Abstract
Psychosocial health predicts and contributes to medical outcomes for patients undergoing hematopoietic stem cell transplantation (HSCT). Yet, there are no standards for psychosocial assessments or support for both patients and caregivers across the care continuum. To examine the current state of psychosocial care, clinicians were sent a survey of their psychosocial assessment practices for patients and caregivers undergoing HSCT via the Listservs of professional organizations. Descriptive statistics and bivariate analyses were performed to summarize the findings. While 96% of participants reported routine pre-HSCT psychosocial assessment of patients, only 10.6% routinely used a validated transplant risk-assessment measure. Just 27% routinely performed follow-up psychosocial assessments. In contrast, only 47% of participants routinely assessed the psychosocial needs of family caregivers pre-HSCT, and 13% routinely performed follow-up assessments for caregivers. Most (90%) reported social workers were the primary providers of assessments. While patient-report measures were used for evaluation, the majority of assessments were clinical interviews. No significant differences were found between programs that treated adult and pediatric patients versus those only treating adult patients. Our findings highlight the lack of standard psychosocial practices for patients and family caregivers undergoing HSCT and we offer recommendations to fill this gap.
BACKGROUND
Hematopoietic stem cell transplantation (HSCT) offers a potential cure for many children and adults with hematologic disorders, metabolic disorders, and bone marrow failure syndromes [1]. However, HSCT is intensive and entails high-dose chemotherapy with treatment-related toxicities, prolonged hospitalization and recovery, and may result in potentially life-threatening complications [2, 3]. Hence, patients undergoing HSCT may endure significant psychological distress throughout the transplant trajectory [4, 5]. Psychological distress in the HSCT population is multifaceted and characterized by numerous symptoms, including depression, anxiety, insomnia, fatigue, delirium, and posttraumatic stress symptoms, each associated with worse health-related outcomes, including diminished quality of life (QOL) [4, 5] and functioning, and increased mortality [6–9].
Despite well-known associations between psychological distress and outcomes in the HSCT population, there are limited data on best practices for assessing psychosocial health in this population. Distress screening is mandated for cancer program accreditation, and the National Comprehensive Cancer Network (NCCN) has provided several resources and validated standardized measures to help cancer programs effectively carry out this mandate [10]. These screening tools are now used by most pediatric and adult HSCT centers [11] to accurately diagnose distress in patients. Since HSCT and its recovery are intense and prolonged, systematic, prospective, and longitudinal assessments are essential to effectively characterize patients’ psychological needs for timely treatment or triage available psychosocial resources. Yet, while distress screening initiatives have increased awareness of the psychological impact of cancer more broadly, limited research exists on best practices for assessing psychosocial health throughout the transplant trajectory in both adult and pediatric populations.
Previous reports focused exclusively on pre-transplant assessment: one identified whether adult and pediatric programs require psychiatric evaluation [12], and another described psychosocial assessment practices in adult programs only [13]. Some studies have found that pre-transplant psychosocial risk-assessment tools such as the Transplant Evaluation Rating Scale (TERS), the Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT), and the Psychosocial Assessment of Candidates for Transplant (PACT) predict post-transplant outcomes, including survival, medical adherence, delirium, intensive care unit transfer, readmissions, hospital length of stay, and QOL [14–22]. Since many psychosocial risk factors (e.g., adherence to medical regimens, cognition, quality of family support) are challenging to obtain from patients’ self-report due to recall bias, these risk assessments are often completed by specialty mental health clinicians. However, there are no standards for pre-transplant assessment of psychosocial and behavioral factors or using these tools [23]. With increasing clinical indications for HSCT for both benign and malignant diseases across the lifespan, it is important to adequately assess and manage psychological health before, during, and after transplant to promote psychological well-being and its impact on health-related outcomes. This assessment is also critical for identifying the needs of the caregivers supporting HSCT patients.
Family and friend caregivers (sometimes called “informal caregivers”) are crucial to every aspect of the HSCT trajectory [24]. HSCT caregivers must navigate a myriad of responsibilities to support patients, including complex medication management, care coordination [25], and transportation of patients to and from weekly follow-up visits once the patient is discharged [26]. Unfortunately, caregivers’ health can be compromised as they grapple with multiple responsibilities, which often add to their distress. Further, parents of pediatric HSCT recipients (i.e., parental caregivers) must learn to manage the needs of their sick child and their other children or family members, which can worsen overall distress. Not surprisingly, patient and caregiver well-being are inextricably linked [27, 28]. Adequate assessment and management of caregiver psychological health impacts both patient and caregiver outcomes [27, 28]. However, there are no established guidelines for how to approach caregiver psychosocial assessment and care throughout the HSCT trajectory [26, 29–31].
Accordingly, this study used a cross-sectional survey of HSCT programs in the United States (U.S.) to determine current practices for psychosocial assessments in patients and their caregivers prior to, during, and post-HSCT. With data from this survey, we aim to inform national guidelines and standardize psychosocial assessment for HSCT patients and caregivers.
METHODS
Study design
We used a web-based self-administered cross-sectional survey. The study was deemed exempt by the National Institute of Health Office of Operation. The survey was fully anonymized, built, and collected using an external, encrypted website (SurveyMonkey). The survey began with background information and objectives related to the study. Two questions followed this introduction, “I agree to participate in this study” and “Do you treat or care for patients undergoing HSCT.” If “no” was provided for either question, the survey ended, and the participants received a “thank you” message for their time. Survey completion by participants indicated informed consent.
In September 2022 and October 2022, we sent the survey to members of the Association of Pediatric Oncology Social Workers, the Society of Pediatric Psychology Hematology/Oncology/Bone Marrow Transplant Special Interest Group (SIG), BMTInfoNet, and the Exchange (an online network of social workers hosted by the National Marrow Donor Program) via their Listservs. In January 2023, we sent the survey to members of the Association of Oncology Social Work through their Listserv. Each group received up to three reminders. Eligible participants were those currently treating or caring for patients undergoing HSCT. The instructions asked for one participant per institution.
Survey development and methods
The survey was designed by an interdisciplinary national group of psychosocial clinicians, researchers, and leaders from diverse training and backgrounds (e.g., psychiatrists, psychologists, social workers, and nurses) who work in HSCT and are members of the Transplantation and Cellular Therapy (TCT) Special Interest Group of the American Psychosocial Oncology Society (APOS).
We designed the 28-item survey to gain a nuanced understanding of psychosocial assessments and practices in the U.S. Questions covered HSCT program characteristics, including location, the age range of patients transplanted, whether pre-transplant assessments are conducted, type of psychosocial provider (available for support and who conducted pre-HSCT assessments), psychosocial assessment tools administered, and overall practices for psychosocial assessments. In addition, we included questions about the timing of assessments collected during follow-up care for patients. The same questions were asked pertaining to caregivers. The survey was beta tested by six members of the TCT SIG to assess question clarity, ease of answering questions, sequence of the questions, and to identify any omissions.
Statistical analysis
The majority of analyses were descriptive: summarizing the frequency of questions completed, how many responses were endorsed, and the mean and standard deviations of all continuous response items. χ2 tests were conducted to compare responses between programs that endorsed treating patients below the age of 18 (in addition to adults) versus programs only treating patients above the age of 18 across dichotomous yes/no responses. The number of responders was used as the denominator for missing responses.
RESULTS
Of the 143 respondents who opened the study link and agreed to participate, each of the survey items was completed by 79–100% of respondents (M = 84.5%). Participants were from 34 US states (see Fig. 1). Most respondents worked at university hospitals (65.3%) that treated both adult and pediatric patients (56.1%). The average minimum age of patients treated was 7.82 (SD = 8.89), and the average maximum age of patients treated was 54.15 (SD = 27.16). Twelve percent of respondents did not provide transplant recipient age ranges.
Fig. 1. HSCT centers of respondents.
Map of the United States showing where HSCT centers of respondents are located in the United States by state. The color blue indicates the states of respondents’ HSCT centers; gray indicates states lacking survey respondents.
Pre-HSCT psychosocial screening
Most respondents (96%) reported that pre-transplant psychosocial evaluations were primarily completed by social workers (90%) and/or psychologists (32.2%), with assessments focusing on high psychosocial risk factors (97.5%), support systems (96.7%), patients’ concrete needs (94.2%), and understanding of the transplant process (81%). An additional 10.7% checked “other,” and the majority of the “other” responses were to assess concrete needs and coping (Table 1). Forty-four percent reported that pre-transplant assessment was conducted as a baseline measure of psychosocial needs. Pre-HSCT assessments consisted of a clinical interview (51.7%) or a combination of a clinical interview along with standardized measure(s) (45%). Standardized measures were quite varied (Fig. 2), with the Patient Health Questionnaire 9 (PHQ-9) most often utilized (59.2%), followed by Generalized Anxiety Inventory-7 (46.9%). Only 10.6% of respondents reported using a validated risk-prediction tool in their setting, and the SIPAT and the TERS were the most commonly used. Additional standardized measures, written as “other,” are listed in Table 2. There were no differences between programs that treated adult and pediatric patients versus those only treating adults in whether they conducted pre-HSCT psychosocial screening χ2 (1, 124) = 1.18, p = 0.28.
Table 1.
“Other” items included in pre-HSCT assessments.
| Items | Number of times reported |
|---|---|
| Coping (ability to adhere to medications, self-efficacy, high-risk behaviors, medical trauma history) | 6 |
| Additional support needs (lodging, financial needs, prescriptions) | 6 |
| Insurance (pre-certification, approval, for post-HSCT) | 4 |
| Advance directives | 2 |
| Sexuality, sexual health | 1 |
Fig. 2. Distribution of standardized screening measures.
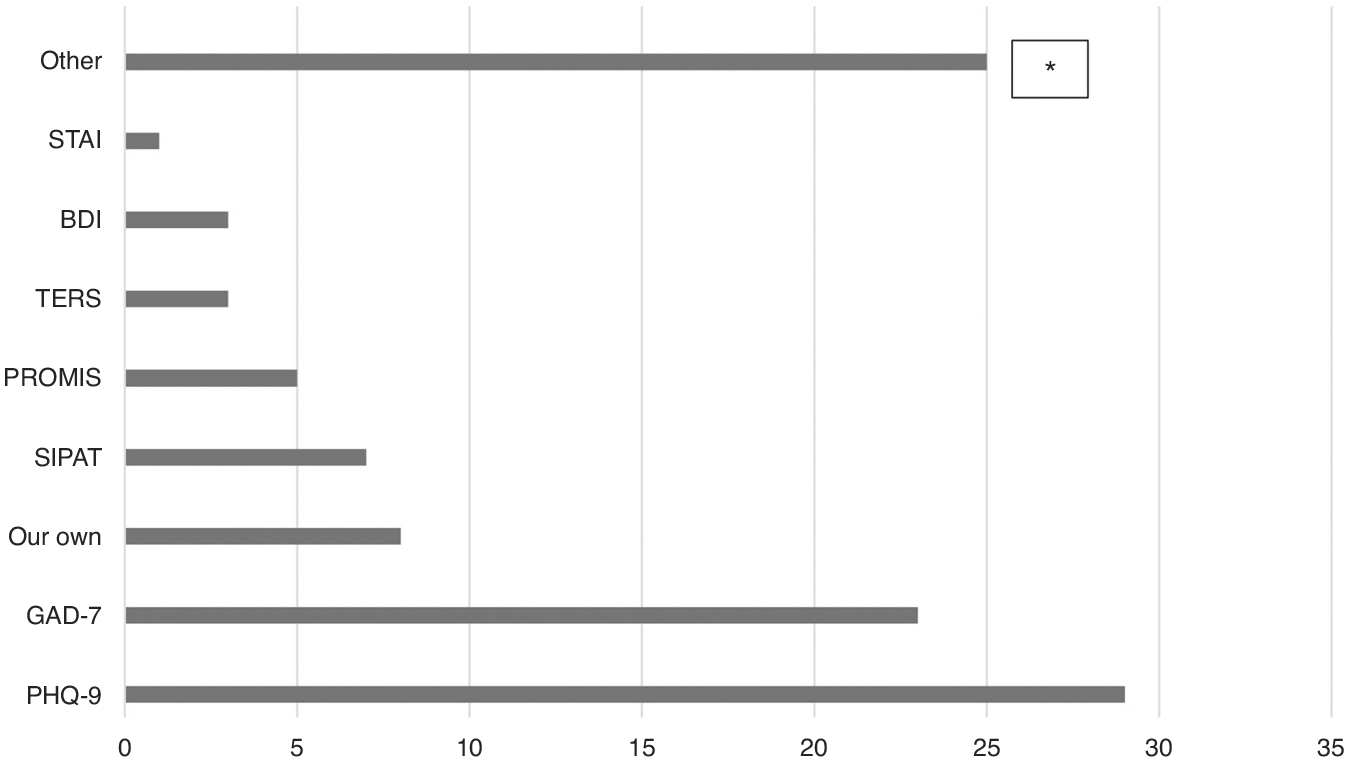
The figure shows the distribution of standardized screening measures used as part of the pre-transplant psychosocial evaluation. *See Table 2.
Table 2.
Additional measures used in HSCT assessments.
| Participants that reported using the measure (N) | |
|---|---|
| Adult measures | |
| Montreal Cognitive Assessment (MoCA) [63] | 6 |
| Alcohol Use Disorders Identification Test (AUDIT) [64] | 5 |
| Psychosocial Assessment of Candidates for Transplant (PACT) [22] | 3 |
| Brief COPE [65] | 2 |
| Functional Assessment of Cancer Therapy (FACT)-BMT [66] | 2 |
| NCCN Distress screening [67] | 2 |
| FACIT Measure of Financial Toxicity (FACIT-COST) [68] | 2 |
| Brief Medical Numbers Test (BMNT) [69] | 1 |
| Multidimensional Health Locus of Control (MHLC) [49] | 1 |
| Beck Anxiety Inventory [70] | 1 |
| Mini Mental State Exam (MMSE) [71] | 1 |
| World Health Organization measuring Quality of Life (WHOQOL-BREF) [72] | 1 |
| Pediatric measures | |
| PedsQL [73] | 2 |
| Psychosocial Assessment Tool (PAT) [74] | 2 |
| PTSD Screener (the specific instrument was not specified by the survey respondent) | 1 |
| Transition Readiness Assessment Questionnaire (TRAQ) (AYA) [75] | 1 |
| Symptom Assessment Pediatrics Tool (SSPedi) [76] | 1 |
| Wechsler Intelligence Scale (WISC or WAIS) [77, 78] | 1 |
| Behavior Assessment System for Children (BASC) [79] | 1 |
HSCT follow-up psychosocial screening
Approximately a quarter of the respondents (26.7%) reported collecting repeat/follow-up clinical assessments following pre-HSCT assessments. These follow-up assessments are administered around day 100 (23.5%), at 6 months (11.8%), and at 1 year (26.5%) post-HSCT. An additional 52.9% of respondents checked “other” for when these assessments were repeated, with most conducted at discharge and subsequent admissions. If a patient reports psychological changes, social workers continue to provide the majority of the follow-up clinical assessments (88.3%), along with psychologists (25%). There were no differences between programs that treated adult and pediatric patients versus those only treating adults in whether they conducted follow-up psychosocial screening χ2 (1, 114) = 6.18, p = 0.43.
Caregiver psychosocial screening
Approximately half of the respondents (49.1%) reported conducting caregiver assessments, and these were primarily completed by social workers (88.3%) and/or psychologists (25%). Psychosocial caregiver screening was almost exclusively done via clinical interview (96.3%), with only 5.4% including any standardized measures. The majority of respondents (94.6%) documented the results of the caregiver screening in the patient’s electronic medical record. There were no differences between programs that treated adult and pediatric patients versus those only treating adults in whether they conducted caregiver psychosocial screening χ2 (1, 115) = 0.76, p = 0.38.
Caregiver follow-up psychosocial screening
Less than a quarter (N = 13, 23.3%) of respondents reported conducting follow-up caregiver assessments during or post-transplant. Caregiver assessments occurred at 100 days following the transplant (N = 4, 28.6%) and 1 year (N = 4, 28.6%). There were no differences between HSCT programs that treated adult and pediatric patients versus those only treating adults in whether they conducted follow-up caregiver screening χ2 (1, 56) = 2.27, p = 0.13.
Psychosocial support services provided during transplant
Routine follow-up support was mostly provided as “the needs arise” (69.8%) though some reported having a set time point for further assessment (26.7%). If the transplant recipient requested psychosocial support services, these were provided by both social work (48.3%), psychology (25%), psychiatry (2.6%), or “other” (24.1%). “Other” services reported in the open text included palliative care, child life specialist, art and/or music therapist, or chaplain. Support consisted of individual counseling (93.9%), peer support (45.2%), support groups (44.4%), family counseling (43.5%), and “other” (20.9%). “Other” reported support services included referrals to community programs, psychiatry, sibling programs, and art/music/massage therapies. Following day 100, 69.6% of respondents reported that transplant recipients received support only “if the need arises.” Less than a quarter of the transplant patients (22.3%) were routinely scheduled to meet with palliative care, as opposed to if clinically indicated. Those who had met with palliative care clinicians did so prior to the transplant or when first admitted for conditioning. Programs that treated adult and pediatric patients were more likely to routinely offer palliative care services than those only treating adult patients χ2 (1, 113) = 7.27, p = 0.007.
Less than half of caregivers (43.9%) were offered psychosocial support during the transplant trajectory. HSCT programs provided even less systematic support for caregivers after day 100 post-transplant (21.9%). When caregivers received support, it was predominately individual counseling (65.7%), support groups (38.9%), family counseling (30.6%), and couples counseling (14.9%) provided by social workers.
DISCUSSION
This interdisciplinary national survey study explored psychosocial assessments and support provided to patients and their caregivers before, during, and after HSCT at pediatric and adult centers. We found that almost all sites include a psychosocial assessment pre-transplant, most often administered by a social worker. Areas prioritized for assessment include: identifying high psychosocial risk factors, current support systems, patients’ concrete needs, and their understanding of the HSCT process, with a limited offering of palliative care services to patients. Half of the pre-HSCT assessments consisted of a clinical interview only, and half consisted of a combination of a clinical interview and standardized measure(s). While close to half of the respondents reported a plan to repeat baseline psychosocial assessments throughout HSCT, only a quarter completed repeat/follow-up clinical assessments. In contrast to previous findings that psychosocial professionals rated assessing the caregiver as one of the most important aspects of the pre-HSCT psychosocial assessment [32], our study found that only 49% of programs administer caregiver psychosocial assessments pre-transplant. Furthermore, only a quarter administer follow-up assessments for caregivers.
We show that pre-HSCT psychosocial assessments were more routine compared to follow-up or longitudinal assessments. Several studies highlight that psychological distress can begin at any point in the HSCT trajectory and worsen over time [33–35]. Hence, a one-time cross-sectional assessment will not capture the changing psychosocial needs of patients and caregivers throughout HSCT recovery, contributing to unmet psychosocial needs. Although we did not obtain explicit information about the lack of follow-up care, persistent shortages of specialty mental health clinicians [36] and inadequate clinical social work and psychology staffing levels [37, 38] are likely contributing factors. Additional factors not captured from our survey, such as insurance requirements that drive the predominance of pre-HSCT assessments, may also provide clues [39–41]. With the increased use of electronic patient-reported outcome (e-PROs) measures in diverse oncological populations [42–44], more work is needed to characterize how e-PROs could facilitate a more longitudinal assessment of psychosocial needs in the pediatric and adult HSCT population [41, 45].
Standardized psychosocial assessments for HSCT recipients and their caregivers are lacking [13]. Clinicians who completed our survey reported using 23 different measures, with the PHQ-9 being the most commonly used. Despite existing validated pre-transplant psychosocial risk-prediction measures, such as the TERS, SIPAT, and PACT, which may predict transplant outcomes and at times show better predictive validity than medical comorbidities for survival and other key outcomes post-transplant [23, 46–48], very few transplant centers reported consistent use of these measures. Therefore, we recommend that all transplant centers incorporate the TERS, SIPAT, and/or PACT in all pre-transplant assessments by psychosocial assessment clinicians. Caregivers of pediatric HSCT recipients have the additional stress of caring for the needs of their children at home, but only one validated measure inquired about siblings [49].
While caregivers’ availability and commitment to overseeing patient care is critical to a successful transplant [24], transplant centers do not routinely assess and manage caregiver psychological distress. Following the pre-transplant workup, less than a quarter of caregivers were re-assessed at any time. Most of these assessments were not completed until 100 days post-transplant, typically at the end of the formal caregiving period. When provided, most interventions given were individual counseling. One way to improve caregiver assessment and intervention may be through improved documentation. We found that documentation of caregiver assessments and interventions was predominantly under the patient’s medical record number (MRN). There is a push for caregivers to have their own MRN [50]. Caregiver MRNs identify caregivers in greatest need of support, help coordinate their care [50, 51], and establish a pathway to bill for caregivers’ own psychosocial or medical care [50]. We provided strategies (Table 3) and a suggested timeline (Fig. 3) for implementing screening and support for patients and caregivers throughout the transplant trajectory.
Table 3.
Recommended strategies.
| Method | Time point | Recommendations |
|---|---|---|
| Assessment | Patient pre-transplant | –Identify psychosocial risk factors for successful transplant outcome, including current mental health presentation/coping, psychosocial history, substance use, medical adherence, understanding/expectations of HSCT, and social support [31] –Use a validated pre-transplant risk-assessment tool (e.g., TERS, SIPAT, PACT) |
| Patient longitudinal | –Hospital: screened/triaged during inpatient rounds –Outpatient: day 80–100, 6 months, 1 year post [74] –Allows assessment of changing psychosocial needs throughout the HSCT trajectory and recovery |
|
| Caregiver pre-transplant | –Identify caregiver(s) availability, ability to meet anticipated needs, understanding/expectation of HSCT, psychosocial/health risk factors, ability to provide care throughout HSCT, social support –Use a validated pre-transplant risk-assessment tool and/or brief clinical assessment tool |
|
| Caregiver longitudinal | –Within 1 week of patient discharge, days 80–100, 6 months, 1 year –Allows assessment of caregiver needs throughout the HSCT trajectory –Use a validated screening tool or brief clinical assessment (e.g., PAT-HCT or CSS-CG) –Document assessments under the caregiver’s MRN |
|
| Follow-up care | Patient support prior to HSCT: as needed, provided by psychosocial and palliative care providers | –Treat psychosocial concerns identified in the assessment that impact transplant or are related to the medical situation, provide an opportunity for advance care planning, help build a social support network to be used during and following HSCT –Psychosocial and palliative providers who are embedded in the HSCT team and integrated for appropriate triage are recommended –Consider peer-to-peer support and group modalities of intervention |
| Patient support provided during HSCT | –Tailor interventions based on an assessment of needs: individual counseling, group education/support, facilitating communication with medical team and family, palliative interventions –Consider peer-to-peer support |
|
| Caregiver support provided during HSCT | –Interventions based on assessments –Consider individual, family, group counseling, and peer-to-peer support –Document caregiver interventions under the caregiver’s MRN |
Fig. 3. Recommendations for psychosocial assessments for HSCT patients and caregivers.
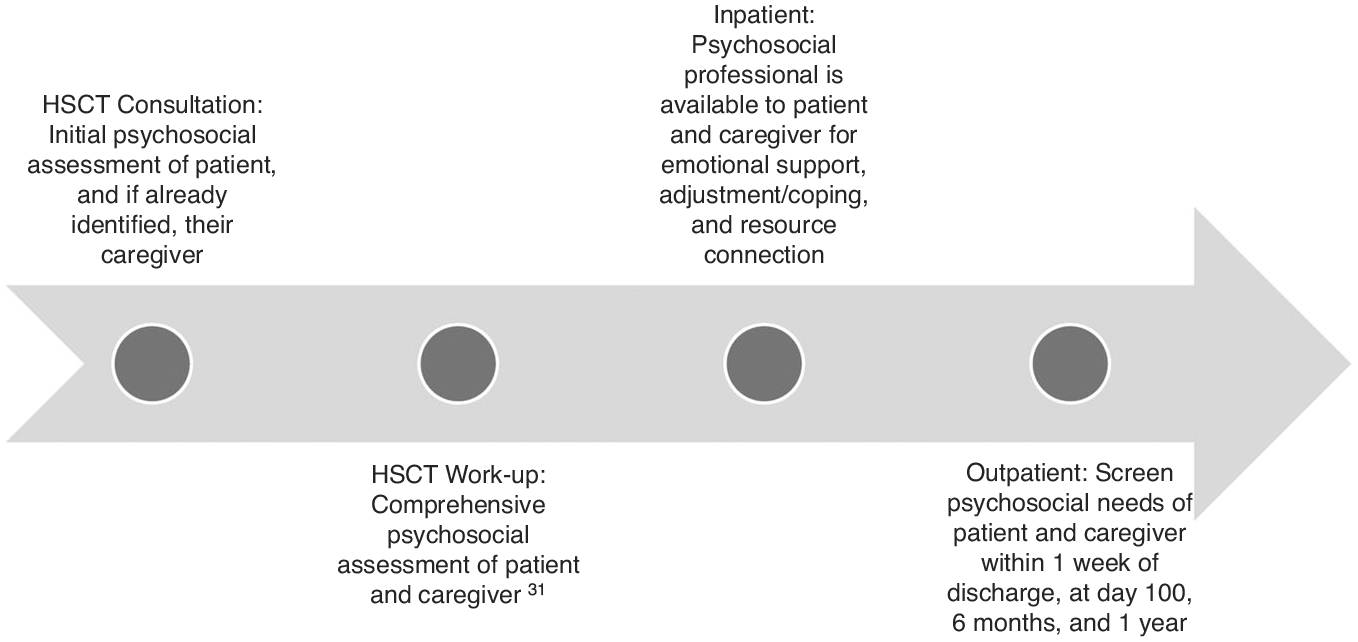
The figure provides recommendations of psychosocial assessments for consideration over the course of the transplant course for both HSCT patients and caregivers.
Participants reported that palliative care is infrequently offered to HSCT recipients despite robust evidence for the benefits of palliative care on various clinical outcomes in oncological populations, including depression and anxiety symptoms, physical symptom burden, and mortality [52–55]. Although randomized trials of integrated palliative care for the HSCT population show improved outcomes [56–58], palliative care use remains limited. Our results indicated that programs treating adult and pediatric patients were more likely to incorporate palliative care services than those only treating adult patients. This may be in concert with national surveys in which pediatric transplant programs overwhelmingly support the integration of palliative care [59]. Persistent shortages of palliative care clinicians and cultural perceptions of palliative care as synonymous with end-of-life care [60] may contribute to inadequate utilization of palliative care for HSCT recipients. We recommend integrating psychosocial and palliative care resources embedded in the HSCT team, where they can work together while maintaining longitudinal relationships with patients, caregivers, and medical providers.
While we provided a timeline for psychosocial assessments that extends to 1 year after HSCT, survivors who are living with graft-versus-host disease (GVHD) would benefit from ongoing assessments. Pediatric HSCT survivors with GVHD have demonstrated slower processing speed and weaker verbal learning [61]. They have frequent absences from school due to medical appointments, or they may be socially isolated due to their immunosuppressed state. Future work will likely build upon identified deficits in survivors (e.g., cognitive functioning) by adding additional assessment tools and timepoints.
There are several significant limitations that warrant discussion. First, we could not determine a traditional response rate due to our use of Listservs to recruit participants and the possibility that some respondents may have been listed in multiple Listservs. Second, although we asked participants to provide one respondent per site, this was not guaranteed. Third, we were unable to determine whether transplant centers exclusively treated pediatric or adult patients due to the wording regarding patients’ age ranges, which limited our ability to conduct subgroup analyses. Fourth, all question responses were optional, with skip patterns built in, and not all participants answered every question, resulting in missing data and possible bias in interpreting the findings. Future surveys may benefit from exploring whether different assessment measures are indicated based on the indication for HSCT. One study found that adolescents and young adults with cancer undergoing HSCT had more advanced care planning discussions than HSCT recipients who were transplanted for a hematologic or immune deficiency [62]. Fifth, we did not capture significant detail about domains covered within less structured, clinical interviews. Future work would benefit from deep, qualitative inquiry on what expert psychosocial clinicians focus during pre-HSCT interview.
Lastly, future surveys may also benefit from more in-depth inquiry about patient socioeconomic status, diversity, languages spoken, and the availability of validated tools for diverse languages. It is critical that we also capture the unique experiences of non-English speaking persons with hematologic and immune dysfunction diagnoses. Cost analyses may be beneficial in depicting the relatively low cost and sustainability of validated psychosocial assessments that mitigate response burden while also reducing or preventing mental healthcare costs following HSCT for patients and their caregivers. In addition, the fiscal benefits of psychosocial assessments in HSCT populations may result in increased palliative care service, social work, and mental healthcare provisions.
In conclusion, this study confirms the lack of standard practice for psychosocial assessment and support for patients undergoing HSCT and their caregivers in the U.S. We recommend the routine use of validated risk-prediction tools for all pre-transplant risk assessments, that caregivers and patients be assessed or screened for distress and other needs prior to transplant and at critical timepoints post-transplant up to and including at 1 year post. We suggest that psychosocial and palliative support be embedded into the transplant team to enhance clinical support during the transplant trajectory. While several national organizations exist that address support in HSCT (e.g., APOS, AOSW, CIBMTR) as well as accrediting bodies (e.g., FACT/JACIE) that require the presence of psychosocial services, there has not been a unified effort, to our knowledge, to define psychosocial assessment or treatment. A comprehensive understanding of the psychological and mental health needs of patients undergoing HSCT and their caregivers allows for more tailored care and improved outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to acknowledge the American Psychosocial Oncology Society (APOS), formed in 1986 to bring together interdisciplinary professionals working on the psychological, behavioral, and social aspects of cancer. APOS’s mission is to advance the development and delivery of equitable and evidence-based psychosocial oncology care through research, practice, education, and advocacy. This work was conducted within APOS’s Transplantation and Cellular Therapy (TCT) Special Interest Group. We also wish to acknowledge all those persons who have gone through HSCT and their caregivers, as well as their healthcare providers, for inspiring this work.
FUNDING
This work was supported (in part) by the Intramural Program of the National Cancer Institute (LW) and through grant K08CA251654 (HLA). JR received research support from the National Cancer Institute institutional training grant T32-CA-236621.
Footnotes
COMPETING INTERESTS
All other authors report no other conflicts of interest. AJA receives support from Blue Note Therapeutics and Beigene.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41409-023-02087-0.
DATA AVAILABILITY
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39. 10.1038/s41409-022-01691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemeke A, Sobczyk-Kruszelnicka M, Kwissa-Gajewska Z. Everyday life following hematopoietic stem cell transplantation: decline in physical symptoms within the first month and change-related predictors. Qual Life Res. 2018;27:125–35. 10.1007/s11136-017-1705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera N Managing survivorship after hematopoietic cell transplantation. Curr Hematol Malig Rep. 2023;18:75–82. 10.1007/s11899-023-00694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevans M, El-Jawahri A, Tierney DK, Wiener L, Wood WA, Hoodin F, et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: the patient-centered outcomes working group report. Biol Blood Marrow Transplant. 2017;23:538–51. 10.1016/j.bbmt.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell SA, Wiener L, Hoag J, Fry A, Bevans MF. Quality of life in adult and pediatric survivors of stem cell transplantation. In: Savani BN, Tichelli A, editors. Blood and Marrow Transplantation Long Term Management: Survivorship after Transplant 2nd ed. West Sussex, UK: Wiley; 2021. pp. 355–383. [Google Scholar]
- 6.Amonoo HL, Massey CN, Freedman ME, El-Jawahri A, Vitagliano HL, Pirl WF, et al. Psychological considerations in hematopoietic stem cell transplantation. Psychosomatics. 2019;60:331–42. 10.1016/j.psym.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amonoo HL, Markovitz NH, Johnson PC, Kwok A, Dale C, Deary EC, et al. Delirium and healthcare utilization in patients undergoing hematopoietic stem cell transplantation. Transplant Cell Ther. 2023;29:334.e1–7. 10.1016/j.jtct.2023.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Jawahri AR, Vandusen HB, Traeger LN, Fishbein JN, Keenan T, Gallagher ER, et al. Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer. 2016;122:806–12. 10.1002/cncr.29818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Jawahri AR, Traeger LN, Kuzmuk K, Eusebio JR, Vandusen HB, Shin JA, et al. Quality of life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer. 2015;121:951–9. 10.1002/cncr.29149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirl WF, Fann JR, Greer JA, Braun I, Deshields T, Fulcher C, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946–54. 10.1002/cncr.28750. [DOI] [PubMed] [Google Scholar]
- 11.Bevans M, Wehrlen L, Prachenko O, Soeken K, Zabora J, Wallen GR. Distress screening in allogeneic hematopoietic stem cell (HSCT) caregivers and patients. Psychooncology. 2011;20:615–22. 10.1002/pon.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland D, Gorman E, Kim S, Rothwell A, Saunders P, Tindle S, et al. Psychiatric evaluations prior to stem cell transplant – a survey of National Marrow Donor Programs. Psychooncology. 2016;25:877–9. 10.1002/pon.3955. [DOI] [PubMed] [Google Scholar]
- 13.Randall J, Anderson G, Kayser K. Psychosocial assessment of candidates for hematopoietic cell transplantation: a national survey of centers’ practices. Psychooncology. 2022;31:1253–60. 10.1002/pon.5919. [DOI] [PubMed] [Google Scholar]
- 14.Solh MM, Speckhart D, Solomon SR, Bashey A, Morris LE, Zhang X, et al. The Transplant Evaluation Rating Scale predicts overall survival after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2020;4:4812–21. 10.1182/bloodadvances.2020002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson DR, Huang Y, McGinty HL, Elder P, Newlin J, Kirkendall C, et al. Psychosocial risk predicts high readmission rates for hematopoietic cell transplant recipients. Bone Marrow Transplant. 2018;53:1418–27. 10.1038/s41409-018-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoodin F, Kalbfleisch KR. Factor analysis and validity of the Transplant Evaluation Rating Scale in a large bone marrow transplant sample. J Psychosom Res. 2003;54:465–73. 10.1016/s0022-3999(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoodin F, Kalbfleisch KR, Thornton J, Ratanatharathorn V. Psychosocial influences on 305 adults’ survival after bone marrow transplantation; depression, smoking, and behavioral self-regulation. J Psychosom Res. 2004;57:145–54. 10.1016/S0022-3999(03)00599-3. [DOI] [PubMed] [Google Scholar]
- 18.Brewer B, Sharma P, Gakhar N, Sannes TS, Joshi TK, Hunter R, et al. Quality of life following cord blood versus matched sibling donor transplantation: pre-transplantation psychiatric and socioeconomic factors significantly impact outcomes. Bone Marrow Transplant. 2022;57:1344–6. 10.1038/s41409-022-01721-7. [DOI] [PubMed] [Google Scholar]
- 19.Mumby PB, Doyle T, Adams W, Hicks EB, Thilges S, Mai HP, et al. Utility of the Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT) in hematopoietic stem cell transplantation (HSCT). J Clin Oncol. 2017;35(15_suppl):10046. 10.1200/JCO.2017.35.15_suppl.10046. [DOI] [Google Scholar]
- 20.Mishkin AD, Shapiro PA, Reshef R, Lopez-Pintado S, Mapara MY. Standardized semi-structured psychosocial evaluation before hematopoietic stem cell transplantation predicts patient adherence to post-transplant regimen. Biol Blood Marrow Transplant. 2019;25:2222–7. 10.1016/j.bbmt.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Mishkin AD, Schmajuk M, Reshef R, Lopez-Pintado S, Mapara MY, Shapiro PA. Standardized semistructured psychosocial evaluation before stem cell transplantation predicts delirium after transplant. J Acad Consult Liaison Psychiatry. 2021;62:440–4. 10.1016/j.jaclp.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Rybicki L, Corrigan D, Dabney J, Hamilton BK, Kalaycio M, et al. Psychosocial Assessment of Candidates for Transplant (PACT) as a tool for psychological and social evaluation of allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant. 2019;54:1443–52. 10.1038/s41409-019-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer S, Scheid C, von Bergwelt M, Hellmich M, Albus C, Vitinius F. Psychosocial pre-transplant screening with the transplant evaluation rating scale contributes to prediction of survival after hematopoietic stem cell transplantation. Front Psychiatry. 2021;12:741438 10.3389/fpsyt.2021.741438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey P, Stinson T, Siston A, Knight SJ, Ferdman E, Traynor A, et al. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant. 2002;30:741–8. 10.1038/sj.bmt.1703676. [DOI] [PubMed] [Google Scholar]
- 25.Volpp KG, Diamond SM, Shrank WH. Innovation in home care: time for a new payment model. JAMA. 2020;323:2474–5. 10.1001/jama.2020.1036. [DOI] [PubMed] [Google Scholar]
- 26.Applebaum AJ, Sannes T, Mitchell HR, McAndrew NS, Wiener L, Knight JM, et al. Fit for duty: lessons learned from outpatient and homebound hematopoietic cell transplantation to prepare family caregivers for home-based care. Transpl Cell Ther. 2023;29:143–50. 10.1016/j.jtct.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sannes TS, Ranby KW, Yusufov M, Brewer BW, Jacobs JM, Callan S, et al. More often than not, we’re in sync: patient and caregiver well-being over time in stem cell transplantation. Health Qual Life Outcomes. 2022;20:6 10.1186/s12955-021-01909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sannes TS, Simoneau TL, Mikulich-Gilbertson SK, Natvig CL, Brewer BW, Kilbourn K, et al. Distress and quality of life in patient and caregiver dyads facing stem cell transplant: identifying overlap and unique contributions. Support Care Cancer. 2019;27:2329–37. 10.1007/s00520-018-4496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng HM, Chuang DM, Yang F, Yang Y, Liu WM, Liu LH, et al. Prevalence and determinants of depression in caregivers of cancer patients: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11863. 10.1097/MD.0000000000011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadalla A, Ginex P, Coleman M, Vrabel M, Bevans M. Family caregiver strain and burden: a systematic review of evidence-based interventions when caring for patients with cancer. Clin J Oncol Nurs. 2020;24:31–50. 10.1188/20.CJON.31-50. [DOI] [PubMed] [Google Scholar]
- 31.Shokri M, Tarjoman A, Borji M, Solaimanizadeh L. Investigating psychological problems in caregiver of pediatrics with cancer: a systematic review. J Child Adolesc Psychiatr Nurs. 2020;33:229–38. 10.1111/jcap.12269. [DOI] [PubMed] [Google Scholar]
- 32.Randall J, Miller JJ. A conceptual framework of the psychosocial elements that should be assessed in candidates for hematopoietic cell transplant: social workers’ and psychologists’ perspectives. J Psychosoc Oncol. 2023;41:303–20. 10.1080/07347332.2022.2104677. [DOI] [PubMed] [Google Scholar]
- 33.Cooke L, Gemmill R, Kravits K, Grant M. Psychological issues of stem cell transplant. Semin Oncol Nurs. 2009;25:139–50. 10.1016/j.soncn.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer. 2008;16:1243–54. 10.1007/s00520-008-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Bernal F, Madva EN, Puckett J, Amonoo HL, Millstein RA, Huffman JC. Relationships between life stressors, health behaviors, and chronic medical conditions in mid-life adults: a narrative review. Psychosomatics. 2019;60:153–63. 10.1016/j.psym.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Deshields T, Asvat Y. The case for accelerating integrated mental health care in the cancer setting. JCO Oncol Pract. 2023;19:231–33. 10.1200/op.22.00840. [DOI] [PubMed] [Google Scholar]
- 37.Stickney Ferguson S, Randall J, Dabney J, Kalbacker ME, Boyle N, Thao V, et al. Perceived workforce challenges among clinical social workers in hematopoietic cell transplantation programs. Biol Blood Marrow Transplant. 2018;24:1063–8. 10.1016/j.bbmt.2017.12.793. [DOI] [PubMed] [Google Scholar]
- 38.Dignan FL, Hamblin A, Chong A, Lee J, Kenyon M, Miller P, et al. Survivorship care for allogeneic transplant patients in the UK NHS: changes centre practice, impact of health service policy and JACIE accreditation over 5 years. Bone Marrow Transplant. 2021;56:673–8. 10.1038/s41409-020-01067-y. [DOI] [PubMed] [Google Scholar]
- 39.Myrvik M, Beverung L, Panepinto J, Igler E, Englebert N, Bingen K. Integration of Electronic Patient-Reported Outcomes (ePROs) into pediatric clinic settings across hematology/oncology/bone marrow transplant. Clin Pract Pediatr Psychol. 2014;2:39 10.1037/cpp0000052. [DOI] [Google Scholar]
- 40.Palos GR, Gilmore KR, Chapman PH, Rodriguez MA. Implementation of ePROs in the care of long-term cancer survivors. J Clin Oncol. 2021;39(28_suppl):334. 10.1200/JCO.2020.39.28_suppl.334. [DOI] [Google Scholar]
- 41.Cusatis R, Flynn KE, Vasu S, Pidala J, Muffly L, Uberti J, et al. Adding centralized electronic patient-reported outcome data collection to an established international clinical outcomes registry. Transpl Cell Ther. 2022;28:e9. 10.1016/j.jtct.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denis F, Krakowski I. How should oncologists choose an electronic patient-reported outcome system for remote monitoring of patients with cancer? J Med Internet Res. 2021;23:e30549. 10.2196/30549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiderlen TR, Schnack A, de Wit M. Essential barriers and considerations for the implementation of electronic patient-reported outcome (ePRO) measures in oncological practice: contextualizing the results of a feasibility study with existing literature. Z Gesundh Wiss. 2022:1–18. 10.1007/s10389-022-01767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patt D, Wilfong L, Hudson KE, Patel A, Books H, Pearson B, et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the texas two-step through a pandemic. JCO Clin Cancer Inform. 2021;5:615–21. 10.1200/cci.21.00063. [DOI] [PubMed] [Google Scholar]
- 45.Shaw BE, Brazauskas R, Millard HR, Fonstad R, Flynn KE, Abernethy A, et al. Centralized patient-reported outcome data collection in transplantation is feasible and clinically meaningful. Cancer. 2017;123:4687–700. 10.1002/cncr.30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30(Suppl):S68–74. 10.1016/j.bbi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Jawahri A, Chen YB, Brazauskas R, He N, Lee SJ, Knight JM, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123:1828–38. 10.1002/cncr.30546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–64. 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 49.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Educ Monogr. 1978;6:160–70. 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 50.Applebaum AJ, Kent EE, Lichtenthal WG. Documentation of caregivers as a standard of care. J Clin Oncol. 2021;39:1955–8. 10.1200/JCO.21.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elko TA, Brown S, Lobaugh S, Devlin S, Jakubowski AA, Perales MA, et al. Characteristics of distress and support group participation in caregivers of older allogeneic hematopoietic cell transplantation patients: a single institution retrospective review. J Adv Pract Oncol. 2023;14:127–37. 10.6004/jadpro.2023.14.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:96–112. 10.1200/jco.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 53.Greer JA, Applebaum AJ, Jacobsen JC, Temel JS, Jackson VA. Understanding and addressing the role of coping in palliative care for patients with advanced cancer. J Clin Oncol. 2020;38:915–25. 10.1200/jco.19.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 55.Temel JS, Petrillo LA, Greer JA. Patient-centered palliative care for patients with advanced lung cancer. J Clin Oncol. 2022;40:626–34. 10.1200/jco.21.01710. [DOI] [PubMed] [Google Scholar]
- 56.El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316:2094–103. 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Jawahri A, LeBlanc TW, Kavanaugh A, Webb JA, Jackson VA, Campbell TC, et al. Effectiveness of integrated palliative and oncology care for patients with acute myeloid leukemia: a randomized clinical trial. JAMA Oncol. 2021;7:238–45. 10.1001/jamaoncol.2020.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBlanc TW, Roeland EJ, El-Jawahri A. Early palliative care for patients with hematologic malignancies: is it really so difficult to achieve? Curr Hematol Malig Rep. 2017;12:300–8. 10.1007/s11899-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 59.Mekelenkamp H, Schröder T, Trigoso E, Hutt D, Galimard J-E, Kozijn A, et al. Specialized pediatric palliative care services in pediatric hematopoietic stem cell transplant centers. Children. 2021;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Jawahri A, Nelson AM, Gray TF, Lee SJ, LeBlanc TW. Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol. 2020;38:944–53. 10.1200/jco.18.02386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phipps S, Rai SN, Leung W-H, Lensing S, Dunavant M. Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol. 2008;25:2027–33. 10.1200/jco.2007.13.6135. [DOI] [PubMed] [Google Scholar]
- 62.Pennarola BW, Fry A, Prichett L, Beri AE, Shah NN, Wiener L. Mapping the landscape of advance care planning in adolescents and young adults receiving allogeneic hematopoietic stem cell transplantation: a 5-year retrospective review. Transpl Cell Ther. 2022;28:164.e1–8. 10.1016/j.jtct.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 64.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–32. 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 65.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4:92–100. 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 66.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–68. 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 67.Cutillo A, O’Hea E, Person S, Lessard D, Harralson T, Boudreaux E. The distress thermometer: cutoff points and clinical use. Oncol Nurs Forum. 2017;44:329–36. 10.1188/17.onf.329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel MR, Zhang G, Heisler M, Song P, Piette JD, Shi X, et al. Measurement and validation of the Comprehensive Score for Financial Toxicity (COST) in a population with diabetes. Diabetes Care. 2022;45:2535–43. 10.2337/dc22-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dykhuis KE, Slowik L, Bryce K, Hyde-Nolan ME, Eshelman A, Miller-Matero LR. A new measure of health numeracy: Brief Medical Numbers Test (BMNT). Psychosomatics. 2019;60:271–7. 10.1016/j.psym.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 71.Pozueta A, Rodríguez-Rodríguez E, Vazquez-Higuera JL, Mateo I, Sánchez-Juan P, González-Perez S, et al. Detection of early Alzheimer’s disease in MCI patients by the combination of MMSE and an episodic memory test. BMC Neurol. 2011;11:78. 10.1186/1471-2377-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–8. 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 73.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–39. 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Alderfer MA, Mougianis I, Barakat LP, Beele D, DiTaranto S, Hwang WT, et al. Family psychosocial risk, distress, and service utilization in pediatric cancer: predictive validity of the Psychosocial Assessment Tool. Cancer. 2009;115:4339–49. 10.1002/cncr.24587. [DOI] [PubMed] [Google Scholar]
- 75.Wood DL, Sawicki GS, Miller MD, Smotherman C, Lukens-Bull K, Livingood WC, et al. The Transition Readiness Assessment Questionnaire (TRAQ): its factor structure, reliability, and validity. Acad Pediatr. 2014;14:415–22. 10.1016/j.acap.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Tomlinson D, Dupuis LL, Gibson P, Johnston DL, Portwine C, Baggott C, et al. Initial development of the Symptom Screening in Pediatrics Tool (SSPedi). Support Care Cancer. 2014;22:71–5. 10.1007/s00520-013-1945-x. [DOI] [PubMed] [Google Scholar]
- 77.Na SD, Burns TG. Wechsler Intelligence Scale for Children-V: test review. Appl Neuropsychol Child. 2016;5:156–60. 10.1080/21622965.2015.1015337. [DOI] [PubMed] [Google Scholar]
- 78.Climie EA, Rostad K. Test review: Wechsler Adult Intelligence Scale. J Psychoeduc Assess. 2011;29:581–6. 10.1177/0734282911408707. [DOI] [Google Scholar]
- 79.Altmann RA, Reynolds CR, Kamphaus RW, Vannest KJ. BASC-3. In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. Springer: International Publishing; 2017. p. 1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.


