Abstract
Objectives
To determine the strength and nature of the association between delirium and incident dementia in a population of older adult patients without dementia at baseline.
Design
Retrospective cohort study using large scale hospital administrative data.
Setting
Public and private hospitals in New South Wales, Australia between July 2001 and March 2020.
Participants
Data were extracted for 650 590 hospital patients aged ≥65 years. Diagnoses of dementia and delirium were identified from ICD-10 (international classification of diseases, 10th revision) codes. Patients with dementia at baseline were excluded. Delirium-no delirium pairs were identified by matching personal and clinical characteristics, and were followed for more than five years.
Main outcome measures
Cox proportional hazards models and Fine-Gray hazard models were used to estimate the associations of delirium with death and incident dementia, respectively. Delirium-outcome dose-response associations were quantified, all analyses were performed in men and women separately, and sensitivity analyses were conducted.
Results
The study included 55 211 matched pairs (48% men, mean age 83.4 years, standard deviation 6.5 years). Collectively, 58% (n=63 929) of patients died and 17% (n=19 117) had a newly reported dementia diagnosis during 5.25 years of follow-up. Patients with delirium had 39% higher risk of death (hazard ratio 1.39, 95% confidence interval 1.37 to 1.41) and three times higher risk of incident dementia (subdistribution hazard ratio 3.00, 95% confidence interval 2.91 to 3.10) than patients without delirium. The association with dementia was stronger in men (P=0.004). Each additional episode of delirium was associated with a 20% increased risk of dementia (subdistribution hazard ratio 1.20, 95% confidence interval 1.18 to 1.23).
Conclusions
The study findings suggest delirium was a strong risk factor for death and incident dementia among older adult patients. The data support a causal interpretation of the association between delirium and dementia. The clinical implications of delirium as a potentially modifiable risk factor for dementia are substantial.
Introduction
Delirium is characterised by inattention and disturbance of awareness that represents a change from baseline cognitive function, and is precipitated by acute events such as illness and surgery. Delirium is a prevalent condition in hospital, with an estimated occurrence of 23% in patients with acute medical conditions1 and up to 45% in patients aged 90 years and older.2 Delirium is associated with adverse outcomes, including death in hospital or in the short to medium term post discharge, prolonged hospital stay, and new admission to a residential institution.3 In 2020, Goldberg and colleagues4 found that delirium was also associated with long term cognitive decline (ie, decrease in objective cognitive scores or new clinical diagnosis of dementia) in their meta-analysis of 24 studies including 10 459 patients. This association persisted in their subgroup analysis of 19 studies examining patients without cognitive impairment at baseline. An association between delirium and incident dementia in patients without dementia at baseline has been reported in a subsequent systematic review and meta-analysis.5 However, included studies were relatively modest in size (between 78 and 329 patients) and variably adjusted for important confounders. Furthermore, studies did not account for the competing risk of death, which is particularly high in this vulnerable population and might contribute to biased risk estimates of incident dementia in relation to delirium.6
Mechanisms linking delirium with incident dementia are under debate. Delirium might be an epiphenomenon, it might uncover unrecognised (preexisting or preclinical) dementia, or it might cause dementia by accelerating underlying neuropathological processes or de novo mechanisms.7 Observational studies are limited in their capacity to validate causality; however, the association between delirium and dementia is not amenable to randomisation. Dose-response analysis might contribute valuable information to the debate about causality. In 2021, the Delirium and Cognitive Impact in Dementia study showed that more than one episode of delirium was associated with a greater risk of incident dementia compared with a single episode in a sample of 173 older hospital patients.8
As the global burden of dementia increases,9 it is important to confirm the extent to which delirium is a potentially modifiable risk factor. We aimed to use large scale hospital administrative data to clarify the strength and nature of the association between delirium and incident dementia in a population of older adult patients without dementia at baseline.
Methods
We undertook a retrospective cohort study using a longitudinal statewide dataset linked by the New South Wales (NSW) Centre for Health Record Linkage.10
Data sources
The NSW Admitted Patient Data Collection records all inpatient episodes of care (defined by separations—discharges, transfers, and deaths) from all NSW public and private hospitals. Data include personal (eg, date of birth, gender, residential address, country of birth), administrative (eg, admission and separation dates), and clinical (eg, diagnoses and procedures) information. For each episode, one primary diagnosis and up to 50 secondary diagnoses are coded using the international classification of diseases, 10th revision (ICD-10).11 Admitted Patient Data Collection records linked to the NSW Registry of Births, Deaths and Marriages data were available from July 2001 onwards. Linked data up to and including 31 March 2020 were available to the research team (>12 million episodes).
Study design and sample
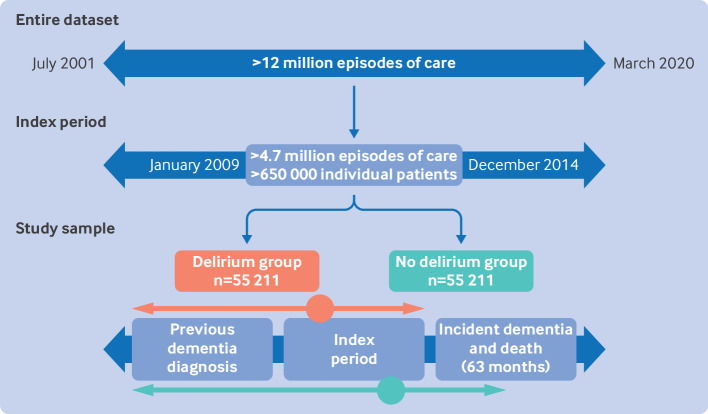
We defined a six year index period (1 January 2009 to 31 December 2014) so that sufficient data were available to determine that patients did not have a previous dementia diagnosis, to calculate a hospital frailty risk score (HFRS) for every patient, and to provide adequate follow-up for all patients (fig 1). A HFRS was calculated for each patient using ICD-10 codes recorded for episodes in the preceding two year period (see supplementary material for further information about HFRS).12 The follow-up period for all patients was 63 months (5.25 years), which was the time between the end of the index period and the end of the dataset. We identified 650 590 patients aged 65 years and older who had one or more episodes of care (total episodes 4 779 584) from NSW hospitals during the index period.
Fig 1.
Study design. Patients in the delirium group (index episode=orange circle) and no delirium group (index episode=green circle) were matched 1:1 according to age, gender, hospital frailty risk score, primary diagnosis, hospital length of stay and intensive care unit length of stay of index episode. Arrows to left of circles represent exclusion of previous dementia diagnosis and two year lookback for hospital frailty risk score calculation. Arrows to right of circles represent 63 month follow-up period
Dementia and delirium diagnoses were extracted from primary and secondary diagnoses data using ICD-10 codes (see supplementary material). In Australian hospitals, a patient presenting to hospital with cognitive impairment, or with an acute change in behaviour or cognitive status in hospital, is assessed for delirium by an appropriately trained clinician, ideally using a validated tool (commonly the 4AT, a diagnostic tool designed specifically for routine clinical use).13 Patients with a dementia diagnosis or episode of delirium recorded before the index period were excluded, as were patients aged >110 years and those with data inconsistencies (eg, implausible dates).
Patients were then categorised into delirium and no delirium groups. For patients in the delirium group, the first episode recording a delirium diagnosis was identified as the index episode. Patients with a dementia diagnosis recorded at or before the index episode were then excluded.
Patients in the delirium group were matched 1:1 to patients in the no delirium group according to patient and episode characteristics with potential to confound the association between delirium and subsequent risk of dementia. The confounders were patient age (in years; continuous variable), gender (man or woman), HFRS (≥0; continuous variable), and the primary diagnosis (ICD-10 code up to seven characters), episode length of stay (in days; discrete variable), and intensive care unit length of stay (in days; continuous variable) of the index episode. In the event that delirium was the primary diagnosis of an index episode, the primary diagnosis variable was not used for matching. Patients in the no delirium group with a dementia diagnosis recorded at or before the index episode were excluded and an alternative match was identified. Matching was without replacement; that is, each patient without delirium was matched to (at most) one patient with delirium.
Outcomes
The primary outcomes were incident dementia and death. The start date of the episode with a newly recorded dementia diagnosis was identified as the event time for incident dementia (see supplementary material for incident dementia ICD-10 codes). Mortality data, including date of death (the other event time), were available in the linked data.
Statistical analysis
Descriptive summary statistics were calculated at baseline separately for patients in the delirium and no delirium groups, and for patients who made up the total eligible sample for comparison. In all statistical models, linear associations were assumed between continuous covariates (age, HFRS, episode length of stay, and intensive care unit length of stay) and study outcomes (death and incident dementia).
When death was the outcome, patient follow-up was from the index episode until death. When dementia was the outcome, patient follow-up was from the index episode until the onset of dementia or death (whichever came first). In both instances, patients who remained event free were censored at 63 months.
We first assessed differences between the delirium group and the no delirium group in the incidence of an outcome (death or dementia). Next, we applied a landmarking approach to determine the presence of a dose-response association between the number of episodes of delirium and the outcome.14 The number of delirium episodes occurring within the first 12 months of follow-up was associated with event incidence rates subsequent to that 12 month period. Delirium episodes were first used as categorical variables in the full sample (categorised as 0 episodes, 1 episode, 2 episodes, and ≥3 episodes) and then as continuous variables in the delirium cohort only. Dose-response models included covariates of age and gender, and the number of hospital episodes recorded within the landmark period (categorised as 1-5, 6-10, 11-20, and >20 episodes), and only patients who remained event free within the landmark period were included.
A different statistical modelling technique was used for each outcome. For death, we used Cox proportional hazards models and expressed the strength of associations as hazard ratios. For dementia, we used Fine-Gray subdistribution hazard models that accounted for the competing risk of death and expressed associations as subdistribution hazard ratios.15 Given the strong association between delirium and risk of death, the competing risks analysis approach improves the accuracy of association estimates.
We conducted several sensitivity analyses to assess the robustness of associations. For both outcomes, we excluded patient pairs with distance values within the top 10% (least matched patients) and simultaneously included as covariates all characteristics used in the matching process (ie, age, gender, HFRS, primary diagnosis ICD-10 category, episode length of stay, and intensive care unit length of stay) to account for any residual differences in characteristics between groups. Additionally, when dementia was the outcome, we repeated analyses after excluding patients who died or had a diagnosis of dementia within 24 months of their index episode to reduce the impact of undetected dementia on the results. To assess the robustness of the dose-response associations, we repeated the analyses while extending the landmark period from 12 to 24 months. Finally, all analyses were conducted in the total matched sample and for men and women separately.
All estimates of associations were accompanied by 95% confidence intervals to represent the uncertainty. Statistical analyses were conducted using R version 4.2.3.
Supplementary analyses
We opted for a matched cohort design to reduce the confounding effects of key clinical variables and to permit sensitivity analyses that adjusted for residual confounding. Matching reduced the computational burden in this large study and allowed more reliable comparison without sacrificing statistical precision. The supplementary material presents an alternative approach using the total eligible sample.
Patient and public involvement
This study was inspired by EHG and REH’s clinical experience as geriatricians. There was no direct patient and public involvement in the study because the analysis of this restricted access administrative dataset was retrospective. However, a consumer representative with lived experienced of delirium who is actively involved in delirium prevention and education programmes in Australia reviewed the manuscript, confirmed the importance and potential impact of the study and its results, and contributed to the dissemination strategy.
Results
Sample characteristics
Of the 650 590 patients, 626 467 were eligible for inclusion in the analytical sample. The supplementary material presents the 80 most frequent ICD-10 codes recorded as primary diagnoses for these patients. The matched study sample included 110 422 patients across the two groups (fig 1). Table 1 presents personal and clinical characteristics for the total eligible sample (n=626 467) and the matched sample (delirium group n=55 211; no delirium group n=55 211). At baseline, matched patients ranged in age from 65 to 109 years and most were older (mean age 83.4 years, standard deviation 6.5 years). Women and men were almost equally represented (52% women, 48% men). Despite matching, the length of stay (for the index episode and in the intensive care unit) was slightly longer for the delirium group than the no delirium group. In the delirium group, 6351 patients had a primary diagnosis of delirium. The supplementary material includes additional results about matching.
Table 1.
Baseline characteristics of study sample
| Characteristics | Total eligible sample | No delirium group | Delirium group |
|---|---|---|---|
| No of patients | 626 467 | 55 211 | 55 211 |
| Age (years), mean (SD) | 78.0 (7.0) | 83.4 (6.5) | 83.5 (6.6) |
| Gender | |||
| Men | 286 430 (46) | 26 339 (48) | 26 339 (48) |
| Women | 340 037 (54) | 28 872 (52) | 28 872 (52) |
| Hospital frailty risk score, median (IQR) | 0.0 (0.0-0.0) | 1.3 (0.0-3.1) | 1.3 (0.0-3.2) |
| Length of stay—index episode (days), median (IQR) | 1 (1.0-5.0) | 6.0 (3.0-12.0) | 9.0 (5.0-17.0) |
| Length of stay—intensive care unit | |||
| None | 605 420 (96.6) | 51 045 (92.5) | 48 299 (87.5) |
| Less than one day | 4668 (0.7) | 814 (1.5) | 1122 (2.0) |
| One day or longer | 16 379 (2.6) | 3352 (6.1) | 5790 (10.5) |
| Primary diagnosis (categories) | |||
| Injury, poisoning, other consequences of external causes | 51 796 (8.3) | 9364 (17.0) | 8714 (15.8) |
| Diseases of the circulatory system | 72 572 (11.6) | 8965 (16.2) | 7989 (14.5) |
| Diseases of the respiratory system | 30 701 (4.9) | 5697 (10.3) | 5160 (9.3) |
| Symptoms, signs, and abnormal findings not classified elsewhere | 62 207 (9.9) | 3306 (6.0) | 5558 (10.1) |
| Neoplasms and blood diseases | 69 349 (11.1) | 4613 (8.4) | 4073 (7.4) |
| Diseases of the musculoskeletal system and connective tissue | 54 344 (8.7) | 4563 (8.3) | 4110 (7.4) |
| Diseases of the genitourinary system | 34 278 (5.5) | 4051 (7.3) | 3764 (6.8) |
| Factors influencing health status and contact with health services | 37 450 (6.0) | 4093 (7.4) | 2896 (5.2) |
| Diseases of the digestive system | 78 548 (12.5) | 3306 (6.0) | 2823 (5.1) |
| Other | 135 222 (21.6) | 7253 (13.1) | 10 124 (18.3) |
Data are numbers (%) unless specified otherwise. IQR, interquartile range; SD, standard deviation.
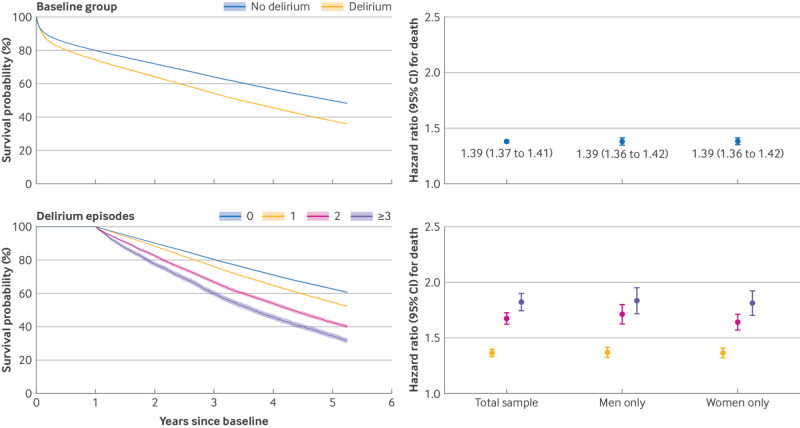
Delirium and risk of death
The rate of death was 1.4 times higher in the delirium group than in the no delirium group (table 2), which equates to a 39% increased risk of death (fig 2, upper panel). The risk was similar for men and women (interaction P=0.62). After excluding the least matched patients and adjusting for all covariates used in the matching process, the association strengthened marginally (hazard ratio 1.41, 95% confidence interval 1.39 to 1.44). When all eligible patients from the total sample were analysed and characteristics used in the matching were included in statistical models as covariates, findings were similar although associations strengthened (see supplementary material).
Table 2.
Delirium and occurrence of death and dementia
| Outcome, statistic, and sample | No delirium group | Delirium group | Delirium episodes in landmark period | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |||
| Death | ||||||
| No of patients at risk | ||||||
| Total | 55 211 | 55 211 | 43 826 | 30 181 | 7463 | 3628 |
| Men | 26 339 | 26 339 | 20 283 | 14 084 | 3215 | 1583 |
| Women | 28 872 | 28 872 | 23 543 | 16 097 | 4248 | 2045 |
| No of events | ||||||
| Total | 28 552 | 35 377 | 17 240 | 14 424 | 4466 | 2475 |
| Men | 14 140 | 17 403 | 8107 | 6823 | 1984 | 1116 |
| Women | 14 412 | 17 974 | 9133 | 7601 | 2482 | 1359 |
| Person years of follow-up | ||||||
| Total | 196 067.3 | 171 404.3 | 148 169.6 | 96 471.6 | 21 165.7 | 9338.7 |
| Men | 90 300.8 | 77 862 | 67 944.0 | 44 616.3 | 8815.0 | 3900.8 |
| Women | 105 766.5 | 93 542.3 | 80 225.6 | 51 855.3 | 12 350.6 | 5438.0 |
| Incidence rate per 100 person years | ||||||
| Total | 14.6 | 20.6 | 11.6 | 15.0 | 21.1 | 26.5 |
| Men | 15.7 | 22.4 | 11.9 | 15.3 | 22.5 | 28.6 |
| Women | 13.6 | 19.2 | 11.4 | 14.7 | 20.1 | 25.0 |
| Dementia | ||||||
| No of patients at risk | ||||||
| Total | 55 211 | 55 211 | 43 037 | 28 694 | 6646 | 2997 |
| Men | 26 339 | 26 339 | 19 970 | 13 484 | 2879 | 1319 |
| Women | 28 872 | 28 872 | 23 067 | 15 210 | 3767 | 1678 |
| No of events | ||||||
| Total | 5151 | 13 966 | 3871 | 6567 | 1979 | 1083 |
| Men | 2161 | 6219 | 1607 | 2979 | 846 | 487 |
| Women | 2990 | 7747 | 2264 | 3588 | 1133 | 596 |
| Person years of follow-up | ||||||
| Total | 189 598.3 | 151 842.2 | 142 331.2 | 85 378.6 | 17 177.4 | 6804.1 |
| Men | 87 873 | 70 195.7 | 65 782.6 | 40 250.6 | 7294.8 | 2918.5 |
| Women | 101 725.3 | 81 646.5 | 76 548.6 | 45 128.1 | 9882.6 | 3885.6 |
| Incidence rate per 100 person years | ||||||
| Total | 2.7 | 9.2 | 2.7 | 7.7 | 11.5 | 15.9 |
| Men | 2.5 | 8.9 | 2.4 | 7.4 | 11.6 | 16.7 |
| Women | 2.9 | 9.5 | 3.0 | 8.0 | 11.5 | 15.3 |
For delirium episodes in first 12 months of follow-up (landmark period), person years of follow-up was calculated as observation time subsequent to landmark period.
Fig 2.
Association of delirium with death by baseline group (upper panel) and episodes of delirium recorded within first 12 months of follow-up (landmark period; lower panel). Associations presented in forest plot in lower panel were adjusted for age and gender at baseline, and number of hospital episodes recorded within landmark period. Total sample data are hazard ratio 1.36 (95% confidence interval 1.33 to 1.39) for one delirium episode, 1.67 (1.61 to 1.72) for two episodes, and 1.82 (1.74 to 1.90) for three or more episodes. Corresponding data for men only are 1.36 (1.32 to 1.40), 1.71 (1.63 to 1.79), and 1.83 (1.72 to 1.95), respectively. Corresponding data for women only are 1.36 (1.32 to 1.40), 1.63 (1.56 to 1.70), and 1.81 (1.70 to 1.91), respectively
When episodes of delirium were counted within the 12 month landmark period and categorised (0 episodes, 1 episode, 2 episodes, ≥3 episodes), more episodes were monotonically associated with a higher risk of death (fig 2, lower panel). These associations strengthened marginally when episodes of delirium were counted within a 24 month landmark period (see supplementary material). Among patients who experienced at least one episode of delirium within the landmark period, each additional episode of delirium was associated with a 10% increased risk of death (hazard ratio 1.10, 95% confidence interval 1.09 to 1.12).
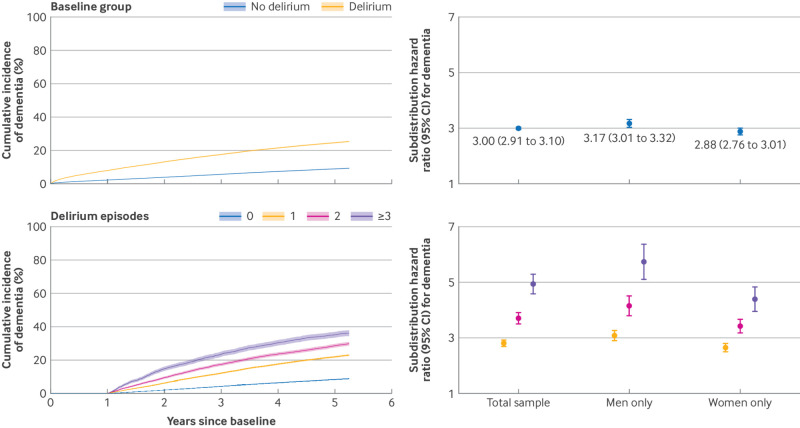
Delirium and risk of dementia
The rate of incident dementia in the delirium group was 3.4 times higher than the no delirium group (table 2). After accounting for the competing risk of death, the risk of incident dementia remained three times higher among the delirium group (fig 3, upper panel). This association was stronger for men than women (subdistribution hazard ratio 3.17 and 2.88, respectively, P=0.004). The association also strengthened marginally after excluding the least matched patients and adjusting for all covariates used in the matching process (3.09, 2.98 to 3.19) and was similar after excluding patients who died or developed dementia within 24 months of the index episode (2.98, 2.86 to 3.11). When all eligible patients from the total sample were analysed and characteristics used in the matching were included as covariates, results were comparable although most associations were weaker (see supplementary material).
Fig 3.
Association of delirium with incident dementia by baseline group (upper panel) and episodes of delirium recorded within first 12 months of follow-up (landmark period; lower panel). Associations presented in forest plot in lower panel were adjusted for age and gender at baseline, and number of hospital episodes recorded within landmark period. Total sample data are subdistribution hazard ratio 2.81 (95% confidence interval 2.70 to 2.92) for one delirium episode, 3.70 (3.50 to 3.91) for two episodes, and 4.91 (4.57 to 5.28) for three or more episodes. Corresponding data for men only are 3.06 (2.88 to 3.25), 4.15 (3.81 to 4.52), and 5.72 (5.12 to 6.38), respectively. Corresponding data for women only are 2.64 (2.51 to 2.78), 3.42 (3.18 to 3.67), and 4.39 (3.99 to 4.83), respectively
In the 12 month landmark analysis, more delirium episodes were monotonically associated with a higher risk of incident dementia (fig 3, lower panel). These associations weakened marginally when episodes of delirium were counted within a 24 month landmark period (see supplementary material). Among patients who experienced at least one episode of delirium within the landmark period, each additional episode of delirium was associated with a 20% increased risk of dementia (subdistribution hazard ratio 1.20, 95% confidence interval 1.18 to 1.23).
Discussion
Principal findings
We found delirium to be a strong risk factor for death and incident dementia in this cohort of older Australian hospital patients. We observed that among patients without dementia at baseline with at least one episode of delirium, the risk of a new dementia diagnosis was about three times higher than for patients without delirium over five years of follow-up. Among patients with at least one episode of delirium, each additional episode of delirium increased that risk by 20%. These associations were observed in a large scale dataset and were robust to several tests of bias and confounding, supporting the hypothesis that delirium has a strong independent effect on dementia risk in this clinical population.
Comparison with other studies
In our study, the rate of death was higher than the rate of incident dementia. Death was an important competing risk—it was an outcome of equal or higher clinical importance than the primary outcome that changed the probability of the primary outcome.6 Leighton and colleagues16 recently estimated the cumulative incidence of new dementia (accounting for competing risk of death without a dementia diagnosis) to be 31% by five years in their sample of 12 949 patients with delirium aged 65 years and older. This proportion is higher than our result (25%), possibly owing to their inclusion of dementia diagnosis at death (18% of patients) in their cumulative incidence calculations.
Recently, two studies conducted competing risk analyses in smaller cohorts of older patients and reported different risk estimates for incident dementia in relation to delirium in patients without dementia at baseline (subdistribution hazard ratio 1.94 and 8.70, respectively).17 18 The studies had many methodological differences, most notably in study design (retrospective v prospective), size (n=390 v 1100), duration of follow-up (median 24 months v mean 82 months), and covariates. While Garcez and colleagues17 accounted primarily for the confounding effects of frailty in their older inpatient population, Rolandi and colleagues18 examined the independent effects of non-modifiable and potentially modifiable risk factors in their population based study. Neither study adjusted for clinical variables relating to illness severity or examined the impact of more than one episode of delirium.
Richardson and colleagues8 recently estimated that older patients with delirium had almost nine times the risk of incident dementia (odds ratio 8.8) compared with patients without delirium and that the risk increased with subsequent episodes of delirium (odds ratio 8.6 and 13.0 for one episode and more than one episode, respectively). These findings are consistent with our study, even though the estimates are higher. This difference might be attributable to the smaller study size (n=135), shorter duration of follow-up (12 months), and an unaccounted for competing risk of death (n=38, 18%).8 Our study and that of Richardson and colleagues8 share some strengths, including adjusting for baseline characteristics such as age, gender, frailty, and measures of illness severity (APACHE II (acute physiology and chronic health evaluation II) v primary diagnosis, episode length of stay, and intensive care unit length of stay). The studies differed in their approach to diagnosis of delirium and dementia. However, the meta-regression of 24 studies by Goldberg and colleagues4 suggested that the approach to diagnosis might not have much impact on variance in results.
Mechanistic understanding and implications for future research
We found that there was a persistent association between delirium and incident dementia years after the episode of delirium (and resolution of the precipitating stressors), which suggests that delirium is not an epiphenomenon or merely a marker of unrecognised dementia or a vulnerable brain. Furthermore, the dose-response association between delirium and incident dementia suggests a causal link between the two conditions. Several hypotheses have been proposed explaining how delirium might cause dementia.7 For example, the sequelae of delirium (drowsiness, agitation, circadian disturbance, and unsafe behaviours) might precipitate a cascade of geriatric syndromes (mobility impairment and falls, pressure ulcers, malnutrition and dehydration), medical complications (electrolyte disturbance, aspiration and respiratory failure, infection and venous thromboembolism) and chemical and physical restraint, all of which might exert a toxic effect on the brain. Alternatively, or additionally, delirium might contribute to neuronal injury and neurodegeneration through a range of disrupted biological mechanisms (see Fong and Inouye7 for a comprehensive review). Associations between systemic inflammatory markers, delirium, and dementia are variable in preclinical and clinical models and appear to be influenced by the presence or absence of dementia pathology. Similarly, markers of neuroinflammation have been associated with both syndromes. Alzheimer’s disease biomarkers (eg, Aβ, tau) have been associated with risk of incident delirium and the association between the APOE genotype and delirium suggests a mediating role of genetic profiles related to systemic inflammation. Neuroimaging studies have identified structural and functional predictors of delirium, such as changes in network connectivity in the posterior cingulate cortex. A direct pathway between delirium and neuronal injury (not mediated by systemic inflammation, for example) has not been established but is theoretically possible. Ultimately, a better understanding of the delirium-dementia pathophysiological pathways might guide the development of new treatments with potential to prevent or reduce neurodegeneration.
In our study, we observed delirium to impart a larger increase in dementia risk in men than women. Despite this difference, in the delirium and no delirium groups, women experienced dementia at a slightly higher rate than men. The literature on sex differences in dementia is rapidly evolving; there is emerging evidence for differences in dementia risk19 and mediating factors20 for men and women. However, one meta-analysis (201 studies, n=998 187) did not find major differences in dementia incidence in men and women except in the oldest old (>90 years).21 For delirium, it remains unclear whether gender is a predisposing risk factor, with both genders being associated with increased risk in various inpatient populations.22
We might hypothesise that the increased risk of incident dementia with delirium in men indicates lower reserve (ie, higher burden of neuropathology). Although this might be unlikely given the higher global prevalence of dementia in older women than men,21 it is increasingly understood that the association between neuropathological burden and clinical dementia is not linear23 and that there are likely to be important sex differences in patterns of neuropathology in people with and without dementia.24 Another hypothesis is that delirium in men might be more severe. However, a recent prospective study of older adults with delirium did not identify any gender differences in clinical phenotypes, course, or response to treatment.25 There might also be fundamental sex differences in the biological mechanisms of delirium that lead to de novo neuronal injury and accelerated neurodegeneration. Future studies might explore these hypotheses to try and identify sex specific targets for intervention.
Pooled data from 14 studies including 2640 patients aged 18 years and older showed that multicomponent non-pharmacological interventions were associated with a reduced incidence of delirium (risk ratio 0.57), a reduced duration of a delirium episode, and reduced hospital length of stay compared with usual care.26 In older adults specifically, a systematic review and meta-analysis of data from studies of a widely disseminated delirium prevention programme (the Hospital Elder Life Program) showed that the intervention was associated with a reduced incidence of delirium (odds ratio 0.47) and falls (odds ratio 0.58), a reduced hospital length of stay and preserved functional status, and reduced healthcare costs.27 Currently, data are lacking about the impact of these interventions on the risk of incident dementia.26 Because the burden of dementia is set to dramatically rise in coming decades and multicomponent non-pharmacological delirium prevention interventions are effective and readily implemented, quantifying the benefit of interventions on dementia incidence rates should be addressed in future clinical trials as a matter of priority.28
Strengths and limitations of this study
In this large study of delirium and incident dementia, we minimised bias by adjusting for important personal and clinical baseline variables, having a long period of follow-up, and accounting for the competing risk of death in our analyses. This approach helped to overcome methodological issues prevalent in the existing literature. Therefore, it is likely that our estimate lies closer to the true effect of delirium on incident dementia in patients without dementia at baseline. The size and granularity of the data afforded precision when conducting adjusted dose-response analyses and the results of predetermined sensitivity analyses showed the robustness of the reported results. By stratifying results by gender, we generated insights with important pathophysiological and clinical implications.
The results should be considered within the context of this study’s limitations. Diagnosis of delirium and dementia depended on clinical coding of medical information from inpatient episodes of care recorded in the administrative dataset used. Differential diagnosis of delirium, dementia, and delirium superimposed on dementia is difficult and conditions might go undetected or be misattributed.7 Under-coding of dementia during hospital admission is a well recognised issue and correlates with lack of documentation of dementia diagnosis in medical notes.29 Similarly, published data suggest that coding for delirium underestimates true delirium rates.30 While the use of routinely collected healthcare data in determining the presence of all cause dementia is supported by positive predictive values between 70% and 90%,31 it is possible that erroneous diagnoses were made (false positives) and other diagnoses were missed (false negatives), which would affect the incident rates reported here. Future studies might combine different administrative data sources (eg, pharmaceutical, primary care, aged care) to improve case detection and reduce the potential for bias.
We matched delirium and no delirium groups 1:1 using important personal and clinical characteristics. However, we were limited to the data available in the administrative dataset and there could be residual confounding effects from unmeasured variables. Differences were found between delirium and no delirium groups for some characteristics (table 1); however, sensitivity analyses that simultaneously included all characteristics used in the matching process as covariates resulted in marginal increases in the risk estimates, suggesting limited residual differences in characteristics between groups.
For our dose-response analysis, data about the duration and severity of delirium episodes were not available, which limited the analysis to the number of episodes of care with coded delirium. It is also possible that the association found between delirium and incident dementia was induced by a confounding variable. For example, incremental increases in frailty in a patient with several hospital admissions (episodes) might underpin the increased risk of incident dementia. Frailty has been shown to affect the association between neuropathological burden and dementia diagnosis in community dwelling adults,23 and gender might have a further impact on this association.20 However, we tried to account for time varying differences in general health status by including the number of episodes (admissions) during the landmark period as a covariate.
While our results are consistent with the hypothesis that delirium might play a causative part in dementia, they are not conclusive owing to the fundamental limitations of observational studies in determining causality. Nevertheless, the results of this study provide valuable insights because prospective randomised controlled trials are unlikely to be conducted.
Conclusions
Using large scale hospital administration data, this study found a strong association between delirium and incident dementia in older adults without dementia at baseline. A dose-response association between delirium and dementia supports a causal pathway between the two conditions, encouraging the search for accelerated and de novo pathways to neuronal injury and the development of new treatment strategies. Differences in the association between delirium and incident dementia in men and women reinforce the need to not only adjust for gender in future studies but also to look for gender specific associations that might have important mechanistic and clinical implications. Delirium is a factor that could triple a person’s risk of dementia. Therefore, delirium prevention and treatment are opportunities to reduce dementia burden globally.
What is already known on this topic
An association might exist between delirium and subsequent dementia; however, the strength and nature of this association are unclear because of limitations in existing observational studies
As the global burden of dementia increases, it is important to confirm the extent to which delirium is a potentially modifiable risk factor
What this study adds
Among patients without dementia at baseline with at least one episode of delirium, the risk of a new dementia diagnosis was about three times higher than for patients without delirium; each additional episode of delirium increased the risk by 20%
The association between delirium and incident dementia seems to be stronger in men than in women
Delirium prevention and treatment could reduce the burden of dementia globally
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: EHG contributed to the study design and interpretation of the results, and drafted the manuscript. HX contributed to the study design, data extraction and analysis, and reviewed the manuscript. DDW contributed to the study design, data analysis and interpretation of the results, drafted the statistical analysis and results sections of the manuscript, and reviewed the manuscript. SB conceptualised the study, contributed to the study design, data analysis and interpretation of the results, and reviewed the manuscript. REH conceptualised the study, contributed to the study design and interpretation of the results, and reviewed the manuscript. HX and SB had access to the entire dataset and study sample dataset. DDW had access to extracted study sample data for the purposes of data analysis. All authors had access to the final study results and accept responsibility to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. EHG is the guarantor.
Funding: HX’s work was supported by the National Health and Medical Research Council: Partnership Centre for Health System Sustainability. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from National Health and Medical Research Council: Partnership Centre for Health System Sustainability for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Study outcomes will be published and made publicly available. A lay summary has also been formulated. Clinician investigators will disseminate results through clinical conferences and clinical networks within hospital and primary health services. The Australian Frailty Network (AFN) will disseminate results to national and international research collaborators, partner organisations (eg, Australasian Association of Gerontology), and consumer networks, including the AFN and EWE (Eat Walk Engage)consumer networks. The key findings will be disseminated by the Partnership Centre for Health Systems Sustainability coordinated by the Australian Institute for Health Innovation at Macquarie University. Investigators will also disseminate results through social media and press releases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the New South Wales Population and Health Service Research Ethics Committee (2019/ETH12806/2019.55). The Committee granted a waiver of the usual requirement of consent for the use of reidentifiable information held by NSW agencies, in line with the State Privacy Commissioner’s Guidelines for Research and the Health Records and Information Privacy Act 2002 (NSW).
Data availability statement
The data used in this study are available to the public through application to the Centre for Health Record Linkage (see www.cherel.org.au for more information). The analysis script (R markdown file) is available for download from https://espace.library.uq.edu.au/view/UQ:dfc4e74
References
- 1. Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing 2020;49:352-60. 10.1093/ageing/afaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gehrke S, Bode L, Seiler A, Ernst J, von Känel R, Boettger S. The prevalence rates and sequelae of delirium at age older than 90 years. Palliat Support Care 2021;19:552-7. 10.1017/S1478951520001297 [DOI] [PubMed] [Google Scholar]
- 3. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443-51. 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 4. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol 2020;77:1373-81. 10.1001/jamaneurol.2020.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira JV, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in older adults is associated with development of new dementia: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2021;36:993-1003. 10.1002/gps.5508 [DOI] [PubMed] [Google Scholar]
- 6. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783-7. 10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fong TG, Inouye SK. The inter-relationship between delirium and dementia: the importance of delirium prevention. Nat Rev Neurol 2022;18:579-96. 10.1038/s41582-022-00698-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richardson SJ, Davis DHJ, Stephan BCM, et al. Recurrent delirium over 12 months predicts dementia: results of the Delirium and Cognitive Impact in Dementia (DECIDE) study. Age Ageing 2021;50:914-20. 10.1093/ageing/afaa244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413-46. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centre for Health Record Linkage. CHeReL Master Linkage Key: NSW Health; 2023. www.cherel.org.au/datasets.
- 11. World Health Organization . ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2nd ed. World Health Organization, 2004. [Google Scholar]
- 12. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775-82. 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Australian Commission on Safety and Quality in Health Care . Delirium Clinical Care Standard. ACSQHC, 2021. [Google Scholar]
- 14. Austin PC, Latouche A, Fine JP. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med 2020;39:103-13. 10.1002/sim.8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 16. Leighton SP, Herron JW, Jackson E, Sheridan M, Deligianni F, Cavanagh J. Delirium and the risk of developing dementia: a cohort study of 12 949 patients. J Neurol Neurosurg Psychiatry 2022;93:822-7. 10.1136/jnnp-2022-328903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcez FB, Apolinario D, Campora F, Curiati JAE, Jacob-Filho W, Avelino-Silva TJ. Delirium and post-discharge dementia: results from a cohort of older adults without baseline cognitive impairment. Age Ageing 2019;48:845-51. 10.1093/ageing/afz107 [DOI] [PubMed] [Google Scholar]
- 18. Rolandi E, Zaccaria D, Vaccaro R, et al. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: a population-based study. Alzheimers Res Ther 2020;12:94. 10.1186/s13195-020-00661-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anstey KJ, Peters R, Mortby ME, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20-76 years. Sci Rep 2021;11:7710. 10.1038/s41598-021-86397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ward DD, Ranson JM, Wallace LMK, Llewellyn DJ, Rockwood K. Frailty, lifestyle, genetics and dementia risk. J Neurol Neurosurg Psychiatry 2022;93:343-50. 10.1136/jnnp-2021-327396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huque H, Eramudugolla R, Chidiac B, et al. Could country-level factors explain sex differences in dementia incidence and prevalence? A systematic review and meta-analysis. J Alzheimers Dis 2023;91:1231-41. 10.3233/JAD-220724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ormseth CH, LaHue SC, Oldham MA, Josephson SA, Whitaker E, Douglas VC. Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open 2023;6:e2249950. 10.1001/jamanetworkopen.2022.49950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol 2019;18:177-84. 10.1016/S1474-4422(18)30371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes LL, Lamar M, Schneider JA. Sex differences in mixed neuropathologies in community-dwelling older adults. Brain Res 2019;1719:11-6. 10.1016/j.brainres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hildenbrand FF, Boettger S, Spiller T, et al. Sex-specific clinical characteristics and treatment responses in delirium management: findings from a prospective cohort study in elderly patients. Int Clin Psychopharmacol 2023;38:384-93; online ahead of print. 10.1097/YIC.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 26.Burton J, Craig L, Yong S, Siddiqi N, Teale E, Woodhouse R, et al. Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2021(11). [DOI] [PMC free article] [PubMed]
- 27. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry 2018;26:1015-33. 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khachaturian AS, Hayden KM, Devlin JW, et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement 2020;16:711-25. 10.1002/alz.12075 [DOI] [PubMed] [Google Scholar]
- 29. Cappetta K, Lago L, Potter J, Phillipson L. Under-coding of dementia and other conditions indicates scope for improved patient management: a longitudinal retrospective study of dementia patients in Australia. Health Inf Manag 2022;51:32-44. 10.1177/1833358319897928 [DOI] [PubMed] [Google Scholar]
- 30. Pendlebury ST, Lovett NG, Thomson RJ, Smith SC. Impact of a system-wide multicomponent intervention on administrative diagnostic coding for delirium and other cognitive frailty syndromes: observational prospective study. Clin Med (Lond) 2020;20:454-64. 10.7861/clinmed.2019-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkinson T, Ly A, Schnier C, et al. UK Biobank Neurodegenerative Outcomes Group and Dementias Platform UK . Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement 2018;14:1038-51. 10.1016/j.jalz.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
The data used in this study are available to the public through application to the Centre for Health Record Linkage (see www.cherel.org.au for more information). The analysis script (R markdown file) is available for download from https://espace.library.uq.edu.au/view/UQ:dfc4e74