Abstract
Background:
Elevated lipoprotein(a) [Lp(a)] and coronary artery calcium (CAC) score are individually associated with increased atherosclerotic cardiovascular disease (ASCVD) risk but have not been studied in combination.
Aim:
To investigate the independent and joint association of Lp(a) and CAC with ASCVD risk.
Methods:
Plasma Lp(a) and CAC were measured at enrolment among asymptomatic participants of Multi-Ethnic Study of Atherosclerosis (MESA, n=4,512) and Dallas Heart Study (DHS, n=2,078) cohorts. Elevated Lp(a) was defined as the highest race-specific quintile and three CAC score categories were studied (0, 1-99, and ≥100). Associations of Lp(a) and CAC with ASCVD risk were evaluated using risk factor-adjusted Cox regression models.
Results:
Among MESA participants (61.9 years, 52.5% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese), 476 incident ASCVD events were observed during13.2 years follow-up. Elevated Lp(a) and CAC score (1-99 and ≥100) were independently associated with ASCVD risk (hazard ratios [HR] 1.29, 95%CI 1.04, 1.61; 1.68, 95%CI 1.30, 2.16; and 2.66, 95%CI 2.07, 3.43; respectively) and Lp(a)-by-CAC interaction was not noted. Compared with participants with non-elevated Lp(a) and CAC=0, those with elevated Lp(a) and CAC ≥100 were at the highest risk (HR 4.71, 95%CI 3.01, 7.40) and those with elevated Lp(a) and CAC=0 were at a similar risk (HR 1.31, 95%CI 0.73, 2.35). Similar findings were observed when guideline recommended Lp(a) and CAC thresholds considered, and findings were replicated in DHS.
Conclusions:
Lp(a) and CAC are independently associated with ASCVD risk and may be useful concurrently for guiding primary prevention therapy decisions.
Keywords: lipoprotein (a), coronary artery calcium, atherosclerotic cardiovascular disease, primary cardiovascular disease prevention
CONDENSED ABSTRACT
Lipoprotein(a) [Lp(a)] and coronary artery calcium (CAC) score are individually associated with atherosclerotic cardiovascular disease (ASCVD) risk. The independent and joint association of these markers with ASCVD is unclear. We studied these relationships among asymptomatic participants of Multi-Ethnic Study of Atherosclerosis (MESA) and Dallas Heart Study (DHS). Elevated Lp(a) and CAC were independently associated with ASCVD risk. Compared with participants with non-elevated Lp(a) and CAC=0, those with elevated Lp(a) and CAC ≥100 were at the highest risk, while those with elevated Lp(a) and CAC=0 were at a similar risk. These observations were similar with guideline recommended Lp(a) and CAC thresholds.
INTRODUCTION
Lipoprotein(a) [Lp(a)] is a low-density lipoprotein (LDL)-like particle with an apolipoprotein B-100 molecule covalently bound to apolipoprotein (a).(1) Circulating Lp(a) levels are primarily genetically determined,(1) and epidemiologic, genome-wide association, and mendelian randomization studies provide robust evidence that elevated Lp(a) levels are causally associated with atherosclerotic cardiovascular disease (ASCVD) risk. (2–4) Coronary artery calcium (CAC) is a highly specific marker of coronary atherosclerosis that is quantified using the Agatston method to yield the CAC score.(5) A CAC score captures the burden of subclinical coronary atherosclerosis and is a guideline-endorsed,(6) independent predictor of ASCVD risk.(7–9)
Current national cholesterol management guidelines consider elevated Lp(a) level (≥50 mg/dL or ≥125 nmol/L) as a risk-enhancing factor, and recommend using the CAC score (≥100 or ≥75th percentile for age/sex/race) as a validated measure to guide decisions regarding primary ASCVD prevention.(6) The cross-sectional associations of Lp(a) and CAC have been previously studied,(10–15) but simultaneous evaluation of the associations of these risk markers with ASCVD risk has not been performed to date. To address this important knowledge gap, we sought to evaluate the independent and joint association of Lp(a) and CAC score with ASCVD risk among asymptomatic participants of two contemporary, multi-ethnic American epidemiologic cohorts: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Dallas Heart Study (DHS). We hypothesized that elevated both Lp(a) level and CAC score have an independent and additive joint association with incident ASCVD.
METHODS
Both MESA and DHS were approved by Institutional Review Boards at the respective coordinating centers, at each field center, and other central agencies. All participants provided written informed consent at enrollment.
Study population
The study designs for MESA and DHS have been previously published. (16,17) These cohort are described in the Supplement. For the purpose of this study, we included participants from both cohorts who were free of clinical ASCVD at baseline and excluded those with missing data on Lp(a) or CAC, statin use at baseline, and those with incomplete follow-up for incident ASCVD events (Supplemental Figure 1A and 1B). The final study population consisted of 4,512 MESA participants and 2,078 DHS participants.
Lp(a) and CAC measurement
In MESA, Lp(a) mass concentration was measured using a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan), and circulating levels were reported in mg/dL.(18) CAC score measurement was performed using an electron-beam CT scanner at three of the six MESA field centers (Chicago, Los Angeles, and New York) and the remaining three field centers (Baltimore, Forsyth County, and St. Paul) used a multidetector CT scanner as previously described.(7) Each participant underwent two phantom-adjusted scans. CAC score was quantified as Agatston units (AU) and the average of the two CAC measurements was reported.(19) In DHS, plasma Lp(a) was measured using a sandwich ELISA, and circulating levels were reported in nmol/L.(14) Notably, the Lp(a) assays used in both cohorts were insensitive to apo(a) isoform size as recommended by the National Lipid Association guidelines.(20) CAC measurements were performed using electron-beam CT as previously described.(21) Two scans were obtained for each participant, and the average CAC score was reported in AU.(14)
Atherosclerotic Cardiovascular Disease events
MESA and DHS participants were prospectively followed for the primary endpoint of time to first adjudicated ASCVD event. ASCVD was defined as coronary heart disease (CHD)-related death, nonfatal MI, or fatal or nonfatal stroke.(22) The ASCVD adjudication criteria used in MESA and DHS are described in the Supplement. The mean follow-up time periods for incident ASCVD were 13.2 ± 4.7 years in MESA and 11.0 ± 1.8 years in DHS.
Statistical analysis
Participants of MESA and DHS were analyzed separately because Lp(a) was measured using different assays in these cohorts. The baseline demographic and clinical characteristics of MESA and DHS participants were described across Lp(a) quintiles and CAC score categories. Lp(a) levels in the general population are distributed in a right-skewed manner and prior literature has shown that Lp(a) levels at the highest quintile are associated with incident cardiovascular disease.(23,24) Black individuals have higher Lp(a) levels as compared with other ethnic groups.(1) Therefore, we stratified our study population across race-specific Lp(a) quintiles similar to a prior report.(24) Participants were also stratified across CAC score categories (0, 1-99, and ≥100 AU) based on current cholesterol management guidelines.(6) Categorical variables are presented as a count (proportion) and continuous variables are presented as mean ± standard deviation or median with interquartile range based on variable distribution. Categorical variables were compared using the chi-squared test, and continuous variables were compared using the Kruskal-Wallis test across Lp(a) and CAC score categories.
The independent association of Lp(a) and CAC with ASCVD was studied using Cox proportional hazard models adjusted for age, sex, race, diabetes, smoking, SBP, antihypertensive medication use, total cholesterol, HDL-C, triglycerides, and body mass index in each cohort. Lp(a) and CAC were analyzed as continuous measures (Ln Lp(a) and Ln [CAC+1], respectively) and as categorical measures (race-specific Lp(a) quintile 5 versus quintiles 1-4 and CAC score categories 1-99 and ≥100 versus zero, respectively). The multiplicative interaction between Lp(a) and CAC was tested in each Cox model. In two sensitivity analyses, family history of MI was added as a covariate to Cox models and incident CHD and stroke events were evaluated as separate outcomes of interest.
The primary analysis involved studying the joint association of Lp(a) and CAC with ASCVD among MESA and DHS participants, which was evaluated by stratifying each cohort into six mutually exclusive groups based on race-specific Lp(a) quintiles (quintile 5 vs. quintiles 1-4) and CAC score categories (0, 1-99, and ≥ 100): Lp(a) quintile 1-4 with CAC=0, Lp(a) quintile 5 with CAC=0, Lp(a) quintile 1-4 with CAC 1-99, Lp(a) quintile 5 with CAC 1-99, Lp(a) quintiles 1-4 with CAC ≥100, and Lp(a) quintiles 5 with CAC ≥100. The 10-year cumulative ASCVD incidence in these groups was studied using the Kaplan-Meier method. The independent associations of these groups with ASCVD risk were studied using Cox proportional hazard models adjusted for traditional cardiovascular risk factors mentioned previously.
Last, the joint association of guideline-recommended Lp(a) and CAC thresholds (≥50 mg/dL and ≥100 AU, respectively) with ASCVD risk was studied among MESA participants using an approach similar to that outlined above. We created four mutually exclusive groups: Lp(a) <50 mg/dL with CAC <100 AU, Lp(a) ≥50 mg/dL with CAC <100 AU, Lp(a) <50 mg/dL with CAC ≥100 AU, and Lp(a) ≥50 mg/dL with CAC ≥100 AU. The 10-year cumulative ASCVD incidence in these groups was studied using the Kaplan-Meier method and the independent association of these groups with ASCVD risk was studied using adjusted Cox proportional hazard models. As a sensitivity analysis, we also considered the alternate CAC score threshold of 100 AU or 75th age/sex/race percentile. The proportional hazards assumption was confirmed using Schoenfeld residuals for all Cox models. Statistical analyses were performed using SAS version 9.4 (SAS, Cary, North Carolina). A two-sided p value of < 0.05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics
The baseline characteristics of MESA participants across race-specific Lp(a) quintiles and CAC score categories are described in Table 1 and Table 2, respectively. Mean age of MESA participants was 61.9 ± 10.4 years, 52.5% were women, 36.8 % White, 29.3 % Black, 22.2 % Hispanic, and 11.7 % Chinese. Similar to prior reports, Lp(a) levels were higher in Blacks as compared with other race groups. The proportion of women and participants with family history of MI increased across race-specific Lp(a) quintiles (Table 1). Importantly, median CAC score and proportion of participants in the three CAC score categories were similar across Lp(a) groups (Table 1).
Table 1:
Baseline characteristics of Multi-Ethnic Study of Atherosclerosis participants stratified by race-specific Lp(a) quintiles
| Characteristics | Quintile 1 (N=898) |
Quintile 2 (N=904) |
Quintile 3 (N=904) |
Quintile 4 (N=905) |
Quintile 5 (N=901) |
p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Lipoprotein(a) range (mg/dl) | ||||||
| White | 0 – 4.6 | 4.7 – 9.8 | 9.9 – 17.1 | 17.2 – 38.2 | 38.5 – 236.3 | |
| Black | 0 – 16.6 | 16.7 – 28.6 | 28.7 – 42.5 | 42.6 – 72.7 | 73.0 – 314.2 | |
| Hispanic | 0 – 4.8 | 4.9 – 9.7 | 9.8 – 17.5 | 17.6 – 34.8 | 35.2 – 316.5 | |
| Chinese | 0 – 6.4 | 6.5 – 10.7 | 10.8 – 16.6 | 16.7 – 29.6 | 29.8 – 131.8 | |
| Median Lipoprotein(a) (mg/dl) | <0.01 | |||||
| White | 2.6 [1.4 – 3.6] | 7.2 [5.9 – 8.7] | 13.2 [11.3 – 14.9] | 24.0 [20.4 – 29.9] | 64.1 [50.2 – 85.3] | |
| Black | 10.4 [5.5 – 13.3] | 23.4 [20.5 – 25.9] | 35.2 [31.6 – 38.6] | 54.3 [47.7 – 61.7] | 94.8 [84.1 – 111.2] | |
| Hispanic | 2.5 [0.9 – 3.8] | 7.7 [6.3 – 8.6] | 13.1 [11.1 – 15.4] | 23.8 [20.2 – 29.3] | 63.2 [46.2 – 87.6] | |
| Chinese | 4.1 [2.5 - 5.1] | 8.7 [7.8 – 9.8] | 13.1 [11.9 – 14.9] | 21.2 [19.1 – 23.5] | 49.7 [36.5 – 72.4] | |
| Age (years) | 61.6 ± 10.6 | 61.9 ± 10.4 | 62.1 ± 10.0 | 62.1 ± 10.5 | 61.9 ± 10.4 | 0.52 |
| Men | 463 (51.6 %) | 449 (49.7 %) | 427 (47.2 %) | 434 (48.0 %) | 372 (41.3 %) | < 0.01 |
| Systolic BP (mm Hg) | 126.2 ± 20.6 | 126.3 ± 21.3 | 125.7 ± 21.7 | 126.7 ± 22.1 | 125.9 ± 21.2 | 0.67 |
| Diastolic BP (mm Hg) | 72.1 ± 10.0 | 72.3 ± 10.0 | 71.8 ± 10.7 | 72.3 ± 10.5 | 71.7 ± 10.3 | 0.37 |
| Antihypertensive use | 317 (35.3 %) | 302 (33.4 %) | 288 (31.9 %) | 279 (30.8 %) | 299 (33.2 %) | 0.17 |
| Diabetes | 93 (10.4 %) | 84 (9.3 %) | 84 (9.3 %) | 85 (9.4 %) | 83 (9.2 %) | 0.80 |
| Smoking | 113 (12.6 %) | 103 (11.4 %) | 133 (14.7 %) | 118 (13.0 %) | 112 (12.4 %) | 0.70 |
| Total cholesterol (mg/dL) | 186.7 ± 34.3 | 191.0 ± 32.2 | 195.5 ±33.4 | 197.4 ± 34.4 | 208.2 ± 35.4 | < 0.01 |
| HDL-cholesterol (mg/dL) | 50.6 ± 14.8 | 50.8 ± 15.3 | 51.3 ± 14.8 | 51.1 ± 14.6 | 52.9 ± 15.7 | < 0.01 |
| Triglycerides (mg/dL) | 112.0 [76.0 – 166.0] | 111.5 [77.0 – 161.0] | 107.5 [73.0 – 156.5] | 103.0 [74.0 – 148.0] | 104.0 [76.0 – 147.0] | < 0.01 |
| LDL-cholesterol (mg/dL) | 110.5 ± 31.1 | 114.6 ± 28.7 | 119.4 ± 30.1 | 122.6 ± 30.9 | 131.5 ± 31.8 | < 0.01 |
| Family History of MI | 343 (47.4 %) | 311 (42.8 %) | 346 (46.7 %) | 349 (48.0 %) | 380 (51.1 %) | 0.03 |
| Body mass index (kg/m2) | 28.2 ± 5.5 | 28.4 ± 5.6 | 28.2 ± 5.4 | 28.3 ± 5.4 | 27.9 ± 5.5 | 0.13 |
| CAC score (AU) | 0 [0 – 81] | 116 [0 – 66] | 0 [0 – 86] | 0 [0 – 65] | 0 [0 – 74] | 0.86 |
| CAC score categories | ||||||
| CAC = 0 | 473 (52.7 %) | 477 (52.8 %) | 475 (52.5 %) | 475 (52.5 %) | 477 (52.9 %) | 0.96 |
| CAC 1-99 | 217 (24.2 %) | 245 (27.1 %) | 216 (23.9 %) | 250 (27.6 %) | 219 (24.3 %) | 0.86 |
| CAC ≥ 100 | 208 (23.2 %) | 182 (20.1 %) | 213 (23.6 %) | 180 (19.9 %) | 205 (22.8 %) | 0.81 |
Values presented are number (proportion) and mean ± standard deviation for normally distributed variables or median [25th-75th percentile] for non-normally distributed variables. All continuous variables are described as mean ± standard deviation, except lipoprotein(a), triglycerides, and CAC score. Divide total cholesterol, HDL-C, and LDL-C by 38.67 and triglycerides by 88.57 to convert mg/dL to mmol/L. Abbreviations: CAC = coronary artery calcium, BP = blood pressure, HDL = high-density lipoprotein, LDL = low-density lipoprotein, MI = myocardial infarction.
Table 2:
Baseline characteristics of Multi-Ethnic Study of Atherosclerosis participants stratified by coronary artery calcium score
| Characteristics | All Participants (N=4512) |
CAC = 0 (N=2377) |
CAC 1-99 (N=1147) |
CAC ≥ 100 (N=988) |
p-value |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 61.9 ± 10.4 | 57.9 ± 9.3 | 64.1 ± 9.8 | 69.2 ± 8.7 | < 0.01 |
| Men | 2145 (47.5 %) | 891 (37.5 %) | 612 (53.4 %) | 642 (65.0 %) | < 0.01 |
| Race | <0.01 | ||||
| White | 1660 (36.8 %) | 770 (32.4 %) | 421 (36.7 %) | 469 (47.5 %) | < 0.01 |
| Black | 1323 (29.3 %) | 785 (33.0 %) | 314 (27.4 %) | 224 (22.7 %) | < 0.01 |
| Hispanic | 1002 (22.2 %) | 565 (23.8 %) | 256 (22.3 %) | 181 (18.3 %) | < 0.01 |
| Chinese | 527 (11.7 %) | 257 (10.8 %) | 156 (13.6 %) | 114 (11.5 %) | 0.27 |
| Systolic BP (mm Hg) | 126.2 ± 21.4 | 122.0 ± 20.4 | 128.9 ± 21.7 | 133.0 ± 21.2 | < 0.01 |
| Diastolic BP (mm Hg) | 72.0 ± 10.3 | 71.3 ± 10.4 | 72.8 ± 10.3 | 72.9 ± 10 | < 0.01 |
| Antihypertensive use | 1485 (32.9 %) | 612 (25.8 %) | 418 (36.4 %) | 455 (46.1 %) | < 0.01 |
| Diabetes | 429 (9.5 %) | 177 (7.5 %) | 110 (9.6 %) | 142 (14.4 %) | < 0.01 |
| Smoking | 579 (12.8 %) | 300 (12.6 %) | 158 (13.8 %) | 121 (12.3 %) | 0.97 |
| Total cholesterol (mg/dL) | 195.8 ± 34.7 | 194.5 ± 34.3 | 197.0 ± 34.2 | 197.4 ± 35.9 | 0.03 |
| HDL-cholesterol (mg/dL) | 51.3 ± 15.1 | 52.7 ± 15.2 | 49.7 ± 14.5 | 49.8 ± 15.3 | < 0.01 |
| Triglycerides (mg/dL) | 107.0 [75.5 – 156.0] | 102.0 [72.0 – 151.0] | 113.0 [82.0 – 161.0] | 111.5 [77.0 – 160.5] | < 0.01 |
| LDL-cholesterol (mg/dL) | 119.7 ± 31.4 | 117.8 ± 30.7 | 121.7 ± 31.8 | 122.1 ± 32.1 | < 0.01 |
| Family History of MI | 1729 (47.2 %) | 815 (42.2 %) | 456 (49.7 %) | 458 (56.3 %) | < 0.01 |
| Body mass index (kg/m2) | 28.2 ± 5.5 | 28.3 ± 5.7 | 28.1 ± 5.4 | 28.1 ± 5.1 | 0.91 |
| Lipoprotein(a) (mg/dL) | 18.2 [8.2 – 40.5] | 19.1 [8.4 – 41.5] | 18.0 [8.4 – 38.5] | 16.2 [7.3 – 39.4] | 0.02 |
Values presented are number (proportion) and mean ± standard deviation for normally distributed variables or median [25th-75th percentile] for non-normally distributed variables. All continuous variables are described as mean ± standard deviation, except lipoprotein(a) and triglycerides. Divide total cholesterol, HDL-C, and LDL-C by 38.67 and triglycerides by 88.57 to convert mg/dL to mmol/L. Abbreviations as in Table 1.
As expected, the burden of traditional cardiovascular risk factors and proportion of White participants increased across CAC score categories (Table 2). Median Lp(a) levels decreased across increasing CAC score categories, which might be related to differences in race composition of each CAC group (Table 2). Baseline characteristics of DHS participants are described in Supplemental Tables 1 and 2. Mean age of DHS participants was 44.5 ± 9.1 years, 56.2% were women, 34.4 % White, 48.3 % Black, and 17.3 % Hispanic.
Independent Association of Lp(a) and CAC with ASCVD
A total of 476 incident ASCVD events were observed among MESA participants (267 CHD and 209 stroke events) and 98 ASCVD events were observed among DHS participants (54 CHD and 44 stroke events) during follow-up. Continuous Lp(a) level (hazard ratio 1.13, 95% confidence interval 1.03, 1.23; and 1.34, 95% CI 1.09, 1.65) and CAC score (HR 1.19, 95% CI 1.14, 1.24; and 1.31, 95% CI 1.19, 1.45) were independently associated with ASCVD events among both MESA and DHS participants, respectively (Supplemental Table 3, Models 1 and 2). Importantly, these independent associations remained significant in both cohorts when Lp(a), CAC, and traditional risk factors were analyzed together in Cox models (Supplemental Table 3, Model 3). Multiplicative interaction between Lp(a) and CAC was not significant in either cohort (p = 0.99 in MESA and p=0.61 in DHS).
In categorical analyses, elevated Lp(a) level (quartile 5 vs. quartiles 1-4) (HR 1.29, 95% CI 1.04, 1.61) and CAC score categories 1-99 (HR 1.68, 95% CI 1.30, 2.16) and ≥100 (HR 2.66, 95% CI 2.07, 3.43) were independently associated with ASCVD events among MESA participants (Table 3). Elevated Lp(a) had a nominal association (HR 1.54, 95% CI 0.96, 2.46) with ASCVD events among DHS participants, perhaps related to smaller sample size (Table 3). CAC score categories 1-99 and ≥100 (HR 3.32, 95% CI 1.74, 6.33; and 5.21, 95% CI 2.48, 10.96, respectively) had an independent association with ASCVD risk in DHS (Table 3). Multiplicative interaction between elevated Lp(a) and CAC categories was not observed in either cohort (Table 3). In sensitivity analyses, the association of elevated Lp(a) and CAC score categories with ASCVD was largely unchanged when family history of MI was added to Cox models (Supplemental Table 4). When examining incident CHD and stroke events separately in both cohorts, elevated Lp(a) level and CAC score categories had a stronger association with CHD (Supplemental Table 5), than with stroke (Supplemental Table 6) risk.
Table 3:
Independent association of elevated Lp(a) and CAC score categories with incident ASCVD among MESA and DHS participants
| MESA* | DHS¶ | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Lp(a) Quintile 5 | 1.29 (1.04 – 1.61) | 0.02 | 1.54 (0.96 – 2.46) | 0.07 |
| Lp(a) Quintile 1-4 | Referent | Referent | ||
| CAC ≥ 100 | 2.66 (2.07 – 3.43) | < 0.01 | 5.21 (2.48 – 10.96) | < 0.01 |
| CAC 1-99 | 1.68 (1.30 – 2.16) | < 0.01 | 3.32 (1.74 – 6.33) | < 0.01 |
| CAC = 0 | Referent | Referent | ||
Cox proportional hazards regression models adjusted for age, sex, race, diabetes, smoking, systolic blood pressure, antihypertensive use, total cholesterol, HDL-cholesterol, triglycerides, body mass index, lipoprotein(a), and CAC score.
CAC ≥100 x Elevated Lp(a) and CAC 1-99 x Elevated Lp(a) p-interaction for ASCVD in MESA = 0.20 and 0.59, respectively.
CAC ≥100 x Elevated Lp(a) and CAC 1-99 x Elevated Lp(a) p-interaction for ASCVD in DHS = 0.25 and 0.93, respectively.
Abbreviations: Lp(a) = lipoprotein(a), HR = hazard ratio, CI = confidence interval, rest of the abbreviations as in Table 1.
Joint Association of Lp(a) and CAC with ASCVD
The 10-year cumulative incidence of ASCVD events among MESA participants stratified by Lp(a) categories (elevated [quintile 5] versus non-elevated [quintile 1-4]) and CAC score categories (0, 1-99, and ≥100) is shown in Figure 1. The highest 10-year ASCVD incidence was observed in the elevated Lp(a) and CAC ≥ 100 group (22.0%, 95% CI 15.9%, 28.0%), while the lowest incidence was observed in the non-elevated Lp(a) and CAC=0 group (2.8%, 95% CI 2.0%, 3.6%). The corresponding Kaplan-Meier survival curve for MESA participants is depicted in Figure 2. Of note, ASCVD incidence among participants with CAC score 0 and 1-99 was similar regardless of elevated or non-elevated Lp(a) level (Figures 1 and 2). The 10-year cumulative incidence of ASCVD events among DHS participants stratified by Lp(a) and CAC categories is shown in Supplemental Figure 2.
Figure 1. Ten-year ASCVD Incidence across lipoprotein(a) (quintiles 1-4, quintile 5) and CAC (0, 1-99, ≥100) groups.
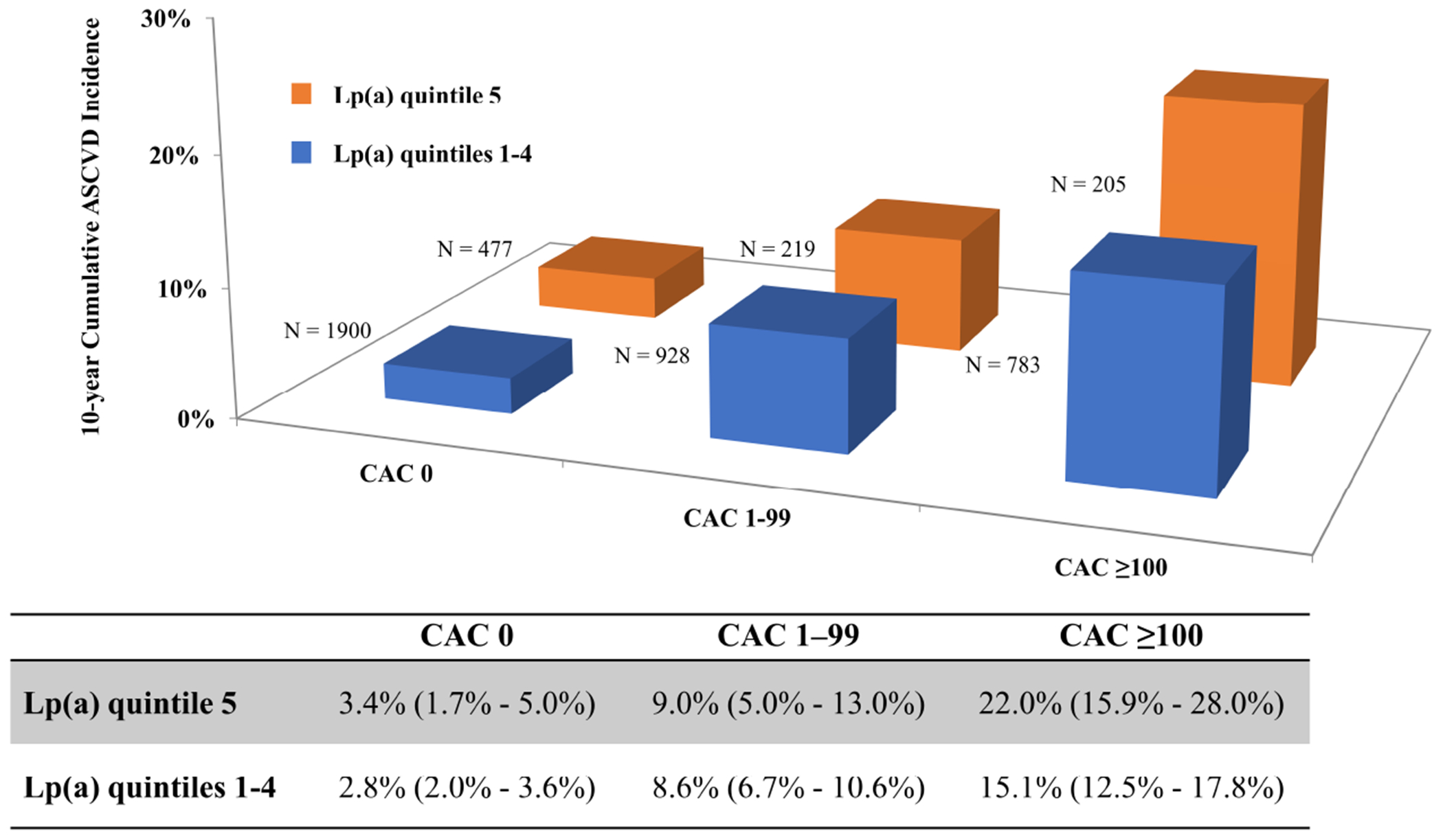
The highest 10-year atherosclerotic cardiovascular disease (ASCVD) incidence among MESA participants was seen in the lipoprotein(a) [Lp(a)] quintile 5 with coronary artery calcium (CAC) ≥ 100 group, while the lowest 10-year ASCVD incidence was evidenced in the Lp(a) quintiles 1-4 with CAC 0 group. A higher 10-year ASCVD incidence was apparent in the Lp(a) quintile 5 group when compared to Lp(a) quintiles 1-4 group only among participants with CAC ≥ 100.
Figure 2. Cumulative ASCVD Incidence across lipoprotein(a) (quintiles 1-4, quintile 5) and CAC (0, 1-99, ≥100) groups.
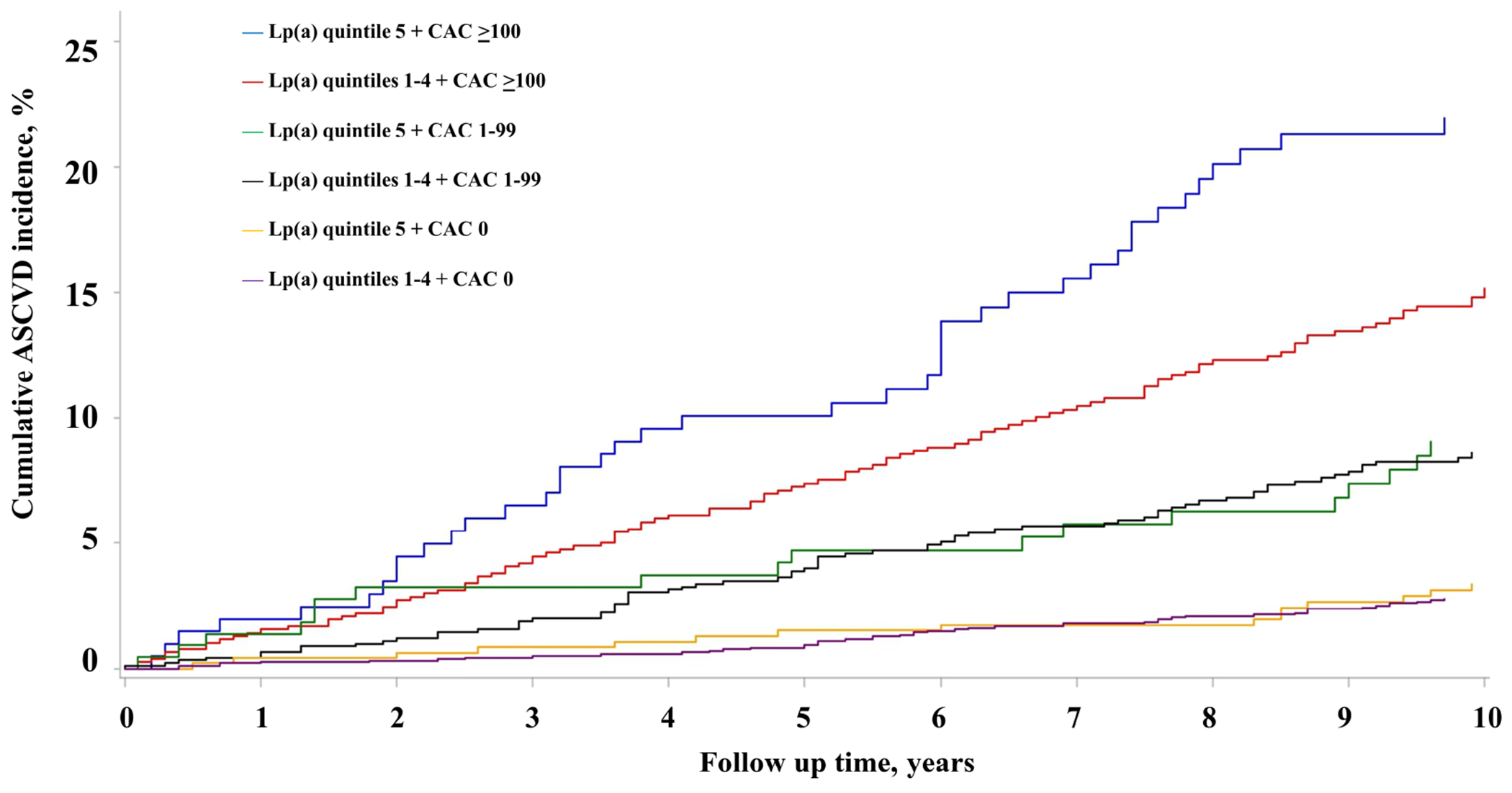
The Kaplan-Meier survival curves for MESA participants illustrates the highest ASCVD incidence in the Lp(a) quintile 5 with CAC ≥ 100 group. Among participants with CAC 0 and 1-99 no difference in ASCVD risk was seen between Lp(a) quintile 5 versus Lp(a) quintiles 1-4 group. Abbreviations as in Figure 1.
The joint association of Lp(a) and CAC score categories with ASCVD events in MESA is described in Table 4. Participants in the elevated Lp(a) and CAC ≥ 100 had a nearly 5-fold increased ASCVD risk as compared to those with non-elevated Lp(a) and CAC=0. Notably, ASCVD hazard was similar across elevated and non-elevated Lp(a) among participants with CAC=0 and CAC score 1-99. Similar findings were observed among DHS participants as described in Supplemental Table 7.
Table 4:
Joint association of elevated Lp(a) and CAC score with incident ASCVD among MESA participants
| HR (95% CI) | p-value | |
|---|---|---|
| Lp(a) quintile 5 and CAC ≥ 100 | 4.71 (3.01 – 7.40) | <0.01 |
| Lp(a) quintiles 1-4 and CAC ≥ 100 | 2.99 (2.06 – 4.33) | <0.01 |
| Lp(a) quintile 5 and CAC 1-99 | 2.35 (1.36 – 4.08) | <0.01 |
| Lp(a) quintiles 1-4 and CAC 1-99 | 2.17 (1.49 – 3.16) | <0.01 |
| Lp(a) quintile 5 and CAC = 0 | 1.31 (0.73 – 2.35) | 0.36 |
| Lp(a) quintiles 1-4 and CAC = 0 | Referent |
Cox proportional hazards regression models adjusted for age, sex, race, diabetes, smoking, systolic blood pressure, antihypertensive use, total cholesterol, HDL-cholesterol, triglycerides, and body mass index. Abbreviations as in Table 3.
Among MESA participants stratified by guideline-recommended Lp(a) and CAC score thresholds (50 mg/dL and 100 AU, respectively), the highest ASCVD incidence was observed among participants with Lp(a) ≥50 mg/dL and CAC ≥100 (Figure 3). Among participants with CAC score <100, ASCVD incidence was similar across Lp(a) categories (Figure 3). The corresponding 10-year cumulative incidence across Lp(a) and CAC score categories is described in Table 5. The highest 10-year ASCVD incidence was observed in the Lp(a) ≥50 mg/dL and CAC ≥100 group (23.2%, 95% CI 17.0%, 29.5%), while the lowest incidence was observed in Lp(a) <50 mg/dL and CAC <100 group (4.6%, 95% CI 3.8%, 5.4%).
Figure 3. Cumulative ASCVD Incidence across lipoprotein(a) (< 50 mg/dL, ≥ 50 mg/dL) and CAC (<100 AU, ≥100 AU) groups.
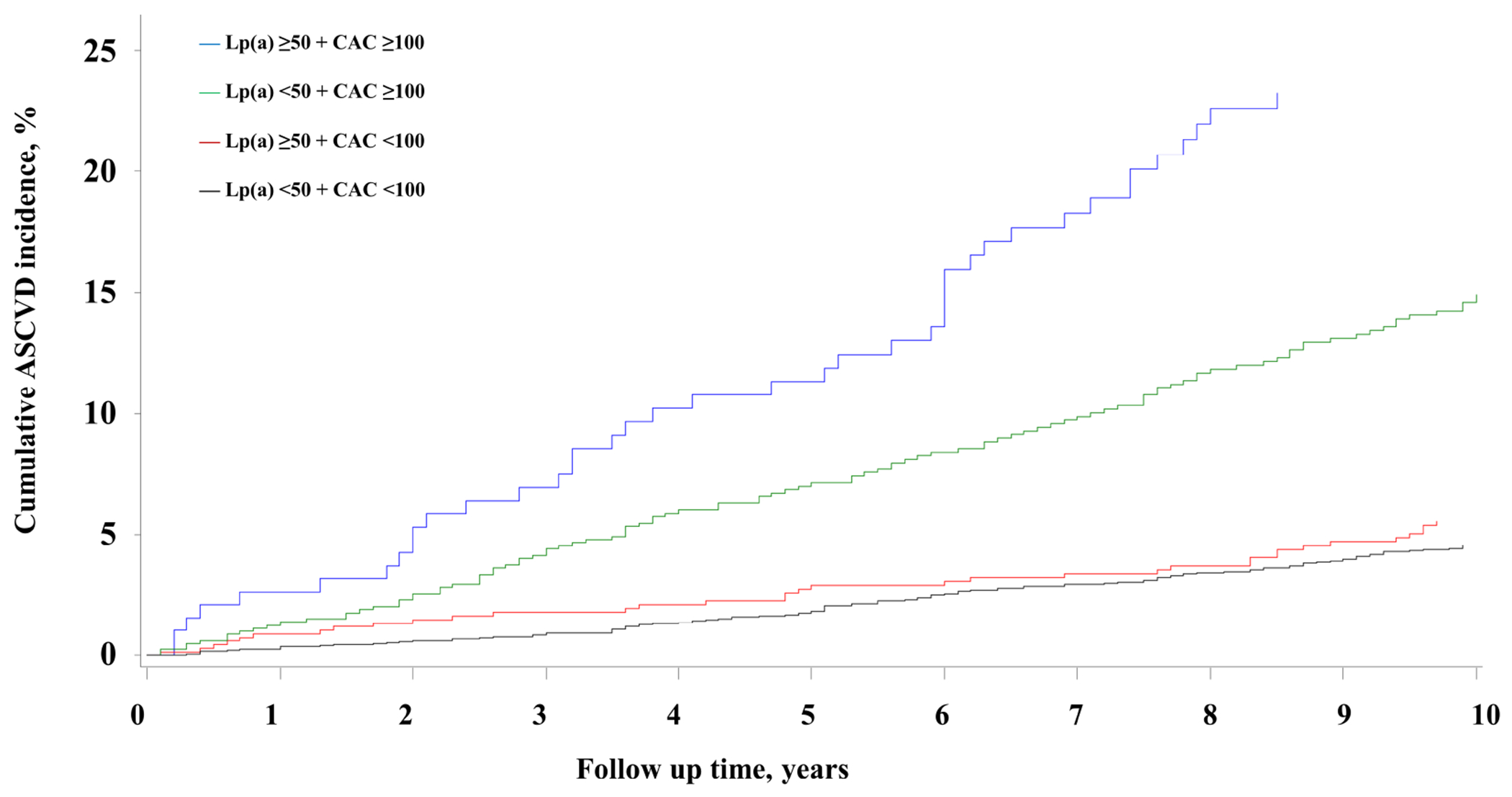
The Kaplan-Meier survival curves for MESA participants illustrates the highest ASCVD incidence in the Lp(a) ≥ 50 mg/dL with CAC ≥ 100 group. For participants with CAC score < 100 AU no difference in cumulative ASCVD incidence was seen between the Lp(a) ≥ 50 mg/dL and < 50 mg/dL groups. Abbreviations as in Figure 1.
Table 5:
Joint association of Lp(a) ≥50 mg/dL and CAC score ≥100 AU with incident ASCVD among MESA participants
| 10-year Cumulative Incidence | HR (95% CI) | p-value | |
|---|---|---|---|
| Lp(a) ≥ 50 mg/dL and CAC ≥ 100 (N=190) | 23.24% [16.97% – 29.51%] | 3.20 (2.19 – 4.69) | <0.01 |
| Lp(a) < 50 mg/dL and CAC ≥ 100 (N=793) | 14.91% [12.26% – 17.56%] | 1.88 (1.42 – 2.50) | <0.01 |
| Lp(a) ≥ 50 mg/dL and CAC < 100 (N=676) | 5.58% [3.78% – 7.38%] | 1.25 (0.85 – 1.85) | 0.26 |
| Lp(a) < 50 mg/dL and CAC < 100 (N=2833) | 4.55% [3.75% – 5.35%] | Referent |
Cox proportional hazards regression models adjusted for age, sex, race, diabetes, smoking, systolic blood pressure, antihypertensive use, total cholesterol, HDL-cholesterol, triglycerides, and body mass index. Abbreviations as in Table 3. CAC ≥100 x Lp(a) ≥50 mg/dl p-interaction for ASCVD = 0.25.
In multivariable-adjusted Cox regression analysis, the multiplicative interaction between Lp(a) and CAC was non-significant (p=0.25) and participants with Lp(a) ≥50 mg/dL and CAC ≥100 were at a 3-fold increased risk of ASCVD during follow-up as compared with those with Lp(a) <50 mg/dL and CAC <100 (referent group). Participants with Lp(a) <50 mg/dL and CAC ≥100 were at a 1.9-fold increased ASCVD risk, while those with Lp(a) ≥50 mg/dL and CAC <100 experienced a similar risk of ASCVD as compared with the referent group (Table 5). Similar results were observed in sensitivity analysis when participants were stratified using Lp(a) threshold 50 mg/dL and CAC score threshold of 100 AU and/or 75th percentile for age/sex/race (Supplemental Table 8 and Supplemental Figure 3).
DISCUSSION
We report several important findings in this study evaluating the independent and joint associations of Lp(a) and CAC score with ASCVD risk among participants of two multi-ethnic American epidemiologic cohorts free of clinical cardiovascular disease at baseline. First, elevated Lp(a) level and CAC score were independently associated with incident ASCVD, after adjusting for traditional risk factors and each other. Second, participants with both elevated Lp(a) and CAC score were at significantly higher ACVD risk compared with those having neither risk marker elevated. Third, elevated Lp(a) level was associated with higher ASCVD risk among individuals with CAC score ≥100, whereas among individuals with CAC <100, ASCVD risk was similar with elevated or non-elevated Lp(a) level. Fourth, individuals with CAC score of zero were at low 10-year ASCVD risk even in the setting of an elevated Lp(a) level.
Lp(a) and CAC are independently associated with cardiovascular risk
Several studies demonstrating an independent association between elevated Lp(a) level and incident cardiovascular disease have been previously published.(2–4) Similarly, the robust independent association of CAC score with cardiovascular risk is also well established.(25–28) A recent study focusing on a smaller subgroup of MESA participants evaluated the utility of CAC score to guide statin therapy allocation according to risk enhancing factors including Lp(a).(29) However, the independent and joint association with ASCVD risk has not been systematically evaluated to date.
In our report, we were able to redemonstrate that the associations of elevated Lp(a) level and CAC score with ASCVD risk are independent of traditional cardiovascular risk factors including family history of MI. A novel finding of our report is that the association of these two risk markers with cardiovascular risk is independent of each other. Importantly, we validated this finding in two distinct multi-ethnic US cohorts. Furthermore, when examining ASCVD subcomponents, CHD and stroke separately, we observed that elevated Lp(a) and CAC have a stronger association with CHD than with stroke risk. These findings are consistent with prior studies that have studied Lp(a) and CAC score individually.(9,30)
The pathophysiologic mechanisms underlying these findings merit consideration. Circulating Lp(a) levels are >90% genetically determined and most children achieve adult levels by 5 years age.(20) Additionally, Lp(a) levels remain fairly stable across the lifespan in the absence of targeted therapeutic interventions.(1) Similar to other cardiovascular risk factors, elevated Lp(a) exerts its effects over time and is thought to mediate ASCVD events through multiple mechanisms including atherogenesis, inflammation, and thrombosis. Lp(a) has the atherogenic properties related to its LDL-like composition but also possesses unique characteristics secondary to its apolipoprotein(a) component.(31) Oxidized phospholipids bound to apolipoprotein(a) promote inflammation and endothelial dysfunction, which play a key role in initiating and propagating atherosclerosis.(32) Apolipoprotein(a) is also thought to be prothrombotic given its structural homology with plasminogen.(31) CAC score on the other hand captures the burden of coronary atherosclerosis that has developed over the lifespan in response to continued exposure to measured and unmeasured atherogenic cardiovascular risk factors.(33) CAC score increases over the lifespan and closely mirrors the natural history of coronary atherosclerosis.(34)
Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score. This is perhaps related to the unique pathways of inflammation and thrombosis that are triggered by elevated Lp(a) and oxidized phospholipid levels. These findings highlight the potential limitation of CAC score for capturing the totality of ASCVD risk in asymptomatic individuals. Widespread Lp(a) testing has been limited in the past due to lack of standardized measurement assays, isoform variation in association with cardiovascular risk, lack of a specific treatment target, and variable reimbursement because of lack of a diagnostic code. These limitations are being gradually addressed and our study findings regarding the joint association of elevated Lp(a) and high CAC with ASCVD risk as discussed below provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process.
Joint Association of Lp(a) and CAC with cardiovascular risk
In addition to studying the independent association of Lp(a) and CAC with ASCVD risk we have also systematically evaluated the joint association of Lp(a) level and CAC score. Given the well-established racial differences in Lp(a) levels, we considered race-specific Lp(a) in our analyses in addition to the guideline-recommended Lp(a) and CAC thresholds. Our results demonstrate an additive joint association between the two risk markers, such that participants with elevated Lp(a) level and CAC score were at a greater risk for incident ASCVD risk when compared to either risk factor elevation alone, providing important prognostic information. This observation was consistent irrespective of whether a race-specific (quintile 5 versus quintiles 1-4) or guideline-recommended cut-off (50 mg/dL) was used to define an elevated Lp(a) level. It is also important to highlight that elevated Lp(a) level did not stratify cardiovascular risk among MESA and DHS participants with zero or low CAC score. We observed that elevated Lp(a) level helped identify individuals at a much higher risk among participants with a significantly elevated CAC score (≥100 AU). This finding has important clinical implications as discussed below.
Clinical Implications
Our study is highly relevant in the context of current guideline recommendations for primary ASCVD prevention and emerging targeted Lp(a)-lowering therapies for ASCVD risk reduction.(6,35) The AHA/ACC/Multisociety Cholesterol Management Guideline recommends using elevated Lp(a) level (≥50 mg/dL or >125 nmol/L) as a risk-enhancing factor and considering CAC score (≥100 or ≥75th percentile for age/sex/race) as a validated subclinical measure of atherosclerosis to guide primary prevention therapy decisions including statin initiation among individuals at borderline or intermediate risk (5-20% 10-year ASCVD risk).(6) However, a recommendation for interpreting these two risk factors together is lacking.
In this context, our findings demonstrate that asymptomatic individuals with concomitant Lp(a) and CAC elevation (≥50 mg/dL and ≥100 Agatston Units, respectively) have a greater than 20% cumulative ASCVD incidence over 10 years, which approaches the rate of events in secondary prevention populations. Elevations of both risk markers together should prompt initiation of high-intensity statin therapy in addition to aggressive lifestyle modification to help reduce ASCVD risk. Aspirin therapy for primary prevention may also be considered in these individuals.(36) However, since statins, lifestyle interventions, and aspirin do not lower Lp(a) significantly, this subgroup of individuals might serve as a good target population for randomized controlled trials in primary prevention,(37) including studies to evaluate the effect of emerging Lp(a) targeted therapies that are currently being studied in the secondary prevention setting.(35)
Our findings also lend support to the guideline-recommended role of CAC score as a robust decision-making aid in primary prevention. Among individuals with CAC=0 or score below the guideline-recommended threshold, we observed that elevated Lp(a) level failed to stratify 10-year ASCVD risk. Notably, half of participants with an elevated Lp(a) had CAC=0. These individuals were at a low 10-year risk of ASCVD regardless of Lp(a) level and this observation can be very helpful in guiding the shared-decision making process for preventive treatment decisions and ‘de-risking’ those with an elevated Lp(a) level. It is important to note that our findings do not preclude the role of measuring Lp(a) in individuals with CAC=0. Our observations are limited to a 10-year ASCVD risk framework and it possible that elevated Lp(a) stratifies long-term or lifetime ASCVD risk in young individuals,(38) who have a high likelihood of having CAC=0. Additionally, previous studies have shown that incident CAC (development of CAC in those with CAC=0) is associated with ASCVD risk,(39,40) and Lp(a)≥50 mg/dL has a nominal association with CAC incidence.(41) Thus, individuals with CAC=0 and Lp(a)>50 mg/dL might be good candidates for repeat CAC scanning,(42) but the association of this relationship with long-term incident ASCVD risk remains to be studied.
Strengths and Limitations
Our study has several notable strengths. We have studied participants of two large, contemporary, multi-ethnic, population-based US cohorts to address a key knowledge gap. Both MESA and DHS participants underwent phenotyping for CAC score measurement, traditional cardiovascular risk factors, and had Lp(a) levels measured using separate apolipoprotein(a)-insensitive assays. We were able to demonstrate and replicate our key findings regarding the independent and joint association of Lp(a) and CAC score with ASCVD risk in MESA and DHS, respectively.
However, the findings presented in this study should be considered in the setting of its limitations. First, our study did not account for prospective changes in CAC, risk factors, and cardiovascular risk reduction therapies including statin therapy. Second, although this study was conducted in two American multi-ethnic contemporary population-based cohorts who were free of prevalent ASCVD at baseline, our results may not be applicable to ethnic groups not represented. Third, our sample size was limited to participants with measured Lp(a) and CAC, which decreased the statistical power of analyses performed in the DHS cohort. Finally, the potential for residual confounding cannot be excluded given the observational nature of this study.
CONCLUSION
Lp(a) and CAC score were independently associated with ASCVD risk among MESA and DHS participants free of clinical ASCVD at baseline. The presence of elevated levels of both markers identified a subgroup at a significantly increased ASCVD risk (Central Illustration). These individuals may benefit more so from aggressive ASCVD risk reduction strategies. In contrast, in those with CAC=0 or low score, elevated Lp(a) levels did not further stratify ASCVD risk (Central Illustration).
Central Illustration. Joint association of Lp(a) and CAC score with ASCVD risk.
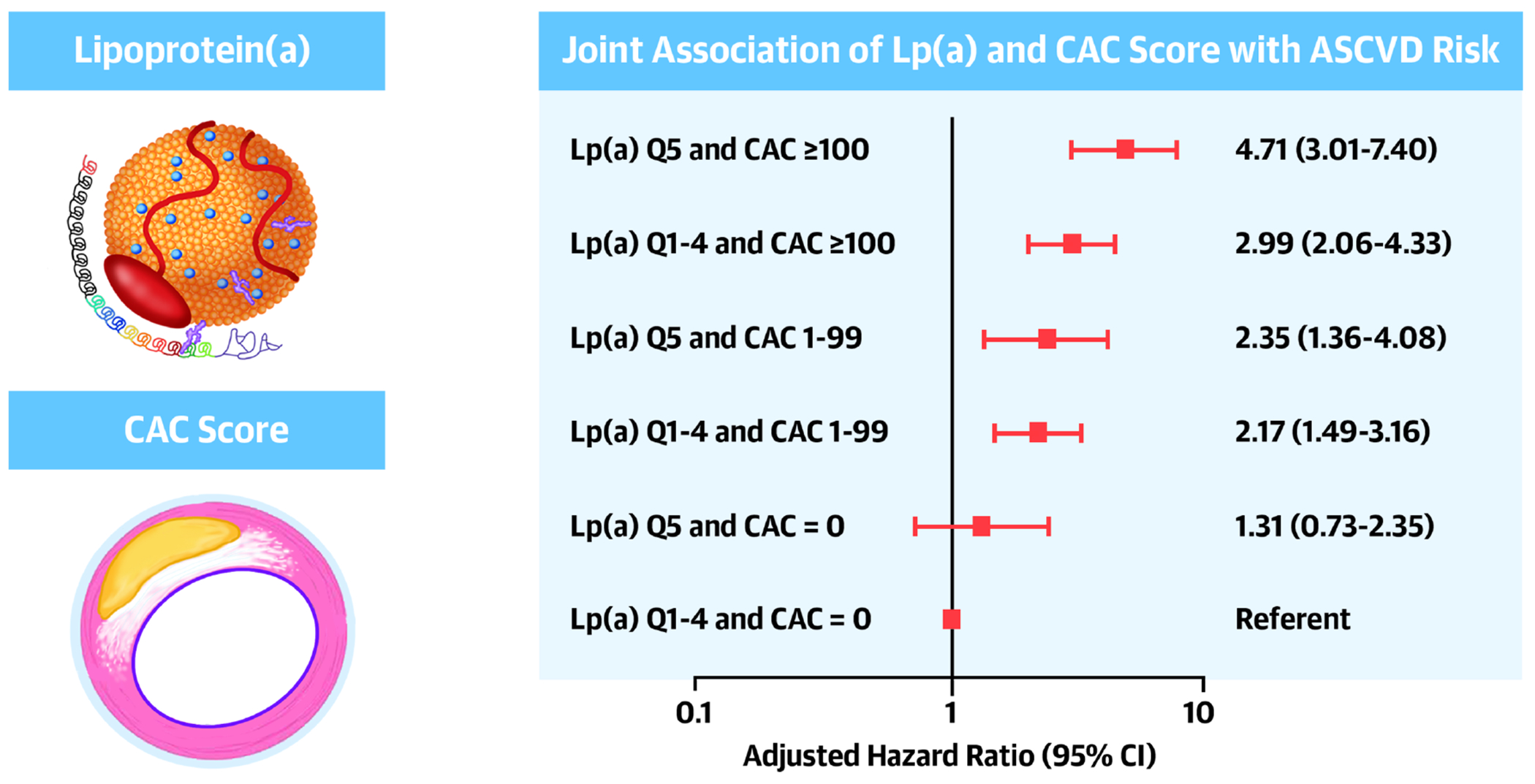
Individuals with elevated Lp(a) and CAC score are the highest ASCVD risk. Elevated Lp(a) level does not stratify ASCVD risk further among those with low CAC score.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care: Elevated plasma concentration of lipoprotein (a) [Lp(a)] and coronary artery calcium (CAC) scores are independently associated with atherosclerotic risk. Patients with both are at highest risk, but Lp(a) level does not further stratify risk among those with a low CAC score.
Translational Outlook: Further research is needed to define algorithms that employ these quantitative assays optimally for cardiovascular risk stratification in appropriate cases.
Acknowledgments:
The authors thank the other investigators, the staff, and the participants of the MESA and DHS for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
The MESA study is supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The DHS was funded by the Donald W. Reynolds Foundation, Las Vegas, Nevada, and partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105.
Conflicts of interest:
Dr. Virani reports grant support from Department of Veterans Affairs, World Heart Federation, Tahir and Jooma Family, and honorarium from American College of Cardiology (Associate Editor for Innovations, acc.org). PHJ reports grant support from the AHA, NASA, Novo Nordisk, consulting income from Bayer and Regeneron, equity in G3 Therapeutics and served as a site investigator with all funds to the institution from GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. None of the other authors have any disclosures.
ABBREVIATIONS
- ASCVD
Atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CHD
Coronary heart disease
- DHS
Dallas Heart Study
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
Lipoprotein (a)
- MESA
Multi-ethnic Study of Atherosclerosis
- MI
myocardial infarction
- PCSK9
proprotein convertase subtilisin/kexin type 9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors C, Erqou S, Kaptoge S et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol 2013;61:1146–56. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Peden JF, Hopewell JC et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–28. [DOI] [PubMed] [Google Scholar]
- 5.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta A, Pandey A, Ayers CR et al. Predictive Value of Coronary Artery Calcium Score Categories for Coronary Events Versus Strokes: Impact of Sex and Race: MESA and DHS. Circ Cardiovasc Imaging 2020;13:e010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pechlivanis S, Mahabadi AA, Hoffmann P et al. Association between lipoprotein(a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification. BMC Med Genet 2020;21:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso R, Mata P, Muniz O et al. PCSK9 and lipoprotein (a) levels are two predictors of coronary artery calcification in asymptomatic patients with familial hypercholesterolemia. Atherosclerosis 2016;254:249–253. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Lehmann N, Churzidse S et al. Gender-specific association of coronary artery calcium and lipoprotein parameters: the Heinz Nixdorf Recall Study. Atherosclerosis 2013;229:531–40. [DOI] [PubMed] [Google Scholar]
- 13.Greif M, Arnoldt T, von Ziegler F et al. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med 2013;24:75–9. [DOI] [PubMed] [Google Scholar]
- 14.Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas Heart Study. Circulation 2005;111:1471–9. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Steffen BT, Budoff M et al. Lipoprotein(a) Levels Are Associated With Subclinical Calcific Aortic Valve Disease in White and Black Individuals: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2016;36:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 17.Victor RG, Haley RW, Willett DL et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 18.Guan W, Cao J, Steffen BT et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DP, Jacobson TA, Jones PH et al. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–392. [DOI] [PubMed] [Google Scholar]
- 21.Paixao AR, Ayers CR, El Sabbagh A et al. Coronary Artery Calcium Improves Risk Classification in Younger Populations. JACC Cardiovasc Imaging 2015;8:1285–93. [DOI] [PubMed] [Google Scholar]
- 22.Goff DC, Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 23.Virani SS, Brautbar A, Davis BC et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012;125:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta A, Virani SS, Ayers CR et al. Lipoprotein(a) and Family History Predict Cardiovascular Disease Risk. J Am Coll Cardiol 2020;76:781–793. [DOI] [PubMed] [Google Scholar]
- 25.Polonsky TS, McClelland RL, Jorgensen NW et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010;303:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erbel R, Mohlenkamp S, Moebus S et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–406. [DOI] [PubMed] [Google Scholar]
- 27.Baber U, Mehran R, Sartori S et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol 2015;65:1065–74. [DOI] [PubMed] [Google Scholar]
- 28.Elias-Smale SE, Proenca RV, Koller MT et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol 2010;56:1407–14. [DOI] [PubMed] [Google Scholar]
- 29.Patel J, Pallazola VA, Dudum R et al. Assessment of Coronary Artery Calcium Scoring to Guide Statin Therapy Allocation According to Risk-Enhancing Factors: The Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langsted A, Nordestgaard BG, Kamstrup PR. Elevated Lipoprotein(a) and Risk of Ischemic Stroke. J Am Coll Cardiol 2019;74:54–66. [DOI] [PubMed] [Google Scholar]
- 31.Mehta A, Shapiro MD. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol 2021. [DOI] [PubMed] [Google Scholar]
- 32.Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol 2019;16:305–318. [DOI] [PubMed] [Google Scholar]
- 33.Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract 2008;62:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Braun LT, Ndumele CE et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation 2019;139:e1162–e1177. [DOI] [PubMed] [Google Scholar]
- 35.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 36.Cainzos-Achirica M, Miedema MD, McEvoy JW et al. Coronary Artery Calcium for Personalized Allocation of Aspirin in Primary Prevention of Cardiovascular Disease in 2019: The MESA Study (Multi-Ethnic Study of Atherosclerosis). Circulation 2020;141:1541–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cainzos-Achirica M, Bittencourt MS, Osei AD et al. Coronary Artery Calcium to Improve the Efficiency of Randomized Controlled Trials in Primary Cardiovascular Prevention. JACC Cardiovasc Imaging 2020. [DOI] [PubMed] [Google Scholar]
- 38.Rossello X. Lifetime Risk Estimation in Atherosclerotic Cardiovascular Disease: Where Inflammation Meets Lipoprotein(a). J Am Coll Cardiol 2021;78:1095–1096. [DOI] [PubMed] [Google Scholar]
- 39.Budoff MJ, Young R, Lopez VA et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann N, Erbel R, Mahabadi AA et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events: Result of the HNR Study (Heinz Nixdorf Recall). Circulation 2018;137:665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2021;15:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzaye O, Dardari ZA, Cainzos-Achirica M et al. Warranty Period of a Calcium Score of Zero: Comprehensive Analysis From MESA. JACC Cardiovasc Imaging 2021;14:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


