Abstract
Borna disease virus (BDV) is a neurotropic nonsegmented negative-stranded RNA virus that persistently infects warm-blooded animals. In horses and other natural animal hosts, infections with BDV cause meningoencephalitis and behavioral disturbances. Experimental infection of adult mice takes a nonsymptomatic course, an observation previously believed to indicate that this animal species is not suitable for pathogenesis studies. We now demonstrate that BDV frequently induces severe neurological disease in infected newborn mice. Signs of neurological disease were first observed 4 to 6 weeks after intracerebral infection. They included a characteristic nonphysiological position of the hind limbs at an early stage of the disease and paraparesis at a later stage. Histological examination revealed large numbers of perivascular and meningeal inflammatory cells in brains of diseased mice and, unexpectedly, no increase in immunoreactivity to glial fibrillar acidic protein. The incidence and severity of BDV-induced disease varied dramatically among mouse strains. While only 13% of the infected C57BL/6 mice showed disease symptoms, which were mostly transient, more than 80% of the infected MRL mice developed severe neurological disorder. In spite of these differences in susceptibility to disease, BDV replicated to comparable levels in the brains of mice of the various strains used. Intracerebral infections of newborn β2-microglobulin-deficient C57BL/6 and MRL mice, which both lack CD8+ T cells, did not result in meningoencephalitis or neurological disease, indicating that the BDV-induced neurological disorder in mice is a cytotoxic T-cell-mediated immunopathological process. With this new animal model it should now be possible to characterize the disease-inducing immune response to BDV in more detail.
Borna disease has been described as a progressive nonpurulent meningoencephalomyelitis of horses and sheep with various clinical symptoms ranging from slightly impaired coordination to paralysis and death (17, 26, 36). This disease is caused by Borna disease virus (BDV), a nonsegmented negative-stranded RNA virus (13, 41, 46). A wide variety of animal species have been successfully infected with BDV, and persistent infection of neuronal tissue is usually achieved (17, 36). Most studies of the pathogenesis of Borna disease were performed with Lewis rats, which are highly susceptible to the deleterious effects of BDV (17, 26, 28, 36, 43). By contrast, persistently infected mice were reported to exhibit only barely detectable clinical symptoms (22, 40). BDV or a related virus may be associated with psychiatric disorders in humans (4, 5, 23, 37, 38, 46).
In rats, the clinical course and histopathology of Borna disease vary with the age of the animal at the time of infection. In adult Lewis rats, BDV infection results in severe encephalitis accompanied by clinical symptoms that include hyperactivity, aggressiveness, and ataxia (17, 36). At a later stage of the disease, surviving animals are apathetic and show signs of dementia and behavioral abnormalities, and their brains show a dramatic loss of neuronal tissue (3, 17, 19, 28, 36). BDV-induced encephalitis can be prevented by immunosuppressive drugs and can be induced in drug-treated rats by adoptive transfer of immune lymphocytes (29, 35, 45), suggesting that immunopathological mechanisms play a key role in the disease process. Borna disease in rats is a CD4+ T-cell-dependent immunopathological disorder in which CD8+ T-cell-mediated mechanisms are operative (32, 33, 35). In newborn Lewis rats, exposure to BDV results in persistent infection of the neuronal tissue and other cell types but few infiltrating lymphocytes are observed histologically (19, 29). Nonetheless, severe hippocampus damage occurs in persistently infected rats and pronounced learning deficiencies are observed (10, 15, 19). Several studies indicated that BDV-induced soluble factors may negatively influence the clinical course of neurological disease. For example, it was found that tolerant, persistently infected Lewis rats developed severe clinical symptoms but only mild encephalitis when connected by parabiosis to rats with Borna disease (10), indicating that cytokines and other soluble factors produced in the brain of the ill animal reached the brain of the acceptor animal and disturbed its function. In fact, potentially toxic nitric oxide and proinflammatory cytokines were detected in the brains of BDV-infected rats (42, 47).
To identify the disease-inducing components of the immune system and the cytokine network, an animal model that is accessible to genetic manipulation is urgently needed. Here we show that infection of newborn but not adolescent mice with BDV can induce a CD8+ T-cell-dependent immunopathological process that results in severe neurological disease.
MATERIALS AND METHODS
Mice.
Breeding pairs of BALB/c, C57BL/6, C3H, and CBA mice were purchased from Charles River, Sulzfeld, Germany. Breeding pairs of wild-type MRL/MpJ and β2-microglobulin-deficient MRL/MpJ-B2m mice were purchased from Jackson Laboratory, Bar Harbor, Maine. Breeding pairs of β2-microglobulin-deficient C57BL/6J-B2m were purchased from Bomholtgard Breeding and Research Centre Ltd, Ry, Denmark. All breedings were done in our local animal facility.
Virus stocks.
A stock of BDV rat He/80 was prepared from brains of persistently infected 4-week-old Lewis rats that were infected with BDV as newborns (44). It represented the fourth continuous passage of the virus in brains of newborn rats. Stocks of BDV mouse He/80 were prepared from brains of 4- to 6-week-old diseased BALB/c mice that were infected with BDV as newborns. The stocks of mouse-adapted BDV used for the experiments described here were from the second, third, and fourth passages of original BDV rat He/80 in mouse brains. To prepare virus stocks, brains were homogenized by douncing in phosphate-buffered saline (PBS) as 10% (wt/vol) suspensions. After freezing and thawing, the material was centrifuged at 4°C for 5 min at 10,000 rpm in an Eppendorf microcentrifuge, and samples of the supernatant were stored as 100-μl aliquots at −70°C. A new aliquot of this virus stock was used for each infection experiment. The stock of BDV rat He/80 titered on C6 rat glioblastoma cells contained approximately 106 focus-forming units (FFU) of virus per ml. The stocks of BDV mouse He/80 contained between 1 × 104 and 2 × 105 FFU of virus per ml.
Titrations of BDV on C6 glioblastoma cells.
Semiconfluent monolayers of C6 cells on glass coverslips in Dulbecco’s modified essential medium supplemented with 10% fetal calf serum were incubated with various dilutions of the virus stocks. After 4 days at 37°C, the cells were fixed with 3% paraformaldehyde for 10 min, permeabilized for 5 min with 0.5% Triton X-100, and analyzed for the presence of cells expressing virus antigen by indirect immunofluorescence (9) using 0.2% serum from BDV-infected BALB/c mice. Virus titers were calculated by assuming that each focus of fluorescent cells originated from infection with a single replication-competent virus particle.
BDV infections of mice.
Samples (approximately 10 μl) of undiluted virus stocks were injected intracerebrally with a Hamilton syringe. Sham infections were performed with brain extracts from uninfected BALB/c mice that were processed as described above for virus stocks (10% [wt/vol] suspensions).
Monitoring infected mice for disease symptoms.
Mice were examined daily for neurological symptoms. To check for the characteristic unphysiological hind limb position of symptomatic animals (see Fig. 1), the mice were held up by the tail for approximately 5 seconds. To monitor the general health status of the infected mice, the animals were weighed daily starting at about 2 weeks postinfection.
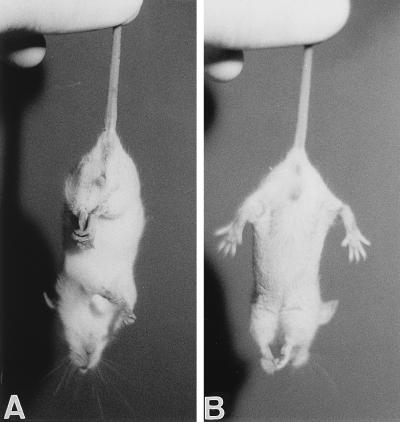
FIG. 1.
(A) Characteristic nonphysiological position of the hind limbs of a BDV-infected MRL mouse with early signs of neurological disease. (B) Position of the hind limbs of a nonsymptomatic littermate.
Analyzing serum samples for BDV-specific antibodies.
Blood samples (3 μl) were taken from the tail veins of infected mice and diluted in 100 μl of PBS. To detect antibodies to viral proteins, the samples were centrifuged and supernatants were allowed to react for 1 h at 25°C with C6 cells persistently infected with BDV (11). The cells were grown on glass coverslips, fixed for 10 min with 3% paraformaldehyde, and permeabilized for 5 min with 0.5% Triton X-100. After being extensively washed with PBS, bound antibodies were visualized with 1% fluorescein-labelled goat anti-mouse immunoglobulin G (IgG). Under these conditions, prominent staining of dot-like structures in the nuclei of infected cells was evident in blood samples containing BDV-specific antibodies.
Northern blot analysis for detecting BDV in mouse brain tissue.
Complete hemispheres of mouse brains were used to prepare total RNA as previously described (12). For Northern blot analysis, 10-μg samples of RNA were electrophoresed through a 1.2% agarose-formaldehyde gel, transferred to a nitrocellulose membrane, and hybridized under standard conditions with a radiolabelled cDNA probe (nucleotides 3 to 1873) that roughly corresponds to the nonpolyadenylated 1.9-kb viral transcript found in BDV-infected cells (7). This cDNA fragment was generated by reverse transcription-PCR with an appropriate pair of primers and RNA from C6 cells persistently infected with BDV (11). The membrane was washed at high stringency and exposed to X-ray film. To verify that a comparable amount of RNA was loaded in each lane, the membrane was stripped and reprobed with a radiolabelled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (30) under standard conditions (2). Quantitation was done by determining the radioactivity that remained bound to the membranes after stringent washing by using a Fujix BAS 1000 bio-imaging analyzer (Raytest, Straubenhardt, Germany). The BDV-specific hybridization signal of each lane was corrected for uneven loading of RNA by normalizing the value to the GAPDH signal.
Histological analysis of brain sections.
Mice were sacrificed under ether anaesthesia. One complete brain hemisphere was fixed in Zamboni’s reagent (4% paraformaldehyde and 15% picric acid in 0.25 M sodium phosphate, pH 7.5) and embedded in paraffin. Sagittal sections approximately 4-μm thick were stained with hematoxylin and eosin and examined under a light microscope. Immunostainings of tissue sections for viral antigen and glial fibrillar acidic protein (GFAP) were done with 0.2% monospecific rabbit antiserum to BDV p40 (a generous gift from I. Lipkin, Irvine, Calif.) and 0.05% polyclonal antibody against bovine GFAP (purchased from Dako, Hamburg, Germany), respectively. After being extensively washed, bound antibodies were identified with peroxidase-labeled antisera with the Vectastain reagent kit (Camon, Wiesbaden, Germany).
RESULTS
Differences among mouse strains in frequency of BDV-induced neurological disease in newborns.
Intracerebral injection of standard preparations of BDV fails to induce disease in adult laboratory mice (22). In a recent study (40), mild signs of neurological disease were observed when mouse-adapted BDV was used to infect adult mice of strain MRL. We therefore examined whether newborn mice might exhibit greater susceptibility to BDV-induced neurological disease than adult mice and whether differences in susceptibility might exist among mouse strains. Newborn mice were given intracerebral injections of 10-μl samples of 10% rat brain homogenates containing about 104 FFU of BDV strain He/80. The injected material was from the fourth passage of the virus in brains of newborn rats. Virus infection of newborn mice resulted in severe neurological disease in some but not all animals (Table 1). Affected animals could easily be identified at an early stage of the virus-induced disease by a characteristic nonphysiological position of the hind limbs: when lifted by their tails, they drew their limbs in towards their bodies (Fig. 1A), in contrast to the full extension of limbs observed with nonsymptomatic littermates (Fig. 1B). A similar disease phenotype was previously observed in mice with targeted disruptions of the neurotrophin-3 receptor gene (24), the A-raf protein kinase gene (34), and the Huntington’s disease gene (27), indicating that this phenotype is a sensitive but rather nonspecific indicator of pathological changes in the brain. The affected animals further exhibited a characteristic hunched posture, had a rough fur, and often presented with tilted heads. Neurological disease typically progressed fast and coincided with a pronounced loss of body weight of usually more than 20% in 4 days (see Fig. 5). Some animals recovered partially or completely from this acute phase of BDV-induced disease, while others failed to regain weight and started to show progressive paraparesis of the hind limbs. If not sacrificed, these animals eventually died, presumably because they were no longer able to eat and drink.
TABLE 1.
Mouse strain differences in susceptibility to BDV-induced neurological diseasea
| Mouse strain | No. of mice | No. (%) of mice with neurological symptoms | Age (days) at onset of symptoms |
|---|---|---|---|
| MRL | 81 | 67 (83) | 27–43 |
| C3H | 17 | 8 (47) | 32–45 |
| BALB/c | 56 | 19 (34) | 22–42 |
| CBA | 11 | 4 (36) | 31–52 |
| C57BL/6 | 40 | 5 (13) | 34–39 |
Newborn mice (less than 24 h old) were given intracerebral injections of 10-μl samples of BDV stock He/80 prepared from brains of infected newborn rats.
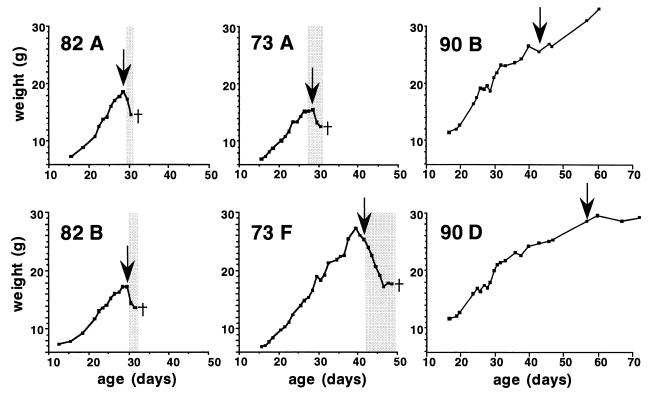
FIG. 5.
Onset of neurological disease in MRL mice coincides with first appearance of serum antibodies to BDV. Newborn wild-type (litters 82 and 73) or β2-microglobulin-deficient (litter 90) MRL mice were infected by the intracerebral route with either rat brain homogenate containing 104 FFU of BDV He/80 (litters 82 and 90) or mouse brain homogenate containing 2 × 103 FFU of BDV He/80 (litter 73). Infected animals were weighed daily and examined for the appearance of signs of neurological disease. Blood samples were analyzed for the presence of BDV-specific antibodies. The charts of two animals from each litter are shown. The shaded areas mark periods of overt neurological disease. The arrows mark the first detection of BDV-specific antibodies in serum samples. The crosses indicate when the diseased animals were euthanatized. The sudden weight gain of animal 90B towards the end of the observation period was due to pregnancy.
The percentages of animals showing the above-described signs of neurological disease differed widely among inbred mouse strains (Table 1). Mice of strain MRL were most susceptible to BDV-induced disease: 83% of the virus-infected animals presented with severe neurological disease between 27 and 43 days postinfection. Although smaller percentages of the infected C3H and CBA mice turned ill (47 and 36%, respectively), the affected individuals of these two strains showed signs of neurological disease that were about as strong as those of diseased MRL mice. About 34 and 13% of the infected BALB/c and C57BL/6 mice, respectively, showed clear signs of neurological disease at 3 to 6 weeks postinfection (Table 1). However, unlike mice of strains MRL, C3H, and CBA, only a small percentage of the infected BALB/c and C57BL/6 mice that exhibited clear signs of neurological disease went on to develop a life-threatening illness. Furthermore, the disease often progressed more slowly in the latter animals, and many BALB/c and C57BL/6 mice recovered partially or completely from the acute phase of disease by about 2 weeks post onset of first symptoms.
When BDV was adapted to grow in mouse brains by serial passage in newborn BALB/c mice, the picture did not change much. Between 36 and 57% of the animals used for each of the four passages came down with disease. When second-passage virus was used to infect newborn MRL mice intracerebrally, 11 of the 12 animals developed signs of severe neurological disease after 27 to 42 days (Table 2). These results indicated that adaptation of BDV to mice had not greatly altered its pathogenicity. To evaluate the possibility that the brain homogenate rather than the virus induced disease in our mice by autoimmunological mechanisms, seven MRL mice were sham infected with 10% extracts prepared from brains of uninfected mice. No neurological disease symptoms were recorded in these mice during the observation period of 10 weeks (data not shown).
TABLE 2.
Mouse brain-passaged BDV retains potential to induce neurological diseasea
| Mouse strain | BDV passage no. | No. of mice | No. (%) of mice with neurological symptoms | Age (days) at onset of symptoms |
|---|---|---|---|---|
| BALB/c | 1 | 7 | 4 (57) | 29–45 |
| BALB/c | 2 | 11 | 4 (36) | 28–35 |
| BALB/c | 3 | 19 | 10 (52) | 23–31 |
| BALB/c | 4 | 7 | 3 (43) | 26–33 |
| MRL | 2 | 12 | 11 (91) | 27–42 |
Neonates were given intracerebral injections of 10-μl samples of stocks of BDV He/80 that were passaged for the indicated times in brains of BALB/c mice.
Lymphocytic meningoencephalitis without increased GFAP immunoreactivity in brains of diseased mice.
Brains of BDV-infected mice were subjected to histological examination at various stages of the disease. Sections through the neocortex revealed signs of severe lymphocytic meningoencephalitis in brains of animals with overt disease. The severely diseased MRL mice presented with serious meningitis and prominent perivascular lymphocytic infiltrations of the brain (Fig. 2B). Furthermore, the numbers of neurons were decreased. The histological features of the cortex in age-matched uninfected mice were inconspicuous (Fig. 2A). Brains of diseased BALB/c mice showed similar but usually milder histopathological features than those of diseased MRL mice (data not shown). Brains of infected mice without noticeable signs of neurological disease rarely contained infiltrating lymphocytes as determined by hematoxylin and eosin staining (data not shown). Thus, the overall extent of inflammation in the brains of the infected animals correlated well with the score of clinical symptoms.
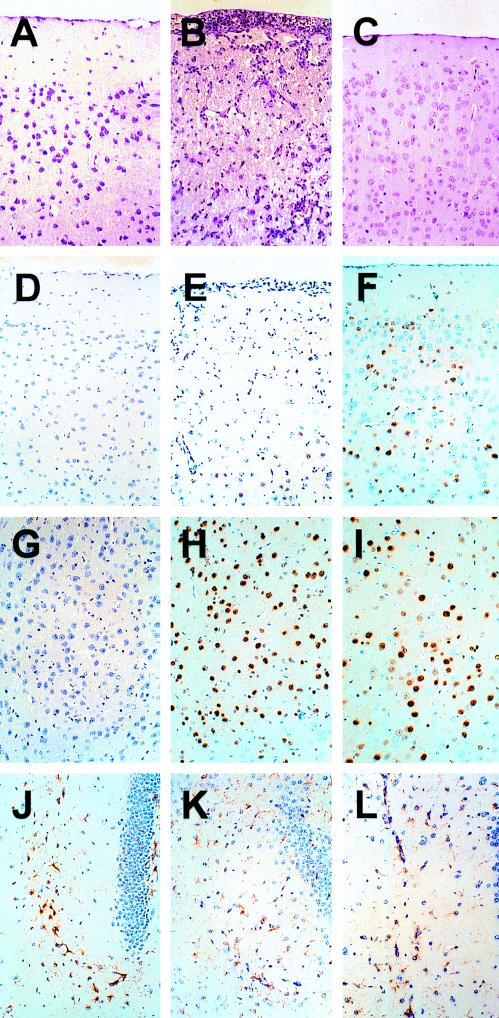
FIG. 2.
Pathological alterations and virus distribution in brains of BDV-infected MRL mice possessing or lacking a functional β2-microglobulin gene. Brain sections from an uninfected healthy MRL control mouse (A, D, G, J), from a BDV-infected wild-type MRL mouse with severe signs of neurological disease (B, E, H, K), and from a BDV-infected healthy MRL mouse lacking β2-microglobulin (C, F, I, L) were compared. Staining with hematoxylin and eosin (panels A to C) revealed strong meningitis and prominent perivascular lymphocytic infiltrates in the neocortex of the diseased animal, whereas the corresponding brain regions of the uninfected healthy control animal and of the infected β2-microglobulin-deficient (β2mo/o) MRL mouse were inconspicuous. Immunostaining of tissue sections for BDV p40 (panels D to I) showed similar numbers of infected neurons in the thalami (panels G to I) of the wild-type and mutant mice. By contrast, fewer infected neurons were present in the neocortex (panels D to F) of the diseased wild-type animal than in the neocortex of the healthy MRL β2mo/o mouse. Immunostaining failed to reveal signs of increased GFAP expression in the brains of BDV-infected wild-type and mutant mice (panels J to L) as usually seen in astrogliosis (16). Corresponding areas of the hippocampus formation are shown.
When the brain sections of diseased MRL mice were immunostained for GFAP to assess the degree of astrogliosis, very little specific staining was observed (Fig. 2K). In fact, GFAP expression in these brains did not exceed the constitutive expression observed in uninfected mice (Fig. 2J). When brain sections were immunostained for the p40 antigen of BDV, we observed that the cortical brain areas of diseased mice which harbored high numbers of lymphocytes contained only a few BDV-infected neurons (Fig. 2E) compared to those contained in the corresponding regions of nonsymptomatic mice (Fig. 2F) or the thalamus region (Fig. 2H), which usually revealed no inflammatory foci. This inverse correlation between lymphocytic infiltrations and presence of BDV-infected neurons suggested that the antiviral immune response had selectively removed infected neurons.
Kinetics of BDV replication in brains of infected newborn mice.
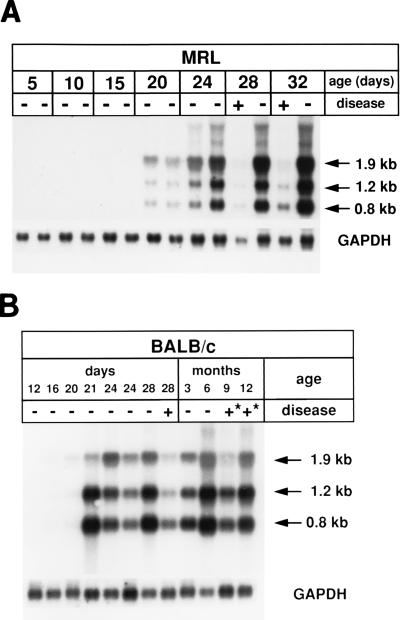
To determine the kinetics of BDV replication in brains of infected mice, we measured the concentrations of BDV-specific transcripts by Northern blot analysis at various times after intracerebral infection of newborns. No or only very low levels of BDV-specific RNAs were observed by this method in brains of MRL or BALB/c mice that were infected for less than 15 days, while viral RNAs could easily be detected in brains of mice that were infected with BDV for 20 days or longer (Fig. 3). The concentration of viral RNAs in brains of infected mice seemed to reach a plateau at approximately 4 weeks postinfection, and it seemed to be maintained at this high level for at least 12 months in nonsymptomatic BALB/c mice (Fig. 3B). In the experiment shown in Fig. 3A, one of the infected MRL mice showed typical signs of severe BDV-induced neurological disease at 28 days postinfection. Quantitative analysis of the Northern blot signals revealed that the diseased animal contained at least sixfold less viral RNA in the brain than a nondiseased littermate that was sacrificed at the same time (Fig. 3A). A similar picture was observed in a pair of diseased and nondiseased MRL mice that was sacrificed at day 32 postinfection (Fig. 3A). Likewise, a BALB/c mouse which showed clear signs of neurological disease at day 28 postinfection contained severalfold less viral RNAs than an infected BALB/c mouse that remained nonsymptomatic for 28 days (Fig. 3B). Similar findings were made in other experiments with pairs of diseased and nondiseased littermates of strains MRL and CBA (Fig. 4). Occasionally, however, as for example in a pair of diseased and nondiseased C3H mice (Fig. 4), the animal showing strong signs of neurological disease contained equal or even slightly higher levels of viral transcripts. Thus, in most but not all cases we found an inverse correlation between the presence of high levels of viral RNAs and signs of neurological disease, suggesting that (i) virus replication in cells of the central nervous system (CNS) per se did not cause the disease which we observed in our infected animals and that (ii) the antiviral activity of the immune system eliminated virus-infected cells and induced disease.
FIG. 3.
Time course of BDV replication in brains of infected MRL (A) and BALB/c (B) mice. Animals were infected as newborns with 104 FFU of BDV He/80 by the intracerebral route. At the indicated times after infection, randomly selected animals were sacrificed, RNA was prepared from complete brain hemispheres, and 10-μg samples were assayed for the presence of BDV-specific transcripts by Northern blot analysis. BDV-specific transcripts of 0.8, 1.2, and 1.9 kb were visualized by hybridization to a radiolabeled cDNA probe comprising nucleotides 2 to 1873 of the BDV genome. After stripping, the membrane was rehybridized to a radiolabeled GAPDH cDNA probe to verify that similar amounts of RNA were present in the various lanes. Animals with (+) and without (−) typical signs of neurological disease were evaluated. The symbol “+*” indicates that the animal had shown typical signs of neurological disease at approximately 5 weeks postinfection. Such animals recovered from the acute phase of disease and were nonsymptomatic at the time of analysis.
FIG. 4.
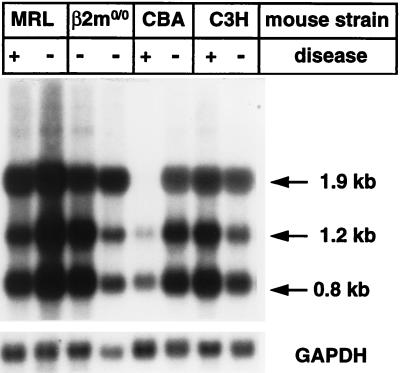
Virus loads in brains of mice of strains differing in resistance to BDV-induced disease. Mice of the MRL, β2-microglobulin-deficient (β2mo/o) MRL, CBA, and C3H strains were infected with BDV as newborns and sacrificed at the age of about 4 to 6 weeks. Brain RNA samples were assayed for the presence of BDV-specific transcripts as described in the legend for Fig. 3. Littermates with (+) and without (−) typical signs of neurological disease were compared.
To determine whether the observed variations in susceptibility of inbred mouse strains to BDV-induced neurological disease simply reflected differences in the rates at which BDV replicates in the CNSs of these animals, we compared the relative levels of viral transcripts in brains from nondiseased animals of strains MRL, BALB/c, CBA, C3H, and C57BL/6 that were infected as newborns and sacrificed at the age of 4 to 6 weeks. Differences in viral RNA contents of the various brains were usually less than fivefold (Fig. 4 and data not shown) and thus not greater than those observed between individuals of two litters of the same mouse strain (reference 18 and unpublished results). These differences probably cannot explain the dramatic differences in susceptibility to BDV-induced disease between C57BL/6 and MRL mice.
Seroconversion coincides with the onset of BDV-induced disease symptoms.
To learn more about the physiological changes immediately preceding disease onset, we carefully monitored health changes and weight gain disturbances of several MRL mice that were infected as newborns with rat- or mouse-passaged BDV (litters 82 and 73, respectively). Starting from around day 18 postinfection, we further collected blood samples for serological analysis every third day. All mice gained weight at a fairly constant rate and looked healthy until about day 28. Thereafter, most animals abruptly stopped gaining weight (Fig. 5 shows the charts of animals 82A, 82B, 73A, and 73F) and started to hold their hind limbs in a characteristic nonphysiological position (Fig. 1). Serological analysis showed that all animals that developed these signs of neurological disease had started to produce antibodies to BDV proteins just prior to the onset of clinical disease (Fig. 5). Neurological disease progressed rapidly in most of these animals so that they had to be euthanatized. Some animals, including 73F which was infected with a mouse-passaged BDV stock, continued to gain weight after 4 weeks of age, showed no signs of neurological disease, and remained seronegative during this period (Fig. 5). However, animal 73F abruptly lost weight at the age of about 6 weeks and started to show strong signs of neurological disease. Serological analysis revealed that, like its littermates which became ill earlier, it had seroconverted shortly before disease onset (Fig. 5). Thus, disease onset coincided with the initial recognition of BDV by the immune system.
No disease in BDV-infected mice lacking CD8+ T cells.
Massive mononuclear cell infiltrates in brains from diseased mice and the fact that serum antibodies to BDV started to appear in infected mice shortly before disease onset both suggested that immunopathological processes were at work. Since CD8+ T cells play a key role in the BDV-induced neurological disease of rats (33), we tested whether the symptoms would be milder or even absent in infected mice lacking this T-cell population. Mice with a targeted disruption of the β2-microglobulin gene fail to express major histocompatibility complex (MHC) class I antigen and, as a consequence, lack CD8+ T cells (25, 48). In a first experiment, we infected newborn β2-microglobulin-deficient C57BL/6 mice with the standard dose of BDV (10 μl of stock He/80 prepared from brains of infected newborn rats) by the intracerebral route. None of the 23 infected animals developed signs of neurological disease within the 12-week observation period. Serum antibodies to viral proteins were detectable in all infected β2-microglobulin-deficient mice, but they appeared later (usually not before 7 weeks postinfection) and reached lower titers than in wild-type mice (data not shown). This latter phenotype can be explained by the fact that β2-microglobulin is a component of the protection receptor for IgG catabolism that enhances the serum half-life of IgG molecules (20): mice lacking β2-microglobulin have greatly reduced levels of IgG but not IgA.
To confirm the notion that BDV-induced neurological disease in mice was dependent on CD8+ T cells, we also infected β2-microglobulin-deficient MRL mice, which genetic background, as shown above, strongly predisposes for BDV-induced disease. All 38 infected MRL mice with this genetic defect remained healthy and did not show signs of neurological disease within the observation period of 6 to 12 weeks, indicating that CD8+ cytotoxic T cells are indeed instrumental for neurologic disorder in BDV-infected mice. Northern blot analysis of brain RNAs indicated that BDV replicated to comparable levels in the CNSs of mutant and wild-type mice (Fig. 4). Unlike wild-type MRL mice, the β2-microglobulin-deficient animals continued to gain weight after seroconversion (Fig. 5), and no inflammation was detected in the brains of infected β2-microglobulin-deficient MRL mice at 6 or 8 weeks postinfection (Fig. 2C). GFAP immunostaining of brain sections of these mice could not be distinguished from that of brain sections from uninfected controls (compare Fig. 2J and L). Immunostaining for BDV p40 showed the presence of many infected neurons in the neocortex (Fig. 2F), the thalamus (Fig. 2I), and other brain regions (data not shown) of the infected β2-microglobulin-deficient MRL mice. Taken together these results strongly support the concept that BDV-induced neurological disease in mice is a CD8+ T-cell-dependent process.
DISCUSSION
Earlier studies suggested that mice are resistant to BDV-induced disease in spite of the fact that the virus grows to high titers in the brains of these animals (22). Mice of strain MRL were moderately susceptible: they exhibited signs of hyperactivity when infected with BDV that was passaged five times in mouse brains (40). Experiments described in this report now demonstrate that mice (in particular mouse strain MRL) are highly susceptible to BDV-induced illness when infected at a very young age. The diseased mice exhibited prominent signs of neurological disease and had numerous infiltrating lymphocytes in the brain. The discrepancy between the earlier reports and our present results can be explained in part by the fact that we used very young animals for our infection experiments. Furthermore, we showed here that genetic factors have a great influence on disease susceptibility. In the earlier studies, the animals were usually not infected as newborns and only a few experiments were performed with MRL mice.
Several of our observations collectively indicate that the BDV-induced neurological disorder of mice is not due to direct cell damage caused by the replicating virus but rather to indirect damage caused by the host immune response. First, BDV grew to comparable levels in the brains of diseased and nondiseased animals. Second, the most striking histopathological feature of brains from diseased mice was the presence of large numbers of infiltrating lymphocytes in the neocortex and other brain regions. Third, by measuring serum antibodies to BDV we observed that the first appearance of neurological signs of disease coincided with the onset of the antiviral immune response. Fourth, and most important, BDV infection of β2-microglobulin-deficient mice that lack CD8+ T cells (25, 48) did not result in neurological disease.
Histological analysis strongly indicated that most of the above-described signs of neurological disease in our BDV-infected mice were due to massive lymphocytic infiltration of the neocortex. We also observed that in animals with terminal disease, the numbers of BDV-infected neurons were usually rather low in brain regions with multiple inflammatory foci, suggesting that the antiviral immune response had successfully destroyed many infected neurons at the cost of severe tissue damage. An interesting finding was that although swelling of astrocytes was evident in brains of diseased mice, this astrogliosis was not accompanied by increased GFAP immunoreactivity. This was surprising considering the fact that most types of brain injuries result in very strong astrogliosis with strong GFAP immunoreactivity (16). In rats experimentally infected with BDV, astrogliosis is usually also not observed in severely inflamed brain regions (2a) but can be found in the hippocampus formation of persistently infected animals that exhibit virus-induced destruction of the dentate gyrus (14, 17).
An important conclusion from this work is that undefined genetic factors determine the susceptibility of mice to BDV-induced disease. The genetic background of MRL mice favored severe disease at a high frequency, while the genetic background of C57BL/6 mice favored only a more moderate course of the disease in a small percentage of infected animals. Although the genetic backgrounds of CBA, C3H, and BALB/c mice mediated disease induction at similar rates we observed clear differences in the severity of BDV-induced disease among these strains. The affected CBA and C3H mice, which, like MRL mice (31), express the H-2k haplotype of the MHC class I antigen, developed very severe disease, reminiscent of that of the MRL mice. By contrast, the BDV-induced neurological disease in most of the affected BALB/c (H-2d) or C57BL/6 (H-2b) mice took a more moderate course. These results suggest that the severity of disease is controlled by alleles of the MHC class I antigen, while the frequency at which BDV induces neurological disease is controlled by other genetic traits. It is of interest in this context that the genetic background of MRL mice strongly predisposes for autoimmune disorders (1). Aged MRL mice (older than 30 weeks) have enhanced titers of autoreactive antibodies and spontaneously develop pancreatitis by a mechanism that involves autoimmune CD4+ T cells (21). Since high susceptibility to BDV was abrogated in β2-microglobulin-deficient MRL mice lacking CD8+ T cells and since BDV-induced immunopathology occurred in 4- to 6-week-old mice, it seems unlikely at first glance that autoimmunity and susceptibility to BDV are mechanistically related. Nonetheless, it is conceivable that the two phenotypes are the result of the same genetic traits. If, as observed in the rat model (32, 33, 35), activation of CD8+ T cells in BDV-infected mice is dependent on help from CD4+ T cells, genetically determined hypersensitivity of the latter cell population in MRL mice might lead to a more potent response of CD8+ T cells and, in turn, to neurological disease at an enhanced frequency. This hypothesis implies that, depending on the antigenic stimulus, the hyperactive CD4+ T cells of MRL mice might predispose to various types of immunopathological disease.
A major difference between the well-established rat model system of experimental Borna disease (17, 36) and the mouse system of experimental Borna disease is that infection of adults induces neurological disease in rats but not in mice (reference 22 and unpublished results). Furthermore, while infection of newborns with BDV leads to immunological tolerance and almost disease-free persistent infection in rats (10, 19), we showed here that this constellation frequently leads to immunopathology in mice. The molecular basis for this unexpected behavior of the mouse is not well understood at present, but differences in the kinetics of BDV spread in infected newborn rats and mice could explain the different outcomes. In infected newborn rats, BDV RNA was detected in the thymus as early as 3 days postinfection (39). Our recent experiments with infected newborn mice indicated that BDV is initially highly neurotropic and that virus RNA cannot be detected outside the CNS during the first 16 days after infection by highly sensitive nested reverse transcription-PCR. If confirmed, these results would suggest that viral antigen does not appear in peripheral sites of infected newborn mice until the immune system has matured. This constellation is expected to induce a T-cell response rather than tolerance.
It is unclear at present why BDV infections of newborn and adult mice have different outcomes. One possibility is that in infected adult mice, BDV multiplication is limited more strictly to the CNS than it is in newborns. In this case, BDV antigen may not be present in sufficient quantities at peripheral sites for efficient priming of T cells. However, since infected adult mice readily produce antibodies to viral proteins, this simple scenario does not seem to be correct. Alternatively, infected adult mice may preferentially mount a Th2-type immune response to BDV antigens, while infected newborn mice might favor a Th1-type response. If true, the cytokine patterns induced by BDV in adults and in newborns should be different. It should then also be possible to induce susceptibility to BDV in adult mice by immunizing with BDV antigen under conditions that favor a Th1-type immune response.
Recent advances in gene targeting (8) were all pioneered in the mouse, and a fast-growing number of mouse strains with defined defects in the various branches of the immune system and the cytokine network are becoming available (6). The mouse model for BDV-induced neurological disorder presented here now allows, for the first time, the performance of a genetic analysis of the Borna disease-promoting processes. Mice with defined gene disruptions should help to determine which of the BDV-induced neuropathological changes are caused by the immune system and which lymphocytes contribute to immunopathology. Our initial experiments with mutant mice have already established that CD8+ T cells are instrumental in this process. By using mice lacking perforin or other T-cell effector molecules, it should now be possible to determine whether neurological disease in MRL mice is due to cytotoxic T-cell activity in the brain or results from cytokines secreted by the infiltrating lymphocytes.
ACKNOWLEDGMENTS
We thank Rosita Frank for expert technical assistance and Hanspeter Pircher, Thomas Bilzer, Ulla Schultz, Georg Kochs, and Otto Haller for technical advice and helpful comments on the manuscript.
This work was supported by a grant from the Zentrum für Klinische Forschung I of the Universitätsklinikum Freiburg.
REFERENCES
- 1.Andrews B S, Eisenberg R A, Theofilopoulos A N, Izui S, Wilson C B, McConahey P J, Murphy E D, Roths J B, Dixon F J. Spontaneous murine lupus-like syndromes: clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2a.Bilzer, T. Personal communication.
- 3.Bilzer T, Stitz L. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 4.Bode L. Human infections with Borna disease virus and potential pathogenic implications. Curr Top Microbiol Immunol. 1995;190:103–130. doi: 10.1007/978-3-642-78618-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 6.Brandon E P, Idzerda R L, McKnight G S. Targeting the mouse genome: a compendium of knockouts. Curr Biol. 1995;5:625–635. doi: 10.1016/s0960-9822(95)00127-8. [DOI] [PubMed] [Google Scholar]
- 7.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capecchi M R. Altering the genome by homologous recombination. Science. 1989;144:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 9.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone K M, Park S W, Rubin S A, Waltrip R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone K M, Rubin S A, Sierra Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschl U, Stitz L, Herzog S, Frese K, Rott R. Determination of immune cells and expression of MHC class II antigen in encephalitic lesions of experimental Borna virus disease. Acta Neuropathol. 1990;81:41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- 15.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;26:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 16.Eng L F, Ghirnikar R S. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 17.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 17a.Hallensleben, W. Unpublished results.
- 18.Hallensleben W, Zocher M, Staeheli P. Borna disease virus is not sensitive to amantadine. Arch Virol. 1997;142:2043–2048. doi: 10.1007/s007050050221. [DOI] [PubMed] [Google Scholar]
- 19.Hirano N, Kao M, Ludwig H. Persistent, tolerant or subacute infection in borna disease virus-infected rats. J Gen Virol. 1983;64:1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- 20.Junghans R P, Anderson C L. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanno H, Nose M, Itoh J, Taniguchi Y, Kyogoku M. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol. 1992;89:68–73. doi: 10.1111/j.1365-2249.1992.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao M, Ludwig H, Gosztonyi G. Adaptation of Borna disease virus to the mouse. J Gen Virol. 1984;65:1845–1849. doi: 10.1099/0022-1317-65-10-1845. [DOI] [PubMed] [Google Scholar]
- 23.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Silos-Santiago I, Smeyne R J, Lira S A, Brambilla R, Bryant S, Zhang L, Snider W D, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates Ia muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- 25.Koller B H, Marrack P, Kappeler J W, Smithies O. Normal development of mice deficient in b2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 27.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S W, Bates G P. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 28.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna disease virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 29.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Pathogenesis of borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Panabieres F, Piechaczyk M, Rainer B, Dani C, Fort P, Riaad S, Marty L, Imbach J L, Jeanteur P, Blanchard J M. Complete nucleotide sequence of the messenger RNA coding for chicken muscle glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun. 1984;118:767–773. doi: 10.1016/0006-291x(84)91461-x. [DOI] [PubMed] [Google Scholar]
- 31.Peng S L, Craft J. MHC class I polymorphism in lupus-prone MRL/Mp mice. Immunogenetics. 1996;44:407–408. [PubMed] [Google Scholar]
- 32.Planz O, Bilzer T, Sobbe M, Stitz L. Lysis of major histocompatibility complex class-I-bearing cells in borna disease virus induced degenerative encephalopathy. J Exp Med. 1993;178:163–174. doi: 10.1084/jem.178.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planz O, Bilzer T, Stitz L. Immunopathologic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pritchard C A, Bolin L, Slattery R, Murray R, McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-raf protein kinase gene. Curr Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 35.Richt J A, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 37.Rott R, Herzog S, Bechter K, Frese K. Borna disease, a possible hazard for man? Arch Virol. 1991;118:143–149. doi: 10.1007/BF01314025. [DOI] [PubMed] [Google Scholar]
- 38.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 39.Rubin S A, Sierra-Honigmann A M, Lederman H M, Waltrip R W, Eiden J J C. Hematologic consequences of Borna disease virus infection of rat bone marrow and thymus stromal cells. Blood. 1995;85:2762–2769. [PubMed] [Google Scholar]
- 40.Rubin S A, Waltrip R W, Bautista J R, Carbone K M. Borna disease virus in mice: host-specific differences in disease expression. J Virol. 1993;67:548–552. doi: 10.1128/jvi.67.1.548-552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 42.Shankar V, Kao M, Hamir A N, Sheng H, Koprowski H, Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with Borna disease virus. J Virol. 1992;66:992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 44.Stitz L, Sobbe M, Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stitz L, Soeder D, Deschl U, Frese K, Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 46.Van de Woude S, Richt J A, Zink M C, Rott R, Narayan O, Clements J E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990;250:1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y M, Schafer M K, Weihe E, Sheng H, Corisdeo S, Fu Z F, Koprowski H, Dietzschold B. Severity of neurological signs and degree of inflammatory lesions in the brains of rats with Borna disease correlate with the induction of nitric oxide synthase. J Virol. 1993;67:5786–5791. doi: 10.1128/jvi.67.10.5786-5791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. β2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]