Abstract
Objectives
To explore the relationship between physical activity over a 10-year period and current symptoms of insomnia, daytime sleepiness and estimated sleep duration in adults aged 39–67.
Design
Population-based, multicentre cohort study.
Setting
21 centres in nine European countries.
Methods
Included were 4339 participants in the third follow-up to the European Community Respiratory Health Survey (ECRHS III), who answered questions on physical activity at baseline (ECRHS II) and questions on physical activity, insomnia symptoms, sleep duration and daytime sleepiness at 10-year follow-up (ECRHS III). Participants who reported that they exercised with a frequency of at least two or more times a week, for 1 hour/week or more, were classified as being physically active. Changes in activity status were categorised into four groups: persistently non-active; became inactive; became active; and persistently active.
Main outcome measures
Insomnia, sleep time and daytime sleepiness in relation to physical activity.
Results
Altogether, 37% of participants were persistently non-active, 25% were persistently active, 20% became inactive and 18% became active from baseline to follow-up. Participants who were persistently active were less likely to report difficulties initiating sleep (OR 0.60, 95% CI 0.45–0.78), a short sleep duration of ≤6 hours/night (OR 0.71, 95% CI 0.59–0.85) and a long sleep of ≥9 hours/night (OR 0.53, 95% CI 0.33–0.84) than persistently non-active subjects after adjusting for age, sex, body mass index, smoking history and study centre. Daytime sleepiness and difficulties maintaining sleep were not related to physical activity status.
Conclusion
Physically active people have a lower risk of some insomnia symptoms and extreme sleep durations, both long and short.
Keywords: sleep medicine, epidemiology, primary care, public health, sports medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The longitudinal study design, in which the exposure (physical activity) is measured 10 years prior to the sleep outcomes, enables an investigation into whether the consistency of physical activity over time has an impact on current symptoms of insomnia, sleep duration and daytime sleepiness.
Data were collected using standardised and validated procedures and instruments, increasing its internal validity.
Data were obtained from nine European countries, increasing the external validity of our findings.
One limitation of our study is that sleep variables are only available at the follow-up, which precluded testing their effect on baseline physical activity.
Insomnia symptoms, sleep durations and daytime sleepiness data were obtained by questionnaire and no sleep disorder diagnoses from medical providers or objective assessments were available.
Introduction
Disturbed sleep is common in the general population and impacts health and quality of life.1–3 Chronic sleep disturbances are associated with cardiovascular disease, metabolic dysfunction, psychiatric disorders and increased mortality.4–6
Physical activity and sleep
Regular exercise is associated with better health and several studies suggest that physical activity (PA) is beneficial to sleep and may improve symptoms of chronic insomnia.7–10 It is, however, unclear how significant these benefits are and which factors may have a moderating effect on them.11 The positive association between PA and sleep may be subject to multiple moderating factors such as gender, age, body mass index (BMI), fitness level, general health and the characteristics of the type of exercise in question. Therefore, sleep and PA probably influence each other through complex, reciprocal interactions including multiple physiological and psychological pathways.7
PA and daytime sleepiness
There is evidence that more PA is associated with less daytime sleepiness.12–17 Cross-sectional studies have shown that low PA is associated with an increased likelihood of excessive daytime sleepiness (EDS)14–16 and that subjects participating in exercise are less likely to have EDS.12 17 In older adults, increasing PA by doing home exercises has been shown to improve EDS and reduce the prevalence of insomnia symptoms,13 while another study showed that increasing PA protected women from future insomnia.18 Other studies have contradictory findings. In an epidemiological study of 4405 Koreans, daytime sleepiness was more common among those in the top quartile of PA compared with those in the lowest quartile group.19 Among patients with obstructive sleep apnoea, increased PA was associated with a lower severity of disease and a 28% decrease in EDS.20 The daily association between PA and sleep duration was described in 2021, based on a systematic review and meta-analysis of 33 peer-reviewed papers, which showed that, on the night following increased PA, there was a lower total sleep time.21
Limitations of previous studies
There is a lack of epidemiological data from long-term follow-up studies of large cohorts exploring the association of PA with sleep length, daytime sleepiness and insomnia symptoms. Previous research on PA and sleep-related outcomes has several important limitations. Most studies are cross-sectional or have a short follow-up interval, preventing the possibility of elucidating whether increased PA improves sleeping outcomes or whether reduced PA is a consequence of sleep problems. Finally, the effects of PA on sleep length, daytime sleepiness and insomnia symptoms have not been studied simultaneously.
Aims of the current study
Therefore, the aim of the present study was to assess the inter-relationship between PA, based on frequency, duration and intensity, and symptoms of insomnia, self-reported sleep durations and daytime sleepiness among middle-aged subjects from 21 centres in nine countries at two moments in time, 10 years apart, providing important longitudinal follow-up data.
Materials and methods
Subjects
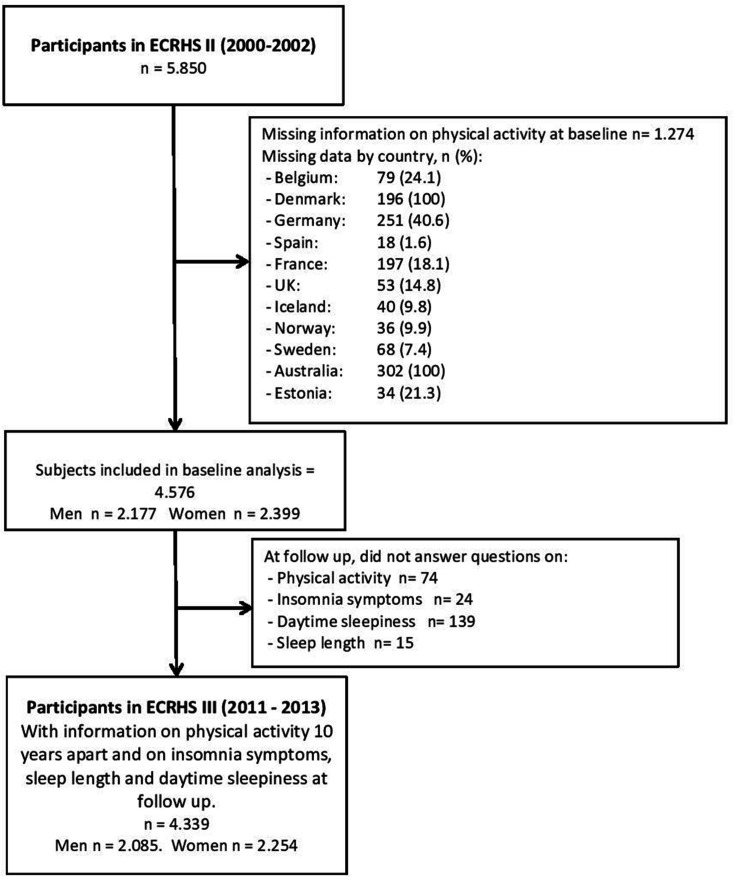
We studied participants from the second and third follow-up surveys of the European Community Respiratory Health Survey (ECRHS II and III, www.ecrhs.org), an international, population-based, multicentre cohort study of asthma and allergic disease, which was first carried out in 1990. Detailed descriptions of the methods used for ECRHS I and ECRHS II have been published elsewhere.22 23 Briefly, participating centres randomly selected samples from subjects aged 20–44 in order to track them for asthma, allergy and lung disease (see: www.ecrhs.org). Participants completed a short postal questionnaire about asthma and asthma-like symptoms and, from those who responded, a random sample was selected to undergo a more detailed clinical examination. In ECRHS II, subjects who had participated in the clinical phase of ECRHS I (performed between 1991 and 1994) were invited to participate in the follow-up study. The clinical phase of ECRHS II was carried out between 1998 and 2002. ECRHS III is the second follow-up study and was carried out from February 2011 to January 2014.22–24 The present study is based on data from ECRHS II and III (see figure 1 for the flow chart).
Figure 1.
Flow chart of the study population in the European Community Respiratory Health Survey (ECRHS).
Health, habits and measurements
Subjects answered the core ECRHS questionnaires, which included questions on lifestyle, respiratory symptoms, smoking history and general health. ‘Current smokers’ were defined as those who smoked tobacco regularly during the last month. ‘Former smokers’ were defined as smokers who denied having smoked regularly in the month prior to the examination. Those who reported no regular smoking at the time of or prior to the examination were defined as ‘never smokers’. The participants’ height and weight were measured and their BMI was calculated.24
Assessment of PA
PA was assessed in ECRHS II and III using replies from questionnaires. The assessment of PA in ECRHS has previously been described in detail, including how both the frequency and duration of PA were used to divide the population into categories.22 In brief, participants were asked how often and for how many hours per week they usually exercised to the point that they became out of breath or sweaty. Participants who exercised two or more times a week, for at least 1 hour/week, were classified as physically active. Changes in activity status from baseline to follow-up were categorised into four PA groups: persistently non-active (non-active at both baseline and follow-up), became inactive (active at baseline and non-active at follow-up), became active (non-active at baseline and active at follow-up) and persistently active (active at both baseline and follow-up).
Sleep questionnaires and measurements
Sleep-related symptoms were assessed using the Basic Nordic Sleep Questionnaire,25 where participants were asked about the frequency of insomnia symptoms. Answers were provided on a scale of 1–5: (1) never or very seldom, (2) less than once a week, (3) once to twice a week, (4) three to five times a week, (5) every day or almost every day of the week. Insomnia symptoms were defined using answers to three questions from the Basic Nordic Sleep Questionnaire: ‘I have difficulties falling asleep at night’ (difficulties initiating sleep), ‘I wake up often during the night’ (difficulties maintaining sleep) and ‘I wake up early in the morning and can’t fall back asleep’ (early morning awakenings). Those who reported these symptoms of insomnia ≥3 times a week (scores 4 and 5) were considered to have the corresponding insomnia subtype. Daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS), a brief questionnaire that measures daytime sleepiness based on the likelihood of falling asleep in eight different situations.26 Participants with an ESS score >10 were considered to have EDS. Participants were asked the question: how much sleep do you estimate that you get on average each night? According to their answers, they were classified as: short sleepers (≤6 hours/night), normal sleepers (6–9 hours/night) or long sleepers (≥9 hours/night).
Patient and public involvement
The study’s design did not involve patients or the general public. However, all participating patients were informed of the research objectives and their informed consent was obtained. The survey was completed by participants voluntarily and no input from patients was sought in interpreting or writing up the results. The results of the research will not be disseminated to the patients.
Statistical analysis
Data are presented as numbers and percentages or mean±SD, depending on distribution. For bivariate analysis, the χ2 test and one-way analysis of variance were used for nominal and continuous variables. Logistic regression was used for multivariable analyses to estimate the association between PA and sleep-related outcomes. The model was adjusted for potential confounders including age, sex, BMI, smoking history and study centre. In the analysis, all variables, including study centre (n=21), were treated as fixed effects. STATA V.16 was used for all statistical analyses.
Results
Participants and level of PA
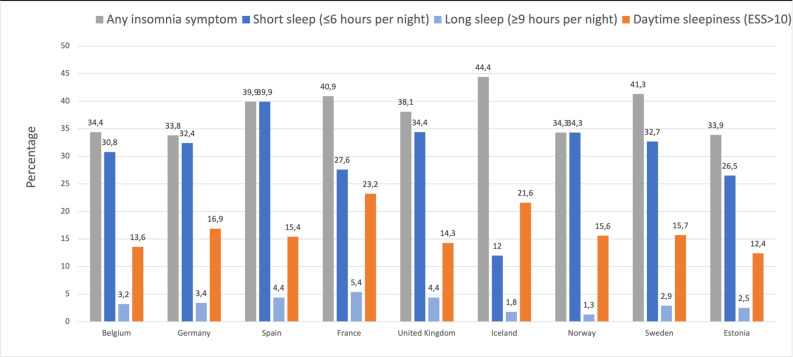
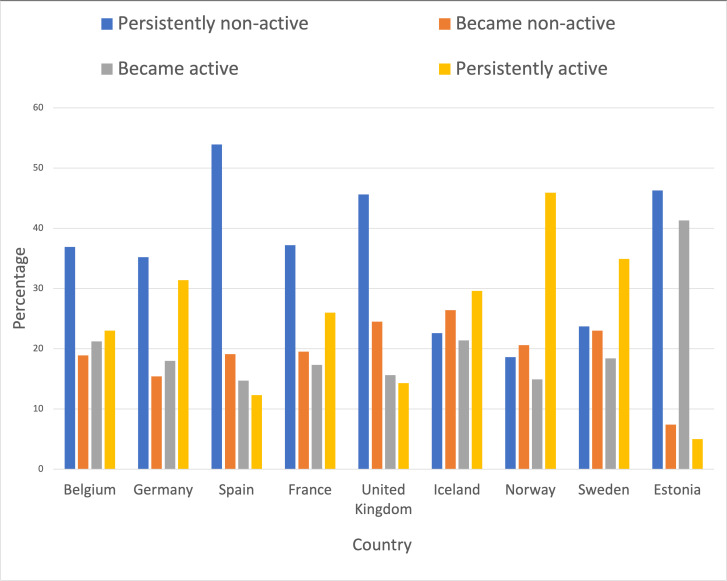
From a total of 5850 participants in ECRHS II, we excluded those with missing data and included a total of 4339 participants (48% men) (see figure 1). Figure 2 shows the prevalence of insomnia symptoms, short and long sleep durations and daytime sleepiness among subjects in the different countries included in the study. From baseline to 10 years later, 36.9% of participants were persistently non-active, 17.9% became physically active at follow-up, 20.3% of participants became inactive and 24.9% were persistently active (table 1). There were geographical differences in the level of PA between the ECRHS countries (figure 3). Participants in Norway were most likely to be persistently active, while participants in Spain, followed by Estonia, were most likely to be persistently non-active (figure 3).
Figure 2.
Prevalence of any insomnia symptom, short sleep duration (≤6 hours/night), long sleep duration (≥9 hours/night) and daytime sleepiness (Epworth Sleepiness Scale (ESS) score >10) by country.
Table 1.
Characteristics and general health of the participants by the level of physical activity
| Persistently non-active | Became inactive | Became active | Persistently active | P value | |
| General characteristics | |||||
| n, % | 1601 (36.9) | 881 (20.3) | 775 (17.9) | 1082 (24.9) | |
| Men, % | 44.3 | 49.0 | 46.8 | 53.7 | <0.001 |
| Age, years | 55.0±7.2 | 54.5±7.1 | 53.4±7.2 | 53.7±7.2 | <0.001 |
| Body mass index, kg/m2 | 27.6±5.2 | 27.1±4.9 | 27.1±4.8 | 27.0±4.4 | 0.007 |
| Currently working, % | 82.7 | 85.8 | 88.9 | 90.4 | <0.001 |
| Smoking history, % | |||||
| Never | 42.4 | 43.4 | 44.3 | 47.6 | <0.001 |
| Former | 34.4 | 39.8 | 37.5 | 40.0 | |
| Current | 23.2 | 16.9 | 18.2 | 12.4 |
Figure 3.
Activity levels by country.
General characteristics and health
Persistently active participants were more often men, they were younger and they had a slightly lower BMI (table 1). They were also less likely to be current smokers and more likely to be currently working (table 1).
Insomnia symptoms
In unadjusted analysis, there was a significant difference in reporting difficulties initiating sleep, early morning awakenings and any insomnia symptom where those persistently active were least likely to report these symptoms. Also, persistently active subjects were the least likely to report having two or three insomnia symptoms (table 2). After adjusting for age, sex, BMI, smoking history and study centre, this negative association remained significant for difficulties initiating sleep (OR 0.58 (0.42–0.77)), any insomnia symptom (OR 0.78 (0.65–0.94)) and reporting two (OR 0.60 (0.43–0.82)) and three (OR 0.63 (0.41–0.98)) insomnia symptoms (table 3). Additionally, in adjusted analysis, persistently active subjects were significantly less likely to report difficulties initiating sleep (OR 0.80 (0.66–0.97)) (table 3). There were also independent associations between insomnia symptoms and age, female gender and BMI (table 4).
Table 2.
Insomnia symptoms, sleep duration and daytime sleepiness by level of physical activity
| Persistently non-active (n=1601) |
Became inactive (n=881) |
Became active (n=775) |
Persistently active (n=1082) |
*P value | |
| Insomnia symptoms | |||||
| Difficulties initiating sleep (%) | 15.4 | 14.0 | 11.7 | 8.2 | <0.001 |
| Difficulties maintaining sleep (%) | 31.9 | 32.1 | 33.0 | 28.5 | 0.128 |
| Early morning awakenings (%) | 18.2 | 18.3 | 15.0 | 13.2 | 0.002 |
| Any insomnia symptom (%) | 41.0 | 41.5 | 39.5 | 34.9 | 0.006 |
| Number of insomnia symptoms (%) | |||||
| 0 | 58.4 | 58.2 | 61.0 | 64.9 | 0.001 |
| 1 | 23.2 | 25.2 | 24.0 | 23.8 | |
| 2 | 11.9 | 10.6 | 10.0 | 7.8 | |
| 3 | 6.6 | 6.1 | 5.1 | 3.6 | |
| Sleep duration | |||||
| Sleep time (hours) | 6.8±1.1 | 6.8±1.0 | 6.9±1.0 | 6.9±0.9 | 0.234 |
| Sleep time, % | <0.001 | ||||
| Short sleepers (≤6 hours) | 35.9 | 31.9 | 20.7 | 26.9 | |
| Normal sleepers (6–9 hours) | 59.2 | 64.6 | 66.9 | 70.9 | |
| Long sleepers (≥9 hours) | 4.9 | 3.5 | 3.4 | 2.2 | |
| Daytime sleepiness | |||||
| Epworth Sleepiness Scale score | 6.8±4.1 | 7.2±4.1 | 6.9±4.1 | 6.9±3.8 | 0.106 |
| Epworth Sleepiness Scale score >10 (%) | 17.2 | 19.4 | 17.7 | 15.6 | 0.176 |
Data are presented as mean±SD or % where indicated. Significant differences are in bold (p<0.05).
*P value from Pearson’s χ2 test (numerical variables) and one-way analysis of variance (continuous variables).
Table 3.
Independent association between the level of physical activity and medical disorders, insomnia symptoms, daytime sleepiness and sleep duration expressed as adjusted* ORs (95% CI) with the persistently non-active group as reference
| Became inactive (n=881) |
Became active (n=775) |
Persistently active (n=1082) |
|
| Insomnia symptoms | |||
| Difficulties initiating sleep | 0.97 (0.75–1.25) | 0.82 (0.62–1.08) | 0.58 (0.42–0.77) |
| Difficulties maintaining sleep | 0.96 (0.80–1.17) | 1.04 (0.85–1.27) | 0.80 (0.66–0.97) |
| Early morning awakenings | 1.09 (0.87–1.38) | 0.86 (0.63–1.03) | 0.80 (0.63–1.03) |
| Any insomnia symptom | 1.02 (0.85–1.22) | 0.95 (0.78–1.14) | 0.78 (0.65–0.94) |
| Number of insomnia symptoms | |||
| 1 | 1.07 (0.86–1.32) | 0.99 (0.79–1.24) | 0.91 (0.74–1.12) |
| 2 | 0.89 (0.66–1.20) | 0.86 (0.63–1.17) | 0.60 (0.43–0.82) |
| 3 | 1.09 (0.74–1.59) | 0.94 (0.62–1.42) | 0.63 (0.41–0.98) |
| Daytime sleepiness | |||
| Epworth Sleepiness Scale score >10 | 1.17 (0.94–1.47) | 1.00 (0.78–1.27) | 0.87 (0.69–1.10) |
| Sleep duration | |||
| Short sleepers (≤6 hours) | 0.89 (0.73–10.7) | 0.85 (0.69–1.03) | 0.71 (0.58–0.85) |
| Normal sleepers (6–9 hours) | 1.18 (0.98–1.42) | 1.21 (1.00–1.47) | 1.55 (1.29–1.87) |
| Long sleepers (≥9 hours) | 0.74 (0.47–1.17) | 0.84 (0.53–1.33) | 0.48 (0.28–0.80) |
Bold text indicates statistical significance.
*Adjusted for age, sex, body mass index (BMI), smoking history and centre.
Table 4.
Associations between age, sex, BMI and smoking history and sleep-related symptoms
| Age | Sex | BMI | Smoking history | |
| Insomnia symptoms | ||||
| Difficulties initiating sleep | 1.02 (1.01–1.03) | 2.16 (1.77–2.64) | 1.02 (1.01–1.05) | 0.81 (0.66–0.99) |
| Difficulties maintaining sleep | 1.04 (1.03–1.05) | 1.80 (1.56–2.07) | 1.01 (1.00–1.03) | 1.09 (0.95–1.26) |
| Early morning awakenings | 1.02 (1.01–1.03) | 1.52 (1.28–1.80) | 1.01 (1.00–1.03) | 1.02 (0.85–1.21) |
| Any insomnia symptom | 1.03 (1.02–1.04) | 1.75 (1.53–1.99) | 1.02 (1.01–1.03) | 1.07 (0.93–1.22) |
| Number of insomnia symptoms | ||||
| 1 | 1.03 (1.01–1.04) | 1.47 (1.26–1.71) | 1.02 (1.00–1.03) | 1.15 (0.98–1.34) |
| 2 | 1.04 (1.02–1.06) | 2.11 (1.69–2.64) | 1.02 (1.00–1.05) | 1.01 (0.80–1.26) |
| 3 | 1.04 (1.02–1.06) | 2.62 (1.93–3.53) | 1.03 (0.99–1.06) | 0.89 (0.66–1.20) |
| Daytime sleepiness | ||||
| Epworth Sleepiness Scale score >10 | 0.99 (0.98–1.00) | 0.95 (0.81–1.12) | 1.01 (0.99–1.03) | 1.28 (1.08–1.52) |
| Sleep duration | ||||
| Short sleepers (≤6 hours) | 1.01 (0.99–1.02) | 0.88 (0.77–1.00) | 1.03 (1.02–1.05) | 0.83 (0.72–0.96) |
| Normal sleepers (6–9 hours) | 0.99 (0.98–0.99) | 1.08 (0.95–1.23) | 0.96 (0.95–0.98) | 1.20 (1.05–1.38) |
| Long sleepers (≥9 hours) | 1.03 (1.01–1.06) | 1.35 (0.96–1.89) | 1.02 (0.99–1.06) | 0.95 (0.67–1.34) |
*Bold text indicates statistical significance.
BMI, body mass index.
Sleep duration and daytime sleepiness
In unadjusted analysis, there was a significant difference in short and long sleep durations between levels of activity. Those who were persistently active were most likely to be normal sleepers while the persistently non-active were least likely to be in that category (70.9% vs 59.2%, respectively) (table 2). After adjusting for age, sex, BMI, smoking history and study centre, these results remained significant for persistently active subjects. They were significantly more likely to be normal sleepers (OR 1.55 (1.29–1.87)) and significantly less likely to be short sleepers (OR 0.71 (0.58–0.85)) or long sleepers (OR 0.48 (0.28–0.80)) (table 3). Additionally, those who became active were more likely to be normal sleepers than those who were persistently non-active (OR 1.21 (1.00–1.47)) (table 3).
However, there was not a significant association between the mean ESS score or percentage with an ESS score >10 and level of PA (tables 2 and 3). Daytime sleepiness was also independently associated with smoking (table 4).
Discussion
The main results of this study were that participants who reported being physically active at the start and end of a 10-year follow-up period were less likely to report insomnia symptoms at the follow-up. We also found that subjects who are persistently active are more likely to sleep the recommended 6–9 hours. This association remained statistically significant after adjusting for sex, age, smoking history and BMI. We also found that persistently active participants were more often men, were younger, had a slightly lower BMI and were less likely to be current smokers and more likely to be currently working.
Our results are in line with previous studies that have shown the beneficial effect of PA on symptoms of insomnia,9 10 but the current study additionally shows the importance of consistency in exercising over time, because the association was lost for initially active subjects who became inactive. A recent meta-analysis examining the effects of acute and regular exercise on a range of sleep variables showed that acute exercise (less than 1 week of exercise) has a small beneficial effect on many objective measures of sleep, such as total sleep time, insomnia symptoms and sleep quality.7 Furthermore, this meta-analysis found greater benefits from regular exercise for both subjective and objective sleep parameters over time. Regular exercise had small beneficial effects on total sleep time and sleep efficiency, small-to-medium beneficial effects on sleep onset latency and moderate beneficial effects on sleep quality.7
There are two recent systematic reviews and meta-analyses on the effects of PA on sleep7 and insomnia,9 both substantially reviewing the same randomised controlled studies. Banno et al included nine studies with a total of 557 participants.7 The majority of participants exercised three times or less per week and the follow-up interval was 4 months or shorter in all the studies except one. Their conclusion was that exercise could improve sleep, but that higher quality research was needed.7 Five studies on insomnia, and, additionally, six on insomnia symptoms, showed shorter sleep latency and higher sleep efficacy, but the authors also acknowledged the small size of the literature and severe methodological limitations, often based on selection bias.9 In addition, most previous studies are cross-sectional, which can also be considered a limitation.
Furthermore, a recent systematic review of PA and sleep showed that moderate exercise had a more promising outcome in terms of sleep quality than vigorous exercise. It is therefore important to study further the impact of the intensity of PA, in the context of age and gender, when exploring any beneficial impact on sleep.27
This study has a long follow-up period (10 years) and indicates strongly that consistency in PA might be an important factor in optimising sleep duration and reducing the symptoms of insomnia. Most other studies have had a much shorter follow-up period,7 which makes it more difficult to assess the consistency of activity over time.
Our results indicate that those who maintain a consistent level of PA are also less likely to be both short (<6 hours) and long sleepers (>9 hours). Those who are physically active in general are also more likely to engage in a healthier lifestyle,28 which can likewise have an effect on sleep. Lifestyle factors, such as a healthy diet and being physically active, are probably part of a phenotype that characterises those individuals who are generally engaged in a healthy lifestyle. A recent review highlighted the importance of focusing on the combination of sleep, diet and exercise when exploring healthy longevity.29
The three groups reporting low PA in either of the ECRHS surveys, or at both points in time, all report a very similar prevalence of insomnia symptoms, extreme sleep lengths and daytime sleepiness. This is somewhat surprising, especially given that those who were active in the follow-up survey but not at the baseline have a very similar symptom profile to those who were inactive in both surveys. Our study found that consistency in a behaviour such as PA for more than a decade is strongly related to a lower incidence of insomnia and a more ‘normal’ sleep length. Important information concerning ‘the healthy phenotype’ would be missed if the PA data were available only at baseline or at follow-up but not at both timepoints.
In a recent review based on 22 randomised controlled trials concerning the effects of regular exercise (lasting at least 2 months on a regular basis) on self-reported sleep quality, insomnia and daytime sleepiness, it was found that regular PA improved subjective sleep quality, insomnia severity and daytime sleepiness as measured with the ESS.30 These results regarding insomnia symptoms are in line with our study, but the results on daytime sleepiness differ from our results. The reason for this discrepancy could be due to different study populations, as there were only two studies in this review that measured daytime sleepiness using the ESS; one study assessed this among the elderly, 60 years and older,13 and the other among overweight and obese men.31 Another recent review of 32 randomised controlled trials on the effects of exercise on improving sleep disturbances showed that exercise is beneficial in improving sleep quality, symptoms of insomnia, restless legs, sleep apnoea and daytime sleepiness. However, exercise only had significant effects on sleepiness if it had lasted for more than 12 weeks, while the exercise period did not matter in regard to the association to sleep quality and insomnia symptoms.32
Another recent study showed that high or increasing levels of PA could protect women from future insomnia.18 Therefore, exercise seems to have a stronger association with sleep quality and insomnia than with sleepiness, which is in line with our results. However, almost all previous studies have the limitation that the definition of sleepiness is limited to the estimate that the likelihood of falling asleep but not the general feeling of sleepiness that we have shown is also an important part of sleepiness.33 34 Another recent review exploring the associations of exercise, sleep and cognitive function among older adults showed that PA is associated with improved cognitive function but the association of sleep and cognitive function seems to be U shaped, as too much or too little sleep is negatively associated with cognitive function.35 We did not explore cognitive function in the current study but it would be interesting for future studies to explore further how cognitive function is affected by the association of PA and sleep.
This study has several strengths such as the population-based nature, the longitudinal study design and the large sample collected in the same manner at many centres in nine different countries. Another strength is the use of standardised and validated procedures and instruments. The long follow-up period is also a strength since data on PA are collected 10 years apart and subjects are categorised according to change in PA. This study is, however, not without limitations. It is not possible to know whether those who are active at both timepoints have been continuously physically active throughout the study period or only at these two timepoints. Furthermore, PA was only measured using a questionnaire. Another limitation of our study is that sleep variables are only available at the follow-up, and we only have information on insomnia symptoms but not the diagnosis of insomnia disorder. Sleep length and daytime sleepiness are also based on subjective data. Therefore, even though the measurement of PA is longitudinal, it may not be entirely appropriate to describe the associations between PA and sleep outcomes as longitudinal. Also, there are potential implications of residual confounders that can influence both PA and sleep which were not explored in the current study (eg, mental health, musculoskeletal disorders/chronic pain) which could influence the study findings.
In conclusion, PA over time is associated with lower prevalence of insomnia symptoms and with sleeping between 6 and 9 hours/night.
Supplementary Material
Footnotes
Contributors: EHT and EB equally drafted, participated in manuscript preparation and were responsible for communications with other coauthors. TG and CJ participated in the design of the study, manuscript preparation and review of the manuscript on several stages. EHT performed the statistical analysis with help from CJ. EL, BB, KF, DJ, PD, JLP, JGA, SD-A, JH, KT, VGL and RJ participated in data collection and/or reviewing of the paper. TG is responsible for the writing of this manuscript accuracy of the data and
accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: Financial support for ECRHS III: Australia: National Health and Medical Research Council. Belgium: Antwerp South, Antwerp City: Research Foundation Flanders (FWO), grant code G041008N10 (both sites). Estonia: Tartu-SF0180060s09 from the Estonian Ministry of Education. France: (All) Ministère de la Santé, Programme Hospitalier de Recherche Clinique (PHRC) National 2010; Bordeaux: INSERM U897, Université Bordeaux Segalen; Grenoble: Comite Scientifique AGIRadom 2011; Paris: Agence Nationale de la Santé, Région Ile de France, Domaine d’intérêt majeur (DIM). Germany: Erfurt: German Research Foundation HE 3294/10-1; Hamburg: German Research Foundation MA 711/6-1, NO 262/7-1. Iceland: Reykjavik, Landspitali University Hospital Research Fund, University of Iceland Research Fund, Icelandic College of Family Physicians Research Fund, ResMed Foundation, California, USA, Orkuveita Reykjavikur (Geothermal plant), Vegagerðin (Icelandic Road Administration, ICERA), Icelandic Research Fund (grant number 173701-052). Italy: All Italian centres were funded by the Italian Ministry of Health, Chiesi Farmaceutici. In addition, Verona was funded by Cariverona Foundation, Education Ministry (MIUR). Norway: Norwegian Research Council (grant number 214123), Western Norway Regional Health Authorities (grant number 911631), Bergen Medical Research Foundation. Spain: Fondo de Investigación Sanitaria (PS09/02457, PS09/00716 09/01511, PS09/02185, PS09/03190), Servicio Andaluz de Salud, Sociedad Española de Neumología y Cirurgía Torácica (SEPAR 1001/2010); Barcelona: Fondo de Investigación Sanitaria (FIS PS09/00716); Galdakao: Fondo de Investigación Sanitaria (FIS 09/01511); Huelva: Fondo de Investigación Sanitaria (FIS PS09/02185) and Servicio Andaluz de Salud; Oviedo: Fondo de Investigación Sanitaria (FIS PS09/03190). Sweden: All centres were funded by the Swedish Heart and Lung Foundation, Swedish Asthma and Allergy Association, Swedish Association Against Lung and Heart Disease, Swedish Research Council for Health, Working Life and Welfare (FORTE); Göteborg: Also received further funding from the Swedish Council for Working Life and Social Research; Umea also received funding from Vasterbotten Country Council ALF grant. Switzerland: Swiss National Science Foundation (grant numbers 33CSCO-134276/1, 33CSCO-108796, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247-065896, 3100-059302, 3200-052720, 3200-042532, 4026-028099), Federal Office for Forest, Environment and Landscape, Federal Office of Public Health, Federal Office of Roads and Transport, Canton’s government of Aargan, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais and Zürich, Swiss Lung League, Canton's Lung League of Basel Stadt/Basel, Landschaft, Geneva, Ticino, Valais and Zurich, SUVA, Freiwillige Akademische Gesellschaft, UBS Wealth Foundation, Talecris Biotherapeutics, Abbott Diagnostics, European Commission 018996 (GABRIEL), Wellcome Trust (WT 084703MA). UK: Medical Research Council (grant number 92091). Support was also provided by the National Institute for Health Research through the Primary Care Research Network.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and ethical approval from the local research ethics committees and written consent from participants were obtained from each site. Australia: Monash University Human Research Ethics Committee (project number CF11/1818-2010001012). Belgium: Comité voor Medische Ethiek (UZA/UA 11/41/288). Denmark: De Videnskabsetiske Komiteer for Region Midtjylland (M-20110106). Estonia: Research Ethics Committee of the University of Tartu (UT REC 209T-17 and 225/M-24). France: Etude ECRHS III: Promotion CHU de Grenoble. Ethical approval from CPP Sud est V 4 mars 2011. Approval from Ministry of Health (AFSSAPS B110053-70) (Paris, Grenoble, Montpellier, Bordeaux). Germany: Ethikkommission der Bayerischen Landesärztekammer (Positive Votum: 10015). Iceland: National Bioethics Committee of Iceland (VSN-11-121-S3). Italy: Ethics Committee of IRCCS ‘San Matteo’ Hospital Foundation, University of Pavia (approval number 24215/2011) (Pavia), ‘Comitato Etico per la sperimentazione dell’Azienda Ospedaliera Universitaria Integrata di Verona’ (N Prog 1393) (Verona). Norway: Regional Ethics Committee West Norway (2010/759). Spain: Ethics Committee of the Parc de Salut Mar, Barcelona (Comité etic d’investigacio clínica, CEIC)–Parc de Salut Mar, Barcelona (approval number 2009/3500/1). Switzerland: Swiss Academy of Sciences. Sweden: Regional Ethical Review Board in Uppsala (decision number 2010/432). UK: NRES Committee London-Stanmore REC (Ref 11/LO/0965). Participants gave informed consent to participate in the study before taking part.
References
- 1. Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv 2005;56:332–43. 10.1176/appi.ps.56.3.332 [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the sleep heart health study. Sleep 2006;29:1009–14. 10.1093/sleep/29.8.1009 [DOI] [PubMed] [Google Scholar]
- 3. Sivertsen B, Lallukka T, Salo P, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT study in Norway. J Sleep Res 2014;23:124–32. 10.1111/jsr.12102 [DOI] [PubMed] [Google Scholar]
- 4. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first national health and nutrition examination survey. Hypertension 2006;47:833–9. 10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 5. Luyster FS, Strollo PJ, Zee PC, et al. Boards of directors of the American Academy of sleep M, the sleep research S. sleep: a health imperative. Sleep 2012;35:727–34. 10.5665/sleep.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child 2006;91:881–4. 10.1136/adc.2005.093013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banno M, Harada Y, Taniguchi M, et al. Exercise can improve sleep quality: a systematic review and meta-analysis. PeerJ 2018;6:e5172. 10.7717/peerj.5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kredlow MA, Capozzoli MC, Hearon BA, et al. The effects of physical activity on sleep: a meta-analytic review. J Behav Med 2015;38:427–49. 10.1007/s10865-015-9617-6 [DOI] [PubMed] [Google Scholar]
- 9. Lowe H, Haddock G, Mulligan LD, et al. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin Psychol Rev 2019;68:1–12. 10.1016/j.cpr.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 10. Passos GS, Poyares D, Santana MG, et al. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med 2010;6:270–5. [PMC free article] [PubMed] [Google Scholar]
- 11. Kelley GA, Kelley KS. Exercise and sleep: a systematic review of previous meta-analyses. J Evid Based Med 2017;10:26–36. 10.1111/jebm.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrianasolo RM, Menai M, Galan P, et al. Leisure-time physical activity and sedentary behavior and their cross-sectional associations with excessive daytime Sleepiness in the French SU.VI.MAX-2 study. Int J Behav Med 2016;23:143–52. 10.1007/s12529-015-9501-3 [DOI] [PubMed] [Google Scholar]
- 13. Brandão GS, Gomes GSBF, Brandão GS, et al. Home exercise improves the quality of sleep and daytime Sleepiness of Elderlies: a randomized controlled trial. Multidiscip Respir Med 2018;13:2. 10.1186/s40248-017-0114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chasens ER, Sereika SM, Weaver TE, et al. Daytime Sleepiness, exercise, and physical function in older adults. J Sleep Res 2007;16:60–5. 10.1111/j.1365-2869.2007.00576.x [DOI] [PubMed] [Google Scholar]
- 15. Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime Sleepiness and mortality in an older community population. J Am Geriatr Soc 1996;44:693–8. 10.1111/j.1532-5415.1996.tb01834.x [DOI] [PubMed] [Google Scholar]
- 16. McClain JJ, Lewin DS, Laposky AD, et al. Associations between physical activity, sedentary time, sleep duration and daytime Sleepiness in US adults. Prev Med 2014;66:68–73. 10.1016/j.ypmed.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 17. Vuori I, Urponen H, Hasan J, et al. Epidemiology of exercise effects on sleep. Acta Physiol Scand Suppl 1988;574:3–7. [PubMed] [Google Scholar]
- 18. Spörndly-Nees S, Åsenlöf P, Lindberg E. High or increasing levels of physical activity protect women from future insomnia. Sleep Med 2017;32:22–7. 10.1016/j.sleep.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 19. Joo S, Baik I, Yi H, et al. Prevalence of excessive daytime Sleepiness and associated factors in the adult population of Korea. Sleep Med 2009;10:182–8. 10.1016/j.sleep.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 20. Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014;192:175–84. 10.1007/s00408-013-9511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atoui S, Chevance G, Romain A-J, et al. Daily associations between sleep and physical activity: A systematic review and meta-analysis. Sleep Med Rev 2021;57:S1087-0792(21)00011-3. 10.1016/j.smrv.2021.101426 [DOI] [PubMed] [Google Scholar]
- 22. Fuertes E, Carsin A-E, Antó JM, et al. Leisure-time vigorous physical activity is associated with better lung function: the prospective ECRHS study. Thorax 2018;73:376–84. 10.1136/thoraxjnl-2017-210947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janson C, Anto J, Burney P, et al. The European Community respiratory health survey: what are the main results so far? European Community respiratory health survey II. Eur Respir J 2001;18:598–611. 10.1183/09031936.01.00205801 [DOI] [PubMed] [Google Scholar]
- 24. Björnsdóttir E, Janson C, Lindberg E, et al. Respiratory symptoms are more common among short sleepers independent of obesity. BMJ Open Respir Res 2017;4:e000206. 10.1136/bmjresp-2017-000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Partinen M, Gislason T. Basic Nordic sleep questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res 1995;4:150–5. 10.1111/j.1365-2869.1995.tb00205.x [DOI] [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime Sleepiness: the Epworth Sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 27. Wang F, Boros S. The effect of physical activity on sleep quality: a systematic review. Europ J Physiother 2021;23:11–8. 10.1080/21679169.2019.1623314 [DOI] [Google Scholar]
- 28. Cobb-Clark DA, Kassenboehmer SC, Schurer S. Healthy habits: the connection between diet, exercise, and locus of control. Journal of Economic Behavior & Organization 2014;98:1–28. 10.1016/j.jebo.2013.10.011 [DOI] [Google Scholar]
- 29. Min S, Masanovic B, Bu T, et al. The association between regular physical exercise, sleep patterns, fasting, and Autophagy for healthy longevity and well-being: A narrative review. Front Psychol 2021;12:803421. 10.3389/fpsyg.2021.803421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie Y, Liu S, Chen XJ, et al. Effects of exercise on sleep quality and insomnia in adults: A systematic review and meta-analysis of randomized controlled trials. Front Psychiatry 2021;12:664499. 10.3389/fpsyt.2021.664499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan X, Alén M, Wiklund P, et al. Effects of aerobic exercise on home-based sleep among overweight and obese men with chronic insomnia symptoms: a randomized controlled trial. Sleep Med 2016;25:113–21. 10.1016/j.sleep.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 32. Amiri S, Hasani J, Satkin M. Effect of exercise training on improving sleep disturbances: a systematic review and meta-analysis of randomized control trials. Sleep Med 2021;84:205–18. 10.1016/j.sleep.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 33. Mazzotti DR, Keenan BT, Thorarinsdottir EH, et al. Is the Epworth Sleepiness scale sufficient to identify the excessively sleepy subtype of OSA? Chest 2022;161:557–61. 10.1016/j.chest.2021.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thorarinsdottir EH, Bjornsdottir E, Benediktsdottir B, et al. Definition of excessive daytime Sleepiness in the general population: feeling sleepy relates better to sleep-related symptoms and quality of life than the Epworth Sleepiness scale score. J Sleep Res 2019;28:e12852. 10.1111/jsr.12852 [DOI] [PubMed] [Google Scholar]
- 35. Mellow ML, Crozier AJ, Dumuid D, et al. How are combinations of physical activity, sedentary behaviour and sleep related to cognitive function in older adults? A systematic review. Exp Gerontol 2022;159:S0531-5565(22)00006-7. 10.1016/j.exger.2022.111698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.