authors believe depth is the driving factor behind the ‘jellied condition’. In Dover sole, a study concluded water content (synonymous with ‘jellied flesh’) to be a function of depth, age and length, but not necessarily reproductive cycle [23]. Age, growth and maturity surveys, conducted by the California Department of Fish and Game (CDFG), similarly report ‘jellied’ Dover sole to be more prevalent in deep-water samples (164–273 m) [47,134].
The fact that not all fish from a given depth are ‘jellied’ would indicate that specific circumstances or a certain exposure time is required to initiate the biochemical changes. By that assumption, older fish would appear more often in the ‘jellied’ category due to the prolonged exposure. Yet, the syndrome also occurs in shallower waters and immature fish. Although physiological adaptation relating to high hydrostatic pressure and low oxygen levels at great depths seems less causative, factors such as temperature, food availability and quality, and activity level could play a role [18,132]. Another possibility is that the correlation with depth simply reflects foraging and movement patterns of affected fish, and not a direct causal relationship.
4.3.3. Demanding reproductive strategy
Several authors attribute the increased water content of ‘jellied’ or 'mushy' muscle tissue to spawning. Protein and lipid depletion of the skeletal muscle tissue during gonadal maturation has been documented in both American plaice, Dover sole and Greenland halibut [23,[135], [136], [137]]. In plaice, studies demonstrate increased white muscle moisture content pre- and post-spawning, with a concurrent decrease in condition factor [138,139], reflecting the energy demand for vitellogenesis. Histological alterations correspond to the ‘jellied condition’, with a distribution pattern indicating that some myofibers in severely hydrated areas maintain their integrity to preserve muscle tone for foraging or predator evasion. A similar tendency has been observed in starved winter flounder (Pleuronectes americanus) [140]. In conjunction with cessation of feeding and elevated cathepsin, spawning can lead to a significant decline in energy reserves and condition [141,142].
In American plaice, the ‘jellied condition’ was assumed due to spawning, but lower temperatures were suspected of exacerbating the condition [21]. The authors argued that warmer temperatures allow for tissue repair and protein accumulation after spawning, which usually occurs in April–May for plaice in the Great Banks. Low temperatures impair post-spawning recovery and as a result, protein declines and water replaces it. This notion is supported by the work of Roff (1982, 1983) [27,143]. Considering the work done on adaptive biology of deep-water fish, lowered metabolism at cold depths with declining prey density could likely delay post-spawning restitution [132,144,145].
4.3.4. Genomic variability
It has been speculated if the ‘jellied’ or ‘mushy’ syndrome is caused by genetic variability [16,18]. Living conditions influence the genetics of fish, and climate change forces fish populations to adapt to new environmental conditions. Studies on genomic differentiation in marine species have revealed that genetic differences are associated with behavioral traits, spawning time and environmental variation, known as functional adaptive divergence [146]. Temperature, oxygen level and pressure are all variables affecting fish physiology, and the histology of ‘jellied’ or 'mushy' white muscle tissue resembles that occurring naturally in some deep-water species [131]. Exposure to certain conditions may have resulted in a ‘jellied' or 'mushy' phenotype in some species; in the Northwest Atlantic, adaptive divergence due to environmental conditions explained up to 51% of the phenotypic differentiation observed between two stocks of Greenland halibut [146]. However, a study on muscle protein polymorphism of sablefish found only weak or limited evidence of population structure [147]. Biological adaptation may also invoke an increase in the mutagenic activity in germ cells [148], which can induce dystrophies in humans. Cellular disorders have not been ruled out as a cause of the syndrome, as was noted in two studies [19,20]. Perhaps the answer lies in the genes, but whether ‘jellied’ or ‘mushy’ fish are genetically distinct has yet to be determined.
4.3.5. Alternative explanations
A viral etiology has not been excluded. Betanodavirus infections can induce encephalopathy in several fish species, and infection causes anorexia and abnormal swimming behavior. This could complicate foraging and lead to malnutrition, resulting in ‘jellied’ or ‘mushy’ musculature [[149], [150], [151]]. Parasitism can alter the body composition of fish, and imposes an energetic cost on the host. While some studies have disproved parasitic infection in the fillets of fish affected by the syndrome, other infectious causes have not been completely ruled out. Specifically for Greenland halibut, we found no publications investigating an infectious etiology, only an online newspaper article referring to unpublished data [152]. As mentioned in the introduction, Kudoa spp. can produce similar myodegenerative effects on fish flesh due to parasitic proteolytic activity, and other myxozoans associated with skeletal muscle distortion count Unicapsula spp [55,153]. Myopathy could also be a secondary effect of parasites that do not elicit a direct degenerative effect on myofibers. Myxobolus groenlandicus induces cartilage distortion in Greenland halibut [154], and infection with the anisakid nematode larvae Contracaecum osculatum can induce chemical alterations in the white musculature similar to those seen in ‘jellied’ or ‘mushy’ specimens. In Baltic cod (Gadus morhua), Contracaecum osculatum infection density correlates positively with fillet and whole fish water. Correspondingly, fillet yield decreases with increased infection levels [155,156]. Infection with performance-reducing parasites such as Ichthyophonus spp. or acanthocephalans could also lead to reduced foraging ability and nutrient utilization [157,158].
Toxin exposure can impact biological processes in animals. Persistent organic pollutants (POPs) and mercury are two examples of contaminants affecting marine wildlife. Mercury can affect biochemical processes and damage cells and tissues in fish [159], while POPs can induce developmental and hepatic toxicity, and possibly impair lipid dynamics [[160], [161], [162]]. Perhaps toxin exposure could impair neuromuscular development and immunity, lower energy reserves, or induce altered foraging behavior, affecting the biochemical composition, muscle structure and overall health of the fish.
4.3.6. Single or several syndromes?
Despite similarities, it is unclear whether the ‘jellied’ and ‘mushy’ syndromes seen in five commercial fish species are single or separate phenomena. While it seems unlikely that syndromes with such similar phenotypic representation would have five unrelated etiologies, it is possible that two different circumstances produce similar effects. This is seen with starvation and spawning, which both exhaust the nutritional reserves of fish. It may also be that determinate factors for developing the condition depend on species and geographic areas.
The case of depth of capture versus reproductive strategy resembles that of “the chicken and the egg”. But the associations may reflect a complex etiology involving both environmental factors and the energetic burden of reproduction – possibly on a nutritionally deficient fish [132,138,163]. Spawning is a costly affair, and inadequate energy reserves are associated with reduced reproductive potential in several fish species [[164], [165], [166]]. If the maturing fish is compromised nutritionally, it will require higher protein mobilization from the somatic energy reserves to spawn [139]. The reported overweight of maturing and spent plaice, sole and sablefish in the ‘jellied’ category indicate that affected fish are in a condition where they can meet the high energetic requirements for spawning, but cannot regenerate either due to unfavorable temperatures or nutritional exhaustion. Yet, this does not explain why the syndrome was reported in young, immature Pacific halibut. A nutritional deficiency may be causative, but it would be striking to see identical dietary imbalances in several fish species of different maturity stages, and in various geographical locations. While a decline in stomach content of American plaice has been noted since the 1990s, it is unknown whether this is associated with the ‘jellied condition’ [167], and we found no systematic recordings of stomach content for other species covered in this review. The conflicting results on maturity status of affected fish would indicate two different, albeit similar syndromes, one driven primarily by a nutritional deficiency and the other by spawning. However, trends in study results need to be verified, and alternate explanations ruled out.
4.3.7. Pathology or ecology?
Is the ‘jellied’ or ‘mushy’ state a pathological or a physiological one, and is it permanent or reversible? Previous work by Love has shown that the increase in white muscle water content due to starvation is reversible upon refeeding [105]. In contrast, the high water content seen in many deep water species is permanent.
Recapture studies conducted by the California Department of Fish and Game (CDFG) revealed that the flesh condition of tagged ‘jellied’ Dover sole was unaltered at recovery, suggesting chronicity. However, the recovery rate of total tagged ‘jellied’ Dover sole was overall low (10%), and the evaluation of flesh condition (‘jellied’ vs. ‘non-jellied’) was based on subjective assessment by individual fishermen [19,47]. Systematic recapture tagging studies would provide insight into the progression or reversibility of the syndrome throughout the life of the affected fish. It would also shed light on the impact on fish condition and longevity, and thus the effect on fish health; although the effect of stress related to capture and tagging on feeding behavior and movement of the fish should be considered in the interpretation of data.
4.3.8. Past findings and future perspectives: limitations and recommendations
All species included in this review are wild-caught, and all studies observational. While observational studies allow for studying correlations, they do not imply causation. The sampling in the studies included in this review is biased by fish selection, inconsistency in sampling method, location and depth, as well as seasonal and seafaring conditions. The first and the latter are hard to change. Experimental studies would be beneficial, but creating a randomized experimental environment that accounts for all possible factors involved in the ‘jellied’ or ‘mushy’ etiology will be costly and difficult to design and reproduce. However, with the developing Dover sole and halibut mariculture, controlled studies could be possible to initiate in the near future. The effect of single parameters such as food deprivation and infection might be elucidated, as has been attempted with the effect of temperature and exercise in the development of ‘chalkiness’ in Pacific halibut [168]. ‘Chalky’ halibut is a condition that occurs when a fish dies in a state of fatigue. Subsequent build-up of lactic acid and lowered pH denatures proteins and gives the flesh a tough, chalky appearance. Contrary to the ‘jellied’ or ‘mushy’ condition, moisture is low while protein increases [76,169,170].
Most studies included in this review are over 30 years old. While they provide historical context, they may not represent current trends. Recent publications is testimony to the continued frustration fishing industries face due to ‘jellied’ or ‘mushy’ fish in catches, and the importance of continued research. Earlier studies have focused on biochemical alterations and associations with size and maturity and depth. Further studies are needed to confirm these trends and investigate associations of occurrence in Greenland and Pacific halibut. Larger correlational studies including essential nutrients and stomach content analysis, protease activity, infection status, and genotyping would provide further insight on causal links. However, genetic investigations are currently limited by incomplete and expensive commercial genomic arrays of wild fish populations. Species-specific reference values for nutrients such as Vitamin E and selenium are also lacking, and while a viral etiology is worth looking into, some viruses require specific and expensive methods for detection.
From a commercial perspective, one might wonder if it is worth investing in studies on a syndrome that may be unavoidable. We would argue that it is vital to minimize, detect and utilize ‘jellied’ and ‘mushy’ products, and that identifying factors associated with the syndrome can be highly useful in a commercial context. Detection is currently complicated by varying degrees of severity of the syndrome. Valid and effective technologies for identifying affected fish would prevent costly processing and distribution of inferior products, which not only causes product rejection and distress for consumers but also expenses related to compensation claims. To develop these technologies, more data on the structural and biochemical properties of affected fish is needed. Hyperspectral imaging appears to be a promising detection tool, but trials are costly to conduct. Until a validated product is commercially available, implementation of cheaper yet precise monitoring methods should be sought. Routine surveys and dedicated reporting on the occurrence and geographic distribution by the fisheries would provide a better understanding of the extent and fluctuations in occurrence, and risks to especially export-driven fisheries. Knowledge on the prevalence in specific fishing zones could enable more efficient quota usage by tailoring fishery strategies. Further characterization of affected products might also allow for exploration of alternate utilization if avoidance is impossible, and increase both the yield and sustainability profile of the fisheries.
5. Conclusion
The ‘jellied condition’ and ‘mushy halibut syndrome’ are both characterized by a high water content of the white skeletal muscle, in conjunction with lowered protein content. Anecdotal reports of jelly-like fish frequently surface, but the phenomenon is under-investigated and the prevalence in several commercially important fish species is unknown. Results from existing studies are biased by inconsistent sampling strategies. Varying methods, geographical locations and in some cases small sample sizes makes it difficult to compare and validate trends (see Table 2). Some studies point to a positive association with depth of capture and gonadal maturity stage, yet the jelly-like syndrome is seen in both young, immature fish in shallow waters and older, mature fish from great depths. It is unclear if the syndrome observed in different species has the same etiology, and whether it is pathological or physiological. The existence of two jelly-like syndromes induced by either nutritional depletion or circumstances related to spawning seems plausible. Species-specific trends such as biochemical composition, infection and toxin levels, and genetic variability of affected fish warrants further studying. Controlled experimental studies might elucidate the contribution of single factors to the development of the syndrome, but are difficult to construct and reproduce. New technologies and adapted fishing strategies are necessary to minimize harmful effects of ‘jellied’ or ‘mushy’ fish in yields or along the supply chain. Recent advances show hyperspectral imaging to be a promising option for early detection, but more data on affected fish species are needed. Until fully implemented, efficient and easily applicable alternative methods could reduce wastage and economic losses. Prevalence studies would increase the ability to predict high occurrences of the syndrome in specific fishing zones, and further characterization of affected fish could permit exploration of alternative processing options. Updated knowledge would help improve quality consistency, strengthen consumer confidence, and improve the overall sustainability of wild-capture fisheries.
Funding
This review is part of a PhD project supported by a grant from the Greenland Institute of Natural Resources (Pinngortitaleriffik). Grant funds are provided by the Danish State's Funds for Arctic Research, Bank of Greenland and Royal Greenland.
The funding sources had no involvement in the writing of this review or in the decision to submit the article for publication.
Data availability statement
The authors confirm that the data supporting the findings of this review are available within the cited references and/or their supplementary materials.
CRediT authorship contribution statement
Natacha Leininger Severin: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Kurt Buchmann: Writing – review & editing, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
The authors wish to thank Royal Greenland and the Fish Pathology Section at Alaska Department of Fish and Game for sharing their observations on ‘jellied’ and ‘mushy’ Greenland and Pacific halibut.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27130.
Unpublished data. Personal communication with Principal Fish Pathologist Theodore Meyers from the Alaska Department of Fish and Game, Pathology Section, Division of Commercial Fisheries, 333 Raspberry Road, Anchorage, AK 99518-1565.
Personal communication Royal Greenland A/S, Qasapi 4, Postal code 1073, 3900 Nuuk, Greenland.
Contributor Information
Natacha Leininger Severin, Email: nls@sund.ku.dk.
Kurt Buchmann, Email: kub@sund.ku.dk.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Dunajski E. Texture of fish muscle. J. Texture Stud. 1980;10(4):301–318. [Google Scholar]
- 2.Daskalova A. Farmed fish welfare: stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int. Aquat. Res. 2019;11(2):113–124. [Google Scholar]
- 3.Jørpeland G., et al. Effects of filleting method, stress, storage and season on the quality of farmed Atlantic cod (Gadus morhua L.) Aquacult. Res. 2015;46:1597–1607. [Google Scholar]
- 4.Rotabakk B.T., et al. Quality assessment of Atlantic cod (Gadus morhua) caught by longlining and trawling at the same time and location. Fish. Res. 2011;112(1):44–51. [Google Scholar]
- 5.Moran J.D.W., Whitaker D.J., Kent M.L. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture. 1999;172(1):163–196. [Google Scholar]
- 6.Lom J., Dyková I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006;53(1):1–36. [PubMed] [Google Scholar]
- 7.Patashnik M., et al. Pacific whiting, Merluccius productus: I. Abnormal muscle texture caused by myxosporidian-induced proteolysis. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 1982:1–12. Seattle, Washington, USA. [Google Scholar]
- 8.Funk V.A., et al. Identification, characterization and deduced amino acid sequence of the dominant protease from Kudoa paniformis and K. thyrsites: a unique cytoplasmic cysteine protease. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;149(3):477–489. doi: 10.1016/j.cbpb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Tsuyuki H., et al. The relationship between acid and neutral protease activities and the incidence of soft cooked texture in the muscle tissue of Pacific hake Merluccius productus infected with Kudoa paniformis and/or K. thyrsites, and held for varying times under different pre-freeze chilled storage conditions. Can. Tech. Rep. Fish. Aquat. Sci. 1982:1–39. Ottawa, Canada. [Google Scholar]
- 10.Ortega S., et al. Early identification of mushy Halibut syndrome with hyperspectral image analysis. LWT. 2023;176 [Google Scholar]
- 11.Ortega S., et al. Characterization of vasskveite (water halibut) syndrome for automated detection. Appl. Food Res. 2023;3(1) [Google Scholar]
- 12.Widera L. Composition of fatty acids in lipids of a gelatinous meat of the black halibut (Reinhardtius hippoglossoides) Medycyna Weterynaryna. 1978;33(6):366–368. [Google Scholar]
- 13.Widera L., Madler J. Amino acid composition of the gelatinous flesh of the Greenland halibut (Rheinhardtius hippoglossoides) Medycyna Weterynaryna. 1977;33(12):753–755. [Google Scholar]
- 14.Meyers T., et al. Diseases of Wild and Cultured Fishes in Alaska. Alaska Department of Fish and Game, Fish Pathology Laboratories; Juneau, Alaska: 2019. Non-infectious diseases: mushy halibut syndrome; pp. 110–111. [Google Scholar]
- 15.Ferriss B.E., Zador S. In: Ecosystem Status Report 2022: Gulf of Alaska, Stock Assessment and Fishery Evaluation Report. North Pacific Fishery Management Council, editor. Anchorage; Alaska: 2022. Ecosystem indicators: Disease & Toxins Indicators - "Mushy" Halibut Syndrome Occurrence; p. 155. [Google Scholar]
- 16.Karinen J.F., Barnett H.J., Masuda M. Soft flesh in sablefish, Anoplopoma fimbria, of southeastern Alaska: relationships with depth, season, and biochemistry. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 2010:26–35. Seattle, Washington, USA. [Google Scholar]
- 17.Norris J.G. In: Wilkins M.E., Saunders M.W., editors. Seattle; Washington: 13-15 April 1993. Adaptive Radiation and sablefish, Anoplopoma fimbria: the soft-textured condition in Teleost fishes; pp. 99–112. (National Oceanic and Atmospheric Administration (NOAA) Technical Report NMFS 130, Biology and Management of Sablefish, Anoplopoma fimbria: Papers from the International Symposium on the Biology and Management of Sablefish). 1997 Seattle, Washington. [Google Scholar]
- 18.Norris J.G., Rowley J., Mathews S.B. Analysis of Four Factors Affecting the Sablefish Soft Fish Problem. Fisheries Research Institute, School of Fisheries, University of Washington; Seattle, Washington, USA: 1987. [Google Scholar]
- 19.Fisher R.A., Fritzsche R.A., Hendrickson G.L. Proceedings of the Fifth Congress of European Ichthyologists, Stockholm 1985. Department of Fisheries, Humboldt State University; Arcata, California: 1987. Histology and ultrastructure of the ‘jellied’ condition in Dover Sole, Microstomus pacificus; pp. 345–350. [Google Scholar]
- 20.Puckett H.M., Hendrickson G.L. IAAAM Conference Proceedings. Department of Fisheries, Humboldt State University; Arcata, California: 1986. Ecology and possible causes of the jellied condition in Dover sole (Microstomus pacificus) [Google Scholar]
- 21.Templeman W., Andrews G.L. Jellied condition in the American plaice Hippoglossoides platessoides (Fabricius) J. Fish. Res. Board Can. 1956;13(2):147–182. [Google Scholar]
- 22.Haard N.F. Protein and non-protein Nitrogen constituents in jellied American plaice Hippoglossoides platessoides. Can. Inst. Food Sci. Technol. J. 1987;20(2):98–101. [Google Scholar]
- 23.Hunter J.R., et al. Bathymetric patterns in size, age, sexual maturity, water content and caloric density of Dover sole, Microstomus pacificus. CalCOFI Reports. 1990;31(9026):132–144. [Google Scholar]
- 24.Toyohara H., et al. Elevated activity of cathepsin L-like protease in the jellied meat of Japanese flounder. Nippon Suisan Gakkaishi. 1993;59(11):1909–1914. [Google Scholar]
- 25.Rideout S.G., Snow G.W. Grand Bank Inspection District. Fisheries and Oceans Canada; Newfoundland, Canada: 1974. A study into the possible utilization of “jellied” flounder of the species Hippoglossoides platessoides by commuting the fillets. [Google Scholar]
- 26.Power H.E. In: Technological Research Laboratory Circular. Fisheries Research Board of Canada, editor. Halifax; Nova Scotia, Canada: 1964. A Report to the Fishing Industry on the Problem of Excess Moisture in Fish. New Series No. 16. [Google Scholar]
- 27.Roff D.A. Reproductive strategies in flatfish: a first Synthesis. Can. J. Fish. Aquat. Sci. 1982;39(12):1686–1698. [Google Scholar]
- 28.St-Hilaire S., et al. A comparative study of muscle texture and intensity of Kudoa thyrsites infection in farm-reared Atlantic salmon Salmo salar on the Pacific coast of Canada. Dis. Aquat. Org. 1997;31:221–225. [Google Scholar]
- 29.Whitaker D.J., Kent M.L. Myxosporean Kudoa thyrsites: a cause of soft flesh in farm-reared Atlantic salmon. J. Aquat. Anim. Health. 1991;3(4):291–294. [Google Scholar]
- 30.Marshall W.L., et al. Long-term epidemiological survey of Kudoa thyrsites (Myxozoa) in Atlantic salmon (Salmo salar L.) from commercial aquaculture farms. J. Fish. Dis. 2016;39(8):929–946. doi: 10.1111/jfd.12429. [DOI] [PubMed] [Google Scholar]
- 31.Barja J.L., Toranzo A.E. Myoliquefaction caused by the myxosporean Kudoa thyrsites in reared Atlantic salmon in Spain. Bull. Eur. Assoc. Fish Pathol. 1996;13(3):86–88. [Google Scholar]
- 32.Prudhomme M., Pantaléon J. Sur un cas de myxosporidiose du saumon. Bull. Acad. Vet. Fr. 1959;112(2):137–140. [Google Scholar]
- 33.Martinez I., et al. Protein expression and enzymatic activities in normal and soft textured Atlantic salmon (Salmo salar) muscle. Food Chem. 2011;126(1):140–148. [Google Scholar]
- 34.Torgersen J.S., et al. Soft texture of Atlantic salmon fillets is associated with glycogen accumulation. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabata Z., Whitaker D.J., Bagshaw J.W. Kudoa thyrsitis (Gilchrist) (Myxosporea: Multivalvulida) in coho salmon, Oncorhynchus kisutch (Walbaum) Can. J. Zool. 1986;64(4):1038–1040. [Google Scholar]
- 36.Langdon J.S. Myoliquefaction post-mortem (‘milky flesh’) due to Kudoa thyrsites (Gilchrist) (Myxosporea: Multivalvulida) in mahi mahi, Coryphaena hippurus L. J. Fish. Dis. 1991;14(1):45–54. [Google Scholar]
- 37.Yokoyama H., et al. Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp. from Chinese sea bass : causative myxozoans of post-mortem myoliquefaction. Fish Pathol. 2004;39:79–85. [Google Scholar]
- 38.Kasai A., et al. Morphological and molecular genetic characterization of two Kudoa spp., K. musculoliquefaciens, and K. pleurogrammi n. sp. (Myxosporea: Multivalvulida), causing myoliquefaction of commercial marine fish. Parasitol. Res. 2016;115(5):1883–1892. doi: 10.1007/s00436-016-4928-2. [DOI] [PubMed] [Google Scholar]
- 39.Greene D.H., Babbitt J.K. Control of muscle softening and protease-parasite interactions in Arrowtooth flounder Atheresthes stomias. J. Food Sci. 1990;55(2):579–580. [Google Scholar]
- 40.Piasecki W. Parasite fauna of Atheresthes stomias (Jordan et Gilbert, 1880) (Pleuronectiformes) from the Northeastern Pacific Ocean. Acta Ichthyol. Piscatoria. 1998;28(1):49–57. [Google Scholar]
- 41.Kristmundsson Á., Freeman M.A. Negative effects of Kudoa islandica n. sp. (Myxosporea: Kudoidae) on aquaculture and wild fisheries in Iceland. Int. J. Parasitol. Parasites Wildl. 2014;3(2):135–146. doi: 10.1016/j.ijppaw.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giulietti L., et al. Distribution of Kudoa thyrsites (Cnidaria, Myxozoa) myoliquefactive stages in Northeast Atlantic mackerel (Scomber scombrus) inferred from qPCR and histology. Parasitol. Res. 2022;121(8):2325–2336. doi: 10.1007/s00436-022-07575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levsen A., Jørgensen A., Mo T.A. Occurrence of postmortem myoliquefactive kudoosis in Atlantic mackerel, Scomber scombrus L., from the North Sea. J. Fish. Dis. 2008;31(8):601–611. doi: 10.1111/j.1365-2761.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- 44.Giulietti L., et al. Long-term investigation of the ‘soft flesh’ condition in Northeast Atlantic mackerel induced by the myxosporean parasite Kudoa thyrsites (Cnidaria, Myxozoa): Temporal trends and new molecular epidemiological observations. Fish. Res. 2022;248 [Google Scholar]
- 45.Højgaard D.P., í Homrum E., Salter I. Prevalence of Kudoa thyrsites (Myxozoa, multivalvulida) in atlantic mackerel, Scomber scombrus L., in the vicinity of the Faroe Islands. Front. Mar. Sci. 2022;9 [Google Scholar]
- 46.Clark E. Jellied condition in Paralichthys squamilentus from the Gulf of Mexico. Q. J. Fla. Acad. Sci. 1958;21(2):187–189. [Google Scholar]
- 47.Quirollo L.F., Kalvass P. 1987. Results of Dover Sole Tagging in Waters off Northern California, 1969-1971. California, USA. [Google Scholar]
- 48.Macdonald G.A., Hall B.I., Vlieg P. Seasonal changes in hoki (Macruronus novaezelandaie) J. Aquat. Food Prod. Technol. 2002;11(2):35–51. [Google Scholar]
- 49.Kasai A., et al. Incidence of three Kudoa spp., K. neothunni, K. hexapunctata, and K. thunni (Myxosporea: multivalvulida), in Thunnus tunas distributed in the western Pacific Ocean. Parasitol. Res. 2017;116(4):1137–1150. doi: 10.1007/s00436-016-5369-7. [DOI] [PubMed] [Google Scholar]
- 50.Huang M.C., et al. Evaluation of biochemical properties of burnt and normal meat in pacific bluefin tuna (Thunnus orientalis) Int. J. Nutr. Food Sci. 2017;6:203–210. [Google Scholar]
- 51.Samaranayaka A.G.P., Ho T.C.W., Li-Chan E.C.Y. Correlation of Kudoa spore counts with proteolytic activity and texture of fish mince from pacific hake (Merluccius productus) J. Aquat. Food Prod. Technol. 2007;15(4):75–93. [Google Scholar]
- 52.Kudo G. Factors affecting cooked texture quality of Pacific whiting, Merluccius productus, fillets with particular emphasis on the effect of infection by the myxosporeans Kudoa paniformis and K. thyrsites. Fish. Bull. (Wash. D. C.) 1987;85(4):745–755. [Google Scholar]
- 53.Sydeman W.J., et al. Puffins reveal contrasting relationships between forage fish and ocean climate in the North Pacific. Fish. Oceanogr. 2017;26(4):379–395. [Google Scholar]
- 54.Thompson W.F. Report of the B.C. Commissioner of Fisheries for 1915; British Columbia, Canada: 1916. A note on a sporozoan parasite of the halibut; pp. 127–129. [Google Scholar]
- 55.Davis H.S. Appendix VIII to the Report of United States Commission on Fisheries 1923. Department of Commerce, Bureau of Fisheries; Washington D.C., USA: 1924. A new myxosporidian parasite, the cause of “wormy” halibut; pp. 1–5. [Google Scholar]
- 56.Li Y.C., et al. Identification of four new Kudoa spp. (myxozoa: myxosporea: multivalvulida) in commercial fishes collected from south China sea, atlantic ocean, and bering sea by integrated taxonomic approach. Parasitol. Res. 2020;119(7):2113–2128. doi: 10.1007/s00436-020-06707-2. [DOI] [PubMed] [Google Scholar]
- 57.Yokoyama H., Itoh N. Two multivalvulid myxozoans causing postmortem myoliquefaction: Kudoa megacapsula n. sp. from red barracuda (Sphyraena pinguis) and Kudoa thyrsites from splendid alfonso (Beryx splendens) J. Parasitol. 2005;91(5):1132–1137. doi: 10.1645/GE-548R.1. [DOI] [PubMed] [Google Scholar]
- 58.Stagg N.J., et al. Autolytic degradation of skipjack tuna during heating as affected by initial quality and processing conditions. J. Food Sci. 2012;77(2):C149–C155. doi: 10.1111/j.1750-3841.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 59.M’Gonigle R.H., Leim A.H. Progress Reports of Atlantic Biological Station, Atlantic Fisheries Experimental Station; Ottawa, Canada: 1937. Jellied Swordfish; pp. 3–5. [Google Scholar]
- 60.Matsumoto K. On the Two New Myxosporidia, Chloromyxum musculoliquefaciens sp. nov. and Neochloromyxum cruciformum gen. et sp. nov., From the Jellied Muscle of Swordfish, Xiphias gladius Linne, and Common Japanese Sea-Bass, Lateolabrax japonicus. Nippon Suisan Gakkaishi. 1954;20(6):469–478. [Google Scholar]
- 61.Kasai A., et al. Morphological and molecular genetic characterization of two Kudoa spp., K. musculoliquefaciens, and K. pleurogrammi n. sp. (Myxosporea: multivalvulida), causing myoliquefaction of commercial marine fish. Parasitol. Res. 2016;115(5):1883–1892. doi: 10.1007/s00436-016-4928-2. [DOI] [PubMed] [Google Scholar]
- 62.Gaglio G., et al. Muscle change due to Kudoa sp. (myxosporea: multivalvulida) in a specimen of swordfish caught in the mediterranean sea. Large Anim. Rev. 2010;16(6):291–293. [Google Scholar]
- 63.Tsuchiya Y., Tatsukawa Y. A chemical study on jellied meat of swordfish. Tohoku J. Agric. Res. 1954;4:251–256. [Google Scholar]
- 64.Arai Y., Matsumoto K. On a New Sporozoa, Hexacapsula neothunni gen. et sp. nou., from the Muscle of Yellowfin Tuna, Neothunnus macropterus. Bull. Jpn. Soc. Sci. Fish. 1953;18(7):293–299. [Google Scholar]
- 65.Davie P.S., Sparksman R.I. Burnt tuna: an ultrastructural study of postmortem changes in muscle of yellowfin tuna (Thunnus albacares) caught on rod and reel and southern bluefin tuna (Thunnus maccoyii) caught on handline or longline. J. Food Sci. 1986;51(5):1122–1128. [Google Scholar]
- 66.Cramer J.L., et al. Burnt tuna: conditions leading to rapid deterioration in the quality of raw tuna. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 1981;43(6):12–16. [Google Scholar]
- 67.Schmitt W.L., et al. Survey of the fishing grounds on the Coasts of Washington and Oregon in 1914Appendix VIII to the Report of the U.S. Comissioner of Fisheries for 1914, Department of Commerce. Bureau of Fisheries; Washington D.C., USA: 1914. [Google Scholar]
- 68.Henning S.S., Hoffman L.C., Manley M. A review of Kudoa-induced myoliquefaction of marine fish species in South Africa and other countries. South Afr. J. Sci. 2013;109(11–12):1–5. [Google Scholar]
- 69.Moran J.D.W., Whitaker D.J., Kent M.L. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture. 1999;172(1–2):163–196. [Google Scholar]
- 70.Kent M.L., et al. Review of Myxosporea of importance in salmonid fisheries and aquaculture in British Columbia. Folia Parasitol. 1994;41(1):27–37. [PubMed] [Google Scholar]
- 71.Michie I. In: Farmed Fish Quality. Kestin S.C., Warriss P.D., editors. Blackwell Science Ltd, Fishing News Books; Oxford, England: 2001. Causes of downgrading in the salmon farming industry; pp. 129–136. [Google Scholar]
- 72.Watson C., Bourke R.E., Brill R.W. A comprehensive theory on the etiology of burnt tuna. Fish. Bull. 1988;86(2):367–372. [Google Scholar]
- 73.Kabata Z., Whitaker D.J. Parasites as a limiting factor in exploitation of pacific whiting, Merluccius productus. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 1985:55–59. Seattle, Washington, USA. [Google Scholar]
- 74.Iverson S., Frost K., Lang S. Fat content and fatty acid composition of forage fish and invertebrates in Prince William Sound, Alaska: factors contributing to among and within species variability. Mar. Ecol. Prog. Ser. 2002;241:161–181. [Google Scholar]
- 75.Nogueira N., Cordeiro N., Aveiro M. Chemical composition, fatty acids profile and cholesterol content of commercialized marine fishes captured in northeastern atlantic. J. Fish. Sci. 2013;7:271–286. [Google Scholar]
- 76.Patashnik M., Groninger J., H. S Observations on the milky condition in some pacific coast fishes. J. Fish. Res. Board Can. 1964;21(2):335–346. [Google Scholar]
- 77.Konagaya S., Bito M., Amano K. Studies on jellied meat of tuna - I. Fractionation of proteins in the jellied meat of yellowfin tuna. Bull. Jpn. Soc. Sci. Fish. 1970;36(6):597–605. [Google Scholar]
- 78.Pitt T.K. ICNAF Res. Bull. Fisheries Research Board of Canada; 1967. Diurnal variation in the catches of American plaice, Hippoglossoides platessoides fabr., from the Grand bank; pp. 53–58. [Google Scholar]
- 79.Lian Y., et al. Diurnal, seasonal and inter-annual variability of fish density and distribution in the Three Gorges Reservoir (China) assessed with hydroacoustics. Limnologica. 2017;63:97–106. [Google Scholar]
- 80.Goetz F.W., et al. Status of sablefish, Anoplopoma fimbria, aquaculture. J. World Aquacult. Soc. 2021;52(3):607–646. [Google Scholar]
- 81.Guzmán J.M., et al. Reproductive life history of sablefish (Anoplopoma fimbria) from the U.S. Washington coast. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter J.R. Fecundity, spawning, and maturity of female Dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish. Bull. (Wash. D. C.) 1992;90(1):101–128. [Google Scholar]
- 83.Hagerman F.B. State of California Department of Fish and Game Bureau of Marine Fisheries Fish Bulletin No. 85; California: California, USA: 1952. p. 48. (The Biology of the Dover Sole, Microstomus pacificus (Lockington)). [Google Scholar]
- 84.Hagen Ø., et al. Biochemical and structural factors contributing to seasonal variation in the texture of farmed atlantic halibut (Hippoglossus hippoglossus L.) flesh. J. Agric. Food Chem. 2007;55(14):5803–5808. doi: 10.1021/jf063614h. [DOI] [PubMed] [Google Scholar]
- 85.Love R.M. Variability in atlantic cod (Gadus morhua) from the northeast atlantic: a review of seasonal and environmental influences on various attributes of the flesh. J. Fish. Res. Board Can. 1975;32(12):2333–2342. [Google Scholar]
- 86.Andersen U.B., Thomassen M.S., Rørå A.M.B. Texture properties of farmed rainbow trout (Oncorhynchus mykiss): effects of diet, muscle fat content and time of storage on ice. J. Sci. Food Agric. 1997;74(3):347–353. [Google Scholar]
- 87.Bailey T.G., Robison B.H. Food availability as a selective factor on the chemical compositions of midwater fishes in the eastern North Pacific. Mar. Biol. 1986;91(1) [Google Scholar]
- 88.Bugeon J., Lefevre F., Fauconneau B. Fillet texture and muscle structure in brown trout (Salmo trutta) subjected to long-term exercise. Aquacult. Res. 2003;34(14):1287–1295. [Google Scholar]
- 89.Ang J.F., Haard N.F. Chemical composition and postmorten changes in soft textured muscle from intensely feeding Atlantic Cod (Gadus morhua, L) J. Food Biochem. 1985;9(1):49–64. [Google Scholar]
- 90.Childress J.J., Nygaard M.H. The chemical composition of midwater fishes as a function of depth of occurrence off southern California. Deep Sea Res. Oceanogr. Abstr. 1973;20(12):1093–1109. [Google Scholar]
- 91.Siebenaller J.F., Yancey P.H. Protein composition of white skeletal muscle from mesopelagic fishes having different water and protein contents. Mar. Biol. 1984;78(2):129–137. [Google Scholar]
- 92.Drazen J.C. Depth related trends in proximate composition of demersal fishes in the eastern North Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2007;54(2):203–219. [Google Scholar]
- 93.Johnston I.A., et al. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture. 2006;256(1):323–336. [Google Scholar]
- 94.Johnston I.A. Quantitative analysis of muscle breakdown during starvation in the marine flatfish Pleuronectes platessa. Cell Tissue Res. 1981;214(2):369–386. doi: 10.1007/BF00249218. [DOI] [PubMed] [Google Scholar]
- 95.Schulman G.E., Love R.M. In: Advances in Marine Biology: the Biochemical Ecology of Marine Fishes. Southward A.J., Tyler P.A., Young C.M., editors. Academic Press; London, England: 1999. Indicators of fish condition; pp. 205–213. [Google Scholar]
- 96.Bar N. Physiological and hormonal changes during prolonged starvation in fish. Can. J. Fish. Aquat. Sci. 2014;71(10):1447–1458. [Google Scholar]
- 97.Foss A., et al. Compensatory growth in Atlantic halibut: effect of starvation and subsequent feeding on growth, maturation, feed utilization and flesh quality. Aquaculture. 2009;290:304–310. [Google Scholar]
- 98.Hagen Ø. Fasting of farmed Atlantic cod (Gadus morhua L.) used as tool to improve fillet texture during the summer. Int. J. Food Sci. Technol. 2010;45:2669–2673. [Google Scholar]
- 99.Einen O., et al. Feed ration prior to slaughter—a potential tool for managing product quality of Atlantic salmon (Salmo salar) Aquaculture. 1999;178(1):149–169. [Google Scholar]
- 100.Ageeva T., et al. Effects of long-term feed deprivation on the development of rigor mortis and aspects of muscle quality in live-stored mature atlantic cod (Gadus morhua L.) J. Aquat. Food Prod. Technol. 2018;27 [Google Scholar]
- 101.Johnston I.A., Goldspink G. Some effects of prolonged starvation on the metabolism of the red and white myotomal muscles of the plaice Pleuronectes platessa. Mar. Biol. 1973;19(4):348–353. [Google Scholar]
- 102.McCue M.D. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2010;156(1):1–18. doi: 10.1016/j.cbpa.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 103.McCormick J.H., Podoliak H.A. Gallbladder color and relative fullness as a field technique for estimating time since last feeding in brook trout. N. Am. J. Fish. Manag. 1984;4(4B):566–568. [Google Scholar]
- 104.Robb A.P. Changes in the gall bladder of whiting (Merlangius merlangus) in relation to recent feeding history. ICES J. Mar. Sci. 1992;49(4):431–436. [Google Scholar]
- 105.Love R.M., Robertson I., Strachan I. Studies on the north sea cod VI.—effects of starvation 4. Sodium and potassium. J. Sci. Food Agric. 1968;19(7):415–422. [Google Scholar]
- 106.Tomlinson N., McBride J.R., Geiger S.E. The sodium, potassium, and water content of the flesh of sockeye salmon (Oncorhynchus nerka) in relation to sexual development and starvation. J. Fish. Res. Board Can. 1967;24(2):243–248. [Google Scholar]
- 107.Sullivan K.M.C., Somero G.N. Size- and diet-related variations in enzymatic activity and tissue composition in the sablefish, Anoplopoma fimbria. Biol Bull. 1983;164(2):315–326. [Google Scholar]
- 108.Lekanda A., Tolimieri N., Nogueira A. The effects of bottom temperature and fishing on the structure and composition of an exploited demersal fish assemblage in West Greenland. ICES J. Mar. Sci. 2021;78(8):2895–2906. [Google Scholar]
- 109.Emblemsvåg M., et al. Recent warming causes functional borealization and diversity loss in deep fish communities east of Greenland. Divers. Distrib. 2022;28(10):2071–2083. [Google Scholar]
- 110.Frainer A., et al. Increased functional diversity warns of ecological transition in the Arctic. Proc. R. Soc. B: Biol. Sci. 2021;288(1948) doi: 10.1098/rspb.2021.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamre K. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquacult. Nutr. 2011;17(1):98–115. [Google Scholar]
- 112.El-Sayed A.M., Izquierdo M. The importance of vitamin E for farmed fish—a review. Rev. Aquacult. 2022;14(2):688–703. [Google Scholar]
- 113.Hamre K., et al. Decreased concentration of hemoglobin, accumulation of lipid oxidation products and unchanged skeletal muscle in Atlantic salmon (Salmo salar) fed low dietary vitamin E. Fish Physiol. Biochem. 1994;12(5):421–429. doi: 10.1007/BF00004306. [DOI] [PubMed] [Google Scholar]
- 114.Bell J.G., et al. Some effects of selenium deficiency on enzyme activities and indices of tissue peroxidation in Atlantic salmon parr (Salmo salar) Aquaculture. 1987;65(1):43–54. [Google Scholar]
- 115.Poston H.A., Combs G.F., Jr., Leibovitz L. Vitamin E and selenium interrelations in the diet of Atlantic salmon (Salmo salar): gross, histological and biochemical deficiency signs. J. Nutr. 1976;106(7):892–904. doi: 10.1093/jn/106.7.892. [DOI] [PubMed] [Google Scholar]
- 116.Castell J.D., et al. Essential fatty acids in the diet of rainbow trout (Salmo gairdneri): growth, feed conversion and some gross deficiency symptoms. J. Nutr. 1972;102(1):77–85. doi: 10.1093/jn/102.1.77. [DOI] [PubMed] [Google Scholar]
- 117.Mommsen T., French C.J., Hochachka P.W. Sites and patterns of protein and amino acid utilization during the spawning migration of salmon. Can. J. Zool. 2011;58:1785–1799. [Google Scholar]
- 118.Squires E.J., Feltham L.A. Isolation, characterization and seasonal variations in the concentration of Nε-(γ-glutamyl)-lysine isodipeptide in the blood plasma of the winter flounder (Pseudopleuronectes americanus) Biochem. J. 1980;185(3):761–766. doi: 10.1042/bj1850761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohanty B., et al. Amino Acid compositions of 27 food fishes and their importance in clinical nutrition. Amino Acids. 2014;2014 doi: 10.1155/2014/269797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shiau C.-Y., et al. Effect of starvation on free histidine and amino acids in white muscle of milkfish Chanos chanos. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;128(3):501–506. doi: 10.1016/s1096-4959(00)00350-x. [DOI] [PubMed] [Google Scholar]
- 121.Wood J.D. Biochemical studies on sockeye salmon during spawning migration: IV. The non-protein nitrogenous constituents of the muscle. Can. J. Biochem. Physiol. 1958;36(8):833–838. [PubMed] [Google Scholar]
- 122.Wood J.D., Duncan D.W., Jackson M. Biochemical studies on sockeye salmon during spawning migration: XI. The free histidine content of the tissues. J. Fish. Res. Board Can. 1960;17(3):347–351. [Google Scholar]
- 123.Jacobson L.D., Hunter J.R. Bathymetric demography and management of dover sole. N. Am. J. Fish. Manag. 1993;13(3):405–420. [Google Scholar]
- 124.Methratta E.T., Link J.S. Ontogenetic variation in habitat associations for four flatfish species in the Gulf of Maine-Georges Bank region. J. Fish. Biol. 2007;70(6):1669–1688. [Google Scholar]
- 125.Saunders M.W., Leaman B.M., McFarlane G. In: National Oceanic and Atmospheric Administration (NOAA) Technical Report NMFS 130, Biology and Management of Sablefish. Wilkins M.E., Saunders M.W., editors. Anoplopoma fimbria: Papers from the International Symposium on the Biology and Management of Sablefish; Seattle, Washington, USA: 1997. Influence of ontogeny and fishing mortality on the interpretation of sablefish, Anoplopoma fimbria, life history; pp. 81–92. Seattle, Washington, 13-15 April 1993. [Google Scholar]
- 126.Bowering W.R., Nedreaas K.H. A comparison of Greenland halibut (Reinhardtius hippoglossoides (Walbaum)) fisheries and distribution in the Northwest and Northeast Atlantic. Sarsia. 2000;85(1):61–76. [Google Scholar]
- 127.Carpi P., et al. Ontogenetic and spawning migration of Pacific halibut: a review. Rev. Fish Biol. Fish. 2021;31(4):879–908. [Google Scholar]
- 128.Somero G.N. Environmental adaptation of proteins: strategies for the conservation of critical functional and structural traits. Comp. Biochem. Physiol. Mol. Integr. Physiol. 1983;76(3):621–633. doi: 10.1016/0300-9629(83)90464-4. [DOI] [PubMed] [Google Scholar]
- 129.Somero G.N. Biochemical ecology of deep-sea animals. Experientia. 1992;48:537–543. doi: 10.1007/BF01920236. [DOI] [PubMed] [Google Scholar]
- 130.Crabtree R.E. Chemical composition and energy content of deep-sea demersal fishes from tropical and temperate regions of the western North Atlantic. Bull. Mar. Sci. 1995;56(2):434–449. [Google Scholar]
- 131.Love R.M., Lavéty J. Wateriness of white muscle: a comparison between cod (Gadus morhua) and jelly cat (Lycichthys denticulatus) Mar. Biol. 1977;43(2):117–121. [Google Scholar]
- 132.Vetter R.D., et al. Depth zonation and metabolic adaptation in Dover sole, Microstomus pacificus, and other deep-living flatfishes: factors that affect the sole. Mar. Biol. 1994;120(1):145–159. [Google Scholar]
- 133.Sullivan K.M., Somero G.N. Enzyme activities of fish skeletal muscle and brain as influenced by depth of occurrence and habits of feeding and locomotion. Mar. Biol. 1980;60(2):91–99. [Google Scholar]
- 134.Jow T. California Department of Fish and Game, Marine Resources Region; California, USA: 1969. Cruise Report 69-S-4: Bottomfish program. [Google Scholar]
- 135.MacKinnon J.C. Summer storage of energy and its use for winter metabolism and gonad maturation in American plaice (Hippoglossoides platessoides) J. Fish. Res. Board Can. 1972;29(12):1749–1759. [Google Scholar]
- 136.Karl H., Numata J., Lahrssen-Wiederholt M. Variability of fat, water and protein content in the flesh of beaked redfish (Sebastes mentella) and Greenland halibut (Reinhardtius hippoglossoides) from artic fishing grounds. J. Consum. Prot, Food Saf. 2018;13:1–7. [Google Scholar]
- 137.Dawson A.S., Grimm A.S. Quantitative seasonal changes in the protein, lipid and energy content of the carcass, ovaries and liver of adult female plaice, Pleuronectes platessa L. J. Fish. Biol. 1980;16(5):493–504. [Google Scholar]
- 138.Burton M.P., Maddock D. Proceedings of the International Symposium on North Pacific Flatfish. Alaska Grant College Program Report; 1995. Reproduction, muscle hydration, and condition cycle variation in northern pleuronectids. [Google Scholar]
- 139.Maddock D.M., Burton M.P.M. Gross and histological observations of ovarian development and related condition changes in American plaice. J. Fish. Biol. 1998;53(5):928–944. [Google Scholar]
- 140.Maddock D.M., Burton M.P.M.M. Some effects of starvation on the lipid and skeletal muscle layers of the winter flounder, Pleuronectes americanus. Can. J. Zool. 1994;72(9):1672–1679. [Google Scholar]
- 141.Wuenschel M.J., Able K.W., Byrne D. Seasonal patterns of winter flounder Pseudopleuronectes americanus abundance and reproductive condition on the New York Bight continental shelf. J. Fish. Biol. 2009;74(7):1508–1524. doi: 10.1111/j.1095-8649.2009.02217.x. [DOI] [PubMed] [Google Scholar]
- 142.Konagaya S. Enhanced protease activity in the muscle of chum salmon during spawning migration with reference to softening or lysing phenomenon of the meat. Bull. Tokai Reg. Fish. Res. Lab. 1983:41–55. Tokai, Japan. [Google Scholar]
- 143.Roff D.A. An allocation model of growth and reproduction in fish. Can. J. Fish. Aquat. Sci. 1983;40(9):1395–1404. [Google Scholar]
- 144.Haedrich R.L., Rowe G.T., Polloni P.T. The megabenthic fauna in the deep sea south of New England, USA. Mar. Biol. 1980;57(3):165–179. [Google Scholar]
- 145.Rowe G.T. In: The Sea: Deep-Sea Biology. Rowe G.T., editor. John Wiley & Sons, Inc.; New York, USA: 1983. Biomass and production of the deep-sea macrobenthos; pp. 97–121. [Google Scholar]
- 146.Ferchaud A.-L., et al. A cold-water fish striving in a warming ocean: insights from whole-genome sequencing of the Greenland halibut in the Northwest Atlantic. Front. Mar. Sci. 2022;9 [Google Scholar]
- 147.Tsuyuki H., Roberts E. Muscle protein polymorphism of sablefish from the eastern pacific ocean. J. Fish. Res. Board Can. 1969;26(10):2633–2641. [Google Scholar]
- 148.Brown A., Thatje S. Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth. Biol. Rev. 2014;89(2):406–426. doi: 10.1111/brv.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Patel S., et al. Nodavirus in farmed Atlantic cod Gadus morhua in Norway. Dis. Aquat. Org. 2007;77(2):169–173. doi: 10.3354/dao01842. [DOI] [PubMed] [Google Scholar]
- 150.Johnson S.C., et al. Identification and characterization of a piscine neuropathy and nodavirus from juvenile Atlantic cod from the Atlantic coast of North America. J. Aquat. Anim. Health. 2002;14(2):124–133. [Google Scholar]
- 151.Johansen R., et al. Acute and persistent experimental nodavirus infection in spotted wolffish Anarhichas minor. Dis. Aquat. Org. 2003;57(1–2):35–41. doi: 10.3354/dao057035. [DOI] [PubMed] [Google Scholar]
- 152.Kvile, K. “Forskerne har analysert ti blåkveiter uten å finne svar på hvorfor den er så vassen” (March 11, 2019), referenced from: https://www.fiskeribladet.no/tekfisk/forskerne-har-analysert-ti-blakveiter-uten-a-finne-svar-pa-hvorfor-den-er-sa-vassen/2-1-561784.
- 153.Lester R.J.G. Unicapsula seriolae n. sp. (myxosporea, multivalvulida) from Australian yellowtail kingfish Seriola lalandi. J. Protozool. 1982;29(4):584–587. [Google Scholar]
- 154.Buchmann K., Skovgaard A., Kania P. Myxobolus groenlandicus n. sp. (Myxozoa) distorting skeletal structures and musculature of Greenland halibut Reinhardtius hippoglossoides (Teleostei: pleuronectidae) Dis. Aquat. Org. 2012;98:133–141. doi: 10.3354/dao02437. [DOI] [PubMed] [Google Scholar]
- 155.Ryberg M.P., et al. Physiological condition of Eastern Baltic cod, Gadus morhua, infected with the parasitic nematode Contracaecum osculatum. Conserv. Physiol. 2020;8(1) doi: 10.1093/conphys/coaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohamed A., et al. Contracaecum osculatum (sensu lato) infection of Gadus morhua in the Baltic Sea: inter- and intraspecific interactions. Int. J. Parasitol. 2020;50(10):891–898. doi: 10.1016/j.ijpara.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Valladão G.M.R., et al. Challenges in the control of acanthocephalosis in aquaculture: special emphasis on Neoechinorhynchus buttnerae. Rev. Aquacult. 2020;12(3):1360–1372. [Google Scholar]
- 158.Kocan R., et al. Ichthyophonus-induced cardiac damage: a mechanism for reduced swimming stamina in salmonids. J. Fish. Dis. 2006;29(9):521–527. doi: 10.1111/j.1365-2761.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 159.Sandheinrich M.B., Wiener J.G. In: Environmental Contaminants in Biota. Beyer N.W., Meador J.P., editors. CRC Press; Boca Raton, Florida, USA: 2011. Recent advances in assessing toxicity of environmentally relevant exposures; pp. 169–192. [Google Scholar]
- 160.Esparza I., et al. Mercury, legacy and emerging POPs, and endocrine-behavioural linkages: implications of Arctic change in a diving seabird. Environ. Res. 2022;212 doi: 10.1016/j.envres.2022.113190. (Part A) [DOI] [PubMed] [Google Scholar]
- 161.Johnson L.L., et al. In: Fish Physiology. Tierney K.B., Farrell A.P., Brauner C.J., editors. Academic Press; 2013. 2 - effects of legacy persistent organic pollutants (POPs) in fish—current and future challenges; pp. 53–140. [Google Scholar]
- 162.Adams S.M., et al. Relationships between physiological and fish population responses in a contaminated stream. Environ. Toxicol. Chem. 1992;11(11):1549–1557. [Google Scholar]
- 163.Lambert Y., et al. Using environmental and biological indices as proxies for egg and larval production of marine fish. J. Northwest Atl. Fish. Sci. 2003;33:115–159. [Google Scholar]
- 164.Marshall C.T., et al. Total lipid energy as a proxy for total egg production by fish stocks. Nature. 1999;402(6759):288–290. [Google Scholar]
- 165.Bell J.D., et al. Spatial variation in reproduction, and occurrence of non-reproductive adults, in orange roughy, Hoplostethus atlanticus Collett (Trachichthyidae), from south-eastern Australia. J. Fish. Biol. 1992;40(1):107–122. [Google Scholar]
- 166.Horwood J.W., Walker M.G., Witthames P. The effect of feeding levels on the fecundity of plaice (Pleuronectes platessa) Mar. Biodivers. Rec. 1989;69(1):81–92. [Google Scholar]
- 167.Hanya G., Chapman C.A. Linking feeding ecology and population abundance: a review of food resource limitation on primates. Ecol. Res. 2013;28(2):183–190. [Google Scholar]
- 168.Foy R.J., Crapo C., Kramer D.E. International Pacific Halibut Commission Technical Report No. 50; Seattle, Washington, USA: 2006. Investigating the Roles of Temperature and Exercise in the Development of Chalkiness in Pacific Halibut. [Google Scholar]
- 169.Tomlinson N., Geiger S.E., Dollinger E. Chalkiness in halibut in relation to muscle pH and protein denaturation. J. Fish. Res. Board Can. 1965;22(3):653–663. [Google Scholar]
- 170.Tomlinson, N., S.E. Geiger, and E. Dollinger, Free Drip, Flesh pH, and Chalkiness in Halibut. J. Fish. Res. Board Can., 1966. 23(5): p. 673-680.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this review are available within the cited references and/or their supplementary materials.