Abstract
Background
Transjugular intrahepatic portosystemic shunt (TIPS) is a minimally invasive therapeutic option to treat the sequelae of portal hypertension. It is unclear whether current international recommendations are reflected in current clinical practice across Australia and the extent of variations in care. This study aimed to address this gap in knowledge and benchmark the current landscape of TIPS services in Australia against international guidelines.
Methods
We designed a 42-item questionnaire according to practice-based recommendations and standards of international guidelines to investigate current landscape of TIPS service across four key domains: (1) service provision, (2) patient selection and indications, (3) best procedure practice, and (4) postoperative care.
Results
Gastroenterologist/hepatologists from 23 major liver centres (67.6%) across Australia currently performing TIPS completed the questionnaire. Between 2017 and 2020, there were 456 elective TIPS insertions. Units offering TIPS service had a low median number of TIPS insertions (n=7 per annum). More than half of respondents (56.5%) did not have institutional clinical practice protocols. There was marked variation in practices across institutions in terms of TIPS indications and patient selection. Despite variations, the success rate of elective TIPS was high at 91.7% (79–100%), with 86.6% (29–100%) for rescue TIPS. There was significant variation in postoperative follow-up and care.
Conclusion
Current TIPS practice in Australia varies significantly across institutions. There is a need for a national consensus clinical practice guidelines to improve access and minimise unwarranted variation. A national registry for TIPS could measure, monitor, and report on quality of clinical care and patient outcomes.
Keywords: PORTAL HYPERTENSION, LIVER CIRRHOSIS, LIVER TRANSPLANTATION
WHAT IS ALREADY KNOWN ON THIS TOPIC
Over recent years, clinical practice guidelines have increasingly recognised advances related to procedural techniques, transjugular intrahepatic portosystemic shunt (TIPS) stent technology, and the expanding list of indications for TIPS. However, little is known about many aspects of TIPS in Australia or whether current practices across institutions are in line with international standards.
WHAT THIS STUDY ADDS
There is marked variation in routine clinical practices across TIPS centres in Australia.
More than half of centres performing TIPS lack institutional practice-based protocols.
Despite robust evidence, survival benefit or clear recommendations, most centres reserve TIPS use for selected indications.
More than one-third of TIPS centres are not providing pre-emptive ‘early’ TIPS for qualifying patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The recency of many practice-based recommendations and practice guideline and/or lack of robust evidence for some high recommendations may explain the discordance in local practice.
The diverse TIPS landscape in Australia is an urgent reminder of the need for establishment of a national registry for TIPS and consensus clinical practice guideline.
Introduction
More than four decades after its inception, transjugular intrahepatic portosystemic shunt (TIPS) has become widely accepted as a minimally invasive therapeutic option for specific complications of portal hypertension.1 Despite a low level of invasiveness when compared with surgical portosystemic shunts, high efficacy, and a favourable safety profile even in vulnerable patients, TIPS uptake appears to be low in Australia. This has likely been fuelled by anecdotal reports of shortcomings combined with local availability of technical TIPS expertise in Australia.2–5 Consequently, little is known about TIPS services in Australia and its outcomes.6–14
Over recent years, clinical practice guidelines have increasingly recognised advances related to procedural techniques, TIPS stent technology, and the expanding list of indications.15–21 However, there remains an absence of up-to-date Australian guidance on TIPS referral pathways and practice guidelines. As a result, it is unclear whether current practices across institutions are in line with international standards.
To address the paucity of real-life data regarding TIPS indications, performance, patient selection, and management, we surveyed TIPS centres in Australia. Survey results were used to assess existing practices and to benchmark the current landscape of TIPS services against international guidelines and standards.
Methods
Questionnaire development
A survey questionnaire was developed to assess and benchmark the current landscape of TIPS services in Australia against agreed international guidelines and protocols.15–19 The online survey included 42 questions across four key domains: (1) service provision, (2) patient selection and indications, (3) best procedure practice, and (4) postoperative care (see online supplemental appendix 1).
bmjgast-2023-001308supp001.pdf (155.4KB, pdf)
Respondents provided consent by completing the initial screening and consent question. The questionnaire was completed anonymously, with the respondents not asked for any identifying details regarding themselves or their institution. If participants opted to provide identifiable information, this information was deleted prior to analysis.
Section 1 included questions concerning the participant’s medical specialty and experience, as well as information about the institution in which they work, and the number of TIPS carried out from 2017 to 2019 (pre-COVID pandemic). Questions regarding the existence of available guidelines or standard of care protocols for TIPS and other questions related to service development were included. This was followed by clinical scenarios to explore institution-specific practices with respect to TIPS indications. Respondents were asked to answer based on the current practice at their institution. Scenarios were designed to include clinical indications for portal hypertensive bleeding, ascites, hepatic hydrothorax, and hepatorenal syndrome (HRS) and Budd-Chiari syndrome (BCS). Furthermore, experts were asked about the utility of TIPS for rarer indications. Participants were given choices regarding what they thought would be usual practice at their workplace.
In the third section, participants were asked about their approach to patient selection, pre-TIPS workup and procedural aspects of TIPS, with a focus on preoperative assessment of hepatic encephalopathy (HE) and preoperative cardiopulmonary and nutritional considerations. Participants were queried for their own individual expert opinion on mandatory investigations before TIPS, contraindications, and best procedural practices. The fourth section addressed postoperative care, regular observations and follow-up of patients after TIPS implantation.
Distribution of the questionnaire and data collection
The questionnaire was distributed by email to all centres performing TIPS in Australia between August and December 2022. Directors of gastroenterology and hepatology departments with expertise in endovascular management of portal hypertension, currently performing TIPS, were invited to participate. To ensure nationwide representation of all TIPS centres, the questionnaire was sent out via the Gastroenterological Society of Australia (GESA) Clinical Research Network (CRN). In total, 34 centres in Australia were identified across all states except the Northern Territory. A total of 23 responses were downloaded from the Qualtrics server in July 2023. If responses were incomplete, they were removed from the dataset (n=9).
Data analysis and presentation
All data analysis was performed using IBM SPSS statistics V.28.01.1. Descriptive data are presented as the total number or percentages of participants responding in each category. Graphs were generated using GraphPad Prism V.9.0 (San Diego, USA). Figures were created with BioRender.com.
Results
Of the 34 invited centres, 23 completed the questionnaire (67.6%). Respondents worked in the specialty of gastroenterology (13/23 (56.5%)) and hepatology (10/23 (48.5%)). TIPS centres were located in New South Wales (NSW; n=7), Victoria (n=6), Queensland (n=3), South Australia (n=2), Western Australia (n=2), Tasmania (n=2), and the Australian Capital Territory (n=1). The majority of respondents (74%) worked in tertiary hospitals without a liver transplantation unit, 26% worked in tertiary hospitals with a unit for liver transplantation.
TIPS service provision in Australia
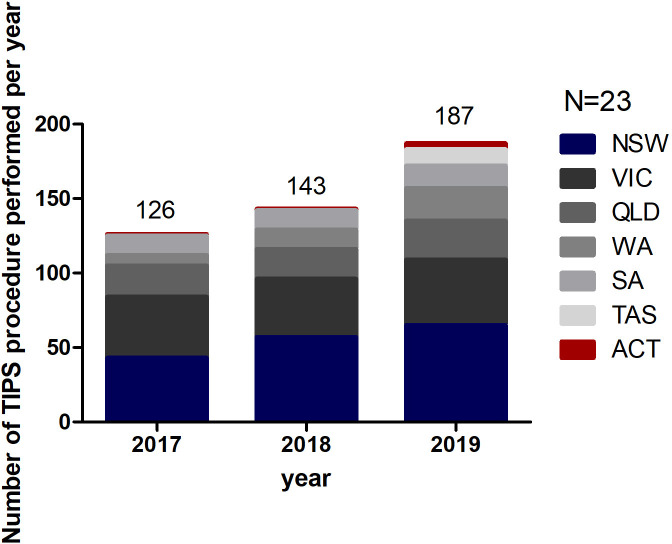
Between 2018 and 2020, there were 456 elective TIPS insertions. Units offering TIPS services had a low median number of TIPS insertion per annum: 7 in 2017; 5 in 2018; and 5 in 2019. TIPS insertion occurred in centres with availability of multidisciplinary services with expertise in interventional radiology, gastroenterology/hepatology, anaesthesia, surgery, critical care medicine and other disciplines as required (haematology, cardiology, nephrology, microbiology, liver transplant unit). The majority of elective insertions were carried out in TIPS centres in NSW (36.2%), Victoria (27.2%) and Queensland (14.7%), representing states with the highest percentage of Australia’s population (78%) (figure 1).
Figure 1.
The number of transjugular intrahepatic portosystemic shunt (TIPS) procedures performed as reported by the respondents between 2017 and 2019 in Australia. ACT, Australian Capital Territory; NSW, New South Wales; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
International guidelines recommend that units offering a TIPS service should perform a minimum of 10 procedures per year due to the relationship between improved specialist expertise and better patient outcomes.22 23 Most of the surveyed TIPS centres indicated that TIPS units should perform a minimum of seven procedures per year (range 2–15) to be considered a TIPS centre. Seven units reported doing more than 10 elective procedures per year. At the same time, other units reported a total number of procedures performed (elective) in a year of one or less, raising the question of numbers required for competency and the need of centralisation of TIPS to high‐volume centres of excellence to improve long‐term outcomes.
A team-based approach to TIPS is recommended for all stages of TIPS planning and management.16 24 25 All respondents agreed that TIPS placement can be performed by a competent proceduralist while decisions to perform a TIPS, in line with international guidelines, should be reached by an expert team made of at least one interventional radiologist and a hepatologist given the need for ongoing liver care as well as the potential need for TIPS revision after insertion.
Institutional clinical practice guidelines or protocols for TIPS
Thirteen respondents (56.5%) reported that their centres do not have a documented TIPS model of care, standard of care protocols, or clinical practice guidance for any aspect of TIPS, while 10 (43.5%) said they have a guideline for some aspects of the TIPS procedure. When asked which of the following aspects of TIPS these guidelines related to, the 10 respondents with available guidelines answered as follows: TIPS indications (7, 30.5%), patient selection (6, 26%), pre-TIPS workup (8, 34.8%), TIPS procedure (7, 30.5%), postoperative complications (4, 17.4%), postoperative care <72 hours (6, 26%), postoperative follow-up >72 hours (4, 17.4%), and post-TIPS anticoagulants (2, 8.7%).
Patient selection and indications
Tables 1 and 2 summarise respondents’ feedbacks regarding scenarios where TIPS should be indicated.
Table 1.
Respondent feedback regarding scenarios where TIPS for hypertensive portal bleeding should be considered
| TIPS for hypertensive portal bleeding | Respondents (%) |
| Salvage TIPS for acute gastro-oesophageal variceal bleeding refractory to endoscopic and drug therapy as defined by Baveno VII criteria, Child-Pugh Score (CPS) <14. | 100 |
| Pre-emptive (early—within 72 hours) TIPS in patients with acute variceal bleeding in haemodynamically stable patients with Child’s C disease C9–C13 or MELD≥19. | 65.2 |
| Secondary prevention of oesophageal variceal bleeding or GOV1 gastric varices. | 26 |
| Secondary prevention of gastric variceal bleeding (IGV1, IGV2, GOV2). | 39.1 |
| For patients with bleeding from ectopic varices refractory to local and pharmacological therapies. | 82.6 |
| For patients with bleeding from portal hypertensive gastropathy (PHG) refractory to non-selective beta blockers (NSBB) and iron therapy. | 52.2 |
| Pre-emptive TIPS for acute variceal bleeding in acute-on-chronic liver failure. | 17.4 |
GOV, gastro-oesophageal varices; IGV, isolated gastric varices; MELD, Model for End-stage Liver Disease; TIPS, transjugular intrahepatic portosystemic shunt.
Table 2.
Respondent feedback regarding scenarios where TIPS should be considered (other indications)
| Indication | Respondents (%) |
| Refractory or current ascites. | 100 |
| Hepatic hydrothorax on maximal medical therapy requiring frequent thoracentesis or with significant clinical symptomatology. | 95.6 |
| Non-refractory ascites. | 13 |
| Hepatorenal syndrome. | 26 |
| Hepatopulmonary syndrome with established indication for TIPS. | 39 |
| Patients with Budd-Chiari syndrome who do not respond to initial medical therapy and hepatic interventions or who are not candidates for percutaneous revascularisation of hepatic venous outflow tract. | 70 |
| Prophylactic TIPS prior to elective non-hepatic surgery in patients with portal hypertension. | 30 |
| Idiopathic non-cirrhotic portal hypertension (portosinusoidal vascular liver disease) with same indications as cirrhotic portal hypertension. | 70 |
| Portal vein thrombosis. | 39 |
| PVT and in the presence of venous cavernoma. | 21.8 |
| Post-liver transplantation. | 13 |
PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt.
TIPS for portal hypertensive bleeding
There was marked variation in response regarding indications of TIPS for portal hypertensive bleeding management and prophylaxis across institutions.
TIPS for ascites, HRS and hepatic hydrothorax
All respondents agreed that TIPS insertion is recommended for selected patients with cirrhosis and refractory or recurrent ascites, provided there is no contraindication to the procedure. The majority (22, 95.6%) indicated that TIPS can be considered in patients with hepatic hydrothorax on maximal medical therapy requiring frequent thoracentesis or with significant clinical symptomatology. There was a noticeable difference between surveyed experts on TIPS consideration for non-refractory ascites. 78.3% (18 centres) commented on the futility of TIPS in patients with ascites that is not refractory; however, three (13%) of respondent advocated that TIPS can be indicated for selected cohorts of patients with no refractory ascites.
In the context of HRS, almost three-quarters of respondents (17, 74%) have not considered TIPS for the management of patients with HRS-acute kidney injury. Only six (26%) centres have performed TIPS in patients with HRS type 1 and/or type 2.
TIPS and hepatopulmonary syndrome
More than half of the respondents (n=14, 61%) pointed out that TIPS is unlikely to have any therapeutic benefit for hepatopulmonary syndrome. In contrast, a narrow proportion of respondents (9, 39%) asserted that TIPS may be considered in patients with hepatopulmonary syndrome who have an established indication for TIPS such as diuretic refractory ascites.
Budd-Chiari syndrome
Nearly a third of respondents (7, 30%) indicated that they do not perform TIPS for patients with BCS at their centres, whereas 16 (70%) of respondents reported that their centres performed TIPS for selected patients with BCS. Of note, out of the 16 TIPS centres who perform TIPS for patients with BCS, six were transplant centres.
Prophylactic TIPS prior to elective non-hepatic surgery in patients with portal hypertension
A large proportion of respondents (16, 70%) were not in favour of performing prophylactic TIPS insertion in patients with compensated cirrhosis undergoing curative surgery for cancer. In contrast, seven (30%) of respondents stated that TIPS can prophylactically be used for patients with cirrhosis necessitating curative surgeries, vascular conditions like abdominal aortic aneurysm-open repair, and other abdominal surgeries.
TIPS and idiopathic non-cirrhotic portal hypertension
Idiopathic non-cirrhotic portal hypertension (INCPH) or portosinusoidal vascular liver disease is a rare cause of intrahepatic presinusoidal portal hypertension.26 27 Approximately one-third of respondents (7, 30%) suggested that TIPS creation should not be considered for patients with INCPH, while the remainder were inclined to consider TIPS for these patients, but exclusively for the same indications as cirrhotic portal hypertension.
Portal vein thrombosis
We found that practices across institutions varied, with nine respondents (39%) finding it appropriate to recommend the TIPS procedure for patients with portal vein thrombosis (PVT) and that the presence of PVT should not be considered as absolute contraindication for TIPS creation. When asked if they would perform TIPS procedures in patients with PVT and in the presence of venous cavernoma, the same experts generally felt uncomfortable to proceed with TIPS (18, 78.2%), likely owing to the presence of cavernoma that is associated with high failure rates and increased morbidity.
TIPS and orthotopic liver transplant
Current opinions demonstrate that whole-graft liver transplantation does not pose a major technical difficulty to TIPS. Only three surveyed centres (13%) indicated that they perform TIPS procedures in patients after orthotopic liver transplants, all of whom were leading TIPS providers in their respective states.
Considerations before TIPS
Patient selection for TIPS is a multidisciplinary decision that entails demographic, clinical, laboratory parameters, and preoperative considerations as well as standard scoring systems such as the Model for End-stage Liver Disease, Child-Pugh Score and the Freiburg Index of Post-TIPS Survival.28 29 Consideration of factors such as encephalopathy, cardiopulmonary function, frailty, and age is necessary to ensure best patient outcomes.
Pre-TIPS assessment of encephalopathy
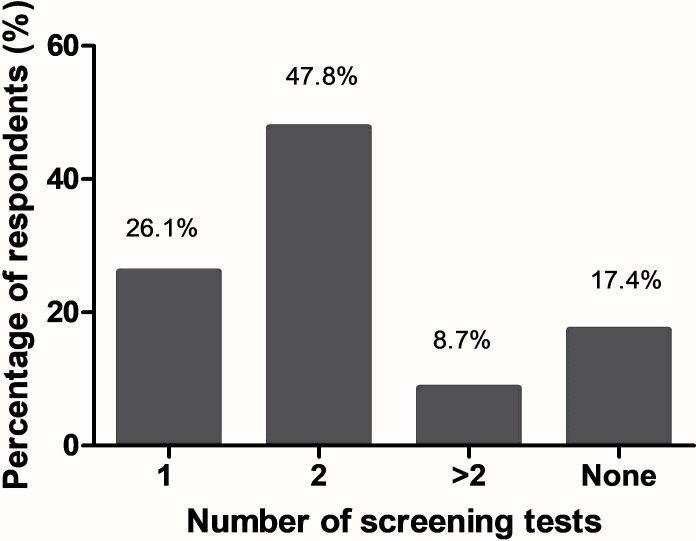
HE is a frequent complication of all portosystemic shunts including TIPS.30 An episode of overt HE can occur in up to 50% of patients after TIPS.31–34 Nearly half of respondents (47.8%) recommend that at least two screening tests for HE should be performed before TIPS placement (figure 2). Almost a quarter of respondents (26.1%) said that they will perform only one test to screen for HE before TIPS, while 56.5% of respondents would recommend two or more tests. Notably, 17.4% reported that they will not screen for HE before TIPS.
Figure 2.
Number of hepatic encephalopathy (HE) screening tests recommended by experts prior transjugular intrahepatic portosystemic shunt (TIPS) procedure (current practice).
Age
The largest proportion of respondents (9, 39.1%) considered TIPS in patients above 70 a risky procedure. On the other hand, only 2 (8.7%) (>65 years), 3 (13%) (>75 years), 2 (8.7%) (>80 years), and 3 (13%) (>85 years) of respondents, respectively, considered these age cut-offs when TIPS becomes a risky procedure. Meanwhile, three (13%) of respondents said that there is no age cut-off when TIPS was perceived as risky procedure and one respondent could not specify an age cut-off for TIPS.
Cardiopulmonary assessment
Overall, respondents have said that candidate patients for TIPS should undertake the following assessments and diagnostic modalities prior elective TIPS insertion: contemporary echocardiographic measurement of both cardiac ventricular function (22, 95.65%); complete cardiopulmonary history and physical examination (21, 91.3%); 12-lead ECG for detection of arrhythmia (15, 65.2%); cardiologist consultation (5, 21.7%); and N-terminal pro-B-type natriuretic peptide (4, 17.4%).
Further, when asked if they mandate Doppler echocardiography prior TIPS, 20 centres (87%) responded that Doppler echocardiography should be undertaken in all patients referred for elective TIPS.
Nutritional assessment
Sarcopenia, frailty, and malnutrition are prevalent among patients with decompensated cirrhosis.35 14 (60.8%) of respondents acknowledged the need for nutritional assessment before TIPS placement, with eight (35%) of respondents recommending pre-TIPS patients undergo anthropometric and functional assessments for sarcopenia such as hand grip and short physical performance battery. Further radiological screening for sarcopenia was indicated by three (13%) of centres (CT, dual-energy X-ray absorptiometry (DEXA), etc).
Alcohol relapse is frequent following TIPS placement.36 37Our data demonstrated marked variation in responses regarding routine alcohol use disorder (AUD) screening prior to TIPS shunt creation across institutions with just 60% of institutions actively screening for AUD.
Pre-TIPS assessments
If a patient is considered an appropriate candidate for TIPS, a comprehensive clinical history and physical examination are necessary. Figure 3 shows routine laboratory and instrumental investigations required prior to elective TIPS placement across institutions in Australia (current practice).
Figure 3.
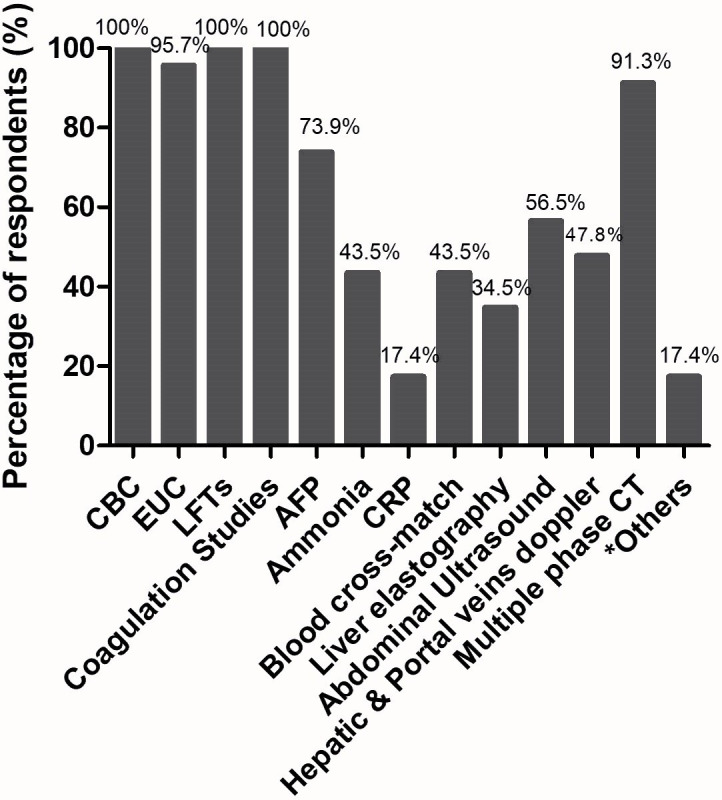
Routine laboratory and instrumental investigations required prior to elective transjugular intrahepatic portosystemic shunt (TIPS) placement across institutions in Australia (current practice). *Other investigations included liver biopsy for selected cases and oesophagogastroscopy (upper endoscopy). Liver scan, also known as transient elastography, is carried using non-invasive device known as FibroScan (Echosens, France). AFP, alpha-fetoprotein; CBC, complete blood count; CRP, C reactive protein; EUC, electrolytes, urea, and creatinine; LFT, liver function test.
Best procedure practice
Absolute contraindications
Respondents enumerated a list of absolute contraindications to TIPS (medical and anatomical). Table 3 summarises TIPS centre responses regarding contraindications to TIPS.
Table 3.
TIPS centre responses to absolute contraindications (medical and anatomical) to elective TIPS creation (current practice)
| Contraindication | Respondents (%) |
| Significant pulmonary hypertension diagnosed on right heart catheterisation (mean pulmonary artery pressure of >45 mm at RCH) despite treatment). | 95.7 |
| Heart failure (ACC/AHA stage C or D, or a documented ejection fraction <50%) or severe cardiac valvular insufficiency (ACC/AHA stage C or D). | 91.3 |
| Rapidly progressive liver failure. | 82.6 |
| Serum creatinine >250 μmol/L. | 39.1 |
| Severe or uncontrolled hepatic encephalopathy (≥2 West Haven Scale). | 95.7 |
| Uncontrolled systemic infection or sepsis. | 87.0 |
| Unrelieved biliary obstruction. | 78.3 |
| Polycystic liver disease precluding TIPS creation. | 52.2 |
| Extensive primary or metastatic hepatic malignancy. | 78.3 |
| Pregnancy or breast feeding. | 47.8 |
| Absence of vascular accesses (technical contraindication). | 87.0 |
RHC, right heart catheterization; TIPS, transjugular intrahepatic portosystemic shunt.
Stents
While bare metal stents were standard in the past, expanded polytetrafluoroethylene-covered stents have become the current gold standard in routine practice mainly due to improved patency, ascites control, rebleeding prevention and cost-effectiveness.38
Centres were asked about the starting diameter of stent deployed during TIPS as this is a critical factor to potentially mitigate postoperative risk of HE. The deployment of controlled expansion stent exhibits incremental and reliable expansion of stent diameter. Only 11 out of 23 centres (39.3%) preferred expandable stents with a ‘dial-able’ diameter of 8 or 10 mm stents. Two centres (7.15%) preferred larger diameter stents (12 mm) to achieve adequate portal pressure reduction. The remaining centres preferred smaller diameter stents such as 8 mm (6, 21.5%), or 10 mm (8, 28.6%) potentially because smaller portosystemic shunts are known to be associated with a lower risk of HE at cost of satisfactory portal pressure reduction.
TIPS access
The technical success of TIPS procedure is determined by effective puncture of the portal vein that does not extend towards the splenic/superior mesenteric vein confluence nor compromise future options for liver transplantation. Around one-third of hepatology representatives (7, 30.4%) reported they do not know the access technique used within their interventional radiology department, perhaps because all respondents self-identified as non-proceduralists (ie, interventional radiologists).
Success rates
In Australia, it is estimated that TIPS success rate of elective procedure according to 20 respondents (87%) is 91.65% (79–100%), while success rate of rescue TIPS is estimated to be 86.55% (29–100%).
Postoperative care
The level of care for postoperative patients with TIPS creation is inherently dictated by patient factors for developing TIPS-related haemodynamic compromise or immediate complication based on intraprocedural events. Based on respondents, patients are monitored in the general inpatient ward after TIPS creation (16, 69.5%), or the high dependence unit (HDU) (6, 20%). Only one centre (4.34%) monitors postoperative patients in an acute care unit after TIPS creation where nurse to patient ratio is usually higher than of HDU.
Testings following TIPS creation
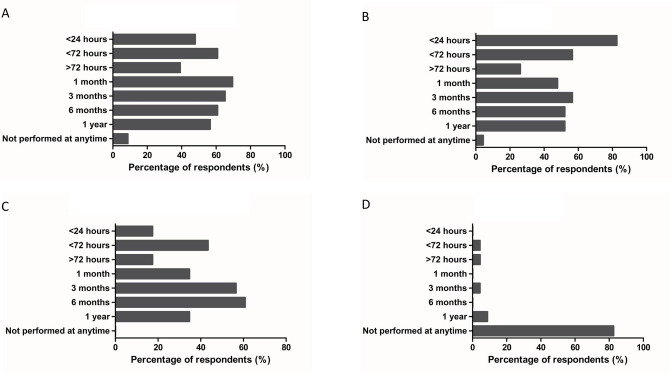
Patients who have undergone TIPS are regularly followed up by hepatologists/gastroenterologists and interventional radiologists to ensure ongoing management of chronic liver disease, postprocedural complications, and to determine any need for potential device revision. Results showed variation in responses regarding routine post-TIPS practices across institutions (figure 4).
Figure 4.
Routine tests performed after elective transjugular intrahepatic portosystemic shunt (TIPS) prior discharge or as part of follow-up. (A) Post-TIPS hepatic encephalopathy (HE) screening. (B) Complete blood count (CBC). (C) Doppler ultrasound. (D) Venography.
Post-TIPS HE screening
Almost half of expert respondents believe that HE screening should start from <24 hours during postoperative period and a significant percentage of respondents (~40%) agreed that this practice should take place during follow-up period (figure 4A).
Routine blood tests
Complete blood count (CBC), Prothrombin time (PT)/ International Normalized Ratio (INR), and metabolic panel usually are undertaken in all patients 24 hours after TIPS insertion. A significant proportion of respondents (82.6%) reported that CBC should be obtained in the first 24 hours after TIPS creation (figure 4B).
Doppler ultrasound
43.4% of respondents said that Doppler ultrasound would routinely be performed less than 72 hours after TIPS (figure 4C). A recent update from American Association for the Study of Liver Diseases (AASLD) guidance on the use of TIPS suggests that the frequency of Doppler ultrasound for TIPS placed for variceal bleeding at 1 week, 3 months and 6 months, and every 6 months thereafter to assess for patency.17
Venography
More than 80% of respondents felt that it was not appropriate to perform venography during postoperative or follow-up periods. Only a narrow proportion of respondents (4.3%) suggested performing venography at <72 hours, >72 hours, 1 month, and 3 months, while 8.7% of respondents suggested performing venography at 1 year (figure 4D).
Early postoperative testings and management
Anticoagulation
Experts were asked about coagulation agents and antiplatelet drugs that are routinely administered after TIPS. Ten respondents (43.47%) said that they would not administer anticoagulants <72 hours postoperatively, while 13 (56.5%) preferred low-molecular weight heparin, and two centres (8.7%) preferred acetylsalicylic acid (aspirin) for postoperative anticoagulation.
Portal pressure gradient measurement
All respondents indicated that they measured portal pressure gradient before and after stent deployment. Respondents reported that in patients with variceal bleeding undergoing TIPS, absolute portal pressure gradient (PPG) reduction to <12 mm Hg or a relative reduction of PPG at least 20% from pre-TIPS baseline was their anticipated target. Recently, Baveno VII criteria have advised that a relative reduction of PPG by at least 50% from pre-TIPS baseline may also be useful.18
Postoperative management
Post-TIPS HE
Treatment strategies for post-TIPS HE vary depending on the clinical presentation. Respondents reported that if a patient develops post-TIPS HE, their pharmacological management will include lactulose alone (23, 100%) as first-line medication, or in combination with rifaximin (22, 95.7%), cessation of proton pump inhibitors (8, 34.8%) and oral branched-chain amino acids (1, 4.34%). The persistence or refractory HE despite optimal medical therapy warrants endovascular shunt reduction, embolisation, or occlusion . Respondents reported that endovascular shunt diameter reduction to mitigate post-TIPS HE was performed in 17 centres (73.9%), while five (21.74%) favoured TIPS occlusion. Other centres (5, 21.74%) said that embolising competing spontaneous shunt may allow maintenance of post-TIPS portosystemic pressure gradient (PSG) above the accepted threshold of TIPS for variceal control, thereby lowers the chances of postprocedural HE and equally minimises the risk of variceal bleeding.
Anticipated discharge time after elective TIPS insertion for uncomplicated cases
The medical decision to discharge patients from one level of care to the next is individualised. Based on Australian centre responses, the anticipated postelective TIPS discharge time is 2.4 days (24 hours–4 days).
Discussion
TIPS is a safe and minimally invasive therapeutic option to treat sequelae of portal hypertension. It is a standard treatment for patients with refractory ascites and variceal bleeding worldwide, providing long-term symptom control and prolonging transplant-free survival.39–41 Improved endovascular techniques and TIPS stent technology have simplified TIPS placement and minimised complications in recent years, yet current attitudes regarding TIPS use in Australia vary enormously across institutions based on experience, knowledge, and risk aversion.
This national study has demonstrated that the TIPS procedure is not widely performed in Australia. Approximately 7.37 TIPS insertions were performed in Australia per million people in 2019 compared with 25.24 insertions per million people in Germany (2018).42 Until late 1990s, only one centre (Royal Prince Alfred Hospital, Sydney, NSW) (population ∼6.5 million in 1990s) performed TIPS.
As TIPS requires a high degree of technical and clinical practice to achieve optimal patient outcomes, numerous studies have explored a link between higher TIPS procedure volume and better outcomes. An American study in 2017 found that the risk of inpatient mortality was lower in hospitals performing ≥20 TIPS per year.22 Consistent with this study, a recent Canadian study found that outcomes improved with units performing a minimum of 10 procedures per year.23 With only seven units performing more than 10 procedures in Australia, there is a need to address centralisation versus decentralisation of services: the advantage of centralised provision of TIPS would provide expert care, high-level infrastructure, state-of-the-art diagnostic tests and therapies. This, however, is challenging in the Australian context given the dispersion of the population over large geographical areas. Patients living in outer regional or remote areas of Australia are likely to face major barriers accessing TIPS centres. In fact, a retrospective study assessing the outcomes of TIPS at a low-volume single centre in South Australia concluded that low volume should not be a contraindication to providing a TIPS service given high technical and clinical success; however, the same study reported on the need for better understanding of institutional factors that may impact quality of service in low-volume centres.43 Ensuring equal access to TIPS centres and determining the extent of centralisation of TIPS provision will be an important aspect of any future regulatory frameworks and guidelines.
A case report published in 1997 described the first successful application of TIPS in Australia on a patient with tense ascites secondary to hepatic vein thrombosis.10 Despite this milestone, a significant proportion of TIPS centres limit TIPS use to routine clinical applications such as refractory ascites and variceal bleedings (ie, no expanding of indications). Moreover, approximately 35% of our respondents were not providing pre-emptive TIPS for qualifying patients (eg, acute variceal bleeding in patients with Child-Pugh class C9–C13) where moderate to high-level evidence recommendations exist and significant improvement in outcomes can be expected.16–18 40 44 45
Our results highlight major challenges regarding available resources and the implementation of changes to practice suggested by the evidence, particularly with respect to patient selection, indication and procedural aspects of TIPS. We found significant variation in preoperative workup and postoperative follow-up. Intriguingly, the clinical standards were significantly different among TIPS centres, suggesting that some updated procedural aspects have not been implemented. This is possibly due to low-level evidence used in some consensus guideline recommendations that, although strongly recommended, have not been updated.
The likelihood of an unfavourable outcome following TIPS can be precipitated by various pre-existing clinical conditions. Patients with active sepsis or severe/uncontrolled HE, for instance, should not undergo TIPS. Meanwhile, the absence of a vascular access represents a technical contraindication to stent placement that can be overcome using alternative, although challenging, techniques to bypass this technical obstacle.46–49 Results of this survey demonstrate that a narrow proportion of centres consider performing TIPS despite these contraindications, highlighting significant knowledge gaps across some centres that have the potential to cause undue harm and complications.
Our study is limited by its small sample size and anonymity of participants, and therefore was not powered to make statistical comparisons between centres. Moreover, participants were not randomly selected but rather invited based on their known expertise in TIPS leading to the possibility of selection bias in responses. The retrospective nature of the study also increases the likelihood of recall bias.
Despite these shortcomings, this study provides valuable information on real-life institutional practices and current TIPS services. Our survey, formulated according to standards set by international guidelines, can be deployed again in the future to capture changes in workforce practice and preferences over time. It can also be repurposed to inform needs for national initiatives targeted to specific specialties or to evaluate change/upskill in their knowledge, practice, or preferences.
It should be highlighted that the international TIPS consensus guidelines from established scientific societies are relatively recent and therefore this can explain the discordance between practice-based recommendations of various international organisations and changes in local Australian practice. In addition, more than half of Australian TIPS centres lack institutional guidance regarding many aspects of TIPS procedures. This work highlights the need to develop a TIPS consensus guideline that will lead to improved practice. Ultimately, adherence to these best practice recommendations and best procedural aspects may lead to system-level improvement in TIPS uptake, quality of care and patient outcomes. The diverse TIPS landscape in Australia is yet another reminder for the need to establish a national registry for TIPS. Such a registry can measure, monitor, and report on the quality of clinical care and patient outcomes. These data will reflect national statistics on the role of TIPS, inform policy concerning health resource utilisation, identify areas of need as well as reduce unwarranted variations in care. Finally, an Australian registry will promote evidence-based clinical practice by assessing compliance with established best practice guidelines.
In conclusion, this study shows significant discrepancies between TIPS guidelines and routine clinical practice in Australia. This underscores the need to collect nationwide evidence on the performance and utilisation of TIPS that will underpin a more uniform approach to service provision in Australia.
Acknowledgments
The authors acknowledge all TIPS centres that took part in this survey.
Footnotes
AM and GA contributed equally.
Contributors: Study concept and design: EK, AM, GA. Writing—original draft preparation: EK. Acquisition of data: EK, GA. Analyses and interpretation of data: EK, GA. Writing—review and editing: EK, AM, GA. Critical revision of the manuscript for important intellectual content: SR, JG, SKR, AM, GA. Supervision: SR, AM, GA. Guarantor: GA. All authors approved the final version of the article.
Funding: This work was supported by the Ainsworth Bequest given to Western Sydney University.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data presented in this study are available on request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethics approval was obtained from the local human research ethics committee of Western Sydney Local Health District (approval number: 2021/ETH10976).
References
- 1. Colapinto RF, Stronell RD, Gildiner M, et al. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol 1983;140:709–14. 10.2214/ajr.140.4.709 [DOI] [PubMed] [Google Scholar]
- 2. Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular Intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol 2008;103:2738–46. 10.1111/j.1572-0241.2008.02102.x [DOI] [PubMed] [Google Scholar]
- 3. Huonker M, Schumacher YO, Ochs A, et al. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut 1999;44:743–8. 10.1136/gut.44.5.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaba RC, Khiatani VL, Knuttinen MG, et al. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol 2011;196:675–85. 10.2214/AJR.10.4819 [DOI] [PubMed] [Google Scholar]
- 5. Busk TM, Bendtsen F, Poulsen JH, et al. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol 2018;314:G275–86. 10.1152/ajpgi.00094.2017 [DOI] [PubMed] [Google Scholar]
- 6. Pateria P, Jeffrey GP, Garas G, et al. Transjugular intrahepatic portosystemic shunt: indications, complications, survival and its use as a bridging therapy to liver transplant in Western Australia. J Med Imaging Radiat Oncol 2017;61:441–7. 10.1111/1754-9485.12563 [DOI] [PubMed] [Google Scholar]
- 7. Kurmis TP. Transjugular intrahepatic portosystemic shunt: an analysis of outcomes. ANZ J Surg 2009;79:745–9. 10.1111/j.1445-2197.2009.05093.x [DOI] [PubMed] [Google Scholar]
- 8. Williams DB, Waugh R, Selby W. Transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of refractory ascites. Aust N Z J Med 1998;28:620–6. 10.1111/j.1445-5994.1998.tb00658.x [DOI] [PubMed] [Google Scholar]
- 9. Armstrong PK, MacLeod C. Infection of transjugular intrahepatic portosystemic shunt devices: three cases and a review of the literature. Clin Infect Dis 2003;36:407–12. 10.1086/346156 [DOI] [PubMed] [Google Scholar]
- 10. Nicoll A, Fitt G, Angus P, et al. Budd-Chiari syndrome: intractable ascites managed by a trans-hepatic portacaval shunt. Australas Radiol 1997;41:169–72. 10.1111/j.1440-1673.1997.tb00706.x [DOI] [PubMed] [Google Scholar]
- 11. Lamanna A, Mitreski G, Maingard J, et al. Ultrasound-guided portal vein puncture during transjugular intrahepatic portosystemic shunt: technique and experience of a quaternary liver transplant hospital. J Med Imaging Radiat Oncol 2022;66:60–7. 10.1111/1754-9485.13288 [DOI] [PubMed] [Google Scholar]
- 12. Hebbard GS, Fitt G, Thomson KR, et al. Transjugular intrahepatic portal-systemic shunts (TIPS)--Initial experience and clinical outcome. Aust N Z J Med 1994;24:141–8. 10.1111/j.1445-5994.1994.tb00549.x [DOI] [PubMed] [Google Scholar]
- 13. Ferral H, Bjarnason H, Wegryn SA, et al. Refractory ascites: early experience in treatment with transjugular intrahepatic portosystemic shunt. Radiology 1993;189:795–801. 10.1148/radiology.189.3.8234706 [DOI] [PubMed] [Google Scholar]
- 14. Duggan A, Waugh RC, Perkins KW, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) for variceal haemorrhage: initial results in 28 patients. Aust N Z J Med 1994;24:136–40. 10.1111/j.1445-5994.1994.tb00548.x [DOI] [PubMed] [Google Scholar]
- 15. Tripathi D, Stanley AJ, Hayes PC, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut 2020;69:1173–92. 10.1136/gutjnl-2019-320221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boike JR, Thornburg BG, Asrani SK, et al. North American practice-based recommendations for transjugular intrahepatic portosystemic shunts in portal hypertension. Clin Gastroenterol Hepatol 2022;20:1636–62. 10.1016/j.cgh.2021.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee EW, Eghtesad B, Garcia-Tsao G, et al. AASLD practice guidance on the use of TIPS, variceal embolization, and retrograde transvenous obliteration in the management of variceal hemorrhage. Hepatology 2024;79:224–50. 10.1097/HEP.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 18. de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - renewing consensus in portal hypertension. J Hepatol 2022;76:959–74. 10.1016/j.jhep.2021.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 20. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis 2017;49:121–37. 10.1016/j.dld.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 21. Mandorfer M, Aigner E, Cejna M, et al. Austrian consensus on the diagnosis and management of portal hypertension in advanced chronic liver disease (Billroth IV). Wien Klin Wochenschr 2023;135:493–523. 10.1007/s00508-023-02229-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarwar A, Zhou L, Novack V, et al. Hospital volume and mortality after transjugular intrahepatic portosystemic shunt creation in the United States. Hepatology 2018;67:690–9. 10.1002/hep.29354 [DOI] [PubMed] [Google Scholar]
- 23. Mah JM, DeWit Y, Djerboua M, et al. Association between institutional factors and long-term survival following transjugular intrahepatic portosystemic shunt. Hepatol Commun 2019;3:838–46. 10.1002/hep4.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases . The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010;51:306. 10.1002/hep.23383 [DOI] [PubMed] [Google Scholar]
- 25. Dariushnia SR, Haskal ZJ, Midia M, et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 2016;27:1–7. 10.1016/j.jvir.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 26. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu . EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol 2016;64:179–202. 10.1016/j.jhep.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Tsao G. Idiopathic noncirrhotic portal hypertension: what is it? Clin Liver Dis (Hoboken) 2015;5:120–2. 10.1002/cld.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patidar KR, Sydnor M, Sanyal AJ. Transjugular intrahepatic portosystemic shunt. Clin Liver Dis 2014;18:853–76. 10.1016/j.cld.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bettinger D, Sturm L, Pfaff L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. Journal of Hepatology 2021;74:1362–72. 10.1016/j.jhep.2021.01.023 [DOI] [PubMed] [Google Scholar]
- 30. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines on the management of hepatic encephalopathy. J Hepatol 2022;77:807–24. 10.1016/j.jhep.2022.06.001 [DOI] [PubMed] [Google Scholar]
- 31. Riggio O, Merlli M, Pedretti G, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci 1996;41:578–84. 10.1007/BF02282344 [DOI] [PubMed] [Google Scholar]
- 32. Zuo L, Lv Y, Wang Q, et al. Early-recurrent overt hepatic encephalopathy is associated with reduced survival in cirrhotic patients after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol 2019;30:148–53. 10.1016/j.jvir.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 33. Bai M, Qi X-S, Yang Z-P, et al. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol 2014;20:2704–14. 10.3748/wjg.v20.i10.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masson S, Mardini HA, Rose JD, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008;101:493–501. 10.1093/qjmed/hcn037 [DOI] [PubMed] [Google Scholar]
- 35. Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the study of liver diseases. Hepatology 2021;74:1611–44. 10.1002/hep.32049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cam I, Gencturk M, Lim N, et al. Alcohol recidivism following transjugular intrahepatic portosystemic shunt placement: frequency and predictive factors. Cardiovasc Intervent Radiol 2021;44:758–65. 10.1007/s00270-020-02754-5 [DOI] [PubMed] [Google Scholar]
- 37. Brensing KA, Textor J, Perz J, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut 2000;47:288–95. 10.1136/gut.47.2.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perarnau JM, Le Gouge A, Nicolas C, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol 2014;60:962–8. 10.1016/j.jhep.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 39. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent Ascites. Gastroenterology 2017;152:157–63. 10.1053/j.gastro.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 40. Larrue H, D’Amico G, Olivas P, et al. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol 2023;79:692–703. 10.1016/j.jhep.2023.04.028 [DOI] [PubMed] [Google Scholar]
- 41. Hernández-Gea V, Procopet B, Giráldez Á, et al. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology 2019;69:282–93. 10.1002/hep.30182 [DOI] [PubMed] [Google Scholar]
- 42. Gu W, Zeleke Y, Hortlik H, et al. Use and outcome of TIPS in hospitalized patients in Germany: a nationwide study (2007-2018). Hepatol Commun 2023;7:e0237. 10.1097/HC9.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tandon B, Ramachandran J, Narayana S, et al. Outcomes of transjugular intrahepatic portosystemic shunt procedures: a 10-year experience. J Med Imaging Radiat Oncol 2021;65:655–62. 10.1111/1754-9485.13168 [DOI] [PubMed] [Google Scholar]
- 44. Nicoară-Farcău O, Han G, Rudler M, et al. Pre-emptive TIPS in high-risk acute variceal bleeding. An updated and revised individual patient data meta-analysis. Hepatology 2024;79:624–35. 10.1097/HEP.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 45. Nicoară-Farcău O, Han G, Rudler M, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute Variceal bleeding: a meta-analysis of individual patient data. Gastroenterology 2021;160:193–205. 10.1053/j.gastro.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 46. Artru F, Moschouri E, Denys A. Direct Intrahepatic Portocaval shunt (DIPS) or Transjugular Transcaval Intrahepatic Portosystemic shunt (TTIPS) to treat complications of portal hypertension: indications, technique, and outcomes beyond Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol 2022;46:101858. 10.1016/j.clinre.2022.101858 [DOI] [PubMed] [Google Scholar]
- 47. Haskal ZJ, Duszak R, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol 1996;7:139–42. 10.1016/s1051-0443(96)70750-9 [DOI] [PubMed] [Google Scholar]
- 48. Hausegger KA, Tauss J, Karaic K, et al. Use of the left internal jugular vein approach for transjugular portosystemic shunt. AJR Am J Roentgenol 1998;171:1637–9. 10.2214/ajr.171.6.9843303 [DOI] [PubMed] [Google Scholar]
- 49. Sze DY, Magsamen KE, Frisoli JK. Successful transfemoral creation of an intrahepatic portosystemic shunt with use of the viatorr device. J Vasc Interv Radiol 2006;17:569–72. 10.1097/01.rvi.0000200054.73714.e1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2023-001308supp001.pdf (155.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data presented in this study are available on request from the corresponding author.