Abstract
Saxitoxin (STX) represents a marine toxin of significant concern due to its deleterious implications for aquatic ecosystems and public food safety. As a potent paralytic agent, the role of STX in obstructing voltage-gated sodium channels (VGSCs) is well-characterized. Yet, the mechanistic details underlying its low-dose toxicity remain largely enigmatic. In the current study, zebrafish embryos and larvae were subjected to subchronic exposure of graded STX concentrations (0, 1, 10, and 100 μg/L) until the 7th day post-fertilization. A tactile stimulus-based assay was employed to evaluate potential behavioral perturbations resulting from STX exposure. Both behavioral and transcription level analyses unveiled a compromised tactile response, which was found to be associated with a notable upregulation in the mRNA of two distinct VGSC isoforms, specifically the scn8aa/ab and scn1Laa/ab transcripts, even at the minimal STX dose. Notably, exposure to this lowest STX concentration also resulted in alterations in the transcriptional patterns of pivotal genes for cholinergic and GABAergic pathways, including ache and gabra1. Furthermore, STX induced a marked decrease in the levels of the neurotransmitter GABA. Our findings underscore that prolonged low-dose STX exposure during early development can significantly compromise the tactile response behavior in zebrafish. This study reveals that chronic low-dose STX exposure of developing zebrafish alters neurotransmission pathways that converge on altered tactile behavior.
Keywords: Saxitoxin, Sensorimotor response, Voltage-dependent sodium channels, Neurotransmission, Zebrafish
1. Introduction
Worldwide, the emergence of harmful algal blooms (HABs), fueled by specific dinoflagellates and cyanobacteria in marine and freshwater ecosystems, poses significant ecological and public health concerns. Saxitoxin (STX), a potent neurotoxin produced during these blooms, inhibits voltage-dependent sodium channels (VGSCs), leading to disrupted nerve impulses and muscle paralysis [1]. The concentration of STX in water samples varies with environmental conditions. For instance, a study by Oluwafemi et al. [2] reported concentrations in water samples ranging from 2.1 to 2.4 mg/mL during wet seasons and 3.0–3.04 mg/mL during dry seasons. Additionally, the study found varying concentrations of STX in shellfishes, highlighting the bioaccumulation of the toxin in organisms. For example, the carapace of Callinectes sapidus showed concentrations of 69.93 ± 0.90 μg/g during wet seasons and 114.71 ± 0.02 μg/g during dry seasons. Furthermore, case studies such as the one conducted near the Irkutsk hydroelectric power station [3] revealed high concentrations of STX (600 ± 100 μg/L) in water samples from areas affected by harmful algal blooms. The study linked this contamination to a bloom of the cyanobacterium Dolichospermum lemmermannii, shedding light on the dynamics of STX release during algal decay. The study conducted by Ledreux [4] provides insights into the prevalence of STX in recreational water bodies. The research detected concentrations higher than 3 μg equiv STX/L in raw water, with Aphanizomenon gracile identified as the likely producer of saxitoxins.
These collective studies underscore the variability in STX concentrations across different environments and highlight the need for a detailed exploration of its presence in aquatic ecosystems. Understanding these concentrations is crucial for assessing the potential harm to fish and other organisms, emphasizing the importance of rigorous monitoring and management strategies. As mentioned above, the HAB occurrences and STX production are modulated by environmental parameters such as water temperature, salinity, and nutrient availability [5,6]. As blooms form, STX bioconcentrates within the food chain, instigating paralytic shellfish poisoning (PSP) and affecting aquatic biota, which holds implications for both ecosystems and global food security [7,8]. Climate change projections suggest exacerbated HAB frequency and severity, emphasizing the need for rigorous HAB management strategies to mitigate STX exposure risks and related ecological and economic repercussions.
To date, numerous studies involving various fish species, including zebrafish, medaka, herring, killifish, and Atlantic salmon, have documented detrimental effects arising from the accumulation and biomagnification of STX [[9], [10], [11], [12], [13]]. These adverse effects encompass a range of sublethal impacts, including, a) Growth Inhibition. For example, fish exposed to STX often exhibit reduced growth rates [9,12,14]. b) Aggressive Behavior, increased aggression can manifest as a consequence of STX exposure [15]. c) Reduced Locomotor Activity, fish may display decreased swimming activity and mobility [9,10]. d) Loss of Appetite, STX-exposed fish often experience a decline in their appetite [14]. e) Antioxidant Activity Reduction, STX can lead to a decrease in antioxidant activity, potentially making fish more vulnerable to oxidative stress [16]. f) Irregular Swimming Patterns, fish may exhibit abnormal swimming patterns and behaviors [13,14]. g) Survival Rate Reduction, the survival rates of fish can decline in response to STX exposure [9,14]. At the molecular level, STX has been associated with a spectrum of adverse effects, including cytotoxicity, genotoxicity, oxidative stress, and immunosuppression [[16], [17], [18]]. Furthermore, at the neuronal level, STX has been observed to up-regulate specific proteins involved in neuronal recovery and proliferation, such as nucleoside diphosphate kinase (NDK) and tubulin family proteins in medaka embryos [12]. These findings collectively underscore the extensive range of negative consequences resulting from STX exposure across multiple biological levels, from behavioral to molecular, highlighting the critical importance of understanding and mitigating the impact of STX on aquatic ecosystems and organisms.
STX is known to be a potent, natural and selective blocker of VGSCs in nerve cells, triggering paralytic effects [1]. Human exposure yields symptoms ranging from facial and extremity numbness to fatal respiratory failure [7]. VGSCs are integral to the generation and propagation of action potentials, thus facilitating communication between excitable cells [19]. These channels, constituted by specific membrane proteins, feature a pore structure that facilitates the rapid and passive diffusion of ions across the lipid bilayer [20]. As action potentials propagate through axons, the activation of VGSCs results in membrane depolarization and the consequent opening of voltage-gated calcium (Ca2+) channels. The resulting Ca2+ influx modulates neurotransmitters release towards postsynaptic neurons or muscle fibers [21]. While acetylcholine release promotes excitatory transmission, the GABAergic system inhibits transmission through the release of GABA. Cumulative evidence shows that STX induces significant perturbations in this intricate system. Specifically, the guanidine group of STX serves as a cationic substitute for sodium, ultimately altering the normal patterns of neural excitation and inhibition [22,23]. On one hand, STX plays strong neurotoxic effects with doses as minimal as 1–4 mg/kg being potentially fatal [5,7]. On the other hand, due to its high specificity, STX has turned into a robust tool to study the structure and function of VGSCs in different conditions, including the development of therapeutic drugs for mental diseases [21,24]. Both mammals and zebrafish express the sodium channel gene (SCNA) responsible for the pore-forming α-subunit proteins (Nav.1) during embryonic stages [25]. Given the structural and genetic parallels between zebrafish and mammalian sodium channels, zebrafish emerge as an invaluable model system to probe toxicant impacts on ion channels [26].
In this study, our primary objective is to comprehensively investigate the toxic effects of STX on fish, with a specific focus on the VGSCs and neurotransmitter pathways. By utilizing zebrafish embryos as a model system, we aim to elucidate the neurotoxicological alterations induced by even sublethal concentrations of STX. Our investigation extends to both behavioral responses and gene expression profiles in zebrafish embryos exposed to varying concentrations of STX. The core purpose of this research is to uncover potential hazards associated with STX exposure and to provide a mechanistic understanding of its neurotoxicological implications, particularly in the context of the modulation of neurotransmission from VGSCs. The implications of our findings go beyond the immediate toxic effects and contribute to a broader understanding of the ecological risks posed by STX in aquatic environments, with a specific emphasis on its impact on fish populations. By correlating behavioral observations with molecular-level analyses of gene expression, we aim to offer a comprehensive perspective on the effects of STX, thereby highlighting its potential ecological consequences. This study contributes valuable insights that can inform not only the understanding of STX toxicity but also the development of strategies for mitigating its impact on aquatic ecosystems. The detailed exploration of STX effects on VGSCs in zebrafish embryos provides a foundation for future research in the broader field of aquatic toxicology and neurobiology.
2. Materials and methods
2.1. Fish maintenance
Adult zebrafish (Danio rerio) were continuously cultured in an automated Aquaneering system with mechanical and biological filters at the Centre for Biotechnology of the University of Concepción (Concepción, Chile). The fish were kept in the controlled system at 28 ± 0.3 °C in a light: dark (L:D) photoperiod of 14:10 h with continuous aeration. The fish were fed twice a day with live brine shrimp metanauplii bred from dry eggs (≈48 h old; Artemia Egg, Great Salt Lake, USA). The physicochemical parameters of the water, such as temperature, pH, and conductivity, were analyzed every day, except for ammonium, nitrite, and nitrites once a week using commercial kits. Embryos for STX exposure experiments were obtained by natural crosses, pairing wild-type (AB strain) adult males with females overnight in specialised spawning chambers. The eggs were collected, cleaned, and inspected with a stereomicroscope, staged according to Kimmel et al. [27], and transferred to Petri dishes containing E3 medium.
2.2. Extraction of STX
Saxitoxin extraction and purification were carried out according to the previously published method by Rubio et al. [28]. Briefly, Mytilus chilensis was collected from different local bays near Punta Arenas, Chile. The toxins were extracted from mussel muscle, which was previously shelled and cleaned. Between 100 and 150 gr of muscle tissue were drained in a net for 5 min and homogenized in a high-speed blender for 60 to 120 sg and 100 gr of homogenate 100 mL of 0.1 N HCl were added. The pH was adjusted to 3 with 0.1 N NaOH or 5 N HCl and gently boiled in an electromantle under a fume hood to avoid excessive evaporation. The sample was cooled in an ice bath to room temperature and the pH was again adjusted to 3.0. The extract was transferred to a test tube and adjusted to a final volume of 200 ml with 0.003 N HCl.
2.3. Interferent removal and purification
To eliminate proteins and condition the mussel extract for purification, solid-phase extraction (SPE) was conducted using C-18 cartridges (Oasis HLB, Waters, MA, USA). A 50 mL volume of the mussel extract was introduced into the SPE cartridge, followed by a 10-min equilibration period. The elution, performed at a rate of 1.3 mL/min, resulted in an 85% by weight removal of proteins. Subsequently, the deproteinized mussel extract underwent purification through preparative high-performance liquid chromatography (HPLC) using a C-18 Kromasil column (250 mm length, 10 mm diameter, 5 μm particle size) with a flow rate of 15.3 mL/min. The mobile phase, adjusted to pH 7.1 with 25% ammonium hydroxide, consisted of 11 mM sodium heptanesulfonate, 16.5 mM o-phosphoric acid, and 11.5% acetonitrile. To eliminate salts and other compounds from the mobile phase, the purified fraction underwent deionization using 6.0 mL Oasis WCX cation-exchange SPE cartridges. The WCX cartridge was washed with deionized water, and saxitoxin was eluted with 5.0% formic acid. Finally, saxitoxin was lyophilized at −52 °C for 24 h and dissolved in 0.003 M HCl. The concentration of saxitoxin was verified using analytical HPLC.
2.4. HPLC analytic method
For the analytical HPLC method, a Kromasil C18 reverse-phase column (5 μm, 300 mm × 4 mm) at room temperature was employed. The mobile phase, in isocratic mode at a flow rate of 1 mL/min, consisted of 11 mM heptanesulfonate, 5.5 mM phosphoric acid, and 11.5% acetonitrile. Post-column derivatization was performed using an oxidizing phase (5 mM periodic acid and 100 mM phosphoric acid) and an acid phase (0.75 mM nitric acid) at flow rates of 0.4 mL/min each. The derivatization reaction occurred in an oven at 80 °C, and toxin detection was achieved by fluorescence at 330 nm excitation and 390 nm emission wavelengths. Quantification of saxitoxin in the samples was performed using an external calibration curve prepared with NIST 8642a MR-SXT standard (103.0 μg/g) (see Fig. S1).
2.5. Exposure to saxitoxin and sensorimotor response assays
Previous experiments showed that exposure to 227 ± 26 μg STX equivalent/L and 426 ± 100 μg STX equivalent/L completely inhibited the response of larvae to a mechanosensory stimulus during 7 days of exposure [9]. As a preliminary experiment, we evaluated the possible toxicity of different concentrations of STX (0, 0.05, 0.1, 0.5, 1, 5, 10, 50, 100, 200 and 600 μg/L) on zebrafish larvae for a period of 7 days (Fig. 1), following the OECD Guideline for Testing of Chemicals, Fish, Acute Toxicity Test (203) (2019) [29]. The different concentrations were diluted in E3 medium. Twenty germ ring stage embryos per treatment were selected and distributed in 4 replicates (five individuals per replicate) in 24 multiwell plates. Embryos were placed in an incubator at 28.5 °C for the duration of the exposure to ensure a constant rate of development. pH (6.5–7.5), conductivity (600–800 μS) and dissolved oxygen (6.6–7.6 mg/L) were monitored at the beginning and end of the treatment. Embryos and larvae were observed daily using a Leica ES2 stereomicroscope, and the touch response, and their mortality were determined.
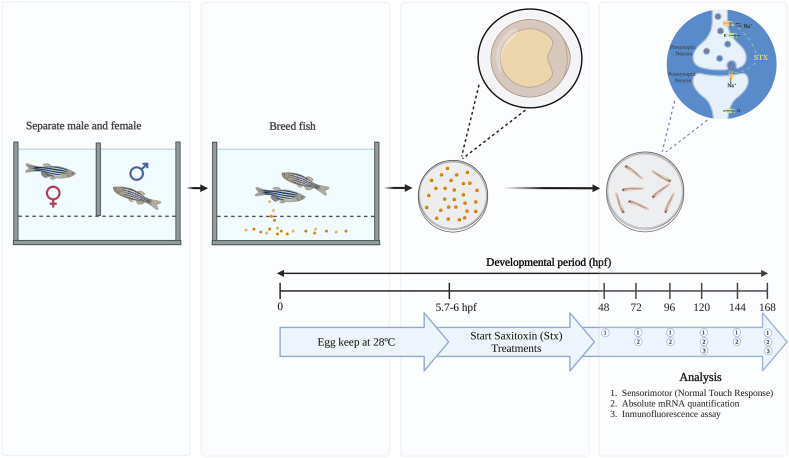
Fig. 1.
Schematic representation of the experimental design. The diagram shows the saxitoxin treatment and analysis during the developmental period (168 hpf) of zebrafish (Danio rerio). Individuals were maintained at 28 °C from 0 hpf until the end of treatment. Treatment was initiated at 5.7–6 hpf when embryos were between the germ-ring and shield stage, lasting until 168 he. Analyses were: 1) Sensorimotor from 48 he; 2) Absolute mRNA quantification from 72 he; and 3) Immunofluorescence assay at 120 and 168 he. Created withBioRender.com.
The touch response was evaluated every day starting at 48 h post-fertilization (hpf), when the larvae were completely dechorionated. Using forceps, a gentle stimulus was applied to the dorsal area of the larval spinal cord, where the mechanosensory neurons are located. This response was recorded according to the method described by Lefebvre et al. [9], with modifications. We define the normal response using the following criteria: (1) the fish responded immediately to the touch stimulus, and (2) the swimming pattern. Initially, the response is described as bursts, and then it transforms into an increasingly more frequent swimming in the form of beat-and-glide, until it develops into a more active swimming from 120 hpf onwards. Therefore, larvae that maintain their swimming pattern in response to the tactile stimulus were considered normal. In contrast, the touch response was recorded as abnormal using the following criteria: (1) delayed response (more than one touch required for the response), (2) larvae do not maintain the swimming pattern in response to the tactile stimulus (fall to the bottom), and (3) no response (paralysis).
2.6. Experimental design of saxitoxin treatment
Based on the mortality and tactile response toxicity data from prior experimentation, three STX concentrations (100, 10, and 1 μg/L) were identified as sublethal and chosen for subsequent analyses. For each of these concentrations, 230 embryos at the germ ring stage were selected for exposure across three replicates. Additionally, a control group comprised of 230 unexposed embryos was established for comparison. The individuals were sampled at the following time points: 48, 72, 96, 120, 144 and 168 h of exposure (he), for each concentration. During the exposure time, the larvae were not fed, and the parameters of ammonia, pH, conductivity, and dissolved oxygen were measured at the beginning and end of each treatment (Fig. 1).
2.7. RNA extraction and quantitative PCR
The transcriptional responses of genes expressed during embryonic and larval development in the nervous system were examined using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Specific gene information and the sequences of primer sets are shown in Table S1. The primers used for qRT-PCR were designed using the NCBI Primer online design software. After exposure to 0, 1, 10, and 100 μg/L of STX, total RNA was isolated from 30 homogenized embryos at 72, 96, 120, 144, and 168 he using TRIZOL Reagent® (0.2 mL; Sigma-Aldrich Missouri, United States) and quantified by absorbance at 260 nm. The integrity of the purified RNA was confirmed by agarose denaturing gel electrophoresis. The cDNA was synthesised from total RNA using the iScript cDNA synthesiser Kit (Bio-Rad). qRT-PCR was performed using SYBR Green Master Mix (Bio-Rad). Each qRT-PCR mixture contained the SYBR Green Master Mix, 2 μL cDNA, 500 nmol/L of each primer, and RNase-free water in a final volume of 10 μL. The amplification was performed in triplicate in 96-well plates with the following thermal cycling conditions: initial activation for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. A series of dilutions made from known concentrations of plasmid containing the PCR inserts was used to calculate absolute copy numbers for each of the genes examined (see Table S1).
2.8. Whole-mount immunofluorescence zebrafish larvae
Zebrafish larvae at 120 he were sampled, sacrificed on ice and fixed in 4% paraformaldehyde for 2 h under agitation and at room temperature. After fixation, larvae were depigmented with 1X PBS, 3% H2O2 and 0.5% KOH for 30 min at room temperature (RT). Subsequently, the larvae were washed with PBS (1X). Then, they were sequentially rehydrated with a decreasing methanol concentration (90%, 75%, 50% and 25% metOH/1X PBS-Tritón 0.1% (PBS-T) and washed with 1X PBS-T for 10 min. Larvae were then immersed in blocking solution (0.2 gr BSA + 10 ml 1X PBS-T) for 2 h at RT in the dark and under continuous agitation. Samples were incubated overnight at 4 °C with Rabbit anti-GABA (Sigma, Cat. No. ab2052, 1:150) and goat anti-ChAT (Sigma, Cat. No. ab144P, 1:100) primary antibodies diluted in blocking buffer. Secondary Alexa 594-conjugated goat anti-rabbit (Abcam, Cat. No. ab15007, 1:1000) and donkey anti-goat secondary antibodies (1:1000) were diluted in blocking buffer and incubated for 3 h at RT in the dark and under continuous agitation, along with DAPI (4′,6-diamidino-2-phenylindole). No immunoreactivity could be observed when the primary antibodies were omitted. Images were captured using a stereomicroscope ZEISS Axio Zoom.V16 and Spectral Confocal Microscope LSM780 NLO, Zeiss.
2.9. Images analysis
Zebrafish larvae were oriented laterally for imaging, which was subsequently processed using Fiji (ImageJ). For GABA quantification, fluorescence intensity was analyzed using the trunk-caudal section of each larva. In contrast, for ChAT analysis, quantification was conducted on the three myotomes of the neuromuscular junctions (NMJ) situated above the posterior vitelline extension. GABA images captured with ZEISS Axio Zoom.V16 were analyzed in the following way: grayscale and channel splitting (Image > Color > Split Channel). The measurements were set with the Analyze > Set Measurement command of Fiji, and the following options were chosen: area, mean gray value, integrated density, and limit to threshold. The image was then thresholded to create a binary image. The area of interest was selected and added to the ROI Manager to apply the selected area to the original image and measure the above-mentioned parameters. Next, an area excluding the larva was marked with the "Polygon selections" tool to record the background levels. This process was continued for all larvae analyzed in both control and saxitoxin-treated conditions.
ChAT confocal images were acquired with a 40× glycerol immersion objective and a total of 243 z-stacks (62 μm thick) were collected and analyzed. From the "Polygon of selections" tool, the area of interest for the three myotomes were plotted and added to the ROI Manager as we moved through the different slides to subsequently interpolate more than one ROI in a stack. The same measurement parameters previously described in the original image were set. The plotted area was then moved excluding the larva to record the background measurements of the stack. Corrected total cell fluorescence (CTCF), in arbitrary units, was measured for each larva as follows: CTCF = integrated density - (area of selected cell/region × mean fluorescence of background readings).
2.10. Data analysis
Statistical analysis was performed using the R-RStudio interface. All values are presented as mean ± standard deviation of means (SD). By using Kaplan-Meier (K-M) curves, survival probabilities were plotted over time for zebrafish larvae exposed to diverse initial concentration of STX. For the tactile response analysis, statistical data were tested using two-way mixed ANOVA. For expression analysis, all data were tested for normality and homogeneity of variance using the Shapiro-Wilk and Levene tests, respectively. Data were then analyses using a two-way ANOVA followed by the Tukey HSD post hoc multiple comparison test, using treatment (with saxitoxin and without saxitoxin) and sample time as independent variables. For image analysis, CTCF differences were statistically tested using the Student's t-test. The results were considered significant with a p-value <0.05. Graphs were plotted and analyses with RStudio.
3. Results
3.1. Saxitoxin (STX) exposure alters developmental trajectory of zebrafish embryos
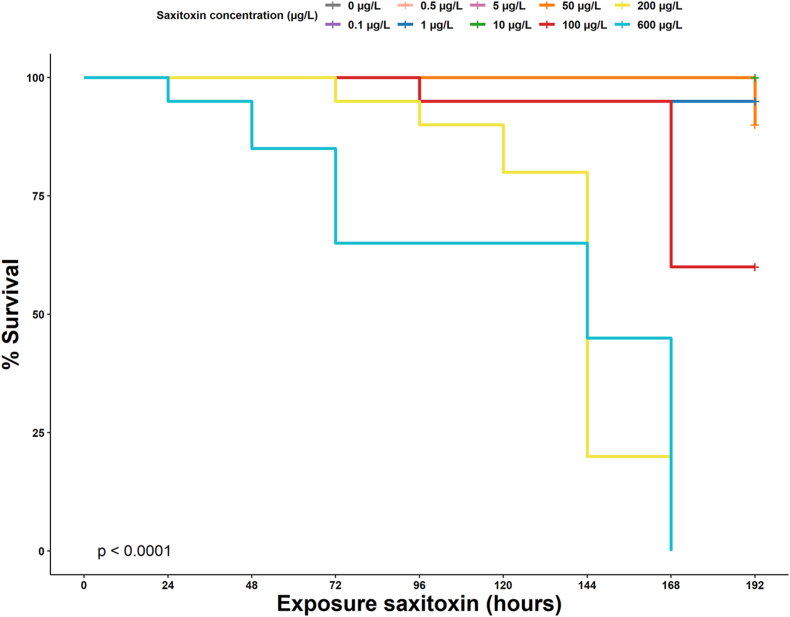
Our first aim was to determine the effect of sublethal doses of STX during subchronic exposure on the survival rates of zebrafish larvae. Typically initiated at 1.0 (indicative of 100% survival probability), the K-M curves observed a decremental pattern, correlating with the increase in event occurrence, specifically death, over time. A significant early divergence was noted in the K-M curves, indicative of considerable differences in survival probabilities among different STX concentration groups (Fig. 2). More specifically, an initial concentration of 600 μg/L gave rise to a marked decrease in survival rate from the initial 24 h of exposure, leading to a 0% survival rate by 168 h. At a lesser initial concentration of 200 μg/L, the survival probability maintained a higher rate until 72 h, but subsequently dropped to 0% by 168 h. Intriguingly, an initial concentration of 100 μg/L resulted in a more gradual decline in the survival rate, maintaining a cumulative value of more than 50% at 168 h. Further analysis using a logarithmic rank test validated these differences in survival probabilities among the concentration groups, showing a statistically significant deviation (p-value <0.05). Hence, our results suggest a direct correlation between STX concentration and survival probability in zebrafish larvae, with higher concentrations leading to a more rapid decline in survival rates, while lower concentrations promote better survival over time.
Fig. 2.
Kaplan-Meier survival curves generated in R of zebrafish larvae during seven days of STX exposure at different initial concentrations. The Y-axis represents the cumulative probability of survival for a given time and the X-axis represents the time in hours post-exposure to STX. The horizontal lines of the curve represent the duration of survival for that time interval, which ends with the occurrence of the death event. The vertical lines represent the change in cumulative probability as the curve progresses.
3.2. Sublethal concentrations of STX induce gradual sensorimotor deficits in early zebrafish development
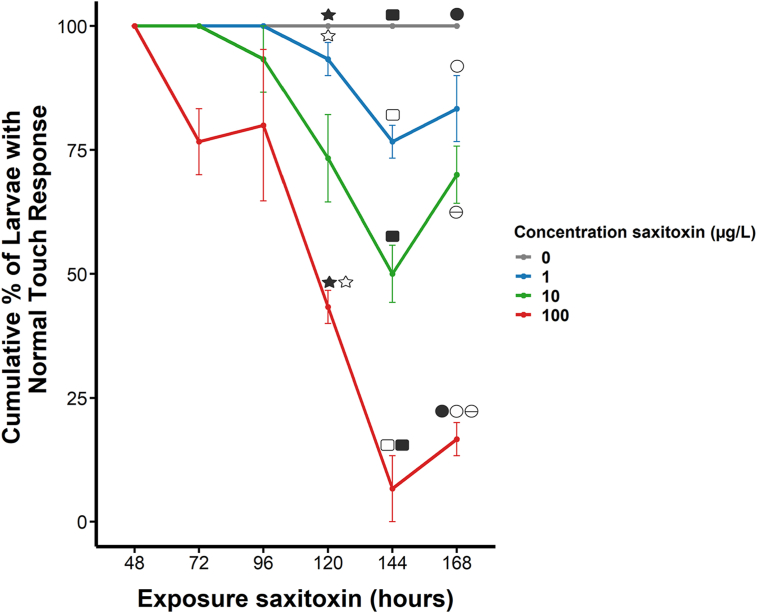
To unravel STX mode of action, we performed a critical assessment of sensorimotor response in zebrafish embryos as a pivotal indicator of behavioral responses. Our findings showed that the exposure to varying STX concentrations induced a significant neurotoxic effect, evidenced as a progressive tactile response decrement over time, as compared to the control untreated group that did not exhibit any significant change in tactile response. Specifically, our observations showed a remarkable 25% decrease in the proportion of embryos showing diminished tactile response following 48 h of exposure to an initial STX concentration of 100 μg/L. Conversely, exposure to lower STX concentrations (10 and 1 μg/L) did not show such effect until later time points, specifically at 96- and 120-h post-exposure, respectively. The 100 μg/L group showed a significant reduction in tactile response compared to the 0 and 1 μg/L STX groups at 120 h. By 144 h, all groups exposed to STX experienced their most severe decline in tactile response. The group exposed to 100 μg/L showed a significant decline compared to the other three groups (0, 1 and 10 μg/L STX). In turn, the group treated with 10 μg/L STX significantly decreased its tactile response compared to the control group (0 μg/L STX). Indeed, only around 7% of the larvae exposed to 100 μg/L STX demonstrated a normal response. However, a comparatively healthier response was noted in larvae exposed to 10 and 1 μg/L STX, displaying 50% and 70% normal tactile response, respectively. In contrast, the normal tactile response exhibited a slight trend of recovery at 168 h in the STX-treated groups, being significant between the highest concentration and all other STX concentrations (Fig. 3, see Table S2). The robustness of these results underscores a critical inference: low-dose STX exposure induces a progressive sensorimotor deficit in zebrafish embryos. Furthermore, the severity of this effect is strongly influenced by both the concentration and the duration of STX exposure.
Fig. 3.
Exposure to sublethal concentrations of saxitoxin decreases the response to tactile stimuli in zebrafish larvae during the first 7 days post-fertilisation. Each of the different treatments consisted of four replicates including 10 embryos. Exposure began when the embryos were in the germ-ring stage (6 hpf). The mechanosensory response is plotted as mean ± SD. Significant differences are plotted by symbols (▲, Δ, ■, □, ●, ○, ɵ) between different STX concentrations at the same point. Equal symbols indicate significant differences.
3.3. Subchronic STX exposure alters expression patterns of sodium channel alpha subunit isoforms
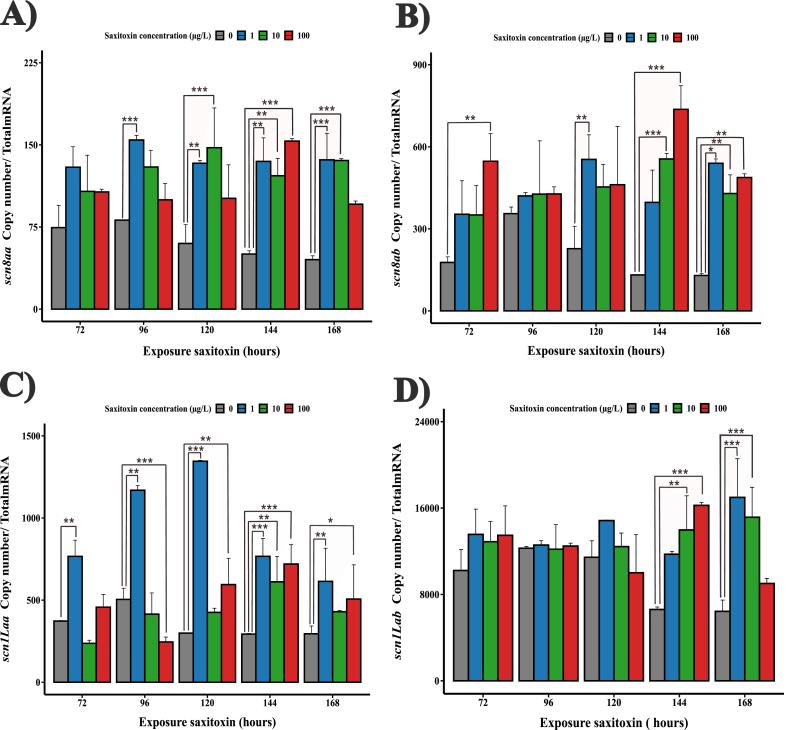
Based on the sensorimotor response decrease observed upon early exposure to STX, we next aimed to unravel potentially involved molecular mechanisms. We focused on the transcription of the pore-forming α subunit of VGSCs (Fig. 4). Following qRT-PCR, we determined the levels of the two sets of duplicated scna genes (scn1Laa & scn1Lab and scn8aa & scn8ab) that are expressed since early zebrafish embryogenesis [25]. Upon exposure to 1 μg/L of STX, we observed a notable increase in the transcript levels of scn8aa starting at 96 h, and scn1Laa from 72 h post-exposure. Additionally, scn8ab levels rose significantly by 120- and 168-h post-exposure, while scn1Lab only at 168 h, when compared to the control condition (Fig. 4A–D). At a higher STX concentration of 10 μg/L, there was a significant elevation in the expression of scn8aa and scn8ab mRNAs from 120- and 144-h post-exposure, respectively. Simultaneously, the transcript level of scn1Laa showed a marked increase at 144 h. Also, scn1Lab mRNA showed a significant increase at both a 144 and 168 h compared to the control (Fig. 4A–D). A STX concentration of 100 μg/L, the most elevated trialed, induced a pronounced overexpression of scn8aa and scn1Lab mRNAs at 144 h and scn8ab transcripts at 72, 144, and 168 h. Interestingly, scn1Laa mRNA expression level followed a unique trajectory, with an initial down-regulation at 96 h, followed by an increase from 120 to 168 h (Fig. 4A–D). With the exception of the scn1Lab mRNA profile, all STX-treated groups displayed significant up-regulation of scn8aa and scn1Laa transcript expression at 144 h¸ and scn8ab at 168 h, when compared to controls (Fig. 4A–D). Notably, marked differences were seen in the expression of scn8aa, scn8ab, scn1Laa and scn1Lab, when comparing the different STX treatments with each other during exposure (see Table S3) (Fig. 4A–D). These findings show that early exposure of zebrafish to STX induce transcriptional changes in genes associated with the VGSC system. The degree of these transcriptional shifts was found to depend on the concentration, time of exposure, and the specific isoform examined.
Fig. 4.
Absolute mRNA level of genes encoding the pore-forming α-subunit of VGSCs during early zebrafish development exposed to different initial concentrations of STX. A)scn8aa gen. B)scn8ab gen. C)scn1Laa gen. D)scn1Lab gen. Error bars indicate mean ± SD of three replicates (n = 10). Two-way ANOVA with Tukey HSD post-hoc test: p < 0.05*, p < 0.01**, p < 0.001***.
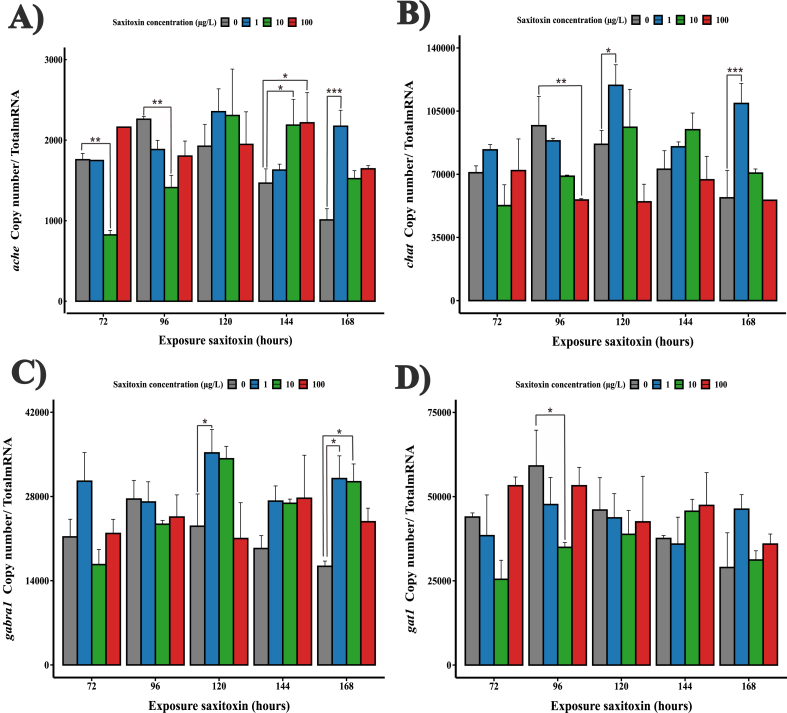
3.4. Low dose subchronic exposure to STX alters transcriptional activity in cholinergic and GABAergic pathways
To dissect how STX could modulate neurotransmission, we analyzed the gene expression profile associated with neurotransmission of cholinergic and gamma-aminobutyric acid (GABAergic) pathways during early zebrafish development. Intriguingly, our analysis revealed differential gene expression patterns responsive to various concentrations of STX. Upon low STX treatment (1 μg/L), we observed a significant increase in the expression of chat and gabra1 transcripts at 120 and 168 h, when compared to the control condition. Furthermore, ache mRNA levels were found to increase at 168 h, while a decrease was observed at 72 and 96 h, while gat1 expression remained largely unaltered (Fig. 5A–D). When we ramped up the STX treatment to 10 μg/L, the abundance of chat mRNA remained without significant differences comparable to control levels at all time points. Nonetheless, ache mRNA expression demonstrated a biphasic response with a down-regulation at 72- and 96-h of exposure and an up-regulation at 144 h (Fig. 5A and B). In contrast, gabra1 transcript showed significant overexpression at 168 h, while gat1 levels were significantly attenuated at 96 h (Fig. 5C and D). After exposure to the highest STX concentration (100 μg/L), chat mRNA abundance significantly declined at 96 h of exposure. Compared to the control, ache mRNA level increased at 144 h. Interestingly, the expression of gabra1 and gat1 transcripts did not exhibit significant changes compared to the control (Fig. 5A–D). Our study further revealed notable differences in the expression levels of chat, ache and gabra1 at the specific time point of 168 h in response to the initial 1 μg/L STX treatment, whereas gat1 levels were not significantly altered (Fig. 5A–D). The levels of chat, ache, gabra1 and gat1 mRNAs also showed significant differences between the different STX treatments during exposure (see Table S4). Collectively, these gene expression patterns suggest that sublethal concentrations of STX can differentially modulate the expression of pivotal genes of cholinergic and GABAergic pathways during early development. Interestingly, the extent and direction of these modulations seem to be highly dependent on the concentration of STX treatment.
Fig. 5.
Absolute mRNA level of genes related to cholinergic and GABAergic pathways during early development of zebrafish exposed to different initial concentrations of STX. A) Cholinergic pathway chat gen. B) Cholinergicpathway ache gen. C) GABAergic pathway gabra1gen. D) GABAergic pathway gat1gen. Error bars indicate mean ± SD of three replicates (n = 10). ANOVA with Tukey post-hoc test: p < 0.05*, p < 0.01**, p < 0.001***.
3.5. Low doses of STX suppress the levels of the neurotransmitter GABA, without affecting choline acetyltransferase (ChAT)
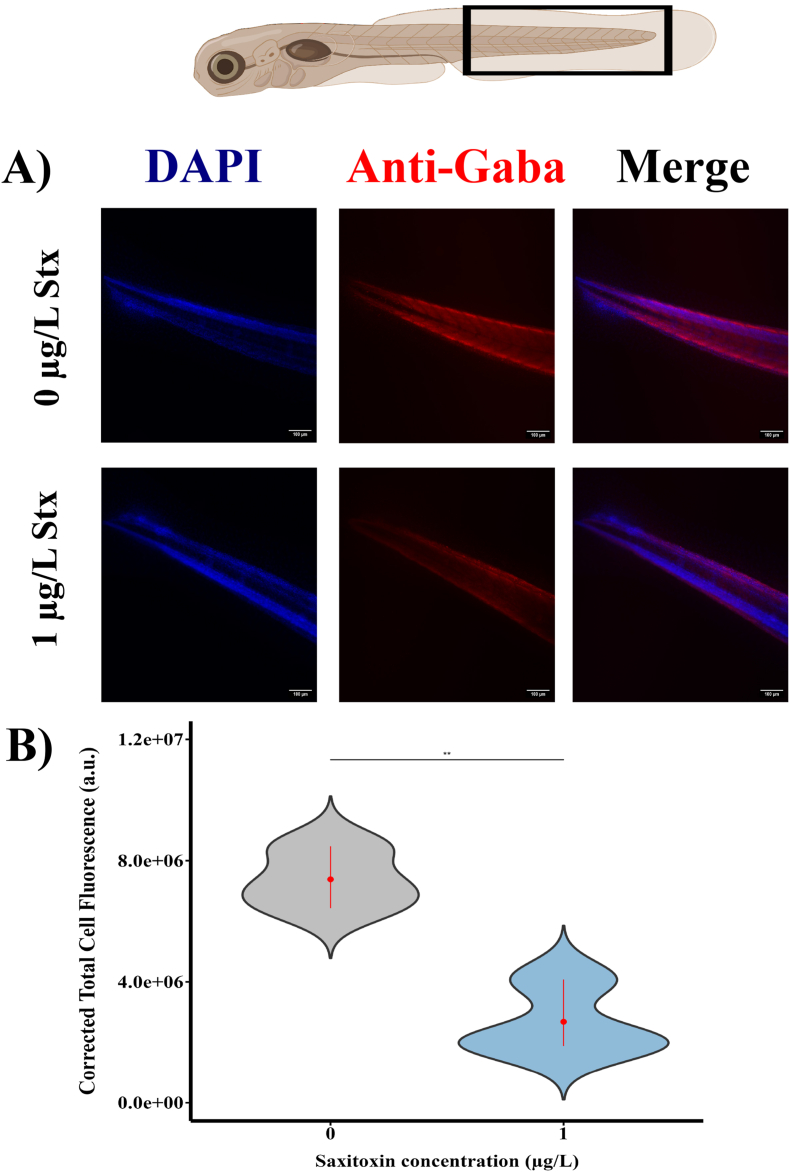
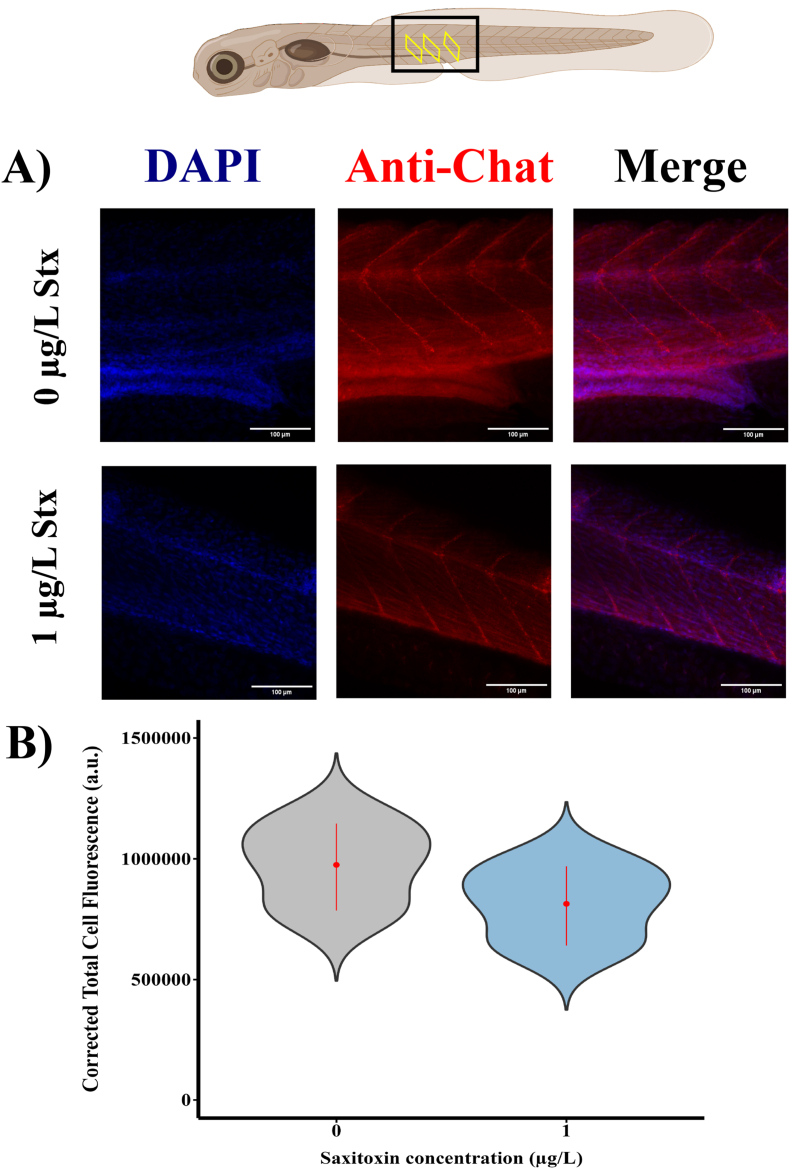
As a first approach to understand how low doses of STX might modulate the cholinergic and GABA pathways we evaluated the levels of the neurotransmitter GABA and ChAT in the trunk-caudal region of zebrafish larvae, the area where we assessed sensorimotor responses, through immunohistochemical analyses. Our findings show that zebrafish exposed to 1 μg/L of STX showed a significant reduction in fluorescence intensity of GABA levels at 120 h exposure (Fig. 6A and B). This finding is consistent with a potential reduction in GABAergic neurotransmission after STX exposure. In contrast, the fluorescence intensity associated with ChAT levels in the neuromuscular junctions did not display significant alterations (Fig. 7). This implies that ChAT activity, and thereby cholinergic neurotransmission, remained largely unaffected by the STX treatment at this dose and time point. Taken together, these results indicate that translational processes, especially those linked to the GABAergic pathway, might be affected by low-dose STX exposure during early stages of zebrafish development. This data provides a clearer picture of the specific impacts that STX has on essential neurotransmission pathways.
Fig. 6.
Exposure to 1 μg/L STX reduced the fluorescence intensity of the neurotransmitter GABA in the caudal trunk of zebrafish larvae at 120 h. A) Co-labelling of DAPI and GABA antibody in control and saxitoxin-treated larvae. B) Quantification of fluorescence in the trunk-caudal part between control and saxitoxin-treated individuals. Y-axis represents corrected total cell fluorescence (CTCF) in arbitrary units. Black rectangle indicates the study area. Student's t-test was used for statistical analysis (p < 0.05). Scale bar is 100 μm.
Fig. 7.
Exposure to 1 μg/L STX does not change the fluorescence intensity of chat fluorescence in neuromuscular junction myotomes at 120 h. A) Co-labelling of DAPI and Chat antibody in control and saxitoxin-treated larvae. B) Fluorescence quantification of three myotomes between control and saxitoxin-treated individuals. Y-axis represents corrected total cell fluorescence (CTCF) in arbitrary units. Black rectangle indicates the study area and yellow rectangles the selected myotomes. Student's t-test (p < 0.05) was used for statistical analysis. Scale bar is 50 μm.
4. Discussion
The presence of STX in aquatic habitats has profound ecological consequences, impacting a range of species from aquatic organisms to mammals, including humans. Strikingly, even concentrations that align with, or are below, the World Health Organisation's guidelines can be harmful. Extended exposure to these "safe" concentrations may result in adverse effects [30,31]. Hence, simply meeting STX guidelines is not necessarily safe, prompting concerns about environmental protection, public health, and conservation. Therefore, it becomes essential to be vigilant about the potential threats posed by STX to eventually implement robust countermeasures.
Various in vitro and in vivo studies have explored the toxicity of this potent neurotoxin, elucidating its impacts across physiological, morphological, and lethal parameters [12,32]. When assessing its toxicity in aquatic species, it becomes vital to account for both the concentration and the exposure duration of STX. Past research has evidenced that STX concentrations of 10 μg/L or higher can result in delayed hatching, anatomical defects, and increased mortality rates in zebrafish (Oberemm et al., 1999). Furthermore, zebrafish larvae exposed to even higher STX concentrations (>100 μg/L) over extended periods have demonstrated elevated mortality [16]. In this way, the present study aligns with these findings, as we were able to pinpoint sublethal concentrations of STX in an aquatic model.
In our study, zebrafish larvae treated with STX during early development exhibited transient sensorimotor anomalies, with the severity directly correlated to the concentration of STX exposure. Behavioral evaluations, frequently employed for toxicity assessments, reinforce the detrimental impacts of STX. We observed that zebrafish larvae displayed reduced responses to external stimuli, corresponding to the STX dose-dependent, with the most significant disruption occurring at 144 h across all experimental groups. This outcome aligns with the known pharmacodynamic properties of the toxin, which primarily induces paralysis, as emphasized by Lefebvre et al. [9]. Notably, a majority of the cases showed recovery in motor activity by the 6th or 7th day post-STX exposure. In a similar way, copepods exposed to STX also experienced a brief reduction in swimming velocity, followed by a return to their usual motility [33].
Our results underscore the dose-dependent effects of STX on sensorimotor functions in zebrafish larvae. At low concentrations, STX appears to elicit disturbances at the neuronal level, as evidenced by mechano-sensorimotor analyses. Intriguingly, STX exposure also led to alterations in the expression profiles of key genes within the VGSC, as well as cholinergic and GABAergic systems. Specifically, all four isoforms of the pore-forming α-subunit of VGSC (scn8aa/ab and scn1Laa/ab) exhibited were mostly up-regulated post-STX treatment. Such modulation in VGSC mRNA levels could stem from altered sodium ion dynamics within excitable cells, prompting neurons to recalibrate their channel density in response to tactile stimuli [34,35]. Scheinman et al. [36] reported analogous observations in rats, where an augmentation in the α-subunit of the VGSC channel correlated with an overproduction of sodium channels in the brain. This channel overproduction temporally aligned with the onset of behavioral alterations following STX exposure, as described by Sashihara et al. [34]. It is worth noting that exposure to phenytoin, a VGSC activity inhibitor, also induced up-regulation of VGSC mRNA levels [35] in a similar fashion than our results. In a parallel context, the exposure of Trigriopus japomicus to STX resulted in up-regulation in genes associated with neuronal action potential and pore formation. In agreement with our present findings, these results emphasize the pivotal role of VGSC alpha-subunits (scn8aa/ab and scn1Laa/ab) in mediating membrane depolarization during neuronal action potentials in response to a neurotoxin, such as STX. Thus, our results show a potential link between STX and neuronal dysregulation, possibly extending to neurological disorders [33].
We also explored the implications of STX exposure on cholinergic signalling in zebrafish embryos. Following STX exposure, we found a marked increase in ache mRNA levels. While a subtle trend towards chat up-regulation was evident at 10 μg/L STX, it did not achieve statistical significance. These findings could be associated to reduced responsiveness to external stimuli, potentially increasing VGSC availability and thereby boosting excitability. This, in turn, could drive increased acetylcholine synthesis via chat [37,38]. Yet, exposure to 100 μg/L STX, resulted in chat down-regulation, likely due to system saturation, which may impede optimal physiological feedback mechanisms.
The present results also show the zebrafish cholinergic dynamics following STX exposure, as we observed an upsurge in ache mRNA levels at both 144-h of exposure with 10 and 100 μg/L STX, while 1 μg/L only at 168-h of exposure. This trend aligns with literature denoting an imbalanced ache and chat dynamic, leading to augmented ACh neurotransmitter concentrations, presenting potential genotoxic effects [39]. Further evidence also underscores that extended STX exposure augments malondialdehyde levels in larvae and induce a lipid peroxidation. The lipid peroxidation triggering oxidative stress, which culminate in DNA damage in fish brains [18,32]. Our results suggest that trace STX exposure levels can perturb the cholinergic system, yielding genotoxic outcomes and spotlighting the susceptibility of aquatic species to such toxins. Similarly, our results also show the modulatory effects of STX on the GABAergic system. At 144 h, neither gabra1 nor gat1 mRNA expressions exhibited discernible shifts across the tested STX concentrations. Yet 1 and 10 μg/L STX exposures triggered a change on gene expression and translational dynamics of gabra1 at 120- and 168- hours, suggesting an involvement of STX in mediating inhibitory synaptic transmission [40]. Our data suggest that the neurological effects of STX, depending on concentration and exposure duration, involve a balance between excitatory and inhibitory molecular responses.
In summary, our study highlights that even minimal concentrations of STX can disrupt neural signaling in excitable cells, potentially through the inhibition of vital sodium channels crucial for action potential transmission. We have elucidated the implications of STX on VGSCs mRNA levels and the functioning of the cholinergic and GABAergic systems. As toxic algal blooms become more prevalent due to environmental shifts [8,41,42], the heightened levels of STX in ecosystems pose escalating health risks. Understanding the mechanisms of STX-dependent neurotoxicity during development is pivotal for effective risk assessment and management strategies. Further comprehensive functional analysis is indispensable for augmenting our current understanding. Among the potential limitations of the present study: a) Concentration-Response Relationship: While our study observed dose-dependent effects of STX on sensorimotor functions, a more detailed exploration of the concentration-response relationship could provide nuanced insights into the dynamics of toxicity; b) Mechanistic Elucidation: Although we highlight alterations in gene expression profiles, further functional studies are needed to fully understand the intricate molecular pathways involved in STX-induced neurotoxicity; c) Single-Species Model: Zebrafish, while valuable, represent one species. The extrapolation of these findings to broader ecosystems requires caution, and additional studies with diverse aquatic species would enhance the generalizability of our results. d) Short-Term Exposure: Our study primarily focused on early developmental stages. Assessing the long-term impacts of STX exposure, especially across different life stages, would provide a more comprehensive understanding of its effects over time; e) Environmental Complexity: While the study was controlled, it simplifies the complex environmental conditions in natural habitats. Real-world scenarios involve multiple interacting factors that could influence the toxicity of STX; f) Toxin Interactions: The study primarily focuses on STX, yet natural environments often harbor multiple toxins. Understanding potential interactions with other toxins is crucial for a comprehensive risk assessment. In conclusion, our study significantly contributes to understanding the neurotoxic effects of STX on aquatic life. However, addressing the complex challenges posed by harmful algal blooms and neurotoxins like STX requires continuous research. Despite the limitations, our findings underscore the urgency of comprehensive risk assessments and management strategies to safeguard both aquatic ecosystems and public health in the face of escalating environmental challenges.
Funding statement
This work was supported by the following grants: FONDECYT 1190627 awarded by CONICYT Chile to SB, and FONDECYT 1221213 to JPH. CONICYT-PCHA/Doctorado Nacional/2018–21181886 to NS, and CONICYT-PCHA/Doctorado Nacional/2019–21190538 to BC.
CRediT authorship contribution statement
Beatriz Carnicero: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ricardo Fuentes: Writing – review & editing, Writing – original draft, Methodology. Nataly Sanhueza: Methodology, Formal analysis. Humberto Mattos: Methodology. Constanza Aguirre-Campos: Methodology. David Contreras: Writing – review & editing, Methodology. Eduardo Troncoso: Writing – original draft, Methodology. Juan Pablo Henriquez: Writing – review & editing, Writing – original draft, Methodology. Sebastián Boltaña: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27874.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Suarez-Isla B.A. In: Marine and Freshwater Toxins. Gopalakrishnakone P., Haddad Jr V., Kem W., Tubaro A., Kim E., editors. Springer; Dordrecht: 2015. Saxitoxin and other paralytic toxins: toxicological profile. [DOI] [Google Scholar]
- 2.Oluwafemi F.T., Akinyemi A.A., Obuotor T.M., Idepefo T.G., Kolapo A.L. Assessment of saxitoxin in water and shellfishes within lagos lagoon, south western Nigeria. EC Microbiology. 2019;15(12):1–12. [Google Scholar]
- 3.Grachev M., Zubkov I., Tikhonova I., Ivacheva M., Kuzmin A., Sukhanova E., Sorokovikova E., Federova G., Suslova M., Netsvetayeva O., Eletkskaya E., Pogadaeva T., Smirnov V., Ivanov A., Shagun V., Minaev V., Belykh O. Extensive contamination of water with saxitoxin near the dam of the Irkutsk hydropower station reservoir (east siberia, Russia) Toxins. 2018;10(10):402. doi: 10.3390/toxins10100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledreux A., Thomazeau S., Catherine A., Duval C., Yéprémian C., Marie A., Bernard C. Evidence for saxitoxins production by the cyanobacterium Aphanizomenon gracile in a French recreational water body. Harmful Algae. 2010;10(1):88–97. doi: 10.1016/j.hal.2010.07.004. [DOI] [Google Scholar]
- 5.Ferreira F.M.B., Soler J.M.F., Fidalgo M.L., Fernández-Vila P. PSP toxins from Aphanizomenon flos-aquae (cyanobacteria) collected in the Crestuma-Lever reservoir (Douro river, northern Portugal) Toxicon. 2001;39:757–761. doi: 10.1016/s0041-0101(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 6.Hallegraeff G.M., Steffensen D.A., Wetherbee R. Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J. Plankton Res. 1988;10(3):533–541. doi: 10.1093/plankt/10.3.533. [DOI] [Google Scholar]
- 7.Testai E., Scardala S., Vichi S., Buratti F.M., Funari E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016;46(5):385–419. doi: 10.3109/10408444.2015.1137865. [DOI] [PubMed] [Google Scholar]
- 8.Starr M., Lair S., Michaud S., Scarratt M., Quilliam M., Lefaivre D., Robert M., Wotherspoon A., Michaud R., Ménard N., Sauvé G., Lessard S., Béland P., Measures L. Multispecies mass mortality of marine fauna linked to a toxic dinoflagellate bloom. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0176299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre K.A., Trainer V.L., Scholz N.L. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat. Toxicol. (Amst.) 2004;66(2):159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre K.A., Elder N.E., Hershberger P.K., Trainer V.L., Stehr C.M., Scholz N.L. Dissolved saxitoxin causes transient inhibition of sensorimotor function in larval Pacific herring (Clupea harengus pallasi) Mar. Biol. 2005;147:1393–1402. doi: 10.1007/s00227-005-0048-8. [DOI] [Google Scholar]
- 11.Bakke M.J., Horsberg T.E. Effects of algal-produced neurotoxins on metabolic activity in telencephalon, optic tectum and cerebellum of Atlantic salmon (Salmo salar) Aquat. Toxicol. (Amst.) 2007;85(2):96–103. doi: 10.1016/j.aquatox.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Tian L., Cheng J., Chen X., Cheng S.H., Mak Y.L., Lam P.K., Chan L.L., Wang M. Early developmental toxicity of saxitoxin on medaka (Oryzias melastigma) embryos. Toxicon: official journal of the International Society on Toxinology. 2014;77:16–25. doi: 10.1016/j.toxicon.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Salierno J.D., Kane A.S. Brevetoxin and saxitoxin alter shoaling dynamics in mummichog, Fundulus heteroclitus. J. Exp. Mar. Biol. Ecol. 2019;520 [Google Scholar]
- 14.Landsberg J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002;10(2):113–390. [Google Scholar]
- 15.Bakke M.J., Hustoft H.K., Horsberg T.E. Subclinical effects of saxitoxin and domoic acid on aggressive behaviour and monoaminergic turnover in rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. (Amst.) 2010;99(1):1–9. doi: 10.1016/j.aquatox.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Haque M.N., Nam S.E., Han Y.S., Park H.S., Rhee J.S. Chronic exposure to sublethal concentrations of saxitoxin reduces antioxidant activity and immunity in zebrafish but does not affect reproductive parameters. Aquatic toxicology (Amsterdam, Netherlands. 2022;243 doi: 10.1016/j.aquatox.2021.106070. [DOI] [PubMed] [Google Scholar]
- 17.Costa P.R., Pereira P., Guilherme S., Barata M., Nicolau L., Santos M.A., Pacheco M., Pousão-Ferreira P. Biotransformation modulation and genotoxicity in white seabream upon exposure to paralytic shellfish toxins produced by Gymnodinium catenatum. Aquat. Toxicol. 2012;106–107:42–47. doi: 10.1016/j.aquatox.2011.08.023. Amsterdam, Netherlands. [DOI] [PubMed] [Google Scholar]
- 18.da Silva C.A., de Morais E.C., Costa M.D., Ribas J.L., Guiloski I.C., Ramsdorf W.A., Zanata S.M., Cestari M.M., Ribeiro C.A., Magalhães V.F., Trudeau V.L., de Assis H.C. Saxitoxins induce cytotoxicity, genotoxicity and oxidative stress in teleost neurons in vitro. Toxicon: official journal of the International Society on Toxinology. 2014;86:8–15. doi: 10.1016/j.toxicon.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Catterall W.A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 20.Strong M., Chandy K.G., Gutman G.A. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol. Biol. Evol. 1993;10(1):221–242. doi: 10.1093/oxfordjournals.molbev.a039986. [DOI] [PubMed] [Google Scholar]
- 21.Hinić-Frlog S. In: Introductory Animal Physiology. Hanley J., Adbalahad N., Laughton S., editors. 2019. Electrical signals. [Google Scholar]
- 22.Hall S., Strichartz G., Moczydlowski E., Ravindran A., Reichardt P.B. In: Hall S., Strichartz G., editors. vol. 418. American Chemical Society; New York, NY, USA: 1990. The saxitoxins—sources, chemistry, and pharmacology; pp. 29–65. (Marine Toxins—Origin, Structure, and Molecular Pharmacology). ISBN 0097-6156. [Google Scholar]
- 23.Kao C.Y., Walker S.E. Active groups of saxitoxin and tetrodotoxin as deduced from actions of saxitoxin analogues on frog muscle and squid axon. J. Physiol. 1982;323:619–637. doi: 10.1113/jphysiol.1982.sp014095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cusick K.D., Sayler G.S. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs. 2013;11(4):991–1018. doi: 10.3390/md11040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak A.E., Taylor A.D., Pineda R.H., Lasda E.L., Wright M.A., Ribera A.B. Embryonic and larval expression of zebrafish voltage-gated sodium channel alpha-subunit genes. Dev. Dynam.: an official publication of the American Association of Anatomists. 2006;235(7):1962–1973. doi: 10.1002/dvdy.20811. [DOI] [PubMed] [Google Scholar]
- 26.Goldin A.L. Diversity of mammalian voltage-gated sodium channels. Ann. N. Y. Acad. Sci. 1999;868:38–50. doi: 10.1111/j.1749-6632.1999.tb11272.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dynam.: an official publication of the American Association of Anatomists. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 28.Rubio D.P., Roa L.G., Soto D.A., Velasquez F.J., Gregorcic N.A., Soto J.A., Martinez M.C., Kalergis A.M., Vasquez A.E. Purification and characterization of saxitoxin from Mytilus chilensis of southern Chile. Toxicon: official journal of the International Society on Toxinology. 2015;108:147–153. doi: 10.1016/j.toxicon.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 29.OECD (2019) OECD Publishing; Paris: 2019. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2. [DOI] [Google Scholar]
- 30.O'Neill K., Musgrave I.F., Humpage A. Extended low-dose exposure to saxitoxin inhibits neurite outgrowth in model neuronal cells. Basic Clin. Pharmacol. Toxicol. 2017;120(4):390–397. doi: 10.1111/bcpt.12701. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q., Chen X., Liu W., Li S., Zhou Y., Yang X., Liu J. Effects of long-term low dose saxitoxin exposure on nerve damage in mice. Aging. 2021;13(13):17211–17226. doi: 10.18632/aging.203199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G., Jia Z., Wang L., Hu T. Effect of acute exposure of saxitoxin on development of zebrafish embryos (Danio rerio) Environ. Res. 2020;185 doi: 10.1016/j.envres.2020.109432. [DOI] [PubMed] [Google Scholar]
- 33.Kang H.M., Lee J., Lee Y.J., Park Y., Lee E., Shin A.Y., Han J., Lee H.S., Lee J.S., Lee K.W. Transcriptional and toxic responses to saxitoxin exposure in the marine copepod Tigriopus japonicus. Chemosphere. 2022;309(Pt 1) doi: 10.1016/j.chemosphere.2022.136464. [DOI] [PubMed] [Google Scholar]
- 34.Sashihara S., Yanagihara N., Kobayashi H., Izumi F., Tsuji S., Murai Y., Mita T. Overproduction of voltage-dependent Na+ channels in the developing brain of genetically seizure-susceptible E1 mice. Neuroscience. 1992;48(2):285–291. doi: 10.1016/0306-4522(92)90490-s. [DOI] [PubMed] [Google Scholar]
- 35.Sashihara S., Yanagihara N., Izumi F., Murai Y., Mita T. Differential up-regulation of voltage-dependent Na+ channels induced by phenytoin in brains of genetically seizure-susceptible (E1) and control (ddY) mice. Neuroscience. 1994;62(3):803–811. doi: 10.1016/0306-4522(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 36.Scheinman R.I., Auld V.J., Goldin A.L., Davidson N., Dunn R.J., Catterall W.A. Developmental regulation of sodium channel expression in the rat forebrain. J. Biol. Chem. 1989;264(18):10660–10666. [PubMed] [Google Scholar]
- 37.de Medeiros L.M., De Bastiani M.A., Rico E.P., Schonhofen P., Pfaffenseller B., Wollenhaupt-Aguiar B., Grun L., Barbé-Tuana F., Zimmer E.R., Castro M.A.A., Parsons R.B., Klamt F. Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for alzheimer's disease studies. Mol. Neurobiol. 2019;56(11):7355–7367. doi: 10.1007/s12035-019-1605-3. [DOI] [PubMed] [Google Scholar]
- 38.Jiang N., Ding J., Liu J., Sun X., Zhang Z., Mo Z., Li X., Yin H., Tang W., Xie S.S. Novel chromanone-dithiocarbamate hybrids as multifunctional AChE inhibitors with β-amyloid anti-aggregation properties for the treatment of Alzheimer's disease. Bioorg. Chem. 2019;89 doi: 10.1016/j.bioorg.2019.103027. [DOI] [PubMed] [Google Scholar]
- 39.Constante J.S., Khateeb J.E.A., Souza A.P., Conter F.U., Lehmann M., Yunes J.S., Dihl R.R. In vitro and in silico assessment of cytotoxicity and chromosome instability induced by saxitoxin in human derived neural cell line. An. Acad. Bras. Cienc. 2022;94(suppl 4) doi: 10.1590/0001-3765202220220029. [DOI] [PubMed] [Google Scholar]
- 40.Saint-Amant L., Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J. Neurosci.: the official journal of the Society for Neuroscience. 2000;20(11):3964–3972. doi: 10.1523/JNEUROSCI.20-11-03964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roggatz C.C., Fletcher N., Benoit D.M., Algar A.C., Doroff A., Wright B., Hardege J.D. Saxitoxin and tetrodotoxin bioavailability increases in future oceans. Nat. Clim. Change. 2019;9(11):840–844. doi: 10.1038/s41558-019-0589-3. [DOI] [Google Scholar]
- 42.Gobler C.J. Climate change and harmful algal blooms: insights and perspective. Harmful Algae. 2020;91 doi: 10.1016/j.hal.2019.101731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.