Abstract
Objective:
Report the 2-year outcomes of a multicenter randomized controlled trial comparing robotic versus laparoscopic intraperitoneal onlay mesh ventral hernia repair.
Background:
Ventral hernia repair is one of the most common operations performed by general surgeons. To our knowledge, no studies have been published to date comparing long-term outcomes of laparoscopic versus robotic ventral hernia repair.
Methods:
The trial was registered at clinicaltrials.gov (NCT03490266). Clinical outcomes included surgical site infection, surgical site occurrence, hernia occurrence, readmission, reoperation, and mortality.
Results:
A total of 175 consecutive patients were approached that were deemed eligible for elective minimally invasive ventral hernia repair. In all, 124 were randomized and 101 completed follow-up at 2 years. Two-year follow-up was completed in 54 patients (83%) in the robotic arm and 47 patients (80%) in the laparoscopic arm. No differences were seen in surgical site infection or surgical site occurrence. Hernia recurrence occurred in 2 patients (4%) receiving robotic repair versus in 6 patients (13%) receiving laparoscopic repair (relative risk: 0.3, 95% CI: 0.06–1.39; P = 0.12). No patients (0%) required reoperation in the robotic arm whereas 5 patients (11%) underwent reoperation in the laparoscopic arm (P = 0.019, relative risk not calculatable due to null outcome).
Conclusions:
Robotic ventral hernia repair demonstrated at least similar if not improved outcomes at 2 years compared with laparoscopy. There is potential benefit with robotic repair; however, additional multi-center trials and longer follow-up are needed to validate the hypothesis-generating findings of this study.
Keywords: hernia repair, laparoscopic surgery, robotic surgery, ventral hernia repair
Ventral hernias are one of the most common pathologies encountered by the general practitioner and surgeon, yet management continues to be a challenge.1 Complications from repair can be as high as 60% and include infection, enterocutaneous fistula, and recurrence which often necessitate reoperation.2 Improved strategies and approaches for treatment are needed.
Robotic surgery was first introduced more than 35 years ago to overcome limitations inherent to both traditional laparoscopic and open surgery. Although evidence at low risk of bias is generally lacking to support the widespread use of robotic surgery, it has seen rapid growth, particularly in the field of general surgery.1,3 Previous studies have demonstrated robotic ventral hernia repair is safe in the short term but few randomized controlled trials (RCTs) have published long-term data.3–6 Our objective was to assess the outcomes of robotic versus laparoscopic ventral hernia repair at 2 years postoperative.
METHODS
After obtaining Institutional Review Board (IRB) approval, we performed a multicenter, blinded RCT comparing robotic to laparoscopic ventral hernia repair in accordance with the ethical standards of the Helsinki Declaration of 1975. Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed.7 We registered this trial on clinicaltrials.gov (NCT03490266).8 We previously published post-operative outcomes and 1-year outcomes.3,4 The study was powered to assess the primary outcome for days in the hospital within 90 days after surgery and this calculation is explained in our previous manuscript.4 We designed this study to validate the findings of a retrospective national database study published by Americas Hernia Society Quality Collaborative showing that robotic ventral hernia repair had a shorter length of stay than laparoscopic repair with comparable outcomes.9 In our study, we found no difference in days in hospital between the groups. There were also no differences in the secondary outcomes of emergency department visits, wound complications, hernia recurrence, or reoperation. However, we found robotic repair had a longer operative duration and increased health care costs.4 We report the 2-year follow-up results in this manuscript.
Setting
This study took place at Lyndon Baines Johnson General Hospital and Memorial Hermann Hospital System in Houston, Texas.
Patient Selection
Adult patients (> 18 years old) with a ventral hernia <12 cm wide on physical examination, able to tolerate pneumoperitoneum, no history of open abdomen or extensive lysis of adhesions for bowel obstruction, and no active infection undergoing elective minimally invasive repair were eligible for inclusion. Patients deemed unlikely to survive beyond 2 years based on their comorbidities as judged by the surgeon, unlikely to follow up, and those who did not speak English and/or Spanish were not eligible for enrollment.8 Our patient selection process for minimally invasive hernia repair is further detailed in our previous works.4,10
Randomization and Blinding
Eligible patients were randomized 1:1 to undergo either robotic (intervention) or laparoscopic (control) ventral hernia repair using computer-generated, variable block randomization, stratified by surgeon. An independent research coordinator randomized patients on the day of surgery. The research coordinator and operating surgeon were unable to be blinded given the nature of the intervention; however, the patients and post-operative outcomes assessor were blinded to the patient’s treatment arm.
Intervention and Control
Three high-volume, minimally invasive surgeons participated in this trial and each performs both laparoscopic and robotic repair. Before the start of this RCT, each center completed a minimum of 50 standardized repairs as a ramp-up period. No consensus existed on the number of repairs a surgeon needs to perform in order to establish proficiency or expertise. The literature has reported wide range of suggested numbers of cases to achieve a plateau in performance.11,12 Based upon the best available evidence, 50 cases were chosen to mitigate any possible effect of a learning curve of the standardized repair technique used and ensure surgeons and operating room staff were optimized. Patients in the ramp-up period were not included in the trial. However, we reviewed and published our data to establish a baseline level of expertise.13
Repairs were performed using conventional approaches recommended by surgical societies.14,15 We utilized the intra-peritoneal onlay mesh for both robotic and laparoscopic repair as was done in prior studies and was more common at the time of trial design than robotic transabdominal preperitoneal repair.9 Steps performed in both robotic and laparoscopic repair included entering the abdomen in the left or right upper abdominal quadrant with an optical trocar. The abdomen was insufflated with CO2 to an intra-abdominal pressure of 10 to 15 mm Hg. Direct visualization was used to place 2 additional trocars and adhesions were dissected free. The mesh was inserted through a 12-mm port and the hernia defect was closed with 0-polydioxanone. We used mid-density, coated polypropylene mesh with at least 5 cm of overlap on all sides relative to the hernia defect and secured it to the anterior abdominal wall using the intraperitoneal onlay mesh technique. Finally, the skin was closed with absorbable sutures. The robotic repair was performed by placing three 8 to 12 mm ports along the lateral abdomen. Scissors and a grasper were used to take down adhesions. The fascial defect was closed with a 0-locking, barbed, polydioxanone suture. The mesh was secured intraperitoneally with running 2-0 barbed polydioxanone suture circumferentially. Lastly, all 12-mm ports were closed with 0-polyglactin 910 suture. Laparoscopic repair was done using three 5 to 12 mm ports. Scissors or vessel sealing device were used to take down adhesions. The fascial defect was closed with 0-polydioxanone sutures for all patients. The mesh was secured intraperitoneally with transfascial sutures and a circumferential single or double crown of permanent tacks.
Outcomes
The primary outcome of this study was days in the hospital up to 90 days which was previously reported and the sample size calculation for the original study was powered to this outcome.4 Clinical and patient-reported outcomes were assessed at 2 years postoperatively. Outcomes included surgical site infection (SSI), surgical site occurrence (SSO), hernia occurrence, readmission, reoperation, and mortality. SSI was defined using the Centers for Disease Control and Prevention guidelines.16 SSO included seroma, hematoma, and wound dehiscence. Hernia occurrence was defined as recurrence or port site hernia and was diagnosed by clinical evaluation by a surgeon blinded to the randomization arm. If the history and physical examination was concerning for possible complications a computed tomography scan of the abdomen and pelvis was ordered for confirmation.
Patient-reported outcomes included functional status, pain, and satisfaction with repair and cosmesis. Functional status was assessed using a validated, hernia-specific survey created from the modified Activities Assessment Scale (mAAS), a 12-question survey with each question using a 10-point Likert scale. Functional status was calculated by totaling the scores of these 12 questions, then normalizing the score to 100—1 indicating poor function and 100 indicating high function, and minimally clinically important difference (MCID) of 5 for a slight difference and 15 for a significant difference.17,18 Pain was assessed using a 10-point visual analog scale with 0 indicating no pain and 10 indicating extreme pain with an MCID of 1. Satisfaction with repair and cosmesis was assessed using a 10-point Likert scale with 0 indicating poor satisfaction and 10 indicating high satisfaction.
Statistical Analysis
Binary outcomes were analyzed using logistic modified Poisson regressions adjusting for randomization variable and stratification variable (surgeon). Among outcomes with no events reported and standard regression could not be completed, Fisher exact test was performed. We used the analysis of covariance to analyze patient-reported outcomes. Patient-reported outcomes at 2 years were adjusted for baseline patient-reported data. Data were analyzed with R version 3.6.0.
RESULTS
A total of 175 eligible patients were approached between April 2018 and February 2019, of which 124 were randomized (65 to robotic repair and 59 to laparoscopic repair). Most patients were female, Hispanic, and obese. The majority of hernias were incisional hernias, small and medium in size. By chance alone, more patients with recurrent hernias were randomized to laparoscopic repair (Table 1).
TABLE 1.
Baseline Demographics
| RVHR (n = 65), n (%) | LVHR (n = 59), n (%) | |
|---|---|---|
| Age, mean ± SD | 50±13 | 48±13 |
| Sex, female | 48 (74) | 37 (63) |
| Race/ethnicity | ||
| Hispanic | 50 (77) | 45 (76) |
| African-American | 6 (9) | 9 (15) |
| White | 7 (11) | 3 (5) |
| Other | 2 (3) | 2 (3) |
| Body mass index (BMI), mean ± SD | 32.4±4.6 | 31.8±5.4 |
| Obese, BMI > 30 | 45 (69) | 41 (69) |
| Recent smoker | 2 (3) | 0 |
| Diabetes mellitus | 15 (23) | 12 (20) |
| ASA score | ||
| 1 | 5 (8) | 5 (8) |
| 2 | 37 (57) | 37 (63) |
| 3 | 23 (35) | 17 (29) |
| 4 | 0 | 0 |
| Prior abdominal surgery | 57 (88) | 43 (73) |
| Hernia type | ||
| Primary | 8 (12) | 16 (27) |
| Incisional | 57 (88) | 43 (73) |
| Recurrent hernia | 8 (12) | 15 (25) |
| Hernia width, median (IQR) | 3.0 (2.0, 5.0) | 3.0 (1.0, 4.5) |
| Small (<4 cm) | 44 (68) | 44 (75) |
| Medium (4–10 cm) | 18 (28) | 11 (19) |
| Large (>10 cm) | 3 (5) | 4 (7) |
ASA indicates American Society of Anesthesiologists; IQR, interquartile range; LVHR, laparoscopic ventral hernia repair; RVHR, robotic ventral hernia repair.
Fifty-four patients (83%) in the robotic arm and 47 patients (80%) in the laparoscopic arm completed follow-up at 2 years (Fig. 1). Median (interquartile range) follow-up for all patients was 2.3 (2.1, 2.6) years.
FIGURE 1.
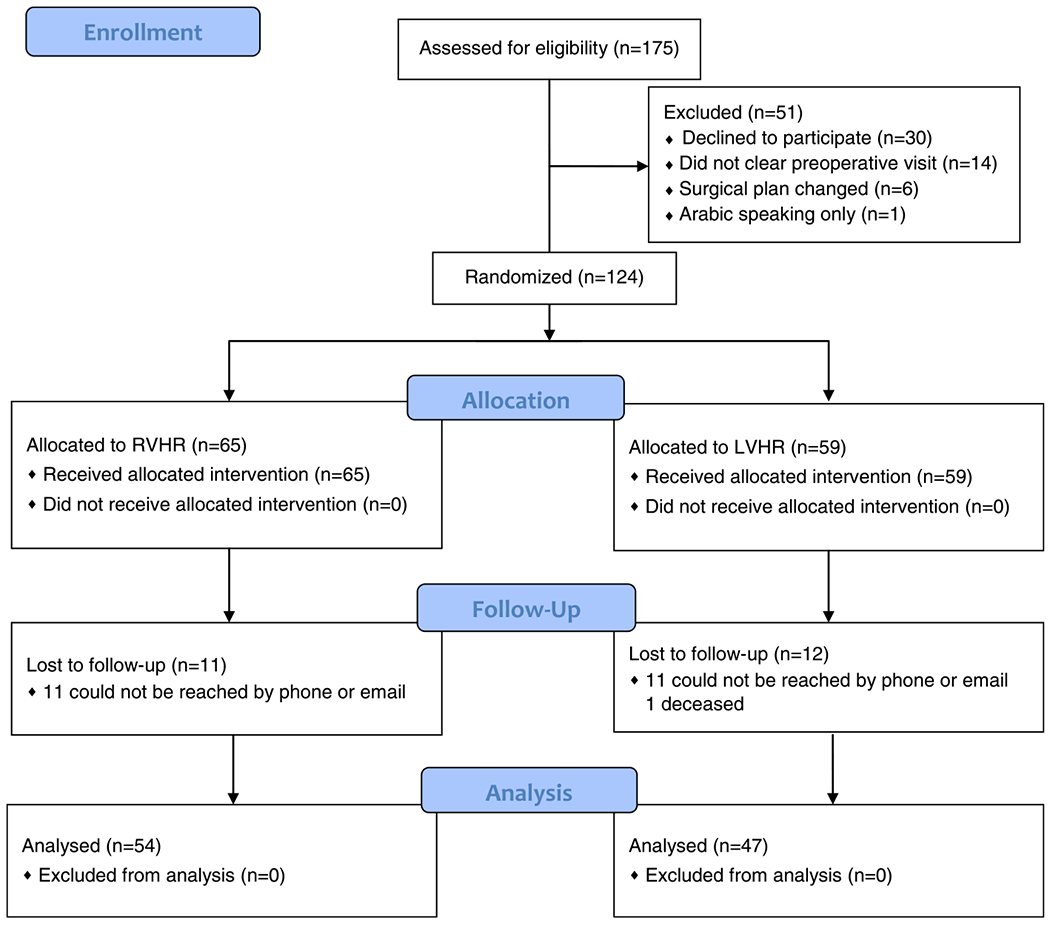
CONSORT flow diagram. LVHR indicates laparoscopic ventral hernia repair; RVHR, robotic ventral hernia repair.
No differences were seen in SSI or SSO. Hernia recurrence occurred in 2 patients (4%) receiving robotic repair versus in 6 patients (13%) receiving laparoscopic repair. No patients (0%) required reoperation in the robotic arm whereas 5 patients (11%) underwent reoperation in the laparoscopic arm. Reasons for reoperation included seroma excision, partial mesh excision, removal of tack for chronic pain, and 3 reoperations for recurrence. One death occurred in a patient receiving laparoscopic repair. Per the family, the cause of death was unknown and occurred 18 months following repair (Table 2).
TABLE 2.
Clinical Outcomes
| RVHR (n = 54), n (%) | LVHR (n = 47), n (%) | P | Relative risk (95% CI) | |
|---|---|---|---|---|
| Follow-up duration, median (IQR) | 2.30 (2.12, 2.62) | 2.26 (2.03, 2.50) | 0.463 | — |
| Surgical site infection | 0 | 0 | 1.000 | — |
| Surgical site occurrence | 7 (13) | 4 (9) | 0.460 | 1.55 (0.48–4.97) |
| Seroma | 7 (13) | 4 (9) | 0.460 | 1.55 (0.48–4.97) |
| Hematoma | 0 | 0 | 1.000 | — |
| Hernia recurrence | 2 (4) | 6 (13) | 0.124 | 0.30 (0.06–1.39) |
| Port site hernia | 0 | 0 | 1.000 | — |
| Readmission | 0 | 3 (6) | 0.097 | — |
| Reoperation | 0 | 5 (11) | 0.019 | — |
| Mortality | 0 | 1 (2) | 0.465 | — |
IQR indicates interquartile range; LVHR, laparoscopic ventral hernia repair; RVHR, robotic ventral hernia repair.
Patients in both robotic and laparoscopic arms reported improved functional status well above the MCID after 2 years. There was a slight clinical difference (MCID > 5); however, there was no statistical difference seen between the 2 groups. Similarly, patients in both groups reported decreased pain scores and were highly satisfied with repair and cosmesis at 2 years (Table 3).
TABLE 3.
Patient-reported Outcomes
| RVHR (n = 54) | LVHR (n = 47) | P | Mean difference (95% CI) | |
|---|---|---|---|---|
| Functional status (mAAS) score* | ||||
| Baseline | 48.7 (29.4, 69.8) | 42.3 (12.0, 63.3) | ||
| At 2 yr | 87.5 (69.9, 94.8) | 80.6 (54.2, 93.8) | 0.167 | 7.2 (−3.1 to 17.5) |
| Pain (VAS) score† | ||||
| Baseline | 3.5 (1.0, 6) | 3.0 (0, 6.0) | ||
| At 2 yr | 1.0 (0, 3.0) | 1.0 (0. 6.0) | 0.218 | −0.8 (−2.0 to 0.5) |
| Satisfaction score‡ | ||||
| VHR satisfaction at 2 yr | 10.0 (8.0, 10.0) | 10.0 (7.8, 10.0) | 0.314 | 0.5 (−0.5 to 1.5) |
| Cosmetic satisfaction at 2 yr | 10.0 (7.0, 10.0) | 10.0 (5.0, 10.0) | 0.409 | 0.5 (−0.8 to 1.8) |
Values are median (interquartile range).
1=poor function, 100=high function.
1=no pain, 10=extreme pain.
1=poor satisfaction, 10=high satisfaction.
LVHR indicates laparoscopic ventral hernia repair; mAAS, modified activities assessment scale; VAS, visual analog scale; RVHR, robotic ventral hernia repair; VHR, ventral hernia repair.
DISCUSSION
To our knowledge, this study is one of the first RCT that provides long-term results of robotic ventral hernia repair. It appears in the hands of high-volume experts, not only is robotic ventral hernia repair safe and effective compared with laparoscopic repair, but there is potential benefit with fewer reoperations seen at 2 years.
Prior studies comparing robotic to laparoscopic ventral hernia repair have demonstrated increased operating room time and cost, with no discernible difference in postoperative outcomes.4,5 Although this is one of the first studies to report longer-term outcomes regarding ventral hernia repair, several studies have previously published long-term outcomes comparing robotic surgery to either laparoscopy or open surgery in the fields of urology and colorectal surgery have found no differences in clinical outcomes at 2 or more years.19–23
Interestingly, our study demonstrated statistically fewer reoperations with robotic surgery at 2 years. By percentage, there were also fewer hernia recurrences. As this study is a secondary analysis of our trial initially powered to detect differences in length of hospital stay at 90 days, we are unable to conclude there are fewer reoperations/recurrences with robotic hernia repair. These findings remain hypothesis generating. It is possible that robotic hernia repair has fewer recurrences thus contributing to fewer reoperations. A large-scale study that is powered to assess this outcome should be performed. It is also possible that increased recurrences/reoperations were due to chance or due to increased number of recurrent hernias in the laparoscopic group. Unfortunately, we are not able to report on the details of why the recurrences happened as we only operated on a few. The cause could be differences due to the platform (robotic repair could be more technically sound), the technique (suturing mesh has better outcomes than tacking mesh), or due to chance. In the one-year results of the Cleveland Clinic hernia group, they found statistically more hernia recurrences with robotic repair with almost 26% recurrence rate.5 Similar to our trial, the primary outcome of the Cleveland Clinic trial was not recurrence and the findings should be considered hypotheses generating. Additional multicenter trials are needed to assess the long-term safety and effectiveness of robotic ventral hernia repair.
It is also possible that the difference in techniques between the laparoscopic and robotic ventral hernia repair may have contributed to the difference in outcomes and may require further investigation. The primary fascial closure was performed with barbed running sutures versus interrupted sutures for robotic and laparoscopic repair, respectively. The mesh was secured with a running suture in the robotic repair and tacks in the laparoscopic repair. It is possible the difference in outcomes was related to technique rather than the platform being used for the repair; however, this is speculative.
There are limitations to this study. First, this study was not powered to detect differences in postoperative outcomes, rather length of index hospital stay, thus these secondary findings should be considered hypothesis generating. We also did not do a cost analysis to see if robotic repair still had higher health care costs at 2 years postoperative. Second, high volume minimally invasive surgeons participated in this study and our results may not be applicable to surgeons with less experience. Third, most patients included in this study were obese with at least one comorbidity and findings may not be generalizable to other patient populations. Finally, most patients presented with hernias that were small or medium-sized and findings may not be applicable to larger or multiple hernias.
CONCLUSIONS
At 2-year follow-up, robotic ventral hernia repair appears to be safe and effective compared with laparoscopic ventral hernia repair with potential benefits of decreased hernia recurrence and reoperation. Future RCTs are needed to validate the hypothesis-generating findings of this study.
ACKNOWLEDGMENTS
This study was possible thanks to Debbie F. Lew, Stephanie Marquez, and Sophia Syed who served as research coordinators.
S.S. receives a research grant and consulting fees paid to the institution from Activ Surgical. The original study was supported by a research grant from Intuitive. The remaining authors report no conflicts of interest.
REFERENCES
- 1.Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3:e1918911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16:179–183. [DOI] [PubMed] [Google Scholar]
- 3.Dhanani NH, Olavarria OA, Holihan JL, et al. Robotic versus laparoscopic ventral hernia repair: one-year results from a prospective, multicenter, blinded randomized controlled trial. Ann Surg. 2021;273:1076–1080. [DOI] [PubMed] [Google Scholar]
- 4.Olavarria OA, Bernardi K, Shah SK, et al. Robotic versus laparoscopic ventral hernia repair: multicenter, blinded randomized controlled trial. BMJ. 2020;370:m2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petro CC, Zolin S, Krpata D, et al. Patient-reported outcomes of robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: the PROVE-IT Randomized Clinical Trial. JAMA Surg. 2021;156:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa TN, Abdalla RZ, Tustumi F, et al. Robotic-assisted compared with laparoscopic incisional hernia repair following oncologic surgery: short- and long-term outcomes of a randomized controlled trial. J Robot Surg. 2023;17:99–107. [DOI] [PubMed] [Google Scholar]
- 7.Consort-statement.org [Internet]. CONSORT Accessed July 27, 2022. http://www.consort-statement.org/.
- 8.Robotic versus laparoscopic ventral hernia repair. Accessed November 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03490266
- 9.Prabhu AS, Dickens EO, Copper CM, et al. Laparoscopic vs robotic intraperitoneal mesh repair for incisional hernia: an americas hernia society quality collaborative analysis. J Am Coll Surg. 2017;225:285–293. [DOI] [PubMed] [Google Scholar]
- 10.Holihan JL, Alawadi ZM, Harris JW, et al. Ventral hernia: patient selection, treatment, and management. Curr Probl Surg. 2016;53:307–354. [DOI] [PubMed] [Google Scholar]
- 11.Pernar LIM, Robertson FC, Tavakkoli A, et al. An appraisal of the learning curve in robotic general surgery. Surg Endosc. 2017;31:4583–4596. [DOI] [PubMed] [Google Scholar]
- 12.Herron DM, Marohn M. SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc. 2008;22:313–325. [DOI] [PubMed] [Google Scholar]
- 13.Dhanani NH, Olavarria OA, Millas S, et al. Is robotic surgery feasible at a safety net hospital? Surg Endosc. 2021;35:4452–4458. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Oleynikov D. The SAGES Manual of Robotic Surgery, 1st ed. International Publishing AG Switzerland: Springer; 2018. [Google Scholar]
- 15.Earle D, Roth S, Saber A, , et al. Guidelines for laparoscopic ventral hernia repair. 2016. Accessed July 22, 2022. https://www.sages.org/publications/guidelines/guidelines-for-laparoscopic-ventral-hernia-repair/. [DOI] [PubMed]
- 16.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 17.McCarthy M, Jonasson O, Chang CH, et al. Assessment of patient functional status after surgery. J Am Coll Surg. 2005;201:171–178. [DOI] [PubMed] [Google Scholar]
- 18.Cherla DV, Moses ML, Viso CP, et al. Impact of abdominal wall hernias and repair on patient quality of life. World J Surg. 2018;42:19–25. [DOI] [PubMed] [Google Scholar]
- 19.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–2536. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19:1051–1060. [DOI] [PubMed] [Google Scholar]
- 21.Khan MS, Gan C, Ahmed K, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol. 2016;69:613–621. [DOI] [PubMed] [Google Scholar]
- 22.Illiano E, Ditonno P, Giannitsas K, et al. Robot-assisted vs laparoscopic sacrocolpopexy for high-stage pelvic organ prolapse: a prospective, randomized, single-center study. Urology. 2019;134:116–123. [DOI] [PubMed] [Google Scholar]
- 23.Bochner BH, Dalbagni G, Marzouk KH, et al. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. Eur Urol. 2018;74:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]


