Abstract
Background
Cavernous malformation (CM) is a well-known cause of epilepsy. Although the location of the CM is usually consistent with the side of seizure onset, some reports have described discrepancies between results from scalp electroencephalography (EEG) and CM location. This study investigated the prevalence and features of patients showing false lateralization (FL). Particularly, we tested the hypothesis that patients showing FL were more likely to have CM in medial and deep areas of the brain than in other areas.
Methods
Patients diagnosed with CM-associated epilepsy in our institution between March 2009 and March 2023 were included in this retrospective analysis. We investigated the presence or absence of FL of interictal epileptiform discharges (IEDs) or ictal discharges against MRI findings or against the true focus as determined from surgical outcomes. We compared the FL group with the non-false-lateralization group (NFL group) to clarify features of CM-associated epilepsy patients showing FL.
Results
Thirty-two epilepsy patients with CM were analyzed. The frequency of FL to MRI was 10.3% for IEDs and 7.7% for ictal discharges, while the frequency of FL to true focus after removal surgery was 10.5% for IEDs and 7.7% for ictal discharges. Regarding the FL of IEDs against MRI findings, the percentage of medial and deep lesions was significantly higher in the FL group (3/3, 100%) than in the NFL group (6/26, 23.1%; p = 0.023). No significant differences in age, sex, seizure type, or size of the CM were seen between groups.
Conclusions
CM-associated epilepsy can also present with FL, particularly if the location of the CM is medial and deep. Caution may be needed in determining the area for resection based solely on scalp EEG findings.
Keywords: Cavernous malformation, Epilepsy, False lateralization, Electroencephalography
1. Introduction
Brain cavernous malformations (CMs) have been reported to cause CM-associated epilepsy in 10–70% of patients, of which 30–70% are cases of drug-resistant epilepsy. The rate of achieving freedom from seizures after epilepsy surgery in such cases is as high as 60–90% [[1], [2], [3]]. As a result, in cases of drug-resistant epilepsy in which CM has been identified, resection is recommended if the site can be resected without sequelae. Since performing resection surgery within a year after seizure onset or at a younger age has been described to induce better seizure outcomes, early surgery has recently been suggested more frequently [[2], [3], [4], [5]]. The perspective of preventing cerebral hemorrhage and a report that anti-seizure medication cannot prevent the psychosocial distress of CM-associated epilepsy may also support early surgery in CM [6].
Although the cerebral cortex near the CM is the epileptogenic lesion in most cases, some authors have documented cases in which scalp electroencephalography (EEG) showed seizure onset contralateral to the location of the CM [[7], [8], [9]]. As with other intractable epilepsies, comprehensive diagnosis of the focus from semiology, scalp EEG, positron emission tomography, single-photon emission computed tomography, and neuropsychological assessment is required before epilepsy surgery for CM-associated epilepsy. Since long-term video-EEG monitoring (LTVEM) is critical among these examinations [10], our institution usually performs preoperative LTVEM to confirm semiology and ictal EEG in patients with frequent seizures. However, we recently encountered a case of CM-associated epilepsy showing false lateralization (FL) of scalp LTVEM findings and cautioned against overconfidence in ictal EEG findings obtained from scalp electrodes [11].
Severe hippocampal sclerosis (burned-out hippocampus) and cortical atrophy have been documented as causes of FL from scalp EEG [12,13]. The mechanism of FL in these cases is considered to involve the amplitude of the ictal EEG being too small to identify. On the other hand, few reports have described FL in CM-associated epilepsy, and its frequency and characteristics remain unknown. This study retrospectively reviewed patients with CM-associated epilepsy in our institution and investigated the percentage and features of patients showing FL. In particular, we verified the hypothesis that patients exhibiting FL would be more likely to have CM in medial and deep regions of the brain than in other regions.
2. Patients and methods
2.1. Study design and participants
The present study was a single-center retrospective investigation carried out in Japan. The Institutional Review Board of Seirei Hamamatsu General Hospital approved the study protocol (study no. 4224). As the study involved minimal risk and used an anonymized retrospective design, the need to obtain written consent was waived and disclosure was provided through opt-out.
We reviewed patients diagnosed with CM-associated epilepsy in our institution between March 2009 and March 2023. The diagnosis of CM was established by magnetic resonance imaging (MRI) in all patients with reference to the criteria published by Al-Shahi Salman et al. [14]. We diagnosed epilepsy patients with CM as having CM-associated epilepsy. We excluded those patients who had not undergone scalp EEG, whose epileptogenic lesions remained unknown, who had bilateral EEG abnormalities in both IED and ictal discharges, who had bilateral abnormalities in IED and no ictal data, or who declined to participate by opting out. Patients with multiple CMs but good seizure outcomes (International League Against Epilepsy [ILAE] class I or II [15]) after resection surgery were also included in this study because we could consider the resected lesion as the epileptogenic lesion.
2.2. Surgical procedure
In our institution, the surgical side is comprehensively determined based on the location of the CM, ictal EEG findings, and semiology. In patients displaying inconsistencies in these findings or multiple CMs, or in cases of reoperation, intracranial electrode implantation is considered prior to resection surgery. We basically consider ictal findings from intracranial EEG as a more reliable indicator of the epileptogenic zone and surgical site than the lesion site. In this study, all patients who underwent intracranial electrode implantation showed seizure onset on the same side as the CM lesion.We conducted CM removal via a transcortical approach, as described in our previous article [5]. The location of the cortical incision was chosen to minimize the surgical trajectory. The cerebral cortex with hemosiderosis was also aspirated using an ultrasonic aspirator as much as possible. However, we did not completely remove hemosiderin deposits, particularly those within the white matter. We submitted specimens from each patient for histopathological examination.
2.3. Outcome measures
We collected age, sex, location and size of the CM, seizure type, EEG findings (scalp EEG performed as an outpatient, LTVEM, and intracranial EEG), and surgical outcomes (ILAE classification) from the electronic medical records. Regarding the size of the CM, the longest diameter of the solid part of the CM, rather than the entire diameter of the hemosiderin ring, was measured on fluid-attenuated inversion recovery (FLAIR) imaging. The first author (K.H.) reviewed outpatient EEG and investigated interictal epileptiform discharges (IEDs) while blinded to patient information. In our institution, since the volume of data from LTVEM and intracranial EEG was excessively large, the parts judged as important, such as ictal discharges, were left and the other parts were deleted. Therefore, although ictal discharges during LTVEM and intracranial EEG could be reviewed, IEDs during these EEGs were judged from EEG reports.
The major outcome measure was the prevalence of FL. In general, “false lateralization” describes a situation in which findings from scalp EEG (IED or ictal discharge) falsely indicate the side of seizure onset opposite to the true seizure focus. However, due to the small number of patients in which the true seizure focus was revealed by intracranial EEG or surgical outcome, we divided FL into two categories, “FL to MRI” and “FL to true focus after removal surgery.” “FL to MRI” means that scalp EEG shows seizure onset contralateral to the location of the CM and was also analyzed with the inclusion of patients who did not undergo epilepsy surgery. In patients with multiple CMs, MRI lesions for determining FL were defined as resected CMs in patients with ILAE class I or II after surgery. “FL to true focus after removal surgery” means that scalp EEG falsely indicated seizure onset opposite to the side of the “true focus” and was therefore analyzed only in patients who underwent epilepsy surgery. In this study, the side on which intracranial EEG revealed seizure onset or the side of resection surgery in patients who achieved ILAE class I or II after surgery was defined as the side of the “true focus.”
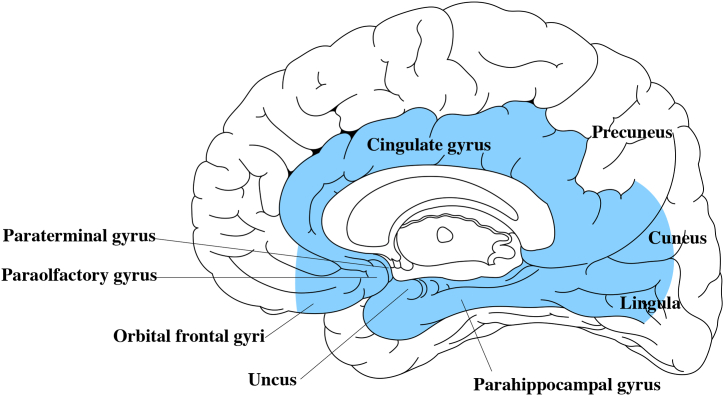
We divided patients into an FL group and a non-false-lateralization group (NFL group), then compared groups regarding age, sex, location and size of the CM, and seizure type. In particular, the location of the CM was also analyzed as “medial and deep” or otherwise. We considered a location to be medial and deep if the CM was located in the hippocampus, amygdala, parahippocampal gyrus, cingulate gyrus, paraolfactory gyrus, paraterminal gyrus, or inner half of the orbital frontal gyrus, cuneus, or lingula (Fig. 1). The medial surface of the superior frontal lobe, paracentral lobule, precuneus, and outer half of the orbital frontal gyrus, cuneus, and lingula were not included as medial and deep regions because these locations may be more likely to show abnormal EEG bilaterally, rather than contralateral to the lesion.
Fig. 1.
Definition of medial and deep location (light blue color). The cingulate gyrus, paraterminal gyrus, paraolfactory gyrus, hippocampus, amygdala, and parahippocampal gyrus were included in this region. The medial halves of the orbital frontal gyri, cuneus, and lingula were also included.
2.4. Statistical analysis
We utilized the χ2 test to compare categorical variables, or Fisher's exact test when the sample size was small and expected values in any of the cells of a contingency table were below 5. Student's t-test or the Mann–Whitney U test was used to compare continuous variables. Two-sided P-values <0.05 were considered statistically significant.
All statistical analyses were performed using Stata/SE version 14.0 software (StataCorp LP, College Station, TX, USA).
3. Results
Forty-two patients were diagnosed with CM-associated epilepsy in our institution during the study period. Two patients for whom the epileptogenic lesions were not revealed due to multiple lesions, two patients with bilateral abnormalities on outpatient EEG and no LTVEM data, and six patients lacking data from scalp EEG were excluded, and the remaining 32 patients were analyzed. Patient characteristics are described in Table 1. Fourteen patients (43.8%) underwent scalp LTVEM, of whom 3 patients never underwent outpatient EEG. Two of the 3 patients who received LTVEM alone (Cases 1 and 6 in Table 2) were not analyzed for IEDs because of a lack of IED findings from EEG reports. One patient with bilateral abnormality of IED and ictal discharge from the right hemisphere (Case 10 in Table 2) was excluded from the IED analysis but included in the ictal discharge analysis. In all 14 patients who underwent scalp LTVEM, we were able to capture the onset of ictal discharges. In one of these 14 patients (Case 17 in Table 2), ictal discharge analysis was not performed because ictal onset was identified from either side. From the above, of the 32 patients, 29 were included in the IED analysis and 13 were included in the ictal discharge analysis. Twenty-two cases (68.8%) underwent resection surgery. Histopathological examination revealed hemangioma in all except two patients (Cases 8 and 23 in Table 2), for whom the specimens were too small to make a diagnosis. Since all cases achieved ILAE class I or II, the true focus was detected in all 22 patients who underwent surgery. In all cases that underwent surgery, the side of the CM was consistent with the side of the true seizure focus. Five patients (Cases 1, 18, 27, 28, and 32 in Table 2) with multiple CMs were included, all of whom underwent resection.
Table 1.
Patient characteristics.
| Variables | Mean ± SD or n (%) |
|---|---|
| Age at diagnosis, years, mean ± SD | 36.1 ± 16.9 |
| Sex, male, n (%) | 22/32 (68.8%) |
| Location of the CM, n (%) | |
| temporal lobe | 19/32 (59.4%) |
| frontal lobe | 8/32 (25.0%) |
| parietal lobe | 3/32 (9.4%) |
| occipital lobe | 1/32 (3.1%) |
| insula | 1/32 (3.1%) |
| Size of the CM, mean ± SD | 18.1 ± 32.7 mm |
| Seizure type, n (%) | |
| FAS | 16/31 (51.6%) |
| FIAS/hyperkinetic | 19/31 (61.3%) |
| FBTCS | 12/31 (38.7%) |
| LTVEM procedure, n (%) | 14/32 (43.8%) |
| Surgery, n (%) | 22/32 (68.8%) |
| Scalp EEG findings, n (%) | |
| IED contralateral to MRI lesion | 3/29 (10.3%) |
| IED contralateral to true focus after removal surgery | 2/19 (10.5%) |
| ictal onset contralateral to MRI lesion | 1/13 (7.7%) |
| ictal onset contralateral to true focus after removal surgery | 1/13 (7.7%) |
CM, cavernous malformation; EEG, electroencephalography; FAS, focal aware seizure; FBTCS, focal to bilateral tonic-clonic seizure; FIAS, focal impaired awareness seizure; IED, interictal epileptiform discharge; LTVEM, long-term video-EEG monitoring; MRI, magnetic resonance imaging; SD, standard deviation.
Table 2.
Detailed information of patients.
| Case | Age (years) | Sex | Location of the CM | Medial & deep location | CM size (mm) | Side of IED | Side of ictal discharge | surgery | True focus | IED |
Ictal discharge |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FL to MRI | FL to true focus | FL to MRI | FL to true focus | ||||||||||
| 1 | 35 | F | Right temporal | 0 | 7 | NA | Unilateral right | yes | right | NA | NA | no | no |
| 2 | 71 | M | Right frontal | 1 | 47.7 | Unilateral left | NA | yes | right | yes | yes | NA | NA |
| 3 | 32 | M | Left posterior quadrant | 0 | 8 | Not detected | Unilateral left | yes | left | no | no | no | no |
| 4 | 38 | M | Left frontal | 0 | 10.8 | Not detected | NA | no | NA | no | NA | NA | NA |
| 5 | 48 | M | Left temporal (medial) | 1 | 7.8 | Unilateral left | Unilateral left | yes | left | no | no | no | no |
| 6 | 25 | M | Right temporal | 0 | 16 | NA | Unilateral right | yes | right | NA | NA | no | no |
| 7 | 9 | M | Left parietal | 0 | 8.5 | Not detected | NA | yes | left | no | no | NA | NA |
| 8 | 32 | F | Left temporal | 0 | 10 | Unilateral left | Unilateral left | yes | left | no | no | no | no |
| 9 | 26 | M | Left insula | 0 | 16.5 | Not detected | NA | no | NA | no | NA | NA | NA |
| 10 | 40 | F | Right frontal | 0 | 7 | Bilateral | Unilateral right | yes | right | NA | NA | no | no |
| 11 | 12 | M | Left frontal | 1 | 7.5 | Not detected | NA | yes | left | no | no | NA | NA |
| 12 | 54 | F | Right temporal | 0 | 5.2 | Not detected | Unilateral right | yes | right | no | no | no | no |
| 13 | 9 | F | Left posterior quadrant | 0 | 10 | Unilateral left | NA | yes | left | no | no | NA | NA |
| 14 | 36 | F | Left frontal | 0 | 4.3 | Not detected | NA | no | NA | no | NA | NA | NA |
| 15 | 25 | F | Left temporal | 0 | 10.9 | Unilateral left | NA | no | NA | no | NA | NA | NA |
| 16 | 69 | M | Left temporal | 0 | 19.6 | Unilateral left | Unilateral left | yes | left | no | no | no | no |
| 17 | 27 | M | Left temporal | 0 | 11 | Unilateral left | Bilateral | yes | left | no | no | NA | NA |
| 18 | 7 | F | Right temporal | 0 | 5 | Unilateral right | NA | no | NA | no | NA | NA | NA |
| 19 | 45 | M | Right temporal | 0 | 10.4 | Unilateral right | NA | yes | right | no | no | NA | NA |
| 20 | 35 | M | Left posterior quadrant | 0 | 6.7 | Not detected | Unilateral left | yes | left | no | no | no | no |
| 21 | 35 | M | Left temporal (medial) | 1 | 5.1 | Not detected | NA | yes | left | no | no | NA | NA |
| 22 | 43 | M | Left temporal | 0 | 5 | Unilateral left | Unilateral left | yes | left | no | no | no | no |
| 23 | 18 | M | Left frontal | 0 | 190 | Unilateral left | NA | yes | left | no | no | NA | NA |
| 24 | 42 | M | Left frontal | 1 | 9.5 | Unilateral right | NA | no | NA | yes | NA | NA | NA |
| 25 | 40 | M | Right temporal (medial) | 1 | 15.9 | Unilateral right | NA | no | NA | no | NA | NA | NA |
| 26 | 25 | M | Left temporal (medial) | 1 | 21 | Unilateral right | Unilateral right | yes | left | yes | yes | yes | yes |
| 27 | 63 | M | Right temporal (medial) | 1 | 10.5 | Not detected | NA | yes | right | no | no | NA | NA |
| 28 | 40 | M | Right temporal | 0 | 1.53 | Unilateral right | Unilateral right | yes | right | no | no | no | no |
| 29 | 63 | M | Left occipital | 0 | 13 | Not detected | NA | no | NA | no | NA | NA | NA |
| 30 | 43 | M | Left frontal | 0 | 36.6 | Not detected | NA | no | NA | no | NA | NA | NA |
| 31 | 17 | F | Left parietal | 0 | 21.7 | Unilateral left | NA | no | NA | no | NA | NA | NA |
| 32 | 52 | F | Right temporal (medial) | 1 | 21 | Unilateral right | Unilateral right | yes | right | no | no | no | no |
CM, cavernous malformation; FL, false lateralization; IED, interictal epileptiform discharge; MRI, magnetic resonance imaging; NA, not available. Shaded cases are patients showing FL.
FL to MRI was found in 3 of 29 cases (10.3%) regarding IEDs and in 1 of 13 cases (7.7%) regarding ictal discharges. FL to the true focus after removal surgery was found in 2 of 19 cases (10.5%) for IEDs and in 1 of 13 cases (7.7%) for ictal discharges.
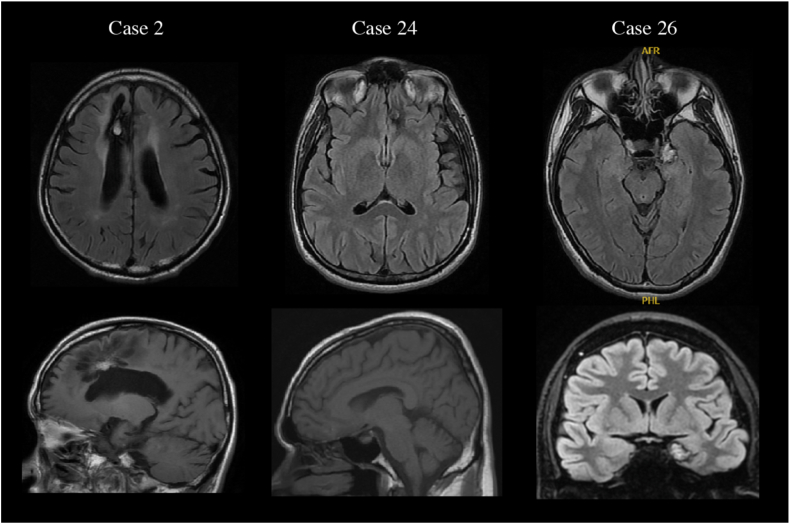
Details of 32 patients are depicted in Table 2. Cases 2, 24, and 26 showed FL and the locations of their CMs were the right cingulate gyrus, left orbital frontal gyrus, and left amygdala, respectively (Fig. 2). In all cases, MRI showed no evidence of cortical atrophy or hippocampal sclerosis. Only Case 26, for whom the CM was located in the left amygdala, received LTVEM. Both IEDs and ictal discharges suggested seizure onset contralateral to the MRI lesion.
Fig. 2.
MRI findings of patients showing FL. CMs in Cases 2, 24, and 26 are revealed to be in the right cingulate gyrus, left orbital frontal gyrus, and left amygdala, respectively. All upper images represent axial-section fluid-attenuated inversion recovery (FLAIR) images. In the lower row, images for Cases 2 and 24 represent sagittal-section T1-weighted images and the image for Case 26 represents a coronal-section FLAIR image.
We compared the FL and NFL groups only regarding the FL of IEDs to MRI. We did not compare these groups regarding the FL of ictal discharges or IEDs to the true focus after removal surgery as the number of such FLs was too low. Regarding the FL of IEDs to MRI findings, the percentage of medial and deep lesions differed significantly between the FL group (3/3, 100%) and the NFL group (6/26, 23.1%; p = 0.023). No significant differences were observed when comparing medial lesions including other-than-deep lesions between the FL group (3/3, 100%) and the NFL group (11/26, 42.3%; p = 0.10). In addition, no differences between groups were evident in terms of age, sex, seizure type, or location or size of the CMs (Table 3).
Table 3.
Comparison between false- and non-false-lateralization groups regarding FL of IEDs compared to MRI findings.
| False-lateralization group | Non-false-lateralization group | p value | |
|---|---|---|---|
| Age, years | 46 ± 23.3 | 35.3 ± 17.2 | 0.33 |
| Sex, male | 3/3 (100%) | 18/26 (69.2%) | 0.54 |
| Location of the CM | 0.44 | ||
| temporal lobe | 1/3 (33.3%) | 16/26 (61.5%) | |
| frontal lobe | 2/3 (66.7%) | 5/26 (19.2%) | |
| parietal lobe | 0 (0%) | 3/26 (11.5%) | |
| occipital lobe | 0 (0%) | 1/26 (3.9%) | |
| insula | 0 (0%) | 1/26 (3.9%) | |
| Medial & deep lesion | 3/3 (100%) | 6/26 (23.1%) | 0.023 |
| Medial lesion | 3/3 (100%) | 11/26 (42.3%) | 0.1 |
| Size of the CM, mm | 26.1 ± 19.6 | 18.2 ± 35.8 | 0.71 |
| Seizure type | |||
| FAS | 1/3 (33.3%) | 13/25 (52.0%) | 1 |
| FIAS | 2/3 (66.7%) | 15/25 (60.0%) | 1 |
| FBTCS | 1/3 (33.3%) | 10/25 (40.0%) | 1 |
CM, cavernous malformation; FAS, focal aware seizure; FBTCS, focal to bilateral tonic-clonic seizure; FIAS, focal impaired awareness seizure; FL, false lateralization; IED, interictal epileptiform discharge MRI, magnetic resonance imaging. Age and size of the CM were analyzed using the t-test, while sex, location of the CM, medial and deep lesion, medial lesion, and seizure type were analyzed using Fisher's exact test.
4. Discussion
This study revealed the following results about CM-associated epilepsy: the ratio of FL to MRI lesions was 10.3% for IEDs and 7.7% for ictal discharges; and the ratio of FL to true focus after removal surgery was 10.5% for IEDs and 7.7% for ictal discharges. We also revealed that a medial and deep localization of the CM may be characteristic of IEDs showing FL when compared to results from MRI.
4.1. FL of ictal discharges
FL of ictal discharges in scalp EEG is considered more likely in cases of severe hippocampal sclerosis (burned-out hippocampus) or cortical atrophy. A previous report on scalp EEG in hippocampal sclerosis documented that the ictal discharges originated from the side of the intact hippocampus in 4.7% of cases, of which 80% had severe hippocampal sclerosis [12]. The rate of FL of ictal discharges in the present study was 7.7%, slightly higher than previously reported. This may be because, despite the small number of cases, we started reviewing data soon after we encountered a patient (Case 26) with FL of ictal discharges.
The mechanism of FL in severe hippocampal sclerosis or cortical atrophy on the side of seizure focus is considered to involve the scalp EEG being unable to adequately depict ictal discharges on the side of the focus due to their low amplitude, while being able to show waves contralateral to the focus because the amplitude of waves from the opposite hemisphere or hippocampus is normal [12,13]. However, since no patients in this study had cortical atrophy or hippocampal sclerosis, this mechanism cannot apply in the present cases. In Case 26, scalp EEG might have falsely revealed seizure onset contralateral to the true focus, if the CM in the left amygdala interrupted the propagation of ictal discharges to the ipsilateral neocortex, as it was described in detail in our previous case report [11]. In the temporal lobe area, ictal discharges are thought to propagate quickly to the contralateral side via the hippocampal commissure or anterior commissure [9,12,16]. Asaadi et al. reported that 23% of ictal discharges originating from the hippocampus did not spread to the ipsilateral neocortex, but instead spread to the contralateral hippocampus [17]. Such propagation through these commissures may have applied in Case 26. We speculate that CM-associated epilepsy in the temporal lobe region might be more prone to false lateralization of seizure onset than other regions, but this was not able to be confirmed in the present study.
4.2. FL of IEDs
IEDs provide a less reliable marker of the side of seizure onset than ictal discharges. The percentage of FL of IEDs has been described as 6.3–25.9%, higher than ictal discharges [[18], [19], [20], [21], [22]]. Our result of 10.3–10.5% in FL of IEDs seems consistent with previous reports. However, the suggestion has been made that if more than 75–90% of IEDs are on one side, the probability of concordance between the true side of the seizure focus and that of IEDs is high, as is the seizure-free rate after resection surgery [18,23].
The characteristics of IED FL have been mentioned to include cerebral atrophy or damage on the side of seizure focus [13,21], hippocampal sclerosis rather than tumor in the case of the temporal lobe [20], and a left-sided focus [24]. From our study, the localization of the CM, particularly with a medial and deep location, appears to be another characteristic of IED FL. We inferred that the contralateral direction of the IED dipole may induce FL and therefore paid attention to medial and deep locations, in which the dipole of IEDs would tend to direct to the contralateral side. For example, as in Case 26, a CM in the left amygdala can produce spikes with the dipole pointing to the right. In this situation, IEDs would be depicted in the right temporal area from scalp EEG. The present study revealed that all patients with IED FL showed the CM in a medial and deep area. However, we could not confirm the mechanism because the spike dipole was not evaluated. Another possible mechanism is early propagation of IED to the more superficial structures contralaterally, similar to ictal discharges. Evidence that the production of IED requires recruitment of more than 10 cm2 of gyral area may support this early propagation mechanism rather than the mechanism of dipole direction [25]. Magnetoencephalography or simultaneous scalp and intracranial EEG is required to obtain suitable evidence for the mechanism of dipole direction.
4.3. Limitations
Some limitations must be considered for this study. First, this study was investigated just after our experience with CM showing FL (Case 26), which may have biased the results. Since only one patient (Case 26) showed FL in ictal discharges, the incidence rate of FL may have been overstated. Second, 2 of the 3 patients with FL of IEDs underwent only outpatient EEG. Those two cases may have exhibited FL coincidentally. LTVEM could have more accurately demonstrated the lateralization of seizure onset. Third, the results obtained in this study may not be applicable to any patients, since this study was conducted in a single institution. Research including larger populations, such as multicenter studies, would increase the generalizability of these findings.
5. Conclusions
CM-associated epilepsy can also show FL, particularly in cases where the CM shows a medial and deep localization. Intracranial EEG before resection surgery seems warranted if a discrepancy is seen among findings of MRI, EEG, and semiology. Further research is desired to validate this study.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Our data have not been deposited into a publicly available repository. The data can be made available upon reasonable request to the corresponding author.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this article.
CRediT authorship contribution statement
Keisuke Hatano: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Keishiro Sato: Conceptualization, Supervision. Tomohiro Nakamura: Data curation, Formal analysis. Ryuya Hotta: Data curation, Writing – review & editing. Shingo Numoto: Formal analysis, Writing – review & editing. Ayataka Fujimoto: Conceptualization, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our medical technologists for their cooperation in EEG measurement and analysis.
References
- 1.Rosenow F., Alonso-Vanegas M.A., Baumgartner C., Blumcke I., Carreno M., Gizewski E.R., Hamer H.M., Knake S., Kahane P., Luders H.O., Mathern G.W., Menzler K., Miller J., Otsuki T., Ozkara C., Pitkanen A., Roper S.N., Sakamoto A.C., Sure U., Walker M.C., Steinhoff B.J. C.o.T.S.o.t.I. Surgical Task Force, cavernoma-related epilepsy: review and recommendations for management--report of the surgical task force of the ILAE commission on therapeutic strategies. Epilepsia. 2013;54:2025–2035. doi: 10.1111/epi.12402. [DOI] [PubMed] [Google Scholar]
- 2.Dammann P., Wrede K., Jabbarli R., Neuschulte S., Menzler K., Zhu Y., Ozkan N., Muller O., Forsting M., Rosenow F., Sure U. Outcome after conservative management or surgical treatment for new-onset epilepsy in cerebral cavernous malformation. J. Neurosurg. 2017;126:1303–1311. doi: 10.3171/2016.4.JNS1661. [DOI] [PubMed] [Google Scholar]
- 3.Kapadia M., Walwema M., Smith T.R., Bellinski I., Batjer H., Getch C., Rosenow J.M., Bendok B.R., Schuele S.U. Seizure outcome in patients with cavernous malformation after early surgery. Epilepsy Behav. 2021;115 doi: 10.1016/j.yebeh.2020.107662. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D.S., Zubay G.P., Goodman R.R. Seizure outcome after lesionectomy for cavernous malformations. J. Neurosurg. 1995;83:237–242. doi: 10.3171/jns.1995.83.2.0237. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto A., Enoki H., Hatano K., Sato K., Okanishi T. Earlier age at surgery for brain cavernous angioma-related epilepsy may achieve complete seizure freedom without aid of anti-seizure medication. Brain Sci. 2022;12 doi: 10.3390/brainsci12030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauschenbach L., Bartsch P., Santos A.N., Lenkeit A., Darkwah Oppong M., Wrede K.H., Jabbarli R., Chmielewski W.X., Schmidt B., Quesada C.M., Forsting M., Sure U., Dammann P. Quality of life and mood assessment in conservatively treated cavernous malformation-related epilepsy. Brain Behav. 2022;12 doi: 10.1002/brb3.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rammo R.A., Greiner H.M., Trout A.T., Leach J.L., Rozhkov L., Fujiwara H., Rose D.F., Mangano F.T. False lateralization of pre-surgical work-up in a child with a cortical cavernous malformation and intractable epilepsy. J. Neurosurg. Sci. 2018;62:91–94. doi: 10.23736/S0390-5616.16.03621-3. [DOI] [PubMed] [Google Scholar]
- 8.Holmes M.D., Wilensky A.J., Ojemann G.A., Ojemann L.M. Hippocampal or neocortical lesions on magnetic resonance imaging do not necessarily indicate site of ictal onsets in partial epilepsy. Ann. Neurol. 1999;45:461–465. doi: 10.1002/1531-8249(199904)45:4<461::aid-ana7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Gaviria Carrillo M., Lopez J., Rodriguez Q.J., Gaona I., Ortiz-Guerrero G., Nava-Mesa M.O. Apparent false lateralization of seizure onset by scalp EEG in temporal lobe epilepsy associated with cerebral cavernous malformation: a case report and overview. Brain Sci. 2020;10 doi: 10.3390/brainsci10090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobulashvili T., Kuchukhidze G., Brigo F., Zimmermann G., Hofler J., Leitinger M., Dobesberger J., Kalss G., Rohracher A., Neuray C., Wakonig A., Ernst F., Braun K.P.J., Mouthaan B.E., Van Eijsden P., Ryvlin P., Cross J.H., Trinka E., consortium E.P. Diagnostic and prognostic value of noninvasive long-term video-electroencephalographic monitoring in epilepsy surgery: a systematic review and meta-analysis from the E-PILEPSY consortium. Epilepsia. 2018;59:2272–2283. doi: 10.1111/epi.14598. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T., Hatano K., Sato K., Enoki H., Fujimoto A. False lateralization of scalp EEG and semiology in cavernous malformation-associated temporal lobe epilepsy: a case report. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mintzer S., Cendes F., Soss J., Andermann F., Engel J., Jr., Dubeau F., Olivier A., Fried I. Unilateral hippocampal sclerosis with contralateral temporal scalp ictal onset. Epilepsia. 2004;45:792–802. doi: 10.1111/j.0013-9580.2004.35703.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto A., Masuda H., Homma J., Sasagawa M., Kameyama S. False lateralization of mesial temporal lobe epilepsy by noninvasive neurophysiological examinations. Neurol. Med.-Chir. 2006;46:518–521. doi: 10.2176/nmc.46.518. [DOI] [PubMed] [Google Scholar]
- 14.Al-Shahi Salman R., Berg M.J., Morrison L., Awad I.A., Angioma Alliance Scientific Advisory B. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Scientific Advisory Board, Stroke. 2008;39:3222–3230. doi: 10.1161/STROKEAHA.108.515544. [DOI] [PubMed] [Google Scholar]
- 15.Wieser H.G., Blume W.T., Fish D., Goldensohn E., Hufnagel A., King D., Sperling M.R., Lüders H., Pedley T.A. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2008;42:282–286. doi: 10.1046/j.1528-1157.2001.35100.x. [DOI] [PubMed] [Google Scholar]
- 16.Mitsuhashi T., Sonoda M., Jeong J.W., Silverstein B.H., Iwaki H., Luat A.F., Sood S., Asano E. Four-dimensional tractography animates propagations of neural activation via distinct interhemispheric pathways. Clin. Neurophysiol. 2021;132:520–529. doi: 10.1016/j.clinph.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaadi T.M., Laxer K.D., Barbaro N.M., Marks W.J., Jr., Garcia P.A. False lateralization by subdural electrodes in two patients with temporal lobe epilepsy. Neurology. 2001;57:532–534. doi: 10.1212/wnl.57.3.532. [DOI] [PubMed] [Google Scholar]
- 18.Holmes M.D., Dodrill C.B., Wilensky A.J., Ojemann L.M., Ojemann G.A. Unilateral focal preponderance of interictal epileptiform discharges as a predictor of seizure origin. Arch. Neurol. 1996;53:228–232. doi: 10.1001/archneur.1996.00550030034020. [DOI] [PubMed] [Google Scholar]
- 19.Vollmar C., Stredl I., Heinig M., Noachtar S., Remi J. Unilateral temporal interictal epileptiform discharges correctly predict the epileptogenic zone in lesional temporal lobe epilepsy. Epilepsia. 2018;59:1577–1582. doi: 10.1111/epi.14514. [DOI] [PubMed] [Google Scholar]
- 20.Hamer H.M., Najm I., Mohamed A., Wyllie E. Interictal epileptiform discharges in temporal lobe epilepsy due to hippocampal sclerosis versus medial temporal lobe tumors. Epilepsia. 1999;40:1261–1268. doi: 10.1111/j.1528-1157.1999.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira R.A., Li L.M., Santos S.L., Amorim B.J., Etchebehere E.C., Zanardi V.A., Guerreiro C.A., Cendes F. Lateralization of epileptiform discharges in patients with epilepsy and precocious destructive brain insults. Arq Neuropsiquiatr. 2004;62:1–8. doi: 10.1590/s0004-282x2004000100001. [DOI] [PubMed] [Google Scholar]
- 22.Adachi N., Alarcon G., Binnie C.D., Elwes R.D., Polkey C.E., Reynolds E.H. Predictive value of interictal epileptiform discharges during non-REM sleep on scalp EEG recordings for the lateralization of epileptogenesis. Epilepsia. 1998;39:628–632. doi: 10.1111/j.1528-1157.1998.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung M.Y., Walczak T.S., Lewis D.V., Dawson D.V., Radtke R. Temporal lobectomy and independent bitemporal interictal activity: what degree of lateralization is sufficient? Epilepsia. 1991;32:195–201. doi: 10.1111/j.1528-1157.1991.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji T., Kawasaki J., Shiba M., Wada M., Yoshimasu F., Kanemoto K. Re-examination of the value of localising aura sensations and lateralising interictal epileptiform discharges in view of structural lesions demonstrated by MRI. Seizure. 2003;12:545–549. doi: 10.1016/s1059-1311(03)00071-2. [DOI] [PubMed] [Google Scholar]
- 25.Tao J.X., Ray A., Hawes-Ebersole S., Ebersole J.S. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data have not been deposited into a publicly available repository. The data can be made available upon reasonable request to the corresponding author.