Abstract
In this study, we show that bovine leukemia virus (BLV)-induced persistent lymphocytosis (PL) results from the in vivo expansion of the CD11b+ B-lymphocyte population. This subset shares phenotypic characteristics with murine and human B-1 cells. BLV interactions with the sheep B-1-like subset were explored. We found that B-1- and B-2-like cells are initially infected to similar extents. However, in long-term-infected sheep, the viral load is higher in B-1-like cells and only B-1- and not B-2-like cells show increased ex vivo survival compared to that in uninfected sheep. Ex vivo viral expression was found in both B-1- and B-2-like cells, indicating that both cell types support viral replication. Finally, cycloheximide and a protein kinase C inhibitor (H7) that blocks the ex vivo activation of viral expression did not affect the increased survival in B-1-like cells, suggesting that resistance to apoptosis is acquired in vivo. Collectively, these results indicate a peculiar susceptibility of sheep B-1-like cells to BLV transforming effects and further support the involvement of increased survival in BLV pathogenesis.
Bovine leukemia virus (BLV), an oncogenic complex retrovirus, is homologous to human T-cell leukemia viruses (HTLV-1 and -2) and simian T-cell leukemia virus. Thirty percent of naturally infected cattle present nonmalignant polyclonal B-cell population expansion in the blood (persistent lymphocytosis [PL]), and 1 to 5% of cows develop lymphocytic leukemia and/or generalized B-cell lymphoma in the 5 to 10 years following infection (for reviews, see references 3 and 30). Since the risk of developing leukemia or lymphoma is greater in cattle with PL than in BLV-seropositive cattle with normal hematological parameters, PL is considered a preneoplastic condition (3). BLV inoculation in sheep is a convenient experimental model for studying the physiopathology of BLV infection because BLV-infected sheep present B-cell lymphoma lesions and B-cell leukemia after a shorter latency period and far more frequently than do cattle (17). We and other authors have reported that some infected sheep also develop nonmalignant B-cell lymphocytosis (7, 20, 28, 31).
In cattle, B-cell lymphocytosis results from an increased number of circulating CD5+ B lymphocytes (6, 18, 19) associated with a lower but significant increase of the CD5− B-cell population (19), whereas lymphomas appear to arise exclusively from the CD5+ B-cell population (6). The provirus has been detected in both CD5+ and CD5− B lymphocytes from long-term-infected animals, with a higher load in CD5+ B cells (19, 29). In contrast, in sheep, involvement of CD5+ B cells at both the PL and the lymphoma stages has not been consistently observed (1, 16, 20).
In mice, the B-lymphocyte population is classically divided into CD5+ B-1a, CD5− B-1b, and “conventional” CD5− B-2 lymphocytes. B-1 cells differ from B-2 cells in many properties (for a review, see reference 9). (i) They display high levels of surface immunoglobulin M (IgM) and low levels of IgD, B220 (CD45RA), and CD11b/CD18, a β2 integrin normally associated with the myelomonocytic lineage. The CD5 marker allows two subpopulations to be distinguished: a CD5+ CD11b+ IgMhigh IgDlow predominant subset named B-1a and a CD5− CD11b+ IgMhigh IgDlow minor “sister” population named B-1b that appears to be otherwise identical to the B-1a subset. (ii) They are larger than conventional B-2 lymphocytes. (iii) They comprise a high number of natural polyspecific-antibody-producing cells and their primary immune repertoire is made around the neonatal period. (iv) They present a capacity for self-renewal. (v) Finally, adoptive transfer of B-1 and B-2 cells strongly supports the hypothesis that they belong to separate lineages, although some reports emphasize that the CD5+ phenotype can be acquired by “conventional” B2 lymphocytes after stimulation through B-cell activation signals (4). Human homologs of murine B-1 cells, CD5+ and/or CD11b+ B cells, have also been described in detail (9, 13). Importantly, murine and human B-1 lymphocytes contribute to several pathological disorders, such as autoimmune diseases (9, 21), B-cell malignancies in mice (9), B-cell lymphocytosis associated with human immunodeficiency virus infection (15), and B-cell chronic lymphocytic leukemia in humans (10).
Our group (31) and Dequiedt et al. (7) recently reported that BLV protects sheep peripheral blood mononuclear cells (PBMCs) from spontaneous ex vivo apoptosis. Both reports also indicated that BLV promotes the survival of infected B lymphocytes. These findings suggested that BLV-induced increased survival in B cells is involved in the development of lymphocytosis.
In this study, we first show that BLV infection in sheep induces PL that results from the accumulation of B cells carrying the CD11b/CD18 integrin with variable coexpression of the CD5 molecule, whereas the CD11b− B-cell population does not significantly expand. We then further describe the interactions of BLV with the sheep CD11b+ B (B-1-like)-cell population. Altogether our data indicate that although both sheep B-1- and B-2-like cells become infected and can express BLV, only B-1 cells show a susceptibility to BLV-associated lymphocytosis and increased survival. Our result also further support the involvement of increased cell survival in the development of lymphocytosis.
B-cell lymphocytosis in BLV-infected sheep mainly results from the expansion of the CD11b+ B (B-1-like)-lymphocyte subset.
As CD5 and CD11b have been detected on B lymphocytes from lymphocytotic cows (18), we analyzed the expression of these markers on blood B cells from sheep infected for 4 to 6 years with BLV. When the experiments were performed, two-thirds of the infected sheep had high levels of PL on the basis of their increased B-cell/T-cell ratio and their absolute B-cell numbers (Table 1).
TABLE 1.
PBMC subpopulations in control and BLV-infected sheep with low- and high-level PLa
| Sheep | B-cell/T-cell ratio | No. of lymphocytes/mm3
|
|||||
|---|---|---|---|---|---|---|---|
| B | CD11b+ B | CD11b− B | CD5+ B | CD5− B | CD11b+ CD5+ Bb | ||
| Controls | |||||||
| 185 | 0.1 | 192 | 111 | 81 | 156 | 36 | 94 (84) |
| 190 | 0.2 | 324 | 264 | 60 | 249 | 74 | 194 (73) |
| 128 | 0.35 | 463 | 314 | 148 | 278 | 185 | 250 (80) |
| 126 | 0.1 | 819 | 368 | 451 | 360 | 458 | 188 (51) |
| Mean ± SD | 0.19 ± 0.1 | 449 ± 270 | 264 ± 110 | 185 ± 181 | 260 ± 84 | 188 ± 190 | 181 ± 64 (72 ± 15) |
| Sheep with low-level PL | |||||||
| 105 | 0.4 | 853 | 648 | 204 | 536 | 315 | 452 (69) |
| 99 | 0.3 | 1,057 | 507 | 550 | 655 | 401 | 200 (39) |
| 120 | 0.5 | 1,166 | 760 | 406 | 291 | 874 | ND |
| Mean ± SD | 0.4 ± 0.1* | 1,025 ± 159** | 638 ± 126** | 386 ± 173* | 494 ± 185* | 530 ± 301* | 326 ± 178* (54 ± 21) |
| Sheep with high-level PL | |||||||
| 142 | 1.0 | 1,610 | 1,461 | 149 | 547 | 1,063 | 450 (30) |
| 84 | 1.7 | 3,118 | 2,619 | 498 | 2,930 | 188 | 2,338 (89) |
| 85 | 2.1 | 3,397 | 2,866 | 531 | 2,275 | 1,121 | 1,936 (67) |
| 121 | 2.9 | 5,000 | 4,559 | 441 | 1,450 | 3,550 | 1,450 (31) |
| 79 | 3.5 | 5,752 | 5,579 | 172 | 4,083 | 1,669 | 4,010 (72) |
| 92 | 3.0 | 6,538 | 5,230 | 1,308 | 1,765 | 4,772 | 1,438 (27) |
| Mean ± SD | 2.3 ± 0.9*** | 4,235 ± 1,846*** | 3,719 ± 1,642*** | 516 ± 421* | 2,175 ± 1,230** | 2,060 ± 1,738* | 1,937 ± 1,196** (53 ± 27) |
*, P < 0.5; **, P < 0.05; ***, P < 0.005. Comparisons are between infected sheep and control sheep and were established with a Student t test.
Values in parentheses are percentages of CD11b+ CD5+ B cells among CD11b+ B lymphocytes. ND, not determined.
B cells were first analyzed with an anti-CD5 monoclonal antibody (MAb) and an anti-sheep pan-B-cell MAb recognizing CD21 (9a) (Table 2). Three subpopulations of B cells presenting a lack of CD5 expression or low or high levels of CD5 expression could be identified (Fig. 1A). In most instances, the distinction between the CD5-negative cells and cells expressing low levels of CD5 was unclear, leading to imprecise estimates (Fig. 1A). An expansion of the CD5+ B-cell population was detected in sheep with a high level of PL (P < 0.05), but its extent variably contributed to the overall proliferation of the total B cells (Table 1). Finally, the relative representations of the CD5+ B cells greatly varied over time for a given sheep. Overall, it can be concluded that, by contrast with the case in cattle, CD5 is not a reliable marker of PL in sheep.
TABLE 2.
MAbs for detection of B-cell and BLV determinants
| Determinant | MAb | Isotype | Reference(s) |
|---|---|---|---|
| CD21 | DU2-104 | IgM | 26, 29 |
| CD5 | CC17 | IgG1 | 22 |
| CD11b | CC125 | IgG1 | 22 |
| CD11b | ILA-130 | IgG2a | 22 |
| IgM | 1H4 | IgG1 | 16 |
| BLV p24 | Pool of 4′F5, 4′G9, and 2′C1 | IgG1 | 7 |
FIG. 1.
Expansion of the CD5+ and CD11b+ B-cell subsets in BLV-infected sheep. (A) The CD5 and CD21 markers were detected on PBMCs from a control sheep and a BLV-infected sheep with PL by incubating the cells with MAbs CC17 and DU2-104 followed by FITC-conjugated F(ab′)2 goat anti-mouse IgG1 (Caltag Laboratories, San Francisco, Calif.) and a phycoerythrin-conjugated F(ab′)2 goat anti-mouse IgM antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The percentages of cells in the different subsets are indicated. (B) Double labeling for CD11b (MAb CC125) and CD21 (MAb DU2-104) detection on PBMCs from the same sheep as for panel A. The percentages of cells in the different subsets are indicated.
The dual labeling of sheep B cells with an anti-CD21 MAb and an anti-CD11b MAb (Table 2) revealed two clearly distinct B-cell subpopulations: one negative and one positive for CD11b expression (Fig. 1B). The sheep with low and high levels of PL presented 2.4-fold (P < 0.05) and 14-fold (P < 0.005) increases in CD11b+ B-cell numbers, respectively, over values for uninfected sheep (Table 1). Interestingly, the absolute number of CD11b− B cells was slightly (2.7-fold) but not significantly increased (P < 0.5) at the highly lymphocytotic stage (Table 1). A regression curve relating the number of total B cells to the number of CD11b+ B cells has a slope equal to 0.9 (r2 = 0.98 [data not shown]). This shows that the increased number of B cells is essentially the result of the expansion of the CD11b+ B-cell subpopulation. Finally, the relative representations of the CD11b+ B cells were quite stable over time for a given sheep.
Triple-fluorescence analyses for the CD21, CD5, and CD11b markers revealed that 30 to 89% of the CD11b+ B lymphocytes from animals with PL coexpressed the CD5 molecule (mean ± standard deviation, 53% ± 27% [Table 1]). In addition, similar to findings for murine B-1 cells relative to B-2 cells, the CD11b+ B lymphocytes from both control and BLV-infected sheep presented higher levels of surface IgM and yielded larger forward- and side-angle scatters than CD11b− B lymphocytes (data not shown).
Overall, these data clearly show that in the BLV-infected sheep in this study, lymphocytosis results mainly from the expansion of the CD11b+ B-cell population, which presents some of the phenotypic characteristics of murine B-1 cells. The CD11b+ B cells in sheep are referred to below as B-1-like cells.
BLV viral loads are similar in B-1- and B-2-like cell subsets from newly infected sheep, and the virus accumulates in B-1-like cells from long-term-infected sheep.
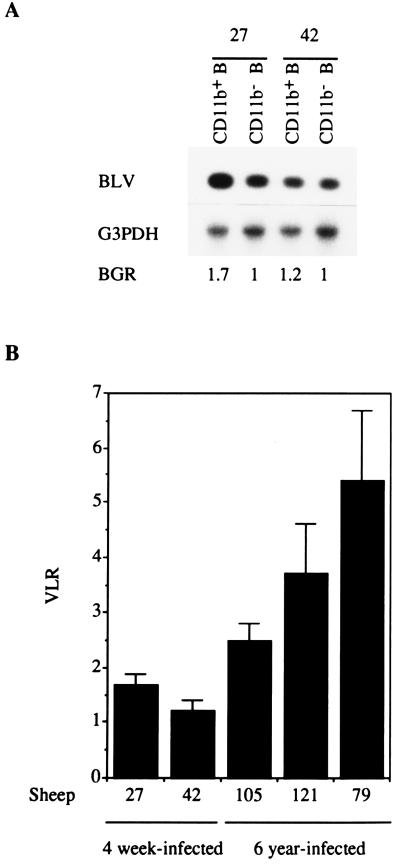
The expansion of the B-1-like cell subset could be the result of a specific propensity of this subset to become infected by replicating BLVs. In order to determine whether this is the case, we looked at the viral loads in B-1- and B-2-like cell subsets from newly infected sheep before the onset of lymphocytosis. Previous reports had indicated that the initial viral replication, around 3 to 4 weeks after infection, was important (12). We thus collected PBMCs from 4-week-infected young sheep, doubly labeled them for CD21 and CD11b detection, and sorted the CD11b+ and CD11b− B cells using flow cytometry. The contamination with cells originating from the theoretically excluded B-cell subset was below 4%. The sorted populations (5 × 104 cells) were lysed and subjected to two independent PCRs, one for BLV detection (forward primer [position 4761], 5′CGCTCTCCTGGCTACTGACC3′; reverse primer [position 5184], 5′ACCGATCTGCCCCCACATAAG3′) and the other for the detection of the endogenous glyceraldehyde-3-phospho-dehydrogenase (G3PDH) gene (forward primer, 5′GACCCCTTCATTGACCTCAACTACA3′; reverse primer, 5′CATGTGGGCCATGAGGTCCACCAC3′). Twenty-seven cycles were performed for both reactions as follows: 45 s at 91°C, 45 s at 58°C, and 45 s at 71°C. The PCR products were transferred onto a nylon membrane, revealed with 32P-labeled probes, and quantitated with the PhosphorImager system (Molecular Dynamics, Sunnyvale, Calif.). The PCRs were performed under strictly defined conditions such that a linear relationship between the initial cell number and the PCR signal intensity was obtained. In order to determine the limit of detection for BLV provirus, we included PCRs with linearized plasmid pBLV13 serially diluted in an uninfected PBMC lysate. The detection limit under our PCR conditions corresponded to 0.5% of cells infected with one viral copy per sample. The intensity of the BLV signal obtained in the two B-cell populations indicated that at most 2.5% of the B cells were infected at that time. Under such conditions, the theoretical signal corresponding to 0.1% of contaminating infected B cells is below the detection level. The BLV signal was normalized between samples by using the G3PDH internal control (Fig. 2A), and a viral load ratio (VLR) for the CD11b+ B cells and the CD11b− B cells was calculated (Fig. 2B). In sheep 27, the VLR was 1.7 ± 0.17 (mean ± standard error of the mean [SEM] from eight independent PCRs), and in sheep 42, the VLR was 1.2 ± 0.2 (n = 8) (Fig. 2B). At 4 weeks after infection, the CD11b+ B-cell number showed 2- and 1.2-fold increases over the values for the day of infection in sheep 27 and sheep 42, respectively, whereas the CD11b− B-cell number was unchanged; this increase probably accounts for the slightly higher viral load detected in the CD11b+ B cells. Overall, these results indicate that there is no restricted or clear preferential viral tropism for the CD11b+ B-cell population compared to the CD11b− B-cell population at the beginning of infection.
FIG. 2.
BLV viral loads in CD11b+ and CD11b− B cells in newly infected and long-term-infected sheep. (A) BLV provirus detection in sorted (by fluorescence-activated cell sorting) CD11b+ B and CD11b− B cells from two 4-week-infected sheep (sheep 27 and 42). Uncultured CD11b+ and CD11b− B cells were sorted, lysed, and analyzed for the detection of BLV provirus and the endogenous G3PDH gene by PCR, followed by Southern blotting and 32P-specific probing. The signals were analyzed with a PhosphorImager. The BGR obtained for each sorted population is shown. (B) VLRs for the BGRs of the CD11b+ and CD11b− B-cell subsets were calculated for newly infected (4-week-infected) sheep and long-term (6-year)-infected sheep. Means and SEMs of the VLRs obtained for 8 (sheep 27 and 42) and 12 (sheep 105, 79, and 121) independent PCR experiments are shown.
In order to assess whether lymphocytosis is associated with the accumulation of BLV-infected CD11b+ B cells, the BLV proviral loads in the CD11b+ and CD11b− B-lymphocyte populations were similarly examined in long-term-infected sheep at different stages of PL. Depending on the animals, the BLV/G3PDH signal ratios (BGRs) indicated that between 8 and 40% of the B-1-like cells were infected (data not shown). The VLRs were 2.5 ± 0.3 for sheep 105 (which had low-level PL) (mean ± SEM, n = 12), 3.7 ± 0.9 for sheep 121 (n = 12), and 5.4 ± 1.3 for sheep 79 (n = 12) (the latter two sheep both had high-level PL) (Fig. 2B). The higher viral load in CD11b+ cells than in CD11b− B cells probably reflects the accumulation of BLV-infected CD11b+ B cells in these long-term-infected sheep. Furthermore, the VLR showed a tendency to increase when the magnitude of the PL increased (Fig. 2B and Table 1).
Altogether, our data show that although BLV infects both CD11b+ and CD11b− B cells at the onset of infection, it preferentially induces an accumulation of infected CD11b+ B cells that is reflected by a higher proviral load in this subset at late stages of infection. The initial viral tropism cannot account for the quasiexclusive involvement of CD11b+ B cells in PL.
BLV-induced protection from apoptosis in B cells mainly affects the CD11b+ B-cell subset.
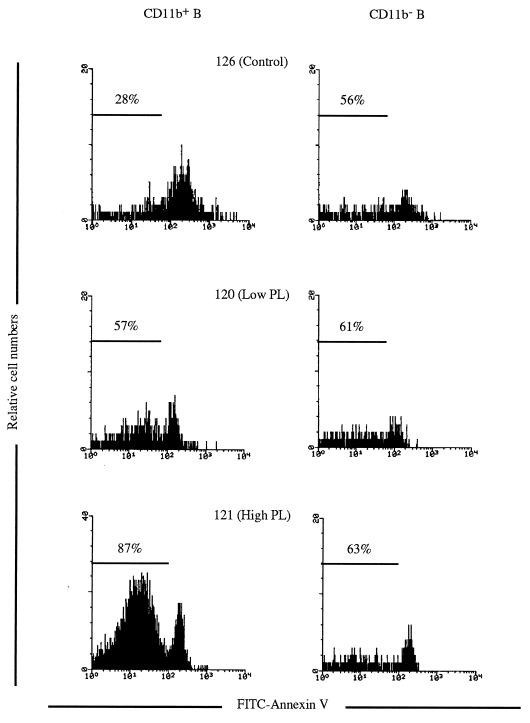
B lymphocytes from BLV-infected sheep were shown to be resistant to spontaneous apoptosis, in contrast to B lymphocytes from uninfected sheep (7, 31). As CD11b+ B lymphocytes are the major contributors to PL, the BLV-induced increase in cell survival was analyzed in CD11b+ and CD11b− B lymphocytes. PBMCs were cultured for 48 h and then triply labeled with an anti-CD21 MAb, an anti-CD11b MAb, and fluorescein isothiocyanate (FITC)-conjugated annexin V (Boehringer Mannheim, Mannheim, Germany); the percentages of surviving (i.e., annexin V negative) among CD21+ CD11b+ and CD21+ CD11b− lymphocytes could thus be established (Fig. 3). As shown in Fig. 3 and Table 3, CD11b+ B lymphocytes from BLV-infected sheep presented a marked increase in survival compared to controls. Conversely, the ex vivo survival of the CD11b− B lymphocytes appeared to be only slightly increased. The data also indicate that the magnitude of the ex vivo survival in B-1-like cells increases with the clinical stage (Table 3).
FIG. 3.
CD11b+ B cells from BLV-infected sheep show increased ex vivo survival. PBMCs from control and BLV-infected sheep with low- and high-level PL were cultured for 48 h and labeled with FITC-annexin V and for detection of the CD11b and the CD21 markers [CC125 followed by a tricolor conjugated F(ab′)2 goat anti-mouse IgG1 antibody (Caltag Laboratories) and DU2-104 followed by a phycoerythrin-conjugated F(ab′)2 goat anti-mouse IgM antibody, respectively). The cells positive for both CD11b and CD21 (CD11b+) were gated, as were the cells positive for CD21 and negative for CD11b (CD11b−). The proportions of surviving cells (annexin V negative) among the gated B cells are indicated.
TABLE 3.
Survival of BLV-infected CD11b+ and CD11b− B cells
| Sheep | % Survivala (mean ± SD)
|
|
|---|---|---|
| CD11b+ B cells | CD11b− B cells | |
| Controls | ||
| 126 | 37 ± 9 | 53 ± 8 |
| 128 | 28 ± 16 | 41 ± 11 |
| 185 | 34 ± 11 | 51 ± 4 |
| 190 | 31 ± 14 | 57 ± 17 |
| Mean ± SD | 33 ± 11 | 51 ± 11 |
| Sheep with low-level PL | ||
| 99 | 40 | 73 |
| 120 | 56 ± 4 | 63 ± 2 |
| 105 | 51 ± 8 | 55 ± 9 |
| Mean ± SD | 51 ± 8 | 60 ± 9 |
| Sheep with high-level PL | ||
| 142 | 69 ± 9 | 60 ± 12 |
| 84 | 59 ± 9 | 58 ± 9 |
| 85 | 77 ± 9 | 66 ± 3 |
| 92 | 71 ± 4 | 58 ± 14 |
| 79 | 69 ± 9 | 59 ± 12 |
| 121 | 81 ± 5 | 58 ± 14 |
| Mean ± SD | 72 ± 9 | 62 ± 10 |
Percentage of annexin V-negative cells.
These results show that BLV infection essentially protects the B-1-like lymphocyte subpopulation from apoptosis, with a marginal effect on the B-2-like cell subpopulation. Although BLV initially infects both B-1- and B-2-like cells, the B-1-like population is the main BLV target for both ex vivo resistance to apoptosis and in vivo lymphocytosis.
BLV-induced protection from apoptosis is not related to restricted viral expression in the CD11b+ B-cell subset.
We (31) and Dequiedt et al. (7) showed previously that the majority of BLV-expressing cells ex vivo were resistant to apoptosis, suggesting a direct role for the virus in the increased lymphocyte life span. The resistance to apoptosis limited to the B-1-like cells that we saw here could have been the result of restricted viral expression in this subpopulation. We thus analyzed viral expression in both CD11b+ and CD11b− B cells ex vivo. PBMCs from sheep 79 cultured for 48 h were labeled for detection of the CD11b and CD21 markers, fixed, permeabilized in 70% methanol, and analyzed for expression of the BLV major capsid protein, p24, by using a pool of anti-BLV p24 MAbs (31). By flow cytometry, the CD11b+ B cells and the CD11b− B cells were gated and were analyzed. Both subsets were found to express p24 (Fig. 4); a higher percentage of CD11b+ B cells (46%) than CD11b− B cells (18%) expressed p24, reflecting the higher proviral load in the CD11b+ B-cell population. Overall, these results demonstrate that the resistance to apoptosis in CD11b+ B cells is not associated with a restriction of the viral expression in this cell subset.
FIG. 4.
In situ detection of BLV p24 expression in CD11b+ and CD11b− B cells. Forty-eight-hour-cultured PBMCs from sheep 79 were labeled with an anti-CD21 MAb (DU2-104 followed by phycoerythrin-conjugated anti-mouse IgM) and an anti-CD11b MAb (ILA-130 followed by FITC-conjugated anti-mouse IgG2a), permeabilized with 70% methanol, and processed for BLV p24 detection (anti-p24 MAbs followed by triply conjugated anti-mouse IgG1). The p24-positive cells among gated CD11b+ and CD11b− B cells are shown. The overlaid left histogram represents background fluorescence of permeabilized CD11b+ and CD11b− B cells obtained with a control mouse IgG1 antibody to mutant p53 (Ctrl).
Resistance to apoptosis in B-1-like lymphocytes is acquired in vivo.
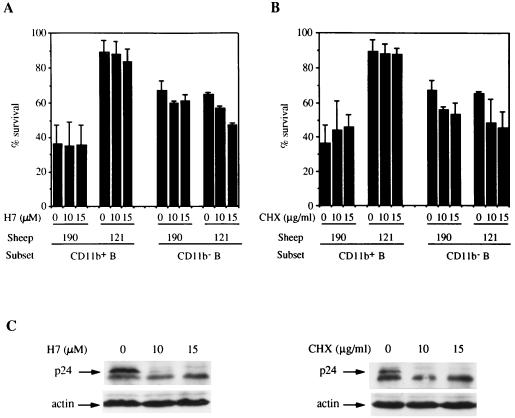
Whereas BLV expression is largely repressed in vivo, a short culturing leads to rapid activation of viral expression (25). The resistance to apoptosis seen in our assay could thus be the result of the high expression of a viral protein that rarely occurs in vivo and/or occurs in small amounts. In order to demonstrate that the activation of viral expression is not required for increased survival in B-1 cells, inhibitors of BLV activation were used in the ex vivo culture. Inhibitors of protein kinase C, such as H7 {[1-(5-isoquinolinylsulfonyl)-3-methylpiperazine dihydrochloride]}, have been previously illustrated to strongly affect the activation of BLV expression (14). We thus treated the cultured PBMCs with H7 (10 and 15 μM; Sigma, St. Louis, Mo.) for 15 h. The time of the culture needed to be shortened to 15 h because H7 induced cell toxicity in the control PBMCs after 48 h of incubation. After 15 h of culturing, the cells from control sheep 190 presented a low level of survival that was not significantly altered by H7 treatments (Fig. 5A). Treatment of sheep 121 PBMCs with H7 was associated with a reduced level of p24 expression at an H7 concentration of 10 μM and with the inability to detect p24 at 15 μM (Fig. 5C). In parallel, H7 did not significantly affect the high survival level of CD11b+ B cells (Fig. 5A). A general inhibitor of protein synthesis, cycloheximide (CHX), was used in order to confirm the results obtained with H7. After 15 h, CHX (Sigma) at 10 and 15 μg/ml slightly but not significantly increased the survival of CD11b+ B cells from the control sheep (Fig. 5B). CHX treatments of sheep 121 PBMCs totally prevented p24 expression as determined by Western blot analysis (Fig. 5C) and fluorescence-activated cell sorter analyses (data not shown). CHX treatments did not modify the survival of CD11b+ B cells from sheep 121.
FIG. 5.
Inhibition of viral activation with H7 or CHX does not alter the increased survival in CD11b+ B cells from BLV-infected sheep with PL. PBMCs from a control sheep (sheep 190) and from a BLV-infected sheep with high-level PL (sheep 121) were cultured for 15 h without or with H7 (A) or with CHX (B). At the end of the culturing, the cells were labeled with FITC-annexin V and for detection of the CD11b (CC125) and CD21 (DU2-104) markers. The percentages of cell survival in each cell subset were obtained for three independent cultures, and means and standard deviations are reported. (C) Inhibition of viral capsid p24 expression with H7 and CHX treatments was analyzed by Western blotting with a pool of anti-BLV p24 MAbs (5 μg/ml) and an antiactin MAb (0.5 μg/ml, clone AC-15; Sigma) as an internal control.
We can conclude from these experiments that the dramatic increase of cell survival induced by BLV in CD11b+ B-1-like lymphocytes is not due to the high ex vivo expression of a viral product that does not occur in vivo. The viral process involved in conferring resistance to apoptosis on CD11b+ B lymphocytes has thus happened in vivo.
Significance of susceptibility and resistance of B-1-like cells to apoptosis in BLV pathogenesis.
B-1 cells in mice and in humans are often present during the development of B-cell leukemia and lymphomas (9), suggesting that these cells are particularly prone to cellular transformation. In the present study, we show that BLV-induced B-cell lymphocytosis in sheep mainly involves a CD11b+ B-cell subpopulation that presents phenotypic similarities to murine B-1 cells. In addition, BLV infection confers a potent resistance to spontaneous apoptosis on B-1-like cells and barely affects B-2-like cells, although both subsets are initially infected and both subsets support viral replication. Collectively, our results suggest that the reactivity of B-1-like cells is not due to restricted viral expression in this subset; rather, B-1-like cells may present a peculiar sensitivity to viral products or to cellular gene activation induced by BLV infection.
Regarding sensitivity to viral products, CD11b+ B lymphocytes may be relatively more responsive than CD11b− B cells to the transforming properties of BLV Tax (33). Actually, the cellular context appears to be important for HTLV-1 Tax-mediated effects: soluble recombinant HTLV-1 Tax induces tumor necrosis factor alpha synthesis in differentiated NT-2 neuronal cells but not in undifferentiated NT-2 cells (5).
BLV infection may modulate the expression of cellular genes interfering with the apoptotic response in CD11b+ B cells specifically. Because the number of known death-modulating genes is increasing, many molecular candidates could be analyzed. In homologous viral infection with HTLV-1, the bcl-2 gene was found to be upregulated in infected endothelial cells (23); however, in our previous report (31), we showed that the bcl-2 mRNA level was not altered in B lymphocytes from sheep with PL. The HTLV-1 Tax protein was also reported to downregulate bax and p53 gene expression (2, 32); we could not detect the corresponding proteins in sheep B-cell lysates in Western blot analyses, probably because of the poor interspecies reactivity of the antibodies. Interestingly, BLV infection in cows was shown to be associated with interleukin-10 (IL-10) mRNA overexpression (27). B-1 cells from mice produce IL-10, which acts as an autocrine growth factor (24): IL-10 is overexpressed in aged mice with B-1-cell proliferation (24), and treatment at birth with antibodies to IL-10 leads to essentially no B-1 cells in the peritoneal cavity but to normal B-cell numbers in the spleen and lymph nodes (11). Consequently, the increased expression of IL-10 in BLV-infected animals may contribute to the preferential expansion of the B-1-like lymphocyte population in sheep.
Yet the hypothesis that B-cell proliferation in BLV-infected animals may affect CD11b− B lymphocytes that acquire the CD11b marker remains. The viral infection would thus lead to both acquisition of the CD11b marker and apoptosis resistance in the same cells; in parallel, the infected B cells that remain CD11b− would show unchanged survival properties. Although possible, this complex scenario is unlikely because the strong ex vivo viral expression in both B-cell subsets is not associated with an increased number of CD11b+ B cells in the culture, which would indicate the induction of CD11b expression by BLV. In addition, in uninfected sheep, the CD11b+ phenotype is associated with a higher relative sensitivity to apoptosis (Table 3).
Three experimental findings in this present work support the idea that increased cell survival is involved in PL development: (i) PL and increased ex vivo survival both affect the B-1-like cell subset, (ii) the magnitude of ex vivo cell survival increases with the clinical stage of PL, and (iii) in vivo viral expression is enough to confer apoptosis resistance on B-1-like cells, as CHX and H7 treatments do not affect the higher survival rate of B-1-like cells. The increased survival is thus not an in vitro artifact of the high expression of viral or cellular gene products. The low level of BLV expression and its transience encountered in animals (8) are thus enough to confer resistance to cell death.
Altogether, our report shows that in sheep, the CD11b+ B-cell subset, a B-1-like cell population, is highly sensitive to BLV-induced in vivo accumulation and to ex vivo increased cell survival. This viral model could be a tool for uncovering the molecular and cellular bases involved in the peculiar sensitivity of the murine and human B-1 subsets to unregulated growth and leukemogenesis.
Acknowledgments
We warmly thank Alain Bernier and Lahcen Souini for taking good care of the sheep herd and for being so patient with us. We are grateful to W. Hein (Basel Institute for Immunology, Basel, Switzerland) for the DU2-104 hybridoma, J. Naessens (International Laboratory for Research on Animal Disease, Nairobi, Kenya) for the ILA-130 MAb, C. Howard (Institute for Animal Health, Compton, United Kingdom) for the CC17 and CC125 hybridomas, M. Pépin (Centre National d’Etudes Vétérinaires et Alimentaires, Sofia Antepolis, France) for the OM1 hybridoma, J. J. Letesson (Namur, Belgium) for the 1H4 hybridoma, and D. Portetelle (Gembloux, Belgium) for the anti-p24 antibodies. We are indebted to B. Polack for his help in many instances, to I. Bouchaert for her expertise in flow cytometry and her involvement in the cell sorting experiments, and to M. Bomsel, B. Schwartz, and M. Mericskay for critical reviews of the manuscript. We thank P. Rodrigues and D. Sitterlin for access to the PhosphorImager.
This work was supported by the Institut National de la Recherche Agronomique.
REFERENCES
- 1.Birkebak T A, Palmer G H, Davis W C, Knowles D P, McElwain T F. Association of GP51 expression and persistent CD5+ B-lymphocyte expansion with lymphomatogenesis in bovine leukemia virus infected sheep. Leukemia. 1994;8:1890–1899. [PubMed] [Google Scholar]
- 2.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 3.Burny A, Bruck C, Chanhenne H, Cleuter Y, Dekegel D, Ghysdael J, Kettmann R, Leclercq M, Leunen J, Mammerickx M, Portetelle D. Bovine leukemia virus: molecular biology and epidemiology. New York, N.Y: Raven Press; 1980. pp. 231–278. [Google Scholar]
- 4.Cong Y-Z, Rabin E, Wortis H H. Treatment of murine CD5− B-cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 5.Cowan E P, Alexander R K, Daniel S, Kashanchi F, Brady J N. Induction of tumor necrosis factor alpha in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax1. J Virol. 1997;71:6982–6989. doi: 10.1128/jvi.71.9.6982-6989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depelchin A, Letesson J J, Lostrie Trussart N, Mammerickx M, Portetelle D, Burny A. Bovine leukemia virus (BLV)-infected B-cells express a marker similar to the CD5 T-cell marker. Immunol Lett. 1989;20:69–76. doi: 10.1016/0165-2478(89)90071-0. [DOI] [PubMed] [Google Scholar]
- 7.Dequiedt F, Hanon E, Kerkhofs P, Pastoret P-P, Portetelle D, Burny A, Kettmann R, Willems L. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J Virol. 1997;71:630–639. doi: 10.1128/jvi.71.1.630-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas L, Divers T, Casey J W. Bovine leukemia virus gene expression in vivo. J Virol. 1992;66:6223–6225. doi: 10.1128/jvi.66.10.6223-6225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa K, Hardy R R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 9a.Hein, W. Personal communication.
- 10.Ikematsu W, Ikematsu H, Okamura S, Otsuka T, Harada M, Niho Y. Surface phenotype and Ig heavy-chain gene usage in chronic B-cell leukemia: expression of myelomonocytic surface markers in CD5− chronic B-cell leukemia. Blood. 1994;83:2602–2610. [PubMed] [Google Scholar]
- 11.Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992;175:1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston E R, Powers M A, Kidd L C, Radke C. Peripheral blood mononuclear cells from sheep infected with a variant of bovine leukemia virus synthesize envelope glycoproteins but fail to induce syncytia in culture. J Virol. 1996;70:6296–6303. doi: 10.1128/jvi.70.9.6296-6303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasaian M T, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5− B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 14.Kerkhofs P, Adam E, Droogmans L, Portetelle D, Mammerickx M, Burny A, Kettmann R, Willems L. Cellular pathways involved in the ex vivo expression of bovine leukemia virus. J Virol. 1996;70:2170–2177. doi: 10.1128/jvi.70.4.2170-2177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouri Y H, Basch R S, Karpatkin S. B-cell subsets and platelet counts in HIV-1 seropositive subjects. Lancet. 1992;339:1445–1446. doi: 10.1016/0140-6736(92)92033-c. [DOI] [PubMed] [Google Scholar]
- 16.Letesson J J, Mager A, Mammerickx M, Burny A, Depelchin A. B cells from bovine leukemia virus (BLV)-infected sheep with hematological disorders express the CD5 T-cell marker. Leukemia. 1990;4:377–379. [PubMed] [Google Scholar]
- 17.Mammerickx M, Palm R, Portetelle D, Burny A. Experimental transmission of leukosis in sheep: latency period of the tumoral disease. Leukemia. 1988;2:103–107. [PubMed] [Google Scholar]
- 18.Matheise J P, Delcommenne M, Mager A, Didembourg C H, Letesson J J. CD5+ B cells from bovine leukemia virus-infected cows are activated cycling cells responsive to interleukin 2. Leukemia. 1992;6:304–309. [PubMed] [Google Scholar]
- 19.Mirsky M L, Olmstead C A, Da Y, Lewin H A. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J Virol. 1996;70:2178–2183. doi: 10.1128/jvi.70.4.2178-2183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami K, Okada K, Ikawa Y, Aida Y. Bovine leukemia virus induces CD5− B cell lymphoma in sheep despite temporarily increasing CD5+ B cells in asymptomatic stage. Virology. 1994;202:458–465. doi: 10.1006/viro.1994.1362. [DOI] [PubMed] [Google Scholar]
- 21.Murakami M, Honjo T. Involvement of B-1 cells in mucosal immunity and autoimmunity. Immunol Today. 1995;16:534–539. doi: 10.1016/0167-5699(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 22.Naessens J, Hopkins J. Introduction and summary to workshop findings. Vet Immunol Immunopathol. 1996;52:213–235. [Google Scholar]
- 23.Nicot T, Astier-Gin T, Guillemain B. Activation of bcl-2 expression in human endothelial cells chronically expressing the human T-cell lymphotropic virus type I. Virology. 1997;236:47–53. doi: 10.1006/viro.1997.8720. [DOI] [PubMed] [Google Scholar]
- 24.O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 25.Powers M A, Radke K. Activation of bovine leukemia virus transcription in lymphocytes from infected sheep: rapid transition through early to late gene expression. J Virol. 1992;66:4769–4777. doi: 10.1128/jvi.66.8.4769-4777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Press C M, Hein W R, Landsverk T. Ontogeny of leukocyte population in the spleen of fetal lambs with emphasis on the early prominence of B cells. Immunology. 1993;80:598–604. [PMC free article] [PubMed] [Google Scholar]
- 27.Pyeon D, O’Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovnak J, Boyd A L, Casey J W, Gonda M A, Jensen W A, Cockerell G L. Pathogenicity of molecularly cloned bovine leukemia virus. J Virol. 1993;67:7096–7105. doi: 10.1128/jvi.67.12.7096-7105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz I, Bensaid A, Polack B, Perrin B, Berthelemy M, Levy D. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J Virol. 1994;68:4589–4596. doi: 10.1128/jvi.68.7.4589-4596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz I, Levy D. Pathobiology of bovine leukemia virus. Vet Res. 1994;25:521–536. [PubMed] [Google Scholar]
- 31.Schwartz-Cornil I, Chevallier N, Belloc C, Le Rhun D, Lainé V, Berthelemy M, Levy D. Bovine leukemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis. J Gen Virol. 1997;78:153–162. doi: 10.1099/0022-1317-78-1-153. [DOI] [PubMed] [Google Scholar]
- 32.Uittenbogaard M N, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type I Tax protein. J Biol Chem. 1985;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 33.Willems L, Heremans H, Chen G, Portetelle D, Billiau A, Burny A, Kettmann R. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 1990;9:1577–1581. doi: 10.1002/j.1460-2075.1990.tb08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]