Abstract
Deep gluteal syndrome (DGS) is a significant cause of posterior hip pain resulting from the compression of the sciatic or other peripheral nerves in the deep gluteal space. Understanding the anatomy of the deep gluteal space and the kinematics of the sciatic nerve, as it passes through this region is crucial for understanding DGS. Despite increasing awareness, DGS is still often overlooked. This review focuses on conditions that specifically contribute to posterior hip pain as a consequence of DGS. Predominantly addressing piriformis syndrome, gemelli-obturator internus syndrome, ischiofemoral impingement syndrome, and proximal hamstring syndrome, the review also touches upon rare cases such as inferior and superior gluteal nerve entrapment.
Keywords: Hip, pain, sciatic nerve
Introduction
The term deep gluteal syndrome (DGS) was first used by McCrory and Bell[1] In the literature, DGS is defined as compression of the sciatic nerve (SN) in the deep gluteal space.[2] To understand the concept of DGS, it is necessary to know the boundaries of the deep gluteal space, the structures passing through it and the kinematics of the SN.
ANATOMY
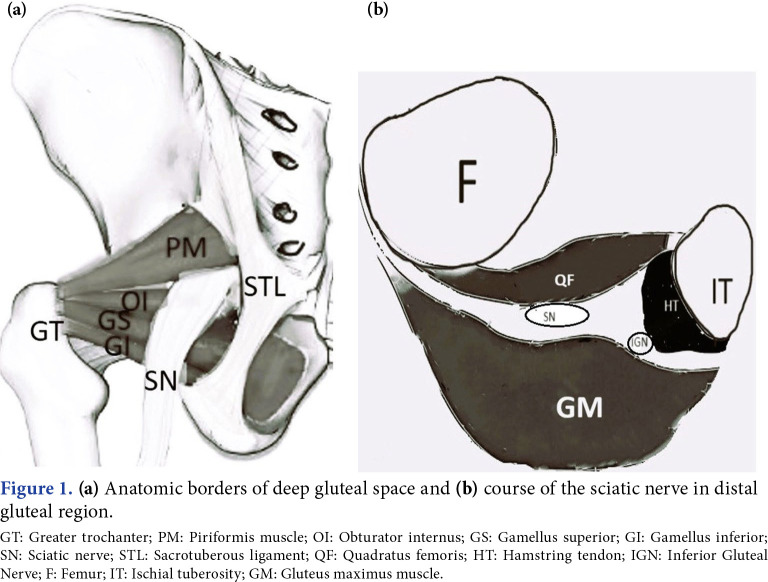
The DGS is located between the middle and deep fascia of the hip. Anatomical borders include gluteus maximus in the posterior, posterior acetabular column, hip joint capsule, and proximal femur in the anterior, lateral lip of linea aspera and greater tuberosity in the lateral, sacrotuberous ligament and falciformis fascia in the medial, inferior margin of the sciatic notch in the superior, proximal origin of the hamstrings and ischial tuberosity in the inferior.[3] It contains, from top to bottom, the piriformis, superior gemellus, obturator internus, inferior gemellus, and quadratus femoris muscles. Neurovascular structures are pudendal nerve, nerve to the obturator internus, posterior femoral cutaneous nerve, SN, inferior gluteal artery, and nerve to quadratus femoris.[4] It also contains connective and fatty tissue (Figure 1).
Figure 1. (a) Anatomic borders of deep gluteal space and (b) course of the sciatic nerve in distal gluteal region. GT: Greater trochanter; PM: Piriformis muscle; OI: Obturator internus; GS: Gamellus superior; GI: Gamellus inferior; SN: Sciatic nerve; STL: Sacrotuberous ligament; QF: Quadratus femoris; HT: Hamstring tendon; IGN: Inferior Gluteal Nerve; F: Femur; IT: Ischial tuberosity; GM: Gluteus maximus muscle.
The SN emerges from the greater sciatic foramen in the pelvic region and passes under the piriformis muscle over the superior gemelli/obturator internus. The nerve, then, follows a distal path posterior to the external rotator muscle complex. It progresses toward the posterior thigh through the ischiofemoral tunnel located between the lesser trochanter and the tuber ischiadicum.[5] It runs deep into the hamstring muscle group. In a study, the ischiofemoral distance (IFD) being below 15 mm was determined as the cut-off value for stenosis,[6] while another study showed the cut-off values of <17 mm for the IFD and <8 mm for the quadratus femoris in patients with symptomatic ischiofemoral impingement (IFI).[7] The quadratus femoris muscle is located in the ischiofemoral space. The SN is located 1.2 cm lateral to where the semimembranosus tendon attaches to the superolateral of the ischial tuberosity, that is, the SN is in close proximity to the proximal part of the hamstring muscle group.[8]
SCIATIC NERVE KINEMATICS
In the deep gluteal space, the SN has mobility that allows it to adapt to pelvic and hip movements in all planes.[5] The SN moves 28 mm during hip flexion movement. During hip flexion, abduction, and external rotation, the tension on the nerve decreases, as the SN slides over the posterior edge of the greater trochanter, and the semimembranosus muscle origin approaches and contacts the posterior edge of the greater trochanter.[9] Knee movements also affect SN movement. When the knee is flexed, the nerve moves posterolaterally, while when the knee is extended, it moves deeply in the ischiofemoral tunnel, and 26% strain develops in the SN.[10] Additionally, during passive internal rotation of the hip, the obturator internus tendon contracts downward, followed by a characteristic curvature of the SN. During passive external rotation, the obturator internus tendon loses its tension and allows the SN to relax. Any reason that disrupts the mobility of the SN during hip joint movement may lead to entrapment. This situation is also called dynamic entrapment.[11] Therefore, a 6% strain resulting from this mobility disorder causes neuropraxia, and a 12% strain leads to a complete blockade.[12]
Deep gluteal syndrome includes other entrapments in this space, such as posterior femoral nerve entrapment, inferior gluteal nerve entrapment, and superior gluteal nerve entrapment,[13] in addition to the SN entrapment in the deep gluteal space, whose anatomical features are mentioned above. However, the subject of this review centers on the DGS as an underestimated cause of posterior hip pain. However, other nerve entrapments, such as the posterior femoral cutaneous nerve, pudendal nerve, or inferior cluneal nerve entrapments, have not been addressed, since these entrapments manifest themselves as groin or anterior hip pain. It should also be kept in mind that entrapment may occur in more than one place in the deep gluteal space.
PREDISPOSING FACTORS AND ETIOLOGY[13]
Anatomical variations between musculotendinous structures and nerves
Congenital anomalies of the piriformis and internal obturator muscle and nerve trajectory variances may cause the nerve entrapment in its path. Smoll[14] reported that this situation was seen at a rate of 16.2%. There are six anatomical variations of the piriformis muscle.[15] There may also be variation in the insertion location of the piriformis muscle. Before the piriformis tendon attaches to the greater trochanter, it may join the superior gamellus and obturator internus tendon (29.5%) or the obturator internus tendon and gluteus medius tendon (13.4%).[16,17] These variations can cause compression of the sciatic nerve by piriformis muscle, particularly during hip internal rotation.
Fibrosis and fibrovascular bands
Due to these bands, the sciatic nerve cannot mobilize adequately or mobilizes to a minimal extent during hip and knee movements, leading to the development of ischemic neuropathy.[13] These bands may be fibrovascular, purely vascular, or purely fibrous. They can be located proximally or distally. There are two types: one that compresses the sciatic nerve and another that tightly adheres to the nerve.
Traumas
Hematoma and edema secondary to trauma, as well as high-energy trauma such as acetabular fractures, ischial tuberosity apophyseal fracture non-union/ malunions or posterior hip dislocations, may cause DGS.[18-20]
Overuse-related conditions[21]
Myofascial pain syndrome, spasm, and hypertrophy may develop in the gluteal, hamstring, piriformis, and deep rotator muscles as a result of cumulative microtrauma due to mechanical overuse, such as longdistance walking, running, cycling, sitting on hard surfaces for a long time. Hypertrophic muscle may cause nerve entrapment.
Abductor tears or weakness of the abductor muscles, may play a role in the pathophysiology of ischiofemoral impingement (IFI).[22-24] Weakness in the hip abductors cause contralateral pelvic drop which may lead to compression of the quadratus femoris (QF) muscle. The instability of the pelvis may result in dynamic IFI over time.[25] Hamstring enthesopathy or rupture is commonly encountered in athletes and can contribute to IFI by narrrowing of the quadratus femoris space (QFS).[26] These muscle pathologies should be either traumatic or mechanical overuse. For instance, repeated movements of the hip (extension, adduction, and external rotation) which may impair the action of the quadratus femoris muscle should cause IFI.
Iatrogenic
Deep gluteal syndrome can be caused not only by postoperative scarring and hematoma but also by protruding hardware, heterotopic ossification, secondary to valgus intertrochanteric osteotomy.[19,27] Studies have demonstrated that during total hip arthroplasty (THA), unrepaired trochanteric bursae can be pulled into the deep gluteal region, leading to increased pressure.[28] Deep gluteal syndrome commonly occurs in individuals with unrepaired trochanteric bursal tissue following THA. Uchida et al.[28] found that the incidence of postoperative DGS after primary hip arthroscopic procedures was 0.9%, with a higher prevalence in females, individuals with a low body mass index, developmental dysplasia of the hip and generalized joint laxity.[28] Therefore, being aware of potential risks for DGS is crucial in patients undergoing hip surgery.
Vascular
Vascular causes such as aneurysms or varicose veins, hematoma and endometriosis compress the SN as a result of the close relationship with the iliac vessels, ovaries, and sacral plexus.[9] Geler Külcü et al.[29] presented a patient who had an endometrial focus compressing SN at left sciatic foramen level that was diagnosed by magnetic resonance neurorrhaphy (MRN).
Biomechanical reasons
In pregnant women, the change in the body's center of gravity, weight gain, and relaxation of the ligaments due to hormonal factors may cause the development of PS.[30] Anatomy of the female pelvis might be a risk factor for IFI in females.[16]
Leg length discrepancy, high femoral/acetabular version and neck-shaft angle, developmental dysplasia of the hip cause IFI.[30-32]
Postural disorders can contribute to DGS, as well. An augmented spinal lordosis and internal rotation of the hip can ˝lock˝ the piriformis muscle against the ilium and the inferior fibers of the gluteus minimus muscle. Additionally, it is important to consider that entrapment may occur at multiple sites within the deep gluteal space.[14-16]
Other
As the SN passes through the sacral foramen and proceeds under the sacroiliac joint, it can be compressed due to many local pathologies such as pelvic soft tissue and bone tumors, presacral abscesses, infectious diseases and inflammatory sacroiliitis.
INCIDENCE OF ETIOLOGICAL CAUSES
Filler et al.[33] presented the most common three sites of SN entrapment as beneath the piriformis muscle (67.8%), sciatic foramen (6%), ischiotunnel (4.7%) in 239 patients. Previously, Martin et al.[34] reported the most common cause of DGS was iatrogenic (30%) followed by PS (26%), and trauma (15%). Obturator internus pathology was detected in only three of the patients who underwent endoscopic decompression, and hamstring pathology was detected in two cases in this systematic review. In another systematic review including 481 patients who underwent surgical treatment, the most common cause was found to be PS (26%), and entrapment due to other muscles was found in 14%.[35] In the light of these data, it appears that the most common cause of DGS is PS. This result is compatible with our clinical observations. However, it should be kept in mind that these reviews included the results of patients who did not respond to conservative treatment and underwent surgery. Considering our clinical practice, although the most common cause is PS, IFI, proximal hamstring tendinopathy (ischiotunnel syndrome) are not as rare as the reports of those systematic reviews. The causes of DGS, such as IFI and ischiotunnel syndrome, are crucial considerations in the differential diagnosis of posterior hip pain. Conducting a study on patients with DGS undergoing conservative treatment can yield clearer insights into the incidence of these conditions.
Distinguishing between these subgroups requires a thorough assessment, involving a detailed medical history, a comprehensive examination, and, when necessary, appropriate imaging methods.
Differential diagnosis
There is no gold standard for the diagnosis of DGS. The diagnosis is established after ruling out other causes of hip or lower extremity pain.[36] Disorders in the spine, such as lumbar radiculopathy, lumbar facet syndrome, and referred pain from trigger points in paraspinal muscles, can lead to pain in the posterior hip.[37] This is because the hip capsule is innervated by the sensory nerves of the L2-S1 roots.[38] Posterior hip pain can also result from intrapelvic problems. It is also important to take urological and gynecological medical history. For instance, cyclic pain may be a sign of endometriosis.[39]
On the other hand, cluneal nerve entrapment, thoracolumbar transition syndrome, sacroiliitis, sacroiliac joint dysfunction, gluteal tendinitis should definitely be considered in the differential diagnosis of DGS. As the causes are multiple, various different symptoms of each disorder may be together in addition to the symptoms of DGS. In a study by Aytaç et al.,[39] the PS and sacroiliac joint dysfunction incidence was found to be 20% in patients after lumbar surgery.
One review examined 11 studies describing the diagnosis of DGS.[2] Deep gluteal syndrome has three typical features: SN pain, non-discogenic nature, and entrapment in the deep gluteal space. The steps in diagnosing DGS are: (i) medical history, (ii) physical examination, (iii) imaging, (iv) diagnostic injection, and (v) provocation tests. History and physical examination should definitely include spinal problems.
The patient's history provides valuable insights into factors that exacerbate or alleviate pain. Specifically, in PS, pain is often aggravated during prolonged periods of sitting, and some individuals may struggle to tolerate a seated position for more than 30 min. Pain may also exacerbate with long periods of walking, running, and squats. Notably, it tends to increase, when the SN is under maximum tension, such as during hip flexion with knee extension. In case of IFI syndrome, characterized by distal sciatic entrapment, patients may be comfortable while sitting. However, they may experience increased pain in the lateral ischium with terminal hip extension during walking, particularly with long strides, Additionally, patients with IFI may report snapping and discomfort in the hip and groin. Snapping should be due to the forceful bypassing of the ischium over the lesser trochanter.
Chronic low back pain can also manifest due to the impact of limited hip range of motion on spinal mobility.[41]
Ischiotunnel syndrome patients typically describe pain in the lateral ischium during the initial heel strike, while walking. Furthermore running and jumping may predispose individuals to hamstring syndrome. Symptoms may be exacerbated by repetitive eccentric hamstring contractions or prolonged forward flexion of the trunk such as during hamstring stretching exercises.[42,43] In more severe cases, fibrosis of the proximal hamstring muscles can compress the SN.[41]
Patients may also describe paresthesia in the affected side extremity. Symptoms usually occur unilaterally. Most patients have a history of trauma. Lumbago and pain at night getting better during the day are other symptoms reported by patients.[43]
Other nerve entrapments in deep gluteal space which cause posterior hip pain should be kept in mind. The inferior gluteal nerve innervates the gluteus maximus muscle. The gluteus maximus is the major extensor of the hip. Pathology of the gluteus maximus would manifest as difficulty in walking up stairs and arising from a chair. Inferior gluteal nerve entrapment may cause gluteus maximus atrophy beside the sciatica. The inferior gluteal nerve should also be injured during THA.[44] Buttock pain, the weakness of hip abduction, tenderness in the region just lateral to the greater sciatic notch with deep palpation may be called the triad of superior gluteal nerve entrapment. The role of hypertrophy of the piriformis muscle, resulting in a narrow suprapiriformis foramen, so far, superior gluteal nerve entrapment has been documented.[45]
PHYSICAL EXAMINATION[13,40,46]
Inspection
During the physical examination, the biomechanical axial alignment and pelvic position deviations are observed while the patient is standing. If the pelvic tilt is impaired, the balance between the hip flexors and extensors may be affected, meaning there may be weakness of the hip muscles. Antalgic gait, leg length difference, and Trendelenburg sign should be observed, while the patient is walking. An antalgic position that avoids putting weight on the painful side while sitting can be followed.
Palpation
On palpation, tenderness in the gluteal and retro-trochanteric region and sciatica like pain, exacerbated with rotation of the hip in flexion and knee extension. Localization of the pain by palpation gives us an idea about the cause. Tenderness in the sciatic notch suggests PS, tenderness in the lateral part of the ischium suggests ischiotunnel syndrome or IFI syndrome, and tenderness in the medial part of the ischium suggests pudendal nerve entrapment.
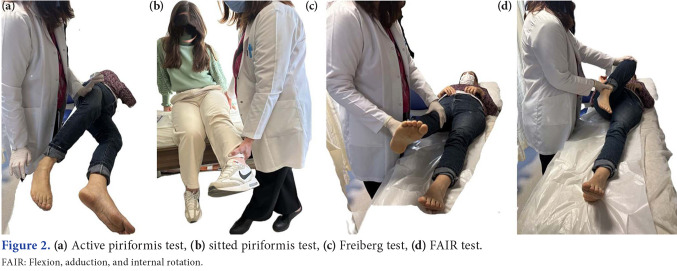
Tests showing SN compression (Laseque, Pace, Freiberg, FAIR, etc.) should be evaluated during the examination. The Lasègue test; the Freiberg sign (in a supine position, internal rotation of the hip); the Pace sign (resisted hip abduction); and the FAIR test (flexion, adduction and internal rotation of the hip in supine position).[47] These provocation tests narrow the space between the piriformis muscle and internal obturator muscle. These tests may also be positive in gemelli-obturator internus syndrome. It has been shown that sensitivity and specificity will increase significantly, particularly when the piriformis stretch test is used in combination with the active piriformis test in the sitting position.[40] In the piriformis stretch test in the sitting position, the examiner moves the patient's knee in the direction of extension and hip in the direction of internal rotation and adduction, while palpating the deep gluteal area. In the active piriformis test, the examiner palpates the piriformis muscle while the patient presses his heel on the examination table in the lateral decubitus position and performs active abduction and external rotation against resistance. In both tests, feeling pain in the deep gluteal area makes the test positive (Figure 2). Due to the tonic contracture of the piriformis muscle, it may be noted that the affected leg of the patient lying on his back is constantly in external rotation position (piriformis sign).[47-49]
Figure 2. (a) Active piriformis test, (b) sitted piriformis test, (c) Freiberg test, (d) FAIR test. FAIR: Flexion, adduction, and internal rotation.
Piriformis syndrome
Piriformis syndrome is diagnosed with pain in the buttock that increases with prolonged sitting, recurrence of pain upon palpation of the greater sciatic notch, or a positive test result of one of the provocation tests described before. The diagnosis can also be confirmed with diagnostic injection.
Gemellus-obturator internus syndrome
The gemellus-obturator internus muscles are considered a complex, as they share structural and functional similarities and are closely located to each other. The SN has a close association with the gemelliobturator internus complex. This proximity leads to a consistent and observable dynamic response of the SN during passive hip rotation. It has also been shown that the tendon of this complex can share a similar function with the piriformis muscle. Therefore, it is extremely difficult to distinguish symptoms and provocation tests from PS. Spasm of these muscles or myofascial pain syndrome, acute strain, tendinitis may also cause DGS independently of the piriformis muscle.[50]
Magnetic resonance imaging (MRI) is useful in the differential diagnosis from PS, particularly in cases resistant to treatment.[51] Murata et al.[52] reported a case of non-discogenic sciatica due to dynamic movement of the obturator internus muscle. Based on this case, they suggested that rapid application of Freiberg and Pace tests may be useful in detecting dynamic changes in obturator internus syndrome. Presumably, in rapid tests, the repetitive and swift anterior traction of the SN by the obturator internus tendon results in a shearing effect, leading to the manifestation of symptoms.
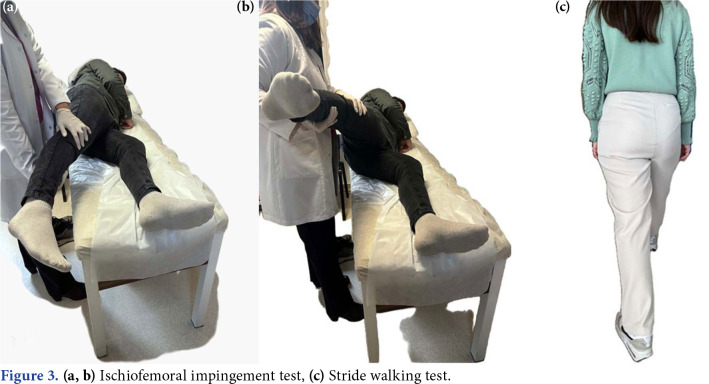
The stride walking test (complaining of pain lateral to the ischium during terminal extension while walking with long strides and relieved by short steps) is a provocation test used to diagnose IFI. The other is the IFI test, and it is expected that the complaints would increase when the patient is in the contralateral decubitus position and the hip is passively extended, while the hip is in adduction or neutral. Passive extension applied while in abduction does not trigger symptoms (Figure 3).[40,53]
Figure 3. (a, b) Ischiofemoral impingement test, (c) Stride walking test.
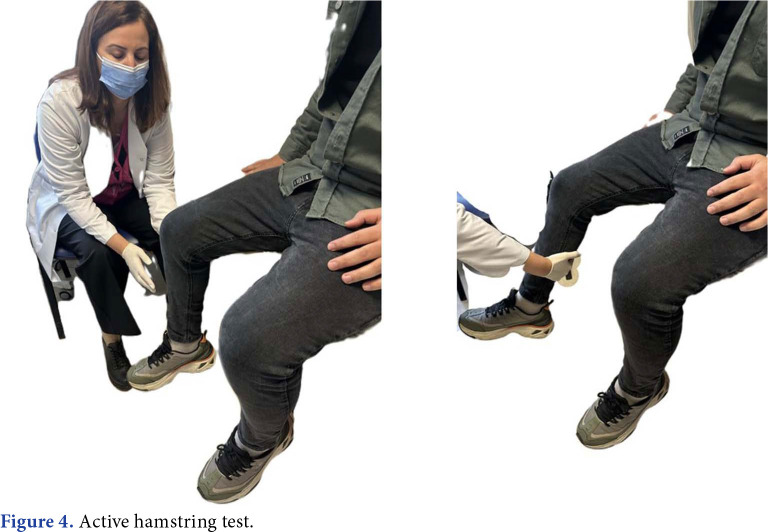
Active hamstring testing is performed to differentiate proximal hamstring tendinopathy or ischiotunnel syndrome. In the active hamstring test, the patient is asked to flex the knee against resistance while sitting with the knee flexed at 30 degrees. Weakness or the appearance of symptoms in this position makes the test positive. When the patient repeats the test with the knee flexed at 90 degrees, he can resist the resistance painlessly (Figure 4).[40,53]
Figure 4. Active hamstring test.
DIAGNOSIS
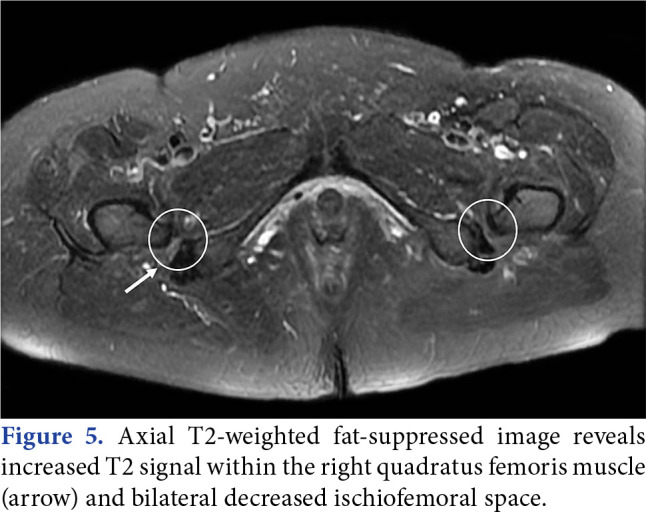
Magnetic resonance imaging is the most useful imaging method in the diagnosis of DGS. To visualize the normal anatomy of the SN and to evaluate extrapelvic causes, T1- and T2-weighted images should be examined in sagittal, axial and coronal planes from L5 to 2 cm distal to the lesser trochanter.[54] Despite it being described for many years, IFI is still frequently misdiagnosed. The accuracy of using MRI to diagnose IFI syndrome is uncertain, as comparable observations may be present in individuals without symptoms. On T2-weighted fat-suppressed MRI, the quadratus femoris muscle may exhibit a high-intensity signal due to impingement between the ischium and a noticeable lesser trochanter (Figure 5). If the MRI examination is evaluated with a preliminary diagnosis of IFI, patient positioning during imaging is critical. For optimal assessment of the ischiofemoral space, the feet should be fixed in a neutral walking position. Otherwise, a misdiagnosis may be made due to a decrease in the ischiofemoral space. The QF muscle signal changes in IFI are observed in MRI. An important current limitation of MRI is in the determination of pathological versus healthy SN anatomy. Some of the SN small branches can be easily mistaken as fibrovascular bands. A 3T MRI with a high resolution, which can reveal the deep gluteal vascular fiber band should be preferred.
Figure 5. Axial T2-weighted fat-suppressed image reveals increased T2 signal within the right quadratus femoris muscle (arrow) and bilateral decreased ischiofemoral space.
It is also possible to evaluate the SN along its entire course using MR neurography. On MR neurography, morphological changes suggestive of neural damage are detected on T2-weighted or short tau inversion recovery (STIR) images.[55] However, its use is not necessary in daily practice. DGS can be considered in patients with suspicion and normal MRI.
Ultrasonography can visualize hematomas, tumors, and abscesses which may cause buttock pain. Recently, the use of ultrasonography has been recommended as a reliable diagnostic tool. Comparing arthroscopic and ultrasound findings, the two matched up in all cases. Therefore, ultrasound was tought to be an essential reference for intraoperative release.
It is concluded that both ultrasonography and MRI can clearly show the piriformis and SN and that both qualitative and quantitative measurements can be used.[56]
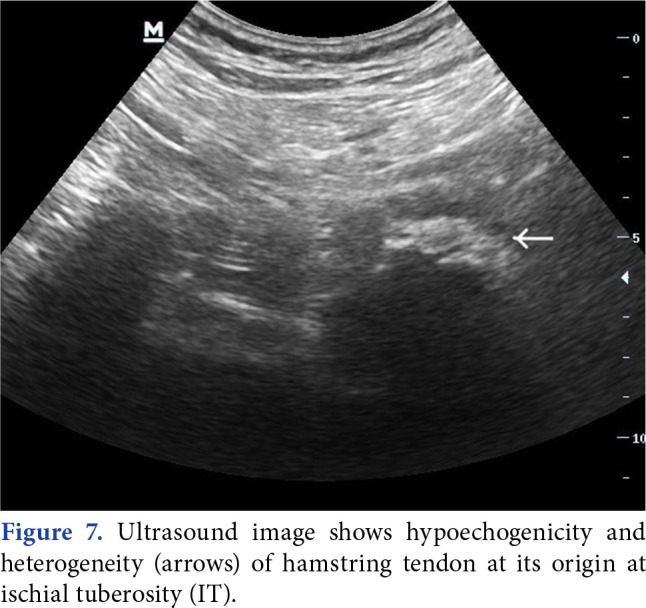
Chang et al.[57] described how to visualize all the anatomic structures in deep gluteal space by sonography in a recent study (Figure 6 and 7 demonstrated PS and hamstring tendinopathy by ultrasonography, respectively).
Figure 6. Hypertrophic piriformis muscle.
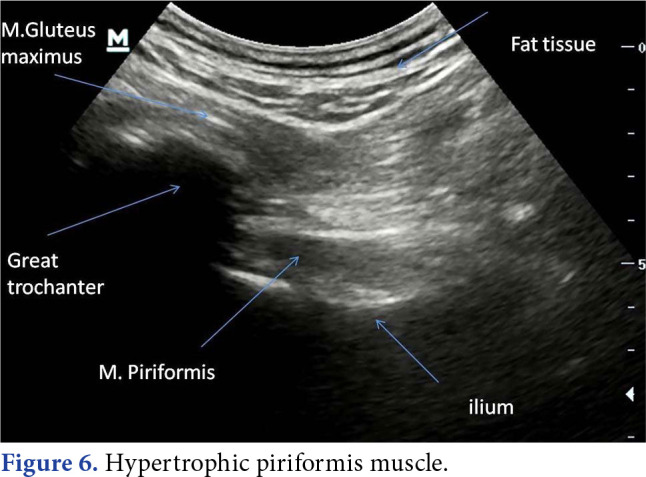
Figure 7. Ultrasound image shows hypoechogenicity and heterogeneity (arrows) of hamstring tendon at its origin at ischial tuberosity (IT).
Diagnostic injection
Diagnostic injection must be accompanied by ultrasonography due to the possibility of pathologies such as possible tumor formations and abscesses.[2]
Periarticular endoscopic examination
Periarticular endoscopic examination of the deep gluteal space can detect the cause of DGS.[58]
Although its diagnostic value is not significant, electromyography (EMG) is a method that can be used to help differentiate from lumbar radiculopathies.
TREATMENT
Understanding the cause of nerve entrapment is essential for successful treatment. In a recent systematic review by Hopayian et al.[58] which included 13 studies on DGS treatment, the common opinion was that the first-line treatment of DGS should primarily include activity modification (such as avoiding crossing one's legs), a conservative treatment consisting of non-steroidal anti-inflammatory drugs and muscle relaxant medications, and physical therapy modalities and exercises. Gabapentin/pregabalin can be used in cases where neuropathic pain is detected.[58,59]
Physiotherapy and exercise
Physical therapy and exercise programs should cover all related factors such as alignment and stabilization of the lumbosacral spine, pelvic floor and intra-articular structures. In the presence of intrapelvic causes, pelvic floor exercises should be added to the treatment plan.[60]
Heat application and analgesic electrical currents can be used in pain treatment. Soft tissue mobilization, strengthening and stretching exercises are prescribed for required muscles individually.
Muscle strengthening exercises: Strengthening specific muscle groups to correct imbalance, posture exercises should be prescribed individually. For instance, strengthening of the hip abductors should be added for patients with IFI.[45]
Gentle and controlled low intensity range of motion exercises can aid in promoting tendon healing and minimizing the development of scar tissue in patients with ischiotunnel syndrome.[61] Progression to the next stage of exercises is recommended when patients can walk without pain and can perform isometric exercises without discomfort. Eccentric knee flexion exercises play a role in encouraging the organization and cross-linking of collagen fibers within tendons, leading to improve mechanical strength and flexibility.
Stretching exercises for deep rotator muscles are strongly recommended.[58] Despite this much emphasis, there is only one randomized-controlled trial in the physiotherapy literature comparing two types of stretching exercises.[62,63] There is no study comparing exercise versus another form of treatment. Thus, it is necessary to carry out studies on this subject.
Nerve mobilization and nerve gliding exercises
Patients can be given a home exercise program consisting of SN mobilization with hip circumduction and nerve gliding exercises provided by rhythmic movement of the superior and inferior segments of the body.[64]
Injection treatment
In the region where the entrapment occurs, a guided injection can be made into the muscle causing the compression. Several medications were used in injection and the most common medications were local anesthetic (e.g. mepivacaine and lidocaine) alone or combined with corticosteroids (e.g. methylprednisolone) in different posologies.[60,65,66]
The studies are usually on PS. There is a limited number of studies evaluating the efficacy of injection treatments in patients with IFI. One study documented the efficacy of 8 mL of 0.25% lidocaine into the quadratus femoris muscle under ultrasound guidance.[67] Another study documented efficacy of ultrasound-guided prolotherapy injection of patients with IFI.[68] Proximal hamstring tendinopathy has been treated with peritendinous steroid injections, shockwave therapy, and percutaneous ultrasoundguided steroid injection in patients with ischiotunnel syndrome.[69] There is a gap about the treatment studies focusing on other causes of DGS rather than PS. Further studies should be planned.
In a study, comparing local anesthetic with local anesthetic-steroid combination in PS, no significant difference was seen between the groups and it was concluded that it was reasonable to apply only local anesthesia.[65] Injection of steroid, in cases with inflammation or spasm of a muscle which leads to irritation of the SN, would be preferred.
In various studies, it has been demonstrated that injections applied around the SN provide relief of symptoms.[70] Rosales et al.,[71] in contrast to others, proposed that ultrasound-guided high-volume injections (1 mL corticosteroid + 4 mL local anesthetic + 20 mL saline) in patients with refractory PS (DGS), not only exhibited anti-inflammatory efficacy similar to injections with lower volumes, but also moved the SN away from anatomical structures that may cause entrapment, suggesting the possibility of achieving results similar to endoscopic decompression. However, in half of the cases, symptoms reappeared approximately five weeks later.
Botulinum toxin (BTX) is effective in patients with myofascial pain syndrome etiology, where hypertrophy is at the forefront, and is preferred in refractory cases. In a study evaluating changes in piriformis muscle morphology after treatment with BTX injections, a significant decrease in the thickness, volume, and fatty infiltration of the piriformis muscle was observed. Botulinum toxin is thought to be effective by causing atrophy and fatty degeneration of the piriformis muscle.[72] In a meta-analysis of studies on BTX applications in PS, although the use of botulinum toxin type A in PS varied between 50 and 100 IU diluted with 1 to 1.5 mL normal saline, the outcome of these studies is limited. The issues are heterogeneous and it is difficult to make a clear judgment.[73]
The use of a "guide" for injections is necessary due to potential anatomical variations along the course of the SN in the deep gluteal space, as well as factors like space-occupying lesions, aneurysms, etc., exerting compression on the nerve. In the literature, various methods such as ultrasonography, computed tomography, MRI, fluoroscopy, and EMG-guided injections have been presented and proven successful. Among these, ultrasound-guided injection stands out as superior, given its easy accessibility and absence of radiation. The guide published by Chang et al.[57] serves as a valuable resource for physicians with an interest in this subject.
Surgery
Surgical treatment may be required in patients with a space-occupying lesion that compresses the SN, particularly in patients with potentially malignant lesions or chronic neurological disorders. For hamstring avulsions, osseous avulsions with retraction of more than 2 cm and complete avulsions of all three tendons (with or without retraction) may be required. Surgical intervention is recommended also in cases that do not respond to conservative treatments, endoscopic or open surgical interventions can be performed.[74] Resection of the lesser trochanter for refractory IFI,[46] piriformis muscle release for PS, arthroscopic sciatic neurolysis are some examples for surgery.[75]
Postoperative rehabilitation
Although its components vary depending on the anatomical structure in which the surgical intervention is performed, the main goal is to prevent adhesions that may occur by providing mobilization in the early period. In this regard, stretching of the SN is prevented and hip joint mobility is increased. Knee braces are often used to limit knee extension to prevent tension on the SN. Partial weight bearing should be provided with support for the first four to eight weeks.[59] It should be kept in mind that exercises to improve lumbopelvic stabilization in the rehabilitation plan are important to protect and increase SN mobility. It is not surprising that most of the studies were conducted in patients diagnosed with PS, since the majority of DGS is PS. However, studies should also be carried out on IFI and ischiotunnel syndrome, there is a gap in the literature on this subject.
In conclusion, according to several authors, the term DGS should be restricted to cases where the syndrome originates from musculoskeletal causes,[58] excluding instances where the SN is compressed by space-occupying lesions such as tumors or post-traumatic or postoperative scarring around the SN. As evident, there is no consensus on exactly what situations the term DGS covers. However, it is more important to be aware of this region in our daily practice, rather than getting stuck in terms and definitions. Consequently, a specialist in physical medicine and rehabilitation must have a solid understanding of anatomy and biomechanics. In the light of this knowledge, the specialist should accurately diagnose patients through medical history and physical examination as part of the differential diagnosis process. Imaging methods, especially 3-T MRI, are highly beneficial for identifying anatomical structures causing entrapment of the SN, in addition to ruling out other diagnoses that may present with a similar clinical picture. Although perineural injections, performed for both diagnostic and therapeutic purposes, are widely used in treatment, physical therapy and exercise modalities should not be overlooked. Since existing studies predominantly focus on injection techniques, there is a need for research on exercise programs.
Footnotes
Conflict of Interest: The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The author received no financial support for the research and/or authorship of this article.
References
- 1.McCrory P, Bell S. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med. 1999;27:261–274. doi: 10.2165/00007256-199927040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kizaki K, Uchida S, Shanmugaraj A, Aquino CC, Duong A, Simunovic N, et al. Deep gluteal syndrome is defined as a non-discogenic sciatic nerve disorder with entrapment in the deep gluteal space: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;28:3354–3364. doi: 10.1007/s00167-020-05966-x. [DOI] [PubMed] [Google Scholar]
- 3.Park MS, Jeong SY, Yoon SJ. Endoscopic sciatic nerve decompression after fracture or reconstructive surgery of the acetabulum in comparison with endoscopic treatments in idiopathic deep gluteal syndrome. Clin J Sport Med. 2019;29:203–208. doi: 10.1097/JSM.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 4.Leite MJ, Pinho AR, Silva MR, Lixa JC, Madeira MD, Pereira PG. Deep gluteal space anatomy and its relationship with deep gluteal pain syndromes. Hip Int. 2022;32:510–515. doi: 10.1177/1120700020966255. [DOI] [PubMed] [Google Scholar]
- 5.Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006;24:1883–1889. doi: 10.1002/jor.20210. [DOI] [PubMed] [Google Scholar]
- 6.Singer AD, Subhawong TK, Jose J, Tresley J, Clifford PD. Ischiofemoral impingement syndrome: A meta-analysis. Skeletal Radiol. 2015;44:831–837. doi: 10.1007/s00256-015-2111-y. [DOI] [PubMed] [Google Scholar]
- 7.Torriani M, Souto SC, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: An entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol. 2009;193:186–190. doi: 10.2214/AJR.08.2090. [DOI] [PubMed] [Google Scholar]
- 8.Miller SL, Webb GR. The proximal origin of the hamstrings and surrounding anatomy encountered during repair. Surgical technique. J Bone Joint Surg Am. 2008;90 Suppl 2:108–116. doi: 10.2106/JBJS.G.01281. [DOI] [PubMed] [Google Scholar]
- 9.Kivlan BR, Martin RL, Martin HD. Defining the greater trochanter-ischial space: A potential source of extraarticular impingement in the posterior hip region. J Hip Preserv Surg. 2016;3:352–357. doi: 10.1093/jhps/hnw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming P, Lenehan B, O'Rourke S, McHugh P, Kaar K, McCabe JP. Strain on the human sciatic nerve in vivo during movement of the hip and knee. J Bone Joint Surg Br. 2003;85:363–365. doi: 10.1302/0301-620x.85b3.13220. [DOI] [PubMed] [Google Scholar]
- 11.Park MS, Yoon SJ, Jung SY, Kim SH. Clinical results of endoscopic sciatic nerve decompression for deep gluteal syndrome: Mean 2-year follow-up. BMC Musculoskelet Disord. 2016;17:218–218. doi: 10.1186/s12891-016-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg Br. 1992;74:126–129. doi: 10.1302/0301-620X.74B1.1732240. [DOI] [PubMed] [Google Scholar]
- 13.Hernando MF, Cerezal L, Pérez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: Anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44:919–934. doi: 10.1007/s00256-015-2124-6. [DOI] [PubMed] [Google Scholar]
- 14.Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequence: A review. Clin Anat. 2010;23:8–17. doi: 10.1002/ca.20893. [DOI] [PubMed] [Google Scholar]
- 15.Beaton L, Anson B. The relation of the sciatic nerve and of its subdivisions to the piriformis muscle. Anat Rec. 2005;70:1–5. doi: 10.1002/ar.1090700102. [DOI] [Google Scholar]
- 16.Windisch G, Braun EM, Anderhuber F. Piriformis muscle: Clinical anatomy and consideration of the piriformis syndrome. Surg Radiol Anat. 2007;29:37–45. doi: 10.1007/s00276-006-0169-x. [DOI] [PubMed] [Google Scholar]
- 17.Arora J, Mehta V, Kumar H, Suri RK, Rath G, Das S. A rare bimuscular conglomeration gluteopiriformis case report. Morphologie. 2010;94:40–43. doi: 10.1016/j.morpho.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Bloom DA, Essilfie AA, Wolfert A, Youm T. Infected hematoma after endoscopic sciatic nerve decompression. e171-4Arthrosc Sports Med Rehabil. 2020;2 doi: 10.1016/j.asmr.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon SJ, Park MS, Matsuda DK, Choi YH. Endoscopic resection of acetabular screw tip to decompress sciatic nerve following total hip arthroplasty. BMC Musculoskelet Disord. 2018;19:184–184. doi: 10.1186/s12891-018-2091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer-Gardner L, Bedi A, Stuart MJ, Larson CM, Kelly BT, Krych AJ. Ischiofemoral impingement and hamstring dysfunction as a potential pain generator after ischial tuberosity apophyseal fracture non-union/malunion. Knee Surg Sports Traumatol Arthrosc. 2017;25:55–61. doi: 10.1007/s00167-015-3812-4. [DOI] [PubMed] [Google Scholar]
- 21.Drăghici NC, Văcăraș V, Bolchis R, Bashimov A, Domnița DM, Iluț S, et al. Diagnostic approach to lower limb entrapment neuropathies: A narrative literature review. Diagnostics (Basel) 2023;13:3385–3385. doi: 10.3390/diagnostics13213385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheterpal AB, Harvey JP, Husseini JS, Martin SD, Torriani M, Bredella MA. Hip abductor tears in ischiofemoral impingement. Skeletal Radiol. 2020;49:1747–1752. doi: 10.1007/s00256-020-03497-7. [DOI] [PubMed] [Google Scholar]
- 23.DiSciullo AA, Stelzer JW, Martin SD. Dynamic ischiofemoral impingement: Case-based evidence of progressive pathophysiology from hip abductor insufficiency: A report of two cases. e107JBJS Case Connect. 2018;8 doi: 10.2106/JBJS.CC.18.00153. [DOI] [PubMed] [Google Scholar]
- 24.Zibis AH, Fyllos AH, Karantanas AH, Raoulis V, Karachalios TS, Arvanitis DL. Quadratus femoris tear as an unusual cause of hip pain: A case report. e7-9Hip Int. 2016;26 doi: 10.5301/hipint.5000304. [DOI] [PubMed] [Google Scholar]
- 25.Jeyaraman M, Murugan J, Maffulli N, Jeyaraman N, Potty AG, Gupta A. Ischiofemoral impingement syndrome: A case report and review of literature. J Orthop Surg Res. 2022;17:393–393. doi: 10.1186/s13018-022-03287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garozzo D, Oluwole J, Iantorno V, Fahad S, Hegazy A, Ibrahim H, et al. Deep gluteal syndrome: Diagnostic assessment and surgical treatment of non-discogenic sciatica in our experience. J Peripher Nerv Surg. 2018;2:10–20. [Google Scholar]
- 27.de Carvalho AD, Garcia FL, Nogueira-Barbosa MH. Ischiofemoral impingement secondary to valgus intertrochanteric osteotomy: A case report. Radiol Bras. 2017;50:335–337. doi: 10.1590/0100-3984.2013.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida S, Kizaki K, Hirano F, Martin HD, Sakai A. Postoperative deep gluteal syndrome after hip arthroscopic surgery. Orthop J Sports Med. 2020;8:2325967120951118–2325967120951118. doi: 10.1177/2325967120951118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geler Külcü D, Öztürk G, Taşdelen N, Aydoğ E. Extragenital endometriosis; a rare casue of sciatica: A case report. Turk J Phys Med Rehab. 2015;61:184–186. [Google Scholar]
- 30.Byrd JW. Piriformis syndrome. Oper Tech Sports Med. 2005;13:71–79. doi: 10.1053/j.otsm.2004.09.008. [DOI] [Google Scholar]
- 31.Morris WZ, Fowers CA, Weinberg DS, Millis MB, Tu LA, Liu RW. Hip morphology predicts posterior hip impingement in a cadaveric model. Hip Int. 2019;29:322–327. doi: 10.1177/1120700018779906. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi Y, Suzuki H, Nakashima H, Murata Y, Matsuda DK, Sakai A, et al. Radiologic correlation between the ıschiofemoral space and morphologic characteristics of the hip in hips with symptoms of dysplasia. AJR Am J Roentgenol. 2018;210:608–614. doi: 10.2214/AJR.17.18465. [DOI] [PubMed] [Google Scholar]
- 33.Filler AG, Haynes J, Jordan SE, Prager J, Villablanca JP, Farahani K, et al. Sciatica of nondisc origin and piriformis syndrome: Diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2:99–115. doi: 10.3171/spi.2005.2.2.0099. [DOI] [PubMed] [Google Scholar]
- 34.Martin HD, Shears SA, Johnson JC, Smathers AM, Palmer IJ. The endoscopic treatment of sciatic nerve entrapment/ deep gluteal syndrome. Arthroscopy. 2011;27:172–181. doi: 10.1016/j.arthro.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Kay J, de Sa D, Morrison L, Fejtek E, Simunovic N, Martin HD, et al. Surgical management of deep gluteal syndrome causing sciatic nerve entrapment: A systematic review. Arthroscopy. 2017;33:2263–2278. doi: 10.1016/j.arthro.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 36.Park JW, Lee YK, Lee YJ, Shin S, Kang Y, Koo KH. Deep gluteal syndrome as a cause of posterior hip pain and sciatica-like pain. Bone Joint J. 2020;102-B:556–567. doi: 10.1302/0301-620X.102B5.BJJ-2019-1212.R1. [DOI] [PubMed] [Google Scholar]
- 37.Kulcu DG, Naderi S. Differential diagnosis of intraspinal and extraspinal non-discogenic sciatica. J Clin Neurosci. 2008;15:1246–1252. doi: 10.1016/j.jocn.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Birnbaum K, Prescher A, Hessler S, Heller KD. The sensory innervation of the hip joint--an anatomical study. Surg Radiol Anat. 1997;19:371–375. doi: 10.1007/BF01628504. [DOI] [PubMed] [Google Scholar]
- 39.Aytaç HD, Külcü DG, Mesci N. Frequency of Sacroiliac Joint Dysfunction (SIJD) in Patients with Failed Back Surgery Syndrome (FBSS), and affecting demographic and surgical factors. Haydarpasa Numune Med J. 2022;62:334–341. doi: 10.14744/hnhj.2020.35492. [DOI] [Google Scholar]
- 40.Martin HD, Reddy M, Gómez-Hoyos J. Deep gluteal syndrome. J Hip Preserv Surg. 2015;2:99–107. doi: 10.1093/jhps/hnv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin HD, Khoury A, Schröder R, Palmer IJ. Ischiofemoral impingement and hamstring syndrome as causes of posterior hip pain: Where do we go next. Clin Sports Med. 2016;35:469–486. doi: 10.1016/j.csm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Fredericson M, Moore W, Guillet M, Beaulieu C. High hamstring tendinopathy in runners: Meeting the challenges of diagnosis, treatment, and rehabilitation. Phys Sportsmed. 2005;33:32–43. doi: 10.3810/psm.2005.05.89. [DOI] [PubMed] [Google Scholar]
- 43.Lempainen L, Sarimo J, Mattila K, Vaittinen S, Orava S. Proximal hamstring tendinopathy: Results of surgical management and histopathologic findings. Am J Sports Med. 2009;37:727–734. doi: 10.1177/0363546508330129. [DOI] [PubMed] [Google Scholar]
- 44.Trescot AM. In: Peripheral nerve entrapments. Trescot AM, editor. Cham: Springer; 2016. Inferior gluteal nerve entrapment; pp. 581–587. [Google Scholar]
- 45.Diop M, Parratte B, Tatu L, Vuillier F, Faure A, Monnier G. Anatomical bases of superior gluteal nerve entrapment syndrome in the suprapiriformis foramen. Surg Radiol Anat. 2002;24:155–159. doi: 10.1007/s00276-002-0048-z. [DOI] [PubMed] [Google Scholar]
- 46.Gollwitzer H, Banke IJ, Schauwecker J, Gerdesmeyer L, Suren C. How to address ischiofemoral impingement? Treatment algorithm and review of the literature. J Hip Preserv Surg. 2017;4:289–298. doi: 10.1093/jhps/hnx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michel F, Decavel P, Toussirot E, Tatu L, Aleton E, Monnier G, et al. Piriformis muscle syndrome: Diagnostic criteria and treatment of a monocentric series of 250 patients. Ann Phys Rehabil Med. 2013;56:371–383. doi: 10.1016/j.rehab.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Hopayian K, Danielyan A. Four symptoms define the piriformis syndrome: An updated systematic review of its clinical features. Eur J Orthop Surg Traumatol. 2018;28:155–164. doi: 10.1007/s00590-017-2031-8. [DOI] [PubMed] [Google Scholar]
- 49.Cass SP. Piriformis syndrome: A cause of nondiscogenic sciatica. Curr Sports Med Rep. 2015;14:41–44. doi: 10.1249/JSR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 50.Balius R, Susín A, Morros C, Pujol M, Pérez-Cuenca D, Sala-Blanch X. Gemelli-obturator complex in the deep gluteal space: An anatomic and dynamic study. Skeletal Radiol. 2018;47:763–770. doi: 10.1007/s00256-017-2831-2. [DOI] [PubMed] [Google Scholar]
- 51.Atıcı A, Geler Külcü D, Akpınar P, Akay Urgun D. A rare cause of non-discogenic sciatica; musculus gemellus inferior: A case report. Turk J Phys Med Rehabil. 2017;63:355–356. doi: 10.5606/tftrd.2017.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murata Y, Ogata S, Ikeda Y, Yamagata M. An unusual cause of sciatic pain as a result of the dynamic motion of the obturator internus muscle. e16-8Spine J. 2009;9 doi: 10.1016/j.spinee.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Ovadia J, Khabyeh-Hasbani N, Amar E, Rath E. Clinical assessment and treatment options for posterior hip pain. Isr Med Assoc J. 2021;23:534–540. [PubMed] [Google Scholar]
- 54.Varenika V, Lutz AM, Beaulieu CF, Bucknor MD. Detection and prevalence of variant sciatic nerve anatomy in relation to the piriformis muscle on MRI. Skeletal Radiol. 2017;46:751–757. doi: 10.1007/s00256-017-2597-6. [DOI] [PubMed] [Google Scholar]
- 55.Chhabra A, Chalian M, Soldatos T, Andreisek G, FaridianAragh N, Williams E, et al. 3-T high-resolution MR neurography of sciatic neuropathy. W357-64AJR Am J Roentgenol. 2012;198 doi: 10.2214/AJR.11.6981. [DOI] [PubMed] [Google Scholar]
- 56.Wu YY, Guo XY, Chen K, He FD, Quan JR. Feasibility and reliability of an ultrasound examination to diagnose piriformis syndrome. e1085-92World Neurosurg. 2020;134 doi: 10.1016/j.wneu.2019.11.098. [DOI] [PubMed] [Google Scholar]
- 57.Chang KV, Wu WT, Lew HL, Özçakar L. Ultrasound imaging and guided injection for the lateral and posterior hip. Am J Phys Med Rehabil. 2018;97:285–291. doi: 10.1097/PHM.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 58.Hopayian K, Mirzaei M, Shamsi M, Arab-Zozani M. A systematic review of conservative and surgical treatments for deep gluteal syndrome. J Bodyw Mov Ther. 2023;36:244–250. doi: 10.1016/j.jbmt.2022.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Martin HD, G´omez-Hoyos J. In: Posterior hip disorders. Martin HD, G´omez-Hoyos J, editors. Cham: Springer; 2019. Deep gluteal syndrome; pp. 167–187. [Google Scholar]
- 60.Vij N, Kiernan H, Bisht R, Singleton I, Cornett EM, Kaye AD, et al. Surgical and non-surgical treatment options for piriformis syndrome: A literature review. e112825Anesth Pain Med. 2021;11 doi: 10.5812/aapm.112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pietrzak JR, Kayani B, Tahmassebi J, Haddad FS. Proximal hamstring tendinopathy: Pathophysiology, diagnosis and treatment. Br J Hosp Med (Lond) 2018;79:389–394. doi: 10.12968/hmed.2018.79.7.389. [DOI] [PubMed] [Google Scholar]
- 62.Saeed Q, Malik AN, Ghulam S. Outcome of specific piriformis stretching technique in females with piriformis syndrome. J Pioneer Med Sci. 2017;7:55–58. [Google Scholar]
- 63.Dakou M, Iakovidis P, Lytras D, Kottaras A, Chasapis G, Kottaras I. The effect of physiotherapy in the treatment of piriformis syndrome: A narrative review. Natl J Clin Orthop. 2021;5:24–26. doi: 10.33545/orthor.2021.v5.i2a.278. [DOI] [Google Scholar]
- 64.Schröder RG, Martin RRL, Bobb VL, Khoury AN, Palmer IJ, Martin HD. Outcomes of non-operative management of deep gluteal syndrome - a case series of six patients. J Musculoskelet Disord Treat. 2016;2:12–12. [Google Scholar]
- 65.Misirlioglu TO, Akgun K, Palamar D, Erden MG, Erbilir T. Piriformis syndrome: Comparison of the effectiveness of local anesthetic and corticosteroid injections: A doubleblinded, randomized controlled study. Pain Physician. 2015;18:163–171. [PubMed] [Google Scholar]
- 66.Blunk JA, Nowotny M, Scharf J, Benrath J. MRI verification of ultrasound-guided infiltrations of local anesthetics into the piriformis muscle. Pain Med. 2013;14:1593–1599. doi: 10.1111/pme.12173. [DOI] [PubMed] [Google Scholar]
- 67.Kim DH, Yoon DM, Yoon KB. Ultrasound-guided quadratus femoris muscle injection in patients with lower buttock pain: Novel ultrasound-guided approach and clinical effectiveness. E863-70Pain Physician. 2016;19 [PubMed] [Google Scholar]
- 68.Kim WJ, Shin HY, Koo GH, Park HG, Ha YC, Park YH. Ultrasound-guided prolotherapy with polydeoxyribonucleotide sodium in ischiofemoral impingement syndrome. Pain Pract. 2014;14:649–655. doi: 10.1111/papr.12215. [DOI] [PubMed] [Google Scholar]
- 69.Dizon P, Jeanfavre M, Leff G, Norton R. Comparison of conservative interventions for proximal hamstring tendinopathy: A systematic review and recommendations for rehabilitation. Sports (Basel) 2023;11:53–53. doi: 10.3390/sports11030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masala S, Crusco S, Meschini A, Taglieri A, Calabria E, Simonetti G. Piriformis syndrome: Long-term followup in patients treated with percutaneous injection of anesthetic and corticosteroid under CT guidance. Cardiovasc Intervent Radiol. 2012;35:375–382. doi: 10.1007/s00270-011-0185-z. [DOI] [PubMed] [Google Scholar]
- 71.Rosales J, García N, Rafols C, Pérez M, Verdugo MA. Perisciatic ultrasound-guided infiltration for treatment of deep gluteal syndrome: Description of technique and preliminary results. J Ultrasound Med. 2015;34:2093–2097. doi: 10.7863/ultra.14.12030. [DOI] [PubMed] [Google Scholar]
- 72.Al-Al-Shaikh M, Michel F, Parratte B, Kastler B, Vidal C, Aubry S. An MRI evaluation of changes in piriformis muscle morphology induced by botulinum toxin injections in the treatment of piriformis syndrome. Diagn Interv Imaging. 2015;96:37–43. doi: 10.1016/j.diii.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 73.Santamato A, Micello MF, Valeno G, Beatrice R, Cinone N, Baricich A, et al. Ultrasound-guided injection of botulinum toxin type a for piriformis muscle syndrome: A case report and review of the literature. Toxins (Basel) 2015;7:3045–3056. doi: 10.3390/toxins7083045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metikala S, Sharma V. Endoscopic sciatic neurolysis for deep gluteal syndrome: A systematic review. e23153Cureus. 2022;14 doi: 10.7759/cureus.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun G, Fu W, Li Q, Yin Y. Arthroscopic treatment of deep gluteal syndrome and the application value of high-frequency ultrasound. BMC Musculoskelet Disord. 2023;24:742–742. doi: 10.1186/s12891-023-06863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]