Abstract
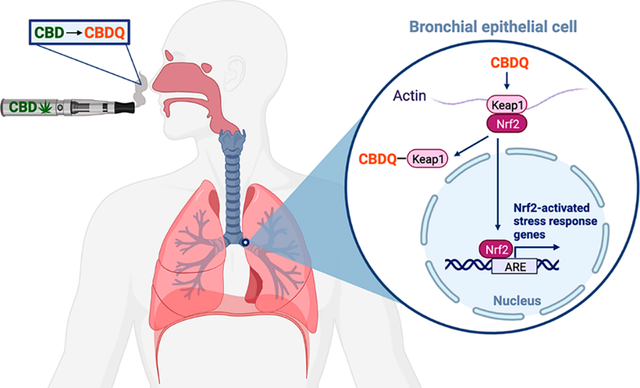
Cannabidiol (CBD) vaping products have become widely available in the U.S. since their legalization in 2018. However, little is known about their respiratory health effects. Here we show that aerosolization of commercial CBD vaping products generates a reactive CBD quinone (CBDQ) which forms adducts with protein cysteine residues. Using click chemistry and a novel in vitro vaping product exposure system (VaPES), we further demonstrate that CBDQ forms adducts with human bronchial epithelial cell proteins including Keap1 and activates KEAP1-Nrf2 stress response pathway genes. These results suggest that vaping CBD alters protein function and induces cellular stress pathways in the lung.
Graphical Abstract
Vaping products containing cannabidiol (CBD) (Figure 1A), a hemp-derived nonpsychoactive cannabinoid, have grown in popularity since they were legalized by the 2018 Farm Bill.1 In a 2019 survey (n = 30,288), 26.1% of U.S. adults reported CBD use in the past 12 months and within that group 18.9% used CBD vaping oils.2 Use of CBD vaping products can be attributed to a wide variety of claims about potential therapeutic effects including help with pain, inflammation, insomnia, anxiety, and depression.2–4 Most CBD vaping products currently on the market can be categorized into either oil distillates (700–1200 mg/mL) or juices (15–50 mg/mL). While oils are usually sold in prefilled cartridges for use in pen-type devices or in disposable vapes, juices can be added to any refillable vaping device including high-heat box mods. CBD vaping products are currently unregulated in the U.S., and measured product concentrations commonly vary ±20% from their labeled values.5 Additionally, analysis of CBD vaping products has shown that they can contain synthetic cannabinoids, Δ9-tetrahydrocannabinol (Δ9-THC) exceeding the legal limit, heavy metals, large amounts of residual solvents, and flavoring chemicals.5–7
Figure 1.
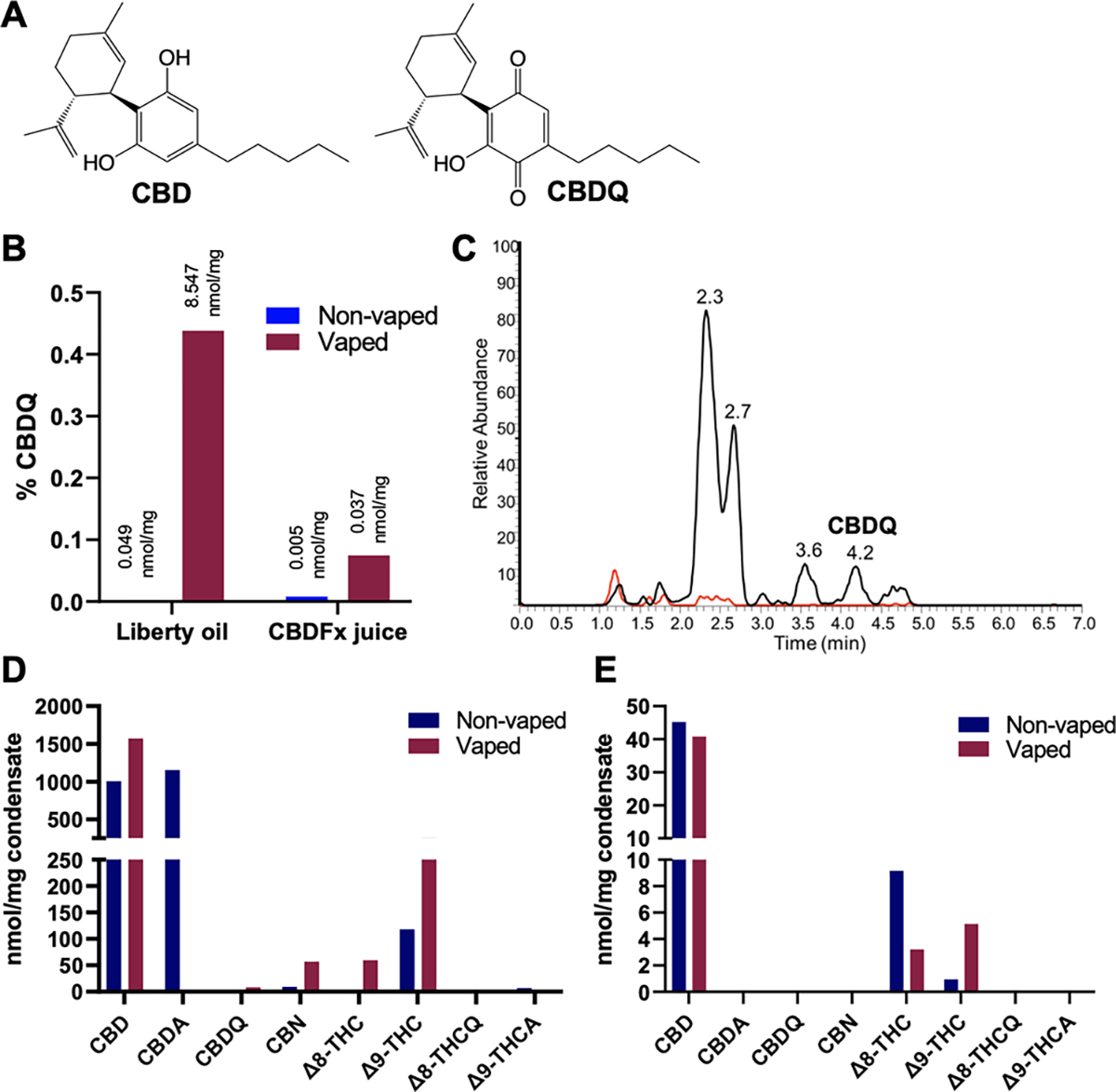
Vaping-induced oxidation of commercial CBD vaping oil (Liberty, Gorilla Glue, 750 mg/mL) and juice (CBDFx, strawberry kiwi, 16.67 mg/mL) to CBDQ and other cannabinoids. (A) Structures of CBD and CBDQ. (B) Generation of CBDQ after vaping; shown as percent CBDQ per total measured cannabinoids of interest (see also Figure S3A). (C) LC chromatograms of CBD oil before (red) and after (black) vaping showing CBDQ formation. Composition of other cannabinoids before and after vaping for (D) Liberty CBD oil (see also Figure S3B) and (E) CBDFx juice.
There is an urgent need to understand the effects of vaping CBD, as 16% of patients within the e-cigarette, or vaping, product use-associated lung injury (EVALI) crisis used CBD vaping products.8 The EVALI outbreak initially included at least 2807 cases and 68 deaths and cases continue to occur.9,10 Despite their association with EVALI as well as the high cannabinoid concentrations and potentially harmful constituents in CBD vaping products, research on the respiratory health effects of vaping CBD has so far been limited. Within the few studies that have evaluated CBD in the context of inhalational exposures, it has been shown that aerosolized CBD induced cell death, pro-inflammatory responses, and reactive oxygen species generation in bronchial epithelial cell lines.11,12 Proteomic analysis has also shown increases in markers of airway epithelial toxicity following CBD aerosol exposure.13 However, the mechanism behind the adverse effects of vaped CBD on respiratory cell function is not clear.
To investigate potential mechanisms of lung injury induced by aerosolized CBD, we explored whether CBD oxidizes to reactive species during vaping. We hypothesized that, due to the oxidation potential of CBD (1.2 V) and the high heat of vaping devices, CBD oxidizes to CBD quinone (CBDQ, also known as HU-331) (Figure 1A) when vaped, which forms adducts with thiol residues through Michael addition, altering protein function. CBDQ is a known oxidation product of CBD that can be formed by reaction with potassium hydroxide (the Beam test) and other oxidizing reagents.14–16 Recently, CBDQ has also been detected in CBD vaping products after long-term storage.17 However, the amount of CBDQ in commercial CBD products is not well characterized and the potential of vaping to promote oxidation of CBD to CBDQ is unknown.
Previously, CBDQ was investigated as a potential anticancer therapeutic and was found to induce cytotoxicity, apoptosis in certain cells types, liver toxicity, and inhibit topoisomerase II and angiogenesis.14,18–20 Based on these findings, CBDQ has the potential to be harmful to the lung epithelium when inhaled and may be responsible for the adverse effects observed from vaping CBD. To explore conversion to CBDQ as a mechanism of toxicity of vaping CBD, we first measured CBDQ and other cannabinoids in commercial CBD vaped condensates and then used alkynyl-tagged CBD and click chemistry to explore the potential for CBDQ and vaped CBD products to form adducts with cellular proteins and modify their function.
To determine whether vaping CBD produces CBDQ, we measured CBDQ and other select cannabinoids in nonvaped and vaped commercial CBD pure distillate oil (Liberty, Gorilla Glue cartridge, 750 mg/mL) and commercial CBD juice (CBDFx, strawberry kiwi, 16.67 mg/mL, 50/50 propylene glycol/vegetable glycerin (PG/VG) base) (Figure 1). Vaped condensates were generated with a CCell Silo battery vaping device (500 mAh, ~3.7 V) and a peristaltic pump (2 L/min) using methods adapted from those previously described.21 A refillable CCell glass cartridge was used for the CBDFx juice. All vaped samples were generated by taking 20 5-s puffs at 1 min intervals, and nonvaped samples were collected from cartridges or liquid that had never been used in a vaping device or otherwise heated. CBDQ, CBD, and other cannabinoids of interest (CBDA, CBN, Δ8-THC, Δ9-THC, Δ8-THCQ, and Δ9-THCA) (Figures S1 and S2) were quantified using LC/MS-MS (Acquity UPLC system equipped with an Agilent Poroshell EC-C18 column) and d9-CBD as a standard.
We found that CBDQ was present in all commercial CBD products and significantly increased after vaping for both the oil (0.049 to 8.547 nmol/mg) and the juice (0.005 to 0.037 nmol/mg) (Figure 1B,C). Based on the estimated density of each product (0.93 g/mL oil; 1.15 g/mL juice), the concentration of CBDQ can be converted to 7.95 mM in the oil and 42.6 μM in the juice after vaping. For the vaping oil (Figure 1D), the amount of CBD and cannabidiolic acid (CBDA) together was 2158 nmol/mg or approximately 678 mg/mL, compared to the advertised concentration of 750 mg/mL. Hemp can be high in CBDA, which is rapidly decarboxylated to CBD and other cannabinoids when heated.22 The amount of CBD measured in the vaping juice (Figure 1E) (45.2 nmol/mg or 16.3 mg/mL) closely matched the advertised concentration of 16.7 mg/mL and slightly decreased after vaping. Interestingly, for both the oil and the juice, we observed that Δ9-THC increased after vaping. We also found that vaping increased the amount of cannabinol (CBN) and Δ8-tetrahydrocannabinol (Δ8-THC) in the oil, while it decreased the amount of Δ8-THC in the juice.
After determining that CBDQ is present in commercial CBD products and increases after vaping, we next investigated whether CBDQ can form protein adducts. Based on the structure of CBDQ, we hypothesized it could form adducts with cysteine residues through Michael addition (Figure 2A). To test this, we combined either synthetic CBDQ (Scheme S1) or vaped commercial CBD oil (Liberty, Gorilla Glue, 750 mg/mL) with a cysteine-containing model peptide TpepKC (AcAVAGKCAGAR) for 2 h at room temperature. We found that both synthetic CBDQ (0.1 mM) (Figure 2B) and vaped CBD oil condensate (Figure 2C) formed adducts with the model peptide exclusively on the cysteine residue (RT: 5.8; m/z 637.0). Although no unmodified peptide was found after reaction with synthetic CBDQ, some unmodified peptide remained after reaction with vaped CBD oil.
Figure 2.
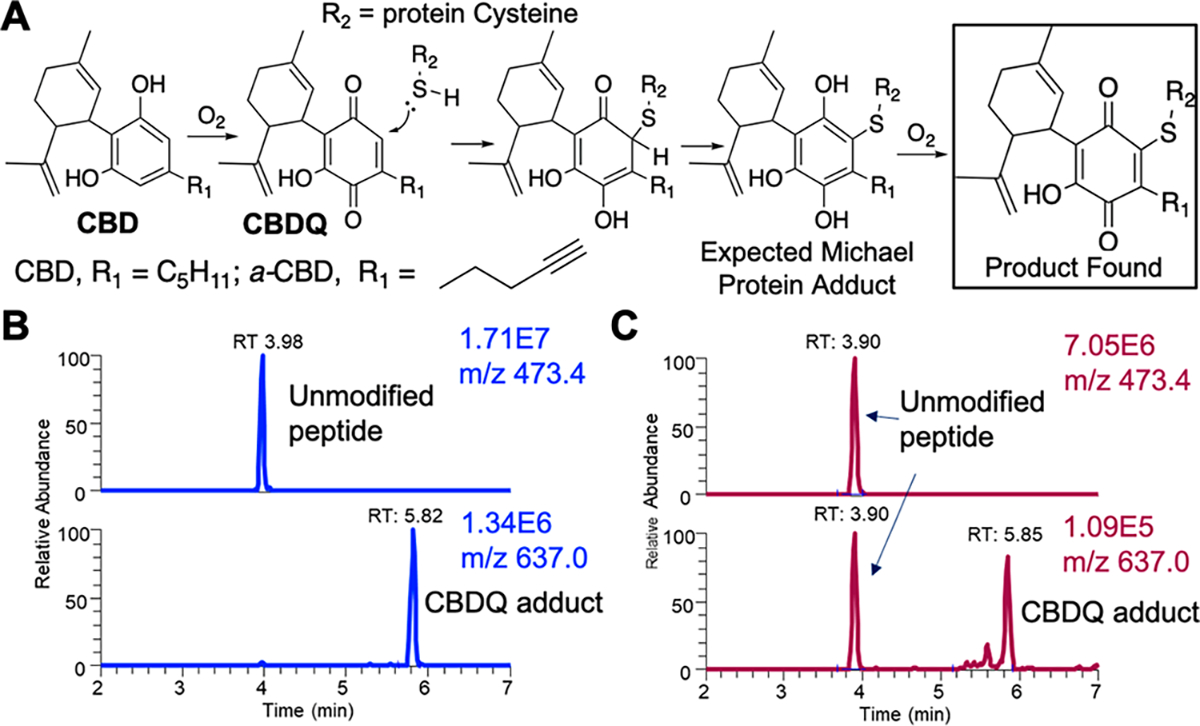
CBDQ forms adducts with cysteine residues. (A) Scheme showing oxidation of CBD and formation of CBDQ-cystine adduct. Spectra of (B) synthetic CBDQ and (C) vaped commercial CBD oil (Liberty, Gorilla Glue, 750 mg/mL) reacted with the cysteine-containing model peptide TpepKC (AcAVAGKCAGAR). For both, adducts formed between CBDQ and the Cys residue exclusively based on b and y ion assignment (Figure S5).
We next explored whether vaping CBD leads to covalent modification of cysteine-containing proteins in vitro and the potential for these modifications to alter protein function. To do this, we first synthesized alkynyl-tagged CBD and CBDQ (a-CBD and a-CBDQ) (Figure 2A) using previously described chemistry23 (Scheme S2). To determine whether pure CBDQ forms adducts with proteins, we exposed a human bronchial epithelial cell line (16HBEs) to synthetic a-CBD or a-CBDQ for 4 h at a range of concentrations (2–35 μM). Fully differentiated primary human bronchial epithelial cells (hBECs; n = 1 donor) grown at air liquid interface were then exposed to vaped lab-made a-CBD juice (33.3 mg/mL a-CBD in 67/33 PG/VG) using the UNC vaping product exposure system (VaPES). VaPES is a gravimetric sedimentation system developed by our group which can be used in a standard laboratory incubator under physiological conditions and provides even deposition that is sufficient for dose–response studies (Figure S4). For these experiments, VaPES was fitted with a JUUL device and refillable pod, and hBECs were exposed to 20 or 40 5-s puffs of lab-made a-CBD juice, each followed by 10 min rests to allow for complete aerosol sedimentation. hBECs exposed to vaped a-CBD were compared to those exposed apically to nonvaped a-CBD (10 μM) in PG/VG and media or a PG/VG in media vehicle control.
Following exposures, cell lysates were reacted with a photocleavable azido biotin reagent under “click” cycloaddition conditions which resulted in the biotinylation of any protein covalently bound to a-CBD derived electrophiles. Lysates were then captured with streptavidin beads, photoreleased, and probed by Western blot. For this study, we focused on targeted analysis of a key protein of interest KEAP1. We chose KEAP1 as an initial target as it is known to have modifiable cysteine residues and is a negative regulator of the KEAP1-Nrf2 stress response pathway, a main cellular mechanism for managing electrophilic and oxidative stress. We found that synthetic a-CBDQ, but not a-CBD, applied directly to cells forms adducts with KEAP1 in a dose-dependent manner starting at 17 μM (Figure 3A,B). Additionally, when vaped, a-CBD formed more adducts with hBEC proteins compared to nonvaped a-CBD or the PG/VG vehicle control (Figure 3C). Targeted analysis of hBEC lysates further revealed that KEAP1 was adducted after 20 and 40 puffs of a-CBD, but not in the nonvaped a-CBD or vehicle control (Figure 3D). Adduction of KEAP1 suggested the KEAP1-Nrf2 stress response pathway may be activated.
Figure 3.
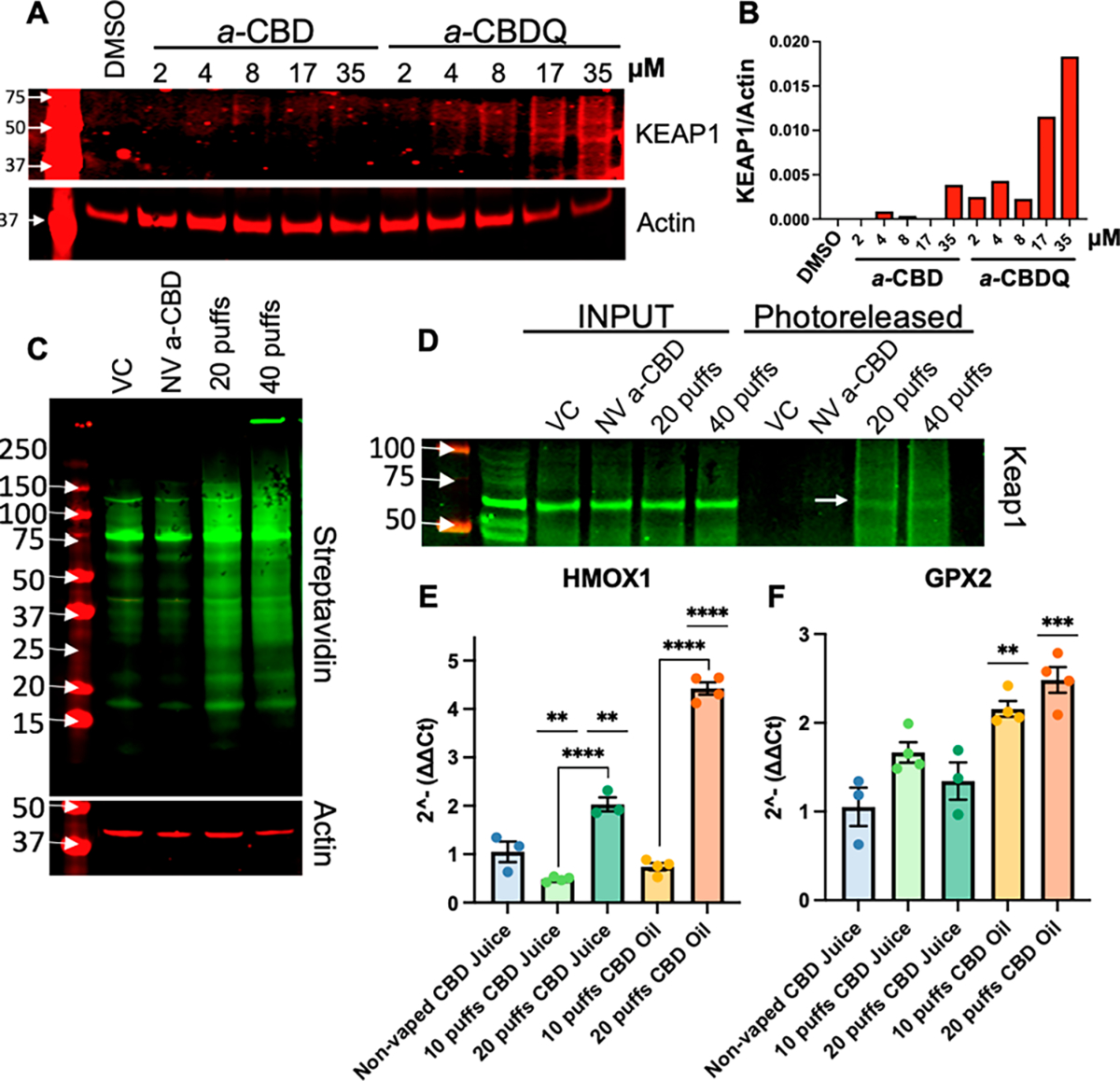
CBDQ and vaped CBD juice form adducts with KEAP1 and activate KEAP1-Nrf2 pathway genes. (A) Western blot and (B) densitometry analysis showing adduction of KEAP1 in 16HBEs incubated with synthetic a-CBD or a-CBDQ for 4 h. (C) Streptavidin blot and (D) Western blot for KEAP1 in primary hBECs exposed apically to PG/VG (VC), nonvaped lab-made a-CBD in PG/VG (NV a-CBD), or vaped lab-made a-CBD juice for 20 or 40 5-s puffs using VaPES. Expression of (E) HMOX1 and (F) GPX2 in primary hBECs exposed to nonvaped CBD commercial juice (20 puff equivalent) or 10–20 puffs commercial CBD juice or oil with VaPES. * p < 0.05 vs nonvaped CBD juice unless otherwise noted (ANOVA with Tukey posthoc).
To confirm that the KEAP1-Nrf2 stress response pathway is activated by vaped CBD aerosols, we measured expression of two prototypic KEAP1-Nrf2 inducible genes, HMOX1 and GPX2, in hBECs (n = 3–4 donors/condition) exposed to commercial CBD oil (Liberty, Gorilla Glue cartridge, 750 mg/mL) and juice (CBDFx, strawberry kiwi, 16.7 mg/mL). VaPES and a CCell Silo battery device were used to take 10 or 20 5-s puffs followed by 10 min rests. Vaped samples were compared to a nonvaped CBD juice control which was a 20-puff equivalent dose based on VaPES deposition testing (Figure S4). We found that the 20-puff dose of CBD juice and oil significantly increased HMOX1 expression compared to nonvaped CBD juice control (Figure 3E). Additionally, both the 10 and 20-puff dose of CBD oil significantly increased GPX2 expression compared to nonvaped CBD juice (Figure 3F). Overall, these data indicated that vaped commercial CBD products activated the KEAP1-Nrf2 stress response pathway.
CBD and other cannabinoid vaping products have become increasingly popular in the U.S. in recent years, partially due to a range of claims about their therapeutic value. However, the respiratory health effects of vaping CBD are not well understood. In this study, we have confirmed that CBDQ is present in CBD vaping liquids17 and have further shown for the first time that the vaping process generates high levels of CBDQ, especially in concentrated vaping oils. We have also demonstrated that both CBDQ and vaped CBD form adducts with cysteine residues and KEAP1, activating KEAP1-Nrf2 stress response pathway genes. Based on the mechanism of action of other electrophiles, we propose that covalent modification of KEAP1 by CDBQ prevents ubiquitination of NRF2, allowing it to translocate into the nucleus where it functions as a transcription factor for a host of antioxidant and cytoprotective genes.24 These obtained data therefore indicate that the CBDQ present in vaped CBD acts as an electrophile and induces cellular stress pathways in the lung.
While we chose KEAP1 as an initial protein target, the reactivity of CBDQ and vaped CBD toward thiols indicates that other proteins with modifiable cystine residues may be adducted, potentially leading to the negative cellular effects previously observed from vaping CBD in vitro.11–13 Additionally, it is possible that other protein targets have higher reactivity with CBDQ at lower concentrations. Future work should therefore focus on identifying all CBDQ protein adducts in an unbiased manner and the biological responses elicited by these adducts. Also of note is that this study only examined one commercial oil and one commercial juice. However, the flavoring chemicals and other additives in commercial products such as metals, terpenes, and solvents have the potential to enhance oxidation of CBD. It will therefore be important to characterize how flavoring profiles and vaping product additives and contaminants alter CBDQ formation during the vaping process.
The results presented here indicate that vaping CBD produces a cytotoxic electrophile CBDQ that activates cellular stress pathways and has the potential to cause injury to the respiratory epithelium. These results are especially important in the context of EVALI which included patients who used CBD vaping products.8 Importantly, EVALI continues to occur, and there have been at least 92 additional cases documented since the CDC stopped reporting in 2020.9 Vitamin E acetate (VEA) was previously identified as a potential causative agent of EVALI.25,26 Recent studies have found that vaping VEA produces potentially harmful thermal degradation products including ketene, duroquinone, and carbonyls;27–29 however, vaping product constituents not derived from VEA may also play an important role in lung injury. Our findings demonstrate that CBDQ and other oxidation-derived cannabinoid quinones, such as those produced when vaping Δ9-THC and Δ8-THC, should be explored as alternative mechanisms for EVALI. Furthermore, this work highlights potential respiratory health risks for the many current users of CBD vaping products as well as the need to evaluate other unregulated CBD-derived vaping products that continue to emerge on the market including Δ10-THC, tetrahydrocannabivarin, THC acetate ester, and hexahydro-cannabinol.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Kevin Schichlein, Maxwell Conolly, and John Kurz for their work on VaPES and the UNC MLI Tissue Procurement and Cell Culture Core and Lisa Dailey for providing hBECs. Table of Contents artwork was created with BioRender.com.
Funding
This work was supported by a T32 Training grant (CAL; National Research Service Award T32 ES007126), a pilot grant from P30ES010126 (P.W.C.) from the National Institute of Environmental Health Sciences, and an administrative supplement to R01HL139369-S2 (I.J. and P.W.C.) from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- CBD
cannabidiol
- Δ9-THC
Δ9 tetrahydrocannabinol
- EVALI
e-cigarette, or vaping, product use-associated lung injury
- CBDQ
CBD quinone
- CBDA
cannabidiolic acid
- CBN
cannabinol
- Δ8-THC
Δ8-tetrahydrocannabinol
- Δ8-THCQ
Δ8-tetrahydrocannabinol quinone
- Δ9-THCA
Δ9-tetrahydrocannabinol carboxylic acid
- a-CBD
alkynyl-tagged CBD
- a-CBDQ
alkynyl-tagged CBD quinone
- PG/VG
propylene glycol/vegetable glycerin
- VaPES
UNC vaping product exposure system
Footnotes
Notes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.3c00038
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.3c00038.
Detailed experimental procedures; structures of compounds of interest; LC/MS-MS chromatogram of CBD and its oxidation products; scheme of synthesis of CBDQ and Δ8-THCQ; scheme of synthesis of a-CBD and a-CBDQ; additional chemical analysis of Liberty CBD oil; demographic data for hBEC lung donors; diagram and deposition data for the UNC Vaping Product Exposure System (VaPES); MS/MS TpepKC adducts (PDF)
Contributor Information
Charlotte A. Love, Curriculum in Toxicology and Environmental Medicine, Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Hye-Young H. Kim, Department of Chemistry and Vanderbilt Institute of Chemical Biology, Vanderbilt University, Nashville, Tennessee 37235, United States
Keri A. Tallman, Department of Chemistry and Vanderbilt Institute of Chemical Biology, Vanderbilt University, Nashville, Tennessee 37235, United States
Phillip W. Clapp, Curriculum in Toxicology and Environmental Medicine, Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Ned A. Porter, Department of Chemistry and Vanderbilt Institute of Chemical Biology, Vanderbilt University, Nashville, Tennessee 37235, United States
Ilona Jaspers, Curriculum in Toxicology and Environmental Medicine, Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
REFERENCES
- (1).Gammon DG; Gaber J; Lee YO CBD Products That Resemble Tobacco Products Enter Traditional Retail Outlets. Tob. Control 2021, 30, 237. [DOI] [PubMed] [Google Scholar]
- (2).Goodman S; Wadsworth E; Schauer G; Hammond D Use and Perceptions of Cannabidiol Products in Canada and in the United States. Cannabis Cannabinoid Res. 2022, 7 (3), 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wheeler M; Merten JW; Gordon BT; Hamadi H CBD (Cannabidiol) Product Attitudes, Knowledge, and Use Among Young Adults. Subst. Use Misuse 2020, 55 (7), 1138–1145. [DOI] [PubMed] [Google Scholar]
- (4).Bhamra SK; Desai A; Imani-Berendjestanki P; Horgan M The Emerging Role of Cannabidiol (CBD) Products; a Survey Exploring the Public’s Use and Perceptions of CBD. Phyther. Res 2021, 35 (10), 5734–5740. [DOI] [PubMed] [Google Scholar]
- (5).Gurley BJ; Murphy TP; Gul W; Walker LA; ElSohly M Content versus Label Claims in Cannabidiol (CBD)-Containing Products Obtained from Commercial Outlets in the State of Mississippi. J. Diet. Suppl. 2020, 17 (5), 599–607. [DOI] [PubMed] [Google Scholar]
- (6).Muthumalage T; Friedman MR; Mcgraw MD; Ginsberg G; Friedman AE; Rahman I Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 2020, Vol. 8, Page 25 2020, 8 (2), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liebling JP; Clarkson NJ; Gibbs BW; Yates AS; O’Sullivan SE An Analysis of Over-the-Counter Cannabidiol Products in the United Kingdom. Cannabis Cannabinoid Res. 2022, 7 (2), 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ellington S; Salvatore PP; Ko J; Danielson M; Kim L; Cyrus A; Wallace M; Board A; Krishnasamy V; King BA; Rose D; Jones CM; Pollack LA; Abbas A; Adebayo A; Atti S; Carter E; Chandra G; Eckhaus L; Fajardo G; Goyal S; Hallowell B; Hamilton J; Israel M; Li Z; Loretan C; Lynfield R; Melstrom P; Pomeroy M; Schrodt C; Soroka S; Thomas K; Wallace BM Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use–Associated Lung Injury — United States, August 2019–January 2020. MMWR. Morb. Mortal. Wkly. Rep 2020, 69 (2), 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rebuli ME; Rose JJ; Noël A; Croft DP; Benowitz NL; Cohen AH; Goniewicz ML; Larsen BT; Leigh N; McGraw MD; Melzer AC; Penn AL; Rahman I; Upson D; Crotty Alexander LE; Ewart G; Jaspers I; Jordt SE; Kligerman S; Loughlin CE; McConnell R; Neptune ER; Nguyen TB; Pinkerton KE; Witek TJ The E-Cigarette or Vaping Product Use-Associated Lung Injury Epidemic: Pathogenesis, Management, and Future Directions: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc 2023, 20 (1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Centers for Disease Control and Prevention. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed 2022-02-24).
- (11).Muthumalage T; Rahman I Cannabidiol Differentially Regulates Basal and LPS-Induced Inflammatory Responses in Macrophages, Lung Epithelial Cells, and Fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Leigh NJ; Goniewicz ML Acute Effect of Electronic Cigarette-Generated Aerosol From Flavored CBD-Containing Refill Solutions on Human Bronchial Epithelial Cells. Front. Physiol. 2020, 11, 592321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Reidel B; Abdelwahab S; Wrennall JA; Clapp P; Beers JL; Jackson KD; Tarran R; Kesimer M Vaping Additives Cannabinoid Oil and Vitamin E Acetate Adhere to and Damage the Human Airway Epithelium. J. Appl. Toxicol. 2022, DOI: 10.1002/jat.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Trac J; Keck JM; Deweese JE Cannabidiol Oxidation Product HU-331 Is a Potential Anticancer Cannabinoid-Quinone: A Narrative Review. J. Cannabis Res 2021, 3 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Caprioglio D; Mattoteia D; Pollastro F; Negri R; Lopatriello A; Chianese G; Minassi A; Collado JA; Munoz E; Taglialatela-Scafati O; Appendino G The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod 2020, 83, 1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Caprioglio D; Mattoteia D; Taglialatela-Scafati O; Muñoz E; Appendino G Cannabinoquinones: Synthesis and Biological Profile. Biomol. 2021, Vol. 11, Page 991 2021, 11 (7), 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Schwarzenberg A; Carpenter H; Wright C; Bayazeid O; Brokl M Characterizing the Degradation of Cannabidiol in an E-Liquid Formulation. Sci. Reports 2022 121 2022, 12 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kogan NM; Schlesinger M; Priel E; Rabinowitz R; Berenshtein E; Chevion M; Mechoulam R HU-331, a Novel Cannabinoid-Based Anticancer Topoisomerase II Inhibitor. Mol. Cancer Ther. 2007, 6 (1), 173–183. [DOI] [PubMed] [Google Scholar]
- (19).Peters M; Kogan NM HU-331: A Cannabinoid Quinone, with Uncommon Cytotoxic Properties and Low Toxicity. Expert Opinion on Investigational Drugs 2007, 16, 1405–1413. [DOI] [PubMed] [Google Scholar]
- (20).Kogan NM; Peters M; Mechoulam R Cannabinoid Quinones-A Review and Novel Observations. Molecules 2021, 26 (6), 1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Olmedo P; Navas-acien A; Hess C; Jarmul S; Rule A A Direct Method for E-Cigarette Aerosol Sample Collection. Environ. Res. 2016, 149, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Citti C; Pacchetti B; Vandelli MA; Forni F; Cannazza G Analysis of Cannabinoids in Commercial Hemp Seed Oil and Decarboxylation Kinetics Studies of Cannabidiolic Acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [DOI] [PubMed] [Google Scholar]
- (23).Gong X; Sun C; Abame A; Shi W; Xie Y; Xu W; Zhu F; Zhang Y; Shen J; Aisa HA Synthesis of CBD and Its Derivatives Bearing Various C4′-Side Chains with a Late-Stage Diversification Method. J. Org. Chem. 2020, 85, 2704. [DOI] [PubMed] [Google Scholar]
- (24).Baird L; Yamamoto M Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway 2020, DOI: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).US Food and Drug Administration. Lung Injuries Associated with Use of Vaping Products. https://www.fda.gov/news-events/public-health-focus/lung-injuries-associated-use-vaping-products (accessed 2021-03-27).
- (26).Blount BC; Karwowski MP; Shields PG; Morel-Espinosa M; Valentin-Blasini L; Gardner M; Braselton M; Brosius CR; Caron KT; Chambers D; Corstvet J; Cowan E; De Jesús VR; Espinosa P; Fernandez C; Holder C; Kuklenyik Z; Kusovschi JD; Newman C; Reis GB; Rees J; Reese C; Silva L; Seyler T; Song M-A; Sosnoff C; Spitzer CR; Tevis D; Wang L; Watson C; Wewers MD; Xia B; Heitkemper DT; Ghinai I; Layden J; Briss P; King BA; Delaney LJ; Jones CM; Baldwin GT; Patel A; Meaney-Delman D; Rose D; Krishnasamy V; Barr JR; Thomas J; Pirkle JL Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382 (8), 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu D; O’Shea DF Potential for Release of Pulmonary Toxic Ketene from Vaping Pyrolysis of Vitamin E Acetate. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (12), 6349–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Canchola A; Ahmed CMS; Chen K; Chen JY; Lin Y-H Formation of Redox-Active Duroquinone from Vaping of Vitamin E Acetate Contributes to Oxidative Lung Injury. Cite This Chem. Res. Toxicol 2022, 35, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Li Y; Dai J; Tran LN; Pinkerton KE; Spindel ER; Nguyen TB Vaping Aerosols from Vitamin e Acetate and Tetrahydrocannabinol Oil: Chemistry and Composition. Chem. Res. Toxicol. 2022, 35 (6), 1095–1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


