Abstract
Background
Cardiovascular diseases (CVDs) are the leading cause of death globally, accounting for almost 18 million deaths annually. People with CVDs have a five times greater chance of suffering a recurrent cardiovascular event than people without known CVDs. Although drug interventions have been shown to be cost‐effective in reducing the risk of recurrent cardiovascular events, adherence to medication remains suboptimal. As a scalable and cost‐effective approach, mobile phone text messaging presents an opportunity to convey health information, deliver electronic reminders, and encourage behaviour change. However, it is uncertain whether text messaging can improve medication adherence and clinical outcomes. This is an update of a Cochrane review published in 2017.
Objectives
To evaluate the benefits and harms of mobile phone text messaging for improving medication adherence in people with CVDs compared to usual care.
Search methods
We searched CENTRAL, MEDLINE, Embase, four other databases, and two trial registers. We also checked the reference lists of all primary included studies and relevant systematic reviews and meta‐analyses. The date of the latest search was 30 August 2023.
Selection criteria
We included randomised controlled trials (RCTs) with participants with established arterial occlusive events. We included trials investigating interventions using short message service (SMS) or multimedia messaging service (MMS) with the aim of improving adherence to medication for the secondary prevention of cardiovascular events. The comparator was usual care. We excluded cluster‐RCTs and quasi‐RCTs.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were medication adherence, fatal cardiovascular events, non‐fatal cardiovascular events, and combined CVD event. Secondary outcomes were low‐density lipoprotein cholesterol for the effect of statins, blood pressure for antihypertensive drugs, heart rate for the effect of beta‐blockers, urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin, adverse effects, and patient‐reported experience. We used GRADE to assess the certainty of the evidence for each outcome.
Main results
We included 18 RCTs involving a total of 8136 participants with CVDs. We identified 11 new studies in the review update and seven studies in the previous version of the review. Participants had various CVDs including acute coronary syndrome, coronary heart disease, stroke, myocardial infarction, and angina. All studies were conducted in middle‐ and high‐income countries, with no studies conducted in low‐income countries. The mean age of participants was 53 to 64 years. Participants were recruited from hospitals or cardiac rehabilitation facilities. Follow‐up ranged from one to 12 months. There was variation in the characteristics of text messages amongst studies (e.g. delivery method, frequency, theoretical grounding, content used, personalisation, and directionality). The content of text messages varied across studies, but generally included medication reminders and healthy lifestyle information such as diet, physical activity, and weight loss. Text messages offered advice, motivation, social support, and health education to promote behaviour changes and regular medication‐taking.
We assessed risk of bias for all studies as high, as all studies had at least one domain at unclear or high risk of bias.
Medication adherence
Due to different evaluation score systems and inconsistent definitions applied for the measurement of medication adherence, we did not conduct meta‐analysis for medication adherence. Ten out of 18 studies showed a beneficial effect of mobile phone text messaging for medication adherence compared to usual care, whereas the other eight studies showed either a reduction or no difference in medication adherence with text messaging compared to usual care. Overall, the evidence is very uncertain about the effects of mobile phone text messaging for medication adherence when compared to usual care.
Fatal cardiovascular events
Text messaging may have little to no effect on fatal cardiovascular events compared to usual care (odds ratio 0.83, 95% confidence interval (CI) 0.47 to 1.45; 4 studies, 1654 participants; low‐certainty evidence).
Non‐fatal cardiovascular events
We found very low‐certainty evidence that text messaging may have little to no effect on non‐fatal cardiovascular events. Two studies reported non‐fatal cardiovascular events, neither of which found evidence of a difference between groups.
Combined CVD events
We found very low‐certainty evidence that text messaging may have little to no effect on combined CVD events. Only one study reported combined CVD events, and did not find evidence of a difference between groups.
Low‐density lipoprotein cholesterol
Text messaging may have little to no effect on low‐density lipoprotein cholesterol compared to usual care (mean difference (MD) −1.79 mg/dL, 95% CI −4.71 to 1.12; 8 studies, 4983 participants; very low‐certainty evidence).
Blood pressure
Text messaging may have little to no effect on systolic blood pressure (MD −0.93 mmHg, 95% CI −3.55 to 1.69; 8 studies, 5173 participants; very low‐certainty evidence) and diastolic blood pressure (MD −1.00 mmHg, 95% CI −2.49 to 0.50; 5 studies, 3137 participants; very low‐certainty evidence) when compared to usual care.
Heart rate
Text messaging may have little to no effect on heart rate compared to usual care (MD −0.46 beats per minute, 95% CI −1.74 to 0.82; 4 studies, 2946 participants; very low‐certainty evidence).
Authors' conclusions
Due to limited evidence, we are uncertain if text messaging reduces medication adherence, fatal and non‐fatal cardiovascular events, and combined cardiovascular events in people with cardiovascular diseases when compared to usual care. Furthermore, text messaging may result in little or no effect on low‐density lipoprotein cholesterol, blood pressure, and heart rate compared to usual care. The included studies were of low methodological quality, and no studies assessed the effects of text messaging in low‐income countries or beyond the 12‐month follow‐up. Long‐term and high‐quality randomised trials are needed, particularly in low‐income countries.
Keywords: Humans, Middle Aged, Bias, Cardiovascular Diseases, Cardiovascular Diseases/prevention & control, Cell Phone, Medication Adherence, Myocardial Infarction, Myocardial Infarction/prevention & control, Randomized Controlled Trials as Topic, Reminder Systems, Secondary Prevention, Secondary Prevention/methods, Stroke, Stroke/prevention & control, Text Messaging
Plain language summary
Can text message reminders help people with heart disease take their medications regularly?
Key messages
Due to a lack of strong evidence, the benefits of text messaging for medication adherence, fatal cardiovascular events (death from heart disease), non‐fatal cardiovascular events (heart complications or stroke), combined cardiovascular events (death from heart disease, heart complications, or stroke), cholesterol, blood pressure, and heart rate are unclear.
Larger and well‐designed studies are needed to measure the longer‐term effects of text messaging on improving medication adherence in people with heart disease, particularly in low‐income countries.
Why is this review important?
At least 523 million people suffer from heart disease worldwide. Medicines are often prescribed to treat the condition. However, the majority of people do not take the medications they need to keep them from having more heart problems. One possible method to improve medication‐taking behaviours is by using text message‐based reminders. Mobile phone text messaging may help people with heart disease take their medications by sending health information and text reminders to these people. However, it is still unclear whether text messaging can help people with heart disease take their medications regularly.
What did we want to find out?
We wanted to find out if text messaging was effective in improving medication adherence in people with heart disease compared to people who did not receive text messages. We were also interested in the effects of text messaging on fatal cardiovascular events (death from heart disease), non‐fatal cardiovascular events (heart complications or stroke), combined cardiovascular events (death from heart disease, heart complications, or stroke), blood pressure, cholesterol, and heart rate.
What did we do?
We searched medical databases for studies looking at the effects of mobile phone text messaging on medication adherence in people with heart disease.
What did we find?
We found 18 studies involving 8136 people with heart disease. The studies took place in 11 countries. All studies compared using text messages to not using text messages.
Main results
All studies took place in middle‐ and high‐income countries, with no studies being performed in low‐income countries. People had various types of heart diseases and were on average 53 to 64 years old. Most people came from hospitals or cardiac rehabilitation facilities. Studies lasted for one to 12 months. The delivery method and frequency of text messages differed amongst studies. Some studies sent text messages customised to patient characteristics and allowed people to reply to the messages. The content of text messages also varied across studies. Generally, text messages included medication reminders and healthy lifestyle information such as diet, physical activity, and weight loss.
The studies used different ways of measuring and definitions of medication adherence, which prevented us from combining the findings of the studies for this outcome. As a result, the combined effects of text messaging on medication adherence are unknown. Of the 18 included studies, 10 studies showed that text messaging was effective in improving medication adherence. The other eight studies showed either a reduction or no difference in medication adherence compared to those people who did not receive text messages. Given that results on medication adherence differed across studies, we are not sure if text messaging can improve medication adherence.
We found that text messaging may make little to no difference to fatal cardiovascular events (death from heart disease). In addition, we are very uncertain whether using text messaging can reduce blood pressure, cholesterol, heart rate, non‐fatal cardiovascular events (heart complications or stroke), and combined cardiovascular events (death from heart disease, heart complications, or stroke) compared with people who did not receive text messages. Two studies reported non‐fatal cardiovascular events, with neither study finding evidence of difference between groups. Only one study reported combined cardiovascular events, and found no evidence of a difference between groups.
What are the limitations of the evidence?
Our confidence in the evidence is low to very low. Three main factors reduced our confidence in the evidence. Firstly, the research methods that the studies used were not of the best quality. It is possible that people in the studies were aware of which treatment they were getting, which could have influenced the results. Also, not all studies provided data about everything that we were interested in. Secondly, the content and delivery method of text messages differed across studies. Thirdly, results were very inconsistent across the different studies, and there were not enough studies to be certain about the results of our outcomes.
How up‐to‐date is this evidence?
This review updates our previous review. The evidence is current to August 2023.
Summary of findings
Summary of findings 1. Mobile phone text messaging compared to usual care for medication adherence in secondary prevention of cardiovascular disease.
| Mobile phone text messaging compared to usual care for medication adherence in secondary prevention of cardiovascular disease | |||||
| Patient or population: people with established arterial occlusive events Setting: hospital/cardiac rehabilitation facility Intervention: mobile phone text messaging Comparison: usual care | |||||
| Outcomes | Anticipated absolute effects | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with usual care | Risk with text messaging interventions | ||||
|
Medication adherence (self‐reported adherence, questionnaire, tablet counts, medication event monitoring systems, pharmacy prescription data) Follow‐up: range 1 to 12 months |
10 out of 18 studies showed a beneficial effect of text messaging on medication adherence compared to usual care. The other 8 studies showed that text messaging resulted in either a reduction or no difference in medication adherence when compared with usual care. Of the 10 studies showing beneficial effects, 4 studies found that text messaging improved the proportion of participants who took medications as prescribed (RR 1.10, RR 1.14, OR 0.43, and OR 0.37, respectively). 3 studies found an improved medication adherence score with text messaging (MD varied from 0.54 to 1.50). 2 studies found that text messaging reduced the risk of being low adherent (RR 4.09) or non‐adherent (OR 0.34). 1 study found that text messaging improved the percentage of correct doses taken by 12.0% (P = 0.02) and percentage of prescribed doses taken on schedule by 9.7% (P = 0.01). |
‐ | 8136 (18 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Fatal cardiovascular events (percentage of people having fatal cardiovascular events) Follow‐up: range 1 to 12 months |
Study population | OR 0.83 (0.47 to 1.45) | 1654 (4 RCTs) |
⊕⊕⊝⊝ Lowa,c |
|
| 36 per 1000 |
30 per 1000 (17 to 52) |
||||
|
Non‐fatal cardiovascular events (percentage of people having non‐fatal cardiovascular events) Follow‐up: range 1 to 12 months |
2 studies reported non‐fatal cardiovascular events, neither of which reported a difference between groups. | ‐ | ⊕⊝⊝⊝ Very lowa,b,c | ||
|
Combined CVD events (percentage of people having combined CVD events) Follow‐up: range 1 to 12 months |
1 study reported combined CVD events and found no difference between groups. | ‐ | ⊕⊝⊝⊝ Very lowa,b,c | ||
|
Low‐density lipoprotein cholesterol (mg/dL) (endpoint low‐density lipoprotein cholesterol reading) Follow‐up: range 1 to 12 months |
The mean low‐density lipoprotein cholesterol score ranged across the control groups from 73.47 to 99.3. | The mean low‐density lipoprotein cholesterol score in the intervention groups was, on average, 1.79 lower (95% CI −4.71 to 1.12). | ‐ | 4983 (8 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c |
|
Blood pressure (mmHg) (endpoint blood pressure reading) Follow‐up: range 1 to 12 months |
Systolic blood pressure | ‐ | 5173 (8 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | |
| The mean systolic blood pressure score ranged across the control groups from 121.0 to 136.0. | The mean systolic blood pressure score in the intervention groups was, on average, 0.93 lower (95% CI −3.55 to 1.69). | ||||
| Diastolic blood pressure | ‐ | 3137 (5 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | ||
| The mean diastolic blood pressure score ranged across the control groups from 72.2 to 84.0. | The mean diastolic blood pressure score in the intervention groups was, on average, 1.00 lower (95% CI −2.49 to 0.50). | ||||
|
Heart rate (beats per minute) (endpoint heart rate reading) Follow‐up: range 1 to 12 months |
The mean heart rate score ranged across the control groups from 67.0 to 69.0. | The mean heart rate score in the intervention groups was, on average, 0.46 lower (95% CI −1.74 to 0.82). | ‐ | 2946 (4 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c |
| Abbreviations: CI: confidence interval; CVD: cardiovascular disease; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded one level for risk of bias: most trials were at unclear or high risk of bias for multiple domains. bDowngraded one level for inconsistency: trial results included large variations in the degree to which the outcome was affected, or substantial heterogeneity (e.g. varying characteristics of the intervention and the comparators, diverse measurement methods, and various definitions of medication adherence). cDowngraded one level for imprecision: very few events, or wide CIs encompassing intervention benefit and harm.
Background
Description of the condition
Worldwide, there are an estimated 17.9 million deaths due to cardiovascular disease (CVD) each year, with over three‐quarters of associated deaths occurring in low‐ and middle‐income countries (WHO 2023). It is estimated that approximately three times as many people will suffer non‐fatal cardiovascular events, and that each year 35 million people have an acute coronary or cerebrovascular event. Worldwide, approximately 523 million people are thought to have prevalent CVD (Nieuwlaat 2013; Perel 2015; Roth 2020). This population has a five times greater chance of suffering a new cardiovascular event than people without known CVD (Kerr 2009).
Description of the intervention
Secondary CVD prevention is defined as health care aimed at preventing recurrent cardiovascular events in people diagnosed with CVD (Perel 2015). It is widely recommended by international guidelines that effective secondary prevention strategies for CVD include appropriate treatments with evidence‐based medications and comprehensive management of risk factors (Taylor 2023). The use of evidence‐based medication, such as antiplatelet therapy, angiotensin‐converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), beta‐blockers, and statins, has been shown to be a cost‐effective intervention for the prevention of fatal and non‐fatal cardiovascular events in people with established atherosclerotic cardiovascular diseases (Perel 2015).
Unfortunately, there is a well‐documented knowledge‐practice gap in the implementation of these proven cost‐effective interventions. For example, the Prospective Urban Rural Epidemiology (PURE) study reported that in low‐ and middle‐income countries, up to 75% of people with known CVD are not using even one recommended medication (Yusuf 2011). Even in high‐income countries, adherence to recommended treatments remains suboptimal. A systematic review showed that amongst people diagnosed with hypertension, 83.7% of people with uncontrolled blood pressure were not adherent to their prescribed antihypertensive medications (Abegaz 2017). Adherence to statin medication was low as well, with approximately 33% to 50% of people discontinuing statin medication within one year (Bosworth 2018).
It has been shown that a considerable proportion of cardiovascular events could be attributed to poor adherence to medication, with 9% of cardiovascular events in Europe attributed to poor adherence to medication (Chowdhury 2013). It is estimated that good adherence to medication may be associated with a 20% lower risk of CVD and a 35% reduction in all‐cause mortality (Chowdhury 2013). This evidence–practice gap might be influenced by different factors, including health system issues such as lack of accessibility and affordability; treatment complexity; or patients' non‐compliance with recommendations (Nieuwlaat 2013).In order to influence non‐compliance, there is a need to develop behaviour change interventions. However, a Cochrane review showed that traditional behaviour change interventions to improve medication adherence are complex and not very effective, which indicates a need for convenient and feasible innovations to improve patient adherence to medication (Nieuwlaat 2014).
The widespread ownership of mobile phones and the possibility of automation leads to a potential to deliver behaviour change interventions to large numbers of people at low cost. The global number of mobile phone subscribers is estimated at 8600 million (Statista 2022). Even in low‐ and middle‐income countries, the penetration rate of mobile phones is over 90% (Feroz 2020). As a basic function of the mobile phone, short message services (SMS) are increasingly being used worldwide for communications. There has been an emergence of studies investigating if medication adherence could be improved by sending messages as reminders for medication‐taking.
How the intervention might work
Mobile phone text messaging has been shown to improve medication adherence for a variety of conditions, including HIV, asthma, and mental illness (Dong 2018; Ibeneme 2021; Simon 2022). The development of messages should follow some theoretical framework, and text messages should be developed specifically for the target population and intervention (MacPherson 2021). Text messages as an intervention are relatively cost‐effective and quick, and do not require that the intended audience search for information, as it is delivered to them (Willems 2023). Two previous systematic reviews addressed the question of using mobile phones for all types of medication adherence (Anglada‐Martinez 2015; Park 2014b). The majority of studies included in the reviews found significant improvement in medication adherence through the use of text messages.
Previous mixed‐methods research has identified three dominant theoretical frameworks as driving behaviour change (including medication adherence) resulting from text messaging programmes for people with CVD. These have been found to include the Information‐Motivation‐Behavior Skills Theoretical Model (Fisher 2006), because participants felt motivated and engaged with the text message programme, it provided advice and skills with solutions and content was considered credible and meaningful (Redfern 2016). Social‐Cognitive Theory, Bandura 1989, was also found to influence adherence if social recognition, improved self‐efficacy, the provision of achievable task‐setting suggestions, practical advice and positive reinforcement were built into the text message programme (Redfern 2016). Furthermore, previous qualitative data suggested that, over time, participants became ‘conditioned’ towards healthy behaviours irrespective of the specific content of the individual messages received at a certain time, which aligns with operant condition (Redfern 2016); that is, through repeated presentation of a stimulus (in this case cardiovascular health‐related text messages), the participants eventually learnt to associate the stimulus with overall cardiovascular health behaviour and consequently messages about diet, also resulting in improved medication adherence and vice versa. In addition, the mechanism of text messaging improving medication adherence could be attributed to the Behaviour Change Techniques proposed by the Capability, Opportunity, Motivation‐Behaviour (COM‐B) framework, which emphasises that capability, opportunity, and motivation are three essential components of behaviour change (MacPherson 2021; Richardson 2019). Overall, the mechanisms associated with text message programmes are complex and multidimensional and varied between and within individuals; however, repeated reminders and positive reinforcement of healthy behaviours serve to influence attention, memory, and decision processes, which also supports medication adherence as part of the overall programme (Redfern 2016).
Why it is important to do this review
Whilst there is a great deal of enthusiasm for mobile health (mHealth) interventions amongst researchers and policymakers, there is still limited evidence for its effectiveness (Unal 2018). Systematic reviews have been conducted on adherence to medications and reported promising results (Anglada‐Martinez 2015; Ershad 2016; Park 2014b; Thakkar 2016; Zhao 2019). The previous version of this review was conducted to evaluate specifically the effect of mobile phone text messaging on medication adherence for secondary CVD prevention and to try to examine how text messages are created and if SMS are tailored based on individual patient characteristics (Adler 2017). However, the effects of text messaging were inconclusive due to the limited number of included studies and lack of meta‐analysis. It remains unclear if text messaging can improve medication adherence and other clinical outcomes (e.g. cardiovascular events, blood pressure, low‐density lipoprotein (LDL) cholesterol, and heart rate). Given that research evolves rapidly in this area, and the findings of the previous review could be changed by the new studies recently published, a review update with introduction of the most recent evidence is warranted.
Objectives
To evaluate the benefits and harms of mobile phone text messaging for improving medication adherence in people with cardiovascular diseases compared to usual care.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs). We excluded cluster‐RCTs because our goal was to focus on interventions aimed at individuals. We also excluded quasi‐RCTs because quasi‐RCTs do not use a full randomisation and limit the study’s ability to conclude a causal association between an intervention and an outcome. A quasi‐randomised trial is defined as a trial allocating participants to different groups using a method of allocation that is not truly random; for example, allocation by date of birth, medical record number, or the order in which participants were recruited (Reeves 2023).Cross‐over trials were eligible for inclusion.
Types of participants
We included participants with established arterial occlusive events, including coronary artery disease, cerebrovascular artery disease, peripheral artery disease, and atherosclerotic aortic disease, for whom antiplatelet, blood pressure‐lowering medications, and lipid‐lowering medications are recommended. We included all studies irrespective of where participants were enrolled (community or clinic). We excluded mixed‐disease populations (e.g. study participants with either CVDs or other diseases). Established arterial occlusive events were defined by authors.
Types of interventions
We included trials comparing interventions using short messaging service (SMS) or multimedia messaging service (MMS) to improve adherence to medication for the secondary prevention of cardiovascular events. We compared mobile phone messaging with usual care. We did not exclude studies based on how the text messages were developed, or if they were sent one way versus two ways. We only included trials that included adherence, but we also included trials of interventions that targeted medication adherence alongside other lifestyle modifications.
Types of outcome measures
For studies with multiple outcome measurements, we selected the longest follow‐up from each study.
Reporting one or more of the outcomes listed below was not an inclusion criterion for this review. Where a published report did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether the outcomes were measured but not reported. Relevant trials that measured these outcomes but did not report the data, or did not report the data in a usable format, were included in the review as part of the narrative.
Primary outcomes
Medication adherence (self‐reported adherence, questionnaire, tablet counts, medication event monitoring systems, pharmacy prescription data).
Fatal cardiovascular events (death caused by cardiovascular events), defined as the proportion of participants with fatal cardiovascular events.
Non‐fatal cardiovascular events (coronary heart disease, revascularisation, stroke), defined as the proportion of participants with non‐fatal cardiovascular events.
Combined CVD event (fatal or non‐fatal CVD events), defined as the proportion of participants with a combined CVD event.
Secondary outcomes
Low‐density lipoprotein (LDL) cholesterol for the effect of statins.
Blood pressure for antihypertensive drugs (systolic blood pressure and diastolic blood pressure).
Heart rate for the effect of beta‐blockers.
Urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin.
-
Adverse effects:
self‐reported road traffic crashes;
repetitive thumb strain.
Patient‐reported experience (utility, acceptability, and satisfaction).
Search methods for identification of studies
Electronic searches
We identified relevant studies through systematic searches of the following bibliographic databases and trial registers on 30 August 2023.
Cochrane Central Register of Controlled Trials (CENTRAL; 2023, Issue 8) in the Cochrane Library (searched 30 August 2023).
MEDLINE In‐Process & Other Non‐Indexed Citations and MEDLINE Ovid (1946 to 30 August 2023).
Embase Classic and Embase Ovid (1947 to 30 August 2023).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; Thomson Reuters; 1990 to 30 August 2023).
CINAHL Complete EBSCO (Cumulated Index to Nursing and Allied Health Literature; 1937 to 30 August 2023).
Scopus Elsevier (1966 to 30 August 2023).
ProQuest Central (1938 to 30 August 2023).
ClinicalTrials.gov (www.clinicaltrials.gov;searched 30 August 2023).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 30 August 2023).
The Cochrane sensitivity‐maximising RCT filter was applied to MEDLINE Ovid and adaptations of it to the other databases, except CENTRAL (Lefebvre 2022). Search strategies are provided in Appendix 1. We searched all databases from their inception to the present, and imposed no restrictions on language of publication, publication date, or publication status.
Searching other resources
We checked the reference lists of all included primary studies and reviewed relevant systematic reviews and meta‐analyses for additional references (Akinosun 2021; Al‐Arkee 2021; Allida 2020; Bond 2021; Chowdhury 2017; Fuller 2018; Fulton 2017; Gandapur 2016; Kassavou 2018; Tam 2021; Treskes 2018; Unal 2018; Xiong 2018; Zhao 2019).
Data collection and analysis
Selection of studies
We used a dual screening process involving two teams of two review authors (team 1: MH & CZ; team 2: QT & NH) for study inclusion and data extraction. Each team was assigned half the number of total records yielded by the searches to assess for eligibility. Each review author independently screened the titles and abstracts for inclusion (therefore, each record was double screened) and decided to retrieve the full‐text copies or to discard them. If there were any disagreements, the other team arbitrated. We retrieved the full‐text study reports/publications, and all four review authors independently screened the full texts and identified studies for inclusion. Any disagreements were resolved by discussion or through arbitration with the other team if necessary. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We completed a PRISMA flow diagram, Stovold 2014, and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form to extract study characteristics and outcome data previously piloted on at least one study in the review. Two review authors (MH, CZ, QT, or NH) independently extracted study characteristics for each included study. We extracted the following study characteristics.
Methods: study design, total duration of study, study setting, withdrawals, and date and country of study.
Participants: number, mean age, age range, gender, condition, diagnostic criteria, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications, how text messages were developed, behaviour change technique, any theoretical framework/s used to develop the intervention, time from arterial occlusive event, if SMS was personalised, mode of delivery (frequency and timing of messaging, duration of programme).
Outcomes: primary and secondary outcomes specified and collected, method of measurement, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We resolved any disagreements by consensus or by involving a third person (MH). One review author (QT) transferred data into Review Manager Web (RevMan Web 2022). We double‐checked the data entry for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
Two of four review authors (MH, CZ, QT, NH) independently assessed the risk of bias for each included study using the RoB 1 tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion with the other two review authors who were not initially involved in the assessment (MH, CZ, QT, NH). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other biases.
We graded each potential source of bias as low, high, or unclear and provided evidence from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
Given the nature of the interventions included in this review, it was likely that blinding of participants and personnel would be impossible, as would blinding of self‐reported outcome assessment, so we expected trials to be categorised at high risk of bias for both of these domains.
For the overall study assessment, we categorised a trial as being at low risk of bias if it was rated as low risk in all the domains listed above (with the exception of blinding of participants and personnel/self‐reported outcome assessment). We categorised trials that were at high or unclear risk of bias for any risk of bias domain (except blinding of participants and personnel/self‐reported outcome assessment) as being at high risk of bias. We assessed trials as at unclear risk of bias when too few details were available to permit a judgement of low or high risk of bias.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
For continuous outcomes, to enable pooling and comparison of the outcomes, mean difference (MD) with 95% confidence interval (CI) was calculated as effect size. For dichotomous outcomes, odds ratio (OR) with 95% CI was calculated as effective size (Higgins 2022). For dichotomous outcomes (e.g. medication adherence) that were reported as risk ratio (RR) in the original study and did not allow for meta‐analysis, we reported RR as per the original reporting.
We entered data presented as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
We did not encounter any unit of analysis issues, so the unit of analysis was the individual.
Dealing with missing data
We contacted investigators to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). If no additional data were provided by the authors, we used available‐case analysis, which included analysis of the available data only (thus ignoring the missing data), assuming the data were missing at random.
Assessment of heterogeneity
We evaluated clinical heterogeneity by assessment of variability in the participants, interventions, and outcomes in studies. We assessed methodological heterogeneity by assessment of variability in study design, outcome measurement tools, and risk of bias. We tested for statistical heterogeneity by inspecting the overlap of CIs and quantifying this using the Chi2 test and the I2 statistic (which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error). We assessed heterogeneity according to the guidance in the Cochrane Handbook (Deeks 2023), using the following thresholds:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; or
75% to 100%: considerable heterogeneity.
We used 40% as a cut‐off value for important heterogeneity, which means that we considered an I2 under 40% as low heterogeneity. When possible, we assessed the potential causes of heterogeneity by sensitivity analyses (Sensitivity analysis).
Assessment of reporting biases
We planned but could not assess the potential publication bias using funnel plots and Egger’s test (Page 2023), as there were fewer than 10 studies within the analysed outcome.
Data synthesis
We planned but did not conduct a meta‐analysis for medication adherence due to a large variation in the way medication adherence was defined and measured. In addition, we aimed to but could not conduct a meta‐analysis for non‐fatal cardiovascular events, combined cardiovascular events, and urinary 11‐dehydrothromboxane B2 due to a lack of reported outcomes in the studies. However, we conducted a meta‐analysis for fatal cardiovascular events, LDL cholesterol, blood pressure, and heart rate. We used a random‐effects model and inverse variance method for meta‐analysis because of foreseen heterogeneity.
We performed a narrative synthesis if quantitative synthesis was deemed inappropriate due to significant statistical, clinical, or methodological heterogeneity.
Subgroup analysis and investigation of heterogeneity
We were not able to conduct our preplanned subgroup analyses due to inadequate data available for analysis (Adler 2015).
Sensitivity analysis
We assessed the robustness of the effect estimate by performing the following sensitivity analyses.
Using a fixed‐effect model.
Excluding outliers.
Including only those studies with a 'low' risk of bias (defined as those studies where there is a low risk of bias classification across all key domains except performance bias, given that it is not feasible to blind participants to a text messaging intervention).
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT software to construct a summary of findings table for the following comparison: text messaging versus usual care (GRADEpro GDT). The summary of findings table includes all primary outcomes (medication adherence, fatal cardiovascular events, non‐fatal cardiovascular events, and combined CVD events) and three secondary outcomes (LDL cholesterol, blood pressure, and heart rate) at follow‐up of one to 12 months. We selected these outcomes because they were the most clinically relevant. We used the methods and recommendations described in Chapter 14 of the Cochrane Handbook (Schünemann 2023).
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence. We downgraded the certainty of the evidence for each outcome up to a maximum of three levels. We rated the certainty of the evidence for each outcome as high, moderate, low, or very low. Two review authors (NH and QT) independently assessed the certainty of the evidence for each outcome, with any differences of opinion resolved by consulting a third review author (MH). We justified all decisions to downgrade the certainty of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
A study flow diagram is shown in Figure 1. The new search of the databases retrieved 15,085 records. Our search of the clinical trial registers and our manual search retrieved an additional 346 and 12 records, respectively. After removal of duplicates, we screened 12,263 records based on title and abstract, excluding 12,197 records as irrelevant. We obtained and assessed the full texts of the remaining 66 reports, and excluded 20 reports (18 studies), resulting in 11 new completed studies (35 reports) and nine ongoing studies (11 reports). After combining the seven completed studies from the original review, the updated search resulted in a total of 18 completed studies and nine ongoing studies.
1.
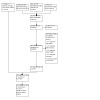
Study flow diagram.
A search for the clinical trial registry numbers of the nine ongoing studies revealed that one study is completed but study results are not published yet (ISRCTN10549665).
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies tables.
We included 18 studies (Bae 2021; Bermon 2021; Chen 2019; Chow 2015; Chow 2022; Dale 2015a; Fang 2016; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Ni 2022; Pandey 2017; Park 2014a; Passaglia 2021; Quilici 2013; Ross 2021; Zheng 2019). In the original review, Pandey 2014 was included but only an abstract was available. The full paper of the abstract was published in 2017 (Pandey 2017), therefore Pandey 2017 replaced Pandey 2014 in this review update. We attempted to make contact with the authors of two studies to obtain information on non‐fatal cardiovascular events (Huo 2019; Zheng 2019), but did not receive a response.
Participants
The sample size of included studies ranged from 34, in Pandey 2017, to 1424, in Chow 2022. The total number of included participants was 8136, of which 7272 completed follow‐up.
All 18 studies targeted people with CVD. Different definitions of CVD were used in the included studies. Six studies included participants with acute coronary syndrome (Chow 2022; Khonsari 2015; Maddison 2021; Passaglia 2021; Quilici 2013; Ross 2021), and seven studies included participants with coronary heart disease (Bae 2021; Chow 2015; Dale 2015a; Huo 2019; Ni 2022; Park 2014a; Zheng 2019). Kamal 2015 reported on participants with stroke. Chen 2019 included participants with chronic heart failure. Pandey 2017 included participants with myocardial infarction. Fang 2016 included participants with chronic stable angina. Bermon 2021 included participants with arterial occlusive events, including acute coronary syndrome, stable angina, ischaemic cerebrovascular disease, peripheral arterial disease, and coronary revascularisation.
The mean age ranged from 53.6 years, in Fang 2016, to 64 years, in Quilici 2013. The proportion of male participants was higher than 70% in all studies. Only three studies had a proportion of male participants of less than 70%: Pandey 2017 (59% males), Kamal 2015 (67.5% males), and Chen 2019 (56.5% males).
Settings
Sixteen studies recruited participants from hospitals including large metropolitan hospitals (Dale 2015a; Fang 2016; Maddison 2021), tertiary teaching hospital (Bae 2021; Chow 2015; Chow 2022; Huo 2019; Kamal 2015; Khonsari 2015; Ni 2022; Passaglia 2021; Ross 2021; Zheng 2019), tertiary referral hospital (Bermon 2021; Chen 2019), and non‐profit community hospital (Park 2014a). One study took place in a cardiac rehabilitation facility (Pandey 2017). In one study the setting was not reported (Quilici 2013).
Seventeen studies reported the country in which they took place: Australia (Chow 2015; Chow 2022), China (Chen 2019; Fang 2016; Huo 2019; Ni 2022; Zheng 2019), Canada (Pandey 2017; Ross 2021), New Zealand (Dale 2015a; Maddison 2021), Brazil (Passaglia 2021), Colombia (Bermon 2021), Korea (Bae 2021), Malaysia (Khonsari 2015), Pakistan (Kamal 2015), and the USA (Park 2014a). Quilici 2013 did not report the country in which the study was conducted, but the author affiliations suggest that it took place in France. Overall, the 18 included studies took place in 11 countries, including six high‐income countries (USA, Australia, Korea, Canada, New Zealand, and France), four upper‐middle‐income countries (China, Malaysia, Brazil, and Colombia), and one lower‐middle‐income country (Pakistan). None of the included studies were from low‐income countries. Of the 18 included studies, nine were conducted in high‐income countries (Bae 2021; Chow 2015; Chow 2022; Dale 2015a; Maddison 2021; Pandey 2017; Park 2014a; Quilici 2013; Ross 2021), eight in upper‐middle‐income countries (Bermon 2021; Chen 2019; Fang 2016; Huo 2019; Khonsari 2015; Ni 2022; Passaglia 2021; Zheng 2019), and one in a lower‐middle‐income country (Kamal 2015).
Development of SMS
All studies used text messaging as a central component of the intervention. The message content was predominantly medication reminders. Thirteen studies reported that the SMS was developed as a reminder to take their medications (Bae 2021; Chen 2019; Chow 2022; Fang 2016; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Ni 2022; Pandey 2017; Park 2014a; Ross 2021; Zheng 2019). Of the 13 studies, 11 studies also included education or lifestyle modification information (e.g. healthy diet and physical activity) as part of the text messages in addition to the medication reminders. Two studies provided access to a supporting website as an additional intervention component (Bae 2021; Dale 2015a). Details regarding the development of SMS are summarised in Table 2.
1. SMS development.
| Study | SMS = reminder | Description of process to design SMS (e.g. did the authors describe the process used to construct the content of the text messages?) | Evaluation of causes for non‐adherence (e.g. did they evaluate causes for non‐adherence in the target population?) | Used psychological theories to develop SMS (e.g. were psychological theories used to develop the messages to target the identified behavioural determinants of non‐adherence?) | Used behaviour change techniques to develop SMS (e.g. were behaviour change techniques employed to develop the messages?) | SMS designed according to participant characteristics (e.g. were different text messages developed according to participants' characteristics?) | Pilot phase to evaluate clarity, grammar of SMS |
| Bae 2021 | Yes, messages contain medication adherence contents | Yes. “The contents of the SMS text messages were based on the Tobacco, Exercise, and Diet Messages (TEXTME) trial and the Australian Heart Foundation Healthy Living Guidelines. The cardiologists, nurses, clinical nutritionists, and preventive medicine experts reviewed the text messages in the TEXTME trial and modified them considering the Asian diet and culture.” (Bae 2021) | No | No information | No information | Yes. “The message sending program delivered semipersonalised text messages considering the smoking status and diet pattern of the participants ‐ vegetarian or not ‐ with their names.” (Bae 2021) | No |
| Bermon 2021 | No, the text messages were not reminders for medication intake or appointments, but an intervention to increase awareness and commitment to medication taking. | Yes. “A protocol was carried out to determine the content, quantity, and frequency of SMS text messages through focus groups, validation of experts, user feedback, and pretest.” (Bermon 2021) | No information | No information | Yes, based on the transtheoretical model of health behaviour change | No | Yes |
| Chen 2019 | Yes, medication reminder | Yes, all contents of text messages were pre‐written and reviewed by heart failure specialists and senior nursing specialists. The contents were developed based on heart failure diagnosis and management guidelines, evidence from review articles, and educational materials from Heart Failure Society of America. | No information | No information | No information | No | No information |
| Chow 2015 | No | Yes, a bank of messages was developed with input from investigators, clinicians, academics, and patients through a multistage iterative process. | No | Yes | Yes | Yes. “The message management program selected messages for each participant at random from the bank of messages from all relevant content areas as per the prespecified algorithms and using baseline data entered into the message management system. e.g. nonsmokers would not be sent smoking messages, and vegetarians would not be sent information about meat. Some messages were merged with patient’s preferred names.” (Chow 2015) | No |
| Chow 2022 | Yes. The medication messages provided information about how medications worked, common side effects, and tips on how to take medication regularly. | Yes | No | Yes | Yes | Yes, the content of the messages was customised to participant characteristics such as medication class prescription, dietary habits (vegetarian or non‐vegetarian), and/or smoking status. Furthermore, messages were ‘personalised’, that is the preferred name of the participant was incorporated into some messages. | Yes |
| Dale 2015a | No | “We created and refined the Text4Heart intervention through formative and pretesting studies following the mHealth Development and Evaluation Framework.” (Dale 2014a) Also, another study that helped inform the physical activity component (Dale 2015b) | No | Messages were based on social cognitive theory and the common sense model (Dale 2014b). | Yes. All messages were coded according to their theoretical construct and corresponding behaviour change techniques. | No, but participants could pick messages on the health behaviour they were most interested in changing (physical activity, healthy eating, smoking cessation, or stress management). Messages were also personalised with participant’s preferred name. | Yes, the healthy eating messages were pilot tested. Feedback from participants was used to refine the messages (Dale 2014a). |
| Fang 2016 | No information | No information | No information | No information | No information | No information | ‐ |
| Huo 2019 | Yes | Yes. “A multidisciplinary team of cardiologists, endocrinologists, psychologists, nurses, linguists, and patients developed the text message bank through a systematic and iterative approach. Messages were drafted based on existing evidence‐based guidelines and standards of care, and they incorporated behavioural change techniques to provide advice, motivation, and support.” (Huo 2019) | No | No | Yes | No. Some messages were personalised with the participant’s preferred name. | Yes. “A pilot study was conducted to elicit patient feedback on the messages, and the text bank was updated to improve clarity and practical usefulness. Messages were also modified to increase their applicability to the Chinese culture and compatibility with Chinese values. For example, some messages used aphorisms and catchy rhyme schemes to make them more appealing to patients.” (Huo 2019) |
| Kamal 2015 | Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khonsari 2015 | Yes | The content of the text messages was based on the WHO multidimensional adherence model (WHO 2003). In constructing the content of the text messages, the study authors focused on the most common reasons for medication non‐adherence based on the WHO model that were unintentional on the patient’s part (forgetfulness and carelessness with medication usage), and included a therapy‐related dimension (misunderstanding of treatment instructions: meds name, dosage and timing) (Gadkari 2012). | According to the study methods, participants were recruited during an admission for ACS prior to discharge from the cardiology ward. This means that all participants were primarily diagnosed with ACS without any experience of taking cardiac medications. Evaluating causes for non‐adherence in the target population was therefore not applicable. | The WHO multidimensional adherence model that guided this study included many different aspects to describe medication non‐adherence behaviour, including psychological factors (WHO 2003). It is
emphasised that no single determinant is responsible for non‐adherence to treatment because the adherence phenomenon is multidimensional and results from the interplay of 5 sets of factors (dimensions), including: A. social and economic factors; B. therapy‐related factors; C. condition‐related factors; D. healthcare team and system‐related factors; and E. patient‐related factors. |
Development of the automated SMS reminder system in this study was useful for deploying spaced repetition strategies via text messaging. Basically, spaced repetition strategy posits that instruction which is repeated at intervals has a great impact on improving a behaviour (Ebbinghaus 1885). | No | The intervention was piloted with 10 cardiac patients during the first stage of the study. During this phase, a variety of test scenarios and clarity of SMS content were analysed. Text messages were further modified to achieve the desired functions. |
| Maddison 2021 | Yes | No | No | Yes. All message content was grounded in psychological theory (Common Sense Model). “The content was focused on modifying people’s perceptions of the symptoms, timeline, cause, consequences, personal control over, and the ability of treatment to prevent cardiovascular disease as well as altering the key mediators of behaviour change, including self‐efficacy, social support, and motivation.” (Maddison 2021) | Yes. All message content was grounded in behaviour change (social cognitive) theory. “The content was focused on modifying people’s perceptions of the symptoms, timeline, cause, consequences, personal control over, and the ability of treatment to prevent cardiovascular disease as well as altering the key mediators of behaviour change, including self‐efficacy, social support, and motivation.” (Maddison 2021) | Yes | Yes. The content of messages was based on the original Text4Heart pilot programme, with some modifications. Message content from weeks 12 to 24 was modified to promote maintenance of the behaviours and relapse prevention. |
| Ni 2022 | Yes, message reminders | No | No | No | No | Yes, personalised reminders | Yes. The interventions were refined based on the pilot study. |
| Pandey 2017 | Yes, medication reminders | No | No | No | No | No | No |
| Park 2014a | Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Passaglia 2021 | No | Yes, text messages were developed by the research group, and reviewed by individuals not involved in the study to check for language issues and evaluation of understanding. The content of the messages was based on the Brazilian Society of Cardiology Guidelines. | No | No | No | No, semi‐personalised | A pilot study was conducted to test the software developed and the initial acceptability of text messages sent. |
| Quilici 2013 | No information | No information | No information | No information | No information | No information | No information |
| Ross 2021 | Yes, medication reminders | Yes. The content of the text messages was developed by a clinical advisory committee (including cardiologists, a general practitioner, a community pharmacist, a cardiac nurse specialist, patient‐users, a programmer, a benefits evaluation specialist, and researchers). Messages were further revised based on the guiding principles, discharge materials, and interviews with patients. | No information | “Instead of conforming to a single one of the many branded theories of behaviour change, the intervention reflects a set of cross‐cutting theoretical domains; the themes in the messages relate to concerns about knowledge, skills, roles and identity, beliefs about capabilities (eg, self‐efficacy), beliefs about consequences, motivation, attention and decision processes (e.g. cues to action such as reminders), environmental context and resources, social influences, emotion, and action plans.” (Ross 2021) | “Instead of conforming to a single one of the many branded theories of behaviour change, the intervention reflects a set of cross‐cutting theoretical domains; the themes in the messages relate to concerns about knowledge, skills, roles and identity, beliefs about capabilities (eg, self‐efficacy), beliefs about consequences, motivation, attention and decision processes (e.g. cues to action such as reminders), environmental context and resources, social influences, emotion, and action plans.” (Ross 2021) | No, but participants received different SMS text messages on 2 occasions based on their smoking status. No other aspects were personalised. | Yes, this was a pilot study. |
| Zheng 2019 | Yes, it reminded people to take their medications and follow‐up. | Yes. “A multidisciplinary team of cardiologists, endocrinologists, psychologists, nurses, linguists, and patients developed the text message bank through a systematic and iterative approach.” (Huo 2019) | No | No | Yes | No, but semi‐personalised with participants' preferred name | Yes. To make the messages more simple, culturally adaptable, and easy to understand, a user test and pilot study were conducted to collect patient feedback on the text bank messages. |
ACS: acute coronary syndrome; SMS: short message service; WHO: World Health Organization
Four studies specified that the automated computer program from which the messages were sent was developed particularly for this study (Chow 2015; Chow 2022; Pandey 2017; Passaglia 2021). Eleven other studies stated that an automated system was used (Bae 2021; Bermon 2021; Chen 2019; Dale 2015a; Fang 2016; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Park 2014a; Zheng 2019), which can also be assumed for two remaining studies whilst not explicitly stated (Quilici 2013; Ross 2021). One study used a mobile app to send messages and was not automated (Ni 2022). In Ni 2022, the study co‐ordinator sent the medication reminders and educational materials through Message Express (a message saving and delivery app) on an encrypted external device.
Seven studies reported on the psychological theory and behaviour change techniques used in the development of their text messages (Chow 2015; Chow 2022; Dale 2015a; Kamal 2015; Khonsari 2015; Maddison 2021; Ross 2021), with Chow 2015 also having an extensive co‐design and development process published (Redfern 2014) (Table 2). Four studies only used behaviour change techniques to develop their text messages without stating a psychological theory (Bermon 2021; Huo 2019; Park 2014a; Zheng 2019). The other seven studies did not specify whether the text messages were supported by theoretical framework.
Nine studies tailored the text messages to the participants' name (Bae 2021; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Khonsari 2015; Maddison 2021; Park 2014a; Zheng 2019). Four studies customised messages according to patients’ medication profile or appointment schedule (Chow 2022; Kamal 2015; Khonsari 2015; Park 2014a). Six studies delivered semi‐personalised text messages based on a consideration of CVD risk factors of participants ‐ smoking status or dietary habits pattern (Bae 2021; Chow 2015; Chow 2022; Maddison 2021; Passaglia 2021; Ross 2021). Two studies stated that the messages were personalised without any further details provided (Ni 2022; Quilici 2013). Two studies did not provide information on whether the messages were tailored (Fang 2016; Pandey 2017). Two studies stated that text messages were not customised according to patient characteristics, and only standardised texts were sent (Bermon 2021; Chen 2019).
Four studies stated that bi‐directional text messaging was employed, and a response from the participants was required (Chow 2022; Dale 2015a; Kamal 2015; Park 2014a). Nine studies stated that unidirectional text messaging was employed (Bae 2021; Bermon 2021; Chen 2019; Chow 2015; Khonsari 2015; Maddison 2021; Pandey 2017; Passaglia 2021; Ross 2021). Two studies stated that messages were predominately unidirectional, with some messages assessing medication usage and blood pressure/glucose level measurements bidirectional (Huo 2019; Zheng 2019). Three studies did not provide information on whether the messages were bi‐directional or unidirectional (Fang 2016; Ni 2022; Quilici 2013).
Thirteen studies provided details on the template texts used for the text messages (Chen 2019; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Khonsari 2015; Maddison 2021; Ni 2022; Pandey 2017; Park 2014a; Passaglia 2021; Ross 2021; Zheng 2019). Ten studies reported that a pilot programme was previously conducted before the formal randomised controlled study (Bermon 2021; Chow 2022; Dale 2015a; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Ni 2022; Passaglia 2021; Zheng 2019). Ross 2021 and Quilici 2013 were pilot studies only. Fang 2016 did not discuss the method or timing of the SMS in the paper.
Interventions
The duration of the intervention ranged from one month (Chen 2019; Park 2014a; Quilici 2013) to 12 months (Bermon 2021; Chow 2022; Pandey 2017). Duration of intervention was six months in eight studies (Bae 2021; Chow 2015; Dale 2015a; Fang 2016; Huo 2019; Maddison 2021; Passaglia 2021; Zheng 2019), two months in four studies (Kamal 2015; Khonsari 2015; Ni 2022; Ross 2021), 12 months in three studies (Bermon 2021; Chow 2022; Pandey 2017), and one month in three studies (Chen 2019; Park 2014a; Quilici 2013). The mean duration of intervention was five months.
The frequency of text messaging delivery varied from twice daily to once weekly. Twelve studies sent text messages at a fixed frequency, including once daily (Kamal 2015; Khonsari 2015; Maddison 2021; Ni 2022; Pandey 2017; Quilici 2013), once per week (Chen 2019; Passaglia 2021; Zheng 2019), four times per week (Bae 2021; Chow 2015), and six times per week (Huo 2019). The remaining studies sent messages at a variable frequency. Park 2014a sent educational messages three times a week and medication reminders twice a day. Dale 2015a sent daily text messages from week zero to 12 weeks, which were reduced in week 13 to week 24 to five messages a week. Ross 2021 sent daily text messages for 36 days, and then every other day until day 60. Bermon 2021 sent daily text messages in the first four weeks, which was gradually reduced to five messages per week in week 5, three messages per week from week 6 to week 8, and one message from week 8 until week 52. Chow 2022 sent four messages per week for the first six months, which was then decreased to three messages per week over the subsequent six months. One study did not report on message frequency (Fang 2016).
The control group included usual care in all 18 studies. However, usual care was not clearly described in nine of the included studies (Chen 2019; Chow 2022; Huo 2019; Pandey 2017; Park 2014a; Passaglia 2021; Quilici 2013; Ross 2021; Zheng 2019). Although the definition of usual care was provided in the remaining nine studies, the definition of usual care varied across studies. Usual care in Bae 2021 included regular follow‐up at the outpatient clinic and education on cardiovascular health and risk factors. The usual care group in Bermon 2021 received text messages for reminding of participation in the study. Chow 2015 defined usual care as community follow‐up and referral to inpatient cardiac rehabilitation. The usual care group in Fang 2016 received monthly phone calls, and in Kamal 2015 received regular follow‐up visits with neurologist. In Ni 2022, the usual care group received educational materials from WeChat. Usual care in Dale 2015a and Maddison 2021 consisted of an outpatient cardiac rehabilitation programme involving health education and supervised exercise. In Khonsari 2015, usual care referred to cardiac rehabilitation and follow‐up appointments with the cardiologists.
Outcomes
Primary outcomes
All included studies measured medication adherence. Five studies measured the overall adherence to several prescribed medications (Bermon 2021; Chow 2022; Khonsari 2015; Maddison 2021; Pandey 2017). Pandey 2017 included participants on a once‐daily regimen of aspirin, a beta‐blocker, an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and a statin using self‐reported logs. Most participants in Khonsari 2015 were on five or more daily medications, and adherence was measured using the Morisky Medication Adherence Scale. Maddison 2021 measured overall adherence to all three medication classes (aspirin, statin, and blood pressure‐lowering medication) and to all four medication classes (aspirin, statin, beta‐blocker, and ACEI/ARB) and adherence to each medication class (statin, aspirin, beta‐blocker, ACEI/ARB) via self‐reported Morisky Medication Adherence Scale. Bermon 2021 measured self‐reported adherence to cardiovascular medications used in secondary prevention with the Medication Adherence Report Scale‐5 (MARS‐5) questionnaire. In contrast to those studies merely using subjective measures, Chow 2022 used a multi‐measure approach including a combination of subjective and objective measures of medication adherence to reduce subjectivity. The subjective measures included measurement of overall adherence to five medication classes (aspirin, beta‐blocker, ACEI/ARB, statin, second antiplatelet) and adherence to each class separately, by self‐reported approach. Objective measures included extraction of prescriptions filled from linked Pharmaceutical Benefits Scheme (PBS) database to validate the self‐reported medication adherence (Department of Health and Aged Care 2023), making it distinct from other studies.
Three studies only measured adherence to a single medication class. Park 2014a measured adherence to antiplatelet and statin medications separately, using both electronic pill bottles and self‐reported adherence. Fang 2016 only looked at adherence to statins by four‐item Morisky Medication Adherence Scale. Quilici 2013 measured aspirin adherence using a self‐reported adherence approach.
Seven studies did not specify which medications the participants were taking and to which medication adherence was measured, but specified that it used self‐reported medication adherence (Bae 2021; Chen 2019; Dale 2015a; Kamal 2015; Ni 2022; Passaglia 2021; Ross 2021).
Three studies reported prescribed medication, including ACEI/ARB, aspirin, beta‐blocker, and statin, and all of these four medications (Chow 2015; Huo 2019; Zheng 2019).
Medication adherence was measured via subjective assessment in 15 of the 18 included studies. In the other three studies, medication adherence was measured and validated via objective measures, including medication possession ratio (MPR) (Maddison 2021), medication event monitoring system (Park 2014a), and data linkage with PBS database (Chow 2022).
Four studies reported on fatal cardiovascular events (Bermon 2021; Chen 2019; Khonsari 2015; Passaglia 2021).
One study reported non‐fatal cardiovascular events and combined CVD event (Chow 2022).
Secondary outcomes
Ten studies provided outcome data for our secondary outcome of blood pressure (Bae 2021; Bermon 2021; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Kamal 2015; Ni 2022; Passaglia 2021; Zheng 2019), and eight studies reported on LDL cholesterol (Bae 2021; Bermon 2021; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Passaglia 2021; Zheng 2019). Five studies reported on heart rate (Bermon 2021; Chow 2015; Chow 2022; Ni 2022; Passaglia 2021).
Thirteen studies reported patient‐reported experience regarding satisfaction, utility, and acceptability of the intervention (Bae 2021; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Park 2014a; Passaglia 2021; Quilici 2013; Ross 2021; Zheng 2019). Only one study measured adverse effects by reporting road traffic crashes (Bermon 2021). None of the included studies reported repetitive thumb strain as an adverse effect.
Four studies did not report on any of our secondary outcomes (Chen 2019; Fang 2016; Khonsari 2015; Pandey 2017). None of the included studies reported on urinary 11‐dehydrothromboxane B2.
Funding
Eleven studies reported the source of funding (Bae 2021; Bermon 2021; Chow 2015; Chow 2022; Dale 2015a; Huo 2019; Maddison 2021; Ni 2022; Park 2014a; Ross 2021; Zheng 2019). Three studies stated that no specific funding was received (Kamal 2015; Khonsari 2015; Passaglia 2021). Four studies did not report if funding was received (Chen 2019; Fang 2016; Pandey 2017; Quilici 2013).
Excluded studies
We excluded 12,197 reports based on title and abstract. We excluded a further 18 studies (20 reports) following full‐text screening. The reasons for exclusion of each study are presented in the Characteristics of excluded studies table. There were four studies that never set out to measure any outcomes of interest (Foccardi 2021; Moradi 2017; Rohde 2021; Santo 2018), and four studies were non‐randomised or single‐arm trials (Carrillo 2021; Chen 2018; Legler 2020; Zhao 2019). We excluded four studies because of ineligible intervention (e.g. text messaging was not a central component of a multicomponent intervention) (IRCT20180911041002N; Santo 2017; Wang 2020; Yan 2021). We excluded the remaining six studies because they did not fulfil the inclusion criteria for cardiovascular disease (Acevedo 2023; Akhu‐Zaheya 2017; Brar 2018; Cheung 2019; Haramiova 2017; Luong 2021).
Ongoing studies
We identified nine ongoing studies (ACTRN12621000754842; CTRI/2021/06/034463; CTRI/2021/10/037432; IRCT2014050617596N1; IRCT2016011025937N1; IRCT2016081125937N2; ISRCTN10549665; Park 2017; Redfern 2019). Three were from high‐income countries (225 participants, USA, Park 2017; 310 participants, Australia, Redfern 2019; 2500 participants, Australia, ACTRN12621000754842), and six were from lower‐middle‐income countries (300 participants, India, CTRI/2021/06/034463; 1200 participants, India CTRI/2021/10/037432; 90 participants, Iran, IRCT2014050617596N1; 116 participants, Iran, IRCT2016011025937N1; 116 participants, Iran, IRCT2016081125937N2; 78 participants, Iran, ISRCTN10549665). Details can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
Details are provided for each study in the risk of bias tables in Characteristics of included studies and in Figure 2 and Figure 3. Overall, studies were assessed as having high or unclear bias across multiple domains, and the certainty of the evidence was deemed to be low or very low (Table 1).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fifteen studies reported adequate random sequence generation and were judged to be of low risk of bias for this domain (Bae 2021; Bermon 2021; Chen 2019; Chow 2015; Chow 2022; Dale 2015a; Fang 2016; Huo 2019; Kamal 2015; Maddison 2021; Ni 2022; Park 2014a; Passaglia 2021; Ross 2021; Zheng 2019). We assessed three studies that provided insufficient information as at unclear risk of bias (Khonsari 2015; Pandey 2017; Quilici 2013). No studies were at high risk of bias for random sequence generation.
Eight studies reported adequate allocation concealment and were judged to be of low risk of bias for this domain (Bermon 2021; Chow 2015; Chow 2022; Dale 2015a; Kamal 2015; Maddison 2021; Park 2014a; Ross 2021). We assessed nine studies that provided insufficient information as at unclear risk of bias (Bae 2021; Chen 2019; Fang 2016; Huo 2019; Khonsari 2015; Pandey 2017; Passaglia 2021; Quilici 2013; Zheng 2019). We judged Ni 2022 to be at high risk of bias because generation of the random allocation sequence, enrolment of participants, and assignment of participants to intervention were all conducted by the first author.
Blinding
Whilst blinding of participants is not possible with this intervention, blinding of outcome assessors could have been done. Three studies clearly stated that no blinding of outcome assessors occurred and were therefore deemed as at high risk of bias (Dale 2015a; Khonsari 2015; Ni 2022). Seven studies did not report on this domain and were assessed as at unclear risk of bias (Chen 2019; Fang 2016; Kamal 2015; Maddison 2021; Pandey 2017; Park 2014a; Quilici 2013). Eight studies clearly stated that outcome assessors were not aware of the study group assignment and were therefore rated as at low risk of bias (Bae 2021; Bermon 2021; Chow 2015; Chow 2022; Huo 2019; Passaglia 2021; Ross 2021; Zheng 2019).
Incomplete outcome data
We assessed most studies (13 studies) as at low risk of attrition bias because dropout rates were low, dropout numbers were balanced between groups, and analysis followed the intention‐to‐treat principle. We judged six studies as at high risk of attrition bias (Bae 2021; Bermon 2021; Kamal 2015; Ni 2022; Passaglia 2021; Ross 2021). In Bae 2021, intention‐to‐treat analysis was not performed. Furthermore, 70/439 (15.9%) and 44/440 (10%) participants were lost to follow‐up in the control and intervention groups, respectively. In Kamal 2015, a large proportion of participants (20%) were lost to follow‐up. In Passaglia 2021, 18.3% of participants were lost to follow‐up, and analysis was not conducted based on the intention‐to‐treat principle. In Ross 2021, there was a significant imbalance between groups for withdrawals or dropouts (5% in the control and 19% in the intervention group). In Ni 2022, 18.4% of the intervention group and 11.2% of the control group did not complete the baseline survey, and analysis was not conducted based on the intention‐to‐treat principle. Bermon 2021 presented imbalances in numbers and reasons for missing data between the intervention group (15.3%) and the control group (11.5%).
Selective reporting
For eight studies, we were able to access the trial protocol or registry, and all prespecified outcomes were reported in the results (Bae 2021; Bermon 2021; Chow 2022; Dale 2015a; Kamal 2015; Maddison 2021; Passaglia 2021; Ross 2021). We therefore judged these eight studies to be at low risk of reporting bias. We judged four studies to be at high risk of reporting bias (Chow 2015; Huo 2019; Quilici 2013; Zheng 2019). For Quilici 2013, the data were minimal (published as a letter to the editor), and details within the report differed. In Huo 2019 and Zheng 2019, death, non‐fatal myocardial infarction, stroke, and rehospitalisation were specified as outcomes of interest in the protocol, but these outcomes were not reported in the findings, representing a deviation from the original stated methodology. In Chow 2015, prespecified outcomes, including psychosocial factors and fruit/vegetable intake, were not reported in the paper. We assessed the remaining six studies as at unclear risk of bias as we did not identify a protocol or trial registration to permit a judgement of reporting bias (Chen 2019; Fang 2016; Khonsari 2015; Ni 2022; Pandey 2017; Park 2014a).
Other potential sources of bias
We assessed 14 studies as at low risk of bias for this domain, as there are no differences between groups at baseline, and they appeared to be free of other sources of bias (Bae 2021; Bermon 2021; Chen 2019; Chow 2015; Chow 2022; Dale 2015a; Fang 2016; Huo 2019; Kamal 2015; Khonsari 2015; Maddison 2021; Ni 2022; Park 2014a; Zheng 2019). We judged four studies as at high risk of other bias (Pandey 2017; Passaglia 2021; Quilici 2013; Ross 2021). In Quilici 2013, the only source of information is a published letter to an editor in which the outcome data for self‐reported non‐adherence differs between the text and Figure 2. Ross 2021 was a pilot study and did not determine a sample size based on power calculations, and thus was likely underpowered to detect clinically important differences. In Passaglia 2021, 20 participants (26.6%) in the intervention group did not receive text messaging though their system confirmed that messages were sent. This may have contributed to loss of study power and raises the possibility of a type II error. In Pandey 2017, there were baseline imbalances between groups in gender: 88% male in the control group and 35% male in the intervention group. Self‐reported outcomes were widely reported in the included studies, which were susceptible to recall bias. We believe such bias could be balanced across the intervention and control groups, and therefore did not include recall bias as an other potential source of bias in this domain.
Effects of interventions
See: Table 1
The results and ratings of the certainty of the evidence for our key outcomes are presented for our main comparison, mobile phone text messaging versus usual care, in Table 1.
Primary outcomes
Medication adherence
All 18 included studies (8136 randomised participants) reported on indirect measures of medication adherence. Of the 18 studies, improvement in medication adherence were observed in 10 studies (Bae 2021; Chen 2019; Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Ni 2022; Pandey 2017; Park 2014a; Quilici 2013). We found significant heterogeneity amongst studies in terms of measurement approach and timing of assessment. First, different evaluation score systems and inconsistent definitions were applied for the measurement of medication adherence. Second, characteristics of text messages varied in terms of content, duration, theoretic framework, personalisation, and directionality. Third, although all included participants were people with CVD, participant characteristics varied across studies.
An overview of the trial results for medication adherence is presented in Table 3. Studies measured the level of medication adherence in different ways, including self‐report questionnaire, personal enquiry, medical record, pill count, and medication event monitoring system.
2. Overview of the trial results for medication adherence.
| Study | Outcome measure | Scale used/measurement tool | Continuous/categorical data | Time point (months) | Number in intervention group | Intervention group effect | Number in control group) | Control group effect | Narrative results |
| Bae 2021 | Medication adherence | 6‐item Modified Morisky Scale (high score indicates better adherence) | Continuous | 6 months | 377 | Median (IQR) = 5 (5 to 5) | 350 | Median (IQR) = 5 (5 to 5) | Mean difference 0.07, 95% CI −0.03 to 0.16; P = 0.19 |
| Proportion of participants taking medications as instructed on > 25 days in the last month | ‐ | Categorical | 6 months | 377 | 98.2% | 350 | 92.1% | Adjusted RR 1.10, 95% CI 1.00 to 1.10; P < 0.001 | |
| Bermon 2021 | Medication adherence | Medication Adherence Report Scale‐5 (high score indicates better adherence) | Continuous | 1 month | 462 | Mean difference (SD) = −0.02 (3.4) | 468 | Mean difference (SD) = 0.2 (3.7) | Adjusted mean difference −0.01, 95% CI −0.40 to 0.40; P = 0.96 |
| Subjective medication intake compliance | ‐ | Continuous | 1 month | 462 | Mean difference (SD) = 0.1 (2.0) | 468 | Mean difference (SD) = 0.1 (1.9) | Adjusted mean difference 0.02, 95% CI −0.20 to 0.20; P = 0.83 | |
| Chen 2019 | Proportion of participants taking medications as prescribed | ‐ | Categorical | 6 months | 209 | 78.9% | 200 | 69.5% | RR 1.14, 95% CI 1.01 to 1.28; P = 0.029 |
| Chow 2015 | Medications‐ ACE inhibitor/ARB | ‐ | Categorical | 6 months | 339 | 66.1% | 354 | 71.8% | P value was not reported. |
| Aspirin | 6 months | 339 | 90.6% | 354 | 93.8% | P value was not reported. | |||
| Beta‐blocker | 338 | 64.5% | 354 | 63.0% | P value was not reported. | ||||
| Statin | 339 | 91.7% | 354 | 92.9% | P value was not reported. | ||||
| At least 3 of the 4 medications (ACE inhibitor/ARB, aspirin, beta‐blocker, and statin) | 338 | 78.1% | 354 | 83.1% | RR 0.94, 95% CI 0.87 to 1.01; P = 0.10 | ||||
| All 4 medications (ACE inhibitor/ARB, aspirin, beta‐blocker, and statin) | 338 | 39.3% | 42.1% | 354 | RR 0.93, 95% CI 0.78 to 1.12; P = 0.46 | ||||
| Chow 2022 | Proportion of participants with proportion of days covered > 80% for 5 medications at both 6 and 12 months | ‐ | Categorical | 12 months | 697 | 50.4% | 682 | 54.3% | No difference in adherence to all recommended medications at 6 and 12 months between the intervention and control groups (RR 0.93, 95% CI 0.84 to 1.03, P = 0.15) |
| Adherence to Aspirin | 671 | 96.3% | 638 | 96.1% | RR 1.00, 95% CI 0.98 to 1.02; P = 0.86 | ||||
| Beta‐blocker | 613 | 84.2% | 581 | 83.6% | RR 1.01, 95% CI 0.96 to 1.06; P = 0.80 | ||||
| ACE inhibitor/ARB | 636 | 76.9% | 621 | 80.2% | RR 0.96, 95% CI 0.90 to 1.02; P = 0.15 | ||||
| Statin | 668 | 94.6% | 655 | 95.3% | RR 0.99, 95% CI 0.97 to 1.02; P = 0.59 | ||||
| Antiplatelet | 636 | 83.6% | 632 | 84.3% | RR 0.99, 95% CI 0.95 to 1.04; P = 0.74 | ||||
| Dale 2015a | Medication adherence | 8‐item Morisky Medication Adherence Questionnaire (high score indicates better adherence) | Continuous | 6 months | 61 | Mean (SD) = 7.3 (0.9) | 62 | Mean (SD) = 6.8 (1.2) | The intervention group reported greater medication adherence score than the control group (mean difference 0.58, 95% CI 0.19 to 0.97; P = 0.004). |
| Fang 2016 | Medication adherence ‐ statin prescriptions | 4‐item Morisky Medication Adherence Scale (high score indicates worse adherence) | Continuous | 12 months | 95 | Not reported | 93 | Not reported | Participants in the SMS group had better adherence than those in the phone group (OR 0.34, 95% CI 0.18 to 0.63). |
| Huo 2019 | Medication ‐ ACE inhibitor/ARB | ‐ | Categorical | 6 months | 251 | 47% | 251 | 42.6% | There was no difference in the proportion of participants who took ACE inhibitor/ARB (P = 0.32). |
| Medication ‐ aspirin | 86.5% | 87.7% | There was no difference in the proportion of participants who took aspirin (P = 0.69). | ||||||
| Medication ‐ beta‐blocker | 62.2% | 58.2% | There was no difference in the proportion of participants who took beta‐blocker (P = 0.36). | ||||||
| Medication ‐ statin | 84.1% | 87.3% | There was no difference in the proportion of participants who took statin (P = 0.31). | ||||||
| Medication ‐ all 4 cardioprotective medications | 30.7% | 23.9% | There was no difference in the proportion of participants who took all 4 cardioprotective medications (P = 0.089). | ||||||
| Medication ‐ insulin | 10.8% | 13.9% | There was no difference in the proportion of participants who took insulin (P = 0.28). | ||||||
| Medication ‐ oral antidiabetic medication | 43% | 44.6% | There was no difference in the proportion of participants who took oral antidiabetic medication (P = 0.72). | ||||||
| Kamal 2015 | Medication adherence | Morisky Medication Adherence Scale (high score indicates better adherence) | Continuous | 2 months | 83 | Mean (SD) = 7.4 (0.93) | 79 | Mean (SD) = 6.7 (1.32) | Adjusted mean difference 0.54, 95% CI 0.22 to 0.85 |
| Khonsari 2015 | Medication adherence | 8‐item Morisky Medication Adherence Scale (high score indicates better adherence) | Categorical | 2 months | 31 | High adherence = 64.5%; medium adherence = 19.4%; low adherence = 16.1% | 31 | High adherence = 12.9%; medium adherence = 29%; low adherence = 58.1% | The risk of being low‐adherent amongst the control group was 4.09 times greater than in the intervention group (RR 4.09, 95% CI 1.82 to 9.18). |
| Maddison 2021 | Medication adherence | 8‐item Morisky Medication Adherence Scale (High score indicates worse adherence) | Continuous | 12 months | 153 | Not reported | 153 | Not reported | Adjusted mean difference 0.30, 95% CI 0.01 to 0.59; P = 0.04 |
| Medication adherence ‐ aspirin + statin + blood pressure‐lowering drug (ACE inhibitor, ARB, beta‐blocker) | Medication possession ratio by linking community pharmacy dispensing records via the National Pharmaceuticals Collection database | Categorical | 12 months | 83 | 54.2% | 104 | 67.9% | Adjusted OR 0.56, 95% CI 0.35 to 0.89; P = 0.01 | |
| Medication adherence ‐ aspirin + statin + beta‐blocker + ACE inhibitor/ARB | 56 | 36.6% | 70 | 45.7% | Adjusted OR 0.68, 95% CI 0.43 to 1.08; P = 0.11 | ||||
| Medication adherence ‐ statin | 119 | 77.7% | 129 | 84.3% | Adjusted OR 0.65, 95% CI 0.36 to 1.16; P = 0.15 | ||||
| Medication adherence ‐ aspirin | 119 | 77.7% | 123 | 80.3% | Adjusted OR 0.85, 95% CI 0.49 to 1.49; P = 0.58 | ||||
| Medication adherence ‐ beta‐blocker | 89 | 58.1% | 102 | 66.6% | Adjusted OR 0.69, 95% CI 0.43 to 1.11; P = 0.13 | ||||
| Medication adherence ‐ ACE inhibitor/ARB | 97 | 63.4% | 123 | 80.3% | Adjusted OR 0.42, 95% CI 0.25 to 0.71; P = 0.001 | ||||
| Medication adherence ‐ blood pressure‐lowering drugs (ACE inhibitor/ARB and/or beta‐blocker) | 113 | 73.8% | 139 | 90.8% | Adjusted OR 0.28, 95% CI 0.15 to 0.55; P < 0.001 | ||||
| Ni 2022 | Medication non‐adherence | 3‐item, 5‐point Voils Extent Scale (high score indicates worse adherence) | Continuous | 3 months | 103 | Mean difference (SD) = −1.58 (2.49) | 93 | Mean difference (SD) = −0.08 (3.15) | The mean decrease in medication non‐adherence score in the intervention group was greater than the mean decrease in the control group (P < 0.001). |
| Pandey 2017 | Average percentage of days covered during 12 months' follow‐up | Logbooks | Categorical | 12 months | 17 | 94% (95% CI 92 to 96) | 16 | 80% (95% CI 73 to 86) | The mean difference in percentage of days covered between groups was 14% (95% CI 7 to 21, P < 0.001). |
| Proportion of participants with proportion of days covered > 80% over the 12 months | 100% | 50% | All intervention group participants were optimally adherent to their prescribed medications during follow‐up compared with 50% (8/16) of control group participants (P < 0.001). | ||||||
| Park 2014a | Medication adherence | Morisky Medication Adherence Scale (high score indicates better adherence) | Continuous | 1 month | 28 | Mean (SD) = 6.43 (1.22) | 28 | Mean (SD) = 6.96 (1.44) | No difference was found between groups over time (P = 0.16). |
| Medication adherence ‐ antiplatelets | Medication event monitoring system (MEMS) | Continuous | 1 month | 24 | Mean doses taken: 28.2 (3.6) Per cent doses taken: 93.7 (11.9) Per cent correct number doses: 88.0 (14.0) Per cent doses taken on schedule: 86.2 (15.4) |
25 | Mean doses taken: 23.7 (8.3) Per cent doses taken: 79.1 (27.7) Per cent correct number doses: 72.4 (27.4) Per cent doses taken on schedule: 69.0 (29.2) |
Participants who received text messages for antiplatelets had a higher percentage of correct doses taken (P = 0.02), percentage number of doses taken (P = 0.01), and percentage of prescribed doses taken on schedule (P = 0.01). | |
| Medication adherence ‐ statins | Mean doses taken: 27.7 (4.2) Per cent doses taken: 92.4 (14) Per cent correct number doses: 85.4 (16.6) Per cent doses taken on schedule: 84.1 (19.4) |
Mean doses taken: 25 (6.4) Per cent doses taken: 83.3 (21.3) Per cent correct number doses: 73.4 (23.8) Per cent doses taken on schedule: 74.4 (21.1) |
No difference between groups for statin medication | ||||||
| Passaglia 2021 | Medication adherence | Treatment Adherence Measure (MAT) form (high score indicates better adherence) | Categorical | 6 months | 75 | 88% | 72 | 93.1% | OR 0.55, 95% CI 0.17 to 1.72; P = 0.30 |
| Quilici 2013 | Proportion of participants with good medication adherence ‐aspirin (good adherence was defined as taking > 95% of prescribed doses in the past 30 days) | ‐ | Categorical | 1 month | 250 | 97.2% | 249 | 92.8% | Intervention improved self‐reported aspirin adherence (OR 0.37, 95% CI 0.15 to 0.90; P = 0.02). |
| Medication adherence ‐ aspirin | Platelet function testing | Categorical | 1 month | 250 | 94.8% | 249 | 88.8% | Controlled non‐adherent patients accounted for 11.2% of the standard care group versus 5.2% in the SMS intervention group (OR 0.43, 95% CI 0.22 to 0.86; P = 0.01). | |
| Ross 2021 | Medication adherence | 8‐item Morisky Medication Adherence Scale (high score indicates better adherence) Low adherence (< 6), medium adherence (6 to 7), or high adherence (8) |
Continuous | 2 months | 32 | Mean (95% CI) = 6.75 (6.34 to 7.16) | 36 | Mean (95% CI) = 7.05 (6.72 to 7.38) | Mean difference −0.30, 95% CI −0.83 to 0.23; P = 0.27 |
| Proportion of high medication adherence | Categorical | 2 months | 32 | 34% | 36 | 42% | "When categorized into low, medium, and high adherence, 34% (11/32) of those in the Txt2Prevent group and 42% (15/36) of those in the usual care group were classified as high‐adherers (χ2 2=2.10, P=.35)." | ||
| Zheng 2019 | Medication ‐ ACE inhibitor/ARB | ‐ | Categorical | 6 months | 411 | 40.9% | 411 | 46.2% | RR 0.96, 95% CI 0.9 to 1.1; P = 0.35 |
| Medication ‐ aspirin | 92% | 90.8% | RR 1.00, 95% CI 0.97 to 1.02; P = 0.78 | ||||||
| Medication ‐ beta‐blocker | 58.2% | 64% | RR 1.00, 95% CI 0.95 to 1.1; P = 0.76 | ||||||
| Medication ‐ statin | 85.4% | 85.2% | RR 1.01, 95% CI 0.96 to 1.05; P = 0.78 | ||||||
| Medication ‐ calcium channel blocker | 17.5% | 19.7% | RR 0.98, 95% CI 0.8 to 1.2; P = 0.83 | ||||||
| Medication ‐ diuretics | 3.2% | 2.9% | RR 1.20, 95% CI 0.7 to 2.1; P = 0.60 | ||||||
| Medication ‐ aspirin + statin | 81.3% | 80.3% | RR 1.00, 95% CI 0.95 to 1.05; P = 0.99 |
ACE: angiotensin‐converting enzyme; ARB: angiotensin II receptor blockers; CI: confidence interval; IQR: interquartile range; OR: odds ratio; RR: risk ratio; SD: standard deviation; SMS: short message service
Validated survey measures
The most commonly used method to assess medication adherence was survey. A total of 11 studies used validated surveys to measure medication adherence. Eight studies measured medication adherence with the validated eight‐item Morisky Medication Adherence Scale (MMAS‐8) (Bae 2021; Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Maddison 2021; Park 2014a; Ross 2021). MMAS‐8 is a patient‐reported metric and validated tool that is widely used in adherence research. Three studies used other validated surveys to measure medication adherence, including the three‐item, five‐point Voils Extent Scale (Ni 2022), Measurement of Adherence to Treatments (MAT) form (Passaglia 2021), and Medication Adherence Report Scale‐5 (MARS‐5) (Bermon 2021).
Dale 2015a followed up 116 participants for six months and found that participants in the intervention group had a greater medication adherence score (mean difference (MD) 0.58, 95% confidence interval (CI) 0.19, 0.97; P = 0.004) compared to usual care. In particular, this was an MMAS‐8 score of 7.3 (standard deviation (SD) 0.9) for the intervention group and 6.8 (SD 1.2) for the control group at six months' follow‐up. Fang 2016, had a three‐arm design with SMS, SMS + micro letter, or telephone calls (follow‐up of six months and 271 participants analysed) and reported that participants given SMS alone had reduced odds of being non‐adherent compared to those who received telephone reminders (odds ratio (OR) 0.34, 95% CI 0.18 to 0.63), and that participants given SMS + micro letter had the lowest odds of being non‐adherent compared to telephone reminders (OR 0.07, 95% CI 0.03 to 0.15). Kamal 2015 (200 participants, two‐month follow‐up) reported higher levels of adherence in the intervention arm (adjusted MD 0.54, 95% CI 0.22 to 0.85). Khonsari 2015 (62 participants) reported that "the risk of being low adherent [(score 3‐8 according to Morisky 1986)] among the control group is 4.09 times greater than the intervention group (Relative Risk (RR) 4.09, 95% CI 1.82 to 9.18, p<0.001)" at eight weeks' follow‐up. The same study also reported that the proportion of participants with low adherence at two‐month follow‐up was 16.1% in the intervention group and 58.1% in the control group. Park 2014a, with the shortest follow‐up of 30 days and 28 participants analysed in each group, reported a baseline MMAS‐8 score of 6.20 (SD 1.66) for the intervention group and 5.85 (SD 2.10) for the control group. At follow‐up, the score had risen for both groups, but was higher for the control group at 6.73 (SD 1.49) than for the intervention group at 6.43 (SD 1.22) (no P value reported). Ni 2022 found that the mean decrease in medication non‐adherence score in the intervention group (−1.58, SD 2.49) was greater than in the control group (−0.08, SD 3.15) at 90 days (P < 0.001). Passaglia 2021 reported no difference in the proportion of participants who adhered to the medications between intervention (88%) and control group (93.1%) at six months' follow‐up (P = 0.297). Ross 2021 showed no differences in medication adherence scores between intervention and control groups at 60 days (MD −0.3, 95% CI −0.83 to 0.23; P = 0.27). Maddison 2021 found a higher medication adherence score in the control group than in the intervention group (adjusted MD 0.3, 95% CI 0.01 to 0.59; P = 0.04). Bermon 2021 did not find differences in medication adherence at 12 months between intervention and control groups (MD −0.01, 95% CI −0.4 to 0.4; P = 0.96). Bae 2021 reported no difference between intervention and control groups at six months (MD 0.07, 95% CI −0.03 to 0.16; P = 0.19).
Objective measures
In addition to the MMAS‐8 score, Park 2014a used a medication event monitoring system (MEMS) (opening of the two electronic pill bottles provided a time‐stamp corresponding with medication self‐administration) to test for medication adherence, with the following results. Antiplatelet doses taken on schedule were 86.2% (SD 15.4) in the intervention group and 85.7% (SD 18.2) in the control group. For statins, 84.1% (SD 19.4) of doses were taken on schedule by the intervention group and 79.7% (SD 19.3) in the control group. The correct number of antiplatelet doses taken were 88.0% (SD 14.0) in the intervention group and 87.2% (SD 16.5) in the control group. For statins, the correct number of doses taken was 85.4% (SD 16.6) in the intervention group and 81.3% (SD 16.4) in the control group.
Maddison 2021 provided an objective assessment of medication adherence at 24 and 52 weeks based on a medication possession ratio (MPR) of 80% or more for three medication classes (statin, aspirin, and blood pressure‐lowering medications). Adherence to the three medication classes was lower in the intervention group than in the control group at 24 weeks (OR 0.60, 95% CI 0.38 to 0.96; P = 0.03) and 52 weeks (OR 0.56, 95% CI 0.35 to 0.89; P = 0.01).
In Chow 2022, self‐reported cardioprotective medication use was validated through prescription data obtained via linkage with the Pharmaceutical Benefits Scheme (PBS), which is a comprehensive database for all prescribed medications in Australia (Department of Health and Aged Care 2023). The authors found no evidence of a difference in medication adherence between the intervention and control groups.
In addition to self‐reported aspirin adherence, Quilici 2013 also objectively measured aspirin adherence by platelet function testing. They found that text messaging might result in a reduction in non‐adherence to medication (OR 0.43, 95% CI 0.22 to 0.86; P = 0.01).
Self‐reported measures
Pandey 2017 assessed medication adherence in 33 participants with self‐reported logs at 12 months. This resulted in 90% adherence in the intervention group compared to 70% in the control group (P < 0.001). Self‐reported data from Quilici 2013 differed between the text and Figure 2, but showed a higher adherence in the intervention group at 30 days' follow‐up (96.4% (text)/97.2% (Figure 2)) than in the control group (93.6% (text)/92.8 (Figure 2)). The OR for self‐reported aspirin non‐adherence as provided in the paper was 0.37 (95% CI 0.15 to 0.90; P = 0.02). The platelet testing confirmed this by showing a 94.8% adherence in the intervention group and 88.8% in the control group. The paper reported the OR for non‐adherence as 0.43 (95% CI 0.22 to 0.86; P = 0.01). Bae 2021 and Chow 2022 measured self‐reported medication adherence by assessing if the proportion of indicated medications taken was > 80% (> 24/30 days in the preceding one month). Bae 2021 resulted in 98.2% adherence in the intervention group compared to 92.1% in the control group (risk ratio (RR) 1.1, 95% CI 1.0 to 1.1; P < 0.001) at six months. Chow 2022 measured adherence to each medication class (aspirin, beta‐blocker, ACEI/ARB, statin, second antiplatelet) and combined five medications at 12 months; however, no evidence of an effect was found. Bermon 2021 measured self‐reported medication adherence through the assessment of participant’s subjective medication intake compliance on days 7 and 30. No differences were found on days 7 and 30. Chen 2019 assessed medication adherence at six months by asking if participants took medication as prescribed without any medication withdrawal and reduction. They found that medication adherence in the intervention group (78.9%) was higher than in the control group (69.5%), with an RR of 1.14 (95% CI 1.01 to 1.28; P = 0.029). Zheng 2019 reported medication usage at six months' follow‐up and did not find any difference in medication usage for each medication class (ACEI/ARB, aspirin, beta‐blocker, calcium channel blockers, diuretics, statin). Similarly, Huo 2019 reported the proportion of participants who were taking secondary preventive medications, insulin, and oral antidiabetic medication at six months; no evidence of differences was found. In addition, Chow 2015 reported the proportion of participants taking cardioprotective medications at six months' follow‐up. There were no differences in the proportion taking cardioprotective medications between groups.
Fatal cardiovascular events
Meta‐analysis using a random‐effects model found that text messaging may have little to no effect on fatal cardiovascular events compared to usual care (OR 0.83, 95% CI 0.47 to 1.45; I2 = 0%; 4 studies, 1654 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 1: Fatal cardiovascular events
When using a fixed‐effect model in our sensitivity analysis, the estimate was exactly the same as the results from a random‐effects model (OR 0.83, 95% CI 0.47 to 1.45; I2 = 0%; 4 studies, 1654 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 2: Fatal cardiovascular events: sensitivity analysis using fixed‐effect model
Non‐fatal cardiovascular events (coronary heart disease, revascularisation, stroke)
Meta‐analysis for non‐fatal cardiovascular events was not possible because of the lack of reported outcomes. We found very low‐certainty evidence that text messaging may have little to no effect on non‐fatal cardiovascular events. Only two studies reported non‐fatal cardiovascular events, and neither reported a difference between groups.
Chow 2022 reported a number of cardiovascular events, including myocardial infarction (intervention: 2.6% versus control: 2.8%), acute coronary syndrome (intervention: 1.1% versus control: 1.2%), new or worsening angina (intervention: 7.3% versus control: 8.1%), new or worsening heart failure (intervention: 1.7% versus control: 1.3%), coronary angiogram or percutaneous coronary intervention (PCI) (intervention: 8.8% versus control: 9.4%), coronary artery bypass graft (intervention: 3.4% versus control: 2.2%), stroke (intervention: 0.9% versus control: 0.6%), transient ischaemic attack (intervention: 0.6% versus control: 0.3%). Chen 2019 reported no difference between the intervention group (27.4%) and the control group (31.9%) in the incidence of heart failure‐related events (OR 0.86, 95% CI 0.66 to 1.12; P > 0.05 as reported by authors, results not shown in the forest plot).
Combined cardiovascular disease (CVD) event (fatal or non‐fatal events)
Meta‐analysis for combined CVD event was not possible because of the lack of reported data. Based on one study, we found very low‐certainty evidence that text messaging may have little to no effect on combined CVD event. Chow 2022 reported no difference between the intervention group (18.2%) and the control group (17.9%) for combined CVD event (RR 1.02, 95% CI 0.81 to 1.28; P = 0.87, results not shown in the forest plot).
Secondary outcomes
Low‐density lipoprotein (LDL) cholesterol for the effect of statins
Of the eight studies evaluating LDL, all of them analysed LDL as a continuous outcome. Meta‐analysis using a random‐effects model found that text messaging may have little to no effect on LDL cholesterol compared to usual care (MD −1.79 mg/dL, 95% CI −4.71 to 1.12; I2 = 61%; 8 studies, 4983 participants; very low‐certainty evidence; Analysis 1.3), but the evidence is very uncertain. We converted mmol/L cholesterol to mg/dL using a multiplier of 38.67, as recommended by Rugge 2011.
1.3. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 3: Low‐density lipoprotein cholesterol
When using a fixed‐effect model in our sensitivity analysis, we found that the evidence is very uncertain for the effect of text messaging on LDL cholesterol compared to usual care (MD −1.76 mg/dL, 95% CI −3.49 to −0.03; I2 = 61%; 8 studies, 4983 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 4: Low‐density lipoprotein cholesterol: sensitivity analysis using fixed‐effect model
When excluding studies at high risk of bias, the evidence is very uncertain for the effect of text messaging on LDL cholesterol compared to usual care (MD −1.60 mg/dL, 95% CI −8.02 to 4.82; I2 = 75%; 3 studies, 1872 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 5: Low‐density lipoprotein cholesterol: sensitivity analysis excluding studies with high risk of bias
Blood pressure for antihypertensive drugs
Systolic blood pressure (SBP)
Of the nine studies recording SBP, all of them reported SBP as a continuous outcome and reported endpoint value. However, one study only reported mean change from baseline within intervention and control groups, and SD for mean change (Ni 2022). Mean/SD at the endpoint were not reported and could not be transformed due to lack of information. Consequently, data from this study could not be pooled, and it was excluded from the meta‐analysis.
Meta‐analysis using a random‐effects model found that text messaging may have little to no effect on SBP compared to usual care (MD −0.93 mmHg, 95% CI −3.55 to 1.69; I2 = 87%; 8 studies, 5173 participants; very low‐certainty evidence; Analysis 1.6), but the evidence is very uncertain.
1.6. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 6: Systolic blood pressure
When using a fixed‐effect model in our sensitivity analysis, the results showed that text messaging may reduce SBP compared to usual care (MD −1.39 mmHg, 95% CI −2.27 to −0.51; I2 = 87%; 8 studies, 5173 participants; very low‐certainty evidence; Analysis 1.7), but the evidence is very uncertain.
1.7. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 7: Systolic blood pressure: sensitivity analysis using fixed‐effect model
When excluding studies at high risk of bias, the results showed that text messaging may have little to no effect on SBP compared to usual care (MD −0.13, 95% CI −2.28 to 2.02; I2 = 46%; 3 studies, 2062 participants; very low‐certainty evidence; Analysis 1.8), but the evidence is very uncertain.
1.8. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 8: Systolic blood pressure: sensitivity of analysis excluding studies with high risk of bias
We detected one outlier where the CI did not overlap with the CI of the summary effect size. When this outlier was removed, the sensitivity analysis showed that the evidence is very uncertain for the effects of text messaging on SBP (MD 0.04, 95% CI −1.26 to 1.34; I2 = 36%; 7 studies, 4463 participants; very low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 9: Systolic blood pressure: sensitivity analysis excluding outlier
Diastolic blood pressure (DBP)
Of the six studies evaluating DBP, all of them analysed DBP as a continuous outcome and reported endpoint value. However, one study only reported mean change from baseline within intervention and control groups, and SD for mean change (Ni 2022). Mean/SD at the endpoint were not reported and could not be transformed due to lack of information. Consequently, data from this study could not be pooled, and it was excluded from the meta‐analysis.
Meta‐analysis using a random‐effects model found that text messaging may have little to no effect on DBP compared to usual care (MD −1.00 mmHg, 95% CI −2.49 to 0.50; I2 = 67%; 5 studies, 3137 participants; very low‐certainty evidence; Analysis 1.10), but the evidence is very uncertain.
1.10. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 10: Diastolic blood pressure
When using a fixed‐effect model in our sensitivity analysis, the results showed that text messaging might reduce DBP compared to usual care (MD −0.91 mmHg, 95% CI −1.63 to −0.19; I2 = 67%; 5 studies, 3137 participants; very low‐certainty evidence; Analysis 1.11), but the evidence is very uncertain.
1.11. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 11: Diastolic blood pressure: sensitivity analysis using fixed‐effect model
When excluding studies at high risk of bias, the results showed that text messaging had little to no effect on DBP compared to usual care (MD 0.09, 95% CI −0.96 to 1.14; I2 = 0%; 2 studies, 1335 participants; very low‐certainty evidence; Analysis 1.12), but the evidence is very uncertain.
1.12. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 12: Diastolic blood pressure: sensitivity analysis excluding studies with high risk of bias
Heart rate for the effect of beta‐blockers
Five studies evaluated heart rate as a continuous outcome. One study, Ni 2022, only reported mean change from baseline within intervention and control groups, and SD for mean change. Mean/SD at the endpoint were not reported and could not be transformed due to lack of information. Consequently, data from this study could not be pooled, and it was excluded from the meta‐analysis.
Meta‐analysis using a random‐effects model found that text messaging may have little to no effect on heart rate compared to usual care (MD −0.46 beats per minute (bpm), 95% CI −1.74 to 0.82; I2 = 57%; 4 studies, 2946 participants; very low‐certainty evidence; Analysis 1.13), but the evidence is very uncertain.
1.13. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 13: Heart rate
When using a fixed‐effect model in our sensitivity analysis, the results showed that text messaging may have little to no effect on heart rate compared to usual care (MD −0.57 bpm, 95% CI −1.34 to 0.21; I2 = 57%; 4 studies, 2946 participants; very low‐certainty evidence; Analysis 1.14), but the evidence is very uncertain.
1.14. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 14: Heart rate: sensitivity analysis using fixed‐effect model
When excluding studies at high risk of bias, only one study was left, which showed that text messaging may have little to no effect on heart rate compared to usual care (MD −0.40 bpm, 95% CI −1.64 to 0.84; 1 study, 1159 participants; very low‐certainty evidence; Analysis 1.15), but the evidence is very uncertain.
1.15. Analysis.

Comparison 1: Text messaging versus usual care, Outcome 15: Heart rate: sensitivity analysis excluding studies with high risk of bias
Urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin
No study reported data on this outcome.
Adverse effects
Only one study evaluated road traffic crashes related to the intervention (Bermon 2021). However, Bermon 2021 stated that no adverse effects were reported by participants, and no traffic accidents occurred in their study.
No study reported repetitive thumb injury related to the intervention.
Patient‐reported experience
Perception of the text messaging intervention was reported in 13 out of 18 studies as utility, acceptability, satisfaction, and recommendation of the programme to other patients. All 13 studies reported high levels of satisfaction, acceptability, and utility with the intervention, expressed strong desire for programme continuation, and demonstrated high interest in the concept. Kamal 2015 reported that 96% of participants were satisfied with the intervention, and 95.6% of participants felt the intervention was acceptable. Park 2014a reported that in the intervention group receiving text messaging reminders and education, 82% participants strongly or moderately agreed that they were satisfied with receiving text messaging for health, and 71% strongly or moderately agreed that it helped them take their medications. Dale 2015a reported that 77% of participants felt the Text4Heart programme (both text messaging and Web access) helped them change their behaviour, and 90% of participants would recommend the programme to other people who had a heart event. Most participants felt that the programme helped them learn about (47/61, 77%) and recover (51/61, 84%) from their heart event. Quilici 2013 reported that 92% of participants found the text messaging support to be valuable. Chow 2015 found that most participants agreed that the text message support programme was useful (91%), easy to understand (97%), and motivated their behaviour change (77%).
The TEXTME study by Chow 2015 also has a parallel, detailed qualitative analysis that summarises engagement and behavioural mechanisms of the texting programme effectiveness (Redfern 2016). The TEXTMEDS study by Chow 2022 reported that most people agreed or strongly agreed that the text message programme was useful (86%), easy to understand (94%), reminded them to take their regular medications (63%), and motivated them to change their lifestyle behaviour (63%). Huo 2019 found that almost all participants believed the text messages were useful (94.1%) and easy to understand (97.1%); 74.1% of participants reported saving messages for further learning, and 94% of participants would like to continue to receive the text messages to improve their knowledge and support disease management in the future. Zheng 2019 found that participants perceived the text messages as useful (96.1%) and easy to understand (98.8%); 69.4% of participants saved messages for further learning, and 94.8% of participants would like to continue to receive the text messages in the future. Maddison 2021 reported that 97.8% of participants would recommend the programme to others. The text messaging programme was perceived to be helpful in managing heart disease (82.6%); learning about heart condition (62.3%); recovery from heart condition (82.6%); and changing behaviour (56.5%). Bae 2021 reported that text messages were perceived to be helpful (82.0%), easy to understand (94.6%), a good motivation for changing behaviour (78.2%), and reminding to take medication (52.7%).
In Khonsari 2015, most participants found that the SMS was useful (93.5%) and helped them take their medications (64.5%). All participants (100%) would recommend the intervention to other patients, and 80.6% of the participants requested that the text messaging reminders be continued. Ross 2021 reported that 94% of participants agreed or strongly agreed that they were satisfied; 74% agreed or strongly agreed that it helped them manage their condition; and 94.4% would recommend the programme to others. Passaglia 2021 found that 79.2% of participants read all SMS; 88.7% totally agree that SMS helped them change daily habits; and 86.8% totally agree that the messages were helpful for their treatment.
Discussion
Summary of main results
We identified 18 studies eligible for inclusion in the review, seven from the original review and 11 newly added in the current update. The number of newly added studies shows the rapid growth in this research area and increased evidence of using text messaging in CVD secondary prevention. A total of 8136 participants were included in the analysis. The 18 included studies were conducted in 11 countries, including six high‐income countries and five middle‐income countries. None of the included studies was from a low‐income country. All 18 studies included participants with CVDs. Participants had various CVDs, including acute coronary syndrome, coronary heart disease, stroke, myocardial infarction, heart failure, and angina. Mean duration of the text messaging was five months. There was variation in the characteristics of text messages amongst studies (delivery method, frequency, theoretic grounding, content used, personalisation, directionality). The message content primarily included mixed functionalities, covering medication reminders, and healthy lifestyle information. We assessed the overall risk of bias for all studies as high, as all studies had at least one domain at unclear or high risk of bias (Figure 3).
This review provides very low‐certainty evidence of the effects of text messaging on medication adherence (Table 1). All 18 studies included in this review reported on medication adherence. Ten out of 18 studies showed a beneficial effect of mobile phone text messaging for medication adherence (Bae 2021; Chen 2019; Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Ni 2022; Pandey 2017; Park 2014a; Quilici 2013). The other eight studies showed that text messaging resulted in either a reduction or no difference in medication adherence when compared with usual care. Due to the heterogeneity with respect to diverse measurement methods and various definitions, medication adherence was not pooled in a meta‐analysis, thus the evidence is uncertain.
The certainty of evidence relating to the effect of text messaging on fatal cardiovascular events, LDL cholesterol, blood pressure, and heart rate was low or very low. Pooled analysis found that text messaging may provide little to no benefit for fatal cardiovascular events, LDL cholesterol, SBP, DBP, and heart rate compared to usual care. Sensitivity analyses removing studies with high risk of bias and outliers revealed similar findings that text messaging has little to no effect on LDL cholesterol, SBP, DBP, and heart rate.
There was insufficient evidence of text messaging interventions for non‐fatal cardiovascular events, combined CVD events, urinary 11‐dehydrothromboxane B2, and adverse events due to sparse data available for synthesis. No study measured urinary 11‐ dehydrothromboxane B2. Only two studies measured non‐fatal cardiovascular events, and only one study measured combined CVD events and adverse events. Studies reported high levels of acceptability, utility, and satisfaction with the text messaging programme.
Overall, these data suggest that the current evidence for text messaging in the CVD population is of low and very low certainty, and therefore insufficient to guide clinical practice and policy. The results showed little to no evidence of an effect of text messaging on medication adherence, fatal cardiovascular events, non‐fatal cardiovascular events, combined CVD events, blood pressure, LDL cholesterol, and heart rate. However, people with CVD tend to have a high level of satisfaction, acceptability, and utility with text messaging. Along with the growth of mobile phone usage globally, future work should also focus on a deep understanding of the enablers and barriers associated with the effectiveness of interventions.
Overall completeness and applicability of evidence
The search strategy identified all relevant studies up until August 2023. The 18 included studies varied in terms of their context, intervention characteristics, and the measurement method and definitions of study outcomes, which makes summarising the data difficult and reduces the certainty of the estimates produced. The studies included in our meta‐analysis had relatively small sample sizes, short study durations, and were of low quality. Small sample size hindered our ability to generalise our findings to a wide population. Short‐term durations prevented evaluation of long‐term intervention effectiveness. The low quality of the included studies reduced our confidence in the certainty of evidence.
The evidence in this review is applicable to a predominantly male population aged between 50 and 65 years. Participants had various CVDs, including acute coronary syndrome, coronary heart disease, stroke, myocardial infarction, heart failure, and angina, therefore indicating an appropriate representation of population.
Eight studies took place in high‐income countries (USA, Australia, Korea, Canada, and New Zealand), and eight took place in upper‐middle‐income countries (China, Malaysia, Brazil, and Colombia). Only one study was conducted in a lower‐middle‐income country (Pakistan) (World Bank 2023). It was uncertain where one study was conducted, but it was likely from a high‐income country (France). It is therefore unclear whether the results would apply to low‐income countries.
All studies used text messaging as a central component of the intervention. However, the characteristics of text messaging (e.g. message frequencies, timing, content, degree of personalisation, ability for patients to respond, and degree of automation) varied across the 18 studies. The various characteristics and delivery method of text messages may have influenced how the intervention might work in a CVD population. In addition, details about the specific theoretical framework, directionality, and personalisation of text messages were lacking in some studies. Seven out of 18 studies did not specify if text messages were designed based on a theoretical approach or framework (e.g. behavioural change techniques or psychological theories). We attempted but were unable to classify the content of messages according to behaviour change techniques due to limited description of behaviour change techniques and text message contents in the studies. Future studies should include comprehensive descriptions of the interventions delivered.
Most of the included studies examined medications and diseases singly, which has implications for the generalisability of results, given that most people may have comorbidities or be on multiple medications. It is worth noting that some studies listed medication adherence as a secondary outcome rather than a primary outcome, which suggests that these studies may not be designed around the focus of our review.
Although all 18 included studies reported the primary outcome of medication adherence, the variability in outcome measures and measurement tools used limited our ability to conduct meta‐analysis. In addition, only a few studies reported data on adverse effects, non‐fatal cardiovascular events, and combined cardiovascular events, and no studies reported on urinary 11‐dehydrothromboxane B2. Insufficient reporting makes it difficult to draw any conclusions for these outcomes.
The most common comparator for the text messaging intervention was reported as "usual care". However, details on usual care were lacking in most of the included studies. In addition, definitions of usual care varied across studies, which were likely to influence the size of the effect estimates.
We identified nine ongoing studies, ranging from 78 participants, ISRCTN10549665, to 2500 participants, ACTRN12621000754842. Three of these nine studies are being conducted in high‐income countries. As these nine studies have not yet reported any results, our findings were limited to those studies that have reported results and could be pooled in meta‐analysis.
There appears to be little or no difference between mobile text messaging and usual care in improving medication adherence for secondary prevention of CVD. There are many potential factors that could contribute to the narrow gap in medication adherence between intervention and control groups. These factors may include low quality of study design, simple intervention design, short duration of intervention delivery, lack of theoretical framework in the development of text messages, small sample size of participants, etc. For example, Ross 2021 only used a simple design of text messaging programme (one‐way messages, prespecified order of SMS text messages that was not personalised), and only sent seven text messages that covered medication‐related topics. Furthermore, random messages received by participants in the control group may remind them to take their medications and lead to biased results. However, most control groups included in this review did not send text messages to participants, and so the impact of this bias on the results was very minimal.
Quality of the evidence
Overall, the summary of findings table shows evidence varying between low and very low certainty.
The included studies were of poor methodological quality, with each study having at least one risk of bias domain judged as unclear or high risk. All studies were at high risk of bias for blinding of participants and personnel (performance bias). Eleven out of 18 studies were at either high or unclear risk of bias for blinding of outcome assessors (detection bias). Only eight studies were at low risk for both allocation concealment and random sequence generation. Hence, we downgraded the certainty of evidence for all outcomes by one level, as the results of the meta‐analysis may have been influenced by the low quality of the included studies. Sensitivity analysis removing low‐quality studies revealed little to no effect for text messaging on LDL cholesterol, SBP, DBP, and heart rate. However, very few studies of high quality were included in the review. Future studies would benefit from having robust study designs.
We identified considerable heterogeneity, including methodological heterogeneity due to differences in the criteria for defining outcome measures and measurement method, and clinical heterogeneity related to the characteristics of text messaging. Although all of the included studies used mobile phones to deliver the intervention, we identified substantial differences in the actual content of the SMS. Seven studies lacked information on whether message content was supported by psychological or behaviour change models. The considerable heterogeneity not only has implications for the applicability of the evidence, but also indicates that the quality of reporting for trials evaluating mobile phone interventions is very poor. Additionally, CIs across studies showed minimal overlap. Consequently, we downgraded the certainty of the evidence one level for inconsistency for all outcomes except fatal cardiovascular events, which had low statistical heterogeneity.
We also downgraded the certainty of the evidence for all outcomes by one level for imprecision because the included studies showed different directions of effect, and CIs crossed the no‐effect line and encompassed positive and negative effects. Indirectness was less of an issue in this review update. All included participants had CVDs, indicating an appropriate representation of population. Publication bias could not be assessed reliably for outcomes due to the small number of studies (< 10) that could be included in the meta‐analysis.
Potential biases in the review process
We acknowledge that, although systematic searches across a number of resources were conducted, any search has limitations for pragmatic reasons. Publication bias with the tendency to report positive findings cannot be excluded. Furthermore, some of the included studies had missing endpoint data, which restricted the inclusion of these studies in the meta‐analysis. Consequently, unpublished and missing data might lead to bias in the pooled effect (Hopewell 2009). We tried to overcome this potential limitation by searching clinical trial registries for data on prospectively registered trials. However, eligible studies published after the last search date could have been missed. In addition, although we did not exclude non‐English articles, non‐English articles published in non‐English journals might have been missed in this review and could have potentially introduced language bias.
We were unable to perform planned subgroup analyses to investigate reasons for heterogeneity due to a lack of details or limited number of studies reporting on the outcome of interest. Study effects may be influenced by the observed heterogeneity. It should be noted that some included studies may not have reported all the data they collected, as study protocols were not available for all studies. As a result, the analysis may be incomplete. Another potential source of bias is that due to variations in approaches used to measure and report outcomes, we were unable to include all reported data in the meta‐analysis. Consequently, internal validity of the meta‐analysis might be affected. For example, studies reporting on medication adherence reported the data as either proportion of days of medication covered or mean change in medication adherence score, making it difficult to group all studies for a comprehensive synthesis and comparison, and limiting our confidence in conclusions on the efficacy of interventions. Furthermore, medication event monitoring system (MEMS) data have their limitations, as patients may unscrew the MEMS cap without actually ingesting the medication. Finally, the use of available data in data analysis may not reflect the entire evidence base and thus threaten the validity of the results. We found two studies that did not report non‐fatal cardiovascular events as per their protocol. We attempted to contact the study authors to obtain the data, but received no response.
Agreements and disagreements with other studies or reviews
This review update includes 11 new studies compared with the original review. Our findings of mixed evidence for the effects of text messaging and no reported harms are consistent with the previous review (Adler 2017). It is worth noting that in the previous review, six of seven included studies showed a beneficial effect on improving medication adherence. However, of the 11 new studies included in the review update, only three studies demonstrated a positive effect on medication adherence. The potential reasons contributing to this interesting finding need further exploration, which may be influenced by participant characteristics and intervention delivery format. Compared with the original review, which included small‐scale studies, the new studies identified in the review update were applied in more countries and larger populations, indicating a higher level of generalisability of study findings.
Limited systematic reviews have examined the effect of text messaging on medication adherence in people with CVD. Zhao 2019 conducted a systematic review and meta‐analysis evaluating the effect of text message reminders on medication adherence in people with coronary heart disease. Their study included only 2 trials with 144 participants for meta‐analysis and found that medication adherence in the intervention group was 2.85 times greater than in the control group. Compared with Zhao 2019, our review update expanded participants to CVD and included more studies for a comprehensive analysis. The mixed results observed in the 18 included studies may reflect the challenges involved in improving adherence, which are possibly attributed to multiple intentional (e.g. cost, side effects, availability) and unintentional (e.g. forgetfulness, lack of health literacy) factors (Thorneloe 2018). It might not be easy to address intentional medication adherence solely by text messaging, which may partly explain the inconsistent results of text messaging across studies. How best to design text messaging interventions to address intentional factors to non‐adherence to medication is important. Our review found that, of the 10 studies reporting benefits on medication adherence, eight studies incorporated medication reminders as straight message contents, which might be considered as a key element of text messaging in overcoming medication non‐adherence (Bae 2021; Chen 2019; Fang 2016; Kamal 2015; Khonsari 2015; Ni 2022; Pandey 2017; Park 2014a). However, there was insufficient power to quantitatively explore heterogeneity due to different content. Another systematic review including nine studies on the use of text messaging interventions for secondary prevention of CVD found that text messaging was effective in improving medication adherence (Unal 2018). However, half of their included studies (four out of nine) included hypertension as the primary condition, which was inconsistent with the inclusion criteria of our review. Our review is comparable to a previous systematic review of mobile phone interventions (e.g. text messaging, mobile apps, or telemonitoring) in the secondary prevention of CVD, which found that text messaging might positively impact the secondary prevention of CVD, but failed to draw any robust conclusions due to high heterogeneity and inability to conduct meta‐analysis (Park 2016).
Whilst there is not a great deal of evidence on mobile text messaging for adherence in secondary prevention, it can be useful to look into research into what has been successful in tackling other chronic conditions. Ershad 2016 conducted a systematic review to examine the effectiveness of mobile phone text messaging in improving medication adherence for people with chronic diseases. They included 34 studies, including 22 RCTs, one quasi‐experimental, two prospective, two observational, and four cohort studies. Although meta‐analysis was not conducted due to the high heterogeneity in the types of included studies, they found that in 85% of included studies, text messaging improved medication adherence in people with chronic diseases. One review on mobile text messaging for medication adherence in all chronic diseases found that mobile text messaging nearly doubled the odds of medication adherence (Thakkar 2016). However, the issue of heterogeneity still existed, as substantial statistical heterogeneity (I2 = 62%) across the included studies was identified. Furthermore, the included RCTs had a short intervention duration (12 weeks), and whether the adherence improvement could translate into the benefits in clinical outcomes was not assessed. In contrast to the positive findings of medication adherence found in these two reviews, our review update did not demonstrate consistent results. It is uncertain whether text messaging is more effective in improving medication adherence for other chronic conditions when compared with people with CVD.
Authors' conclusions
Implications for practice.
Overall, this review suggests that the current evidence for the use of text messaging in people with cardiovascular disease (CVD) is of low or very low certainty. Half of the included studies (10/18) showed that text messaging interventions improved medication adherence. The remaining eight studies showed that text messaging resulted in either a reduction or no difference in medication adherence when compared with usual care. Due to the heterogeneous nature of study outcomes, we could not conduct meta‐analysis for medication adherence. Consequently, there is insufficient evidence to determine the effects of text messaging on medication adherence for people with CVD.
Due to limited evidence, we are uncertain if text messaging reduces fatal and non‐fatal cardiovascular events, and combined cardiovascular events in people with CVD when compared to usual care. Furthermore, there may be little to no impact of text messaging on low‐density lipoprotein cholesterol, blood pressure, and heart rate for people with CVD. The findings of this review therefore cannot provide important implications for practices, and further evaluation is warranted.
Implications for research.
The results of the review update should be interpreted with caution, as pooling of the results may be influenced by methodological and clinical heterogeneity. More large‐scale, theory‐based randomised trials that are adequately powered and of high quality are therefore needed to provide more precise estimates of the effect of interventions. Future studies should synthesise data from high‐quality studies in order to provide stronger evidence.
Given that the effectiveness of text messaging may be strongly influenced by the context in which it is applied, the need to perform process evaluation and qualitative studies to enhance contextual understanding of randomised controlled trial findings and identify the individual and organisational‐level factors affecting the implementation, adoption, and effectiveness of interventions is highlighted. There is a lack of evidence from low‐income countries, as all of the included studies were conducted in middle‐ and high‐income countries. More research should be conducted in low‐income countries to bridge the knowledge gap and to assess whether text messaging is beneficial in that setting. No studies included in our review update investigated the long‐term effects (> 12 months) of text messaging. Given that in most cases lifelong adherence to medications is required, a long‐term sustainability (> 12 months) of text messaging should be explored in future studies. The findings of high utility, feasibility, and satisfaction for the delivery of text messaging are encouraging for future studies of mobile text messaging to support secondary prevention goals.
We were unable to perform subgroup analysis (e.g. directionality of text messaging, personalisation, intervention modality) in this updated review due to insufficient data. Whilst there has been growing research in this area, there are still a lot of unsolved questions. This review identified that large variability exists with regard to the content of text messages. It suggests that future reviews should carry out content analysis of text messages to ascertain the optimal content of text messages. In addition, seven out of 18 studies did not specify if text messages were designed based on a theoretical approach or framework (e.g. behavioural change techniques or psychological theories). Consequently, there is a need for improvement in intervention design through incorporation of theory‐based tailoring strategies to address medication non‐adherence. Furthermore, future reviews need to explore the optimal frequency and timing of intervention delivery and use process evaluations to assess the mechanisms by which messages have an effect. In future studies, the form of text messaging might be changed into graphics, video, or audio that is delivered via multimedia messaging services. Future studies should therefore provide sufficient detailed description of the intervention to allow identification of the effective intervention components and to enable rigorous evaluations to be performed. In the future development of text messaging interventions, a wide range of factors influencing adherence that may be amenable to change needs to be fully considered. In addition, cost‐effectiveness of text messaging was seldom evaluated amongst the included studies; in future studies, cost efficiency should be considered.
We found that substantial variability exists with regard to the definition of medication adherence and methods of adherence assessment across studies, making it difficult to group studies for comprehensive comparison. The vast majority of the studies evaluating medication adherence were reliant on participants’ self‐report and subjective measurement, which are subject to recall bias and social desirability bias. It is therefore of particular importance that standardised and objective approaches to quantify medication adherence (e.g. development of free and validated scores, pill counts or prescription refill rates) are used to improve comparability of outcome measures across studies for a more reliable and rigorous assessment of the effectiveness of text messaging for CVD. Although there is a lack of high‐quality evidence of supporting the intervention effects in this review update, the high number of ongoing studies indicate that the evidence will continue to evolve over time. Consequently, we recommend that review updates be performed regularly to provide nuanced insight and exert new evidence to guide policy and research.
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2024 | New search has been performed | We updated our search to 30 August 2023. Eleven new studies have been added. Conclusions are not changed. |
| 27 March 2024 | New citation required but conclusions have not changed | This is a review update based on the previous version that was searched in November 2016. |
History
Protocol first published: Issue 8, 2015 Review first published: Issue 4, 2017
Acknowledgements
We thank the staff of Cochrane Heart Group for their assistance with this review. We also acknowledge the contribution of previous authors (Carlos D Tajer, Onikepe O Owolabi, and Javier Mariani), who were not listed as the authors of this review update.
Editorial and peer‐reviewer contributions
Cochrane Heart supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Rui Providencia, Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, London;
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Joanne Duffield, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, and supported the editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service;
Copy Editor (copy editing and production): Lisa Winer, Cochrane Central Production Service;
Peer reviewers (provided comments and recommended an editorial decision): Professor Gerry Molloy, School of Psychology, University of Galway, Ireland (clinical/content review), Dr Azimuddin Azim Siraj, Ministry of Health, Brunei Darussalam (consumer review), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review), Jo Platt, Information Specialist, Cochrane Central Editorial Service (search review). One additional peer reviewer provided clinical/content peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
CENTRAL
#1MeSH descriptor: [Reminder Systems] this term only #2MeSH descriptor: [Telemedicine] this term only #3MeSH descriptor: [Cell Phones] explode all trees #4sms #5mms #6short near/6 messag* #7text near/6 messag* #8texting #9telemedicine* #10reminder next/6 (text* or system* or messag*) #11telehealth #12mobile near/6 (health* or phone*) #13mhealth #14telemonitor* #15#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16MeSH descriptor: [Cardiovascular Diseases] explode all trees #17cardio* #18cardia* #19heart* #20coronary* #21angina* #22ventric* #23myocard* #24pericard* #25isch?em* #26emboli* #27arrhythmi* #28thrombo* #29atrial next fibrillat* #30tachycardi* #31endocardi* #32(sick near/2 sinus) #33MeSH descriptor: [Stroke] explode all trees #34stroke or strokes #35cerebrovasc* #36cerebral next vascular #37apoplexy #38brain near/2 accident* #39(brain* or cerebral or lacunar) near/2 infarct* #40peripheral next arter* next disease* #41aortic* #42arterial near/2 occlus* #43infarct* #44#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 #45#15 and #44
MEDLINE OVID
1. Reminder Systems/ 2. Telemedicine/ 3. exp Cell Phones/ 4. sms.tw. 5. mms.tw. 6. (short adj messag*).tw. 7. (text adj messag*).tw. 8. texting.tw. 9. telemedicine*.tw. 10. (reminder adj (text* or system* or messag*)).tw. 11. telehealth.tw. 12. (mobile adj (health* or phone*)).tw. 13. mhealth.tw. 14. telemonitor*.tw. 15. or/1‐14 16. exp Cardiovascular Diseases/ 17. cardio*.tw. 18. cardia*.tw. 19. heart*.tw. 20. coronary*.tw. 21. angina*.tw. 22. ventric*.tw. 23. myocard*.tw. 24. pericard*.tw. 25. isch?em*.tw. 26. emboli*.tw. 27. arrhythmi*.tw. 28. thrombo*.tw. 29. atrial fibrillat*.tw. 30. tachycardi*.tw. 31. endocardi*.tw. 32. (sick adj sinus).tw. 33. exp Stroke/ 34. (stroke or strokes).tw. 35. cerebrovasc*.tw. 36. cerebral vascular.tw. 37. apoplexy.tw. 38. (brain adj2 accident*).tw. 39. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 40. peripheral arter* disease*.tw. 41. aortic*.tw. 42. (arterial adj occlus*).tw. 43. infarct*.tw. 44. or/16‐43 45. 15 and 44 46. randomized controlled trial.pt. 47. controlled clinical trial.pt. 48. randomized.ab. 49. placebo.ab. 50. drug therapy.fs. 51. randomly.ab. 52. trial.ab. 53. groups.ab. 54. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 55. exp animals/ not humans.sh. 56. 54 not 55 57. 45 and 56
Embase OVID
1. reminder system/ 2. telemonitoring/ 3. mobile phone/ 4. sms.tw. 5. mms.tw. 6. (short adj messag*).tw. 7. (text adj messag*).tw. 8. texting.tw. 9. telemedicine*.tw. 10. (reminder adj (text* or system* or messag*)).tw. 11. telehealth.tw. 12. (mobile adj (health* or phone*)).tw. 13. mhealth.tw. 14. telemonitor*.tw. 15. or/1‐14 16. exp cardiovascular disease/ 17. cardio*.tw. 18. cardia*.tw. 19. heart*.tw. 20. coronary*.tw. 21. angina*.tw. 22. ventric*.tw. 23. myocard*.tw. 24. pericard*.tw. 25. isch?em*.tw. 26. emboli*.tw. 27. arrhythmi*.tw. 28. thrombo*.tw. 29. atrial fibrillat*.tw. 30. tachycardi*.tw. 31. endocardi*.tw. 32. (sick adj sinus).tw. 33. cerebrovascular accident/ 34. (stroke or strokes).tw. 35. cerebrovasc*.tw. 36. cerebral vascular.tw. 37. apoplexy.tw. 38. (brain adj2 accident*).tw. 39. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 40. peripheral arter* disease*.tw. 41. aortic*.tw. 42. (arterial adj occlus*).tw. 43. infarct*.tw. 44. or/16‐43 45. 15 and 44 46. random$.tw. 47. factorial$.tw. 48. crossover$.tw. 49. cross over$.tw. 50. cross‐over$.tw. 51. placebo$.tw. 52. (doubl$ adj blind$).tw. 53. (singl$ adj blind$).tw. 54. assign$.tw. 55. allocat$.tw. 56. volunteer$.tw. 57. crossover procedure/ 58. double blind procedure/ 59. randomized controlled trial/ 60. single blind procedure/ 61. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 62. (animal/ or nonhuman/) not human/ 63. 61 not 62 64. 45 and 63
Conference Proceedings Citation Index – Science
#5 #4 AND #3 #4 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) #3 #2 AND #1 #2 TS=( cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or "atrial fibrillat*" or tachycardi* or endocardi* or "sick sinus" or stroke or strokes or cerebrovasc* or "cerebral vascular" or apoplexy or "brain accident*" or infarct* or "peripheral arter* disease*" or aortic* or "arterial occlus*") #1 TS=(sms or mms or "short messag*" or "text messag*" or texting or telemedicine* or "reminder text*" or "reminder system*" or "reminder messag*" or telehealth or "mobile health*" or " mobile phone*" or mhealth or telemonitor*)
CINAHL
S1. TI (Reminder Systems or Telemedicine or Cell Phones or sms or mms or short messag* or text messag* or texting or telemedicine* or reminder text* or reminder system* or reminder messag* or telehealth or mobile health* or mobile phone* or mhealth or telemonitor*) S2. AB (Reminder Systems or Telemedicine or Cell Phones or sms or mms or short messag* or text messag* or texting or telemedicine* or reminder text* or reminder system* or reminder messag* or telehealth or mobile health* or mobile phone* or mhealth or telemonitor*) S3. MH ‘text messaging’ S4. MH ‘mobile phone’ S5. MH ‘telehealth’ S6. S1 OR S2 OR S3 OR S4 OR S5 S7. TI (Cardiovascular Diseases or cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or atrial fibrillat* or tachycardi* or endocardi* or (sick adj sinus) or stroke or (stroke or strokes) or cerebrovasc* or cerebral vascular or apoplexy or (brain adj2 accident*) or (brain* or cerebral or lacunar) adj2 infarct* or peripheral arter* disease* or aortic* or (arterial adj occlus*) or infarct*) S8. AB (Cardiovascular Diseases or cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or atrial fibrillat* or tachycardi* or endocardi* or (sick adj sinus) or stroke or (stroke or strokes) or cerebrovasc* or cerebral vascular or apoplexy or (brain adj2 accident*) or (brain* or cerebral or lacunar) adj2 infarct* or peripheral arter* disease* or aortic* or (arterial adj occlus*) or infarct*) S9. MH ‘cardiovascular disease’ S10. S7 OR S8 OR S9 S11. PT randomized controlled trial S12. PT randomised controlled trial S13. PT controlled clinical trial S14. AB randomized S15. AB randomised S16. AB placebo S17. AB drug therapy S18. AB randomly S19. AB trial S20. AB groups S21. S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S22. S6 AND S10 AND S21
Scopus
( TITLE‐ABS‐KEY ( sms or mms or "short messag*" or "text messag*" or texting or telemedicine* or "reminder text*" or "reminder system*" or "reminder messag*" or telehealth or "mobile health*" or " mobile phone*" or mhealth or telemonitor* ) ) AND ( TITLE‐ABS‐KEY ( cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or "atrial fibrillat*" or tachycardi* or endocardi* or "sick sinus" or stroke or strokes or cerebrovasc* or "cerebral vascular" or apoplexy or "brain accident*" or infarct* or "peripheral arter* disease*" or aortic* or "arterial occlus*") ) AND ( TITLE‐ABS‐KEY ( random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) )
ProQuest
S1. AB,TI (sms or mms or "short messag*" or "text messag*" or texting or telemedicine* or "reminder text*" or "reminder system*" or "reminder messag*" or telehealth or "mobile health*" or " mobile phone*" or mhealth or telemonitor*) S2. AB,TI (cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or "atrial fibrillat*" or tachycardi* or endocardi* or "sick sinus" or stroke or strokes or cerebrovasc* or "cerebral vascular" or apoplexy or "brain accident*" or infarct* or "peripheral arter* disease*" or aortic* or "arterial occlus*") S3. S1 AND S2 S4. AB,TI (random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) S5. S3 AND S4
Clinicaltrials.gov
Advanced search: study type: interventional studies conditions: cardiovascular interventions: text
WHO ICTRP
text AND cardio*
Data and analyses
Comparison 1. Text messaging versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Fatal cardiovascular events | 4 | 1654 | Odds Ratio (IV, Random, 95% CI) | 0.83 [0.47, 1.45] |
| 1.2 Fatal cardiovascular events: sensitivity analysis using fixed‐effect model | 4 | 1654 | Odds Ratio (IV, Fixed, 95% CI) | 0.83 [0.47, 1.45] |
| 1.3 Low‐density lipoprotein cholesterol | 8 | 4983 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐4.71, 1.12] |
| 1.4 Low‐density lipoprotein cholesterol: sensitivity analysis using fixed‐effect model | 8 | 4983 | Mean Difference (IV, Fixed, 95% CI) | ‐1.76 [‐3.49, ‐0.03] |
| 1.5 Low‐density lipoprotein cholesterol: sensitivity analysis excluding studies with high risk of bias | 3 | 1872 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.02, 4.82] |
| 1.6 Systolic blood pressure | 8 | 5173 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐3.55, 1.69] |
| 1.7 Systolic blood pressure: sensitivity analysis using fixed‐effect model | 8 | 5173 | Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐2.27, ‐0.51] |
| 1.8 Systolic blood pressure: sensitivity of analysis excluding studies with high risk of bias | 3 | 2062 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐2.28, 2.02] |
| 1.9 Systolic blood pressure: sensitivity analysis excluding outlier | 7 | 4463 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.26, 1.34] |
| 1.10 Diastolic blood pressure | 5 | 3137 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.49, 0.50] |
| 1.11 Diastolic blood pressure: sensitivity analysis using fixed‐effect model | 5 | 3137 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.63, ‐0.19] |
| 1.12 Diastolic blood pressure: sensitivity analysis excluding studies with high risk of bias | 2 | 1335 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.96, 1.14] |
| 1.13 Heart rate | 4 | 2946 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.74, 0.82] |
| 1.14 Heart rate: sensitivity analysis using fixed‐effect model | 4 | 2946 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.34, 0.21] |
| 1.15 Heart rate: sensitivity analysis excluding studies with high risk of bias | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bae 2021.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: 2 tertiary and university teaching hospitals, Korea Recruitment period: April 2017 to May 2020 Length of intervention: 6 months Study start and end dates: April 2017 to November 2020 |
|
| Participants |
Inclusion criteria: CHD and underwent PCI for the first time Exclusion criteria: < 18 years of age; had no mobile phone; or difficulty reading SMS text messages Randomised: total: n = 879, intervention: n = 440, control: n = 439 Number available for follow‐up: total: n = 760, intervention: n = 392 (4 deaths; 44 lost to follow‐up), control: n = 368 (1 death and 70 lost to follow‐up) Mean age in years (SD): total:60.4 (10.5), intervention: 60.1 (10.6), control: 60.7 (10.4) Sex (% male): total: 83.30%, intervention: 83.60%, control: 82.90% |
|
| Interventions |
Intervention group: "access to a supporting website and received 4 SMS text messages per week for 6 months regarding a healthy diet, physical activity, smoking cessation, and cardiovascular health." (Bermon 2021, p 1) Text type: automated, unidirectional Control group: usual care including regular follow‐up at the outpatient clinic and education on cardiovascular health and risk factors provided by nurses |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: received funding from the National Research Foundation of Korea (NRF) (NRF‐2017R1C1B5017736) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized randomization program was developed for a random 1:1 allocation whose sequence was generated in a block size of 8." (Bae 2021, p 4) |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the nature of the intervention it was impossible to blind participants. Care provider was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome evaluator were blinded to the assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was not performed for primary outcomes. There were differences between groups in withdrawals. 70/439 (15.9%) and 44/440 (10%) participants were lost to follow‐up in the control and intervention groups, respectively. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but pre‐specified outcomes in trial registry were reported. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Bermon 2021.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: a tertiary hospital serving as a reference centre for cardiovascular diseases in Northeastern Colombia Recruitment period: NR Length of intervention: 12 months Study start and end dates: NR |
|
| Participants |
Inclusion criteria: aged ≥ 18 years, with a history of at least 1 of the following: arterial occlusive events (acute coronary syndrome, stable angina, ischaemic cerebrovascular disease, peripheral arterial disease, or coronary revascularisation), own a mobile phone, able to read the SMS text message Exclusion criteria: "had a known contraindication to take cardiovascular secondary prevention medications" (Bermon 2021, p 3) Randomised: total: n = 930, intervention: n = 462, control: n = 468 Number available for follow‐up: total: n = 805, intervention: n = 414 (54 lost to follow‐up), control: n = 391 (71 lost to follow‐up) Mean age in years (SD): total: 63.5 (9.8), intervention: 64 (9.7), control: 63.1 (10) Sex (% male): total: 78.40%, intervention: 76.40%, control: 80.30% |
|
| Interventions |
Intervention group: received SMS text messages daily for the first 4 weeks, 5 SMS text messages on week 5, 3 SMS text messages each in weeks 6 and 7, and 1 SMS text message weekly from week 8 until week 52 Text type: automated, unidirectional Control group: received text messages for reminding participation in the study |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: this work was supported by the Ministerio de Ciencia Tecnología e Innovación (code: 656672553352; grants 899‐2015 and 753 de 2016); Fundación Cardiovascular de Colombia, Floridablanca; UK Medical Research Council Funded Reference (reference number: MR/N021304/1); and the Universidad Pontificia Bolivariana, Bucaramanga Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was used, with block sizes of 5 patients each in a 1:1 allocation ratio, and assignment was done automatically using a remote computer‐based randomization." (Bermon 2021, p 4) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally using the CommCare platform after eligibility criteria was confirmed, informed consent signed and baseline information collected. Randomised allocation was not revealed until after a participant was formally entered into the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators handling and analyzing the data were blinded to the intervention assigned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers or reasons for missing data across groups: 54/468 (11.5%) control and 71/462 (15.3%) intervention |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Chen 2019.
| Study characteristics | ||
| Methods |
Design: parallel, 3‐arm, RCT Setting: large tertiary referral hospital, China Recruitment period: December 2011 and March 2015 Length of intervention: 1 month Study start and end dates: December 2011 to September 2015 |
|
| Participants |
Inclusion criteria: diagnosed with chronic heart failure Exclusion criteria: were deceased in hospital, unwilling to participate, < 18 years of age, unable to read in Chinese, do not own a phone, discharged to a long‐term care facility, were planning to receive cardiac surgery within 6 months, were waiting for heart transplantation, have malignancy or other critical illness with a life expectancy of < 1 year, have severe mental disorders, and were participating in other research Randomised: total: n = 767, intervention group 1: n = 252, intervention group 2: n = 255, control: n = 260 Number available for follow‐up: total: n = 489, intervention group 1: n = 241 (11 lost to follow‐up), intervention group 2: n = 248 (7 lost to follow‐up), control: n = 241 (19 lost to follow‐up) Mean age in years (SD): total: 61 (15), intervention group 1: 60 (15), intervention group 2: 62 (14), control: 61 (15) Sex (% male): total: 56%, intervention group 1: 57.5%, intervention group 2: 54.5%, control: 57.30% |
|
| Interventions |
Intervention group 1: participants and their caregivers received both educational and reminder text messages. The educational messages aimed to improve health knowledge, whilst the reminder messages aimed to remind participants to take their medications. "All educational messages were sent within the first 10 days after discharge, and then the reminder messages were sent weekly for 1 month. Patients were informed not to reply to the messages." (Chen 2019, p 165) Intervention group 2: "received one structured phone call from research nurses within 30 days after discharge" (Chen 2019, p 165) Text type: automated, one‐way Control group: usual care after discharge |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: NR Declaration of interest: no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random sequence list was generated and encrypted with Excel 2010 and kept by a statistician who had no access to patient information during the trial." (Chen 2019, p 165) |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in three groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol found to compare planned with reported outcomes. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Chow 2015.
| Study characteristics | ||
| Methods |
Design: parallel‐group, single‐blind, RCT Setting: outpatients from large tertiary referral centre and university teaching hospital, Australia Recruitment period: September 2011 to November 2013 Length of intervention: 6 months Study start and end dates: September 2011 to May 2014 |
|
| Participants |
Inclusion criteria: > 18 years of age, documented CHD, able to provide informed consent Exclusion criteria: did not have an active mobile phone, insufficient English language proficiency, referred for congenital heart disease or coronary anomalies Randomised: total: n = 710, intervention: n = 352, control: n = 358 Number available for follow‐up: total: n = 693, intervention: n = 339 (9 unable to contact, 4 died), control: n = 354 (3 unable to contact, 1 died) Mean age in years (SD): total: 57.6 (9.2), intervention: 57.9 (9.1), control: 57.3 (9.3) Sex (% male): total: 82%, intervention: 81.5%, control: 82.4% |
|
| Interventions |
Intervention group: received 4 messages per week for 24 weeks. Each message was sent on 4/5 randomly selected workdays. Text messages provided advice, health information, motivational reminders, and support to change lifestyle behaviours. Messages for each participant were randomly selected from the bank of messages based on participant characteristics (e.g. smoking). Text type: automated, one‐way Control group: community follow‐up and referral to inpatient cardiac rehabilitation |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: grants from the National Heart Foundation of Australia Grant‐in‐Aid (G10S5110) and a BUPA Foundation Grant Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization occurred via a computerized randomization program that was accessed through a secure web interface. The random allocation sequence was in a uniform 1:1 allocation ratio with a block size of 8 and was concealed from study personnel." (Chow 2015, p 1256) |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed from study personnel. "Study staff enrolled patients by entering data into the secure web interface." (Chow 2015, p 1256) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded assessments were conducted at baseline and 6 months. Study personnel taking follow‐up assessments were blinded to parallel group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers between groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | High risk | Psychosocial factors and fruit/vegetable intake were not reported as per the study trial. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Chow 2022.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: 18 public teaching hospitals, Australia Recruitment period: NR Length of intervention: 12 months Study start and end dates: NR |
|
| Participants |
Inclusion criteria: diagnosis of ACS; own an operational texting‐capable mobile phone, able to read text messages in English, life expectancy > 6 months, and able to provide informed consent Exclusion criteria: NR Randomised: total: n = 1424, intervention: n = 716, control: n = 708 Number available for follow‐up: total: n = 1298, intervention: n = 641 (1 withdrew consent; 1 did not meet criteria; 54 requested messages stop; 9 unable to contact; 10 died), control: n = 657 (5 withdrew consent; 21 requested stop; 16 unable to contact; 2 moved; 1 unwell; 1 no reason; 5 died during usual care period) Mean age in years (SD): total: 58 (10.7), intervention: 58 (10.4), control: 58 (10.9) Sex (% male): total: 79.20%, intervention: 79.50%, control: 79% |
|
| Interventions |
Intervention group: received 4 messages per week for the first 6 months and 3 messages per week over the subsequent 6 months. Text messages provided health information on general secondary prevention and support on medications and lifestyle modification. The text messages were customised to participant characteristics including aspects of their diet, physical activity capacity, and types of medications they were taking. Text messages were personalised by incorporating the participant’s name and the hospital at which the participant was treated. Text type: automated, two‐way communication (text or telephone) Control group: usual care (secondary prevention as determined by the treating clinician) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: supported by the National Health and Medical Research Council (APP1042290) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was in a 1:1 allocation ratio stratified by site through a computerized randomization program." (Chow 2022, p 1445) |
| Allocation concealment (selection bias) | Low risk | Central allocation via a centralised, computerised randomisation programme was used. Therefore, investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study coordinators and research assistants conducting the assessments and statisticians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers between groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned in protocol. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Dale 2015a.
| Study characteristics | ||
| Methods |
Design: parallel, 2‐arm RCT Setting: 2 large metropolitan hospitals, Auckland, New Zealand Recruitment period: 2013 to 2014 Length of intervention: 6 months Study start and end dates: start date: 2013, end date: NR |
|
| Participants |
Inclusion criteria: English‐speaking adults, diagnosis of CHD (myocardial infarction, angina, or revascularisation), access to the internet (e.g. at home, work, or library) Exclusion criteria: untreated ventricular tachycardia, severe heart failure, life‐threatening co‐existing disease with life expectancy less than 1 year, and/or significant exercise limitations for reasons other than CHD Randomised: total: n = 123, intervention: n = 61, control: n = 62 Number available for follow‐up: total: n = 116, intervention: n = 57 (4 withdrawals: 2 for medical reasons, 2 being too busy), control: n = 59 (3 could not be contacted) Mean age in years (SD): total: 59.9 (11.1), intervention: 59.0 (10.5), control: 59.9 (11.8) Sex (% male): total: 81.3%, intervention: 79%, control: 84% |
|
| Interventions |
Intervention group: received text messages for 24 weeks and had access to a supporting website. Messages were tailored to participant name and preferred time of day to receive messages. Participants received daily text messages for the first 12 weeks and then 5 messages per week from weeks 13 to 24. Text type: automated, bi‐directional Control group: usual care (outpatient cardiac rehabilitation programme involving health education and supervised exercise) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: government body (National Institute for Health Innovation, the University of Auckland) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization sequence was computer generated by a statistician independent to the project using a block size of 6" (Dale 2015a, p 4) |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed in sequentially numbered, opaque, sealed envelopes. Participant enrolment and assignment to the intervention were completed by a trained research assistant" (Dale 2015a, p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the nature of the intervention, participants and outcome assessors were not blinded to their treatment allocation." (Dale 2015a, p 4) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Because of the nature of the intervention, participants and outcome assessors were not blinded to their treatment allocation." (Dale 2015a, p 4) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers between groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Fang 2016.
| Study characteristics | ||
| Methods |
Design: parallel, 3‐arm RCT Setting: Chengdu City, China Recruitment period: over 10 months in 2013 Length of intervention: 6 months Study start and end dates: NR |
|
| Participants |
Inclusion criteria: adult patients with CAD treated in the General Medicine Department at West China Hospital. All participants had chronic stable angina consistent with the criteria of the Chinese Medical Association of Cardiovascular Disease Guide. Exclusion criteria: "(1) nonconformance with the diagnostic standards for chronic stable angina established by the Chinese Medical Association of Cardiovascular Epidemiology, (2) history of mental illness, (3) infection, fever, operation, serious heart failure, respiratory failure or acute stroke in the prior month and (4) inability to use a mobile phone that accepts SMS" (Fang 2016, p 666) Randomised: total: n = 280, intervention group 1: n = 95, intervention group 2: n = 92, control: n = 93 Number available for follow‐up: total: n = 271, intervention group 1: n = 91, intervention group 2: n = 90, control: n = 90 Mean age in years (SD): intervention group 1: 53.73 (7.20), intervention group 2: 53.69 (7.74), control: 53.50 (7.62) Sex (% male): intervention group 1: 70.33%, intervention group 2: 67.78%, control: 67.78% |
|
| Interventions |
Intervention group 1: the SMS group received medication reminders and educational materials via SMS Intervention group 2: the SMS + Micro Letter group received medication reminders via SMS and educational materials via Micro Letter. A public Micro Letter platform was established for the study. CAD‐related information (e.g. prevention of hyperlipidaemia, medication use, side effects of medication) was regularly relayed to the platform. Text type: NR Control group: received monthly telephone call to remind participant of medication schedule and upcoming appointments |
|
| Outcomes |
Primary outcomes
|
|
| Notes |
Funding: NR Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed but given nature of intervention unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Unclear risk | Only one outcome, but no protocol |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Huo 2019.
| Study characteristics | ||
| Methods |
Design: multicentre, parallel‐group, single‐blind RCT Setting: 34 hospitals (tertiary and secondary), China Recruitment period: August 2016 to April 2017 Length of intervention: 6 months Study start and end dates: start date: August 2016, end date: NR |
|
| Participants |
Inclusion criteria: > 18 years of age, documented CHD (acute MI or PCI) and diabetes within the prior 3 years, access to a mobile phone to read and send text messages Exclusion criteria: "had cognitive or communication disorders that prevented them from comprehending, detecting, or applying language when attempting to speak or communicate with others; or were unable to provide informed consent" (Huo 2019, p 3) Randomised: total: total: n = 502, intervention: n = 251, control: n = 251 Number available for follow‐up: total: n = 500, intervention: n = 250 (1 lost to follow‐up), control: n = 250 (1 lost to follow‐up) Mean age in years (SD): total: 59.5 (9.3), intervention: 59.5 (9.4), control: 59.5 (9.1) Sex (% male): total: 82.5%, intervention: 82.9%, control: 82.1% |
|
| Interventions |
Intervention group: received 6 messages per week for 6 months. Messages were randomly selected from software system and sent at 1 of 3 random times (9 am, 12 pm, 4 pm) on all days except Monday. Participants "received one of each of the following message types each week: information about CHD and DM, glucose monitoring and control, blood pressure control, medication adherence, physical activity, lifestyle recommendations, including diet and foot care". (Huo 2019, p 3) Text type: automated. "Most of the text messages were unidirectional, with no reply anticipated. Bidirectional text messages requesting blood glucose measurements and reports on medication usage were sent at weekly intervals to assess patient engagement." (Huo 2019, p 3) Control group: usual care |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: "This project was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Science (2016‐I2M‐1–006 and 2017‐I2M‐B&R‐02), the Research Special Fund for Public Welfare Industry of Health (201502009) from the National Health and Family Planning Commission of China, the National Key Research and Development Program (2017YFC1310803) from the Ministry of Science and Technology of China, and the 111 Project (B16005) from the Ministry of Education of China." (Huo 2019, p 9) Declaration of interest: "Dr Krumholz is the recipient of a research grant from Medtronic and Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing; chairs a cardiac scientific advisory board for United Health; works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures that are publicly reported; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science and the Physician Advisory Board for Aetna; and is the founder of Hugo ‐ a personal health information platform. Dr Spatz receives support from the Centers for Medicare & Medicaid Services to develop publicly reported quality measures, the Food and Drug Administration to support projects within the Yale‐Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI), the National Institute on Minority Health and Health Disparities (U54MD010711‐01) to study precision based approaches to diagnosing and preventing hypertension, and the National Institute of Biomedical Imaging and Bioengineering (R01 EB028106‐01) to study a cuff‐less blood pressure device. Dr Masoudi has a contract with the American College of Cardiology for his role as Chief Scientific Advisor of NCDR. He has received travel expenses from the China Oxford Centre. The other authors report no conflicts." (Huo 2019, p 9) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation system and a strstified randomisation approach based on age, gender, acute myocardial infarction history, education and medical insurance type were used. |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. Recruiting personnel and clinicians were blinded to assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study minimized any potential bias by not disclosing the group allocation of patients to data collectors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | High risk | Death, non‐fatal myocardial infarction, stroke and rehospitalisaiton were not reported as per the trial protocol. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Kamal 2015.
| Study characteristics | ||
| Methods |
Design: parallel, 2‐arm RCT Setting: Karachi, Pakistan Recruitment period: NR Length of intervention: 2 months Study start and end dates: NR |
|
| Participants |
Inclusion criteria: "age greater than 18 years old; history of stroke(s) confirmed by neuroimaging at the time of the episode; > 1 month since last episode of stroke; use of at least two drugs such as (but not limited to) antiplatelets, statins, anti‐hypertensives to control risk factors of stroke; modified Rankin Score of 3 or less (so that they are able to operate mobile phones); possession of a personal cell phone that the patient has access to at all times. In the case of patients who do not own or are unable to use mobile phones, they must have a caregiver available at all times who possesses a cell phone; ability to receive, comprehend and reply to an SMS in English, Nastaleeq Urdu (local Urdu script) or Roman Urdu. In the case of patients who themselves are unable to receive, comprehend or reply to an SMS, they must have caregivers available at all times who could perform the above mentioned tasks." (Kamal 2015, p 2) Exclusion criteria: "biological impairment in reading or responding to SMS in the caregiver such as (but not limited to) loss of vision, visual field cuts, aphasia in case the patient himself/herself is supposed to receive SMS; diagnosed organ dysfunction or malignancy such as hepatic, renal or malignancy; plans to travel outside the country inside the two months following enrolment" (Kamal 2015, pp 2‐3) Randomised: total: n = 200, intervention: n = 100, control: n = 100 Number available for follow‐up: total: n = 162, intervention: n = 83 (10 unwilling to come, 2 sick, 3 out of station, 2 discontinued intervention), control: n = 79 (17 unwilling to come, 4 out of station) Mean age in years (SD): total: 56.85 (SD not reported), intervention: 56 (1.5), control: 57.6 (1.3) Sex (% male): total: 67.5%, intervention: 71%, control: 64% |
|
| Interventions |
Intervention group: received automated SMS reminders customised to participant's individual prescription. "The participants were required to respond to the SMS stating if they have taken their medicines. Moreover, twice weekly health information SMS were also sent to the intervention group. Health information SMS were customised according to medical and drug profile of every patient by the research team. The messages were designed in a weekly schedule at preset days of the week for total 8 weeks e.g., Wednesday and Saturday week 1 for patient X. The timings were decided according to the prescription so that health messages do not collide with the reminder messages for that day. Usually 5 pm was found feasible for most participants. These messages did not ask for a reply." (Kamal 2015, p 3) Text type: automated, two‐way Control group: "patients received the usual standard of care provided at the centre for stroke patients. This primarily consisted of regular follow‐up visits (as advised by their neurologist) with their stroke neurologist. In general, these were at 1, 3, 5, 9 and 12 months after a stroke. Each patient was provided with a telephone number that could be used to reach the stroke team in case of an emergency and each patient was also reminded of their clinic appointments 1‐2 days prior via SMS and/or phone." (Kamal 2015, p 3) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: no specific funding was reported for this study Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomised computer‐generated sequence. The staff who randomised, assessed and delivered the intervention were separate. |
| Allocation concealment (selection bias) | Low risk | Concealed in white envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed but based on intervention high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only mention is that "The staff who randomized and those who assessed and those who delivered the intervention were separate". (Kamal 2015, p 3) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A large number of participants (20%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported on. Blood pressure not mentioned in protocol, but acceptability and patient satisfaction were. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Khonsari 2015.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: tertiary teaching hospital, Kuala Lumpar, Malaysia Recruitment period: 23 January 2013 to 23 February 2013 Length of intervention: 2 months Study start and end dates: December 2012 to April 2013 |
|
| Participants |
Inclusion criteria: ACS Exclusion criteria: "did not have cell phones to receive related text‐messages; were not discharged during the specified study timeline or were discharged to a care facility or transferred to another health care institution; were illiterate or unable to read text‐messages; were not available for the two‐month period of the study (including being unavailable by phone and/or travelling out the country); or had been diagonsed with cognitive impairment so that the informed consent process might be incomprehensible." (Khonsari 2015, p 171) Randomised: total: n = 62, intervention: n = 31, control: n = 31 Number available for follow‐up: total: n = 60, intervention: n = 31, control: n = 29 (2 deaths) Mean age in years (SD): total: 57.9 (12.64), intervention: 56 (11.3), control: 59 (13.9) Sex (% male): total: 85.5%, intervention: 87.1%, control: 83.9% |
|
| Interventions |
Intervention group: "received text‐message reminders based on the following template before every medication intake, starting the day after discharge: ‘‘[Mr/Ms] [Patient’s Name], please take [Medication Quantity] tablet of [Medication Name] at [Time]’’. When the course of medication was completed (patients were given a 30‐day dosage), a message was sent to remind the patients to come to the hospital and have their prescribed cardiac medications refilled. The SMS reminder service was continued until two months after discharge. The system is a web‐based software where all tasks are handled automatically. Reminders were generated and sent to each participant in the intervention group before every cardiac medication intake in an 8‐week programme. The researcher also followed up with the participants in the SMS group via telephone calls once per two weeks during the study to reassure the delivery of text messages, to enquire whether any emergency readmission was needed as well as to show up for their appointments." (Khonsari 2015, pp 171‐2) Text type: automated, one‐way Control group: "usual care for ACS post‐discharge, including cardiac rehabilitation and follow‐up appointments with the cardiologist, usually occurring at six or eight weeks following discharge." (Khonsari 2015, p 171) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: "This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors." (Khonsari 2015, p 178) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the nature of the intervention, it was impossible to blind either the subjects or the researchers to the study group assignment." (Khonsari 2015, p 171) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "To prevent potential bias in the results of the study, all participants were visited by cardiologists and cardiac rehabilitation specialists who were unaware of the study group assignment to assess the participants' heart function status based on the New York Heart Association Functional Classification (NYHA) at the endpoint of the study". (Khonsari 2015, p 171) However, NYHA class was not an outcome of this review and no blinding of outcome assessors was done in relation to the other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol found to compare planned with reported outcomes. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Maddison 2021.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: 2 large metropolitan hospitals, New Zealand Recruitment period: July 2016 and September 2018 Length of intervention: 6 months Study start and end dates: July 2016 and November 2019 |
|
| Participants |
Inclusion criteria: "adults with an ACS (including those who had undergone a percutaneous coronary revascularization procedure), clinically stable, able to read English, and able to provide informed consent" (Maddison 2021, p 2) Exclusion criteria: "had untreated ventricular tachycardia, severe heart failure, life‐threatening coexisting disease with life expectancy of less than 1 year, and significant exercise limitations other than cardiovascular disease" (Maddison 2021, p 2) Randomised: total: n = 306, intervention: n = 153, control: n = 153 Number available for follow‐up: total: n = 267, intervention: n = 130 (20 lost to follow‐up, 3 discontinued intervention), control: 134 (19 lost to follow‐up) Mean age in years (SD): total: 61 (11), intervention: 61 (11), control: 61 (11) Sex (% male): total: 77.10%, intervention: 73.80%, control: 80.40% |
|
| Interventions |
Intervention group: "Text4HeartII comprised a personalised, automated program of self‐management that was delivered via SMS text messages over 24 weeks." "Text4HeartII included core Heart Health content comprising education and support to encourage regular taking of medication, eat a healthy diet (including moderating alcohol consumption), manage stress, and exercise regularly (total 126 messages). Additional SMS text messages were delivered based on the suboptimal behavior participants wanted to modify (e.g. physical activity, heart healthy diet, stress management, and stop smoking); each module contained 35 text messages. Participants were only able to choose one additional module; however, smokers were prioritized to receive messages providing cessation support. Participants received a minimum of 1 core heart message per day for 24 weeks, with an additional 35 messages sent over the first 12 weeks; all messages were sent from a centralised server." (Maddison 2021, p 3) Text type: automated, predominantly unidirectional Control group: usual care (outpatient cardiac rehabilitation programme involving health education and supervised exercise) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: funded by the Health Research Council of New Zealand and the National Heart Foundation Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization sequence was generated by a biostatistician and block randomisaiton with block sizes of 2 or 4 was used. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed by a centralised computer system that revelaed treatment allocation only after submission of baseline data. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Ni 2022.
| Study characteristics | ||
| Methods |
Design: 2‐arm parallel RCT Setting: university‐affiliated hospital, China Recruitment period: began May 2018; end date of recruitment not reported Length of intervention: 2 months Study start and end dates: May 2018 to December 2018 |
|
| Participants |
Inclusion criteria: CHD, ≥ 18 years of age, had an antihypertensive medication regimen that would last at least > 90 days beyond enrolment, can read text messages through a mobile phone, had a mobile phone that could receive WeChat messages, was capable of giving consent, had an electronic blood pressure cuff to check blood pressure and heart rate Exclusion criteria: NR Randomised: total: n = 230, intervention: n = 116, control: n = 114 Number available for follow‐up: total: n = 215, intervention: n = 110 (5 lost to follow‐up; 1 discontinued), control: n = 105 (4 lost to follow‐up; 5 discontinued) Mean age in years (SD): total: 61 (11), intervention: 61 (11), control: 62 (11) Sex (% male): total: 80.1%, intervention: 80.6%, control: 79.6% |
|
| Interventions |
Intervention group: "received reminders to take medication and educational materials from Message Express and WeChat, respectively." "Educational materials sent to the intervention group were specifically related to CHD and medication adherence and included information on the cardioprotective medications, negative consequences of medication non‐adherence, and reasons why people with CHD fail to take them." The intervention group received daily reminders to take medication. "The educational materials and reminders were sent through Message Express on an encrypted external device." (Ni 2022, p 3) Text type: not automated Control group: only received educational materials from WeChat. "Materials sent to the control group only contained general medical information that was not specifically related to CHD or medication adherence." (Ni 2022, p 3) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: supported by the Duke University Global Health Institute (2018 Duke Global Health Doctoral Certificate Fieldwork Grant); Duke University Graduate School (2017 International Dissertation Research Travel Award); and the Duke University School of Nursing (2018 PhD Student Pilot Study Fund) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random allocation sequence was generated using SAS software version 9.4 by the first author, who also enrolled participants and assigned participants to interventions." (Ni 2022, p 3) |
| Allocation concealment (selection bias) | High risk | The first author generated the random allocation sequence, enrolled participants and assigned participants to interventions. Therefore, the first author enorlling participants could foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants, data collectors, and data analysts were aware of which treatment arms participants had been assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18.4% of the intervention group and 11.2% of the control group did not complete baseline survey. Analysis was not conducted based on intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Unclear risk | Published protocol was not found to compare planned with reported outcomes. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Pandey 2017.
| Study characteristics | ||
| Methods |
Design: single‐centre, open‐label, 2‐arm RCT Setting: cardiac rehabilitation facility, Canada Recruitment period: NR Length of intervention: 12 months Study start and end dates: NR |
|
| Participants |
Inclusion criteria: ≥ 18 years of age, discharged after MI in last 2 weeks, and enrolled in cardiac rehabilitation Exclusion criteria: patients taking medications in dosing regimens of more than once daily were excluded Randomised: total: n = 34, intervention: n = 17, control: n = 17 Number available for follow‐up: total: n = 33, intervention: n = 17 (0 withdrew), control: n = 16 (1 withdrew, reason not reported) Mean age in years: total: NR, intervention: 64.6 (11.5), control: 62.1 (11.0) Sex (% male): total: 60.6%, intervention: 35%, control: 88% |
|
| Interventions |
Intervention group: received daily text message reminders at the times they were to take their prescribed medication. An example of text message was "Please remember to take your morning medications now." (Pandey 2017, p 2) Text type: automated, one‐way Control group: usual care |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: NR Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details reported but we made the assumption that given the nature of the intervention it was impossible to blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant in the control group dropped out. Analysis was performed based on the intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol found to compare planned with reported outcomes. |
| Other bias | High risk | There were baseline imbalances between groups in gender; 88% male in the control group and 35% in the intervention group. |
Park 2014a.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 3‐arm RCT Setting: non‐profit community hospital, Northern California, USA Recruitment period: April 2012 to March 2013 Length of intervention: 1 month Study start and end dates: start date: April 2012; end date: NR |
|
| Participants |
Inclusion criteria: ≥ 21 years of age, hospitalised for MI or PCI, prescribed antiplatelet or statin medication, owned a mobile phone with text messaging capability, and was able to speak, read, and understand English Exclusion criteria: cognitive impairment, and inability to operate a mobile phone Randomised: total: n = 90, intervention group 1: n = 30, intervention group 2: n = 30, control: n = 30 Number available for follow‐up: total: n = 84, intervention group 1: n = 28 (2 lost to follow‐up), intervention group 2: n = 28 (2 lost to follow‐up: 1 withdrew due to busy schedule and 1 withdrew due to illness), control group: n = 28 (2 lost to follow‐up: 1 due to privacy request and 1 was unable to contact) Mean age in years (SD): total: 59.2 (SD not reported), intervention group 1: 58.2 (10.6), intervention group 2: 58.3 (8.5), control group: 61.1 (9.1) Sex (% male): total: 76%, intervention group 1: 76.7%, intervention group 2: 66.7%, control group: 83.3% |
|
| Interventions |
Intervention group 1: text messages for medication reminders and health education. The participants received 74 messages over 1 month. "Personalised reminders were delivered at times selected by the patients that correlated with their medication schedule. The medication reminders were two‐way, requiring patients to respond back to confirm receipt." (Park 2014a, p 263) Intervention group 2: text messages for health education. The participants received 14 messages over 1 month. Health education messages were one‐way educational health messages on cardiovascular risk reduction on Monday, Wednesday, and Friday. Text type: sent from a customisable program through CareSpeak Communications ‘‘mobile Health manager’’ platform. Two‐way text messages for intervention group 1 and one‐way text messages for intervention group 2 Control group: no text messages |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: a grant from the Graduate Division of University of California, San Francisco and a scholarship from the University of California/Hartford Center of Geriatric Nursing Excellence Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was generated by random allocation sequence using blocks of six that was prepared by a biostatistician." (Park 2014a, p 262) |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes"; "The prinicple investigator assigned patients to their groups by distributing envelopes in consecutive, numbered order." (Park 2014a, p 262) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the nature of the study design, the prinicple invesitgator and patients could not be blinded to the intervention once group assignment was determined." (Park 2014a, p 262) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol available |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
Passaglia 2021.
| Study characteristics | ||
| Methods |
Design: parallel, double‐blind, 2‐arm RCT Setting: tertiary university hospital, Brazil Recruitment period: December 2017 to December 2018 Length of intervention: 6 months Study start and end dates: start date: December 2017; end date: NR |
|
| Participants |
Inclusion criteria: age ≥ 18 years, hospitalised with a diagnosis of ACS, discharged for outpatient follow‐up, and able to receive SMS on their own mobile phone Exclusion criteria: refusal or inability to sign the informed consent, inability to read and write Randomised: total: n = 180, intervention: n = 90, control: n = 90 Number available for follow‐up: total: n = 147, intervention: 75 (2 died, 13 lost to follow‐up), control: 72 (1 died, 17 lost to follow‐up) Age in years (median (interquartile range)): total: 58 (51 to 64), intervention: 57.5 (50.7 to 63), control: 58 (51 to 65) Sex (% male): total: 74.4%, intervention: 72.2%, control: 76.7% |
|
| Interventions |
Intervention group: text messages were sent to participants based on their baseline characteristics (subgroup 1: non‐smokers and free of diabetes, subgroup 2: non‐smokers and diabetic patients, subgroup 3: smokers and non‐diabetic patients, and subgroup 4: smokers and diabetic patients). Participants received text messages 4 times per week for 6 months. Text type: automated, one‐way Control group: usual care (standard discharge care after ACS) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: no specific funding was obtained Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used. A blocked randomization was provided in blocks of four patients each, following the date of patient enrollment, following a uniform 1:1 fashion. |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. However, the researchers, data collectors, and attending physicians were blind to the treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers blinded to treatment allocation collected the data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of withdrawals (18.3% lost to follow‐up). Analysis was not conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol |
| Other bias | High risk | 20 participants (26.6%) in the intervention group reported that they did not recevie text messages, which may contributed to the loss of study power and raised the possibility of a type II error. |
Quilici 2013.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: NR Recruitment period: NR Length of intervention: 1 month Study start and end dates: NR |
|
| Participants |
Inclusion criteria: undergone coronary stenting for ACS with good in‐hospital aspirin response defined by arachidonic acid‐induced platelet aggregation lower than 30%, owned a mobile phone with ability to communicate via SMS Exclusion criteria: NR Randomised: total: n = 521, intervention: n = 262, control: n = 259 Number available for follow‐up: total: n = 499, intervention: n = 250 (12 withdrew, no reasons), control: n = 249 (10 withdrew, no reasons) Mean age in years (SD): total: 64 (14), intervention: 64 (10), control: 64 (14) Sex (% male): total: 76.6%, intervention group: 78%, control group: 75.1% |
|
| Interventions |
Intervention group: 1 month personalised SMS reminding of aspirin intake. Text messages with different formulation were sent to the participants every day. Text type: personalised, computer‐generated Control group: usual care |
|
| Outcomes |
Primary outcomes: aspirin adherence at 1‐month follow‐up (measured by self‐report questionnaire and platelet function testing; good adherence was defined as more than 95% of prescribed doses in the past 30 days) Secondary outcomes: NR |
|
| Notes |
Funding: NR Declaration of interest: NR Publication: published letter to the editor; discrepancy in outcome data for self‐reported non‐adherence between text and Figure 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details reported but we made the assumption that given the nature of the intervention it was impossible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | High risk | Minimal data, no trial protocol found |
| Other bias | High risk | Outcome data for self‐reported non‐adherence differ between text and Figure 2 |
Ross 2021.
| Study characteristics | ||
| Methods |
Design: prospective, parallel, 2‐arm RCT Setting: tertiary care hospital, Canada Recruitment period: June 2015 and October 2016 Length of intervention: 16 months Study start and end dates: start date: June 2015; end date: NR |
|
| Participants |
Inclusion criteria: "a diagnosis of ACS, as identified by clinical staff, were recruited from St. Paul’s Hospital, a tertiary care hospital, in Vancouver, Canada between June 2015 and October 2016. Patients were eligible to participate if they had ACS (unstable angina or any type of myocardial infarction) as their primary admitting diagnosis, had daily access to a phone with SMS text messaging capabilities, were able to provide informed consent, and were able to read and understand English." (Ross 2021, p 3) Exclusion criteria: "coronary artery bypass graft surgery as a treatment for the ACS admission, had a prescheduled surgery within the study period, had a possibility of death during the study due to non‐CVD reasons, being discharged to a long‐term care centre, or living outside the province of British Columbia." (Ross 2021, p 3) Randomised: total: n = 76, intervention: n = 38, control: n = 38 Number available for follow‐up: total: n = 67, intervention: n = 31 (6 lost to follow‐up; 1 discontinued intervention), control: n = 36 (1 lost to follow‐up; 1 discontinued intervention) Mean age in years (SD): total: NR, intervention: 59.5 (9.1), control: 61.1 (9.6) Sex (% male): total: 72.30%, intervention: 73%, control: 74% |
|
| Interventions |
Intervention group: a total of 48 one‐way, automated messages were received over a period of 60 days. Text messages were sent daily for the first 36 days and then every other day until day 60. SMS text messages covered a range of topics, including (i) time‐sensitive information regarding their recovery (e.g. timely follow‐up with their healthcare professional), and (2) general healthy living advice such as physical activity, diet, and psychosocial health. Text messages were delivered in a prespecified order. Participants received 2 personalised text messages based on their smoking status. No other aspects were personalised. Text type: automated, one‐way Control group: usual care |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: from the Canadian Institutes of Health Research (CIHR) through a Catalyst Grant for eHealth Innovations (application number 316822) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician not associated with the study generated a random allocation schedule, which randomized participants in a 1:1 ratio using variable block sizes, stratified by sex (Ross 2021, p 3). |
| Allocation concealment (selection bias) | Low risk | Central allocation via a web‐based randomisation service was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers or reasons for missing data across groups (19% loss to follow‐up in the intervention group and 5% loss to follow‐up in the control group) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol |
| Other bias | High risk | As it was a pilot study, it did not determine our sample size based on power calculations and were likely underpowered to detect clinically important differences. |
Zheng 2019.
| Study characteristics | ||
| Methods |
Design: multicentre, single‐blinded RCT Setting: 37 hospitals (tertiary and secondary), China Recruitment period: August 2016 to March 2017 Length of intervention: 7 months Study start and end dates: start date: August 2016; end date: NR |
|
| Participants |
Inclusion criteria: ≥ 18 years of age, had CHD (AMI or PCI), no diabetes mellitus, had access to a mobile phone to read and send text messages, were willing to participate in the study Exclusion criteria: had cognitive or communication disorders, were unable to provide informed consent Randomised: total: n = 822, intervention: n = 411, control: n = 411 Number available for follow‐up: total: n = 806, intervention: n = 402 (2 moved to other provinces; 4 other reasons; 1 died; 2 lost to follow‐up), control: n = 404 (1 moved to other provinces; 5 other reasons; 1 lost to follow‐up) Mean age in years (SD): total: NR, intervention: 56.25 (9.3), control: 56.56 (9.7) Sex (% male): total: NR, intervention: 85.90%, control: 85.90% |
|
| Interventions |
Intervention group: "received 6 text messages/week for 6 months delivered by an automated computerised system. The messages provided educational and motivational information related to disease‐specific knowledge, risk factor control, physical activity, and medication adherence." (Zheng 2019, p 2) Text type: most messages were automated and unidirectional. However, weekly bi‐directional messages assessing medication adherence and blood pressure/glucose level measurements were sent to participants to evaluate their engagement. Control group: usual care |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: "supported by the National Key Research and Development Program (2016YFE0103800) from the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences Innovation Fund for Medical Science (2016‐I2M‐1–006), the National Key Research and Development Program (2017YFC1310803) from the Ministry of Science and Technology of China, and the 111 Project (B16005) from the Ministry of Education of China" (Zheng 2019, p 9) Declaration of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation system and a stratified randomisation approach based on age, gender, acute myocardial infarction history, education and medical insurance type were used. |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, the participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study minimized any potential bias by not disclosing the group allocation of patients to data collectors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups. Analysis was conducted following intention‐to‐treat principle. |
| Selective reporting (reporting bias) | High risk | Death, non‐fatal myocardial infarction, stroke and rehospitalisaiton were not reported as per the trial protocol. |
| Other bias | Low risk | The study appears to be free from other sources of bias. There were no differences between the groups at baseline. |
ACE(I): angiotensin‐converting enzyme (inhibitors) ACS: acute coronary syndrome AMI: acute myocardial infarction ARB: angiotensin receptor blockers BMI: body mass index BUPA: British United Provident Association CAD: coronary artery disease CHD: coronary heart disease CVD: cardiovascular disease LDL‐C: low‐density lipoprotein cholesterol MI: myocardial infarction MMAS‐8: Morisky Medication Adherence Survey NR: not reported PCI: percutaneous coronary intervention RCT: randomised controlled trial SAS: Statistical Analysis System SD: standard deviation SMS: short message service
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acevedo 2023 | Ineligible population (participants without cardiovascular disease) |
| Akhu‐Zaheya 2017 | Ineligible population (most participants were diagnosed with hypertension) |
| Brar 2018 | Ineligible population (participants with 1 or more chronic conditions including diabetes, hypertension, and/or high cholesterol) |
| Carrillo 2021 | Ineligible study design (1‐arm study) |
| Chen 2018 | Ineligible study design (pre‐post pilot study) |
| Cheung 2019 | Ineligible population (participants with diabetes or coronary heart disease) |
| Foccardi 2021 | Ineligible outcomes (study did not set out to measure any outcomes of interest) |
| Haramiova 2017 | Ineligible population (participants with hypertension) |
| IRCT20180911041002N | Ineligible intervention (text messaging was not the main intervention component) |
| Legler 2020 | Ineligible study design (1‐arm pilot study) |
| Luong 2021 | Ineligible population (a large proportion of participants had hypertension or diabetes) |
| Moradi 2017 | Ineligible outcomes (study did not set out to measure any outcomes of interest) |
| Rohde 2021 | Ineligible outcomes (study did not set out to measure any outcomes of interest) |
| Santo 2017 | Ineligible intervention (medication reminder application) |
| Santo 2018 | Ineligible outcomes (study did not set out to measure any outcomes of interest) |
| Wang 2020 | Ineligible intervention (a smartphone‐based application including cardiac health education, medication reminders, and cardiologist‐based follow‐up service) |
| Yan 2021 | Ineligible intervention (text messaging was not the main intervention component) |
| Zhao 2019 | Ineligible study design (a systematic review and meta‐analysis) |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12621000754842.
| Study name | TeleClinical Care Cardiac (TCC‐Cardiac): Efficacy and safety of adjunctive virtual models of care in the secondary prevention of cardiovascular events in adults discharged from hospital after myocardial infarction or decompensated heart failure |
| Methods |
Design: multicentre RCT Setting: Prince of Wales Hospital, The Sutherland Hospital, Coffs Harbour Base Hospital, Liverpool Hospital, Port Macquarie Base Hospital, Royal North Shore Hospital, St George Hospital, St Vincent’s Hospital, Wollongong Hospital, Concord Repatriation Hospital, Bankstown‐Lidcombe Hospital, Australia |
| Participants |
Estimated enrolment: 2500 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: supportive text messages will be delivered to the participants 3 times a week. Text messages will be tailored to the participants (e.g. diagnosis, smoking status) and varied according to time from discharge. Control: follow‐up by participant's general practitioner and cardiologist, referral as appropriate to local cardiac rehabilitation services and a referral to the NSW Get Healthy programme. |
| Outcomes |
Primary outcomes: unplanned hospital readmission at 6 months using patient medical records and linked health administrative data Secondary outcomes: death, incidence of MI, incidence of stroke, incidence of unplanned coronary revascularisation, unplanned hospital readmission, unplanned cardiac hospital readmissions, cardiac rehabilitation participation rates, cardiac rehabilitation completion rates, prescription of guideline‐recommended medications, maximum doses of recommended medications and cost‐effectiveness (at 30 days, 6 and 12 months post‐hospital discharge, measured by patient medical records and linked health administrative data) |
| Starting date | 16 July 2021 |
| Contact information |
Name: Sze Yuan Ooi Affiliation: Prince of Wales Hospital |
| Notes |
Status: recruiting Sponsor: New South Wales Ministry of Health, Australia |
CTRI/2021/06/034463.
| Study name | Drug adherence in persons after stunting and the effect of text messages on drug adherence |
| Methods |
Design: RCT Setting: hospital, India |
| Participants |
Estimated enrolment: 300 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: mobile phone text messages reminding participants of medication compliance and healthy habits Control: routine follow‐up without any mobile messages |
| Outcomes |
Primary outcomes: medication adherence (measured at 4 to 6 months after allocation) Secondary outcomes: major adverse cardiovascular events (MI, stroke, target vessel revascularisation, heart failure, and death) (measured at 4 to 6 months after allocation) |
| Starting date | 29 June 2021 |
| Contact information |
Name: Rajesh Vijayvergiya Affiliation: Post Graduate Institute of Medical Education and Research, Chandigarh |
| Notes |
Status: not yet recruiting Sponsor: NR |
CTRI/2021/10/037432.
| Study name | Prevention of secondary stroke by risk factor control and medication adherence |
| Methods |
Design: cluster‐RCT Setting: India |
| Participants |
Estimated enrolment: 1200 participants Inclusion criteria: all ischaemic or hemorrhagic strokes within 1 year of onset of stroke Exclusion criteria: patients with survival less than 6 months |
| Interventions |
Intervention: participants will receive text messages on medication adherence and risk factor control. The messages will be sent once a week for a period of 6 months. Control: routine care |
| Outcomes |
Primary outcomes: risk factor control and medication adherence (measured at baseline, 3 and 6 months) Secondary outcomes: NR |
| Starting date | 1 November 2021 |
| Contact information |
Name: PN Sylaja Affiliation: Sree Chitra Tirunal Institute for Medical Sciences and Technology |
| Notes |
Status: recruiting Sponsor: NR |
IRCT2014050617596N1.
| Study name | Comparison of telephone and SMS follow up on treatment regimen adherence in patients with coronary artery bypass surgery |
| Methods |
Design: parallel RCT Setting: hospital, Iran |
| Participants |
Estimated enrolment: 90 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: participants will receive SMS messages once daily for 8 weeks. The SMS will include adherence to treatment (diet, exercise, medication regimen) information. Control: not stated |
| Outcomes |
Primary outcomes: treatment regimen adherence at 2 months' follow‐up (measured by adherence to treatment questionnaire) Secondary outcomes: not stated |
| Starting date | 22 April 2016 |
| Contact information |
Name: Maryam Jadid Milani Affiliation: Shahed University |
| Notes |
Status: recruitment is complete Sponsor: NR |
IRCT2016011025937N1.
| Study name | The effect of follow up using short message service on illness perception and medication adherence in patients under coronary angioplasty: a one blind randomized control trial |
| Methods |
Design: RCT Setting: Cardiac Clinic of Tabriz University of Medical Sciences, Iran |
| Participants |
Estimated enrolment: 116 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: participants will receive follow‐up text messages after angioplasty. Text messages will contain educational information and reminders to take medication (5 times a week at 10 am except Thursdays and Fridays) for 3 months. Control: routine follow‐up |
| Outcomes |
Primary outcomes: medication adherence at 3 months (measured by Morisky Medication Adherence Questionnaire) and illness perception at 3 months (measured by Brief Illness Perception Questionnaire) Secondary outcomes: NR |
| Starting date | 3 April 2016 |
| Contact information |
Name: Atefeh Allahbakhshian Affiliation: Tabriz University of Medical Sciences |
| Notes |
Status: recruitment is complete Sponsor: Vice Chancellor for Research of Tabriz University of Medical Sciences |
IRCT2016081125937N2.
| Study name | Effect of a reminder system using a web based short message service on medication adherence in patients with acute coronary syndrome following coronary angioplasty |
| Methods |
Design: RCT Setting: Shahid Madani Hospital, Iran |
| Participants |
Estimated enrolment: 116 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: reminder messages will be sent based on physician‐recommended medication regimen and on drug name and dose, in specified intervals for 6 months Control: routine follow‐up |
| Outcomes |
Primary outcomes: medication adherence at 6 months (measured by MMAS‐8) Secondary outcomes: NR |
| Starting date | 22 July 2016 |
| Contact information |
Name: Atefeh Allahbakhshian Affiliation: Tabriz University of Medical Sciences |
| Notes |
Status: recruitment is complete Sponsor: Vice Chancellor for Research of Tabriz University of Medical Sciences |
ISRCTN10549665.
| Study name | Improving medication taking in patients with coronary heart disease using a mobile health technology, a feasibility study |
| Methods |
Design: RCT Setting: Tehran Heart Centre, Iran |
| Participants |
Estimated enrolment: 78 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: receive daily text message reminders for 12 weeks. The researcher will follow up with the participants in the intervention group via telephone calls once every 2 weeks during the study to reassure the delivery of reminders and to enquire about any patient’s emergency readmission. Control: usual care with no text reminders |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 1 February 2016 |
| Contact information |
Name: Sahar Khonsari Affiliation: School of Health in Social Care, Edinburgh Napier University, UK |
| Notes |
Status: study has been completed, but no results identified by search Sponsor: NR |
Park 2017.
| Study name | Mobile health strategies for veterans with coronary heart disease |
| Methods |
Design: RCT Setting: John Muir Medical Center, VA Palo Alto Health Care System, San Francisco Veterans Affairs Medical Center, North Florida/South Georgia Veterans Health System, VA North Texas Health Care System, USA |
| Participants |
Estimated enrolment: 225 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: the 'Text message group' will use the VA 'Annie' text messaging programme to remind participants to take antiplatelet medications Control: the 'Website‐Control group' will be offered the American Heart Association patient education website (My Life Check ‐ 7 Steps To Healthy Living) |
| Outcomes |
Primary outcomes: change in medication adherence over 12 months (measured by medication event monitoring system, Adherence to Refills and Medications Scale, Medication Recall Questionnaire, VA Corporate Data Warehouse refill data, and Peoplechart Meds Incontext refill data, separately) Secondary outcomes: NR |
| Starting date | December 2016 |
| Contact information |
Name: Linda Park Affiliation: San Francisco Veterans Medical Center; University of California |
| Notes |
Status: recruiting Sponsor: San Francisco Veterans Affairs Medical Center |
Redfern 2019.
| Study name | Impact of integrated text messaging (ITM) on the efficacy of rehabilitation programs for chronic respiratory and cardiovascular disease |
| Methods |
Design: RCT Setting: Royal Prince Alfred Hospital, Concord Repatriation Hospital, Balmain Hospital, Canterbury Hospital, Westmead Hospital, Royal North Shore Hospital, Australia |
| Participants |
Estimated enrolment: 310 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: participants will receive a 26‐week text message programme (5 messages/week, one‐way communication) in addition to cardiac or pulmonary rehabilitation. The messages will be semi‐personalised where some contain the participant’s preferred name and are tailored for individual circumstances and preferences (e.g. non‐smoker, vegetarian, physical activity level). Control: usual chronic disease management programme |
| Outcomes |
Primary outcomes: exercise capacity at 6 months measured by 6‐minute walking distance Secondary outcomes: the percentage of participants attending and completing a chronic disease management programme (retrieved from attendance records), medication adherence (self‐report), quality of life (measured by 12‐Item Short‐Form Health Survey), lifestyle change (measured by a case report form), hospital readmissions (measured by patient information and medical records), well‐being (measured by COPD Assessment Test), and depression and anxiety (measured by Hospital Anxiety and Depression Scale). All outcomes will be measured at 6 months' postrandomisation. |
| Starting date | 1 May 2017 |
| Contact information |
Name: Julie Redfern Affiliation: The University of Sydney, Australia |
| Notes |
Status: recruitment of participants is complete Sponsor: National Heart Foundation 2015 NSW Cardiovascular Research Network Research Development Project Grant |
ACS: acute coronary syndrome CHD: coronary heart disease COPD: chronic obstructive pulmonary disease MI: myocardial infarction MMAS‐8: 8‐item Morisky Medication Adherence Scale NR: not reported NSW: New South Wales PCI: percutaneous coronary intervention RCT: randomised controlled trial SMS: short message service
Differences between protocol and review
Differences between protocol and the previous version of the review
Due to heterogeneity between studies with respect to participants, methods, and outcome measures, we did not pool the results in a meta‐analysis, but instead described the results narratively.
Changes in the current version of the review
In order to appropriately reflect the findings of the review, we revised the title to 'Mobile phone text messaging for medication adherence in secondary prevention of cardiovascular disease'.
In the Methods section, we removed the inclusion of cluster‐RCTs because the aim was to focus on interventions aimed at individuals. We also excluded quasi‐RCTs, as they do not use full randomisation and limit the study’s ability to conclude a causal association between an intervention and an outcome.
In the protocol (Adler 2015), the comparators were no treatment and other modes of communication. We changed the comparator to usual care to align with the reporting of the included studies.
We changed the inclusion criteria of participants. In the protocol, an eligible study must have had at least 50% of participants with established CVD. In order to reduce heterogeneity in the study populations, we removed the cut‐off value of 50%. We excluded mixed‐disease populations (e.g. participants with either CVD or other diseases).
For the primary outcome, we changed "adherence to treatment" to "adherence to medication" in order to accurately reflect the study aim. We also added patient‐reported experience of using text messaging as a further secondary outcome. Reporting patient experience of using text messaging could contribute to a deep understanding of text messaging in terms of utility, acceptability, and satisfaction.
In order to consider all relevant literature and to reduce publication bias, we added three databases (CINAHL Complete, Scopus Elsevier, and ProQuest Central) to our literature search.
Due to the considerable heterogeneity in the reporting method of continuous outcomes measured by scales, we were not able to combine these data, therefore we did not compute standardised mean difference for continuous outcomes measured on different scales.
Given that we excluded cluster‐RCTs from the current review update, we did not need to use our preplanned methods to either perform an appropriate analysis that accounts for the cluster design or calculate correct estimates using the intracluster correlation coefficient.
In future updates of this review, should we find studies with multiple intervention groups and a meta‐analysis is possible, we will combine all relevant experimental intervention groups of the study into a single group, and combine all relevant control intervention groups into a single control group, to make pairwise comparisons.
To align with the latest version of the Cochrane Handbook, we modified the cut‐off value of important heterogeneity from 50% to 40% (Deeks 2023). In the current review, we used 40% as a cut‐off value for important heterogeneity, with an I2 < 40% considered as low heterogeneity. This differed from the protocol, which specified a cut‐off value of 50%. In addition to the assessment of statistical heterogeneity, we added assessment methods for clinical heterogeneity and methodological heterogeneity. Addition of assessment methods for clinical and methodological heterogeneity contributed to a comprehensive assessment of variability amongst studies and provided insights into the suitability of performing a meta‐analysis.
We planned to assess for potential publication bias using funnel plots and Egger’s test (Page 2023). However, an insufficient number of studies (< 10) within the analysed outcomes precluded this assessment.
We planned to conduct a meta‐analysis if studies did not show sufficient heterogeneity in the types of intervention, its delivery, and study design. However, due to large variations in the way medication adherence was defined and measured, we did not conduct a meta‐analysis for medication adherence. Furthermore, we aimed to conduct a meta‐analysis for non‐fatal cardiovascular events, combined cardiovascular events, and urinary 11‐dehydrothromboxane B2, but were unable to do so because of a lack of reported data in the studies.
We planned to carry out the following subgroup analyses, which were precluded due to insufficient data.
Baseline CVD condition (i.e. coronary artery disease, cerebrovascular artery disease, peripheral artery disease, and atherosclerotic aortic disease).
Age (not older people versus older people, i.e. 64 or more years old).
Gender comparison (male versus female).
In order to present a comprehensive summary of study findings, we included additional outcomes (fatal cardiovascular events, non‐fatal cardiovascular events, combined CVD events, LDL cholesterol, blood pressure, and heart rate) in the summary of findings table.
Contributions of authors
JR: conception, design and co‐ordination of the review, interpretation of data, writing of the review
QT: conception, design and co‐ordination of the review, literature search, study selection, data extraction, assessment of risk of bias, arbitration of disagreement, data analysis, interpretation of findings, assessment of the certainty of the body of evidence, writing of the review
KH: data analysis, interpretation of findings
MH: study selection, data extraction, assessment of risk of bias, arbitration of disagreement, assessment of the certainty of the body of evidence, writing of the review
NH: study selection, data extraction, assessment of risk of bias, arbitration of disagreement, assessment of the certainty of the body of evidence, writing of the review
CZ: study selection, data extraction, assessment of risk of bias, arbitration of disagreement, writing of the review
CF: conception and design of the review, writing of the review
PP: conception and design of the review, writing of the review
CKC: writing of the review
JR is the guarantor for the review.
Sources of support
Internal sources
-
No sources of support supplied, Other
No sources of support supplied
External sources
-
National Health and Medical Research Council (NHMRC), Australia
NHMRC Investigator Grants held by JR, KH, CKC. NHMRC Synergy Grant held by JR
Declarations of interest
JR holds a National Health and Medical Research Council (NHMRC) Fellowship (investigator‐initiated), which supports part of her salary that includes academic time spent working on this review; and an NHMRC Synergy Grant, paid to institution. JR was one of the authors of Chow 2015 and Chow 2022. JR was not involved in assessing the eligibility of the studies, extracting data, or assessing risk of bias or the certainty of the evidence for this review update. These tasks were performed by two independent review authors (QT, NH). Chow 2015 was supported by grants from the National Heart Foundation of Australia Grant‐in‐Aid (G10S5110) and a BUPA Foundation Grant. Chow 2022 was supported by the NHMRC (APP1042290). JR retained complete control over the design, methods, data analysis, and reporting of both studies. JR did not receive the funds personally for either study and did not benefit financially from the payment or have access to or control of the funds. JR reports a trademark for the TEXTCARE software that delivers text message programmes for research, which is owned by the University of Sydney. It is not patented and was not used to deliver any of the research projects for this Cochrane review. JR has not benefitted financially from the trademark, and the University of Sydney does not intend to commercialise it.
QT: none known.
KH reports a grant from the National Health and Medical Research Council, which is a government‐funded fellowship and is not related to the topic. KH retains complete control over all study designs, methods, data analysis, and reporting related to her grant.
MH: none known.
NH: none known.
CZ: none known.
CF is a former editor of Cochrane Heart but was not an editor of Cochrane Heart in the 36 months prior to this review update. CF was not involved in the editorial process for this review. CF was also involved in a study eligible for inclusion in the review (Bermon 2021). The study was supported by the Ministerio de Ciencia Tecnología e Innovación (code: 656672553352; grants 899‐2015 and 753 de 2016); Fundación Cardiovascular de Colombia, Floridablanca; UK Medical Research Council Funded Reference (reference number: MR/N021304/1); and the Universidad Pontificia Bolivariana, Bucaramanga. However, CF was not involved in assessing the eligibility of the study, extracting data, or assessing risk of bias or the certainty of the evidence for this review update; these tasks were performed by two independent review authors (QT, NH). CF’s institution did not receive any payments for this study, and CF had complete control over the study design, methods, data analysis, and reporting.
PP has published an opinion on the topic of text messaging, Adler 2018, and several editorials, but these were unrelated to the current review. PP is affiliated with the World Heart Federation, and was and remains affiliated to London School of Hygiene & Tropical Medicine, but the organisation received no financial contribution for PP's work on this Cochrane review and had no involvement in the protocol or published work. PP is a former editorial advisor of the Cochrane Heart Group and was not involved in the editorial process for this review update. PP was also involved in the Bermon 2021 study, which was eligible for inclusion in the review. The study was supported by the Ministerio de Ciencia Tecnología e Innovación (code: 656672553352; grants 899‐2015 and 753 de 2016); Fundación Cardiovascular de Colombia, Floridablanca; and UK Medical Research Council Funded Reference (reference number: MR/N021304/1). However, PP was not involved in assessing the eligibility of the study, extracting data, or assessing risk of bias or the certainty of the evidence for this review update; these tasks were performed by two independent review authors (QT, NH). PP’s institution did not receive any payments for this study, and PP had complete control over the study design, methods, data analysis, and reporting.
CKC was one of the authors of Chow 2015 and Chow 2022. CKC was not involved in assessing the eligibility of the studies, extracting data, or assessing risk of bias or the certainty of the evidence for this review update. These tasks were performed by two independent review authors (QT, NH). Chow 2015 was supported by grants from the National Heart Foundation of Australia Grant‐in‐Aid (G10S5110) and a BUPA Foundation Grant. Chow 2022 was supported by the National Health and Medical Research Council (NHMRC) (APP1042290). CKC was the Principal Investigator for both of these studies, and, as such, payments were made to her institution, University of Sydney, for delivery of the research projects (such as paying research assistants and to perform measurements and analyses). However, both studies were investigator‐initiated, and CKC received no direct financial gain and retained complete control over the study design, methods, data analysis, and reporting. CKC holds an NHMRC Fellowship (investigator‐initiated), which supports part of her salary that includes academic time spent working on this review; paid to institution, but CKC benefitted or had control over the funds. CKC is a Cardiologist at Westmead Hospital in Australia. CKC reports a trademark for the TEXTCARE software that delivers text message programmes for research, which is owned by the University of Sydney. It is not patented and was not used to deliver any of the research projects for this Cochrane review. CKC has not benefitted financially from the trademark, and the University of Sydney does not intend to commercialise it. CKC has also published an opinion piece on the topic of text messaging (Klimis 2021).
These authors should be considered joint first author.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bae 2021 {published data only}
- Bae JW, Woo SI, Lee J, Park SD, Kwon SW, Choi SH, et al. mHealth interventions for lifestyle and risk factor modification in coronary heart disease: randomized controlled trial. JMIR mHealth and uHealth 2021;9(9):e29928. [DOI: 10.2196/29928] [DOI] [PMC free article] [PubMed] [Google Scholar]
- KCT0005087. Effect of text messaging program on lifestyle and clinical outcomes in patients with cardiovascular disease. cris.nih.go.kr/cris/search/detailSearch.do/19282 (first received 11 September 2019).
Bermon 2021 {published data only}
- Bermon A, Uribe AF, Pérez-Rivero PF, Prieto-Merino D, Saaibi JF, Silva FA, et al. Efficacy and safety of text messages targeting adherence to cardiovascular medications in secondary prevention: TXT2HEART Colombia randomized controlled trial. JMIR mHealth and uHealth 2021;9(7):e25548. [DOI: 10.2196/25548] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermon A, Uribe-Rodríguez AF, Pérez-Rivero PF, Prieto-Merino D, Rivera DIC, Guio E, et al. Evaluation of the efficacy and safety of text messages targeting adherence to cardiovascular medications in secondary prevention: the txt2heart Colombia randomised controlled trial protocol. BMJ Open 2019;9:e028017. [DOI: 10.1136/ bmjopen-2018-028017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03098186. TXT2HEART COLOMBIA: evaluation of the efficacy and safety of text messages to improve adherence to cardiovascular medications in secondary prevention [TXT2HEART COLOMBIA: evaluation of the efficacy and safety of text messages to improve adherence to cardiovascular medications in secondary prevention]. clinicaltrials.gov/ct2/show/NCT03098186 (first received 3 March 2017).
Chen 2019 {published data only}
- Chen C, Li X, Kang Y, Liu H, Liang YD, Zhang Q. Post-discharge short service message improves short-term clinical outcome and self-care behavior in Chinese patients with chronic heart failure. European Heart Journal 2018;39(Suppl 1):797-8. [DOI: 10.1093/eurheartj/ehy563.P3770] [DOI] [Google Scholar]
- Chen C, Li X, Sun L, Cao S, Kang Y, Hong L, et al. Post-discharge short message service improves short-term clinical outcome and self-care behaviour in chronic heart failure. ESC Heart Failure 2019;6(1):164-73. [DOI: 10.1002/ehf2.12380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChiCTR-INR-17012316. Effect of post-discharge text messages on clinical outcomes in patients with heart failure [Effect of post-discharge text messages on clinical outcomes in patients with heart failure]. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-17012316 (first received 9 August 2018).
- Li X, Chen C, You GY, Zhang Q. Post-discharge text messaging intervention improved clinical outcomes in patients with heart failure. European Heart Journal 2016;37(Suppl 1):727-8. [DOI: 10.1093/eurheartj/ehw433] [DOI] [Google Scholar]
Chow 2015 {published data only}
- ACTRN12611000161921. Tobacco, EXercise and dieT MEssages (TEXT ME): the effect of semi-personalised lifestyle reminder text message intervention on cardiovascular disease risk [Tobacco, EXercise and dieT MEssages (TEXT ME): The effect of a semi-personalised lifestyle reminder text message intervention on cardiovascular risk factors in patients with cardiovascular disease and those who are at high risk of cardiovascular disease]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=ACTRN12611000161921 (first received 3 February 2011).
- Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314(12):1255-63. [DOI: 10.1001/jama.2015.10945] [DOI] [PubMed] [Google Scholar]
- Chow CK, Redfern J, Thiagalingam A, Jan S, Whittaker R, Hackett M, et al. Design and rationale of the tobacco, exercise and diet messages (TEXT ME) trial of a text message-based intervention for ongoing prevention of cardiovascular disease in people with coronary disease: a randomised controlled trial protocol. BMJ Open 2012;2(1):e000606. [DOI: 10.1136/bmjopen-2011-000606] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern J, Santo K, Coorey G, Thakkar J, Hackett M, Thiagalingam A, et al. Factors influencing engagement, perceived usefulness and behavioral mechanisms associated with a text message support program. PLOS ONE 2016;11(10):e0163929. [DOI: 10.1371/journal.pone.0163929] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar J, Redfern J, De Keizer L, Thiagalingam A, Chow C. Patient perceptions of text message-based intervention for secondary prevention of cardiovascular events. Heart, Lung and Circulation 2013;22(Suppl 1):S264. [DOI: 10.1016/j.hlc.2013.05.630] [DOI] [Google Scholar]
- Thakkar J, Redfern J, Thiagalingam A, Chow CK. Patterns, predictors and effects of texting intervention on physical activity in CHD – insights from the TEXT ME randomized clinical trial. European Journal of Preventive Cardiology 2016;23(17):1894-902. [DOI: 10.1177/2047487316664190] [DOI] [PubMed] [Google Scholar]
Chow 2022 {published data only}
- ACTRN12613000793718. TEXT messages to improve MEDication adherence & Secondary prevention – TEXTMEDS [TEXT messages to improve MEDication adherence & Secondary prevention in patients with acute coronary syndrome - TEXTMEDS]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364448&isReview=true (first received 18 June 2013).
- Chow CK, Klimis H, Thiagalingam A, Redfern J, Hillis GS, Brieger D, et al. Text messages to improve medication adherence and secondary prevention after acute coronary syndrome: the TEXTMEDS randomized clinical trial. Circulation 2022;145(19):1443-55. [DOI: 10.1161/CIRCULATIONAHA.121.056161] [DOI] [PubMed] [Google Scholar]
- Chow CK, Thiagalingam A, Santo K, Kok C, Thakkar J, Stepien S, et al. TEXT messages to improve MEDication adherence and Secondary prevention (TEXTMEDS) after acute coronary syndrome: a randomised clinical trial protocol. BMJ Open 2018;8:e019463. [DOI: 10.1136/bmjopen-2017-019463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2015a {published data only}
- ACTRN12613000901707. Text4Heart: a text message and internet-based comprehensive cardiac rehabilitation intervention [A randomised controlled trial to determine the efficacy of a text message and Internet-based comprehensive cardiac rehabilitation programme to improve adherence to lifestyle change compared with usual care in New Zealand adults with a diagnosis of cardiovascular disease]. anzctr.org.au/ACTRN12613000901707.aspx (first received 9 August 2013).
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self-management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials 2014;15(71):1-9. [DOI: 10.1186/1745-6215-15-71] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self-management: results from the Text4Heart randomized controlled trial. Journal of Medical Internet Research 2015;17(10):e237. [DOI: 10.2196/jmir.4944] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2016 {published data only}
- Fang R, Li X. Electronic messaging support service programs improve adherence to lipid-lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. Journal of Clinical Nursing 2016;25(5-6):664-71. [DOI: 10.1111/jocn.12988] [DOI] [PubMed] [Google Scholar]
Huo 2019 {published data only}
- Huo X, Krumholz HM, Bai X, Spatz ES, Ding Q, Horak P, et al. Effects of mobile text messaging on glycemic control in patients with coronary heart disease and diabetes mellitus: a randomized clinical trial. Circulation Cardiovascular Quality and Outcomes 2019;12(9):e005805. [DOI: 10.1161/CIRCOUTCOMES.119.005805] [DOI] [PubMed] [Google Scholar]
- NCT02883842. Cardiovascular Health and Text Messaging-Diabetes Mellitus (CHAT-DM) Study [Effectiveness of text messaging on risk factors control and medication adherence among patients with Coronary Heart Disease and Diabetes in China: cardiovascular health andtext messaging-Diabetes Mellitus (CHAT-DM) study]. clinicaltrials.gov/ct2/show/NCT02883842 (first received 30 August 2016).
Kamal 2015 {published data only}
- Kamal AK, Shaik QN, Pasha O, Azam I, Islam M, Memon AA, et al. Improving medication adherence in stroke patients through Short Text Messages (SMS4Stroke)-study protocol for a randomized, controlled trial. BMC Neurology 2015;15:157. [DOI: 10.1186/s12883-015-0413-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AK, Shaikh Q, Pasha O, Azam I, Islam M, Memon AA, et al. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored short messaging service (SMS)-SMS4Stroke study. BMC Neurolology 2015;15:212. [DOI: 10.1186/s12883-015-0471-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khonsari 2015 {published data only}
- Khonsari S, Subramanian P, Chinna K, Latif LA, Ling LW, Gholami O. Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. European Journal of Cardiovascular Nursing 2015;14(2):170-9. [DOI: 10.1177/1474515114521910] [DOI] [PubMed] [Google Scholar]
Maddison 2021 {published data only}
- ACTRN12616000422426. Text4Heart Partnership: a text messaging program to enhance self-management of cardiovascular disease [Text messaging to enhance self-management of cardiovascular disease]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000422426 (first received 23 March 2016).
- Maddison R, Jiang Y, Stewart R, Scott T, Kerr A, Whittaker R, et al. An intervention to improve medication adherence in people with heart disease (Text4HeartII): randomized controlled trial. JMIR mHealth and uHealth 2021;9(6):e24952. [DOI: 10.2196/24952] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison R, Stewart R, Doughty R, Scott T, Kerr A, Benatar J, et al. Text4Heart II - improving medication adherence in people with heart disease: a study protocol for a randomized controlled trial. Trials 2018;19(1):70. [DOI: 10.1186/s13063-018-2468-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2022 {published data only}
- NCT02793830. A mobile phone based medication reminder program [A mobile phone based medication reminder program for patients in China with coronary heart disease: a pilot study]. clinicaltrials.gov/ct2/show/NCT02793830 (first received 8 June 2016).
- Ni Z, Wu B, Yang Q, Yan LL, Liu C, Shaw RJ. An mHealth intervention to improve medication adherence and health outcomes among patients with coronary heart disease: randomized controlled trial. Journal of Medical Internet Research 2022;24(3):e27202. [DOI: 10.2196/27202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2017 {published data only}
- NCT02783287. The impact of text messaging on medication adherence and exercise regimen among post-myocardial infarction patients. clinicaltrials.gov/ct2/show/NCT02783287 (first received 26 May 2016).
- Pandey A, Choudhry N. An m-health application of daily text messages to address forgetfulness as contributor to non-adherence to medications post-myocardial infarction. Circulation 2015;132:A18641. [DOI: 10.1161/circ.132.suppl_3.18641] [DOI] [Google Scholar]
- Pandey A, Krumme AA, Patel T, Choudhry NK. The impact of text messaging on medication adherence and exercise among postmyocardial infarction patients: randomized controlled pilot trial. JMIR mHealth and uHealth 2017;5(8):e110. [DOI: 10.2196/mhealth.7144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Choudhry N. Text message reminders to address medication non-adherence in post-MI patients: a one year intervention study. Canadian Journal of Cardiology 2014;30(10):S179. [DOI: 10.1016/j.cjca.2014.07.280] [DOI] [Google Scholar]
Park 2014a {published data only}
- Park LG, Howie-Esquivel J, Chung M, Dracup K. A text messaging intervention improves medication adherence for patients with coronary heart disease: a randomized controlled trial. Circulation 2013;128(Suppl 22):A15249. [DOI: 10.1161/circ.128.suppl_22.A15249] [DOI] [PubMed] [Google Scholar]
- Park LG, Howie-Esquivel J, Chung ML, Dracup K. A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Education and Counseling 2014;94(2):261-8. [DOI: 10.1016/j.pec.2013.10.027] [DOI] [PubMed] [Google Scholar]
- Park LG, Howie-Esquivel J, Whooley MA, Dracup K. Psychosocial factors and medication adherence among patients with coronary heart disease: a text messaging intervention. European Journal of Cardiovascular Nursing 2015;14(3):264-73. [DOI: 10.1177/1474515114537024] [DOI] [PubMed] [Google Scholar]
Passaglia 2021 {published data only}
- NCT03414190. Impact of text messages to promote secondary prevention after acute coronary syndrome [Impact of a mobile phone text messages intervention on the secondary prevention of cardiovascular events after acute coronary syndrome]. clinicaltrials.gov/show/NCT03414190 (first received 29 January 2018).
- Passaglia LG, Brant LC, Nascimento BR, Ribeiro AL. Impact of text messages in a middle-income country to promote secondary prevention after acute coronary syndrome (IMPACS): a randomized trial. Medicine 2019;98(22):e15681. [DOI: 10.1097/MD.0000000000015681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaglia LG, Brant LC, Silva JL, Nascimento BR, Ribeiro AL. Text messages to promote secondary prevention after acute coronary syndrome (IMPACS trial). International Journal of Cardiovascular Sciences 2021;35(2):202-13. [DOI: 10.36660/ijcs.20200378] [DOI] [Google Scholar]
Quilici 2013 {published data only}
- Quilici J, Fugon L, Beguin S, Morange PE, Bonnet JL, Alessi MC, et al. Effect of motivational mobile phone short message service on aspirin adherence after coronary stenting for acute coronary syndrome. International Journal of Cardiology 2013;168(1):568-9. [DOI: 10.1016/j.ijcard.2013.01.252] [DOI] [PubMed] [Google Scholar]
Ross 2021 {published data only}
- NCT02336919. The use of texting messaging to improve the hospital-to-community transition period in cardiovascular disease patients (Txt2Prevent) [The use of texting messaging to improve the hospital-to-community transition and prevent readmission in patients with cardiovascular disease (Txt2Prevent)]. clinicaltrials.gov/ct2/show/NCT02336919 (first received 13 January 2015).
- Ross ES, Sakakibara BM, Mackay MH, Whitehurst DG, Singer J, Toma M, et al. The use of SMS text messaging to improve the hospital-to-community transition in patients with acute coronary syndrome (Txt2Prevent): results from a pilot randomized controlled trial. JMIR mHealth and uHealth 2021;9(5):e24530. [DOI: 10.2196/24530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ES, Sakakibara BM, Mackay MH, Whitehurst DG, Singer J, Toma M, et al. The use of text messaging to improve the hospital-to-community transition in acute coronary syndrome patients (Txt2Prevent): intervention development and pilot randomized controlled trial protocol. JMIR Research Protocols 2017;6(5):e91. [DOI: 10.2196/resprot.6968] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2019 {published data only}
- Huo X, Spatz ES, Ding Q, Horak P, Zheng X, Masters C, et al. Design and rationale of the Cardiovascular Health and Text Messaging (CHAT) study and the CHAT-Diabetes Mellitus (CHAT-DM) study: two randomised controlled trials of text messaging to improve secondary prevention for coronary heart disease and diabetes. BMJ Open 2017;7(12):e018302. [DOI: 10.1136/bmjopen-2017-018302] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Spatz ES, Bai X, Huo X, Ding Q, Horak P, et al. Effect of text messaging on risk factor management in patients with coronary heart disease: the CHAT randomized clinical trial. Circulation Cardiovascular Quality and Outcomes 2019;12(4):e005616. [DOI: 10.1161/CIRCOUTCOMES.119.005616] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acevedo 2023 {published data only}
- Acevedo M, Varleta P, Casas-Cordero C, Berríos A, Navarrete C, Valentino G, et al. Mobile-phone text messaging to promote ideal cardiovascular health in women. Open Heart 2023;10(1):e002214. [DOI: 10.1136/openhrt-2022-002214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhu‐Zaheya 2017 {published data only}
- Akhu-Zaheya LM, Shiyab WY. The effect of short message system (SMS) reminder on adherence to a healthy diet, medication, and cessation of smoking among adult patients with cardiovascular diseases. International Journal of Medical Informatics 2017;98:65-75. [DOI: 10.1016/j.ijmedinf.2016.12.003] [DOI] [PubMed] [Google Scholar]
Brar 2018 {published data only}
- Brar Prayaga R, Jeong EW, Feger E, Noble HK, Kmiec M, Prayaga RS. Improving refill adherence in Medicare patients with tailored and interactive mobile text messaging: pilot study. JMIR mHealth and uHealth 2018;6(1):e30. [DOI: 10.2196/mhealth.8930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrillo 2021 {published data only}
- Carrillo A, Huffman JC, Kim S, Massey CN, Legler SR, Celano CM. An adaptive text message intervention to promote well-being and health behavior adherence for patients with cardiovascular disease: intervention design and preliminary results. Journal of the Academy of Consultation-Liaison Psychiatry 2021;62(6):617-24. [DOI: 10.1016/j.jaclp.2021.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen S, Gong E, Kazi DS, Gates AB, Bai R, Fu H, et al. Using mobile health intervention to improve secondary prevention of coronary heart diseases in China: mixed-methods feasibility study. JMIR mHealth and uHealth 2018;6(1):e9. [DOI: 10.2196/mhealth.7849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2019 {published data only}
- Cheung NW, Redfern J, Thiagalingam A, Hng TM, Islam SMS, Haider R, et al. Text messaging support for patients with diabetes or coronary artery disease (SupportMe): protocol for a pragmatic randomised controlled trial. BMJ Open 2019;9(6):e025923. [DOI: 10.1136/bmjopen-2018-025923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung NW, Redfern J, Thiagalingam A, Hng TM, Marschner S, Haider R, et al. Effect of mobile phone text messaging self-management support for patients with diabetes or coronary heart disease in a chronic disease management program (SupportMe) on blood pressure: pragmatic randomized controlled trial. Journal of Medical Internet Research 2023;25:e38275. [DOI: 10.2196/38275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foccardi 2021 {published data only}
- Foccardi G, Vecchiato M, Neunhaeuserer D, Mezzaro M, Quinto G, Battista F, et al. Effectiveness of text messaging as an incentive to maintain physical activity after cardiac rehabilitation: a randomized controlled pilot study. International Journal of Environmental Research and Public Health 2021;18(12):6645. [DOI: 10.3390/ijerph18126645] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haramiova 2017 {published data only}
- Haramiova Z, Stasko M, Hulin M, Tesar T, Kuzelova M, Morisky DM. The effectiveness of daily SMS reminders in pharmaceutical care of older adults on improving patients' adherence to antihypertensive medication (SPPA): study protocol for a randomized controlled trial. Trials 2017;18(1):334. [DOI: 10.1186/s13063-017-2063-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20180911041002N {published data only}
- IRCT20180911041002N. Effect of patient education and follow up with short massage on patient's therapeutic adherence with heart disease. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180911041002N1 (first received 22 January 2019).
Legler 2020 {published data only}
- Legler S, Celano CM, Beale EE, Hoeppner BB, Huffman JC. Use of text messages to increase positive affect and promote physical activity in patients with heart disease: the promoting activity in cardiac patients via text messages (PACT) pilot study. Current Psychology 2020;39:648-55. [DOI: 10.1007/s12144-018-9785-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luong 2021 {published data only}
- Luong P, Glorioso TJ, Grunwald GK, Peterson P, Allen LA, Khanna A, et al. Text message medication adherence reminders automated and delivered at scale across two institutions: testing the nudge system: pilot study. Circulation Cardiovascular Quality and Outcomes 2021;14(5):e007015. [DOI: 10.1161/CIRCOUTCOMES.120.007015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moradi 2017 {published data only}
- Moradi A, Moeini M, Sanei H. The effect of interactive text message follow-up on health promoting lifestyle of patients with acute coronary syndrome. Iranian Journal of Nursing and Midwifery Research 2017;22(4):287-93. [DOI: 10.4103/ijnmr.IJNMR_89_16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohde 2021 {published data only}
- Rohde LE, Hoffmann Filho CR, Rover MM, Rabelo-Silva ER, Lopez L, Passos LCS, et al. Design of a multifaceted strategy based on automated text messaging in patients with recent heart failure admission. ESC Heart Failure 2021;8(6):5523-30. [DOI: 10.1002/ehf2.13516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santo 2017 {published data only}
- Santo K, Chow CK, Thiagalingam A, Rogers K, Chalmers J, Redfern J. MEDication reminder APPs to improve medication adherence in Coronary Heart Disease (MedApp-CHD) Study: a randomised controlled trial protocol. BMJ Open 2017;7(10):e017540. [DOI: 10.1136/bmjopen-2017-017540] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo K, Singleton A, Rogers K, Thiagalingam A, Chalmers J, Chow CK, et al. Medication reminder applications to improve adherence in coronary heart disease: a randomised clinical trial. Heart 2019;105(4):323-9. [DOI: 10.1136/heartjnl-2018-313479] [DOI] [PubMed] [Google Scholar]
Santo 2018 {published data only}
- Santo K, Hyun K, Keizer L, Thiagalingam A, Hillis GS, Chalmers J, et al. The effects of a lifestyle-focused text-messaging intervention on adherence to dietary guideline recommendations in patients with coronary heart disease: an analysis of the TEXT ME study. International Journal of Behavioral Nutrition and Physical Activity 2018;15(1):45. [DOI: 10.1186/s12966-018-0677-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang J, Zeng Z, Dong R, Sheng J, Lai Y, Yu J, et al. Efficacy of a WeChat based intervention to adherence to secondary prevention in patients undergoing coronary artery bypass graft in China: a randomized controlled trial. Journal of Telemedicine and Telecare 2020;28(9):653-66. [DOI: 10.1177/1357633X20960639] [DOI] [PubMed] [Google Scholar]
Yan 2021 {published data only}
- Yan LL, Gong E, Gu W, Turner EL, Gallis JA, Zhou Y. Effectiveness of a primary care-based integrated mobile health intervention for stroke management in rural China (SINEMA): a cluster-randomized controlled trial. PLOS Medicine 2021;18(4):e1003582. [DOI: 10.1371/journal.pmed.1003582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2019 {published data only}
- Zhao YY, Dang FP, Zhai TT, Li HJ, Wang RJ, Ren JJ. The effect of text message reminders on medication adherence among patients with coronary heart disease: a systematic review and meta-analysis. Medicine 2019;98(52):e18353. [DOI: 10.1097/MD.0000000000018353] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12621000754842 {published data only}
- ACTRN12621000754842. Efficacy and safety of adjunctive virtual models of care in the secondary prevention of cardiovascular events in adults discharged from hospital after myocardial infarction or decompensated heart failure. anzctr.org.au/ACTRN12621000754842.aspx (first received 16 June 2021).
CTRI/2021/06/034463 {published data only}
- CTRI/2021/06/034463. Drug adherence in persons after stunting and the effect of text messages on drug adherence. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/06/034463 (first received 29 June 2021).
CTRI/2021/10/037432 {published data only}
- CTRI/2021/10/037432. Prevention of secondary stroke by risk factor control and medication adherence. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/10/037432 (first received 21 October 2021).
IRCT2014050617596N1 {published data only}
- IRCT2014050617596N1. Comparison of telephone and SMS follow up on treatment regimen adherence in patients with coronary artery bypass surgery. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014050617596N1 (first received 7 August 2014).
IRCT2016011025937N1 {published data only}
- IRCT2016011025937N1. The effect of follow up using short message service on illness perception and medication adherence in patients under coronary angioplasty. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2016011025937N1 (first received 20 February 2016).
IRCT2016081125937N2 {published data only}
- IRCT2016081125937N2. Effect of a reminder system on medication adherence. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2016081125937N2 (first received 29 August 2016).
ISRCTN10549665 {published data only}
- ISRCTN10549665. Improving medication taking in patients with coronary heart disease using a mobile health technology, a feasibility study. www.isrctn.com/ISRCTN10549665 (first received 26 November 2019). [DOI: 10.1186/ISRCTN10549665] [DOI]
Park 2017 {published data only}
- NCT03022669. Mobile health strategies for veterans. clinicaltrials.gov/study/NCT03022669 (first received 16 January 2017).
- Park LG, Collins EG, Shim JK, Whooley MA. Comparing mobile health strategies to improve medication adherence for veterans with coronary heart disease (Mobile4Meds): protocol for a mixed-methods study. JMIR Research Protocols 2017;6(7):e134. [DOI: 10.2196/resprot.7327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Redfern 2019 {published data only}
- ACTRN12616001167459. Impact of integrated text messaging (ITM) on the efficacy of rehabilitation programs for chronic respiratory and cardiovascular disease. anzctr.org.au/ACTRN12616001167459.aspx (first received 26 August 2016).
- Redfern J, Hyun K, Singleton A, Hafiz N, Raeside R, Spencer L, et al. ITM support for patients with chronic respiratory and cardiovascular diseases: a protocol for a randomised controlled trial. BMJ Open 2019;9(3):e023863. [DOI: 10.1136/bmjopen-2018-023863] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abegaz 2017
- Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine 2017;96(4):e5641. [DOI: 10.1097/MD.0000000000005641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adler 2018
- Adler AJ, Casas JP, Martin N, Free C, Perel P. Cochrane corner: text messaging to improve adherence to drugs for secondary prevention of cardiovascular disease. Heart 2018;104(22):1814-6. [DOI: 10.1136/heartjnl-2017-312888] [DOI] [PubMed] [Google Scholar]
Akinosun 2021
- Akinosun AS, Polson R, Diaz-Skeete Y, De Kock JH, Carragher L, Leslie S, et al. Digital technology interventions for risk factor modification in patients with cardiovascular disease: systematic review and meta-analysis. JMIR mHealth and uHealth 2021;9(3):e21061. [DOI: 10.2196/21061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Arkee 2021
- Al-Arkee S, Mason J, Lane DA, Fabritz L, Chua W, Haque MS. Mobile apps to improve medication adherence in cardiovascular disease: systematic review and meta-analysis. Journal of Medical Internet Research 2021;23(5):e24190. [DOI: 10.2196/24190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allida 2020
- Allida S, Du H, Xu X, Prichard R, Chang S, Hickman LD, et al. mHealth education interventions in heart failure. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD011845. [DOI: 10.1002/14651858.CD011845.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anglada‐Martinez 2015
- Anglada-Martinez H, Riu-Viladoms G, Martin-Conde M, Rovira-Illamola M, Sotoca-Momblona JM, Codina-Jane C. Does mHealth increase adherence to medication? Results of a systematic review. International Journal of Clinical Practice 2015;69(1):9-32. [DOI: 10.1111/ijcp.12582] [DOI] [PubMed] [Google Scholar]
Bandura 1989
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, 1986. [LC: 85009310] [Google Scholar]
Bond 2021
- Bond Z, Scanlon T, Judah G. Systematic review of RCTs assessing the effectiveness of mHealth interventions to improve statin medication adherence: using the behaviour-change technique taxonomy to identify the techniques that improve adherence. Healthcare 2021;9(10):1282. [DOI: 10.3390/healthcare9101282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosworth 2018
- Bosworth HB, Ngouyombo B, Liska J, Zullig LL, Atlani C, Beal AC. The importance of cholesterol medication adherence: the need for behavioral change intervention programs. Patient Preference and Adherence 2018;12:341-8. [DOI: 10.2147/PPA.S153766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdhury 2013
- Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. European Heart Journal 2013;34(38):2940-8. [DOI: 10.1093/eurheartj/eht295] [DOI] [PubMed] [Google Scholar]
Chowdhury 2017
- Chowdhury FM, Ayala C, Dalmat D, Shantharam S, Chang T, Russell Z, et al. Effectiveness of telehealth on hypertension management among disparate populations: a systematic review. Circulation Cardiovascular Quality and Outcomes 2017;10(Suppl 3):A216. [DOI: 10.1161/circoutcomes.10.suppl_3.216] [DOI] [Google Scholar]
Dale 2014a
- Dale LP, Whittaker R, Eyles H, Ni Mhurchu C, Ball K, Smith N, et al. Cardiovascular disease self-management: pilot testing of an mHealth healthy eating program. Journal of Personalized Medicine 2014;4(1):88-101. [DOI: 10.3390/jpm4010088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2014b
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials 2014;15(71):1-9. [DOI: 10.1186/1745-6215-15-71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2015b
- Dale LP, Whittaker R, Dixon R, Stewart R, Jiang Y, Carter K, et al. Acceptability of a mobile health exercise-based cardiac rehabilitation intervention: a randomized trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2015;35(5):312-9. [DOI: 10.1097/HCR.0000000000000125] [DOI] [PubMed] [Google Scholar]
Deeks 2023
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Department of Health and Aged Care 2023
- Department of Health and Aged Care. Pharmaceutical Benefits Scheme (PBS). www.pbs.gov.au/pbs/home (accessed 30 August 2023).
Dong 2018
- Dong J, Reeves L, Ali A, Freeman MK, Adunlin G. A systematic review of the effectiveness of text message reminders on asthma medication adherence. Innovations in Pharmacy 2018;9(3):1-7. [DOI: 10.24926/iip.v9i3.1370] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbinghaus 1885
- Ebbinghaus H. Über das Gedächtnis: Untersuchungen zur experimentellen Psychologie. Leipzig: Dunker, 1885. [OCLC: (OCoLC)5876733] [Google Scholar]
Ershad 2016
- Ershad Sarabi R, Sadoughi F, Jamshidi Orak R, Bahaadinbeigy K. The effectiveness of mobile phone text messaging in improving medication adherence for patients with chronic diseases: a systematic review. Iranian Red Crescent Medical Journal 2016;18(5):e25183. [DOI: 10.5812/ircmj.25183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feroz 2020
- Feroz A, Jabeen R, Saleem S. Using mobile phones to improve community health workers performance in low-and-middle-income countries. BMC Public Health 2020;20(49):1-6. [DOI: 10.1186/s12889-020-8173-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2006
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology 2006;25(4):462-73. [DOI: 10.1037/0278-6133.25.4.462] [DOI] [PubMed] [Google Scholar]
Fuller 2018
- Fuller RH, Perel P, Navarro-Ruan T, Nieuwlaat R, Haynes RB, Huffman MD. Improving medication adherence in patients with cardiovascular disease: a systematic review. Heart 2018;104(15):1238-43. [DOI: 10.1136/heartjnl-2017-312571] [DOI] [PubMed] [Google Scholar]
Fulton 2017
- Fulton RL, Kroll T, McMurdo MET, Molloy GJ, Witham MD. Interventions to enhance medication adherence in older heart failure patients - a systematic review. Age and Ageing 2017;46(Suppl 1):i25-6. [DOI: 10.1093/ageing/afx059.104] [DOI] [Google Scholar]
Gadkari 2012
- Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: how unintentional is it really. BMC Health Services Research 2012;12:98. [DOI: 10.1186/1472-6963-12-98] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandapur 2016
- Gandapur Y, Kianoush S, Kelli HM, Misra S, Urrea B, Blaha MJ, et al. The role of mHealth for improving medication adherence in patients with cardiovascular disease: a systematic review. European Heart Journal - Quality of Care & Clinical Outcomes 2016;2(4):237-44. [DOI: 10.1093/ehjqcco/qcw018] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 20 October 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibeneme 2021
- Ibeneme SC, Ndukwu SC, Myezwa H, Irem FO, Ezenwankwo FE, Ajidahun AT, et al. Effectiveness of mobile text reminder in improving adherence to medication, physical exercise, and quality of life in patients living with HIV: a systematic review. BMC Infectious Diseases 2021;21(1):859. [DOI: 10.1186/s12879-021-06563-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassavou 2018
- Kassavou A, Sutton S. Automated telecommunication interventions to promote adherence to cardio-metabolic medications: meta-analysis of effectiveness and meta-regression of behaviour change techniques. Health Psychology Review 2018;12(1):25-42. [DOI: 10.1080/17437199.2017.1365617] [DOI] [PubMed] [Google Scholar]
Kerr 2009
- Kerr AJ, Broad J, Wells S, Riddell T, Jackson R. Should the first priority in cardiovascular risk management be those with prior cardiovascular disease. Heart 2009;95(2):125-9. [DOI: 10.1136/hrt.2007.140905] [DOI] [PubMed] [Google Scholar]
Klimis 2021
- Klimis H, Chow CK. Are digital health services the key to bridging the gap in medication adherence and optimisation? Heart, Lung & Circulation 2021;30(7):943-946. [DOI: 10.1016/j.hlc.2021.04.010] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
MacPherson 2021
- MacPherson MM, Cranston KD, Locke SR, Bourne JE, Jung ME. Using the behavior change wheel to develop text messages to promote diet and physical activity adherence following a diabetes prevention program. Translational Behavioral Medicine 2021;11(8):1585-95. [DOI: 10.1093/tbm/ibab058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morisky 1986
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care 1986;24:67-74. [DOI: 10.1097/00005650-198601000-00007] [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2013
- Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease. European Heart Journal 2013;34(17):1262-9. [DOI: 10.1093/eurheartj/ehs481] [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD000011. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2023
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Pandey 2014
- Pandey AK, Choudhry N. Text message reminders to address medication non-adherence in post-MI patients: a one year intervention study. Canadian Journal of Cardiology 2014;1:S179. [DOI: 10.1016/j.cjca.2014.07.280] [DOI] [Google Scholar]
Park 2014b
- Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. Journal of Advanced Nursing 2014;70(9):1932-53. [DOI: 10.1111/jan.12400] [DOI] [PubMed] [Google Scholar]
Park 2016
- Park LG, Beatty A, Stafford Z, Whooley MA. Mobile phone interventions for the secondary prevention of cardiovascular disease. Progress in Cardiovascular Diseases 2016;58(6):639-50. [DOI: 10.1016/j.pcad.2016.03.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perel 2015
- Perel P, Avezum A, Huffman M, Pais P, Rodgers A, Vedanthan R, et al. Reducing premature cardiovascular morbidity and mortality in people with atherosclerotic vascular disease: the World Heart Federation roadmap for secondary prevention of cardiovascular disease. Global Heart 2015;10(2):99-110. [DOI: 10.1016/j.gheart.2015.04.003] [DOI] [PubMed] [Google Scholar]
Redfern 2014
- Redfern J, Thiagalingam A, Jan S, Whittaker R, Hackett ML, Mooney J, et al. Development of a set of mobile phone text messages designed for prevention of recurrent cardiovascular events. European Journal of Preventive Cardiology 2014;21(4):492-9. [DOI: 10.1177/2047487312449416] [DOI] [PubMed] [Google Scholar]
Redfern 2016
- Redfern J, Santo K, Coorey G, Thakkar J, Hackett M, Thiagalingam A, et al. Factors influencing engagement, perceived usefulness and behavioral mechanisms associated with a text message support program. PLOS ONE 2016;11(10):e0163929. [DOI: 10.1371/journal.pone.0163929] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2023
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Richardson 2019
- Richardson M, Khouja CL, Sutcliffe K, Thomas J. Using the theoretical domains framework and the behavioural change wheel in an overarching synthesis of systematic reviews. BMJ Open 2019;9:e024950. [DOI: 10.1136/bmjopen-2018-024950] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2020
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. Journal of the American College of Cardiology 2020;76(25):2982-3021. [DOI: 10.1016/j.jacc.2020.11.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rugge 2011
- Rugge B, Balshem H, Sehgal H, Relevo R, Gorman P, Helfand M. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism. Report No.: 11(12)-EHC033-EF.AHRQ Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality, 2011. [PMID: ] [PubMed] [Google Scholar]
Schünemann 2023
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Simon 2022
- Simon E, Edwards AM, Sajatovic M, Jain N, Montoya JL, Levin JB. Systematic literature review of text messaging interventions to promote medication adherence among people with serious mental illness. Psychiatric Services 2022;73(10):1153-64. [DOI: 10.1176/appi.ps.202100634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Statista 2022
- Statista. Number of mobile (cellular) subscriptions worldwide from 1993 to 2021. www.statista.com/statistics/262950/global-mobile-subscriptions-since-1993 (accessed 30 August 2022).
Stovold 2014
- Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Systematic Reviews 2014;3(54):1-5. [DOI: 10.1186/2046-4053-3-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tam 2021
- Tam HL, Wong EM, Cheung K, Chung SF. Effectiveness of text messaging interventions on blood pressure control among patients with hypertension: systematic review of randomized controlled trials. JMIR mHealth uHealth 2021;9(9):e24527. [DOI: 10.2196/24527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2023
- Taylor RS, Fredericks S, Jones I, Neubeck L, Sanders J, Stoutz ND, et al. Global perspectives on heart disease rehabilitation and secondary prevention: a scientific statement from the Association of Cardiovascular Nursing and Allied Professions, European Association of Preventive Cardiology, and International Council of Cardiovascular Prevention and Rehabilitation. European Heart Journal 2023;44(28):2515-25. [DOI: 10.1093/eurheartj/ehad225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thakkar 2016
- Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, et al. Mobile phone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Medicine 2016;176(3):340-9. [DOI: 10.1001/jamainternmed.2015.7667] [DOI] [PubMed] [Google Scholar]
Thorneloe 2018
- Thorneloe RJ, Griffiths CE, Emsley R, Ashcroft DM, Cordingley L, Barker J, et al. Intentional and unintentional medication non-adherence in psoriasis: the role of patients’ medication beliefs and habit strength. Journal of Investigative Dermatology 2018;138(4):785-94. [DOI: 10.1016/j.jid.2017.11.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treskes 2018
- Treskes RW, Van der Velde ET, Schoones JW, Schalij MJ. Implementation of smart technology to improve medication adherence in patients with cardiovascular disease: is it effective. Expert Review of Medical Devices 2018;15(2):119-26. [DOI: 10.1080/17434440.2018.1421456] [DOI] [PubMed] [Google Scholar]
Unal 2018
- Unal E, Giakoumidakis K, Khan E, Patelarou E. Mobile phone text messaging for improving secondary prevention in cardiovascular diseases: a systematic review. Heart & Lung 2018;47(4):351-9. [DOI: 10.1016/j.hrtlng.2018.05.009] [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Chapter V - Towards the solution. In: Adherence to Long-Term Therapies: Evidence for Action. World Health Organization, 2003:27-38. [ ISBN: 9241545992] [Google Scholar]
WHO 2023
- World Health Organization. Cardiovascular diseases (CVDs). www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed 30 August 2023).
Willems 2023
- Willems R, Annemans L, Siopis G, Moschonis G, Vedanthan R, Jung J, et al. Cost effectiveness review of text messaging, smartphone application, and website interventions targeting T2DM or hypertension. NPJ Digital Medicine 2023;6(150):1-15. [DOI: 10.1038/s41746-023-00876-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2023
- World Bank. The world by income and region. datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed 30 August 2023).
Xiong 2018
- Xiong S, Berkhouse H, Schooler M, Pu W, Sun A, Gong E, et al. Effectiveness of mHealth interventions in improving medication adherence among people with hypertension: a systematic review. Current Hypertension Reports 2018;20(10):86. [DOI: 10.1007/s11906-018-0886-7] [DOI] [PubMed] [Google Scholar]
Yusuf 2011
- Yusuf S, Islam S, Chow CK, Rangarajan S, Daenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high income, middle-income and low income countries (the PURE study): a prospective epidemiological survey. Lancet 2011;378:1231-43. [DOI: 10.1016/S0140-6736(11)61215-4] [DOI] [PubMed] [Google Scholar]
Zhao 2019
- Zhao YY, Dang FP, Zhai TT, Li HJ, Wang RJ, Ren JJ. The effect of text message reminders on medication adherence among patients with coronary heart disease: a systematic review and meta-analysis. Medicine 2019;98(52):e18353. [DOI: 10.1097/MD.0000000000018353] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Adler 2015
- Adler AJ, Martin N, Mariani J, Tajer CD, Serrano NC, Casas JP, et al. Mobile phone text messaging to improve adherence to cardiovascular disease secondary prevention interventions. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD011851. [DOI: 10.1002/14651858.CD011851] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adler 2017
- Adler AJ, Martin N, Mariani J, Tajer CD, Owolabi OO, Free C, et al. Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD011851. [DOI: 10.1002/14651858.CD011851.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


