Abstract
Objectives
The study aimed to compare the effect of cranial electrical stimulation (CES) and transcranial direct current stimulation (tDCS) in improving cognition among individuals with mild traumatic brain injury.
Patients and methods
The pretest-posttest randomized controlled study was conducted between November 2020 and March 2022. Seventy-two patients (64 males, 8 females; mean age: 40.5±9.5 years; range, 18 to 45 years) experiencing cognitive impairment within three months of traumatic brain injury were recruited. Participants were randomly assigned into two groups: Group 1 (CES with cognitive training, n=36) and Group 2 (tDCS with cognitive training, n=36). Participants were blinded in the study. Both groups received 30-min sessions of neuromodulation along with 30 min of cognitive training five days a week for four weeks. The patients were assessed at baseline and at the end of two and four weeks of intervention. The primary outcome measure was the Montreal Cognition Assessment (MoCA), and the secondary outcome measure was the Galveston Orientation Amnesia Test (GOAT).
Results
Demographic and baseline characteristics depicted normal distribution for both groups (p>0.05). Within group analyses of both groups demonstrated significant differences for both outcome measures (MoCA: p=0.001; GOAT: p=0.001). Between group analyses of MoCA showed significant improvement with p-value of 0.001 while GOAT exhibited p-value of 0.002 showing significant difference between the two groups. Time group interaction effect and covariance analyses depicted significant improvement with p-value of 0.001 for both outcome measures with excellent effect size >0.80.
Conclusion
Cranial electrical stimulation was a more effective noninvasive neuromodulatory device than tDCS in improving cognition among individuals with traumatic brain injury.
Keywords: Brain stimulation, cranial electrical stimulation, cognitive dysfunction, head trauma, neuromodulation, transcranial direct current stimulation.
Introduction
Mild traumatic brain injury (mTBI) is the most common type of traumatic insult to the brain, where symptoms may present as irritability, headaches, anxiety, fatigue, depression, and impaired cognition. Survivors may continue to exhibit cognitive difficulties in functioning relative to optimal levels at work, home, or in the community even years after injury.[1] The persistence of injury-related cognitive impairments can have devastating consequences for everyday function and can deleteriously interfere with patients’ return to optimum living standards. Sixty-five percent of moderate-to-severe TBI cases displayed persisting cognitive deficits pertaining to common ailments in attention, memory, and executive functions.[2] Amnesia is the most common cognitive illness in individuals with head injury; perceptual memory, including the combination of retrograde memory, meta-cognitive functions, and executive functions, is frequently impaired following brain trauma.[3]
Rehabilitation strategies aim at improving cognitive difficulties in individuals with mTBI to gain faster independence and facilitate community integration[4] through training on cognitive tasks set within the context of carrying out daily life functions. Numerous pharmacological and nonpharmacological interventions for perceptual and cognitive deficits are present in the literature.[1] Pharmacological interventions have failed to show realizable benefits in mitigating cognitive decline. As a result, there is growing interest in exploring the benefits of nonpharmacological interventions for this domain.[5]
Cognitive rehabilitation directly targets domains such as cognition and psychosocial functioning, and improvements in cognitive functions could indirectly lead to improvements in physical functioning.[4] It may include REHACOP (http://rehacop.deusto. es),[5] physical activity, cognitive training, functional task exercise,[6] computer-assisted problem-solving program,[7] compensatory cognitive training, CogSMART (Cognitive Symptom Management and Rehabilitation Therapy),[8] functional cognitive training software, virtual reality training,[9] computer based cognitive training,[10] lifestyle modifications (nutrition and exercise),[11] attention process training, compensatory strategy training, including internalized strategy training (e.g., visual imagery) and external memory compensations (e.g., memory notebooks and assistive technologies [AT] tools), vision restoration therapy, constraint-induced aphasia therapy, melodic intonation therapy, Lee Silverman voice treatment, metacognitive strategy training, cognitive behavioral therapy, and family therapy.[12] These methods are widely used in different populations of cognition.[13] Some of the neuromodulatory techniques such as deep brain stimulation[6] and repetitive noninvasive brain stimulation,[12] such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation (tDCS),[3] was also found to be effective in improving cognition. Cranial electrotherapy stimulation (CES) is another neuromodulatory technique that has been increasingly used for various neuropsychological domains.[14]
There are various neuropsychological outcome tools and scales available to assess cognition, such as the Rancho Los Amigos scale, Montreal Cognition Assessment (MoCA), and Galveston Orientation Amnesia Test (GOAT). The clinician selects the outcome based on applicability, accessibility, and ease of administration so that the quantification of the data can be done. Montreal Cognition Assessment evaluates attention, memory, concentration, language, executive functions, concentration, abstraction, orientation, calculation, and visuospatial skills and has a total score of 30.[15] The GOAT assesses posttraumatic amnesia and retrograde amnesia and consists of 10 items presented by the patient orally. The total score of GOAT ranges from 0 to 100.[16,17]
Beneficial effects of tDCS have been shown in the management of cognition in individuals with mTBI.[3] However, to the best of our knowledge, no previous research has evaluated the efficacy of CES in improving cognition in individuals with mTBI and compared these two treatment modalities. This study aimed to investigate and compare the therapeutic effects of CES and tDCS for the treatment of cognition in patients with mTBI. It was hypothesized that individuals with traumatic brain injury would benefit more from CES than tDCS in improving cognition.
Patients and Methods
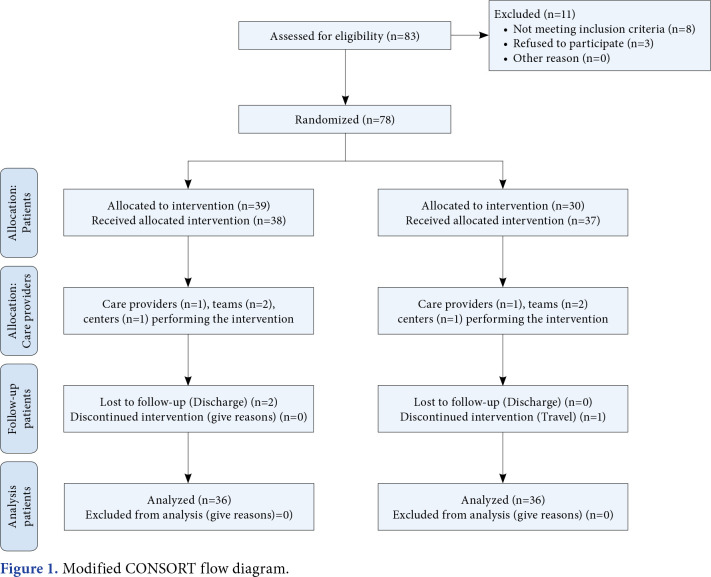
Seventy-two mTBI patients (64 males, 8 females; mean age: 40.5±9.5 years; range, 18 to 45 years) were enrolled in this randomized controlled study conducted at the Maharishi Markandeshwar Superspeciality Hospital, Department of Neurosurgery between November 2020 and March 2022. The inclusion criteria were as follows: individuals within three months of injury, a MoCA score <25, a GOAT score <66, and a Glasgow Coma Scale score between 12 and 15. Individuals with a metal implant at the site of stimulation, concomitant neurological disorders, individuals with previous history of TBI, psychiatric disorders, drug or alcohol abuse, seizure disorders, cardiac pacemaker, hypertension, and medical illness that may affect cognitive functions (thyroid, renal, or hepatic disorder), presence of wounds in the skin of the skull, and pregnant women were excluded. Demographic characteristics and outcomes were obtained from all individuals before intervention. Study participation and enrolment are detailed in Figure 1.
Figure 1. Modified CONSORT flow diagram.
A random allocation software was utilized to randomly allocate the individuals into Group 1 (CES with cognitive training) and Group 2 (tDCS with cognitive training) via block randomization technique using the sequentially numbered opaque sealed envelope method with a matrix size of 12x6. Afterward, subjects were equally allocated to the groups by the assessor. Subjects were blinded in this study, given that all procedures and outcome measures were conducted and assessed by the therapist.
Individuals in each group received 20 sessions of intervention over a four-week period (five sessions per week). Individuals were in a sitting position. Both groups were assessed at baseline and at the end of two and four weeks of intervention.
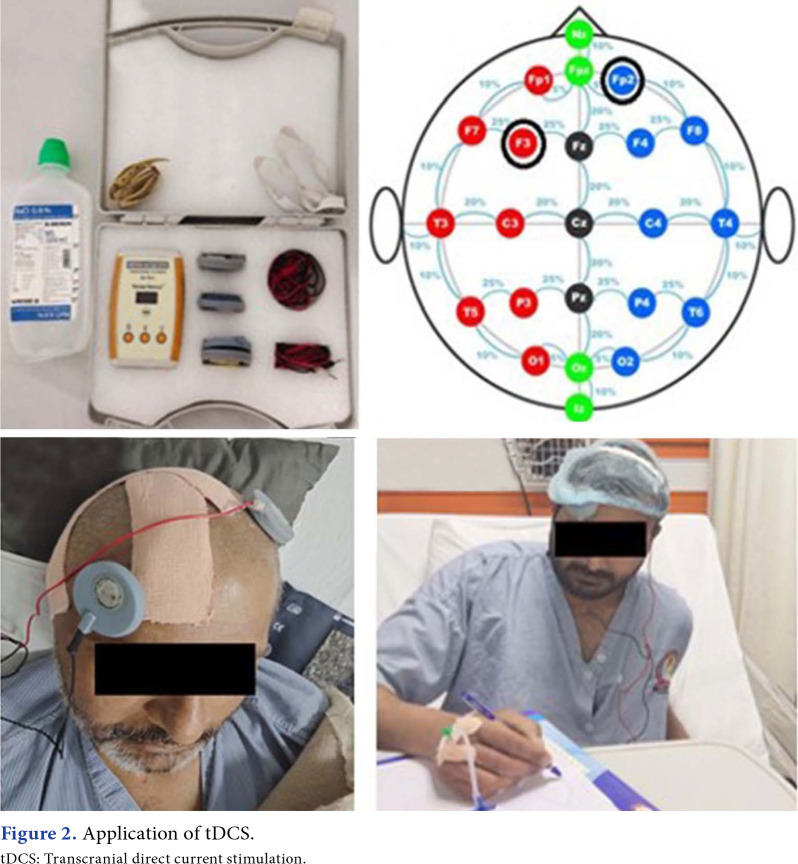
In Group 1, the Alpha-Stim® 100 microcurrent and cranial electrotherapy stimulator (Electromedical Products International, Mineral Wells, TX, USA) was utilized. The Alpha-Stim® 100 generates bipolar asymmetric rectangular waves with a frequency of 0.5, 1.5, or 100 Hz and a constantly adjustable current intensity to deliver between 10 and 600 A (http://www.alpha-stim.com). This device stimulates the brain electrically. As these pulse frequencies are most frequently applied in clinical treatment, we tested 0.5- and 100-Hz pulse frequencies. Due to technology limitations, the maker adjusted the gadget such that it would automatically alternate between "on" blocks of 22 sec and "off" blocks of 22 sec for the duration of the experiment. Copper wires were used to link the instrument to nonferromagnetic adhesive electrodes with a contact area of 1.5 cm on the participants' left and right earlobes, respectively.
In Group 2, a battery-driven tDCS (Walnut Medical, Punjab, India) was used with its anode placed over the F3 region of the cortex to stimulate the left dorsolateral prefrontal cortex and a cathode placed over the contralateral supraorbital region. A constant current with an intensity of 2 mA was applied for 30 min through electrodes (anode & cathode) with a surface area of 28.3 cm2 and covered with saline-soaked sponges. Electrodes were placed according to the 10-20 electroencephalogram system of electrode placement.
Both groups received cognitive training from a therapist in addition to brain stimulation. Cognitive training consisted of attention and concentration exercises (e.g., drawing exercise, calculation, and activities by the nondominant hand); memory skills (e.g., picture recall, naming therapy, and card recall), and executive functional training (e.g., series of elements, series of real-life situations, and list of words in pair; Table 1, Figures 2, 3).
Table 1. Cognitive training.
| Domain | Intervention & Strategies | Activity |
| Attention & Concentration | Drawing exercise | Drawing of hexagon, pentagon and octagon were observed by the patient and asked to reproduce them exactly. |
| Calculation | Patients were asked to pick any 2-digit number, then asked to add 3 to that number 3 times, then 5 was subtracted from that final number 3 times. | |
| Non dominant hand activities | Patients were asked to use non- dominant hand in doing activities like feeding, combing, medication, hand shake. | |
| Memory skills | Picture Recall | Pictures (Lion, Camel and Rhinoceros) illustrated in MoCA were shown to the patients and then questions are asked to confirm some details of the image. |
| Naming Therapy | Patients were ought to read the list of the words given in assessment form of MoCA. After 5 min, patient tries to remember as much words as possible. | |
| Card Recall | 10 cards were chosen from a deck; placed on the table so as to be observed by the patient. After 2 min, they were turned down. Then patients were ought to recall the cards and arrange them in ascending/descending order. | |
| Executive functional training includes | Series of elements | (a,c,e,g,_); (2,4,6,8,_); (6,5,4,3,_) were provided to the patients and asked to indicate which is the next element, following the logical sequence. |
| Series of real-life situations | Situations were presented to the patients like (when someone surprises you, when someone greets you, when someone orders you to do something, etc.) and were asked to what would be their reactions and why. | |
| List of words in pair | (orange-banana; bicycle-car; pen-pencil; camel-cow; tea-water) were presented to patients and were asked to indicate the relationship between the pairs i.e. similarities and dissimilarities. | |
| MoCA: Montreal cognitive assessment. | ||
Figure 2. Application of tDCS. tDCS: Transcranial direct current stimulation.
Figure 3. Application of CES. CES: Cranial electrical stimulation.
Individuals’ functions were assessed by MoCA and GOAT, which were measured at baseline and two and four weeks after the end of the last session. Montreal Cognitive Assessment is a useful, applicable, and psychometrically valid tool for neuropsychological outcomes in TBI patients with adequate content and construct validity and reliability [Cronbach’s alpha=0.78 (0.73; 0.80), composite reliability=0.86].[15] Montreal Cognitive Assessment has 66.7% specificity and 87.9% sensitivity for detecting cognitive impairment and is proven to be a highly valid tool for this population,[15] and GOAT has excellent interrater reliability (r=0.99).[16,17]
Statistical analysis
Sample size was calculated in accordance with the study of Stienen et al.[18] The sample size was calculated with the following formula, where n was the sample size required in each group, S was the standard deviation of the primary outcome variable, and D was the size of difference of clinical importance: n=2[(Zα+Zβ) S/D]2. It was calculated that 16 participants in each group were required, with a level of significance of 95% (1.96) and a power of 80% (0.84). Moreover, considering a 40% dropout rate, a final sample of 23 in each group was determined. Nonetheless, the sample size was increased to 72 individuals, with 36 in each group, to minimize the marginal errors and achieve accurate results.
Data were analyzed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to assess the normality of the demographic and baseline characteristics of each group. The effects of both groups (from baseline to after intervention) were assessed with repeated measure analysis of variance (ANOVA). The MoCA and GOAT scores were measured with the independent sample t-test and analysis of covariance (ANCOVA) for between-group analyses. Time and group interaction was analyzed using two-way ANOVA. Effect sizes were determined with Cohen’s d [(M1-M2)/spooled], according to Cohen’s[19] criteria. Effect sizes >0.8 were considered large, 0.5- 0.8 moderate, and 0.2-0.5 small. A p-value <0.05 was considered statistically significant.
Results
No significant differences were observed between Group 1 and Group 2 in terms of individuals’ demographic and baseline characteristics (Table 2).
Table 2. Patients’ demographic and baseline characteristics.
| Variables | CES+CT (Group 1) (n=36) | tDCS+CT (Group 2) (n=36) | ||||||
| Mean±SD | Median | Range | p | Mean±SD | Median | Range | p | |
| Age (year) | 39.3±10.5 | 0.653* | 41.7±8.4 | 0.243* | ||||
| Weight (kg) | 77.10±8.57 | 0.059* | 74.44±10.77 | 0.560* | ||||
| Height (cm) | 167.90±4.76 | 0.060* | 169.23±4.01 | 0.577* | ||||
| BMI (kg/m2) | 27.38±3.18 | 0.009 | 25.94±3.33 | 0.575* | ||||
| GCS | 13 | 11-15 | 0.039 | 13 | 11-15 | 0.364* | ||
| Duration of injury (days) | 5.5 | 3-9 | 0.709* | 6 | 4-12 | 0.092* | ||
| MoCA at baseline | 12 | 6-18 | 0.606* | 14 | 5-20 | 0.868* | ||
| GOAT at baseline | 46 | 38-58 | 0.095* | 49.5 | 31-64 | 0.171* | ||
| CES: Cranial electrotherapy stimulation; CT: Cognitive training; tDCS: Transcranial direct current stimulation; SD: Standard deviation; MoCA: Montreal cognitive assessment; GOAT: Galveston orientation and amnesia teel; * Level of significance (p-value) was set at >0.05; Shapiro-Wilk test was used to analyze normality of each group. | ||||||||
Within-group analyses showed significant improvement in both groups regarding all outcomes when comparing the baseline values to after four weeks of intervention (Table 3). However, there was no significant improvement at the end of two weeks of intervention.
Table 3. Analysis of outcome measures for Group 1 and Group 2.
| Outcome measure | Time line | Group 1 | Group 2 | Between group analysis# | |||||
| Mean±SD | 95% CI | p | Mean±SD | 95% CI | p | Mean difference | p | ||
| MoCA | Baseline | 12.89±3.9 | 9.83-15.95 | 0.001* | 12.78±4.8 | 9.04-16.52 | 0.001* | 0.11 | 0.958 |
| 2nd week | 16.22±4.35 | 12.88-19.57 | 19.78±4.9 | 15.98-23.58 | -3.56 | 0.570 | |||
| 4th week | 19.22±3.9 | 16.18-22.27 | 27.56±2.1 | 25.88-29.24 | -8.34 | 0.001‡ | |||
| GOAT | Baseline | 47.89±6.58 | 42.83-52.95 | 0.001* | 47.22±12.8 | 37.35-57.09 | 0.001* | 0.67 | 0.892 |
| 2nd week | 56.89±4.93 | 53.09-60.68 | 62.56±12.1 | 53.23-71.88 | -5.67 | 0.938 | |||
| 4th week | 67.56±5.76 | 63.12-71.99 | 77.89±11.2 | 69.29-86.49 | -10.33 | 0.002‡ | |||
| SD: Standard deviation; CI: Confidence interval; MoCA: Montreal cognitive assessment; GOAT: Galveston orientation and amnesia test; Repeated Measure ANOVA was used for within-group and between-group analyses, and interaction effect and independent sample t-test were used for between-group analysis at baseline; ANCOVA was used for analysis at two and four weeks; * Level of significance (p-value) measured through Repeated Measure ANOVA was set at <0.05; # Significance level for time*group interaction by ANOVA was set at <0.05; ‡ Level of significance by ANCOVA at two and four weeks was set at <0.05. | |||||||||
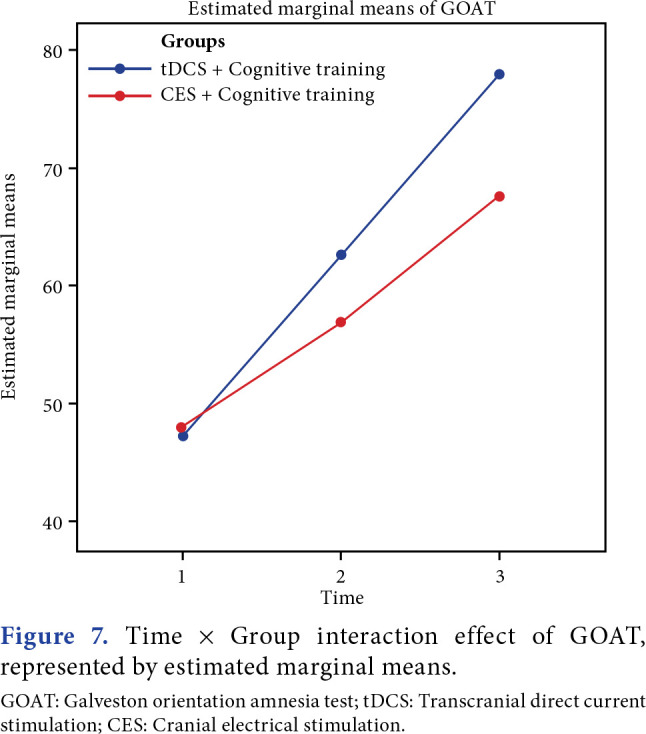
Between-group analyses demonstrated a highly significant improvement in MoCA (p=0.001), and a significant improvement in GOAT was observed (p=0.02). Time and group interaction effect was found to be significant for both outcomes between the groups (Figures 4-7).
Figure 4. Between group analyses of MoCA scores, represented by Bars and Error bars in 95% CI. MoCA: Montreal cognition assessment; tDCS: Transcranial direct current stimulation; CES: Cranial electrical stimulation; CI: Confidence interval.
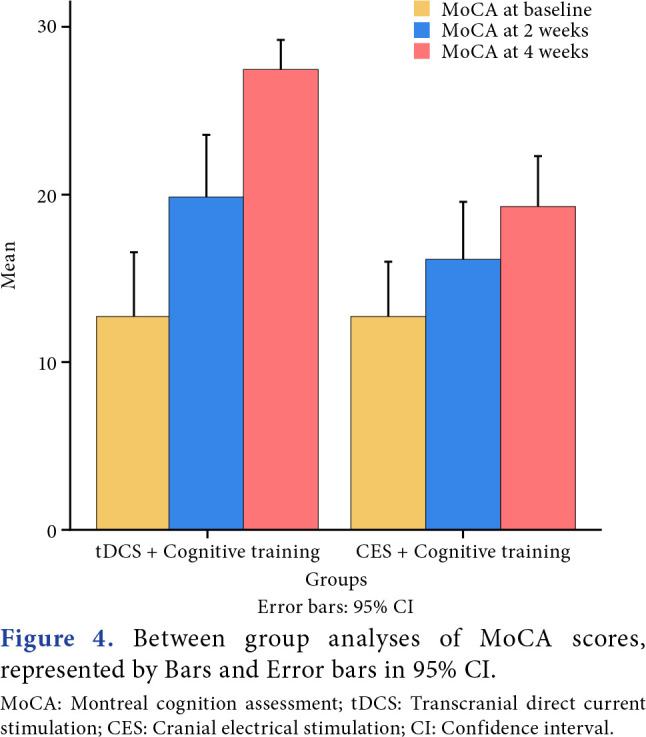
Figure 5. Between group analyses of GOAT scores, represented by Bars and Error bars in 95% CI. GOAT: Galveston orientation amnesia test; tDCS: Transcranial direct current stimulation; CES: Cranial electrical stimulation; CI: Confidence interval.
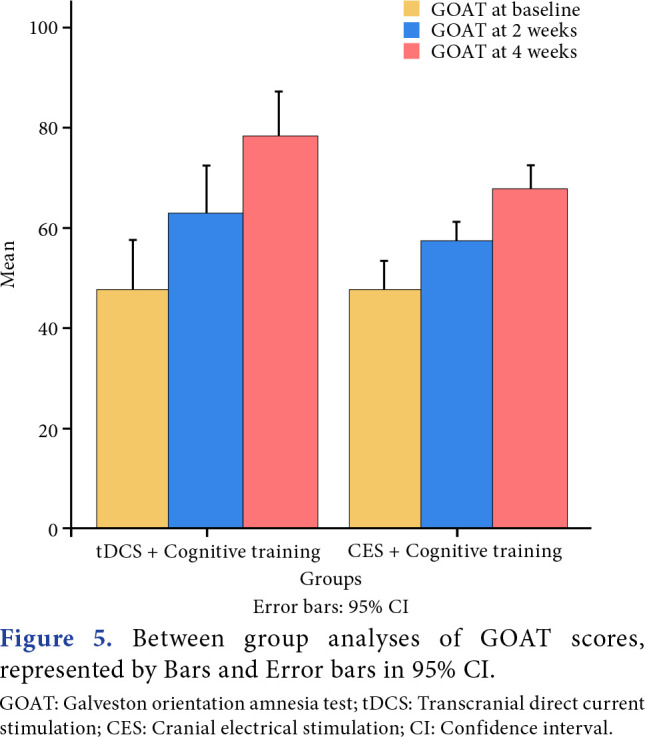
Figure 6. Time x Group interaction effect of MoCA, represented by estimated marginal means. MoCA: Montreal cognition assessment; tDCS: Transcranial direct current stimulation; CES: Cranial electrical stimulation.
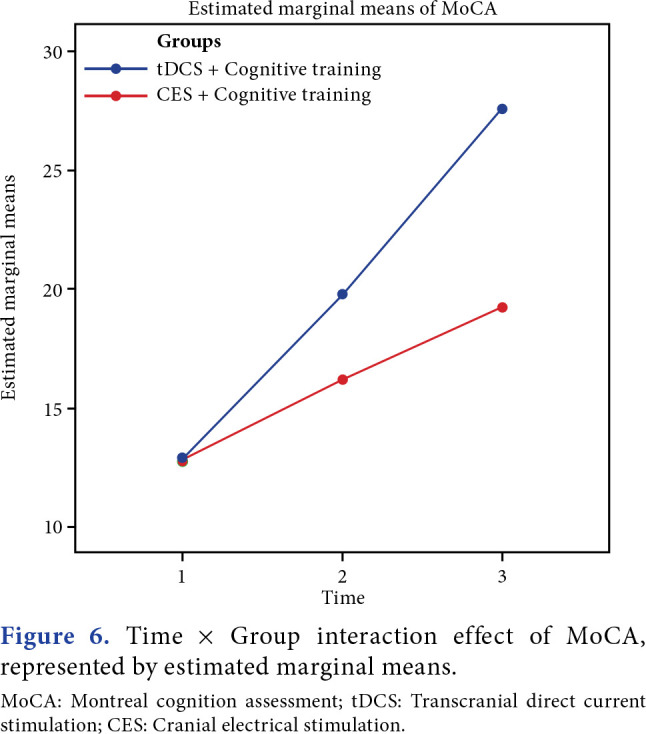
Figure 7. Time x Group interaction effect of GOAT, represented by estimated marginal means. GOAT: Galveston orientation amnesia test; tDCS: Transcranial direct current stimulation; CES: Cranial electrical stimulation.
Effect size [(Mpre-Mpost)/SDpre] and effect size index (ESI) [(Mpre-Mpost)/SDpooled] were calculated for both groups. Group 1 had an effect size and ESI of 1.59 for MoCA. Group 1 had an effect size of 2.99 and ESI of 3.18 for GOAT. Group 2 exhibited a large effect size (3.04) and ESI (2.55) for MoCA, while a small effect size (2.39) and ESI (2.55) was observed for GOAT in Group 2, although the overall effect size (2.45 for MoCA; 2.54 for GOAT) and ESI (2.12 for MoCA; 2.51 for GOAT) were found to be large between the groups.
Time and group interaction and covariance analysis for both outcome measures depicted that there was significant improvement (p=0.001) for each timeline among both the groups where treatment effect was observed at each time. Covariance analysis was conducted by taking baseline measurement as a covariate. It depicted a significant effect from two weeks to four weeks, with excellent effect size measured through partial Eta-squared for covariance analysis.
Discussion
This study evaluated the effect of CES and tDCS, which are noninvasive neuromodulatory techniques, on cognitive functions in TBI patients. According to the findings of this study, both CES and tDCS combined with cognitive training improved cognition (evaluated by MoCA and GOAT) following one month of intervention. However, there was no significant improvement in both groups after two weeks of intervention.
The brain functions electrochemically and can be readily modulated by electrical intervention.[14] Neuromodulators and nonintrusive brain stimulation techniques promote modifiable neuroplasticity via neurotransmitters, such as serotonin, dopamine, acetylcholine, and histamine. They help to improve clinical recovery by speeding up structural and functional neuronal changes, increasing their connections and dendritic spines, and improving clinical outcomes beyond ubiquitous rehabilitation, and helping patients who do not respond to typical therapies. They are easily operated and painless.[13] Noninvasive brain stimulation techniques, including tDCS and CES, are painless and a relatively safe form of neuromodulatory interventions.[20] Transcranial DCS uses a low-intensity electrical current delivered by electrodes placed on the scalp over a targeted cortical area to induce sustained changes in neuronal activity by facilitating or inhibiting neuronal circuits depending on the polarity of stimulation.[21] It induces neuroplasticity by applying a 0.5 to 2 mA electric current, boosts adaptive neuroplasticity, and reduces pathological sequelae following TBI. It also has been shown to improve neuroplasticity and cognitive outcomes in neurological conditions, including TBI.[22] Cranial electrical stimulation transcutaneously applies a pulsed, alternating microcurrent (1000 µA) to the head via electrodes placed on the earlobes, mastoid processes, zygomatic arches, or the maxillo-occipital junction[23] and has few side effects (≤1%).[14] Stimulation alters intrinsic connectivity networks, such as dorsal fronto-parietal network and the sensorimotor network.[23] Cranial electrical stimulation increases blood plasma levels of beta-endorphins, adrenocorticotrophic hormone, serotonin, melatonin, norepinephrine, cholinesterase, and dopamine[24] and decreases serum cortisol levels, which enhances the ability to focus and improves attention span, concentration, short-term memory, and creativity.[14]
Shaker et al.[25] showed that there was a significant improvement in attention, concentration, figural memory, logical reasoning, behavior, and functional activities after receiving tDCS, along with a positive impact on daily activities. Nitsche et al.[26] showed the effects of tDCS stimulation on polarity-dependent cortical activities and on excitability enhancements or inhibition, modulating resting membrane potentials, which emerge during stimulation, causing neuroplastic variations of cortical activity. This forms the basis of memory and learning. Anodal tDCS was also observed to enhance alpha and theta activity to improve memory. Facilitatory effect of alpha modulates motor learning and high gamma frequency-enhanced contrast perception,[27] whereas mode 3 of CES modulates the activities of alpha.[24]
After the intervention, the results clearly demonstrated that both groups showed statistically significant improvement in their outcomes. In order to establish the clinical significance, a minimal clinically important difference value is observed, which is 18 for MoCA,[28] and, fortunately, Group 1 achieved this value. Group 1 scored 19 in comparison to Group 2, which scored 13. Hence, it can be stated that a clinical significance was found in Group 1 (the individuals receiving CES) compared to Group 2 (individuals who received tDCS, with conventional exercises being constant in both groups).
The MoCA showed significant changes both clinically and statistically in both groups. Hence, conclusion can be drawn that both tDCS and CES are effective in enhancing the cognition of patients after TBY in hospital-based settings. Thus, both modalities are recommended for future use in improving cognition. The effect size between the groups interpreted larger effect size for GOAT compared to MoCA. After intervention, the mean difference was 44 in GOAT in Group 2 and 42 in Group 1.
Previous studies established that tDCS has positively impacted the effect of the modality on consciousness and on the cognitive domain.[5,29-33] However, the effect of CES on this domain is not well explored, and thus, establishing a direct correlation between the treatment and cognitive domain becomes a necessity. Although there have been studies in the literature regarding the efficacy of CES on anxiety and depression in individuals with brain injury,[29] this is the first clinical trial to use CES on the cognitive domain in individuals with TBI. Transcranial DCS has also shown evident and significant effects on the cognitive domain.[33]
A previous study showed that the potential role of anodal tDCS applied to the left dorsolateral prefrontal cortex along with cognitive training improves attention in individuals with TBI.[33] The study showed the same sequence of patterns within the results section, where significant values were achieved for both within-group and between-group analyses. Another study showed that tDCS is a secure and harmless non-invasive neuromodulatory device with the least side effects that can be given as monotherapy to improve cognition, but it can also be used with other strategies, such as cognitive rehabilitation and physical therapy, to show further clinical significant improvement in cognition.[34]
There are several limitations to this study. The male dominance of the study population makes it less likely to generalize. The level of education might have influenced the cognitive domain, and thus, generalization to the entire population is not valid. Another limitation was the lack of a control group, which could have further solidified the results. Future studies with a larger sample size, evaluation of different CES protocols, and comparison of CES+tDCS with CES, tDCS, and a sham intervention are recommended for better evaluation of these modalities. Further studies should also take into account the severity of TBI.
In conclusion, CES is an effective and safe neurorehabilitation tool that significantly improves posttraumatic cognitive impairment compared to tDCS. This study helps enhance the understanding about the impact of CES on cognition.
Acknowledgments.
The authors would like show their gratitude towards the teaching hospital for providing the infrastructure and permission to conduct the research in the hospital based setting.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Conceptualization, planning, methodology, writing-original draft preparation, data curation, data compilation, investigation: K.K.; Visualization, conceptualization, planning, data curation and interpretation, writing-content, review and editing, final approval of manuscript, formal analysis: N.S.; Writing-content, review and editing, formal analysis of data: P.K.; Content writing, revision, data analysis and curation, review and editing: S.K.; Drafted the article, data analysis and compilation: G.K.; Content writing, data curation, investigation: A.G.; All the authors approved the manuscript.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Krawczyk DC, Han K, Martinez D, Rakic J, Kmiecik MJ, Chang Z, et al. Executive function training in chronic traumatic brain injury patients: Study protocol. Trials. 2019;20:435–435. doi: 10.1186/s13063-019-3526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser EE, Downing MG, Biernacki K, McKenzie DP, Ponsford JL. Cognitive reserve and age predict cognitive recovery after mild to severe traumatic brain injury. J Neurotrauma. 2019;36:2753–2761. doi: 10.1089/neu.2019.6430. [DOI] [PubMed] [Google Scholar]
- 3.Fleming J, Ownsworth T, Doig E, Hutton L, Griffin J, Kendall M, et al. The efficacy of prospective memory rehabilitation plus metacognitive skills training for adults with traumatic brain injury: Study protocol for a randomized controlled trial. Trials. 2017;18:3–3. doi: 10.1186/s13063-016-1758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: Assessment to treatment. Mt Sinai J Med. 2009;76:173–181. doi: 10.1002/msj.20099. [DOI] [PubMed] [Google Scholar]
- 5.Das N, Spence JS, Aslan S, Vanneste S, Mudar R, Rackley A, et al. Cognitive training and transcranial direct current stimulation in mild cognitive impairment: A randomized pilot trial. Front Neurosci. 2019;13:307–307. doi: 10.3389/fnins.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law LLF, Mok VCT, Yau MMK. Effects of functional tasks exercise on cognitive functions of older adults with mild cognitive impairment: A randomized controlled pilot trial. Alzheimers Res Ther. 2019;11:98–98. doi: 10.1186/s13195-019-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peña J, Ibarretxe-Bilbao N, Sánchez P, Uriarte JJ, Elizagarate E, Gutierrez M, et al. Mechanisms of functional improvement through cognitive rehabilitation in schizophrenia. J Psychiatr Res. 2018;101:21–27. doi: 10.1016/j.jpsychires.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Howe EI, Langlo KS, Terjesen HCA, Røe C, Schanke AK, Søberg HL, et al. Combined cognitive and vocational interventions after mild to moderate traumatic brain injury: Study protocol for a randomized controlled trial. Trials. 2017;18:483–483. doi: 10.1186/s13063-017-2218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsychol Soc. 2016;22:120–137. doi: 10.1017/S1355617715000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130:1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 11.Barman A, Chatterjee A, Bhide R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J Psychol Med. 2016;38:172–181. doi: 10.4103/0253-7176.183086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz DI, Ashley MJ, Shanick GJO, Connors SH. Cognitive rehabilitation: The evidence, funding and case for advocacy in brain injury. McLean, VA: Brain Injury Association of America. 2006 [Google Scholar]
- 13.Rodríguez-Blanco L, Lubrini G, Vidal-Mariño C, Ríos-Lago M. Efficacy of cognitive rehabilitation of attention, executive functions, and working memory in psychotic disorders: A systematic review. Actas Esp Psiquiatr. 2017;45:167–178. [PubMed] [Google Scholar]
- 14.Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36:169–176. doi: 10.1016/j.psc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Vissoci JRN, de Oliveira LP, Gafaar T, Haglund MM, Mvungi M, Mmbaga BT, et al. Cross-cultural adaptation and psychometric properties of the MMSE and MoCA questionnaires in Tanzanian Swahili for a traumatic brain injury population. BMC Neurol. 2019;19:57–57. doi: 10.1186/s12883-019-1283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreutzer JS, DeLuca J, Caplan B. Encyclopedia of clinical neuropsychology. New York: Springer; 2011. [DOI] [Google Scholar]
- 17.Moin P, Khalighinejad N, Yusefi A, Farajzadegan Z, Barekatain M. Converting three general-cognitive function scales into persian and assessment of their validity and reliability. Int J Prev Med. 2011;2:82–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Stienen MN, Geisseler O, Velz J, Maldaner N, Sebök M, Dannecker N, et al. Influence of the intensive care unit environment on the reliability of the montreal cognitive assessment. Front Neurol. 2019;10:734–734. doi: 10.3389/fneur.2019.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863–863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rushby JA, De Blasio FM, Logan JA, Wearne T, Kornfeld E, Wilson EJ, et al. tDCS effects on task-related activation and working memory performance in traumatic brain injury: A within group randomized controlled trial. Neuropsychol Rehabil. 2021;31:814–836. doi: 10.1080/09602011.2020.1733620. [DOI] [PubMed] [Google Scholar]
- 21.Rudroff T, Workman CD. Transcranial direct current stimulation as a treatment tool for mild traumatic brain injury. Brain Sci. 2021;11:806–806. doi: 10.3390/brainsci11060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Freitas DJ, De Carvalho D, Paglioni VM, Brunoni AR, Valiengo L, Thome-Souza MS, et al. Effects of transcranial direct current stimulation (tDCS) and concurrent cognitive training on episodic memory in patients with traumatic brain injury: A double-blind, randomised, placebocontrolled study. e045285BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feusner JD, Madsen S, Moody TD, Bohon C, Hembacher E, Bookheimer SY, et al. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav. 2012;2:211–220. doi: 10.1002/brb3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.User’s manual, Repose: cranial electrotherapy stimulation. India: Walnut Medical; V1.1a 1:1-25 [Google Scholar]
- 25.Shaker HA, Sawan SAE, Fahmy EM, Ismail RS, Elrahman SAEA. Effect of transcranial direct current stimulation on cognitive function in stroke patients. Egypt J Neurol Psychiatr Neurosurg. 2018;54:32–32. doi: 10.1186/s41983-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 27.Wong GK, Ngai K, Lam SW, Wong A, Mok V, Poon WS. Validity of the Montreal Cognitive Assessment for traumatic brain injury patients with intracranial haemorrhage. Brain Inj. 2013;27:394–398. doi: 10.3109/02699052.2012.750746. [DOI] [PubMed] [Google Scholar]
- 28.de Guise E, Alturki AY, LeBlanc J, Champoux MC, Couturier C, Lamoureux J, et al. The Montreal Cognitive Assessment in persons with traumatic brain injury. Appl Neuropsychol Adult. 2014;21:128–135. doi: 10.1080/09084282.2013.778260. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Chan SSM, Chan WC, Lin C, Cheng CPW, Linda Chiu Wa L. Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Ann Clin Transl Neurol. 2019;6:1938–1948. doi: 10.1002/acn3.50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissim NR, O’Shea A, Indahlastari A, Kraft JN, von Mering O, Aksu S, et al. Effects of transcranial direct current stimulation paired with cognitive training on functional connectivity of the working memory network in older adults. Front Aging Neurosci. 2019;11:340–340. doi: 10.3389/fnagi.2019.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashir S, Al-Hussain F, Hamza A, Asim Niaz T, Albaradie R, Habib SS. Cognitive function assessment during 2 mA transcranial direct current stimulation in DLPFC in healthy volunteers. e14264Physiol Rep. 2019;7 doi: 10.14814/phy2.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz Gonzalez P, Fong KNK, Brown T. The effects of transcranial direct current stimulation on the cognitive functions in older adults with mild cognitive impairment: A pilot study. Behav Neurol. 2018;2018:5971385–5971385. doi: 10.1155/2018/5971385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang EK, Kim DY, Paik NJ. Transcranial direct current stimulation of the left prefrontal cortex improves attention in patients with traumatic brain injury: A pilot study. J Rehabil Med. 2012;44:346–350. doi: 10.2340/16501977-0947. [DOI] [PubMed] [Google Scholar]
- 34.Zaninotto AL, El-Hagrassy MM, Green JR, Babo M, Paglioni VM, Benute GG, et al. Transcranial direct current stimulation (tDCS) effects on traumatic brain injury (TBI) recovery: A systematic review. Dement Neuropsychol. 2019;13:172–179. doi: 10.1590/1980-57642018dn13-020005. [DOI] [PMC free article] [PubMed] [Google Scholar]