Abstract
Persistent Borna disease virus infection of the brain can be prevented by treatment of naive rats with a virus-specific CD4+ T-cell line prior to infection. In rats receiving this treatment, only a transient low-level encephalitis was seen compared to an increasingly inflammatory reaction in untreated infected control rats. Virus replication was found in the brain for several days after infection before the virus was cleared from the central nervous system. The loss of infectivity from the brain was confirmed by negative results by reverse transcription-PCR with primers for mRNA, by in situ hybridization for both genomic and mRNA, and by immunohistology. Most importantly, in vitro assays revealed that the T-cell line used for transfusion had no cytotoxic capacity. The kinetics of virus clearance were paralleled by the appearance of CD8+ T cells and the expression of perforin in the brain. Testing of lymphocytes isolated from the brains of CD4+ T-cell-treated rats after challenge revealed high cytotoxic activity due to the presence of CD8+ cytotoxic T cells at time points when brain lymphocytes from infected control rats induced low-level cytolysis of target cells. Neutralizing antiviral antibodies and gamma interferon were shown not to be involved in the elimination of virus from the brain.
Borna disease (BD) is a naturally occurring or experimentally induced meningoencephalitis caused by infection with BD virus (BDV), a single-stranded RNA virus with a remarkably wide host spectrum (25). Recent data suggest that BDV can infect humans and might be related to psychiatric disease (6, 9, 10, 26, 27). In experimentally infected rats (14, 18, 32) as well as diseased horses (3), BD is based on an immunopathological reaction in the brain. Both CD4+ and CD8+ T cells have been found in the brains of infected rats and ungulates and participate in the inflammatory response (3, 11, 24, 29). However, their effects on the development and consequences of the encephalitic reaction appear to be quite distinct. There is an increasing body of evidence that CD4+ T cells act as T helper cells, whereas CD8+ T cells exert effector functions by destroying virus-infected cells leading to a severe degenerative disease of the brain (reviewed in references 5 and 29). The presence of CD8+ T cells in vivo and the detection of major histocompatibility complex (MHC) class I-restricted cytotoxicity in brain lymphocyte preparations in vitro could be correlated with the presence of MHC class I antigen in the brain, the onset of disease and, finally, the appearance of cellular degeneration of brain cells, including virus-infected neurons (4, 8, 20, 21, 28). The important role of cytotoxic lymphocytes in cytodestructive mechanisms resulting in massive degeneration of brain cells was demonstrated by adoptive transfers of lymphocytes isolated from the brains of diseased rats. Transfer of brain lymphocytes caused an early onset of disease in infected recipients, as represented by severe neurological symptoms and a marked spongiform degeneration with premature cortical brain atrophy (28). In the same study, besides an exceedingly high cytotoxic activity exerted by CD8+ T cells, we demonstrated the entry of cells from perivascular areas into the brain parenchyma after adoptive transfer. As for the role of CD4+ T cells in BD, so far we have found no evidence that this T-cell population directly participates in brain tissue destruction; e.g., we found no evidence for MHC class II-restricted cytotoxic activity in isolated brain lymphocytes or virus-specific CD4+ T-cell lines (20, 21, 28). Furthermore, the distribution pattern of CD4+ and CD8+ T cells supports an effector role for CD8+ T cells; the latter are found predominantly in the brain parenchyma, whereas the vast majority of CD4+ T cells accumulate perivascularly (4, 20). Despite the vigorous local cellular immune response in the brain, the virus is not eliminated from the host. An explanation for this finding might be that the immune response is induced and/or recruited too slowly to the brain. Passive immunization with a BDV-specific CD4+ T-cell line was shown by Richt et al. to inhibit virus replication, and rats were protected from immune-mediated disease (23). This particular virus-specific CD4+ T cell exhibited MHC class II-restricted lysis in vitro, but the CD4 T-cell-mediated effects on virus elimination were not analyzed further. Here, we report on experiments with a noncytolytic BDV-specific CD4+ T-cell line that is able to confer protection against BDV infection and disease by enhancing the activity of virus-specific CD8+ T cells in the brain.
MATERIALS AND METHODS
Virus and experimental animals.
Giessen strain He/80 of BDV was used for this study. Female Lewis rats were infected at the age of 5 weeks by injection into the left hemisphere with 5 × 103 50% tissue culture infective doses (TCID50) of BDV.
Clinical evaluation.
All experimental animals were examined daily and weighed, and disease symptoms were scored by two independent observers on an arbitrary scale from 0 to 3, based on the rats’ general state of health (0.25 to 0.5, ruffled fur and hunchback) and the appearance of neurologic symptoms (1, slight incoordination and fearfulness; 2, distinct ataxia or slight paresis; 3, paresis or paralysis). The percentage of change from weight at the day of infection (100%) was calculated.
Infectivity assay and antigen detection.
Assays were done essentially as described before (30). Briefly, virus infectivity from brain homogenates was determined on rabbit embryo brain indicator cells by immunocytochemical staining with rat hyperimmune sera or BDV-specific monoclonal antibody (MAb) (33) and anti rat or anti-mouse peroxidase conjugates. The reaction was visualized by the addition of amino-9-ethylcarbazol. The detection limit of this assay is 10 TCID50 (1 log10).
Antibody titration and neutralization assay.
All sera were tested in twofold dilution in a solid-phase enzyme-linked immunosorbent assay (ELISA) with a purified antigen from BDV-infected rat brains containing the most abundant BDV-specific proteins, namely, p40 (nucleoprotein) and p24 (phosphoprotein) (32, 33).
Virus neutralization was performed essentially as described previously (12). Briefly, 50 TCID50 of BDV were incubated with serial twofold dilutions of heat-inactivated serum (at 56°C for 30 min) for 1 h. The reaction mixture was added to rabbit embryonal brain cells and incubated for 6 days. The dilution of serum required to reduce the TCID50 by 50% was defined as the neutralization titer (NT50). In all assays, titers of a serum pool from rats infected for 15 weeks or longer (12) were at an NT50 of ≥1:1,024.
Propagation of the T-cell lines.
The induction and propagation of T-cell lines were done essentially as reported before (21). Briefly, 8- to 10-week-old Lewis rats were immunized in both hind footpads with virus-specific antigen containing p24 and p40 which were purified from BDV-infected rat brain by affinity chromatography. Ten to 12 days later, the animals were anesthetized and the popliteal lymph nodes were collected. Lymphocytes were separated by Lympholyte R (Cedarlane, Hornsby, Canada) gradient centrifugation. In a secondary in vitro restimulation, 106 cells together with the same number of irradiated syngeneic thymocytes were cultured in the presence of 30 μg of the virus-specific antigen per ml for 4 days. Thereafter, 5 × 105 lymphocytes were cultured repeatedly in Iscove’s modified Dulbecco medium (IMDM) supplemented with 15% interleukin-2 (IL-2)-containing medium and 5% rat serum in 6-day cycles together with 5 × 106 irradiated syngeneic thymocytes in the presence of the virus-specific antigen. After the fifth restimulation in vitro, when sufficient numbers of cells and stable cultures had been established, the cells were restimulated in the presence of either purified p24 plus p40 virus-specific proteins or recombinant p24 or recombinant p40 alone. In all cases, the T-cell cultures were restimulated alternately with and without IL-2.
Transfusion of the BDV-specific T-cell line.
Various numbers of BDV-specific CD4+ T cells (5 × 105 to 3 × 106) were injected intravenously into the tail veins of rats at different time points prior to BDV infection.
Proliferation assay.
To determine the antigen specificity of the T-cell cultures, proliferation assays were performed. Therefore, 5 × 104 T cells were cultured in the presence of 5 × 105 irradiated syngeneic thymocytes with 30 μg of recombinant p24 or p40 (provided by W. I. Lipkin, Irvine, Calif.) per ml, BDV-specific protein, and influenza virus nucleoprotein (provided by H. Becht, Giessen, Germany) or without antigen in flat-bottomed 96-well microtiter plates for 60 h. Thereafter, 0.2 μCi of [3H]thymidine per well was added, and after 12 more h the cells were collected and the incorporation of [3H]thymidine was measured.
Isolation of effector cells.
Lymphocytes from the brains of BDV-infected control rats or CD4+ T-cell-treated rats were isolated by a method previously described by Irani and Griffin (15) and modified for the BDV infection of rats (20). The animals were anesthetized with ketaminehydrochloride and perfused with balanced salt solution (BSS). The brain tissue was carefully homogenized through stainless steel mesh and collected in BSS containing collagenase D (0.05%), trypsin inhibitor (TLCK; 0.1 μg/ml), DNase I (10 μg/ml), and HEPES (10 mM). The cell suspension was stirred at room temperature for 1 h and allowed to settle for 30 min. The supernatant was pelleted at 200 × g for 5 min. The pellet was resuspended in 10 ml of Ca-Mg-free phosphate-buffered saline. Five milliliters of the suspension was layered on top of 10 ml of a modified RPMI medium-Ficoll gradient and centrifuged at 500 × g for 30 min. The pellet containing the lymphocytes was resuspended in IMDM with 2% fetal calf serum, and the cells were counted for further use in cytotoxicity assays.
In vitro cell-mediated cytotoxicity.
Aliquots of 107 virus-infected (BDV-F10) and noninfected (F10) histocompatible astrocytes (the astrocytic cell line cloned from a primary Lewis astrocyte culture was kindly provided by H. Wekerle, Munich, Germany) or virus-infected (BDV-Lou) and noninfected (Lou) histocompatible skin fibroblasts from Louvain rats were labeled with 0.2 mCi of 51Cr at 37°C for 1 h and washed three times with medium. Target cells were coincubated with effector cells from BDV-infected rats at various effector-to-target ratios in a final volume of 200 μl/well. In some experiments, target cells were pretreated with rat gamma interferon (IFN-γ) for 72 h to induce expression of MHC class II antigen (20). Some tests were performed in the presence of MAb directed against MHC class I (OX-18) or MHC class II (OX-6) (both from Serotec, Cambridge, United Kingdom) determinants. After 9 h, 50 μl of sample was collected and counted in a gamma counter. The percentage of 51Cr release was calculated according to the following formula: 100 × [(test release − spontaneous release)/(maximal release − spontaneous release)], where test release is in the presence of effector cells, spontaneous release is in the presence of medium alone, and maximal release is in the presence of 1 N HCl.
RT-PCR analysis.
For reverse transcription-PCR (RT-PCR), total cellular RNA was isolated from brain homogenates of BDV-infected rats. Magnetic beads (Dynabeads; Dianova, Hamburg, Germany) were used to separate mRNA from the total cellular RNA according to a procedure described by the manufacturer. RNA was reverse transcribed with oligo(dT) primer and murine leukemia virus reverse transcriptase before resuspension to a final volume of 20 μl. Reverse-transcribed mRNA was amplified in a 100-μl reaction mixture volume containing 70 ng of each oligonucleotide primer per μl, 10 mM (each) dATP, dTTP, dGTP, and dCTP (Pharmacia, Freiburg, Germany), 500 mM KCl, 250 mM Tris-HCl (pH 8.3), 100 mM MgCl2, and 5 U of ampli-Taq DNA polymerase (Amersham) per μl. The reaction was performed in a Biometra thermocycler for 35 total cycles at 95°C for 1 min, 65°C for 2 min, and 72°C for 3 min, after which 10 μl of the reaction mixture was loaded onto a 1% agarose minigel and visualized by ethidium bromide staining.
The following primers, used for the detection of mRNAs of BDV, CD8, perforin, and cytokines, have been described before (21, 28): BDV p40 antisense, 5′-GGGTAGCATCCATACATTCTGCGAGG-3′; BDV p40 sense, 5′-CAGTAACGCCCAGCCTTGTGTTTC-3′; CD8 antisense, 5′-CATGAAGTGAATCCGGGCTCTCCTCCGC-3′; CD8 sense, 5′, CTCCTTCAGACTCCTTCATCCCTGCTGGTT-3′; perforin antisense, 5′-CCGGGGATTGTTATTGTTCC-3′; perforin sense, 5′-AGCCCCTGCACACATTACTG-3′; β-actin antisense, 5′-AGCATTTGCGGTGCACGATGGAGGG-3′; and β-actin sense, 5′-ATGCCATCCTGCGTCTGGACCTGGC-3′. The sensitivity of our assay had a titer of 102 to 103 molecules of in vitro-synthesized BDV p40 RNA in 1 ml. Samples of uninfected rat brain were used as negative controls. As a positive RNA control, primers for β-actin were used.
Cytofluorometry.
Unstained and stained T-cell lines were scanned on an Epics Elite laser flow cytometer (Coulter Electronics, Hialeah, Fla.). During acquisition, the T-cell population was gated to exclude debris and 104 cells were counted per sample. Cells were incubated with various fluorescein isothiocyanate-conjugated MAbs specific for the following leukocyte differentiation markers (Camon, Wiesbaden, Germany): W3/13 (T cells), OX-33 (B cells), W3/25 (CD4+ T cells), OX-8 (CD8+ T cells), and P 12520 (anti-CD49d; α4-integrin) and R73 (α/β T-cell receptor) (Dianova).
In situ hybridization.
Digoxigenin-labeled RNAs complementary to BDV mRNAs were prepared from the BDV clone pAF4 (kindly provided by W. I. Lipkin). Brains from experimental animals were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Five-micrometer sagittal sections were mounted on slides, and paraffin was removed with xylene. After treatment with proteinase K and 0.05 N HCl to facilitate penetration of the probe, hybridization was carried out overnight at 65°C with 20 ng of probe per slide by the standard protocol (Boehringer, Mannheim, Germany).
Histology and immunohistochemistry.
Immediately after the rats were killed at different time points after infection, brain samples were obtained. Materials were either frozen in isopentane at −150°C or fixed in buffered paraformaldehyde. All tissue sections were stained with hematoxylin and eosin. Encephalitic infiltrates were scored with an arbitrary scale ranging from 0 to 3, based on the number of infiltrates per section and the number of cell layers in each infiltrate (1, up to 5 small infiltrates/section; 2, more than 5 small infiltrates/section or more than 3 infiltrates with multiple layers; 3, more than 10 small infiltrates or more than 5 infiltrates with multiple layers). Immunohistochemistry was carried out on cryostat sections for the presence of lymphocyte subsets and macrophages and microglia. The following MAbs were used: Ox-8 (anti-CD8+ T cells), OX-38 (anti-CD4+ T cells), and ED1 (macrophages) (Serotec) and anti-tumor necrosis factor alpha and anti-IFN-γ (Genzyme, Cambridge, Mass.).
RESULTS
Cultivation and characterization of BDV-specific CD4+ T-cell line K38.24.
Lymphocytes from the politeal lymph nodes of Lewis rats immunized with a mixture of affinity-purified BDV-specific antigen containing the two major proteins p40 and p24 were harvested 11 days after local immunization. Lymphocytes were repeatedly restimulated in vitro in the presence of irradiated syngeneic thymocytes and BDV-specific antigen containing either both major virus-specific proteins or only p40 or p24. To mimic the situation in vivo, for this study we chose a T-cell line (K38.24) that was maintained in the presence of both virus-specific proteins. The specificity of this T-cell line was determined in proliferation assays at various time points of cultivation in the presence of virus-specific proteins. In all of the experiments described, T cells from the 7th through the 13th restimulation cycle, when stimulation indices (SI) for both virus-specific proteins remained high (SI of 41 to 55), were used, whereas the irrelevant hemagglutinin antigen from influenza virus did not induce proliferation (SI of 1; data not shown). Furthermore, the K38.24 T-cell line was phenotypically characterized in fluorescence-activated cell sorter analyses, revealing the phenotype of a CD4+ T-cell line, namely, W3/13+ OX 33− W3/25+ OX 8−. In addition, this T-cell line was shown to carry the α/β T-cell receptor and the adhesion molecule α4-integrin (VLA-4). The presence of the latter marker was important, since T cells expressing VLA-4 are capable of entering the brain (2, 13, 34). The characterization of this T-cell line included the determination of the cytokine profile by RT-PCR analysis and revealed the presence of IL-2, IL-4, and IFN-γ but not IL-6 or IL-10 (data not shown). Most importantly, cytotoxicity assays on a persistently BDV-infected astrocytic target cell line (BDV-F10) uniformly revealed the absence of lytic activity, even after IFN-γ treatment of target cells to upregulate MHC class II antigen expression in BDV-infected cells (data not shown) (20). Characterization of the CD4+ T-cell line K38.24 in vivo revealed the induction of typical BD symptoms after adoptive transfer into BDV-infected immunosuppressed recipient rats (data not shown).
T-cell treatment prior to infection results in absence of disease symptoms and elimination of virus.
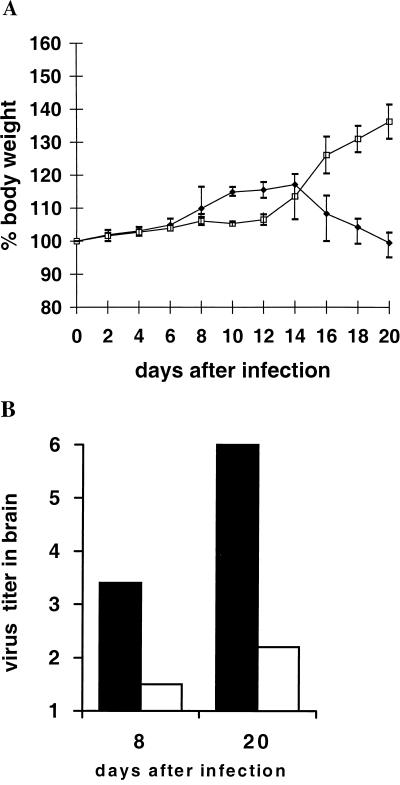
Various protocols were followed in T-cell treatment experiments with T-cell line K38.24. In the first experiment, 5 × 105 cells were used for transfusion at day 12 before intracranial (i.c.) challenge and 2 × 106 cells were used for day 2 before i.c. challenge (Fig. 1 and Table 1). The treated rats showed a faster antibody kinetic, no disease symptoms, and an early onset of a slight inflammatory reaction during the 20-day observation period (Table 1). Health status is reflected by an increase in body weight after day 14, when infected control rats showed decreases in body weight (Fig. 1A), moderate to severe clinical symptoms, and a strong encephalitic reaction (Table 1). At both time points, the virus titers in the brains of T-cell-pretreated rats were considerably lower than those in untreated infected controls (Fig. 1B).
FIG. 1.
Transfusion of CD4+ T-cell line at days 12 (5 × 105) and 2 (2 × 106) before BDV infection. (A) Body weight curves for T-cell-treated rats (□) and untreated control rats (⧫). (B) Virus titers (mean of values listed in Table 1) for T-cell-treated rats (□) and untreated control rats (▪). Virus titers are given as log10.
TABLE 1.
Data for transfusion of CD4+ T-cell line at days 12 (5 × 105) and 2 (2 × 106) before BDV infectiona
| T-cell transfer | Day p.i. | Symptoms | Encephalitis | Antibody titer (log2) | Virus titer (log10) | Presence of mRNA
|
||
|---|---|---|---|---|---|---|---|---|
| BDV | CD8 | Perforin | ||||||
| − | 8 | 0, 0, 0 | 0, 0, 0 | <1, <1, <1 | 2.5, 3.0, 3.8 | +, +, + | −, −, − | −, −, − |
| + | 8 | 0, 0 | 0.5, 0.5 | 2, 5 | 1.0, 1.7 | +, + | +, + | −, + |
| − | 20 | 0.5, 1.5, 1.0 | 1.5, 1.5, 2.0 | 6, 7, ≥8 | 5.9, 5.9, 5.9 | +, +, + | +, +, + | +, +, + |
| + | 20 | 0, 0 | 0.25, 0.5 | ≥8, ≥8 | 1.7, 2.5 | +, + | +, + | +, + |
Data are for individual rats. Encephalitis and clinical scores were on a scale ranging from 0 to 3.
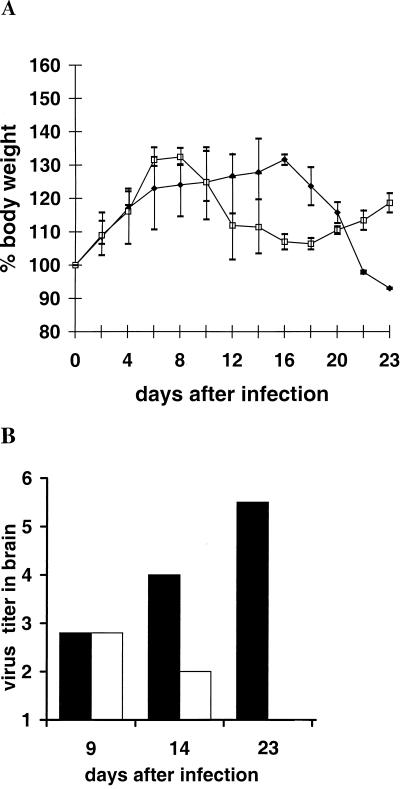
Next, K38.24 CD4+ T cells were transfused into naive rats 13 days (2 × 106 or 3 × 106 cells) and again 3 or 2 days (106 cells) before rats were infected i.c. with BDV, and the observation period was prolonged (Fig. 2 and Table 2 and Fig. 3 and Table 3). Whereas control animals showed the beginnings of clinical disease after day 14 and full-blown disease at later time points, T-cell-treated rats had clinical scores that were transiently very low or exhibited no clinical symptoms (Tables 2 and 3). Interestingly, in T-cell-treated rats, a low-level encephalitic reaction was again seen by day 9, whereas the controls did not show any evidence of inflammatory cell aggregation at this time point. The body weight curves reflect the rats’ general health status. BDV-infected control rats had steadily decreasing body weights, whereas rats that received T cells showed only a transient weight loss or even increased body weights (Fig. 2A and 3A). The rats were killed at various time points after infection and were tested for the presence of pathological alterations and virus in the brain.
FIG. 2.
Transfusion of CD4+ T-cell line at days 13 (2 × 106) and 3 (1 × 106) before BDV infection. Symbols are the same as those in the legend for Fig. 1.
TABLE 2.
Data for transfusion of CD4+ T-cell line at days 13 (2 × 106) and 3 (1 × 106) before BDV infectiona
| T-cell transfer | Day p.i. | Symptoms | Encephalitis | Antibody titer (log2) | Virus titer (log10) | Presence of mRNA
|
||
|---|---|---|---|---|---|---|---|---|
| BDV | CD8 | Perforin | ||||||
| − | 9 | 0, 0 | 0, 0 | <1, <1 | 2.5, 3.0 | +, + | −, − | −, − |
| + | 9 | 0, 0 | 0.5, 1.0 | 1, 4 | 2.5, 3.0 | +, + | +, + | +, + |
| − | 14 | 0, 0.5 | 0.5, 1.0 | 6, ≥8 | 3.0, 5.3 | +, + | +, + | +, + |
| + | 14 | 0.5, 0.5 | 0.5, 1.0 | ≥8, ≥8 | 1.0, 3.0 | +, + | +, + | +, + |
| − | 23 | 1.5, 1.5 | 1.5, 2.0 | ≥8, ≥8 | 5.3, 5.3 | +, + | +, + | +, − |
| + | 23 | 0, 0 | 0, 0 | ≥8, ≥8 | <1.0, <1.0 | −, − | +, + | +, + |
Data are for individual rats. Encephalitis and clinical scores were on a scale ranging from 0 to 3.
FIG. 3.
Transfusion of CD4+ T-cell line at days 13 (3 × 106) and 2 (1 × 106) before BDV infection. Symbols are the same as those in the legend for Fig. 1.
TABLE 3.
Data for transfusion of CD4+ T-cell line at days 13 (3 × 106) and 2 (1 × 106) before BDV infectiona
| T-cell transfer | Day p.i. | Symptoms | Encephalitis | Antibody titer (log2) | Virus titer (log10) | Presence of mRNA
|
||
|---|---|---|---|---|---|---|---|---|
| BDV | CD8 | Perforin | ||||||
| − | 6 | 0, 0, 0 | 0, 0, 0 | <1, <1, 1 | <1.0, <1.0, 1.7 | −, −, + | −, −, − | −, −, − |
| + | 6 | 0, 0, 0 | 0, 0, 0 | <1, 1, 2 | <1.0, 1.0, <1.0 | −, +, − | −, −, − | −, −, − |
| − | 12 | 0, 0, 0, 0 | 0, 0, 0, 0 | <1, 5, 1, <1 | 4.5, 5.0, 5.0, 4.5 | +, +, +, + | +, +, −, + | −, −, −, − |
| + | 12 | 0, 0 | 0.5, 1.0 | 7, 7 | 1.0, 2.5 | +, + | +, + | +, + |
| − | 32 | 1.5, 2.0, 3.0 | 2.0, 2.0, 2.5 | 7, ≥8, ≥8 | 5.9, 5.9, 5.9 | +, +, + | +, +, + | +, +, + |
| + | 32 | 0, 0, 0, 0 | 0.25, 1.0, 0.25, 0.25 | ≥8, ≥8, ≥8, ≥8 | <1.0, <1.0, <1.0, 1.0 | −, −, −, + | −, −, −, − | −, −, −, − |
Data are for individual rats. Encephalitis and clinical scores were on a scale from 0 to 3.
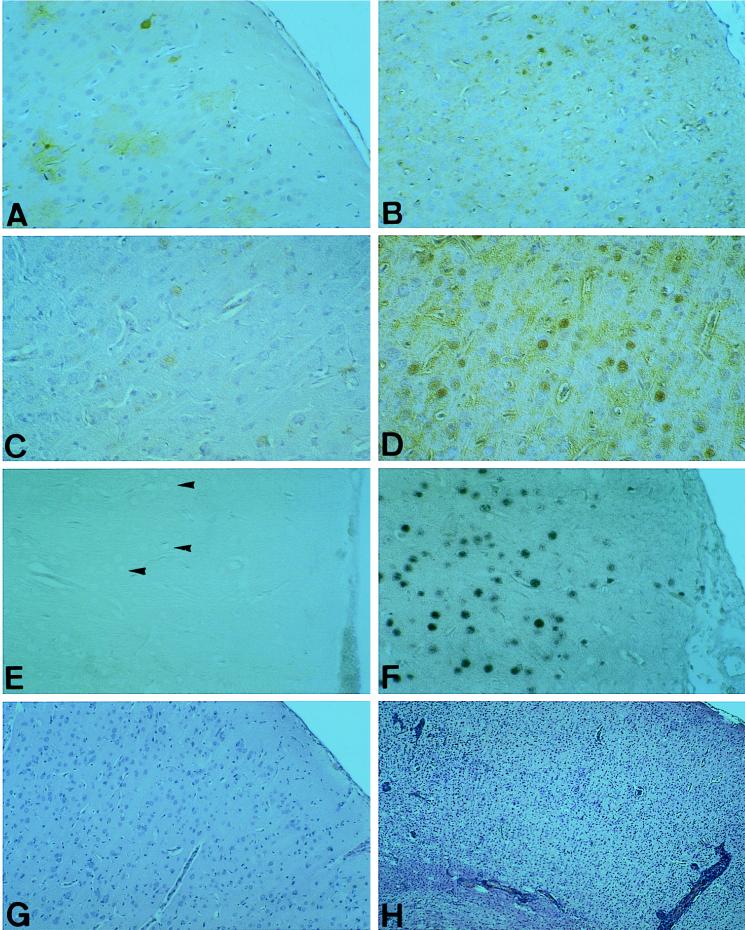
The inflammatory reactions in the brains of rats that received T cells prior to infection were in general less pronounced and never received a score higher than 1 (see Materials and Methods). However, both T-cell-treated rats killed at day 9 after infection had a low-level encephalitic reaction with CD4+ and CD8+ T cells, whereas the infected control rats did not (Table 2). At later time points, infected controls without T-cell treatment exhibited significant brain inflammation and obvious clinical signs of BD (Tables 2 and 3). Most interestingly, with a single exception, rats which had been injected with the CD4+ T-cell line did not reveal infectious virus in cortical brain tissue on day 21 or 32. With one exception (Table 2, day 9), virus titers in the brains of treated rats at all time points tested were at least 2 log10 units lower than those in untreated infected rats (Fig. 2A and 3A; Tables 2 and 3). Also, depending on the time period after infection, T-cell-treated rats lacking virus in the brain exhibited virus-specific antigen below detectable levels or at drastically reduced levels, as demonstrated by staining of rare single cells, compared to untreated infected controls showing widespread dissemination of virus-specific antigen (Fig. 4A through D). Corresponding with these findings, in general, no virus-specific mRNA was detected by RT-PCR in the brain beyond day 23 after infection (Tables 1 to 3), and the same was true for in situ hybridization experiments (Fig. 4E and F). In addition, the morphological structure in the brains of T cell-treated rats was not altered visibly, whereas infected control rats had severe degenerative defects in the cortical areas (Fig. 4G and H). Most strikingly, immunohistological examination also revealed that in all T-cell recipients, including those in which virus had been eliminated from the cortex, virus-specific antigen and an inflammatory reaction were restricted apparently exclusively to the hippocampal area, but no overt disease was seen (data not shown). In total, 23 rats were tested for the presence of virus after T-cell treatment, and they generally had reduced virus titers (n = 14) or no virus (n = 6) in the brain (data for 19 rats are shown in Fig. 1 to 3 and Tables 1 to 3). Treatment with the described CD4+ T-cell line resulted in protection from severe encephalitis and disease and in clearance of virus from the brain.
FIG. 4.
Clearance of BDV from the brains of CD4+ T-cell-treated rats; reduced expression of BDV-specific p40 in T-cell-treated (A and C) versus untreated, infected control (B and D) rats at day 10 (A and B) and day 20 (C and D); in situ hybridization in the cortical area of T-cell-treated (E) and untreated, infected control (F) rats with a probe for p40 mRNA at day 32 p.i.; absence of BDV RNA in T-cell-treated rats. Note the uninfected, unstained pyramidal neurons in T-cell-treated rats (E) (arrowheads). In contrast, most neurons and many astrocytes are infected with BDV in untreated control rats (F). Almost-intact brain morphology of a T-cell-treated rat (G) at day 32 p.i. in the neocortex and inflamed brain with morphological alterations in an infected control rat (H). (A through D) Immunohistology with the anti-p40 specific MAb 38/17C1; (E and F) in situ hybridization; (G and H) hematoxylin and eosin staining. Magnifications: A, B, E, F, and G, ×50; C, ×100; D, ×120; H, ×30.
Elimination of virus from the brain correlates with the early presence of CD8+ T cells and perforin.
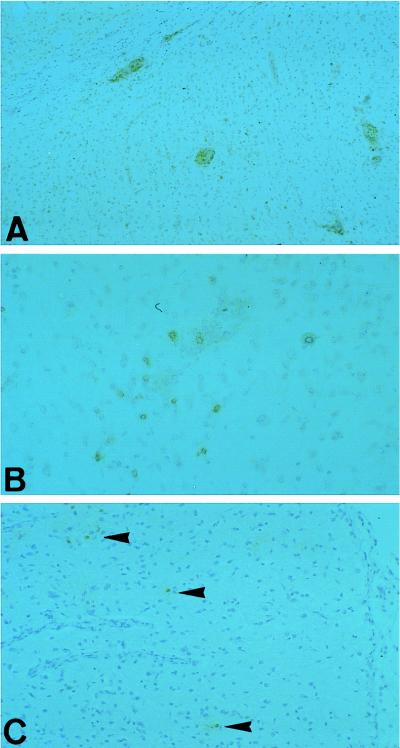
In earlier studies we showed that virus-specific CD8+ T cells are present in the brains of BDV-infected and otherwise untreated rats (4, 20, 28). Therefore, we investigated the appearance of CD8+ T cells in the brains of T-cell-treated rats versus those of control rats. Though only CD4+ T cells had been used for treatment, numerous CD8+ cells as well as CD4+ T cells were found in all recipient rats (Fig. 5A and B and Tables 1 to 3). At very early time points after infection (day 6, Table 3), no CD8 mRNA was detectable in any rat tested. Slightly later, CD8+ T cells were detected in treated but not in control rats by RT-PCR specific for the mRNA of CD8 (day 8, Table 1; day 9, Table 2; day 10, Fig. 5B). Furthermore, at late time points after challenge, in the absence of infectious virus and virus-specific mRNA, very few (day 20, Table 1 and Fig. 5C) or no (day 32, Table 3) CD8+ T cells were found in the brain.
FIG. 5.
Presence and localization of T-cell populations in CD4+ T-cell-treated rats: perivascular distribution of CD4+ T cells (A, day 10) and dissemination of CD8+ T cells (B and C) in the brains of T-cell-treated rats. Note the difference in the numbers of CD8+ T cells at day 10 (B) and day 20 (C). In infected control rats, very few cells are found at day 10 (data not shown). Magnifications: A, ×50; B, ×10; C, ×60.
Since perforin has been identified as the major effector molecule in cytolysis by CD8+ T cells, and since we have demonstrated the presence of mRNA for perforin in BDV-infected rats (28), we tested the brains of T-cell-treated and untreated infected rats for the presence of perforin mRNA by RT-PCR. First, in T-cell recipients there was a clear correlation between the early presence of CD8+ T cells and perforin mRNA in the brain (Tables 1 and 2). When no CD8 mRNA was detectable, no perforin mRNA was found either (Table 3). In some cases, discrepancies between the kinetics of the presence of CD8+ T cells and perforin in vaccinated and control rats appear to be of great importance (e.g., day 9, Table 2). In this case, RT-PCR analyses for CD8 and perforin mRNA produced positive results for both rats which received the CD4+ T-cell line, while results were negative for infected and untreated control rats. The results for day 8 after challenge were essentially the same (Table 1).
Presence of CD8+ T cells and perforin correlates with cytotoxicity in T-cell-treated rats.
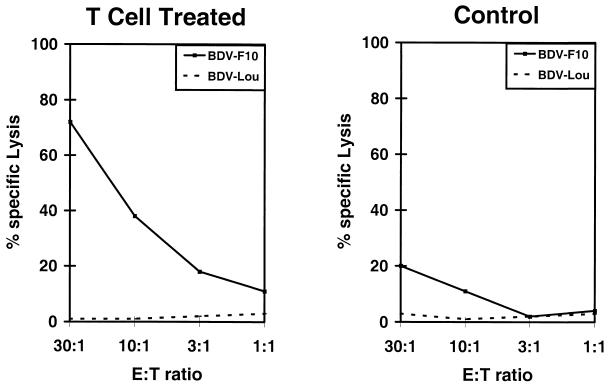
After having established the early presence of CD8+ T cells and the major effector molecule of cytotoxicity in CD4+ T-cell-treated rats, we questioned whether cytotoxic activity could be found in lymphocytes isolated from the brains of treated and untreated infected controls at early time points after infection. Therefore, lymphocytes isolated from the brains of Lewis rats after T-cell treatment and from infected Lewis controls on day 9 postinfection (p.i.) were tested by conventional cytotoxicity assays (Fig. 6). Cytotoxic activity was detectable in brain lymphocyte preparations isolated from infected and untreated control rats. The BDV-infected syngeneic astrocytic target cell line (BDV-F10) was moderately lysed after an 8-h incubation period by day 9 lymphocytes, but allogeneic BDV-infected cells (BDV-Lou skin cells) were not killed (Fig. 6, right panel). Pretreatment of target cells with IFN-γ to enhance the expression of MHC class II antigen of target cells did not result in increased lysis, even in a 16-h assay (data not shown). In contrast, day 9 brain lymphocyte preparations from CD4+ T-cell-treated rats elicited significantly higher lysis of BDV-infected syngeneic target cells (Fig. 6, left panel). Here again, despite the higher cytolytic capacity of lymphocytes from T-cell-treated rats, allogeneic infected target cells were not lysed, and treatment of syngeneic targets with IFN-γ did not change the results, i.e., it did not cause MHC class II-restricted lysis. Essentially the same results were obtained after the coincubation period was increased to 16 h to enable lysis from MHC class II-restricted killer cells due to the generally observed delayed kinetics for CD4+ T-cell-mediated killing in rats (data not shown). Again, lysis of target cells was comparable whether or not targets were treated with IFN-γ. These data confirm earlier results (20, 28).
FIG. 6.
Nine-hour cytotoxicity assay with lymphocytes isolated from the brain of a T-cell-treated rat (left panel) versus an untreated infected control rat (right panel) at day 9 p.i. Spontaneous release of syngeneic BDV-F10 (24%) and allogeneic BDV-Lou (Louvain, 19%). E:T, effector cell to target cell.
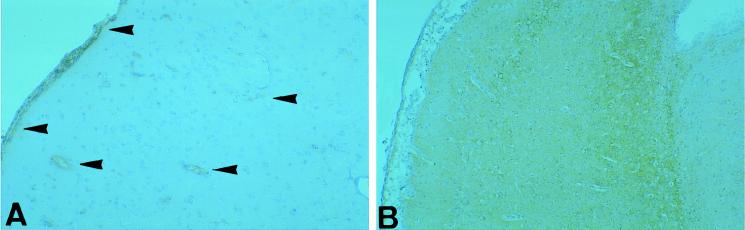
To exclude a possible role of locally synthesized IFN-γ in virus elimination from the brain, we looked for the presence of this cytokine by immunohistochemistry with sections processed in parallel (Fig. 7). However, staining in T-cell-treated rats was even less intense than that in untreated infected rats.
FIG. 7.
Immunohistological detection of IFN-γ in T-cell-treated (A, ×50) and untreated control (B, ×30) rats. Note the lower staining activity for IFN-γ (arrowheads) in T-cell-treated rats in panel A. Magnifications: A, ×50; B, ×30.
No evidence for importance of neutralizing antibodies after CD4+ T-cell vaccination.
In all T-cell-treated rats, the kinetics of virus-specific antibody synthesis were enhanced Tables 1 to 3). In most cases, titers as determined by ELISA were two- to fourfold higher than those for untreated infected controls. Since antiviral antibodies can have neutralizing activity which might interfere with virus replication or neutralize extracellular virus, sera from rats transfused with the CD4+ T-cell line and rats infected without T-cell treatment were tested in neutralization assays. Titers of neutralizing activity in all sera remained below the detection level (NT50 < 1:32) independently of the antibody titers found in binding assays (ELISA). Therefore, we conclude that none of the infected control rats and none of the T-cell-treated and infected rats synthesized detectable neutralizing antibodies during the 32-day observation period. This finding is in good agreement with the earlier observation that neutralizing antibodies are detectable only after 10 to 15 weeks p.i. (12).
DISCUSSION
In this study we show that transfusion of virus-specific CD4+ T cells results in the termination of a viral infection initiated after transfusion. These results support and extend earlier experimental data obtained in the same virus system by Richt et al. (23). Moreover, they provide a mechanistic basis for the understanding of the termination of a potentially persistent viral infection and protection from virus-induced immunopathology. We argue that the observed phenomena are not due to an effector mechanism mediated by transfused virus-specific CD4+ T cells but, rather, present strong evidence for a virus-specific CD8+ cytotoxic T-cell response induced by transfusion with a CD4+ T helper cell.
During recent years, BDV infection of rats has been established as an important model of an immunopathological disease in the brain resulting in severe neurological symptoms such as abnormal behavior, disturbances of motility and, finally, signs of debility and dementia (5, 17, 29). These changes have been correlated with the initial invasion of the brain by mononuclear cells that results in severe inflammatory reactions throughout this organ as well as degenerative alterations of various cell types, including neurons (4, 20, 28, 30). Ultimately, a significant proportion of brain tissue, especially the cortex, is destroyed; this is represented by cortical brain atrophy (4, 17, 28). Since the basis of this disease is a persistent infection of the central nervous system, one could argue that the elimination of the virus and therefore the absence of viral replication and the lack of virus-specific antigens would also prevent immunopathology and disease. However, despite a vigorous T-cell-mediated immune response defined by the cytolytic activity of classical MHC class I-restricted T cells, the virus persists in the central nervous systems of infected individuals (7, 18, 20). In addition, the mere presence of virus-specific antibodies, even those with neutralizing activity, does not prevent infection or limit or eliminate the virus from the host (12). Therefore, the finding that the transfusion of virus-specific CD4+ T cells prior to infection eliminates the virus and prevents disease appears to be rather important (23). However, the mechanism of this phenomenon remained unanswered. The following differences between the experiments reported by Richt et al. (23) and those described here may be important: first, the use of a T-cell line that induced cytolysis of MHC class II-bearing target cells and did not allow Richt et al. to decide whether the transfused cytolytic CD4+ T cells or other mechanisms were responsible for the observed virus elimination; second, these authors did not employ methods to demonstrate or phenotype infiltrating cells such as CD4+ or CD8+ T cells; and third, these researchers did not include functional assays. In contrast to Richt et al., we have never found any argument for the operativeness of MHC class II-restricted cytolysis in this disease (20, 21, 28). Nevertheless, after transfusion with virus-specific CD4+ T-cell lines that lack cytotoxic activity in vitro, we were able to induce BD (21; this study). We could show that these BD-specific T cells, obviously acting as helpers, were sufficient to cause disease via the recruitment of CD8+ T cells to the brain, whereas recipient rats depleted of CD8+ T cells or all T cells by T-cell-specific antibodies did not show neurological symptoms or destructive encephalitis (21). Therefore, in the study reported here, we decided to use the CD4+ T-cell line K38.24, which does not induce cytolytic activity in vitro and therefore could not be directly responsible for the lysis of infected cells in vivo. However, the effect of CD4+ T-cell transfusion prior to i.c. virus challenge on virus titers and clinical disease was quite impressive. If given CD4+ T cells before virus challenge, rats were capable of limiting and even abrogating virus replication, resulting in reduced virus titers and even in an absence of detectable virus at later time points after challenge. In contrast, if given after infection, the same cell line was capable of inducing disease (data not shown). The lack of virus in T-cell-treated rats was demonstrated by the generally complete absence of infectious virus, virus-specific antigen, and virus-specific RNA as shown by RT-PCR and in situ hybridization. In all experiments, transient virus replication was seen in the brains of the challenged rats. At early time points after infection, virus titers in rats treated with T cells were equal to or lower than those from controls.
These results are in agreement with the proposed role of cytotoxic T cells in BD; earlier, we provided several lines of evidence that CD8+ T cells are involved in the immunopathogenesis and degenerative encephalopathy that occur after infection with BDV (5, 29). First, MHC class I-restricted lysis can be detected in lymphocytes isolated from the brains of diseased rats (20, 28). Second, the appearance of CD8+ T cells in the brain coincides with the onset of disease and the destruction of brain cells (4, 28, 30). Third, the absence of CD8+ T cells results in the prevention of disease (21, 30, 31). Fourth, the adoptive transfer of brain lymphocytes with high cytolytic capacity results in the early onset of severe degenerative alterations in the brain, as represented by spongiform degeneration; and fifth, cytodestruction is linked with the presence of perforin mRNA (perforin is a major pathway of CD8-mediated cellular destruction [28]).
In the experiments described in the present study, we found a direct correlation between the enhanced kinetics of CD8+ T cells, MHC-restricted cytotoxicity, the presence of perforin mRNA, and the loss of virus from cortical brain areas compared to those in control rats exhibiting immunopathological reactions and disease. Interestingly, the time points when CD8+ T cells and perforin mRNA could be detected in the brains of T cell-treated rats and untreated rats differed. In addition, rats receiving virus-specific CD4+ T cells prior to infection had CD8+ T cells predominantly in parenchymal locations, whereas CD4+ T cells appeared to be stringently restricted to perivascular locations, as demonstrated by immunohistochemistry. This finding again supports our concept and is in agreement with the distribution patterns of T cells at early time points after infection, when the local activity of CD8+ T cells commences. Furthermore, considerably more CD8+ T cells were found in cortical areas of T-cell recipient rats at 10 days p.i. than at day 20 or 32, when the virus load was drastically reduced or even eliminated. In this respect, it is worth mentioning that the conventional histology of the cortexes of T-cell-treated rats did not reveal any gross morphological changes by day 20 or 32, whereas infected control rats showed strong degenerative alterations in addition to severe generalized inflammation.
The observed presence of CD8+ T cells, the upregulation of perforin mRNA, and the elimination of virus were most obvious in the neocortex. Interestingly, in all T-cell-treated rats that were killed after day 20 p.i., virus-specific antigen and a severe inflammatory reaction were found in the hippocampus. However, in situ hybridization with probes specific for both genomic RNA and mRNA revealed no signal. Therefore, it appears that the infectious virus was eliminated and the viral antigen was the remains of an earlier productive infection that resulted in localized inflammation without visible neurological symptoms. We do not presently have a valid explanation for this finding.
The present study clearly shows that persistent virus infection in an organ can be controlled and that virus can be eliminated by CD8+ cytotoxic T lymphocytes if they are activated prior to the considerable spread of the virus. In the presented model of BD, the activity of the CD8+ cytotoxic T-cell response appears to be induced by the presence of virus-specific CD4+ T cells, which might thus be defined as helper cells. This conclusion is supported by faster antibody kinetics in T-cell recipients. In contrast to CD8+ T cells, even BD virus-specific antisera with neutralizing activity do not seem to eliminate the virus from persistently infected hosts (unpublished data). This conclusion is supported by various studies on persistent viral infections in which neutralizing antibodies are synthesized too late to prevent disease (1, 12, 16), B cells producing neutralizing antibodies are killed by virus-specific T cells (22), or neutralizing antibodies have no effect on an ongoing or established persistent infection for various reasons (19). Furthermore, IFN-γ does not appear to play a critical role in BDV elimination.
Our results suggest that protection from disease caused by viral infections or cellular autoimmune reactions that is afforded by treatment with CD4+ T cells might be due to their helper activity for CD8+ cytotoxic T cells. Finally, the present findings might suggest that, at least in protection from persistent viral infections, efforts to induce a cytotoxic T-cell response by using, for example, defined T-cell epitopes for vaccination should be enhanced.
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Sti 71/2-1 to L.S., Sti 71/2-2 to L.S. and O.P., and Bi 323/2-2 to T.B.) and an EU grant (CHRX-CT94-0670 to L.S.).
We thank Martin Sobbe for help and valuable discussions and Silke Gommel for outstanding technical assistance.
Footnotes
Dedicated to Professor Hermann Becht.
REFERENCES
- 1.Alberti A, Cavalletto D, Pontisso P, Chemello L, Tagariello G, Belussi F. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet. 1988;i:1421–1424. doi: 10.1016/s0140-6736(88)92237-4. [DOI] [PubMed] [Google Scholar]
- 2.Baron J L, Madri J A, Ruddle N H, Hashim G, Janeway C A., Jr Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilzer T, Planz O, Lipkin W I, Stitz L. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with Borna disease virus-induced encephalitis. Brain Pathol. 1995;5:223–230. doi: 10.1111/j.1750-3639.1995.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 4.Bilzer T, Stitz L. Immune-mediated brain atrophy: CD8+ T cells contribute to tissue destruction during Borna disease. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 5.Bilzer T, Stitz L. Immunopathogenesis of virus diseases affecting the central nervous system. Crit Rev Immunol. 1996;16:145–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- 6.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 7.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone K M, Moench T R, Lipkin W I. Borna disease virus replicates in astrocytes Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991;50:205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 9.De la Torre J C, Bode L, Durrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 10.De la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grasser F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 11.Deschl U, Stitz L, Herzog S, Frese K, Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol. 1990;81:41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- 12.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1995;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey W F, Hsu B L, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 14.Hirano N, Kao M, Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus infected rats. J Gen Virol. 1983;64:1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- 15.Irani D N, Griffin D E. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. J Immunol Methods. 1991;139:223–227. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- 16.Moore J P, McCutchan F E, Poon S W, Mascola J, Liu J, Cao Y, Ho D D. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68:8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan O, Herzog S, Frese K, Scheefers K, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 18.Narayan O, Herzog S, Frese K, Scheefers K, Rott R. Behavioral disease in rats caused by immunopathological response to persistent Borna disease virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 19.Nash A A, Cambouropoulos P. The immune response to herpes simplex virus. Semin Virol. 1993;4:181–186. [Google Scholar]
- 20.Planz O, Bilzer T, Sobbe M, Stitz L. Lysis of MHC class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J Exp Med. 1993;178:163–174. doi: 10.1084/jem.178.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planz O, Bilzer T, Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies. Nature. 1996;382:726–729. doi: 10.1038/382726a0. . (Erratum, 384:288.) [DOI] [PubMed] [Google Scholar]
- 23.Richt J A, Schmeel A, Frese K, Carbone K M, Narayan O, Rott R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J Exp Med. 1994;179:1467–1473. doi: 10.1084/jem.179.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richt J A, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 26.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 27.Sauder C, Muller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, De la Torre J C, Grasser F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobbe M, Bilzer T, Gommel S, Nöske K, Planz O, Stitz L. Induction of degenerative brain lesions after adoptive transfer of brain lymphocytes from Borna disease virus-infected rats: presence of CD8+ T cells and perforin mRNA. J Virol. 1997;71:2400–2407. doi: 10.1128/jvi.71.3.2400-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 30.Stitz L, Planz O, Bilzer T, Frei K, Fontana A. Transforming growth factor-β modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J Immunol. 1991;147:3581–3586. [PubMed] [Google Scholar]
- 31.Stitz L, Sobbe M, Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stitz L, Soeder D, Deschl U, Frese K, Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus infected rats by cyclosporine A. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 33.Thiedemann N, Presek P, Rott R, Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992;73:1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- 34.Wekerle H, Ketelsen U P, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymus: morphological and serological characterization. J Exp Med. 1980;151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]