Abstract
Background:
Even though worldwide death rates from coronavirus disease 2019 (COVID-19) have decreased, the threat of disease progression and death for high-risk groups continues. Few direct comparisons between the available severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antivirals have been made.
Objective:
We aimed to compare two SARS-CoV-2 antivirals (nirmatrelvir/ritonavir and remdesivir) against all-cause hospitalization or death.
Design:
This is a propensity score-matched cohort study.
Methods:
We included all high-risk outpatients with COVID-19 in a tertiary referral center in Mexico City from 1 January 2022 to 31 July 2023. The primary outcome was all-cause hospitalization or death 28 days after symptom onset. The secondary outcome was COVID-19-associated hospitalization or death 28 days after symptom onset. Logistic regression analysis for characteristics associated with the primary outcome and a multi-group comparison with Kaplan–Meier survival estimates were performed.
Results:
Of 1566 patients analyzed, 783 did not receive antiviral treatment, 451 received remdesivir, and 332 received nirmatrelvir/ritonavir. The median age was 60 years (interquartile range: 46–72), 62.5% were female and 97.8% had at least one comorbidity. The use of nirmatrelvir/ritonavir was associated with an absolute risk reduction of 8.8% and a relative risk reduction of 90% for all-cause hospitalization or death. The use of remdesivir was associated with an absolute risk reduction of 6.4% and a relative risk reduction of 66% for all-cause hospitalization or death. In multivariable analysis, both antivirals reduced the odds of 28-day all-cause hospitalization or death [nirmatrelvir/ritonavir odds ratio (OR) 0.08 – 95% confidence interval (CI): 0.03–0.19, remdesivir OR 0.29 – 95% CI: 0.18–0.45].
Conclusion:
In high-risk COVID-19 outpatients, early antiviral treatment with nirmatrelvir/ritonavir or remdesivir was associated with lower 28-day all-cause hospitalization or death.
Keywords: COVID-19, early treatment, nirmatrelvir/ritonavir, remdesivir
Plain language summary
Nirmatrelvir/ritonavir and remdesivir against symptomatic treatment in high-risk COVID-19 outpatients
In this study, we included high-risk non-hospitalized patients with confirmed mild COVID-19. We compared those who received antiviral treatment (nirmatrelvir/ritonavir or remdesivir) against those who only received symptomatic treatment. The aim was to detect differences in hospitalization or death 28 days after symptom onset. We analyzed 1566 patients: 783 did not receive antiviral treatment, 451 received remdesivir, and 332 received nirmatrelvir/ritonavir. Most patients were female and over 60 years old. The most common comorbidities were chronic hypertension (44%), diabetes mellitus (26%), and autoimmune diseases (25%); systemic immunosuppression was registered in 35% of patients. Hospitalization or death 28 days after symptom onset occurred in 168 patients (136 in the symptomatic treatment group, 27 in the remdesivir group, and 5 in the nirmatrelvir/ritonavir group). Considering multiple variables like age, sex, comorbidities, and previous vaccination, both antivirals significantly reduced the odds of hospitalization or death (nirmatrelvir/ritonavir odds ratio 0.08, 95% confidence interval 0.03-0.19; remdesivir odds ratio 0.29, 95% confidence interval 0.18-0.45).
Introduction
On 5 May 2023, the World Health Organization declared the end of coronavirus disease 2019 (COVID-19) as a public health emergency of international concern. 1 Even though worldwide death rates from COVID-19 have decreased, the threat of disease progression and death for high-risk groups such as immunosuppressed or multimorbid patients continues. Early antiviral treatment against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has proven effective in reducing hospitalization and death in clinical trials and real-life settings.2–6 Global and multiple local COVID-19 treatment guidelines now include nirmatrelvir/ritonavir, remdesivir, and molnupiravir as part of the standard of care for patients at high risk for disease progression.7,8
The continuing evolution of the virus has led to the emergence of variants with decreased susceptibility to antivirals. 9 Antiviral resistance is a constant threat, especially in light of the newest Omicron subvariants. Despite relevant mutations in the regions encoding SARS-CoV-2 RNA-dependent RNA polymerase and main protease, molnupiravir, nirmatrelvir, and remdesivir were recently shown to be efficacious in vitro against the most recently described BQ.1.1 and XBB Omicron subvariants. 10
The COVID-19 global case fatality rate has dramatically declined. 11 There are numerous factors affecting this phenomenon, including viral evolution, vaccine coverage, and treatment availability. The rapidly evolving nature of the COVID-19 pandemic highlights the relevance of the continuous study of therapeutic strategies. Few direct comparisons between the recommended antivirals against SARS-CoV-2 have reported similar outcomes.12,13 The present study aims to compare the real-world effectiveness of two SARS-CoV-2 antivirals (nirmatrelvir/ritonavir and remdesivir) against all-cause hospitalization or death in a referral center.
Materials and methods
Study design and population
This is an observational propensity score-matched study conducted at a tertiary care referral center located in Mexico City.
We included all high-risk outpatients diagnosed with COVID-19 from 1 January 2022 to 31 July 2023. We excluded patients with incomplete information during follow-up. High risk was defined as having at least one or more of the following characteristics: age ⩾60, body mass index ⩾30 kg/m2, diabetes mellitus, chronic hypertension, ischemic heart disease, chronic lung disease, cirrhosis, active malignant disease, autoimmune disease, solid organ or hematopoietic stem cell transplant, and systemic immunosuppression in the previous 30 days. Vaccination was considered when patients had received at least two doses of any available SARS-CoV-2 vaccine.
Per local guidelines, patients with at least one high-risk condition and with room air oxygen saturation ⩾90% are considered candidates for the COVID-19 outpatient treatment program (Figure 1). Patients with up to 5 days from symptom onset received nirmatrelvir/ritonavir, patients with up to 7 days from symptom onset received remdesivir, and patients with more than 7 days from symptom onset received symptomatic treatment. Antiviral treatment decisions were made by the medical staff considering pharmacological interactions and local guidelines. 14 Remote 28-day follow-up to assess hospitalization or death is regularly made in all patients who are considered candidates for the COVID-19 outpatient treatment program. Nirmatrelvir/ritonavir and remdesivir are the only SARS-CoV-2 treatments available in our center.
Figure 1.
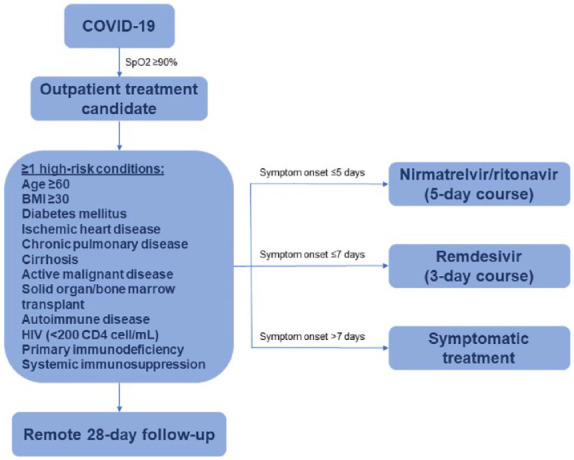
A treatment decision algorithm for mild–moderate high-risk COVID-19 outpatients.
The primary study outcome was all-cause hospitalization or death 28 days after symptom onset. The secondary outcome was COVID-19-related hospitalization or death 28 days after symptom onset.
Statistical analysis
Considering three groups (no antiviral treatment, nirmatrelvir/ritonavir, and remdesivir), the sample size was calculated using the formula for the one-way ANOVA test. A sample size of at least 967 subjects (323 per group) was calculated to identify an effect size of 0.1 with a power of 90% at a significance level of 0.05. Categorical variables were reported as frequencies and proportions. Continuous variables were reported using mean and standard deviation or median and interquartile range (IQR) values. Univariate analysis for associations between clinical characteristics and the primary outcome was made using Fisher’s exact test or exact T-test.
Logistic regression analysis for characteristics associated with the primary outcome was performed. Variables with a p value <0.15 in the univariate analysis and those with biological plausibility were included in the model. Multi-group comparisons with Kaplan–Meier survival estimates were plotted. A two-sided p value <0.05 was considered statistically significant. To account for confounding between patients receiving antiviral treatment and patients not receiving it, propensity score matching using the nearest neighbor method and a 1:1 ratio was done using the R ‘MatchIt’ package. Characteristics considered in the propensity score matching were age, sex, obesity, diabetes mellitus, chronic hypertension, ischemic heart disease, chronic pulmonary disease, liver cirrhosis, malignant neoplastic disease, autoimmune disease, organ transplant, and systemic immunosuppression. Missing data were not replaced. Statistical analysis was done using R version 4.3.1.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 15
Results
During the study period, 2116 high-risk COVID-19 outpatients were evaluated. For this study, 165 were excluded due to incomplete information in the electronic medical record system, leaving 1952 eligible COVID-19 outpatients. After propensity score matching, 1566 patients were analyzed: 783 did not receive antiviral treatment, 451 received remdesivir, and 332 received nirmatrelvir/ritonavir (there were no unmatched treatment units). Overall, the median age was 60 years (IQR 46–72), 62.5% were female (979/1566) and 97.8% had at least one comorbidity (1532/1566). Ischemic heart disease, chronic pulmonary disease, hematopoietic stem cell transplant, and solid organ transplant were more frequent in the remdesivir group. The rest of the baseline clinical characteristics are detailed in Table 1.
Table 1.
Baseline clinical characteristics and outcomes.
| Characteristics | Overall n = 1566 | No treatment n = 783 | Remdesivir n = 451 | Nirmatrelvir/ritonavir n = 332 | p |
|---|---|---|---|---|---|
| Age, median (IQR) | 60 (46–72) | 60 (47–73) | 55 (42–69) | 64 (53–74) | <0.001 |
| Sex female, n (%) | 979 (62.5) | 492 (62.8) | 265 (58.8) | 222 (66.9) | 0.07 |
| Obesity, n (%) | 328 (20.9) | 172 (22) | 86 (19.1) | 70 (21.1) | 0.48 |
| Diabetes mellitus, n (%) | 407 (26.0) | 206 (26.3) | 126 (27.9) | 75 (23.4) | 0.23 |
| Chronic hypertension, n (%) | 685 (43.7) | 351 (44.8) | 197 (43.7) | 137 (42.8) | 0.55 |
| Ischemic heart disease, n (%) | 112 (7.2) | 48 (6.1) | 49 (10.9) | 15 (4.7) | 0.001 |
| Chronic pulmonary disease, n (%) | 67 (4.3) | 17 (2.2) | 36 (8.0) | 14 (4.4) | <0.001 |
| Cirrhosis, n (%) | 75 (4.8) | 40 (5.1) | 22 (4.9) | 13 (4.1) | 0.69 |
| Solid organ malignancy, n (%) | 192 (12.3) | 98 (12.5) | 56 (12.4) | 38 (11.9) | 0.88 |
| Hematological malignancy, n (%) | 98 (6.3) | 53 (6.8) | 29 (6.4) | 16 (5.0) | 0.46 |
| Autoimmune disease, n (%) | 394 (25.2) | 193 (24.6) | 117 (25.9) | 84 (26.2) | 0.88 |
| Solid organ transplant, n (%) | 133 (8.5) | 39 (5) | 88 (19.5) | 6 (1.9) | <0.001 |
| Hematopoietic stem cell transplant, n (%) | 11 (0.7) | 2 (0.3) | 8 (1.8) | 1 (0.3) | 0.005 |
| Systemic immunosuppression, n (%) | 543 (34.6) | 244 (31.2) | 231 (51.2) | 67 (20.9) | <0.001 |
| SARS-CoV-2 vaccine, n (%) | 1438 (93.3) | 710 (91.3) | 426 (94.5) | 302 (96.8) | 0.002 |
| All-cause hospitalization or death, n (%) | 168 (10.7) | 136 (17.4) | 27 (6.0) | 5 (1.5) | <0.001 |
| Hospitalization, n (%) | 168 (10.7) | 136 (17.4) | 27 (6.0) | 5 (1.5) | |
| Death, n (%) | 32 (2) | 28 (3.6) | 14 (1.0) | 0 (0) | |
| COVID-19-related hospitalization or death, n (%) | 94 (6.0) | 76 (9.7) | 15 (3.3) | 3 (0.9) | <0.001 |
| Hospitalization, n (%) | 94 (6.0) | 76 (9.7) | 15 (3.3) | 3 (0.9) | |
| Death, n (%) | 20 (1.3) | 18 (2.3) | 2 (0.4) | 0 (0) |
IQR, interquartile range.
The use of nirmatrelvir/ritonavir was associated with an absolute risk reduction of 8.8% and a relative risk reduction of 90% for all-cause hospitalization or death. The use of remdesivir was associated with an absolute risk reduction of 6.4% and a relative risk reduction of 66% for all-cause hospitalization or death. In multivariable analysis (Table 2), characteristics positively associated with the primary outcome were male sex [odds ratio (OR): 1.70, 95% confidence interval (CI): 1.19–2.41], diabetes mellitus (OR: 1.46, 95% CI: 1.0–2.1), solid organ malignancy (OR: 1.6, 92% CI: 1.0–2.6), and hematological malignancy (OR: 2.5, 95% CI: 1.5–4.3). The only characteristic negatively associated with the primary outcome was SARS-CoV-2 vaccination (OR: 0.46, 95% CI: 0.27–0.79). After adjusting for sex, diabetes mellitus, age, SARS-CoV-2 vaccination, and comorbidities related to the primary outcome, both antiviral treatments showed a statistically significant reduced odds of 28-day all-cause hospitalization or death (nirmatrelvir/ritonavir OR: 0.08 – 95% CI: 0.03–0.19, remdesivir OR: 0.29 – 95% CI: 0.18–0.45).
Table 2.
Risk factors for 28-day all-cause hospitalization or mortality.
| Univariate analysis aOR (95% CI) | p | Multivariate analysis aOR (95% CI) | p | |
|---|---|---|---|---|
| Age | 1.007 (0.998–1.016) | 0.131 | 1.002 (0.993–1.014) | 0.55 |
| Sex male | 1.878 (1.381–2.590) | <0.001 | 1.701 (1.199–2.412) | 0.003 |
| Obesity | 0.802 (0.530–1.215) | 0.299 | ||
| Diabetes mellitus | 1.585 (1.128–2.227) | 0.008 | 1.462 (1.000–2.137) | 0.05 |
| Chronic hypertension | 1.192 (0.865–1.642) | 0.284 | ||
| Ischemic heart disease | 0.998 (0.536–1.859) | 0.996 | ||
| Chronic pulmonary disease | 0.812 (0.346–1.907) | 0.632 | ||
| Cirrhosis | 1.993 (1.089–3.646) | 0.025 | 1.598 (0.816–3.130) | 0.17 |
| Solid organ malignancy | 1.659 (1.082–2.543) | 0.02 | 1.669 (1.052–2.647) | 0.03 |
| Hematological malignancy | 2.621 (1.583–4.340) | <0.001 | 2.553 (1.472–4.426) | <0.001 |
| Autoimmune disease | 0.564 (0.369–0.861) | 0.008 | 0.848 (0.529–1.357) | 0.49 |
| Solid organ transplant | 0.812 (0.439–1.503) | 0.507 | ||
| Hematopoietic stem cell transplant | 0.831 (0.106–6.531) | 0.86 | ||
| Systemic immunosuppression | 1.184 (0.851–1.648) | 0.315 | ||
| SARS-CoV-2 vaccine | 0.413 (0.250–0.682) | <0.001 | 0.463 (0.272–0.788) | 0.005 |
| Antiviral treatment | <0.001 | <0.001 | ||
| No treatment | Ref | Ref | ||
| Nirmatrelvir/ritonavir | 0.073 (0.030–0.179) | 0.084 (0.034–0.207) | ||
| Remdesivir | 0.303 (0.197–0.466) | 0.301 (0.194–0.468) |
aOR, adjusted odds ratio; CI, confidence interval.
Kaplan–Meier survival estimates (Figure 2) showed an increased probability of the primary and secondary outcomes in the group not receiving antiviral treatment when compared to those receiving it. There were no differences between the groups receiving either remdesivir or nirmatrelvir/ritonavir.
Figure 2.
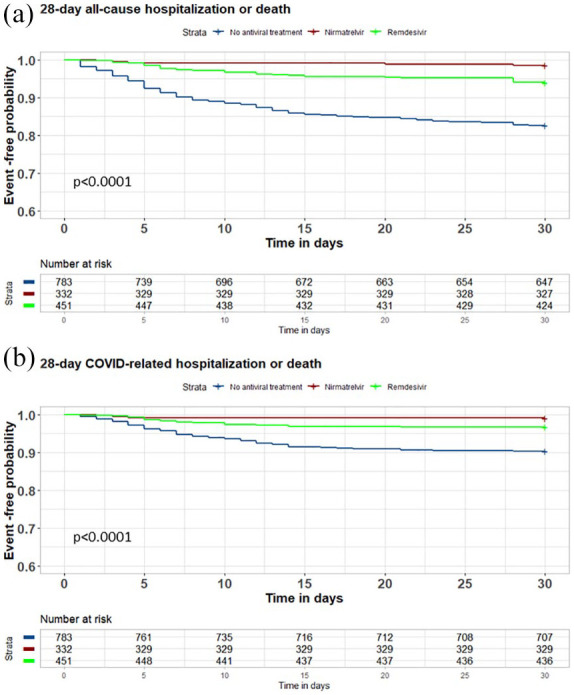
Kaplan–Meier 28-day hospitalization or death estimates.
Discussion
Authors in different countries have reported that, compared to the Delta variant, the Omicron variant is related to less severe disease.16,17 However, due to the widespread availability and known efficacy of nirmatrelvir/ritonavir and remdesivir for the treatment of COVID-19, there are no placebo-controlled studies assessing their use in the Omicron era. The findings of this study add to the real-world evidence regarding the use of early outpatient antiviral treatments against SARS-CoV-2 in high-risk patients, with the added benefit of assessing adverse COVID-19 outcomes during Omicron circulation. Our findings suggest that COVID-19-related adverse outcomes in high-risk groups are common, even with the Omicron variant, and strategies that prevent progression to severe disease are still needed.
Relevant differences in baseline clinical characteristics between the remdesivir and the nirmatrelvir/ritonavir groups were age, solid organ transplant, systemic immunosuppression, and ischemic heart disease. Age, systemic immunosuppression, and solid organ transplant may be directly related to each other, since transplant recipients are generally younger, and systematically receive at least one systemic immunosuppressant drug. Tacrolimus is one the most commonly used immunosuppressors for solid organ transplant recipients in our institution, while clopidogrel is the standard of care for patients with ischemic heart disease. Both drugs have an important drug–drug interaction with nirmatrelvir/ritonavir and, as expected, patients taking them may have been more likely to receive remdesivir. SARS-CoV-2 vaccine uptake was statistically significantly lower in the no-treatment group. However, the recorded vaccine coverage was above 90% in the three groups making this difference unlikely related to the outcomes.
Vaccination was significantly associated with lower odds of the primary outcome. Although data on the timing and the type of vaccines were not registered, in Mexico the only available vaccines are those designed with the original SARS-CoV-2 variant. The observations from this study suggest an important role of ancestral vaccines in protecting against severe COVID-19, even with Omicron viral variants.
As presented in other reports,12,13 both antivirals were highly effective in reducing the risk of COVID-19 adverse outcomes. We observed that the frequency of all-cause and COVID-19-related hospitalization or death was slightly greater in the remdesivir than in the nirmatrelvir/ritonavir group. Possible explanations for this are that the limit from symptom onset to use remdesivir is 7 days, compared to 5 days in nirmatrelvir/ritonavir and that there was a higher number of solid organ transplant patients in the remdesivir group, which was a characteristic found to be strongly correlated with the primary outcome in multivariable analysis.
Two of the major challenges faced by our institution in the implementation of these COVID-19 outpatient treatment programs were the administration of remdesivir and the drug interactions of nirmatrelvir/ritonavir. Despite the logistical and economical caveats of using a parenteral antiviral such as remdesivir, our group and others have demonstrated its feasibility and low rate of complications.4,18,19 Although not reported, drug interactions were frequently mentioned as a reason not to prescribe nirmatrelvir/ritonavir in the population seen at our center. Imbalances in baseline characteristics between groups could be related to this.
The main limitation of this study is that the control group originated from late diagnosis. Other limitations include its non-randomized nature, the limited availability of antivirals (molnupiravir not used in Mexico), the lack of long-term follow-up, and the absence of data regarding specific antiviral-associated adverse events and drug–drug interactions that may have contraindicated the use of nirmatrelvir/ritonavir. However, the main strength of the study is the minimization of potential confounding in treatment indication by propensity score matching. Other strengths include having a control group without antiviral treatment, assessing COVID-19 outcomes in high-risk patients during the Omicron era, and including a wide group of immunocompromised patients.
Conclusion
Early antiviral treatment for high-risk COVID-19 outpatients with nirmatrelvir/ritonavir or remdesivir effectively reduces the 28-day risk of all-cause and COVID-19-related hospitalization or death.
Acknowledgments
The authors thank and recognize the hard work of the internal medicine and infectious diseases residents, the emergency department staff, and the molecular virology and clinical microbiology laboratories, without whose work this study could not have been done.
Footnotes
ORCID iD: Sandra Rajme-López  https://orcid.org/0000-0001-7809-9619
https://orcid.org/0000-0001-7809-9619
Contributor Information
Sandra Rajme-López, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Bernardo A. Martinez-Guerra, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
Carla M. Román-Montes, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
Karla M. Tamez-Torres, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
Andrea C. Tello-Mercado, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
Karen M. Tepo-Ponce, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
Zurisadai Segura-Ortíz, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Abigail López-Aguirre, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Orianlid del Rocío Gutiérrez-Mazariegos, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Oswaldo Lazcano-Delgadillo, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Rafael Nares-López, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
María F. González-Lara, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico
David Kershenobich-Stalnikowitz, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
José Sifuentes-Osornio, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Alfredo Ponce-de-León, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico City, Mexico.
Guillermo M. Ruíz-Palacios, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Vasco de Quiroga #15, Belisario Domínguez Sección XVI, Tlalpan, Ciudad de México 14080, México.
Declarations
Ethics approval and consent to participate: This study was reviewed and approved by the Research and Ethics in Research Committees at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (reference number 4793). Informed consent was waived by these committees due to the retrospective nature of the study.
Consent for publication: Not applicable.
Author contributions: Sandra Rajme-López: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Bernardo A. Martínez-Guerra: Data curation; Formal analysis.
Carla M. Román-Montes: Data curation.
Karla M. Tamez-Torres: Data curation.
Andrea C. Tello-Mercado: Data curation.
Karen M. Tepo-Ponce: Data curation.
Zurisadai Segura-Ortíz: Data curation.
Abigail López-Aguirre: Data curation.
Orianlid del Rocío Gutiérrez-Mazariegos: Data curation.
Oswaldo Lazcano-Delgadillo: Data curation.
Rafael Nares-López: Data curation.
María F. González-Lara: Project administration; Supervision.
David Kershenobich-Stalnikowitz: Conceptualization; Funding acquisition; Project administration; Resources.
José Sifuentes-Osornio: Conceptualization; Project administration; Resources.
Alfredo Ponce-de-León: Conceptualization; Project administration; Resources; Supervision; Writing – review & editing.
Guillermo M. Ruíz-Palacios: Conceptualization; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study did not receive private funding. Remdesivir and nirmatrelvir/ritonavir were provided to our Institution by the Ministry of Health, as part of a COVID-19 outpatient treatment program. All other resources and expenses were covered by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author, GMRP, upon reasonable request.
References
- 1. WHO. WHO chief declares end to COVID-19 as a global health emergency, https://www.who.int/news-room/speeches/item/who-director-general-s-opening-remarks-at-the-media-briefing—5-may-2023 (accessed 1 October 2023).
- 2. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386: 1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajme-López S, Martinez-Guerra BA, Zalapa-Soto J, et al. Early outpatient treatment with remdesivir in patients at high risk for severe COVID-19: a prospective cohort study. Open Forum Infectious Diseases 2022; 9: ofac502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torti C, Olimpieri PP, Bonfanti P, et al. Real-life comparison of mortality in patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrelvir plus ritonavir during the Omicron era in Italy: a nationwide, cohort study. Lancet Reg Health Eur 2023; 31: 100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2021; 386: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO. Therapeutics and COVID-19: living guideline. Geneva: World Health Organization, 2023, p. 145. [PubMed] [Google Scholar]
- 8. COVID-19TreatmentGuidelinesPanel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. National Institutes of Health, 2023. [PubMed] [Google Scholar]
- 9. ECDC. SARS-CoV-2 variant mutations conferring reduced susceptibility to antiviral drugs and monoclonal antibodies: a non-systematic literature review for surveillance purposes, 2023. [Google Scholar]
- 10. Imai M, Ito M, Kiso M, et al. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med 2022; 388: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horita N, Fukumoto T. Global case fatality rate from COVID-19 has decreased by 96.8% during 2.5 years of the pandemic. J Med Virol 2023; 95: e28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basoulis D, Tsakanikas A, Gkoufa A, et al. Effectiveness of oral nirmatrelvir/ritonavir vs. intravenous three-day remdesivir in preventing progression to severe COVID-19: a single-center, prospective, comparative, real-life study. Viruses 2023; 15: 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiseo G, Barbieri C, Galfo V, et al. Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: The PISA outpatient clinic experience. Infect Dis Ther 2023; 12: 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CENAPRECE. Lineamiento operativo para el uso de emergencia de Paxlovid (Nirmatrelvir/ritonavir) en grupos de riesgo para COVID-19 en México, https://coronavirus.gob.mx/tratamiento-covid/ (accessed 3 October 2023).
- 15. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 16. Hyams C, Challen R, Marlow R, et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom. Lancet Reg Health Eur 2023; 25: 100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrone D, Mateo-Urdiales A, Sacco C, et al. Reduction of the risk of severe COVID-19 due to Omicron compared to Delta variant in Italy (November 2021–February 2022). Int J Infect Dis 2023; 129: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramos-Rincón JM, Pinargote-Celorio H, Llenas-García J, et al. A retrospective real-world study of early short-course remdesivir in non-hospitalized COVID-19 patients at high risk for progression: low rate of hospitalization or death, regardless of immunocompetence status. Front Pharmacol 2023; 14: 1218650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgakopoulou VE, Gkoufa A, Makrodimitri S, et al. Early 3-day course of remdesivir for the prevention of the progression to severe COVID-19 in the elderly: a single-centre, real-life cohort study. Exp Ther Med 2023; 26: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]


