Abstract
Background
Older adults receiving chemotherapy are at a risk of hospitalization. Predictors of unplanned hospitalization among older adults receiving cancer chemotherapy were recently published utilizing data from a study conducted by the Cancer and Aging Research Group (CARG). This study aimed to externally validate these predictors in an independent cohort including older adults with advanced cancer receiving chemotherapy.
Methods
This validation cohort included patients (n =369) from GAP 70+ Trial (NCT02054741; PI: Mohile) usual care arm. Enrolled patients were aged 70+ with incurable cancer and starting a new line of chemotherapy. Previously identified risk factors proposed by the CARG study were ≥3 comorbidities, albumin <3.5 g/dl, creatinine clearance ≤60 mL/min, gastrointestinal cancer, ≥5 medications, requiring assistance with daily activities (ADL), and having someone available to take them to the doctor. The primary outcome was unplanned hospitalization within 3 months of treatment initiation. Multivariable logistic regression was applied including the seven identified risk factors. Discriminative ability of the fitted model was performed by calculating area under the receiver-operating characteristic curve (AUC).
Results
Mean age was 77 years; 45% were females; and 29% experienced unplanned hospitalization within the first 3 months of treatment. The proportions of hospitalized patients among those with 0-3, 4-5, 6-7 identified risk factors were 24%, 28%, and 47%, respectively (p=0.04). Impaired ADL (OR 1.76, 95% confidence interval (CI) 1.04-2.99) and albumin level <3.5mg/dl (OR 2.23, CI, 1.37-3.62) were significantly associated with increased odds of unplanned hospitalization. The AUC of the model including the 7 identified risk factors was 0.65 (CI, 0.59-0.71).
Conclusion
The presence of higher number of risk factors was associated with increased odds of unplanned hospitalization. This association was largely driven by impairment in ADL and low albumin level. Validated predictors of unplanned hospitalization can help with counselling and shared decision-making with patients and their caregivers.
Introduction
Older adults with cancer are at a higher risk for hospitalization, which can be a significant burden for patients, caregivers, and the healthcare system.1 Recent data suggest that 34% of patients with cancer aged 66-75 have unplanned hospitalizations in the first year after cancer diagnosis2. This percentage substantially increases to 43% among those over the age of 75.2 Unplanned hospitalization negatively impact quality of life and increase the risk functional decline and loss of independence.3,4 Moreover, hospitalization is associated with increased healthcare expenses and significant financial burden for older adults with cancer and their families.5-7 Identification of validated risk factors for hospitalization among older adults could inform treatment and care delivery interventions to minimize risk.
Compared to younger adults, older adults with cancer are more vulnerable to adverse events with cytotoxic chemotherapy. This leads to higher risk of hospitalization due to their aging-related conditions and lower physiological reserve with organ function.8,9 However, risk factors associated with unplanned hospitalization are not well defined among older adults with cancer receiving chemotherapy. The utility of the geriatric assessment (GA) or its components in predicting unplanned hospitalization has previously been investigated. These studies have identified geriatric impairments, such as functional dependency, poor nutrition, and polypharmacy, to be associated with increased risk of hospitalization among older adults with cancer receiving chemotherapy.10-12 However, no predictive model has been externally validated for these studies.
A recent study published by the Cancer and Aging Research Group (CARG) identified risk factors associated with unplanned hospitalizations among older patients receiving chemotherapy for cancer.13 Seven risk factors were identified including type of cancer, number of comorbidities, polypharmacy, below normal creatinine clearance, below normal albumin level, dependence in Activities of Daily Living (ADL), and availability of social support. The purpose of the current analysis is to: 1) externally validate the identified risk factors in an independent cohort of older adults with advanced cancer; and 2) explore additional risk factors associated with unplanned hospitalization in older adults with advanced cancer receiving chemotherapy.
Methods
Development cohort
A recent study published by CARG, identified seven risk factors for unplanned hospitalization among adults age ≥65 years with any cancer stage receiving chemotherapy13. This analysis used data collected in a prospective longitudinal study of 750 patients age ≥65 years initiating a new chemotherapy regimen that was evaluating predictors of chemotherapy toxicity.8,14 Details of the parent cohort study are published elsewhere.8 The seven identified risk factors by included a combination of clinical, laboratory, and GA measures.
Study design
In the current analysis, external validation of the identified risk factors was conducted using data from a nationwide, multicenter, cluster-randomized study that assessed whether providing information regarding GA plus GA-driven recommendations to community oncologists reduced clinician-rated grade 3-5 toxicity in patients aged ≥70 years with incurable cancer starting a new cancer treatment regimen (Geriatric Assessment for Patients [GAP70+] study; University of Rochester Cancer Center (URCC) 13059, PI: Mohile; NCT02054741).15 In the GAP70+ Study, community practices within the NCI’s Community Oncology Research Program (NCORP) were randomized to the intervention group (oncologists received GA summary & recommendations) or usual care (no summary or recommendations given except alerts for impaired scores on depression or cognitive status). Since the GAP70+ study showed that unplanned hospitalization was lower in the intervention arm, the current analysis used data from patients in the usual care only to avoid the possible influence of the intervention. Eligible criteria for this analysis were 1) patients aged ≥70 years, 2) diagnosed with an incurable stage III/IV solid tumor or lymphoma, 3) ≥1 GA domain impairment, and 4) planning to start a new cancer treatment regimen including a chemotherapy drug or other agents that have a similar prevalence of toxicity (e.g., tyrosine kinase inhibitors such as sorafenib and erlotinib). Eligible regimens were determined based on enrolling physicians' discretion and were reviewed at the primary coordinating site.
Outcome variable
The primary outcome of this analysis was the proportion of participants who experienced treatment-related unplanned hospitalization(s) within 3 months of starting a new treatment regimen (an overnight hospital stay for any reason that was not scheduled). Any planned or scheduled admissions were excluded from the analysis. Data on hospitalization were prospectively captured by practice staff. Clinic notes and discharge summaries were reviewed by blinded clinicians at the research base at URCC and the treating physician was queried if there is any discrepancy.
Predictor variables
For the primary aim, we focused on validation of the seven identified risk factors proposed by Klepin et al. Similar to the development cohort, all predictors were treated as categorical dichotomous variables to ease interpretation of the predictors. These risk factors included cancer type (gastrointestinal [GI] versus other types of cancer), comorbidity (≥3 self –reported comorbid conditions on the Older Americans Resources and Services Physical Health subscale versus <3), polypharmacy (≥5 concomitant medications versus <5), creatinine clearance (≤60 ml/min versus >60 Creatinine clearance; calculated using the Jelliffe formula with ideal body weight), albumin level (<3.5 g/dl versus ≥3.5), requiring assistance with ADL (yes versus no), and having someone available to take them to the doctor most or all of the time (yes versus no). All predictor variables were captured at baseline prior to starting a new line of cancer treatment.
For the secondary aim of the study, we collected information on the following baseline variables and assessed them in relation to hospitalization: 1) Demographic variables including age, gender, race, education, income; 2) clinical characteristics including cancer stage, treatment regimen (standard versus non-standard), palliative treatment line (first versus ≥second-line); and 3) GA variables including Geriatric Depression Scale-15 (GDS-15) to assess psychological status, Mini-Cog as a cognitive screening assessment, Mini Nutrition Assessment (MNA) to assess nutrition, and history of falls in the past 6 months to assess physical function. All GA variables were previously defined and described in the primary manuscript.15 The assessed baseline variables were found to be associated with hospitalization or other chemotherapy adverse-events among older adults in prior studies.9,16-18
Statistical analysis
Descriptive statistics (proportions for categorical variables and means for continuous variables) were performed to summarize and compare demographics, GA measures, clinical characteristics, and outcome measures between the development and validation cohorts.
For the primary aim, multivariable logistic regression modelling was applied including the seven identified risk factors. Discriminative ability of the fitted model was assessed by composing receiver operating characteristic (ROC) and calculating the area under the curve. To investigate additional risk factors unique to our study population, we first ran bivariate analyses using chi-square tests for categorical variables and t-tests for continuous variables examining the relationship of other baseline demographic, clinical, and geriatric variables with hospitalization. Subsequently, variables with p-values <0.1 were added to the model with the seven a priori risk factors and model performance was reassessed.
For all the analyses, two-sided p-values of 0.05 or less were considered statistically significant. All data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Table 1 includes patient characteristics for the validation and development cohorts. Mean age for participants in the validation cohort was 77.2 years (standard deviation [SD], 5.2) and 73.1 years (SD, 6.0) in the development cohort; 45.3% of patients in the validation cohort were females compared to 55.9% in the development cohort. Lung cancer was the most common cancer type among both validation (31.4%) and development cohorts (27.6%). The validation cohort included more patients with metastatic (stage 4) disease compared to the development cohort (87.8% versus 58.1%).
Table 1:
Patient and Treatment Characteristics in Development and Validation Cohorts
| Variable | Category | Development cohort |
Validation cohort |
P value |
|---|---|---|---|---|
| N=750 (100%) | N=369 (100%) | |||
| Socio-demographic variables | ||||
| Age | Mean (SD) | 73.1 (6.0) | 77.2 (5.2) | <0.01 |
| Gender | Male | 331 (44.1%) | 202 (54.7%) | <0.01 |
| Female | 419 (55.9%) | 166 (45.3%) | ||
| Race | White | 632 (84.3%) | 350 (95.1%) | <0.01 |
| | Black | 62 (8.3%) | 12 (3.3%) | |
| Others | 56 (7.5%) | 6 (1.6%) | ||
| Education | High school or less | 289 (38.5%) | 178 (48.3%) | <0.01 |
| College or above | 470 (61.4%) | 290 (51.5%) | ||
| Baseline clinical variables | ||||
| Cancer type | GI | 203 (27.1%) | 114 (30.9%) | <0.01 |
| Lung | 207 (27.6%) | 116 (31.4%) | ||
| GU | 80 (10.7%) | 53 (15.2%) | ||
| Others | 260 (34.7%) | 86 (23.3%) | ||
| Cancer stage | Stage 1 | 33 (4.4%) | -- | -- |
| Stage 2 | 99 (13.2%) | -- | ||
| Stage 3 | 175 (23.3%) | 35 (9.5%) | ||
| Stage 4 | 436 (58.1%) | 324 (87.8%) | ||
| Line of chemotherapy | First line | 531 (70.8%) | 275 (74.5%) | 0.19 |
| Second line or more | 219 (29.2%) | 94 (25.5%) | ||
| Standard chemotherapy | Yes | 548 (73.1%) | 240 (65.0%) | <0.01 |
| No | 177 (23.6%) | 129 (35.0%) | ||
| Number of chemotherapy agents | Single | 223 (29.7%) | 174 (47.3%) | <0.01 |
| Poly | 527 (70.3%) | 194 (52.7%) | ||
| Geriatric assessment and laboratory variables | ||||
| Falls in past 6 months | yes | 145 (19.4%) | 76 (20.7%) | 0.26 |
| no | 603 (80.6%) | 292 (79.3%) | ||
| Number of comorbidities | Median (range) | 2 (0-12) | 3 (0-9) | -- |
| Polypharmacy | < 5 medications | 384 (52.2%) | 72 (19.5%) | <0.01 |
| >= 5 medications | 352 (47.8%) | 297 (80.5%) | ||
| Difficulty with ADL | yes | 74 (9.9%) | 90 (24.5%) | <0.01 |
| no | 676 (90.1%) | 278 (75.5%) | ||
| Have someone take them to the doctor (social support) | yes | 671 (89.8%) | 352 (95.4%) | <0.01 |
| no | 79 (10.2%) | 17 (4.6%) | ||
| Impairment on GDS-15 | yes | NA | 84 (22.8%) | -- |
| no | -- | 285 (77.2%) | ||
| Cognitive impairment* | yes | 46 (6.1%) | 119 (32.2%) | -- |
| no | 703 (93.9%) | 250 (67.8%) | ||
| Albumin | Median (range) | 3.9 (1.0-5.0) | 3.6 (1-6.9) | -- |
| Creatinine clearance | Median (range) | 58.1 (12.3-122.9) | 64.2 (9.6-188) | -- |
Abbreviations: ADL, activities of daily living; GDS, Geriatric Depression Scale; GI, gastrointestinal; GU, genitourinary; KPS, Karnofsky Performance Scale; NA, not assessed; NR, not reported; SD, standard deviation.
Cognitive impairment was assessed through Blessed Orientation Memory Concentration test in the development cohort and Mini-Cog test in the validation cohort
Regarding the treatment characteristics, while all patients in the development cohort (100%) received chemotherapy agents, we found that 10% of patients in the validation cohort received agents that are considered non-chemotherapy drugs but have similar prevalence of toxicity (e.g., tyrosine kinase inhibitors such as sorafenib and erlotinib). In addition, the development cohort included more patients who received standard of care regimens compared to the validation cohort (73.1% versus 65.0%). Similarly, the development cohort included more patients who received combination chemotherapy agents compared to the validation cohort (70.3% versus 52.7%).
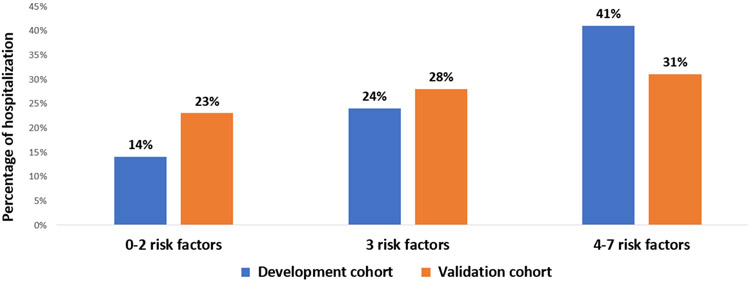
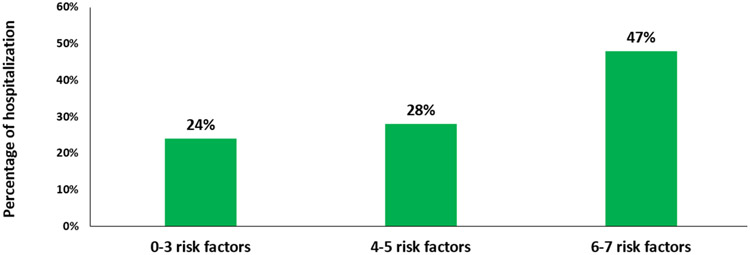
Distribution of the identified risk factors in the validation cohort was as follows: diagnosis of GI cancer (n=114, 31.0%); ≥3 or more comorbid conditions (n=234, 63.0%); receiving ≥5 medications (n=224, 61.0%); creatinine clearance <60 ml/min (n=154, 42.0%); albumin level <3.5 g/dl (n=155, 42.0%); requiring assistance with ADL(n=90, 24.5%); and having someone available to take them to the doctor most or all of the time (n=352, 95.0%). Twenty nine percent of patients in the validation cohort (n=107) experienced unplanned hospitalization with first 3 months of initiation of treatment (compared to 25.0% in the development cohort). When we used the same cut points as the development cohort (0-2, 3, and 4-7 risk factors), the proportions of hospitalized patients were 23%, 28%, and 31%, respectively (figure 1). Because none of the patients in the validation cohort had zero risk factors, we also evaluated alternate cut points fitted this population. In this cohort, a categorization of 0-3, 4-5, 6-7 identified risk factors corresponded to hospitalization rates of 24%, 28%, and 47% , respectively, p=0.04 (figure 2). Presence of higher number of risk factors was associated with 23% increased odds of unplanned hospitalization (odds ratio 1.23, 95% confidence interval (CI) 1.05-1.51, p=0.02).
Figure 1:
Proportion of older adults hospitalized during chemotherapy by presence of number of identified risk factors among development and validation cohort (using original cutoff values of risk factors; 0-2, 3, and 4-7). Risk factors included GI cancer, comorbidity, polypharmacy, below normal creatinine clearance and albumin levels, requiring assistance with activities of daily living, and having someone available to take them to the doctor most or all of the time.
Figure 2:
Proportion of older adults hospitalized during chemotherapy by presence of number of identified risk factors among validation cohort (using new cutoff values of risk factors; 0-3, 4-5, and 6-7). Risk factors included GI cancer, comorbidity, polypharmacy, below normal creatinine clearance and albumin levels, requiring assistance with activities of daily living, and having someone available to take them to the doctor most or all of the time.
In bivariate analysis, when examining other baseline variables in relation to hospitalization, history of falls in the past 6 months (p=0.01) and impairment on GDS-15 (p<0.01) were found to be significantly associated with unplanned hospitalization. Other variables including age, gender, race, education, income, cancer stage, treatment regimen, line of palliative treatment, mini-cog test, and MNA were not associated with unplanned hospitalization within 3 months (p values >0.1) (supplementary table 1).
In multivariable analysis, in the model with the seven identified risk factors, we found that impaired ADL (OR 1.76, 95% confidence interval (CI) 1.04-2.99) and albumin level <3.5mg/dl (OR 2.23, CI, 1.37-3.62) were significantly associated with increased odds of unplanned hospitalization (table 2). When we extended the model to include other significant baseline variables in bivariate analysis, we found that history of falls in the past 6 months (OR, 1.76, CI, 0.98-1.15) and impairment on GDS (OR, 1.84, CI 1.04-3.27) were also associated with increased odds of unplanned hospitalization (supplementary table 2).
Table 2:
Multivariable analysis for risk factors associated with hospitalization including the seven identified risk factors
| Risk factor | Odds ratio |
95% Confidence Limits | |
|---|---|---|---|
| GI cancer | 1.37 | 0.80 | 2.33 |
| Impaired ADL | 1.76* | 1.04 | 2.99 |
| Impaired polypharmacy | 0.95 | 0.57 | 1.56 |
| Creatinine Clearance (<60) | 1.21 | 0.74 | 1.96 |
| Impaired comorbidity (3 or more) | 1.20 | 0.72 | 2.01 |
| Albumin level (<=3.5) | 2.23* | 1.37 | 3.62 |
| Have someone take them to the doctor | 0.92 | 0.28 | 3.05 |
Abbreviations: ADL, activities of daily living; GI, gastrointestinal
P<0.05
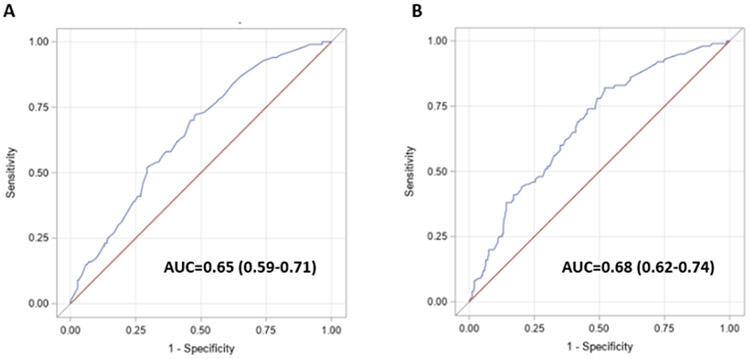
The AUC of the model including the seven a priori risk factors (≥3 comorbidities, albumin <3.5 g/dl, creatinine clearance ≤60 mL/min, GI cancer, ≥5 medications, requiring assistance with ADL, and having someone available to take them to the doctor) was 0.65 (CI, 0.59-0.71) (figure 3A).
Figure 3:
Receiver operating characteristic curves for the examined risk factors in relation to unplanned hospitalization.
After extending this model to include history of falls in the past 6 months and impaired GDS, the AUC increased to 0.68 (CI, 0.62-0.74) (figure 3B). The multivariable effect estimates for risk factors associated with hospitalization in the validated and extended models are shown in table 2 and supplementary table 2.
Discussion
This study aimed to validate a group of clinical, laboratory, and GA risk factors for unplanned hospitalization among older adults with advanced cancer receiving chemotherapy, identified in a prior study conducted by CARG. We found that presence of higher number of risk factors was associated with increased odds of unplanned hospitalization. This association was largely driven by impairment in ADL and low albumin level. In addition, we demonstrated that evaluating these risk factors together has the ability to assist in discriminating the hospitalization risk in older adults with cancer during their treatment course . Furthermore, we explored additional risk factors unique to older adults with advanced cancer and found that risk of unplanned hospitalization was higher among patients with history of falls in the past 6 months and those with who screened positive on GDS.
To our knowledge, this is the first study to externally validate predictors for unplanned hospitalization among older adults with cancer receiving chemotherapy. Validated risk factors of unplanned hospitalization are of particular interest for oncologists. First, they would allow clinicians to estimate the risk of hospitalization before cancer treatment is planned. Second, validated predictors of unplanned hospitalization can help with counselling and shared decision-making with patients and their caregivers. Moreover, identifying these predictors can guide the development of interventions to reduce the risk of hospitalization and improve both patient and caregiver outcomes.
Despite the differences in treatment characteristics between the 2 cohorts (i.e. more patients in the development cohort received standard of care regimens and were on combination treatment), the current analysis demonstrated that incidence of unplanned hospitalization was greater among the validation cohort (29%) compared to the incidence of hospitalization in the development cohort (25%).13 We found that the majority of risk factors identified in the development cohort were not significantly associated with hospitalization when we assessed their individual effect estimates in our validation cohort. These findings could be attributed to the difference in the characteristics between the two cohorts. While the development cohort included patients with different cancer stages (I-IV), the validation cohort was restricted to patients with incurable cancers (stages III-IV), who are typically frailer and have more aging-related conditions.19 In addition, all the participants in the validation cohort were impaired on at least one geriatric domain per trial eligibility. Accordingly, the proportion of patients who had 4-7 identified risk factors (i.e., intermediate and most frail groups) was greater among the validation cohort compared to the development cohort (59.0% versus 32.0%).
Despite these differences, the current analysis demonstrated a significant association between increased number of these risk factors and the risk of hospitalization. In addition, when we classified the seven clinical, laboratory and geriatric identified risk factors into different risk categories that better fit our frail and homogenous population (i.e. 0-3, 4-5, 6-7), we found that, compared with patients in the low-risk category (0-3 risk factors), the odds of experiencing unplanned hospitalization were more than two times greater for patients in the high-risk category. This reinforces the hypothesis that these risk factors (i.e., aging-related conditions) are not considered discrete diseases and are closely linked with each other.
It is worth noting that we observed only a modest discriminative ability when we compared the hospitalization risk among the different risk categories used in the development cohort (0-2, 3, and 4-7 risk factors; 23%, 28%, and 31% respectively). This loss of discrimination in external validation cohorts has been described previously14 but, in this case may be largely attributable to known differences between the study populations. We observed the biggest difference in performance of the prediction tool between the development and this validation cohort in the incidence of outcome in low-risk patients . This could be explained by all the participants in the validation cohort having impairment on at least one geriatric domain per trial eligibility, which reflects a frailer cohort compared to the development cohort in the low risk group. Specifically, the validation cohort excluded truly low risk patients by design. Despite its modest discriminative ability, this model still provides some risk stratification to support it use in the clinical setting where providers will see a heterogeneous population including those who match the validation cohort population as well as those who are fit and were represented in the development cohort.
In our validation cohort, the association between the number of risk factors and increased odds of unplanned hospitalization was largely driven by impairment in ADL and low albumin level before treatment. Difficulty performing daily activities such as bathing and dressing (i.e. functional impairment) affected approximately one quarter of our validated cohort. Older adults who develop such difficulties, commonly caused by frailty and other age-related conditions, are at increased risk of chemotherapy adverse events including unplanned hospitalization9,19,20. Moreover, previous data have shown that reduction in serum albumin, which is more pronounced in older patients with poor nutrition (38% of the study participants) may lead to increased risk of chemotherapy adverse events including unplanned hospitalization13,21. The reduction in serum albumin increases the free fraction of the drug in plasma, which was reported with multiple chemotherapeutic agents such as cisplatin, etoposide, and taxanes21,22.
In our analysis, we found that having history of falls in the past 6 months was associated with increased risk of hospitalization. Prior studies have shown that patients hospitalized for cancer have higher frequencies of falls, when compared to hospitalized patients who do not have cancer23,24. Some chemotherapeutic drugs, such as platinum compounds and taxanes are known to be neurotoxic, resulting in peripheral neuropathy, which can cause gait and balance issues, as well as an increased risk of falling25,26. We also noticed a positive association between impairment on the GDS-15 scale and increased risk of hospitalization. Studies suggested that psychological impairments including depression are common among older adults with cancer.27 Moreover, depression was associated with adverse outcomes such as functional impairment and poor survival in this population.9,28
It is worth noting that the majority of the risk factors predicting unplanned hospitalization are part of the GA. This underscores the importance of performing geriatric screening prior to initiation of chemotherapy among older adults with cancer and aging related conditions. One advantage of the risk factors predicting unplanned hospitalization in our study is that they can be easily obtained and gathered during routine clinical practice. Accordingly, they can be easily implemented in daily oncology care compared to a full GA which may be difficult to perform within the time constraints of busy clinical practices in limited resource settings.
A major strength of this study is its inclusion of a population that is that is typically marginalized in oncology trials – older adults with advanced cancer receiving care in community oncology (i.e., real-world) practices. Additionally, the prospective capturing of hospitalization data limited the problem of recall bias. Our study also has some limitations. First, because the patients in this study were enrolled as part of a GA intervention clinical trial, this may have introduced bias upon the population selecting to participate, which may have limited the study's generalizability. Second, because our patients were primarily non-Hispanic White and well-educated, our findings may not be applicable to patients of other races or with lower levels of education.
In conclusion, this study contributes to informed clinical decision-making regarding planning treatment and expectation of adverse outcomes in this vulnerable population. The identified and validated clinical and GA predictors can be used to identify high risk patients to guide interventions to reduce hospitalization in older adults with cancer.
Supplementary Material
References
- 1.Brooks GA, Kansagra AJ, Rao SR, Weitzman JI, Linden EA, Jacobson JO. A Clinical Prediction Model to Assess Risk for Chemotherapy-Related Hospitalization in Patients Initiating Palliative Chemotherapy. JAMA Oncol 2015;1:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitney RL, Bell JF, Tancredi DJ, et al. Unplanned Hospitalization Among Individuals With Cancer in the Year After Diagnosis. Journal of oncology practice 2019;15:e20–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drageset J, Sandvik RK, Eide LSP, Austrheim G, Fox M, Beisland EG. Quality of life among cancer inpatients 80 years and older: a systematic review. Health and Quality of Life Outcomes 2021;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonna S, Chiang L, Liu J, Carroll MB, Flood K, Wildes TM. Geriatric assessment factors are associated with mortality after hospitalization in older adults with cancer. Support Care Cancer 2016;24:4807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila J, Jupiter D, Chavez-MacGregor M, de Oliveira C, Kaul S. High-Cost Hospitalizations Among Elderly Patients With Cancer. J Oncol Pract 2019;15:e447–e57. [DOI] [PubMed] [Google Scholar]
- 6.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008;100:630–41. [DOI] [PubMed] [Google Scholar]
- 7.Arastu A, Patel A, Mohile SG, et al. Assessment of Financial Toxicity Among Older Adults With Advanced Cancer. JAMA Network Open 2020;3:e2025810–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Abbema DL, van den Akker M, Janssen-Heijnen ML, et al. Patient- and tumor-related predictors of chemotherapy intolerance in older patients with cancer: A systematic review. Journal of Geriatric Oncology 2019;10:31–41. [DOI] [PubMed] [Google Scholar]
- 10.Williams GR, Dunham L, Chang Y, et al. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. Journal of oncology practice 2019;15:e399–e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silay K, Akinci S, Silay YS, et al. Hospitalization risk according to geriatric assessment and laboratory parameters in elderly hematologic cancer patients. Asian Pac J Cancer Prev 2015;16:783–6. [DOI] [PubMed] [Google Scholar]
- 12.Chiang LY, Liu J, Flood KL, et al. Geriatric assessment as predictors of hospital readmission in older adults with cancer. J Geriatr Oncol 2015;6:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepin HD, Sun CL, Smith DD, et al. Predictors of Unplanned Hospitalizations Among Older Adults Receiving Cancer Chemotherapy. JCO Oncol Pract 2021;17:e740–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. Journal of Clinical Oncology 2016;34:2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. The Lancet 2021;398:1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamri F, Alsofayan Y, AlRuthia Y, et al. Predictors of Hospitalization Among Older Adults with COVID-19 in Saudi Arabia: A Cross-Sectional Study of a Nationally Representative Sample. Risk Manag Healthc Policy 2021;14:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcusson J, Nord M, Dong H-J, Lyth J. Clinically useful prediction of hospital admissions in an older population. BMC Geriatrics 2020;20:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodewijckx E, Kenis C, Flamaing J, et al. Unplanned hospitalizations in older patients with cancer: Occurrence and predictive factors. J Geriatr Oncol 2021;12:368–74. [DOI] [PubMed] [Google Scholar]
- 19.Kadambi S, Loh KP, Dunne R, et al. Older adults with cancer and their caregivers — current landscape and future directions for clinical care. Nature Reviews Clinical Oncology 2020;17:742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lage DE, El-Jawahri A, Fuh CX, et al. Functional Impairment, Symptom Burden, and Clinical Outcomes Among Hospitalized Patients With Advanced Cancer. J Natl Compr Canc Netw 2020;18:747–54. [DOI] [PubMed] [Google Scholar]
- 21.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Lara K, Diaz-Romero C, Motola-Kuba D, et al. Albumin serum levels and malnutrition are associated with toxicity secondary to paclitaxel-cisplatin chemotherapy in patients with advanced non-small cell lung cancer: A prospective study. Journal of Clinical Oncology 2008;26:9623-. [Google Scholar]
- 23.Jun MD, Lee KM, Park SA. Risk factors of falls among inpatients with cancer. Int Nurs Rev 2018;65:254–61. [DOI] [PubMed] [Google Scholar]
- 24.Hendrich A, Nyhuis A, Kippenbrock T, Soja ME. Hospital falls: development of a predictive model for clinical practice. Appl Nurs Res 1995;8:129–39. [DOI] [PubMed] [Google Scholar]
- 25.Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci 2019;20:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer 2012;20:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohile SG, Epstein RM, Hurria A, et al. Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncol 2020;6:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. Journal of the American Geriatrics Society 2004;52:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.