Abstract
Traumatic brain injury (TBI) is a major global health problem that affects both civilian and military populations worldwide. Post-injury acute, sub-acute, and chronic progression of secondary injury processes may contribute further to other neurodegenerative diseases. However, there are no approved therapeutic options available that can attenuate TBI-related progressive pathophysiology. Recent advances in preclinical research have identified that mitochondria-centric redox imbalance, bioenergetics failure and calcium dysregulation play a crucial role in secondary injury progression after TBI. Mitochondrial antioxidants play an important role in regulating redox homeostasis. Based on the proven efficacy of preclinical and clinical compounds and targeting numerous pathways to trigger innate antioxidant defense, we may be able to alleviate TBI pathology progression by primarily focusing on preserving post-injury mitochondrial and cerebral function. In this review, we will discuss novel mitochondria-targeted antioxidant compounds, which offer a high capability of successful clinical translation for TBI management in the near future.
Keywords: traumatic brain injury, mitochondria, free radicals, oxidative stress, cell death, antioxidants, therapeutics, neuroprotection
1. Introduction
Traumatic brain injury (TBI) is caused by a mechanical blow, penetration, bump, or jolt to the head subsequently leading to tissue and cellular damage, and ultimately resulting in alteration of physiological and behavioral functions. TBI represents a major contributor to morbidity and mortality amongst civilian and military populations across the world. There were over 69,000 TBI-related deaths in the United States alone in 2021, accounting for about 190 deaths per day [1]. TBI also has a big global impact, with annual TBI incidence estimated to be 27 to 69 million [2,3]. These injuries have both short-term and long-term effects on individuals, their families, and society and their financial cost is enormous. Many survivors live with significant disabilities, resulting in major socioeconomic burden. The economic impact of TBI in the United States is estimated to be about USD 76.5 billion for survivors [4,5]. Clinically, TBI is categorized as mild, moderate, or severe injury based on the Glasgow Coma Scale (GCS) scores range between 3 to 15, with a lower score indicating more severe brain damage and a poorer prognosis. The GCS describes the level of consciousness of an individual after acute brain trauma [6,7]. Nevertheless, across all TBI severities, the consequences of TBI may lead to long-term disability, including cognitive and motor function limitations/impairments, and decreased psychosocial health.
TBI-induced neuronal tissue damage manifests in primary and secondary injuries. The primary injury stems from the initial mechanical impacts to the brain [8]. The primary injury is considered an irreversible injury resulting from brain tissue compression, displacement, stretching, shearing, tearing, crushing of the brain parenchyma, brain hemorrhage, and blood–brain barrier (BBB) disruption [8]. Following the primary mechanical insult, the downstream sequelae of molecular events activate complex secondary injury pathophysiological cascades such as excitotoxicity, intracranial hypertension, edema, elevated calcium, metabolic dysregulation, mitochondrial dysfunction (energy crisis, antioxidant depletion, and free-radical generation), inflammation, and ischemic injury, which occur at the acute (i.e., minutes to hours) and sub-acute (i.e., hours to weeks) phases of progressive TBI [9,10,11,12]. Consequently, brain functions are first disrupted at the injury site and subsequently disrupted at distal interconnected regions. Despite the advancements in TBI research, the precise mechanisms leading to the progression of TBI pathophysiology are yet to be fully elucidated.
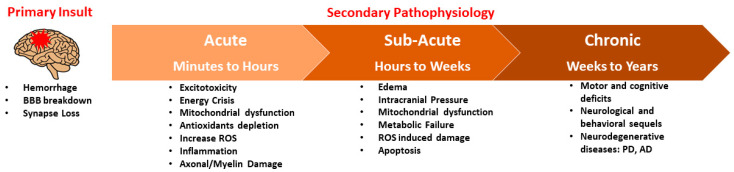
The chronic progression of post-TBI secondary injury responses (i.e., weeks to years) further affects TBI patients’ neuronal ability to maintain their long-term physiological and behavioral functions. TBI progression is linked to the etiology of many neurodegenerative diseases such as Alzheimer’s disease (AD), Amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), Multiple sclerosis (MS), and Parkinson’s disease (PD) [13]. However, the specific epidemiological factors and pathophysiological mechanisms that underlie this association between TBI and specific neurodegenerative pathologies remain unclear. Notably, reports indicate a 63–96% increased risk of all-cause dementia following TBI [14]. Additionally, the risk of PD may go up by at least 1.8 times following moderate to severe TBI [14,15]. Meta analysis indicates an increased risk of ALS following TBI [16]. Additionally, patients with TBI have a higher risk of developing MS [17]. Furthermore, a World War II study suggested that early-adulthood TBI increases the likelihood of developing AD later in life by 2.3 to 4.5 times, respectively, for moderate and severe injuries [14,15]. These reports suggest that TBI is a major risk factor for the onset of neurodegenerative disorders in later life (Figure 1).
Figure 1.
Progressive pathology of TBI. The primary mechanical impact may lead to brain damage in the form of brain hemorrhage, blood–brain barrier (BBB) breakdown and synapse loss, thereby subsequent secondary injury processes beginning immediately after post-injury, and sometimes may sustained over lifetime. During these acute (minutes to hours) and sub-acute (hours to weeks) processes, mitochondria-centric mechanisms play a key role in the further progression of TBI pathology. During the chronic (weeks to years) period of secondary brain injury, neurological behavior deficits in terms of cognitive and motor functions are evident, and may further contribute to neurological diseases such as AD, PD, HD, ALS and MS.
Much of our understanding of the pathobiology of TBI has arisen from animal models that simulate features of human TBI. Multiple preclinical TBI models, including models of penetrating traumatic brain injury (PTBI), controlled cortical impact (CCI) injury, blast-induced traumatic brain injury (BTBI) and closed head injury (CHI) have ascertained that mitochondrial dysfunction is a common and immediate indicator of cellular damage [18,19,20,21] that may even play a critical role in secondary excitotoxic post-injury events. Several detailed previous reports have highlighted preclinical models and cellular mechanisms of TBI [22,23,24,25,26,27,28,29,30]. Mitochondria-centered cellular mechanisms involve calcium homeostasis, energy homeostasis, and redox homeostasis. Their imbalance subsequently may prompt downstream cellular processes such as cell death pathways and neuronal death and alter behavior outcomes in TBI.
Mitochondria are key organelles in all eukaryotic cells and play a central role in cellular energy homeostasis through the metabolism of carbohydrates, fats, and/or proteins. Brain cells manage higher cellular energy (i.e., adenosine triphosphate, ATP) demands by oxidizing their metabolic substrates through respiration and oxidative phosphorylation. Mitochondrial dysfunction following TBI has been shown to be devastating for neuronal cell survival [31,32,33,34]. Several time-course studies of mitochondrial bioenergetics in preclinical models of TBI have suggested that mitochondrial energy failure is the key pathological event that is initiated immediately (e.g., within 30 min) and remains evident for up to 2 weeks after injury [11,12,33,34,35,36].
Interestingly, under normal physiological conditions, intracellular calcium levels are modulated by mitochondria to maintain cellular homeostasis at a certain threshold; however, rapid increase in cellular calcium following TBI may lead to excitotoxicity. Under physiological conditions during ATP production, mitochondria also maintain calcium homeostasis and regulate mitochondrial permeability transition (MPT) pore formation. Several reports have identified impaired mitochondrial calcium-buffering capacity and early mitochondrial MPT opening following acute and sub-acute phases of TBI [37]. Therefore, mitochondrial MPT is considered as the “biological on/off switch” that determines the fate of cells in response to noxious excitotoxic stimuli [38,39,40,41].
Additionally, mitochondrial dysfunction in response to secondary injury elevates the oxidative stress response. Post-TBI oxidative damage leads to structural functional alteration in cellular and subcellular components. This, coupled with the impairment of mitochondrial bioenergetics, initiates a vicious cycle of free-radical formation and apoptosis. In this review, we evaluate the detailed mechanisms of mitochondrial redox homeostasis and discuss potential antioxidant strategies to mitigate oxidative damage following TBI. The aim of this review is to provide a comprehensive overview of antioxidant therapy for TBI to the scientific research community, categorized into different classes, and systematically discussed in the following sections.
2. Mitochondrial Redox Mechanisms in TBI
Mitochondria are vital organelles present in all eukaryotic cells, that consume approximately 98% of body’s total oxygen supply. Efficiently utilizing this oxygen, mitochondria produce energy through oxidative phosphorylation processes linked by respiration via the electron transport chain (ETC) complex proteins. In normal physiological conditions, oxygen slippage estimated from 3 to 5% can occur during the utilization of oxygen in the mitochondrial ETC complexes I and III [42,43,44]. This oxygen slippage results in the generation of the superoxide radical (O2•−), a highly reactive unstable singlet oxygen, which, in turn, can lead to the production of other reactive oxygen species (ROS). Mitochondria also contain antioxidants to manage elevated levels of free radicals as part of normal repair mechanisms. Highly reactive superoxide (O2•−) is rapidly converted into less-reactive ROS, hydrogen peroxide (H2O2) by superoxide dismutase (SOD), which is then further decomposed/neutralized into water by catalase (CAT) or peroxiredoxin (Prx) or thioredoxin (Trx) complex enzyme systems. However, O2•− also generates hydroxyl (•OH) radicals through the Fenton reaction, which can be further converted to peroxynitrite (ONOO−), and subsequently other reactive nitrogen species (RNS) such as nitrogen dioxide and peroxynitrous acid (ONOOH). Normally, mitochondria maintain redox homeostasis with antioxidant activities to scavenge ROS–RNS [45,46]. However, under pathophysiological conditions, elevated levels of ROS–RNS have been observed as early as 30 min after TBI [47,48].
Moreover, these harmful ROS–RNS molecules can further oxidize and damage cellular proteins, lipids, nucleic acids, and extracellular matrix components. The oxidized protein adducts, 3-nitrotyrosine (3-NT), protein carbonylation (PC), and the lipid peroxidation adduct 4-hydroxynonenal (4-HNE) are the hallmarks of peroxynitrite-mediated oxidative stress. Additionally, ROS–RNS may further induce nuclear and mitochondrial DNA damage and affect gene expression responses. ROS–RNS overproduction may inducedamage to ETC subunits, which may further exaggerate the vicious cycle of mitochondrial dysfunction.
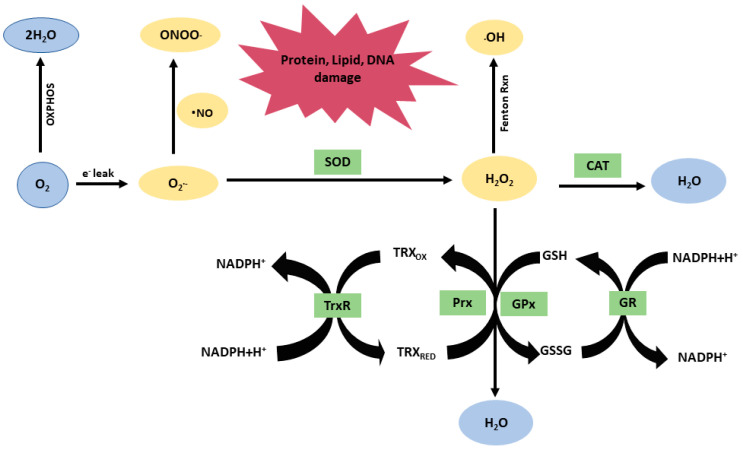
Following TBI, higher redox footprints with respect to elevated free radicals (ROS–RNS), together with altered lipid, protein, and DNA adducts have been observed during the acute and sub-acute phases of secondary injury. If not mitigated, these elevated redox mediators may further contribute to other chronic neurological disease pathologies. The protective antioxidant defense system has potential to mitigate the TBI-induced oxidative stress response is further discussed in detail (Figure 2).
Figure 2.
Generation and scavenging of reactive oxygen species (ROS) using the antioxidant defense system. Electrons released from the mitochondrial ETC and produced by NADPH oxidases are the major source of endogenous reactive oxygen species. The oxidative stress generated via this mechanism can be countered via the antioxidant defense system. Oxygen (O2) is reduced to superoxide (O2•−), which can be reduced to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Nitric oxide radicals (•NO) form the potent oxidant peroxynitrite (ONOO−) following reaction with O2•−. The H2O2 can undergo the Fenton reaction and transformed into hydroxyl radicals (•OH) or reduced to water (H2O) by catalase (CAT), or the glutathione (GSH)/glutathione peroxidase (GPx) or peroxiredoxin (Prx) systems. The oxidized form of thioredoxin (Trx) is reduced back by the reaction with Trx reductase (TrxR), while that of Grx is reduced back by GSH and terminal NADPH oxidation. An oxidized GSH (GSSG) is reduced back to two GSH molecules through the enzymatic reaction of GSH reductase (GR). Both Trx and Grx reduce protein disulfides. These enzymatic antioxidant defense systems counter free-radical-induced stress, and maintain cellular redox homeostasis. The reactive oxygen species may further lead to protein, lipid, and DNA damage.
3. Mitochondrial Antioxidants in TBI
TBI is a highly heterogeneous condition, with patients exhibiting diverse patterns of injury, severity, and outcomes. Oxidative stress plays a crucial role in the development of acute brain injury and acts as a key mediator in the secondary injury cascade of TBI pathology. Oxidative stress leading to oxidative damage represents a state where oxygen levels, combined with oxygen-derived free radicals overwhelm the scavenging antioxidant system.
Following TBI, excitotoxicity occurs, where an excess of Ca2+ further promotes ROS–RNS production. The increased ROS–RNS levels, coupled with depleted antioxidant levels after TBI, lead to elevated oxidative stress, wherein protective mechanisms, such as antioxidants, fail to control these radicals, resulting in oxidative stress and subsequent neuronal death. Post TBI, various complications such as brain edema, mitochondrial dysfunction, BBB breakdown, sensory–motor dysfunction, and secondary neuronal injury have been proposed to be linked to oxidative stress [49,50,51]. Our recent findings indicate decreased mitochondrial antioxidants and increased oxidative stress markers during the acute phase of TBI [37], a trend supported by numerous researchers highlighting the role of oxidative stress following TBI [33,52,53,54,55,56,57]. This underscores oxidative stress redox mechanisms as a valid therapeutic target for TBI. Furthermore, antioxidant intervention emerges as a logical therapeutic approach for achieving neuroprotection after TBI. Both elevated free-radical-mediated oxidative stress and depleted endogenous antioxidant responses have been observed during acute and sub-acute phases preclinically; and only limited reports have noted duringchronic phase of TBI [37,52,58].
Antioxidants are substances which scavenge or neutralize free radicals in cells, thereby prevent oxidative damage. Antioxidants may be able to reduce the risk of the onset of chronic diseases. Antioxidant therapy emerge as a novel approach to preventing and treating neurodegenerative conditions where oxidative stress acts as a major contributing factor to the pathogenesis and/or progression of the diseases. There are two main approaches by which antioxidant levels can be replenished in brain cells, and these may serve as options for therapeutic interventions to limit free-radical generation and oxidative stress responses. This, in turn, improves the balance of redox homeostasis after brain trauma. In an injured brain, antioxidants may be able to modulate redox mechanisms through (a) scavenging or detoxifying excessive ROS–RNS using natural or synthetic antioxidants and restrict free-radical overproduction, and (b) modulating cell signaling pathways that favor endogenous antioxidant synthesis and balanced redox homeostasis.
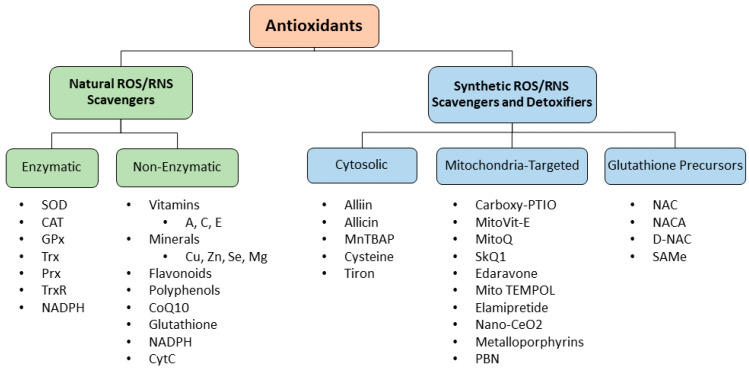
This review highlights each category of antioxidants that may serve as future therapeutic options to restrict/stimulate the mechanisms listed above and favor balanced redox homeostasis following the secondary injury phases of TBI. Unfortunately, there are no FDA-approved treatment options that are currently available to restrain multifaceted TBI pathophysiology, leaving a critical gap unfilled. Therefore, more preclinical research efforts are warranted to identify novel therapeutic targets. Additionally, repetitive injuries aggravate secondary injuries and lead to early neurological deficit. Collaborative efforts between preclinical and clinical communities under regulatory guidance of the FDA are ongoing to conduct better-designed clinical studies, and gain rapid approvals of therapeutic products for TBI. This review is intended to provide an overview on comprehensive information about antioxidant therapy for TBI to the scientific research community, classified into different categories (Figure 3), and discussed below.
Figure 3.
Classification of different antioxidants discussed in the current review. As shown in this illustration, antioxidants are further classified into natural and synthetic ROS–RNS scavengers and detoxifiers. Among the natural antioxidants, they are further sub-divided into enzymatic and non-enzymatic forms, whereas in the group of synthetic antioxidants, they are further sub-divided into three categories, namely non-targeted cytosolic, mitochondria-targeted, and glutathione precursors, based on their sub-cellular target, pharmacological properties, and abundance. Examples of each category of antioxidants are listed and discussed in detail.
4. ROS–RNS Scavengers
The ROS–RNS scavengers/detoxifiers are further categorized as natural (e.g., endogenous enzymatic or non-enzymatic) and synthetic (e.g., drug molecules and dietary supplements), as described below.
4.1. Natural ROS–RNS Scavengers
Among natural enzymatic antioxidants, the mitochondria-specific superoxide dismutase (SOD) isoform (e.g., manganese SOD or Mn-SOD) plays a critical role in scavenging mitochondrial production of O2•− at the mitochondrial ETC complex I and III sites [59,60]. Another isoform of SOD (i.e., cytosolic copper–zinc SOD or Cu-Zn-SOD) scavenges O2•− in the cytosolic compartment. The mitochondrial ETC is a primary site of O2•− generation; therefore, scavenging O2•− at the mitochondrial ETC level offers the greatest benefit. Catalase (CAT) is an additional important ROS scavenger that neutralizes H2O2 and converts it into water [61]. The redoxin family, including peroxiredoxin (Prx) and thioredoxin (Trx) enzyme systems, together with other non-enzymatic reducing cofactors, nicotinamide adenine dinucleotide phosphate (NADPH) and/or glutathione, also plays an important role in scavenging ROS [37,61]. They help to convert H2O2 into water (Table 1, and Figure 2).
Table 1.
Natural ROS scavengers.
| ROS Scavengers | Properties and Mechanisms of Action |
|---|---|
| Enzymatic ROS scavengers | |
| Superoxide dismutase (SOD) | Enzyme. Converts superoxide radicals into oxygen and H2O2. |
| Catalase (CAT) | Enzyme in the peroxisomes. Neutralizes H2O2 in water. |
| Glutathione peroxidase (GPx) Thioredoxin system: Thioredoxin (Trx), Peroxiredoxin (Prx), Thioredoxin reductase (TrxR) |
Thiol-dependent enzymatic antioxidants. Neutralize H2O2 and are recycled by nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. |
| Non-enzymatic ROS scavengers | |
| Vitamin A (retinol) or carotenoids | Fat-soluble antioxidant. Donates electrons to neutralize free radicals. |
| Vitamin E (tocopherols and tocotrienols) | Fat-soluble antioxidant. Scavenges lipid peroxyl radicals. |
| Vitamin C (ascorbic acid) | Water-soluble antioxidant. Donates electrons to neutralize free radicals. Scavenge superoxide. |
| Carotenoids | Found in various fruits and vegetables. Of the ~600 types of carotenoids, some can synthesize Vitamin A. Neutralizers of ROS. |
| Polyphenols | Ubiquitously present in fruits and vegetables. Free-radical scavenger. |
| Flavonoids | Phytochemicals present in plants, fruits, and vegetables. Scavengers of ROS. |
| Pycnogenol (PYC) | Combination of bioflavonoids with robust capacity to scavenge free radicals. |
| Alliin | Found in both natural and synthetic compounds. A bioactive compound derived from garlic. Superoxide scavenger. |
| Allicin | Synthesized from alliin. Inhibits superoxide, nitric oxide (NO) and hydroxyl radicals. |
| Minerals (copper, zinc and selenium, magnesium) | Precursors to antioxidants that help regulate free radicals. |
| Coenzyme Q10 (CoQ10), coenzyme Q (CoQ) | Lipid antioxidant. Essential component of the ETC. Protects cells from oxidative damage. |
| Glutathione | Tripeptide. Detoxifies ROS. Maintains redox balance. |
| NADPH | NADPH, as a cofactor independently and with redoxins, plays a crucial role in ROS detoxification. |
| Cytochrome C | Endogenous heme protein located in mitochondria. Oxidized cytochrome C is able to scavenge superoxide radicals. |
Chronic exposure to oxidative stress may further lead to activation of the first line of antioxidant defense by increasing SOD-, CAT-, and GPx-mediated protective feedback mechanisms that may be able to help mitigate oxidative stress responses to some extent. Interestingly, we observed a depletion of SOD protein expression during the acute period following PTBI [37]. Other studies have also detected diminished SOD activity during the acute phase of TBI that remained low for at least several weeks post TBI [60,62]. Depletion of SOD after TBI could make injured tissue more susceptible to increased O2•− formation, amplifying post-injury oxidative damage over time. In contrast, studies have reported an increase in CAT during the acute and sub-acute phases following TBI; however, the precise mechanism by which brain injury leads to increased CAT protein expression is currently unknown [37]. Earlier studies have reported decreased SOD, CAT, and GPx antioxidant enzyme activity in AD patients [63,64]. Interestingly, in an AD mouse model, the overexpression of the SOD protein showed great promise in relation to improvement in neurological outcomes [65]. Therefore, in therapeutic applications, SOD and SOD mimetics have great potential to serve as a drug to ameliorate TBI-related oxidative damage.
The other class of natural antioxidants are the non-enzymatic antioxidants, which are mainly acquired from dietary sources; these are also called natural ROS scavengers. The most common dietarily derived antioxidants are Vitamin A (retinol), Vitamin C (ascorbate), Vitamin E (α-tocopherol), carotenoids (carotene, zeaxanthin, lutein, lycopene, cryptoxanthin, retinoids), polyphenols, and flavonoids, among others.
Vitamin A is obtained from dietary sources such as green and yellow vegetables, dairy products, fruits, and meats. Vitamin A can act as a chain-breaking antioxidant by combining with reactive radicals before these radicals can propagate peroxidation in the lipid phase of the cell and generate H2O2 [66]. Likewise, Vitamin C is acquired from dietary sources such as fruits and vegetables, and is available as a dietary supplement. Vitamin C has been used as an antioxidant to treat mitochondrial diseases; additionally, it can act as an electron transfer mediator to bypass complex III in combination with Vitamin K at the ETC [67,68]. The oxidized form of Vitamin C is transported into the mitochondria via glucose transporter 1, which helps to maintain a healthy mitochondrial membrane potential and inhibits mitochondrial membrane depolarization [69]. Additionally, Vitamin C facilitates electron movement, favoring energy production [70]. Vitamin E is mainly found in vegetable oil and its derivatives, nuts and seeds. Vitamin E interrupts the chain reaction of oxidant generation and oxidative damage by capturing free radicals.
Carotenoids are another class of antioxidants, and their main dietary sources are red vegetables and fruits (carrots, tomatoes, apricots, plums) and green leafy vegetables (spinach, kale). Indeed, carotenoids are important precursors of Vitamin A [71]. Carotenoids are very efficient quenchers of singlet oxygen and potent scavengers of other ROS–RNS. Similarly, polyphenol antioxidants (flavanols, anthocyanins, isoflavones, phenolic acid), mainly found in fruits (apples, berries, grapes), vegetables (celery, kale, onions), legumes (beans, soybeans), nuts, wine, tea, coffee, and cocoa, can be obtained from nutritional sources. Polyphenol acts as an antioxidant via a direct ROS-scavenging mechanism and the modulation of antioxidant enzymes. Flavonoids are phenolic structures containing natural substances mainly found in fruits, vegetables, grains, bark, roots, stems, flowers, tea, and wine. Flavonoids exert antioxidant, anti-inflammatory, and anti-cholinesterase activities. Flavonoids act as potent inhibitors for several enzymes, such as xanthine oxidase (XO), cyclo-oxygenase (COX), lipoxygenase, and phosphoinositide 3-kinase [72,73]. Pycnogenol (PYC) is a combination of bioflavonoids that is extracted from the bark of the French maritime pine tree (Pinus maritima), and has a robust capacity to scavenge free radicals. The neuroprotective effects of PYC have been explored in a rodent model of TBI [74]. Additionally, alliin, a garlic-derivative compound, reacts with O2•− and scavenges by utilizing the xanthine/xanthine oxidase system [75]. Allicin, a derivative of alliin, also inhibits O2•−, nitric oxide (NO•), and hydroxyl (•OH) radical production [75,76,77].
Some antioxidants are produced by cells that chelate and/or bind to redox metals, thus protecting the cells against oxidative stress indirectly. Micronutrients such as metal and trace elements (zinc, iron, selenium, and copper) possess antioxidant properties. Supplementation with either selenium or zinc has been found to restore the alterations of mitochondrial parameters, including ETC enzymes and antioxidant enzymes, in several diseases [78].
The membrane-bound coenzyme Q10 (CoQ10) is an important antioxidant that is part of the mitochondrial ETC. It shuttles electrons from complexes I/II to complex III. CoQ10 prevents the generation of free radicals and modifications of proteins, lipids, and DNA. Thus, CoQ10 markedly regulates the cellular redox balance.
Among other non-enzymatic ROS scavengers, one of the key cellular antioxidants is glutathione (e.g., γ-L-glutamyl-L-cysteinylglycine). Glutathione is synthesized from the amino acids L-cysteine, L-glutamic acid, and glycine. Glutathione is an important antioxidant which reacts with ROS using thiol-SH groups of cysteine. Glutathione is a ubiquitously distributed tripeptide antioxidant abundantly present in all cells in millimolar concentrations (~5 mM) [79]. The reduced form of glutathione (i.e., GSH) is involved in various cell functions, including the detoxification of oxidized amino acids/proteins, the biosynthesis of proteins and DNA precursors, amino acid transport, and the maintenance of redox balance. During this process, the endogenously generated oxidized glutathione (GSSG) can be recycled back to GSH by the endogenous Grx system. The GSH/GSSG ratio remains an important indicator of redox homeostasis and imbalance in cell oxidative metabolism.
Another antioxidant, NADPH (e.g., nicotinamide dinucleotide phosphate), works closely with glutathione and other redoxin enzymes to protect against ROS–RNS-induced cell damage. In redoxin systems, NADPH serves as a cofactor, used for transferring and preserving redox potential for multiple antioxidants such as glutathione, Prx, and Trx. This NADPH-induced conversion reactivates the functions of antioxidant molecules. We found that NADPH levels significantly decreased following TBI [37]. This reinforces the importance of exogenous NADPH treatment following TBI to increase the effectiveness of antioxidant proteins as the scavengers of oxidants. Additionally, in cells, endogenous cytochrome C (Cyt C) may act as an O2•− scavenger since it is reduced by O2•− and oxidized by H2O2 [80]. Cyt C seems to be an ideal antioxidant since Cyt C can regenerate and avoid being damaged during antioxidant reactions [81].
Additionally, several dietary or nutritional supplements serve as conventional (non-targeted) antioxidants in cells. However, all of these natural antioxidants have limited effectiveness in scavenging mitochondrial ROS–RNS and oxidative stress due to their limited ability to cross the mitochondrial biomembranes [82,83]. In the next section, we compile a list of mitochondria-targeted synthetic antioxidants, which may serve as better options to combat ROS–RNS and oxidative stress and may offer neuroprotection.
4.2. Synthetic ROS–RNS Scavengers
Novel synthetic ROS–RNS scavengers targeted towards preventing or minimizing oxidative damage have contributed new insights into potential neuroprotective therapies (Table 2). Superoxide (O2•−) scavengers are important antioxidants due to their ability to mitigate oxidative stress during the acute post-injury phase. One such synthetic compound is Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), an ROS scavenger. MnTBAP is both an SOD mimetic and peroxynitrite (ONOO−) scavenger [84,85]. Other compounds that can donate electrons to O2•− are ascorbic acid, cysteine (via the sulfhydryl group), tiron, and carboxy-PTIO (a nitric oxide scavenger), which can also react with superoxide radicals [86,87,88,89,90,91,92,93,94]. Tiron is a Vitamin E-analog antioxidant that can enhance NF-κB-dependent gene transcription with an anti-apoptotic effect [95]. Carboxy-PTIO is an imidazole-derived free-radical scavenging compound that inactivates NO• and NO2, subsequently reacting with water to form nitrite and nitrate. Phenelzine (PZ) is an FDA-approved drug for the management of treatment-resistant depression, panic disorder, and social anxiety disorder that functions as an MAO inhibitor [96]. PZ has aldehyde-scavenging properties. PZ administration was also shown to significantly improve mitochondrial respiration following TBI [96].
Table 2.
Synthetic ROS scavengers and detoxifiers.
| ROS Scavengers and Detoxifiers | Properties and Mechanisms of Action |
|---|---|
| Non-targeted compounds | |
| MnTBAP | O2•− scavenger. Possesses SOD- and catalase-like activity. Also scavenges ONOO−. |
| Cysteine | Amino acid. O2•− scavenger. |
| Tiron | Reduced and oxidized Tiron species. Reacts with O2•− radical. |
| Carboxy-PTIO | Specific NO scavenger. Reacts with O2•− radical. |
| Phenelzine | FDA-approved drug. MAO inhibitor. Aldehyde-scavenging properties partially protect against oxidative damage. |
| Mitochondria-targeted compounds | |
| MitoVit-E | Vitamin E attached to TPP. Reduces mitochondrial oxidative damage. |
| MitoQ | CoQ10 derivative linked with TPP. Scavenges mitochondrial ROS. |
| Plastoquinone (SkQ1) | Targeted antioxidant. Scavenges mitochondrial ROS. |
| Edaravone | Used clinically as a neuroprotective compound. Reduces oxidative damage and lipid peroxidation. |
| Mito TEMPOL | Cell permeable, stable nitroxide. SOD mimetic. |
| Elamipretide (SS-31) | Cationic tetrapeptide freely permeable to the mitochondria. Reduces the production of toxic ROS. |
| Cerium oxide nanoparticles (Nano-CeO2) | Cerium atoms linked by oxygen atoms. Scavengers of ROS. |
| Metalloporphyrins | Manganese and iron complexes. Synthetic catalytic antioxidants that mimic the body’s own antioxidant enzymes. |
| Phenyl-tert-butylnitrone (PBN) | Nitroxide radical. ROS-scavenging properties. |
| Glutathione precursors | |
| NAC | A cysteine prodrug. Replenishes intracellular glutathione level. |
| NACA | N-acetyl cysteine (NAC) analog.Glutathione precursor. |
| D-NAC | Dendrimer-tagged NAC. Serves as a prodrug to synthesize glutathione. |
| S-adenosyl methionine (SAMe) | SAMe is processed stepwise into cysteine synthesis, and ultimately synthesize glutathione. |
Moreover, to overcome the limited effectiveness of natural ROS scavengers, several synthetic mitochondrial ROS scavengers have been designed to cross the BBB and accumulate in neuronal mitochondria. These compounds are formulated to target mitochondria at the injured region to neutralize ROS and promote the mitigation of oxidative damage, together with improving bioenergetic function. The development of antioxidants capable of restoring mitochondrial function following brain injury is highly significant since redox homeostasis dysregulation is a critical factor in the cell death pathway during the acute, sub-acute, and chronic phases of TBI. Also, specific mitochondrial targeting leads to more precise and effective mitigation of redox homeostasis. We have compiled a list of such covalently modified compounds in this table.
The synthetic mitochondrial-targeted Vitamin E compound (MitoVit-E) is created by covalently attaching natural Vitamin E (α-tocopherol) to a triphenylphosphonium (TPP+) cation. MitoVit-E facilitates the accumulation of TPP+ in the mitochondrial matrix against the negatively charged mitochondrial membrane potential (ΔΨm). This unique feature makes MitoVit-E an effective mitochondria-targeted ROS scavenger. By utilizing the concentration gradients of ΔΨm, MitoVit-E decreases ROS production and apoptosis in aortic endothelial cells via peroxide-induced oxidative stress and apoptosis [97,98]. One disadvantage of Vitamin E is that it is not a catalytic antioxidant and therefore its scavenging activity is not regenerated. Another well-studied mitochondria-targeted antioxidant is mitoquinone (MitoQ), a ubiquinone derivative conjugated to the TPP+ cation that serves as a potent reactive oxygen species (ROS) scavenger. MitoQ has structural similarity with endogenous components of the mitochondrial ETC ubiquinone; therefore, it may help in assisting efficient electron transfer through the ETC [99,100]. The active form of MitoQ, i.e., ubiquinolis able to scavenge ROS and is being modified into its inactive form, ubiquinone. This inactive form is continuously recycled back into its active form by the mitochondrial complex II. This reduction–oxidation cycle enables MitoQ to maintain an efficient chain-breaking antioxidant capacity. MitoQ treatment has been shown to inhibit mitochondrial oxidative damage in rodent models of cardiac ischemia and reperfusion injury [101]. The antioxidant properties of MitoQ were further demonstrated in several preclinical models of TBI, where it increased the activity of antioxidant enzymes and reduced oxidative damage [102,103]. Plastoquinonyl-decyl-triphenylphosphonium bromide (SkQ1) is another class of mitochondria-targeted antioxidant [104]. In the case of SkQ1, the phosphorus cation bound to three phenyl rings (TPP+) is conjugated to plastoquinol via a decyl linker. The binding of this cation to the phenyls ensures the ability of SkQ1 to penetrate membranes [105]. A positive electrical charge leads to a thousand-fold accumulation of SkQ1 in the mitochondrial membrane’s inner layer [105]. SkQ1 is able to reduce cardiac ischemic injury, and is well known for lipid peroxidation inhibition [106,107,108,109].
Edaravone is a free-radical scavenger that can quench hydroxyl radicals and hydroxyl radical-dependent lipid peroxidation. It is an FDA-approved compound for the treatment of acute ischemic strokes and amyotrophic lateral sclerosis (ALS) [110]. Additionally, edaravone has shown promising beneficial effects in a wide range of diseases, such as PD, AD, atherosclerosis, chronic heart failure, and diabetes mellitus [111,112,113,114]. Other potential synthetic antioxidants designed to reduce oxidative damage effectively include Mito TEMPOL, elamipretide (SS-31), cerium oxide nanoparticles (Nano-CeO2), metalloporphyrins, and phenyl-tert-butylnitrone (PBN) [115,116,117,118,119,120,121]. However, their roles in TBI have not yet been investigated. Research on the evaluation of ROS–RNS scavengers in TBI is ongoing, and the field continues to explore novel approaches and compounds to mitigate oxidative stress and improve behavioral outcomes following TBI [52,58,122,123]. Indeed, several preclinical studies have shown the therapeutic efficacy of mitochondria-targeted antioxidants by improving cognitive and functional recovery post TBI [122,123]. Thus, this strategy may offer new hope for treating TBI patients.
Amongst synthetic ROS scavengers and detoxifiers, novel precursors of glutathione play a significant role. Glutathione, a ubiquitous reducing sulfhydryl tripeptide, plays a major role in ROS–RNS detoxification. Many studies have reported a depletion of glutathione and its precursors, namely cysteine, methionine, and glycine, in brain tissue and cerebrospinal fluid (CSF) following TBI [37,49,50,124]. Therefore, several strategies have explored boosting glutathione levels following TBI to protect neurons against oxidative damage. One approach is to administer glutathione directly. Glutathione injections have been used in the past to boost glutathione levels in blood and skin; however, there was no systemic study available to prove its efficacy [125]. Direct enhancement of glutathione comes with its own challenges like short half-life, absorption, BBB permeability, and limited brain bioavailability [125].
The de novo synthesis of glutathione is primarily controlled by the cellular concentration of cysteine. In keeping with this, NAC and its analogs, such as the cysteine supplement, are effective at raising levels of glutathione in various neurological diseases and injuries, preclinically and clinically [126,127,128,129]. Therefore, various glutathione prodrugs or antioxidant supplements to boost innate glutathione levels have been investigated.
N-acetyl cysteine (NAC) is perhaps the most widely studied glutathione precursor to act as an antioxidant. NAC has been approved by the FDA for treating hepatotoxic doses of acetaminophen (Tylenol). Additionally, NAC has been widely used because of its mucolytic effects, taking part in the therapeutic protocols of cystic fibrosis. Over the past decade, studies have documented the positive outcome of NAC treatment for many CNS diseases, including TBI [130]. Additionally, NAC’s ability to replenish glutathione, maintain cellular homeostasis, and support mitochondrial function has been successfully demonstrated in TBI [131,132,133,134,135].
Clinical treatment with NAC has been shown to upregulate glutathione-centered pathways in the CSF of severe TBI pediatric patients (ClinicalTrials.gov NCT01322009) [136,137,138]. NAC treatment was evaluated in U.S. service members who had been exposed to a blast-induced mild TBI [139]: the outcome of this study demonstrated NAC as safe and effective pharmaceutical agent for acute countermeasure. NAC treatment has beneficial effects on the injury severity, and resolution of post-traumatic sequelae of blast-induced mild TBI (ClinicalTrials.gov NCT00822263) [139]. Furthermore, NAC’s neuroprotective effects are mediated by both antioxidant and anti-inflammatory mechanisms [140,141,142,143]. These multimodal neuroprotective properties of NAC may confer significant benefits on the complex and heterogenous nature of TBI pathology.
The BBB permeability of NAC is limited by its physiochemical properties, such as its acidic nature and negative charge [144,145]. Notably, numerous studies evaluating the neuroprotective properties of NAC have yielded inconsistent results, which may be due to its low bioavailability [144,145]. A potential strategy for overcoming the low bioavailability of NAC is to use an NAC analog where the carboxyl group of NAC is neutralized, thus making it more hydrophobic and increasing its BBB permeability [146]. In this regard, the preparation of NAC analogs, such as N-acetylcysteine amide (NACA), is very attractive and may have advantages over NAC in treating CNS pathologies due to the improved stability and bioavailability [147]. For instance, there are studies reporting the neuroprotective efficacy of NACA in neurological diseases including PD, AD, and HIV-associated neurological disorders [148,149,150]. In the same line of effort, we have demonstrated that NACA effectively reduces oxidative damage, maintains the glutathione level, and improves mitochondrial bioenergetics following TBI [128,129,151]. Other studies have reported similar outcomes in spinal cord injury (SCI) patients [129]. Thus, NACA may offer neuronal protection by reducing oxidative stress and supporting cellular pathways to limit mitochondrial dysfunctions following TBI.
To enhance NAC’s bioavailability and address neurological conditions, researchers are investigating an alternative intranasal route for its direct delivery to the CNS via neuronal pathways, thus minimizing the BBB permeability issues [152]. However, the optimal dosing regimen for this intranasal route of NAC administration still needs to be further investigated at the preclinical level for TBI.
Recently, researchers have used nanoparticle delivery systems, such as dendrimers, to ensure targeted and effective drug delivery to the CNS. Hydroxyl-terminated polyamidoamine (PAMAM) dendrimer, a dendrimer linked with NAC (D-NAC), has shown to be a promising route of drug delivery to injury sites within the brain. In particular, D-NAC has been investigated as a drug delivery system to target cells involved in neuroinflammation [153]. In the presence of a brain injury, D-NAC traverses the BBB and localizes specifically in activated microglia and astrocytes, and the extent of its uptake correlates with the extent of the injury [154,155]. D-NAC also has been shown to be effective in improving myelination and motor functions in cerebral palsy [156,157]. The protective role of D-NAC has been established in ischemic brain injury, asphyxia brain injury [158,159,160], and other CNS pathologies like choroidal and retinal neovascularization [161]. Collectively, novel dendrimer-based delivery methods, such as D-NAC, appear to be promising avenues for targeting therapeutic agents in CNS diseases.
Similarly, another compound that aids in restoring glutathione synthesis by recycling its precursor cysteine is S-adenosyl methionine (SAMe). SAMe has been studied for its potential neuroprotective efficacy in several CNS diseases [162,163]. Besides providing amino acids during methyltransferase reactions for glutathione synthesis, SAMe serves as a key metabolite in many biochemical reactions, and is available as a dietary supplement. Depletion of methionine and its crucial metabolites has been reported in TBI; therefore, restoring methionine metabolites with SAMe supplementation may improve its outcome [124].
A thorough understanding of methods to replenish glutathione and the application of innovative technology to advance targeted therapy in research is critically important when considering therapies to combat TBI secondary pathogenesis. Similarly, the development of neuroprotective formulations to enhance signaling pathways to upsurge innate antioxidants as a potential tool for the therapeutic treatment of neurological diseases represents an important goal for current neuroscience research.
5. Signaling Pathway Modulators for Cellular Antioxidant Synthesis
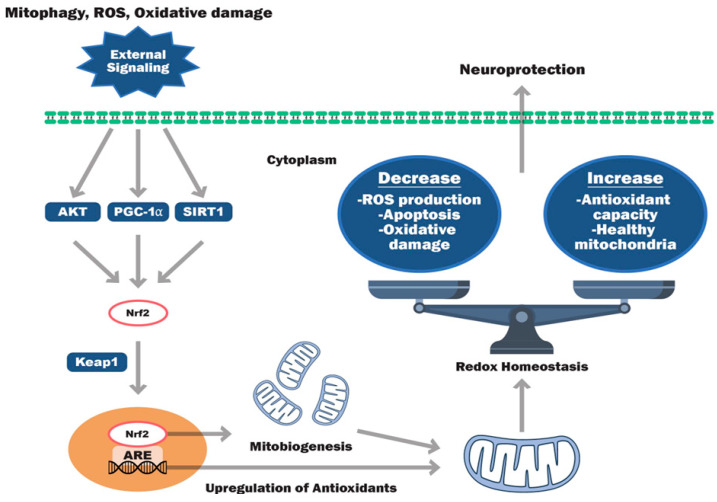
The initiation of redox homeostasis originates from extracellular or intracellular signals via nuclear receptors and mitochondria-mediated pathways. There are intra- and extracellular signaling pathways that activate the protective mechanisms that particularly trigger the endogenous synthesis of antioxidants. Inducers such as ROS, oxidative stress, mitophagy, apoptosis, excitotoxicity, ischemic insults, calcium, neurotransmitters, exercise, or therapeutic treatment (agonists/antagonists) may trigger the onset of signal transduction via modulating several transcription factors in the nucleus, thereby activating gene expression of downstream protein expression. More specific to the current review topic, there are several inducers listed below that may be able to modulate notable antioxidant signaling pathways, such as the Nrf2, AKT, SIRT1, PGC1α, and mTOR signaling pathways (Table 3). The Nrf2 pathway centers around the broad-reaching transcription factor Nrf2, which modulates the transcription of a myriad of endogenous antioxidants. Protein kinase B, a serine/threonine kinase (AKT), is the main mediator of the downstream effector protein phosphoinositide 3-kinase (PI3K). AKT serves as the central component in numerous signaling pathways regulating cell metabolism, growth, proliferation, and survival. Thus, activating AKT can help preserve typical mitochondrial function across several disease conditions [164]. Additionally, AKT regulates Nrf2 to affect the transcription of pro- and antioxidant enzymes and maintain the cellular redox state [165]. Likewise, SIRT1 is a deacetylase that controls the expression of a multitude of antioxidants and oxidative stress modulators like PGC-1α, which plays a major role in the antioxidant defense system. The rapamycin (mTOR) signaling pathway integrates both intracellular and extracellular signals, and serves as a regulator of cellular metabolism, growth, proliferation, and survival. These pathways, in turn, modulate gene expression and the protein biosynthesis of downstream targets, such as antioxidants and mitochondrial biogenesis proteins. Thus, these pathways may be able to modulate ROS–RNS levels, keep the redox balance in check, and maintain cellular integrity. An overview of cell signaling pathways favoring cellular antioxidants synthesis and neuroprotection is illustrated in detail (Figure 4) and discussed below.
Table 3.
Signaling pathway modulators.
| Pathway Modulators | Properties and Mechanisms of Action |
|---|---|
| Nrf2 activators | |
| Omaveloxolone (RTA-408) |
Synthetic compound. FDA-approved for the treatment of FA. Prevents Nrf2 degradation. |
| Dimethyl fumarate (DMF) | Synthetic compound. Activates the Nrf2 pathway and AKT pathway. |
| Curcumin | Derived from turmeric. Activates the Nrf2 pathway. |
| Sulforaphane | Naturally found in cruciferous vegetables. Activates Nrf2 by inhibiting Keap1. |
| Epigallocatechin gallate (EGCG) | Abundant in green tea. Activates the Nrf2 pathway and has antioxidant and anti-inflammatory properties. |
| Quercetin | Present in various fruits, vegetables and grains. Activates Nrf2 and SIRT1. |
| Oltipraz | Synthetic compound. Activates Nrf2 by modifying Keap1. |
| Bardoxolone methyl | Synthetic compound. Activates the Nrf2 pathway. |
| SIRT1, PGC-1α, and mTOR modulators | |
| Resveratrol | Natural polyphenol compound. Most-relevant SIRT1 and mTOR modulator, AKT activator, Nrf2 activator and PGC-1α activator. |
| Naringenin | Natural citrus flavonoid. Modulates SIRT1. |
| SRT2104 | Synthetic compound. SIRT1 activator. |
| 1,4-dihydropyridine derivative | Synthetic compound. SIRT1 activator. |
| Naphthofuran derivative | Synthetic compound. SIRT1 activator. |
| Bisarylaniline derivative | New synthetic analog. SIRT1 activator. |
| Berberine | Small molecule isolated from various plants, mainly used in Chinese traditional medicine. PGC-1α activator. |
| Metformin | Anti-diabetic drug. Activator of AMPK, which further regulates PGC-1α. |
| Rapamycin/Sirolimus | Bacterial origin natural product. mTOR inhibitor and increases antioxidant defense. |
| Everolimus | Newly developed mTOR inhibitor. Rapamycin analog. |
| Temsirolimus | Newly developed mTOR inhibitor. Rapamycin analog. |
Figure 4.
Illustration of signaling pathway modulators involved in endogenous cellular antioxidant synthesis. Extracellular stimulants such as mitophagy, ROS, and oxidative damage may lead to the activation of cell signaling pathways such as AKT, PGC-1α, mTOR, and SIRT. These signaling pathways are involved in upregulating endogenous antioxidant homeostasis by activating nuclear antioxidant response element (ARE) signaling via the activation of common transcription factors Nrf-2 and Keap1. Activation of the nuclear ARE gene upregulates mitochondrial biogenesis. Additionally, ARE gene expression activation may also leads to activation of several mitochondrial antioxidant transcription factors, thereby protein biosynthesis and protect cells against external stimuli. Increased antioxidant levels further balance redox homeostasis by decreasing cellular ROS, oxidative stress, and apoptotic cell death response together by improving the cellular antioxidant capacity and overall health of mitochondria. By activating these pathways, therapeutic compounds may be further able to offer neuroprotection following TBI and CNS diseases.
5.1. Nrf2 Activators
One of the main cellular signaling and oxidative stress defense pathways is the nuclear factor erythroid 2 (Nrf2)-dependent transcriptional mechanism. Nrf2 is responsible for regulating an extensive panel of antioxidant enzymes involved in the detoxification of oxidative stress. Several strategies have been proposed to activate this pathway to counter ROS production and promote neuroprotection. Nrf2 is a transcription factor responsible for regulating the expression of various downstream genes that modulate the oxidative stress response through regulating the antioxidant response element (ARE). Thus, Nrf2-targeted genes affect many vital antioxidants through ARE gene regulation, such as SOD, CAT, and GPX, among others, which help in combatting ROS [166]. Additionally, Nrf2 downregulation supports the decreased efficiency of mitochondrial oxidative phosphorylation. It is widely thought that the Nrf2 pathway plays an important role in TBI pathogenesis, and even in other neurological diseases like AD and PD [167,168,169]. In a mouse model of TBI, Nrf2 was found to be downregulated in cortical tissue, leading to increased oxidative stress, inflammation, and apoptosis [168]. Therefore, targeting and activating Nrf2 signaling is a potential novel target following the oxidative stress-centered pathology of TBI. Owing to its excellent therapeutic potential in CNS diseases in both preclinical and clinical settings, recently, several Nrf2 activators have been approved by the FDA.
Omaveloxolone (RTA-408) and dimethyl fumarate (DMF) are both FDA-approved Nrf2 activators used to treat various neurological conditions like FA and MS. Other Nrf2 activators like curcumin, sulforaphane, epigallocatechin gallate (EGCG), quercetin, oltipraz, and bardoxolone methyl also hold promise as therapeutic agents due to their antioxidant properties [170,171].
RTA-408 is one of the FDA-approved Nrf2 activators for treating FA, a progressive neurodegenerative condition. RTA-408 affects the Nrf2 pathway by preventing Nrf2 ubiquitination and degradation, leading to Nrf2 translocation to the nucleus and increased antioxidant expression. RTA-408 therapy has been found to enhance mitochondrial function and improve neurological symptoms, cognitive impairment, and neuroinflammation in multiple preclinical and clinical models of CNS conditions like epilepsy [172]. RTA-408’s efficacy in minimizing CNS pathology and its mitochondria-protective properties makes it a potential candidate for treating TBI and other neurological diseases [173,174,175].
Similarly, dimethyl fumarate (DMF) is another Nrf2 activator approved by the FDA for treating MS [176,177]. DMF influences the Nrf2 pathway by modifying Keap1, thus promoting Nrf2 nuclear translocation. DMF also activates AKT pathways and thus promotes neuroprotection [178]. DMF upregulates several antioxidants including glutathione, bolstering the downstream antioxidant capacity in CNS conditions like MS, cerebral edema, TBI, and intracerebral hemorrhage [179,180,181,182,183]. DMF treatment in clinical settings has shown long-term efficacy in reducing relapse rates and minimizing lesion formation in relapsing forms of MS [184]. Furthermore, DMF treatment was found to increase neuronal mitochondrial biogenesis via Nrf2 regulation along with improved mitochondrial function and neurological symptoms in a preclinical model of MS [185]. Additionally, DMF has shown to improve cognitive functions in animal models of AD and PD [186]. Together, this evidence emphasizes that DMF has broader therapeutic applicationfor MS, and other neurodegenerative diseases.
Several Nrf2 activators listed here exhibit potential health benefits. Curcumin, found in turmeric, a commonly used spice in Indian cuisine and in traditional medicine, activates the Nrf2 pathway, leading to increased antioxidant and detoxifying enzyme production [187]. Curcumin has shown to be effective against cancer, cardiovascular diseases, and various metabolic and neurological conditions [188]. Sulforaphane, another compound, is mainly found in cruciferous vegetables such as broccoli, cabbage, and brussels sprouts. It activates Nrf2 by inhibiting the protein Keap1 [189]. It similarly enhances endogenous antioxidants and detoxifying enzymes. Sulforaphane has shown therapeutic potential against neurodegenerative diseases [190]. Epigallocatechin gallate (EGCG) is a catechin present in green tea. It activates Nrf2, and it is known for its antioxidant and health-promoting properties [191]. Research suggests that ECCG may help protect neurons from oxidative damage and improve cognitive function [192]. Quercetin, found in fruits and vegetables, is an Nrf2 pathway activator that reduces inflammation and improves antioxidant defense mechanisms. The therapeutic effects of quercetin have been investigated in cancer, cardiovascular diseases, and neurodegenerative conditions [193,194]. Oltipraz, a synthetic compound, activates Nrf2 and reduces oxidative stress in cancer, and additionally has shown neuroprotective benefits [195]. Bardoxolone methyl, another synthetic compound, activates the Nrf2 pathway and stimulates antioxidant enzyme production [196,197]. It has been found to be effective against various disease conditions. Although Nrf2 compounds have shown promising protective effects in various health conditions, further research is warranted to confirm and fully understand their safety and efficacy.
5.2. SIRT, PGC-1α, AKT, and mTOR Modulators
There are other critical regulatory mechanisms in redox homeostasis, such as the Silent Information Regulator (SIRT) genes, also known as Sirtuins, which stimulate antioxidant expression of several enzymes. One of the members of the SIRT family, SIRT1, is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase that plays a wide range of roles in transcriptional regulation, inflammation, cell survival, and repair mechanisms. It guards against oxidative stress by activating the gene transcription of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) via the removal of the acetyl group [198]. PGC-1α is a transcriptional coactivator that is able to upregulate mitochondrial biogenesis, and plays a central role in regulating the oxidative stress defense [199]. SIRT1 is described as a complex target for multiple strategies addressed for the prevention/treatment of several chronic age-related diseases and CNS diseases. Natural and synthetic SIRT1 modulators have been examined. This review examines compounds of a natural origin that have recently been found to upregulate SIRT1 activity, such as polyphenolic products in fruits, vegetables, and plants, including resveratrol, quercetin, and curcumin.
Resveratrol is a natural polyphenol found in various plant sources, such as grapes, berries, and peanuts, which acts as an antioxidant by activating SIRT1. SIRT1 is involved in various cellular processes, including mitochondrial biogenesis [200,201,202]. Resveratrol also activates the Nrf2 signaling pathway to ameliorate oxidative stress and improve mitochondrial function [203]. Moreover, resveratrol activates the PI3K/AKT pathway. On the other hand, resveratrol modulates the recently identified mammalian target of the rapamycin (mTOR) and Janus kinase/signal transducer and activator of transcription (Jak/STAT) pathways to enhance antioxidant defense and positively modulate mitochondrial function. Resveratrol has been suggested to influence mitochondrial dynamics by modulating the balance between mitochondrial fusion and fission, thus regulating mitophagy. Proper regulation of fusion/fission processes is crucial for maintaining mitochondrial health and function. Therefore, resveratrol helps preserve mitochondrial integrity.
Moreover, resveratrol has been shown to be beneficial in neurological diseases like AD, PD, HD, and ALS [201,202,204]. The evidence supports resveratrol’s role in attenuating TBI-associated behavioral abnormalities, brain edema, and pathophysiology [205,206,207,208,209,210]. In a TBI preclinical model, resveratrol improved mitochondrial biogenesis and function by activating the PGC-1α signaling pathway [210]. PGC-1α is central modulator of cell metabolism, where it regulates mitochondrial biogenesis and oxidative metabolism, and controls the expression of antioxidants. It is important to note that while numerous preclinical studies and some clinical trials have explored the potential benefits of resveratrol, the findings are often mixed, and the optimal dose and duration of resveratrol supplementation for specific health conditions remain areas of ongoing research. Other compounds like berberine and metformin which activate PGC-1α may be more useful in neurodegenerative diseases conditions [211,212,213,214].
Despite the potential positive health benefits of resveratrol, it exhibits low CNS bioavailability. This unfavorable pharmacokinetic profile of natural SIRT1 modulators has prompted the development of novel compounds that can positively modulate SIRT1 activity and display better neuroprotective efficacy profiles. Numerous synthetic SIRT1 modulators have been formulated, such as SRT2104, 1,4-dihydropyridine derivative, naphthofuran derivative, and bisarylaniline derivative. However, studies to confirm the pharmacokinetic profiles of these compounds are ongoing. These compounds may have implications in CNS therapeutic development. Additionally, mTOR modulators like everolimus and temsirolimus might regulate ROS through mTOR-mediated antioxidant defense [215]. The mTOR signaling pathway is at the core of many metabolic activities; its activation improves oxidative stress adaptation by activating Nrf2-associated antioxidant signaling [216].
6. Challenges and Future Approach
Despite significant strides in characterizing TBI pathophysiology and identifying therapeutic interventions, the landscape is marred by numerous clinical trial failures, even after promising preclinical success [217,218,219,220]. Several conceptual and methodological issues have undoubtedly contributed to the hitches in translating the preclinical results of antioxidant therapy to a clinical setting. Major challenges lie in the inherent heterogeneity of traumatic brain injuries, the complex and multifaced pathology of brain injuries, the limited information on their molecular pathology, the clinical predictiveness/relevance of animal models, the adequacy of pharmacological methodology, the ill-defined category of TBI, and the outcome measures used. Herein, we reviewed some of these critical problems and potential solutions.
The complex and multifaceted nature of TBI pathophysiology complicates treatment, rendering it challenging to address comprehensively with a single targeted drug. A drug targeting multiple components of the secondary TBI cascade may have superior potency compared to a drug that has a single target. For instance, the Nrf2 pathway activator discussed above broadly modulates intracellular and mitochondria-mediated oxidative and inflammatory responses and may support multiple innate defense mechanisms against TBI pathology. Therefore, any drug with pleiotropic mechanisms of action may be advantageous for TBI research [221].
It has been suggested that the complex pathophysiology of TBI may even possibly be addressed through a combination of therapeutic interventions [222]. The need for integrated multitargeted treatments for TBI has been recognized [223]. At the mitochondrial level, we have identified significant impairment of multitargeted homeostasis, including bioenergetics, calcium, apoptosis, and redox mechanisms, post TBI [11,37]. Providing acetate supplements such as glyceryl triacetate (GTA) and acetyl-L-carnitine (ALC) to boost energy production could contribute to neuronal repair and recovery in the energy deprivation-related pathophysiology of TBI [224,225]. Combining acetate therapy with antioxidants may have additive or synergetic mitochondrial mechanism-targeted neuroprotective efficacy compared to monotherapy in attenuating TBI pathology or promoting recovery. Thus, an effective approach to interrupt post-injury oxidative brain damage might involve the combined treatment of antioxidants with mechanistically complementary energy substrates that simultaneously provide a boost in their antioxidant capacity. Ideally, numerous combination therapies should undergo preclinical testing, with the best combinations chosen for further clinical exploration. An efficient and validated screening platform for candidate therapeutics, sensitive and clinically relevant biomarkers and outcome measures, and standardization and data sharing across centers would greatly facilitate the development of successful combination therapies for TBI [221].
There remains a strong need for rigorous studies to understand the temporal profile of oxidative injury mechanisms following preclinical heterogeneous models of TBI, which may identify novel targets for evaluating neuroprotective therapeutics. As the pathophysiology of secondary injury evolves over time, antioxidant interventions must be able to adapt to evolution in the molecular causes of injury; each compound is likely to have a unique therapeutic time window based on the molecular timeline of secondary injury, during which it is most effective and outside of which it may lack significant benefit [222]. Thus, it is crucial to determine the most efficacious therapeutic window for initiating each antioxidant based on its physiochemical properties and molecular targets in TBI.
Many pharmacological methodological issues have limited the clinical application of antioxidant therapies. Failure to demonstrate sufficient CNS penetration, inadequate dose optimization, or failure to show effectiveness with the treatment delays common in human studies represent some key issues. Nanotechnology, including dendrimers and structural modifications like TPP, discussed earlier, offers excellent potential to increase the efficiency and efficacy of antioxidant therapeutics as their customizable size, stealthy chemistry, and multifunctionality allow them to enhance drug penetration through the BBB. One strategy to improve the delivery of antioxidants to the brain involves the use of the nose-to-brain route, with administration of the antioxidant in specific nasal formulations and its passage to the CNS mainly through the olfactory nerve route [226,227].
Outcomes between individuals following TBI greatly vary, making antioxidant treatment or other treatments for TBI so challenging [228]. The “one-size-fits-all” approach to TBI medicine that has been followed for many years is questionable. Due to this, many researchers have begun to investigate the possibility of using precision medicine techniques to address TBI treatment [228]. TheFDA-approved novel biomarkers for TBI screening, such as GFAP and UCH-L1, which are released from the brain into the bloodstream within 12 h of injury [229]. Notably, personalized stratification based on recently discovered biomarkers can account for individual variability, forming a practical tool that can be used to assist clinical decision making for early TBI diagnosis, and evaluation of therapeutics intervention. This approach holds the potential to overcome the challenges posed by TBI heterogeneity, offering a more tailored and effective strategy for treating TBI patients. An increased understanding of additional biomarkers across the TBI spectrum is needed to improve antioxidant precision medicine in TBI. We stress the importance of further research into this area to improve the clinical efficacy of antioxidant therapy for TBI in the future.
7. Holistic Approach to Improve TBI Outcomes
A holistic approach to provide support that looks at the whole person, not just their CNS health, should be taken into consideration for TBI management. TBI alone or in combination with polytraumatic injuries (i.e., TBI + polytrauma) heavily impacts the body, damaging the brain tissue and shifting homeostasis in many bodily systems such as the immune system, GI system, lungs, heart, and gut microbiota [11,12,230]. This systemic insult can result in changes throughout the body that can increase morbidity and even mortality following TBI [231]. Herein, we reviewed a bidirectional relationship between the gut microbiome and the brain, which also plays a role in TBI-associated pathology. Damage to the brain alters the composition of the microbiome; the altered microbiome affects TBI severity, neuroplasticity, and metabolic pathways through various bacterial metabolites [232]. Significant changes in the gut microbiome within two hours following a TBI was demonstrated in rats, and dysbiosis persisted throughout the study period of 7 days [233]. Furthermore, gut dysbiosis was associated with neuronal loss 3 months after TBI [234]. Notably, emerging research indicates a potential link between the gut microbiome and neurological health [232,233]. The interaction of the CNS and gut signaling pathways includes chemical, neural, metabolic, immune, and endocrine routes, and imbalances in these pathways have been associated with neurological disorders like PD, MS, and AD [235]. Therefore, microbiota manipulation has been proposed as a treatment target for such diseases [236]. While this field of research evolves, maintaining a healthy gut through diet and lifestyle may positively impact outcomes following TBI.
The gut–brain axis suggests that a bidirectional communication between the gut and the brain may influence neurological conditions. A balanced microbiome may contribute to antioxidant production, potentially influencing our body’s ability to combat oxidative damage [237]. Recently, it has been shown that the intake of antioxidant compounds might modulate the composition of beneficial microbial species in the gut, and these commensal bacteria often exhibit antioxidant properties [238]. Thus, the antioxidant supplements and balanced microbiome complement each other due to their mutualistic associations. Probiotic-derived metabolites such as butyrate, propionate, and acetate may serve as alternative energy sources for an injured brain and may improve mitochondrial function following TBI [230,239]. Further supporting the benefit of antioxidants, polyphenol antioxidants such as quercetin, resveratrol, and flavonoid intervention have shown to selectively inhibit pathogenic bacteria in the gut [240]. Additionally, short-chain fatty acids (SCFAs), the main metabolites produced in the colon by bacterial fermentation may contribute to host energy production and ROS modulation [241]. Furthermore, the gut microbiota has been shown to regulate key transcriptional co-activators, transcription factors and enzymes involved in mitochondrial biogenesis, such as the PGC-1α, SIRT1, and AMPK genes [241]. Thus, metabolites produced by commensal gut microbiota, including the beneficial SCFAs, might influence key mitochondrial functions related to TBI pathobiology such as energy production, mitochondrial biogenesis, and redox balance, making them a potential therapeutic target.
Due to the high energy demands exist during the repair of an injured brain; and growing our understanding of brain-gut microbiota crosstalks for the host’s overall health, we have briefly highlighted the existence of interactions between the brain, gut microbiota and mitochondrial redox homeostasis. However, the underlying mechanisms through which antioxidants might influence the gut–brain axis to exert neuroprotection in TBI is yet to be fully elucidated. This knowledge gap is of paramount clinical significance.
8. Conclusions
Emerging evidence indicates that mitochondrial homeostasis is central to the secondary injury cascade in TBI pathology, which lacks approved therapy. Loss of this homeostasis, including redox imbalance, excitotoxicity, calcium overload, bioenergetics failure, and apoptosis, are the main participants in mitochondria-centered damage following TBI, contributing to neuronal death and long-term neurobehavioral sequelae. Thus, mitochondria-targeted antioxidant strategies in TBI have been increasingly studied, as their maintenance could potentially preserve neuronal homeostasis and crucial brain functions. Properly selecting mitochondria-targeted antioxidants, greater understanding of the underlying injury mechanisms, better-tailored treatments, and the application of novel pharmacological methodology offer new insights into the successful management of TBI, and its translation from bench to bed. Therefore, the antioxidants reviewed here could be a viable therapeutic option to minimize secondary damage and improve the quality of life after TBI. However, further research using antioxidants as a treatment for TBI is necessary in order to move towards adding them into routine care for TBI.
Acknowledgments
No experimental studies were conducted to generate any preclinical or clinical data for this manuscript. We thank visual information specialist Christopher S. Nititham, Department of Strategic Communications at WRAIR for providing outstanding graphical design support for this work.
Author Contributions
All authors contributed to the discussion and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
This material has been reviewed by the Walter Reed Army Institute of Research. There are no objections to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Conflicts of Interest
All authors have declared no conflicts of interest.
Funding Statement
The research conducted here was supported by the US Army Combat Casualty Care Research Program (CCCRP) H_001_2018_WRAIR (FY18-23) and ongoing research support CO240012_WRAIR (FY24-26).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.CDC National Center for Health Statistics. [(accessed on 8 September 2023)];2022 Available online: https://wonder.cdc.gov/mcd.html.
- 2.James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., Abbasi N., Abdulkader R., Abraha H.N., Adsuar J.C., et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung Y.-C., Punchak M., Agrawal A., Adeleye A.O., Shrime M.G., Rubiano A.M., et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018;130:1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein E.A., Corso P.S., Miller T.R. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; New York, NY, USA: 2006. [DOI] [Google Scholar]
- 5.Coronado V.G., McGuire L.C., Faul M., Sugerman D.E., Pearson W.S. Traumatic brain injury epidemiology and public health issues. Brain Inj. Med. Princ. Pract. 2012;84:84–100. [Google Scholar]
- 6.Nagalakshmi, Sagarkar S., Sakharkar A.J. Epigenetic mechanisms of traumatic brain injuries. Prog. Mol. Biol. Transl. Sci. 2018;157:263–298. doi: 10.1016/bs.pmbts.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Mena J.H., Sanchez A.I., Rubiano A.M., Peitzman A.B., Sperry J.L., Gutierrez M.I.M., Puyana J.C. Effect of the Modified Glasgow Coma Scale Score Criteria for Mild Traumatic Brain Injury on Mortality Prediction: Comparing Classic and Modified Glasgow Coma Scale Score Model Scores of 13. J. Trauma. 2011;71:1185–1192; Discussion 1193. doi: 10.1097/TA.0b013e31823321f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves W.M., Marshall L.F. Handbook of Neuroemergency Clinical Trials. Elsevier; Amsterdam, The Netherlands: 2006. Traumatic brain injury; pp. 61–79. [Google Scholar]
- 9.Başkaya M.K., Doğan A., Rao A.M., Dempsey R.J. Neuroprotective effects of citicoline on brain edema and blood—Brain barrier breakdown after traumatic brain injury. J. Neurosurg. 2000;92:448–452. doi: 10.3171/jns.2000.92.3.0448. [DOI] [PubMed] [Google Scholar]
- 10.Chodobski A., Zink B.J., Szmydynger-Chodobska J. Blood–Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandya J.D., Leung L.Y., Hwang H.M., Yang X., Deng-Bryant Y., Shear D.A. Time-Course Evaluation of Brain Regional Mitochondrial Bioenergetics in a Pre-Clinical Model of Severe Penetrating Traumatic Brain Injury. J. Neurotrauma. 2021;38:2323–2334. doi: 10.1089/neu.2020.7379. [DOI] [PubMed] [Google Scholar]
- 12.Pandya J.D., Leung L.Y., Flerlage W.J., Gilsdorf J.S., Bryant Y.D., Shear D. Comprehensive profile of acute mitochondrial dysfunction in a preclinical model of severe penetrating TBI. Front. Neurol. 2019;10:605. doi: 10.3389/fneur.2019.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., O W., Li W., Jiang Z.-G., Ghanbari H.A. Oxidative Stress and Neurodegenerative Disorders. Int. J. Mol. Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett B.L., Gardner R.C., Godbout J., Dams-O’connor K., Keene C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry. 2022;91:498–507. doi: 10.1016/j.biopsych.2021.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plassman B., Havlik R., Steffens D., Helms M., Newman T., Drosdick D., Phillips C., Gau B., Welsh–Bohmer K., Burke J., et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/WNL.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 16.Liu G., Ou S., Cui H., Li X., Yin Z., Gu D., Wang Z. Head Injury and Amyotrophic Lateral Sclerosis: A Meta-Analysis. Neuroepidemiology. 2021;55:11–19. doi: 10.1159/000510987. [DOI] [PubMed] [Google Scholar]
- 17.Kang J.-H., Lin H.-C. Increased Risk of Multiple Sclerosis after Traumatic Brain Injury: A Nationwide Population-Based Study. J. Neurotrauma. 2012;29:90–95. doi: 10.1089/neu.2011.1936. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard W.B., Joseph B., Spry M., Vekaria H.J., Saatman K.E., Sullivan P.G. Acute Mitochondrial Impairment Underlies Prolonged Cellular Dysfunction after Repeated Mild Traumatic Brain Injuries. J. Neurotrauma. 2019;36:1252–1263. doi: 10.1089/neu.2018.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilbaugh T.J., Karlsson M., Byro M., Bebee A., Ralston J., Sullivan S., Duhaime A.-C., Hansson M.J., Elmér E., Margulies S.S. Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain. Exp. Neurol. 2015;271:136–144. doi: 10.1016/j.expneurol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandya J.D., Pauly J.R., Nukala V.N., Sebastian A.H., Day K.M., Korde A.S., Maragos W.F., Hall E.D., Sullivan P.G. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J. Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan P.G., Rabchevsky A.G., Keller J.N., Lovell M., Sodhi A., Hart R.P., Scheff S.W. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 2004;474:524–534. doi: 10.1002/cne.20130. [DOI] [PubMed] [Google Scholar]
- 22.Andriessen T.M.J.C., Jacobs B., Vos P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010;14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullock M.R., Povlishock J.T. Guidelines for the management of severe traumatic brain injury. Editor’s Commentary. J. Neurotrauma. 2007;24((Suppl. S1)):2 p preceding S1. doi: 10.1089/neu.2007.9998. [DOI] [PubMed] [Google Scholar]
- 24.Maas A.I.R., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 25.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., et al. Clinical Trials in Head Injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Povlishock J.T., Katz D.I. Update of Neuropathology and Neurological Recovery After Traumatic Brain Injury. J. Head Trauma Rehabilit. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Prins M., Greco T., Alexander D., Giza C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013;6:1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vespa P., Bergsneider M., Hattori N., Wu H.-M., Huang S.-C., Martin N.A., Glenn T.C., McArthur D.L., Hovda D.A. Metabolic Crisis without Brain Ischemia is Common after Traumatic Brain Injury: A Combined Microdialysis and Positron Emission Tomography Study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshino A., Hovda D.A., Kawamata T., Katayama Y., Becker D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-K. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lifshitz J., Sullivan P.G., Hovda D.A., Wieloch T., McIntosh T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan P.G., Krishnamurthy S., Patel S.P., Pandya J.D., Rabchevsky A.G. Temporal Characterization of Mitochondrial Bioenergetics after Spinal Cord Injury. J. Neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- 33.Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., Hall E.D. Time Course of Post-Traumatic Mitochondrial Oxidative Damage and Dysfunction in a Mouse Model of Focal Traumatic Brain Injury: Implications for Neuroprotective Therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y., Gu Q., Peterson P., Muizelaar J., Lee C. Mitochondrial Dysfunction and Calcium Perturbation Induced by Traumatic Brain Injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 35.Gilmer L.K., Roberts K.N., Sullivan P.G., Miller K., Scheff S. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma. 2009;219:1. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandya J.D., Pauly J.R., Sullivan P.G. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Pandya J.D., Musyaju S., Modi H.R., Cao Y., Flerlage W.J., Huynh L., Kociuba B., Visavadiya N.P., Kobeissy F., Wang K., et al. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free. Radic. Biol. Med. 2023;198:44–58. doi: 10.1016/j.freeradbiomed.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Pt 2Biochem. J. 1999;341:233–249. doi: 10.1042/bj3410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halestrap A.P. Mitochondrial calcium in health and disease. Biochim. Biophys. Acta (BBA)—Bioenerg. 2009;1787:1289–1290. doi: 10.1016/j.bbabio.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Halestrap A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., Springer J.E. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- 42.Chance B., Sies H., Walker C.L., Pomatto L.C.D., Tripathi D.N., Davies K.J.A., Ninsontia C., Phiboonchaiyanan P.P., Kiratipaiboon C., Chanvorachote P., et al. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 43.Halliwell B., Gutteridge J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 45.Berg J.M., Tymoczko J.L., Stryer L. Biochemistry. 5th ed. W. H. Freeman and Company; New York, NY, USA: 2002. [Google Scholar]
- 46.Chance B., Williams G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 47.Deng Y., Thompson B.M., Gao X., Hall E.D. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh I.N., Sullivan P.G., Hall E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- 49.Bayir H., E Kagan V., Tyurina Y.Y., Tyurin V., A Ruppel R., Adelson P.D., Graham S.H., Janesko K., Clark R.S.B., Kochanek P.M. Assessment of Antioxidant Reserves and Oxidative Stress in Cerebrospinal Fluid after Severe Traumatic Brain Injury in Infants and Children. Pediatr. Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Ansari M.A., Roberts K.N., Scheff S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free. Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall E.D., Detloff M.R., Johnson K., Kupina N.C. Peroxynitrite-Mediated Protein Nitration and Lipid Peroxidation in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 52.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant Therapies for Traumatic Brain Injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall E.D., Wang J.A., Miller D.M. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 2012;238:176–182. doi: 10.1016/j.expneurol.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill R.L., Kulbe J.R., Singh I.N., Wang J.A., Hall E.D. Synaptic Mitochondria are More Susceptible to Traumatic Brain Injury-induced Oxidative Damage and Respiratory Dysfunction than Non-synaptic Mitochondria. Neuroscience. 2018;386:265–283. doi: 10.1016/j.neuroscience.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 55.Abdul-Muneer P.M., Chandra N., Haorah J. Interactions of Oxidative Stress and Neurovascular Inflammation in the Pathogenesis of Traumatic Brain Injury. Mol. Neurobiol. 2015;51:966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petronilho F., Feier G., de Souza B., Guglielmi C., Constantino L.S., Walz R., Quevedo J., Dal-Pizzol F. Oxidative Stress in Brain According to Traumatic Brain Injury Intensity. J. Surg. Res. 2010;164:316–320. doi: 10.1016/j.jss.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Cornelius C., Crupi R., Calabrese V., Graziano A., Milone P., Pennisi G., Radak Z., Calabrese E.J., Cuzzocrea S. Traumatic Brain Injury: Oxidative Stress and Neuroprotection. Antioxid. Redox Signal. 2013;19:836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 58.Fernández-Gajardo R., Matamala J.M., Carrasco R., Gutiérrez R., Melo R., Rodrigo R. Novel Therapeutic Strategies for Traumatic Brain Injury: Acute Antioxidant Reinforcement. CNS Drugs. 2014;28:229–248. doi: 10.1007/s40263-013-0138-y. [DOI] [PubMed] [Google Scholar]
- 59.Kitada M., Xu J., Ogura Y., Monno I., Koya D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020;11:755. doi: 10.3389/fphys.2020.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suthammarak W., Somerlot B.H., Opheim E., Sedensky M., Morgan P.G. Novel interactions between mitochondrial superoxide dismutases and the electron transport chain. Aging Cell. 2013;12:1132–1140. doi: 10.1111/acel.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holley A.K., Clair D.K.S., Huang C.-L., Yokomise H., Miyatake A., Setyawati M.I., Tay C.Y., Leong D.T., Schumacher B., Gartner A., et al. Watching the watcher: Regulation of p53 by mitochondria. Futur. Oncol. 2009;5:117–130. doi: 10.2217/14796694.5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeKosky S.T., Taffe K.M., Abrahamson E.E., Dixon C.E., Kochanek P.M., Ikonomovic M.D. Time Course Analysis of Hippocampal Nerve Growth Factor and Antioxidant Enzyme Activity following Lateral Controlled Cortical Impact Brain Injury in the Rat. J. Neurotrauma. 2004;21:491–500. doi: 10.1089/089771504774129838. [DOI] [PubMed] [Google Scholar]
- 63.Pappolla M.A., Omar R.A., Kim K.S., Robakis N.K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer’s disease. Am. J. Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- 64.Zemlan F.P., Thienhaus O.J., Bosmann H.B. Superoxide dismutase activity in Alzheimer’s disease: Possible mechanism for paired helical filament formation. Brain Res. 1989;476:160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]
- 65.Massaad C.A., Washington T.M., Pautler R.G., Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palace V.P., Khaper N., Qin Q., Singal P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free. Radic. Biol. Med. 1999;26:746–761. doi: 10.1016/S0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez M.J., Miranda-Massari J.R., Olalde J. Molecular Nutrition and Mitochondria. Elsevier; Amsterdam, The Netherlands: 2023. Vitamin C and mitochondrial function in health and exercise; pp. 225–242. [Google Scholar]
- 68.Eleff S., Kennaway N.G., Buist N.R., Darley-Usmar V.M., A Capaldi R., Bank W.J., Chance B. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc. Natl. Acad. Sci. USA. 1984;81:3529–3533. doi: 10.1073/pnas.81.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kc S., Càrcamo J.M., Golde D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Gluti) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19:1657–1667. doi: 10.1096/fj.05-4107com. [DOI] [PubMed] [Google Scholar]
- 70.González M.J., Miranda J.R., Riordan H.D. Vitamin C as an Ergogenic Aid. J. Orthomol. Med. 2005;20:100–102. [Google Scholar]
- 71.U.S. Department of Health & Human Services Vitamin A and Carotenoids. Fact Sheet for Health Professionals. [(accessed on 29 April 2022)]; Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- 72.Hayashi T., Sawa K., Kawasaki M., Arisawa M., Shimizu M., Morita N. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988;51:345–348. doi: 10.1021/np50056a030. [DOI] [PubMed] [Google Scholar]
- 73.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansari M.A., Roberts K.N., Scheff S.W. Dose- and Time-Dependent Neuroprotective Effects of Pycnogenol® following Traumatic Brain Injury. J. Neurotrauma. 2013;30:1542–1549. doi: 10.1089/neu.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung L.Y. The Antioxidant Properties of Garlic Compounds: Allyl Cysteine, Alliin, Allicin, and Allyl Disulfide. J. Med. Food. 2006;9:205–213. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz I.F., Hershkovitz R., Iaina A., Gnessin E., Wollman Y., Chernichowski T., Blum M., Levo Y., Schwartz D. Garlic attenuates nitric oxide production in rat cardiac myocytes through inhibition of inducible nitric oxide synthase and the arginine transporter CAT-2 (cationic amino acid transporter-2) Clin. Sci. 2002;102:487–493. doi: 10.1042/cs1020487. [DOI] [PubMed] [Google Scholar]
- 77.Nadeem M.S., Kazmi I., Ullah I., Muhammad K., Anwar F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants. 2021;11:87. doi: 10.3390/antiox11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adebayo O.L., Adenuga G.A., Sandhir R. Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci. 2016;152:145–155. doi: 10.1016/j.lfs.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Pizzorno J. Glutathione! Integr. Med. 2014;13:8–12. [PMC free article] [PubMed] [Google Scholar]
- 80.Korshunov S.S., Krasnikov B.F., O Pereverzev M., Skulachev V.P. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/S0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 81.Pereverzev M.O., Vygodina T.V., Konstantinov A.A., Skulachev V.P. Cytochrome c, an ideal antioxidant. Pt 6Biochem. Soc. Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- 82.Sokol R.J., McKim J.M., Goff M., Ruyle S.Z., Devereaux M.W., Han D., Packer L., Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164–174. doi: 10.1016/S0016-5085(98)70644-4. [DOI] [PubMed] [Google Scholar]
- 83.Gilgun-Sherki Y., Melamed E., Offen D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/S0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 84.Batinić-Haberle I., Cuzzocrea S., Rebouças J.S., Ferrer-Sueta G., Mazzon E., Di Paola R., Radi R., Spasojević I., Benov L., Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free. Radic. Biol. Med. 2009;46:192. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zahmatkesh M., Kadkhodaee M., Moosavi S.M.S., Jorjani M., Kajbafzadeh A., Golestani A., Ghaznavi R. Beneficial effects of MnTBAP, a broad-spectrum reactive species scavenger, in rat renal ischemia/reperfusion injury. Clin. Exp. Nephrol. 2005;9:212–218. doi: 10.1007/s10157-005-0359-6. [DOI] [PubMed] [Google Scholar]
- 86.Kim J.-H., Jang H.-J., Cho W.-Y., Yeon S.-J., Lee C.-H. In vitro antioxidant actions of sulfur-containing amino acids. Arab. J. Chem. 2020;13:1678–1684. doi: 10.1016/j.arabjc.2017.12.036. [DOI] [Google Scholar]
- 87.Nandi A., Chatterjee I.B. Scavenging of superoxide radical by ascorbic acid. J. Biosci. 1987;11:435–441. doi: 10.1007/BF02704692. [DOI] [Google Scholar]
- 88.E Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991;54:S1119–S1124. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]
- 89.Hussain S., Slikker W., Jr., Ali S.F. Role of Metallothionein and other Antioxidants in Scavenging Superoxide Radicals and their Possible Role in Neuroprotection. Neurochem. Int. 1996;29:145–152. doi: 10.1016/0197-0186(95)00114-X. [DOI] [PubMed] [Google Scholar]
- 90.Schneider M.P., Delles C., Schmidt B.M., Oehmer S., Schwarz T.K., Schmieder R.E., John S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005;18:1111–1117. doi: 10.1016/j.amjhyper.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Taiwo F.A. Mechanism of tiron as scavenger of superoxide ions and free electrons. Spectroscopy. 2008;22:491–498. doi: 10.1155/2008/953692. [DOI] [Google Scholar]
- 92.Wright V.P., Klawitter P.F., Iscru D.F., Merola A.J., Clanton T.L. Superoxide scavengers augment contractile but not energetic responses to hypoxia in rat diaphragm. J. Appl. Physiol. 2005;98:1753–1760. doi: 10.1152/japplphysiol.01022.2004. [DOI] [PubMed] [Google Scholar]
- 93.Pfeiffer S., Leopold E., Hemmens B., Schmidt K., Werner E.R., Mayer B. Interference of Carboxy-PTIO with Nitric Oxide- and Peroxynitrite-Mediated Reactions. Free. Radic. Biol. Med. 1997;22:787–794. doi: 10.1016/S0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- 94.Lilley E., Gibson A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J., Su Y., Richmond A. Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic. Biol. Med. 2007;42:1369–1380. doi: 10.1016/j.freeradbiomed.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill R.L., Singh I.N., Wang J.A., Kulbe J.R., Hall E.D. Protective effects of phenelzine administration on synaptic and non-synaptic cortical mitochondrial function and lipid peroxidation-mediated oxidative damage following TBI in young adult male rats. Exp. Neurol. 2020;330:113322. doi: 10.1016/j.expneurol.2020.113322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith R.A.J., Porteous C.M., Coulter C.V., Murphy M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 98.Davidson S.M. Endothelial mitochondria and heart disease. Cardiovasc. Res. 2010;88:58–66. doi: 10.1093/cvr/cvq195. [DOI] [PubMed] [Google Scholar]
- 99.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta (BBA)—Bioenerg. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 100.Murphy M.P., Smith R.A. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 101.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cochemé H.M., Murphy M.P., Dominiczak A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 102.Zhou J., Wang H., Shen R., Fang J., Yang Y., Dai W., Zhu Y., Zhou M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018;10:1887–1899. [PMC free article] [PubMed] [Google Scholar]
- 103.Tabet M., El-Kurdi M., Haidar M.A., Nasrallah L., Reslan M.A., Shear D., Pandya J.D., El-Yazbi A.F., Sabra M., Mondello S., et al. Mitoquinone supplementation alleviates oxidative stress and pathologic outcomes following repetitive mild traumatic brain injury at a chronic time point. Exp. Neurol. 2022;351:113987. doi: 10.1016/j.expneurol.2022.113987. [DOI] [PubMed] [Google Scholar]
- 104.Liberman E.A., Topaly V.P., Tsofina L.M., Jasaitis A.A., Skulachev V.P. Mechanism of Coupling of Oxidative Phosphorylation and the Membrane Potential of Mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 105.Skulachev V.P., Vyssokikh M.Y., Chernyak B.V., Averina O.A., Andreev-Andrievskiy A.A., Zinovkin R.A., Lyamzaev K.G., Marey M.V., Egorov M.V., Frolova O.J., et al. Mitochondrion-targeted antioxidant SkQ1 prevents rapid animal death caused by highly diverse shocks. Sci. Rep. 2023;13:4326. doi: 10.1038/s41598-023-31281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amemiya S., Kamiya T., Nito C., Inaba T., Kato K., Ueda M., Shimazaki K., Katayama Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur. J. Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 107.Toyoda K., Fujii K., Kamouchi M., Nakane H., Arihiro S., Okada Y., Ibayashi S., Iida M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J. Neurol. Sci. 2004;221:11–17. doi: 10.1016/j.jns.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 108.Abe K., Yuki S., Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke. 1988;19:480–485. doi: 10.1161/01.STR.19.4.480. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto T., Yuki S., Watanabe T., Mitsuka M., Saito K.-I., Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–242. doi: 10.1016/S0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 110.Homma T., Kobayashi S., Sato H., Fujii J. Edaravone, a free radical scavenger, protects against ferroptotic cell death in vitro. Exp. Cell Res. 2019;384:111592. doi: 10.1016/j.yexcr.2019.111592. [DOI] [PubMed] [Google Scholar]
- 111.Yuan W.J., Yasuhara T., Shingo T., Muraoka K., Agari T., Kameda M., Uozumi T., Tajiri N., Morimoto T., Jing M., et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiao S.-S., Yao X.-Q., Liu Y.-H., Wang Q.-H., Zeng F., Lu J.-J., Liu J., Zhu C., Shen L.-L., Liu C.-H., et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc. Natl. Acad. Sci. USA. 2015;112:5225–5230. doi: 10.1073/pnas.1422998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xi H., Akishita M., Nagai K., Yu W., Hasegawa H., Eto M., Kozaki K., Toba K. Potent free radical scavenger, edaravone, suppresses oxidative stress-induced endothelial damage and early atherosclerosis. Atherosclerosis. 2007;191:281–289. doi: 10.1016/j.atherosclerosis.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 114.Higashi Y., Jitsuiki D., Chayama K., Yoshizumi M. Edaravone (3-Methyl-1-Phenyl-2-Pyrazolin-5-one), A Novel Free Radical Scavenger, for Treatment of Cardiovascular Diseases. Recent Pat. Cardiovasc. Drug Discov. 2006;1:85–93. doi: 10.2174/157489006775244191. [DOI] [PubMed] [Google Scholar]
- 115.Shetty S., Anushree U., Kumar R., Bharati S. Mitochondria-targeted antioxidant, mito-TEMPO mitigates initiation phase of N-Nitrosodiethylamine-induced hepatocarcinogenesis. Mitochondrion. 2021;58:123–130. doi: 10.1016/j.mito.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Nhu N.T., Xiao S.-Y., Liu Y., Kumar V.B., Cui Z.-Y., Lee S.-D. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front. Integr. Neurosci. 2021;15:747901. doi: 10.3389/fnint.2021.747901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rzigalinski B.A., Carfagna C.S., Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1444. doi: 10.1002/wnan.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liang L.-P., Waldbaum S., Rowley S., Huang T.-T., Day B.J., Patel M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol. Dis. 2012;45:1068–1076. doi: 10.1016/j.nbd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peterson S.L., Purvis R.S., Griffith J.W. Comparison of Neuroprotective Effects Induced by α-Phenyl-N-tert-butyl nitrone (PBN) and N-tert-Butyl-α-(2 sulfophenyl) nitrone (S-PBN) in Lithium-Pilocarpine Status Epilepticus. NeuroToxicology. 2005;26:969–979. doi: 10.1016/j.neuro.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Deletraz A., Zéamari K., Hua K., Combes M., Villamena F.A., Tuccio B., Callizot N., Durand G. Substituted α-phenyl and α-naphthlyl-N-tert-butyl nitrones: Synthesis, spin-trapping, and neuroprotection evaluation. J. Org. Chem. 2020;85:6073–6085. doi: 10.1021/acs.joc.0c00563. [DOI] [PubMed] [Google Scholar]
- 121.Chamorro B., Diez-Iriepa D., Merás-Sáiz B., Chioua M., García-Vieira D., Iriepa I., Hadjipavlou-Litina D., López-Muñoz F., Martínez-Murillo R., Gonzàlez-Nieto D., et al. Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived HomoBisNitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020;10:14150. doi: 10.1038/s41598-020-70690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Pietro V., Yakoub K.M., Caruso G., Lazzarino G., Signoretti S., Barbey A.K., Tavazzi B., Lazzarino G., Belli A., Amorini A.M. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants. 2020;9:260. doi: 10.3390/antiox9030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis C.K., Vemuganti R. Antioxidant therapies in traumatic brain injury. Neurochem. Int. 2022;152:105255. doi: 10.1016/j.neuint.2021.105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dash P.K., Hergenroeder G.W., Jeter C.B., Choi H.A., Kobori N., Moore A.N. Traumatic Brain Injury Alters Methionine Metabolism: Implications for Pathophysiology. Front. Syst. Neurosci. 2016;10:36. doi: 10.3389/fnsys.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sonthalia S., Daulatabad D., Sarkar R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 2016;82:262–272. doi: 10.4103/0378-6323.179088. [DOI] [PubMed] [Google Scholar]
- 126.Holmay M.J.B., Terpstra M., Coles L.D., Mishra U., Ahlskog M.B., Öz G., Cloyd J.C., Tuite P.J. N-acetylcysteine Boosts Brain and Blood Glutathione in Gaucher and Parkinson Diseases. Clin. Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alkandari A.F., Madhyastha S., Rao M.S. N-Acetylcysteine Amide against Aβ-Induced Alzheimer’s-like Pathology in Rats. Int. J. Mol. Sci. 2023;24:12733. doi: 10.3390/ijms241612733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., Sullivan P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014;257:106–113. doi: 10.1016/j.expneurol.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel S.P., Sullivan P.G., Pandya J.D., Goldstein G.A., VanRooyen J.L., Yonutas H.M., Eldahan K.C., Morehouse J., Magnuson D.S., Rabchevsky A.G. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol. 2014;257:95–105. doi: 10.1016/j.expneurol.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berk M., Malhi G.S., Gray L.J., Dean O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Baki S.G.A., Schwab B., Haber M., Fenton A.A., Bergold P.J. Minocycline Synergizes with N-Acetylcysteine and Improves Cognition and Memory Following Traumatic Brain Injury in Rats. PLoS ONE. 2010;5:e12490. doi: 10.1371/journal.pone.0012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hicdonmez T., Kanter M., Tiryaki M., Parsak T., Cobanoglu S. Neuroprotective Effects of N-acetylcysteine on Experimental Closed Head Trauma in Rats. Neurochem. Res. 2006;31:473–481. doi: 10.1007/s11064-006-9040-z. [DOI] [PubMed] [Google Scholar]
- 133.Thomale U.-W., Griebenow M., Kroppenstedt S.-N., Unterberg A.W., Stover J.F. The effect of N-acetylcysteine on posttraumatic changes after controlled cortical impact in rats. Intensiv. Care Med. 2006;32:149–155. doi: 10.1007/s00134-005-2845-4. [DOI] [PubMed] [Google Scholar]
- 134.Xiong Y., Peterson P., Lee C. Effect of N-Acetylcysteine on Mitochondrial Function Following Traumatic Brain Injury in Rats. J. Neurotrauma. 1999;16:1067–1082. doi: 10.1089/neu.1999.16.1067. [DOI] [PubMed] [Google Scholar]
- 135.Thomale U.W., Griebenow M., Kroppenstedt S.N., Unterberg A.W., Stover J.F. The antioxidant effect of N-acethylcysteine on experimental contusion in rats. Acta Neurochir. Suppl. 2005;95:429–431. doi: 10.1007/3-211-32318-x_88. [DOI] [PubMed] [Google Scholar]
- 136.Hagos F.T., Empey P.E., Wang P., Ma X., Poloyac S.M., Bayir H., Kochanek P.M., Bell M.J., Clark R.S.B. Exploratory Application of Neuropharmacometabolomics in Severe Childhood Traumatic Brain Injury*. Crit. Care Med. 2018;46:1471–1479. doi: 10.1097/CCM.0000000000003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clark R.S.B., Empey P.E., Bayır H., Rosario B.L., Poloyac S.M., Kochanek P.M., Nolin T.D., Au A.K., Horvat C.M., Wisniewski S.R., et al. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS ONE. 2017;12:e0180280. doi: 10.1371/journal.pone.0180280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clark R.S.B., Empey P.E., Kochanek P.M., Bell M.J. N-Acetylcysteine and Probenecid Adjuvant Therapy for Traumatic Brain Injury. Neurotherapeutics. 2023;20:1529–1537. doi: 10.1007/s13311-023-01422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoffer M.E., Balaban C., Slade M.D., Tsao J.W., Hoffer B. Amelioration of Acute Sequelae of Blast Induced Mild Traumatic Brain Injury by N-Acetyl Cysteine: A Double-Blind, Placebo Controlled Study. PLoS ONE. 2013;8:e54163. doi: 10.1371/journal.pone.0054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khan M., Sekhon B., Jatana M., Giri S., Gilg A.G., Sekhon C., Singh I., Singh A.K. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J. Neurosci. Res. 2004;76:519–527. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 141.Sekhon B., Sekhon C., Khan M., Patel S.J., Singh I., Singh A.K. N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003;971:1–8. doi: 10.1016/S0006-8993(03)02244-3. [DOI] [PubMed] [Google Scholar]
- 142.Gilgun-Sherki Y., Rosenbaum Z., Melamed E., Offen D. Antioxidant Therapy in Acute Central Nervous System Injury: Current State. Pharmacol. Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 143.Pahan K., Sheikh F.G., Namboodiri A.M., Singh I. N-Acetyl Cysteine Inhibits Induction of No Production By Endotoxin or Cytokine Stimulated Rat Peritoneal Macrophages, C6 Glial Cells and Astrocytes. Free. Radic. Biol. Med. 1998;24:39–48. doi: 10.1016/S0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 144.Aitio M.L. N-acetylcysteine—Passe-partout or much ado about nothing? Br. J. Clin. Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tenório M.C.D.S., Graciliano N.G., Moura F.A., Oliveira A.C.M.D., Goulart M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants. 2021;10:967. doi: 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen W., Ercal N., Huynh T., Volkov A., Chusuei C.C. Characterizing N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) binding for lead poisoning treatment. J. Colloid Interface Sci. 2012;371:144–149. doi: 10.1016/j.jcis.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Atlas D. Emerging therapeutic opportunities of novel thiol-amides, NAC-amide (AD4/NACA) and thioredoxin mimetics (TXM-Peptides) for neurodegenerative-related disorders. Free Radic. Biol. Med. 2021;176:120–141. doi: 10.1016/j.freeradbiomed.2021.08.239. [DOI] [PubMed] [Google Scholar]
- 148.Offen D., Gilgun-Sherki Y., Barhum Y., Benhar M., Grinberg L., Reich R., Melamed E., Atlas D. A low molecular weight copper chelator crosses the blood–brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. 2004;89:1241–1251. doi: 10.1111/j.1471-4159.2004.02428.x. [DOI] [PubMed] [Google Scholar]
- 149.Grinberg L., Fibach E., Amer J., Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free. Radic. Biol. Med. 2005;38:136–145. doi: 10.1016/j.freeradbiomed.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 150.Bahat-Stroomza M., Gilgun-Sherki Y., Offen D., Panet H., Saada A., Krool-Galron N., Barzilai A., Atlas D., Melamed E. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s disease. Eur. J. Neurosci. 2005;21:637–646. doi: 10.1111/j.1460-9568.2005.03889.x. [DOI] [PubMed] [Google Scholar]
- 151.Bhatti J., Nascimento B., Akhtar U., Rhind S.G., Tien H., Nathens A., Da Luz L.T. Systematic Review of Human and Animal Studies Examining the Efficacy and Safety of N-Acetylcysteine (NAC) and N-Acetylcysteine Amide (NACA) in Traumatic Brain Injury: Impact on Neurofunctional Outcome and Biomarkers of Oxidative Stress and Inflammation. Front. Neurol. 2017;8:744. doi: 10.3389/fneur.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kurano T., Kanazawa T., Iioka S., Kondo H., Kosuge Y., Suzuki T. Intranasal Administration of N-acetyl-L-cysteine Combined with Cell-Penetrating Peptide-Modified Polymer Nanomicelles as a Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis. Pharmaceutics. 2022;14:2590. doi: 10.3390/pharmaceutics14122590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kannan S., Balakrishnan B., Nance E., Johnston M.V., Rangaramanujam K. Nanomedicine in cerebral palsy. Int. J. Nanomed. 2013;8:4183–4195. doi: 10.2147/IJN.S35979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nance E., Zhang F., Mishra M.K., Zhang Z., Kambhampati S.P., Kannan R.M., Kannan S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials. 2016;101:96–107. doi: 10.1016/j.biomaterials.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang F., Nance E., Alnasser Y., Kannan R., Kannan S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflamm. 2016;13:65. doi: 10.1186/s12974-016-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lesniak W.G., Mishra M.K., Jyoti A., Balakrishnan B., Zhang F., Nance E., Romero R., Kannan S., Kannan R.M. Biodistribution of Fluorescently Labeled PAMAM Dendrimers in Neonatal Rabbits: Effect of Neuroinflammation. Mol. Pharm. 2013;10:4560–4571. doi: 10.1021/mp400371r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kannan S., Dai H., Navath R.S., Balakrishnan B., Jyoti A., Janisse J., Romero R., Kannan R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nance E., Porambo M., Zhang F., Mishra M.K., Buelow M., Getzenberg R., Johnston M., Kannan R.M., Fatemi A., Kannan S. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J. Control. Release. 2015;214:112–120. doi: 10.1016/j.jconrel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mishra M.K., Beaty C.A., Lesniak W.G., Kambhampati S.P., Zhang F., Wilson M.A., Blue M.E., Troncoso J.C., Kannan S., Johnston M.V., et al. Dendrimer Brain Uptake and Targeted Therapy for Brain Injury in a Large Animal Model of Hypothermic Circulatory Arrest. ACS Nano. 2014;8:2134–2147. doi: 10.1021/nn404872e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Modi H.R., Wang Q., Olmstead S.J., Khoury E.S., Sah N., Guo Y., Gharibani P., Sharma R., Kannan R.M., Kannan S., et al. Systemic administration of dendrimer N-acetyl cysteine improves outcomes and survival following cardiac arrest. Bioeng. Transl. Med. 2022;7:e10259. doi: 10.1002/btm2.10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kambhampati S.P., Bhutto I.A., Wu T., Ho K., McLeod D.S., Lutty G.A., Kannan R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release. 2021;335:527–540. doi: 10.1016/j.jconrel.2021.05.035. [DOI] [PubMed] [Google Scholar]
- 162.Wilson A. S-Adenosyl Methionine (SAMe) for Depression in Adults. Issues Ment. Heal. Nurs. 2019;40:725–726. doi: 10.1080/01612840.2017.1392161. [DOI] [PubMed] [Google Scholar]
- 163.Guo T., Chang L., Xiao Y., Liu Q. S-Adenosyl-L-Methionine for the Treatment of Chronic Liver Disease: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0122124. doi: 10.1371/journal.pone.0122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xie X., Shu R., Yu C., Fu Z., Li Z. Mammalian AKT, the Emerging Roles on Mitochondrial Function in Diseases. Aging Dis. 2022;13:157–174. doi: 10.14336/AD.2021.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li H., Tang Z., Chu P., Song Y., Yang Y., Sun B., Niu M., Qaed E., Shopit A., Han G., et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: Involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radic. Biol. Med. 2018;120:228–238. doi: 10.1016/j.freeradbiomed.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 166.He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., León R. Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Bhowmick S., D’mello V., Caruso D., Abdul-Muneer P.M. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J. Mol. Med. 2019;97:1627–1641. doi: 10.1007/s00109-019-01851-4. [DOI] [PubMed] [Google Scholar]
- 169.Abdul-Muneer P.M. Nrf2 as a Potential Therapeutic Target for Traumatic Brain Injury. J. Integr. Neurosci. 2023;22:81. doi: 10.31083/j.jin2204081. [DOI] [PubMed] [Google Scholar]
- 170.Wu A.-G., Yong Y.-Y., Pan Y.-R., Zhang L., Wu J.-M., Zhang Y., Tang Y., Wei J., Yu L., Law B.Y.-K., et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022;2022:1015791. doi: 10.1155/2022/1015791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dong W., Yang B., Wang L., Li B., Guo X., Zhang M., Jiang Z., Fu J., Pi J., Guan D., et al. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 2018;346:28–36. doi: 10.1016/j.taap.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 172.Lalitha A., Nanjundeshwari G., Swetha M., Swetha R. A Review On Omaveloxolone. Int. J. Pharm. Sci. 2023;1:447–456. doi: 10.5281/zenodo.8383431. [DOI] [Google Scholar]
- 173.Lynch D.R., Farmer J., Hauser L., Blair I.A., Wang Q.Q., Mesaros C., Snyder N., Boesch S., Chin M., Delatycki M.B., et al. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2019;6:15–26. doi: 10.1002/acn3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lynch D.R., Chin M.P., Delatycki M.B., Subramony S.H., Corti M., Hoyle J.C., Boesch S., Nachbauer W., Mariotti C., Mathews K.D., et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study) Ann. Neurol. 2021;89:212–225. doi: 10.1002/ana.25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abeti R., Baccaro A., Esteras N., Giunti P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018;12:188. doi: 10.3389/fncel.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wingerchuk D.M., Carter J.L. Multiple Sclerosis: Current and Emerging Disease-Modifying Therapies and Treatment Strategies. Mayo Clin. Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 177.Maldonado P.P., Guevara C., Olesen M.A., Orellana J.A., Quintanilla R.A., Ortiz F.C. Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways. Antioxidants. 2022;11:1146. doi: 10.3390/antiox11061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abd El-Fatah I.M., Abdelrazek H.M., Ibrahim S.M., Abdallah D.M., El-Abhar H.S. Dimethyl fumarate abridged tauo-/amyloidopathy in a D-Galactose/ovariectomy-induced Alzheimer’s-like disease: Modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, adiponectin/Adipo1R, and NF-κB/IL-1β/ROS trajectories. Neurochem. Int. 2021;148:105082. doi: 10.1016/j.neuint.2021.105082. [DOI] [PubMed] [Google Scholar]
- 179.Bresciani G., Manai F., Davinelli S., Tucci P., Saso L., Amadio M. Novel potential pharmacological applications of dimethyl fumarate—An overview and update. Front. Pharmacol. 2023;14:1264842. doi: 10.3389/fphar.2023.1264842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kunze R., Urrutia A., Hoffmann A., Liu H., Helluy X., Pham M., Reischl S., Korff T., Marti H.H. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood–brain barrier integrity. Exp. Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 181.Casili G., Campolo M., Paterniti I., Lanza M., Filippone A., Cuzzocrea S., Esposito E. Dimethyl Fumarate Attenuates Neuroinflammation and Neurobehavioral Deficits Induced by Experimental Traumatic Brain Injury. J. Neurotrauma. 2018;35:1437–1451. doi: 10.1089/neu.2017.5260. [DOI] [PubMed] [Google Scholar]
- 182.Zhao X., Sun G., Zhang J., Ting S.-M., Gonzales N., Aronowski J. Dimethyl Fumarate Protects Brain From Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke. 2015;46:1923–1928. doi: 10.1161/STROKEAHA.115.009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Krämer T., Grob T., Menzel L., Hirnet T., Griemert E., Radyushkin K., Thal S.C., Methner A., Schaefer M.K.E. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J. Neurochem. 2017;143:523–533. doi: 10.1111/jnc.14220. [DOI] [PubMed] [Google Scholar]
- 184.Gold R., Arnold D.L., Bar-Or A., Fox R.J., Kappos L., Mokliatchouk O., Jiang X., Lyons J., Kapadia S., Miller C. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: Final ENDORSE study results. Mult. Scler. J. 2022;28:801–816. doi: 10.1177/13524585211037909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hayashi G., Jasoliya M., Sahdeo S., Saccà F., Pane C., Filla A., Marsili A., Puorro G., Lanzillo R., Brescia Morra V., et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017;26:2864–2873. doi: 10.1093/hmg/ddx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Majkutewicz I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022;926:175025. doi: 10.1016/j.ejphar.2022.175025. [DOI] [PubMed] [Google Scholar]
- 187.Ashrafizadeh M., Ahmadi Z., Mohammadinejad R., Farkhondeh T., Samarghandian S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020;20:116–133. doi: 10.2174/1566524019666191016150757. [DOI] [PubMed] [Google Scholar]
- 188.Monroy A., Lithgow G.J., Alavez S. Curcumin and neurodegenerative diseases. BioFactors. 2013;39:122–132. doi: 10.1002/biof.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dinkova-Kostova A.T., Fahey J.W., Kostov R.V., Kensler T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017;69(Pt B):257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schepici G., Bramanti P., Mazzon E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:8637. doi: 10.3390/ijms21228637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Talebi M., Talebi M., Farkhondeh T., Mishra G., Ilgün S., Samarghandian S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother. Res. 2021;35:3078–3112. doi: 10.1002/ptr.7033. [DOI] [PubMed] [Google Scholar]
- 192.Afzal O., Dalhat M.H., Altamimi A.S.A., Rasool R., Alzarea S.I., Almalki W.H., Murtaza B.N., Iftikhar S., Nadeem S., Nadeem M.S., et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules. 2022;27:7604. doi: 10.3390/molecules27217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chiang M.-C., Tsai T.-Y., Wang C.-J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023;24:6328. doi: 10.3390/ijms24076328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Elumalai P., Lakshmi S. Role of Quercetin Benefits in Neurodegeneration. Adv. Neurobiol. 2016;12:229–245. doi: 10.1007/978-3-319-28383-8_12. [DOI] [PubMed] [Google Scholar]
- 195.Dinkova-Kostova A.T., Kostov R.V., Kazantsev A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang D.D. Bardoxolone Brings Nrf2-Based Therapies to Light. Antioxidants Redox Signal. 2013;19:517–518. doi: 10.1089/ars.2012.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hisamichi M., Kamijo-Ikemori A., Sugaya T., Hoshino S., Kimura K., Shibagaki Y. Role of bardoxolone methyl, a nuclear factor erythroid 2-related factor 2 activator, in aldosterone- and salt-induced renal injury. Hypertens. Res. 2018;41:8–17. doi: 10.1038/hr.2017.83. [DOI] [PubMed] [Google Scholar]
- 198.Ren Z., He H., Zuo Z., Xu Z., Wei Z., Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019;24:36. doi: 10.1186/s11658-019-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Halling J.F., Pilegaard H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020;45:927–936. doi: 10.1139/apnm-2020-0005. [DOI] [PubMed] [Google Scholar]
- 200.Tellone E., Galtieri A., Russo A., Giardina B., Ficarra S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol. Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bastianetto S., Ménard C., Quirion R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015;1852:1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 203.Kim E.N., Lim J.H., Kim M.Y., Ban T.H., Jang I.A., Yoon H.E., Park C.W., Chang Y.S., Choi B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging. 2018;10:83–99. doi: 10.18632/aging.101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wahab A., Gao K., Jia C., Zhang F., Tian G., Murtaza G., Chen J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules. 2017;22:1329. doi: 10.3390/molecules22081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sönmez Ü., Sönmez A., Erbil G., Tekmen I., Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 206.Singleton R.H., Yan H.Q., Fellows-Mayle W., Dixon C.E. Resveratrol Attenuates Behavioral Impairments and Reduces Cortical and Hippocampal Loss in a Rat Controlled Cortical Impact Model of Traumatic Brain Injury. J. Neurotrauma. 2010;27:1091–1099. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Salberg S., Yamakawa G., Christensen J., Kolb B., Mychasiuk R. Assessment of a nutritional supplement containing resveratrol, prebiotic fiber, and omega-3 fatty acids for the prevention and treatment of mild traumatic brain injury in rats. Neuroscience. 2017;365:146–157. doi: 10.1016/j.neuroscience.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 208.Cong P., Wang T., Tong C., Liu Y., Shi L., Mao S., Shi X., Jin H., Liu Y., Hou M. Resveratrol ameliorates thoracic blast exposure-induced inflammation, endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-κB signaling pathway. Injury. 2021;52:2795–2802. doi: 10.1016/j.injury.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 209.Ateş O., Çayli S., Altinoz E., Gurses I., Yucel N., Sener M., Kocak A., Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell. Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 210.Zhou J., Yang Z., Shen R., Zhong W., Zheng H., Chen Z., Tang J., Zhu J. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021;8:620683. doi: 10.3389/fmolb.2021.620683. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Jiang W., Li S., Li X. Therapeutic potential of berberine against neurodegenerative diseases. Sci. China Life Sci. 2015;58:564–569. doi: 10.1007/s11427-015-4829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Javadipour M., Rezaei M., Keshtzar E., Khodayar M.J. Metformin in contrast to berberine reversed arsenic-induced oxidative stress in mitochondria from rat pancreas probably via Sirt3-dependent pathway. J. Biochem. Mol. Toxicol. 2019;33:e22368. doi: 10.1002/jbt.22368. [DOI] [PubMed] [Google Scholar]
- 213.Rotermund C., Machetanz G., Fitzgerald J.C. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front. Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang Y., An H., Liu T., Qin C., Sesaki H., Guo S., Radovick S., Hussain M., Maheshwari A., Wondisford F.E., et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019;29:1511–1523.e5. doi: 10.1016/j.celrep.2019.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fanoudi S., Hosseini M., Alavi M.S., Boroushaki M.T., Hosseini A., Sadeghnia H.R. Everolimus, a mammalian target of rapamycin inhibitor, ameliorated streptozotocin-induced learning and memory deficits via neurochemical alterations in male rats. EXCLI J. 2018;17:999–1017. doi: 10.17179/excli2018-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shiau J.-P., Chuang Y.-T., Cheng Y.-B., Tang J.-Y., Hou M.-F., Yen C.-Y., Chang H.-W. Impacts of Oxidative Stress and PI3K/AKT/mTOR on Metabolism and the Future Direction of Investigating Fucoidan-Modulated Metabolism. Antioxidants. 2022;11:911. doi: 10.3390/antiox11050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stein D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29:1259–1272. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ikonomidou C., Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/S1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 219.Menon D.K. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 220.Maas A.I.R., Roozenbeek B., Manley G.T. Clinical Trials in Traumatic Brain Injury: Past Experience and Current Developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Margulies S., Hicks R. Combination therapies for traumatic brain injury: Prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lynch D.G., Narayan R.K., Li C. Multi-Mechanistic Approaches to the Treatment of Traumatic Brain Injury: A Review. J. Clin. Med. 2023;12:2179. doi: 10.3390/jcm12062179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Somayaji M.R., Przekwas A.J., Gupta R.K. Combination Therapy for Multi-Target Manipulation of Secondary Brain Injury Mechanisms. Curr. Neuropharmacol. 2018;16:484–504. doi: 10.2174/1570159X15666170828165711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Arun P., Ariyannur P.S., Moffett J.R., Xing G., Hamilton K., Grunberg N.E., Ives J.A., Namboodiri A.M. Metabolic Acetate Therapy for the Treatment of Traumatic Brain Injury. J. Neurotrauma. 2010;27:293–298. doi: 10.1089/neu.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Scafidi S., Racz J., Hazelton J., McKenna M.C., Fiskum G. Neuroprotection by Acetyl-L-Carnitine after Traumatic Injury to the Immature Rat Brain. Dev. Neurosci. 2010;32:480–487. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bonferoni M.C., Rassu G., Gavini E., Sorrenti M., Catenacci L., Giunchedi P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics. 2020;12:1246. doi: 10.3390/pharmaceutics12121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pandya J.D., Musyaju S., Modi H.R., Okada-Rising S.L., Bailey Z.S., Scultetus A.H., Shear D.A. Intranasal delivery of mitochondria targeted neuroprotective compounds for traumatic brain injury: Screening based on pharmacological and physiological properties. J. Transl. Med. 2024;22:167. doi: 10.1186/s12967-024-04908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Giarratana A., Reddi S., Thakker-Varia S., Alder J. Status of precision medicine approaches to traumatic brain injury. Neural Regen. Res. 2022;17:2166–2171. doi: 10.4103/1673-5374.335824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Su Y.S., Schuster J.M., Smith D.H., Stein S.C. Cost-Effectiveness of Biomarker Screening for Traumatic Brain Injury. J. Neurotrauma. 2019;36:2083–2091. doi: 10.1089/neu.2018.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rice M.W., Pandya J.D., Shear D.A. Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Front. Neurol. 2019;10:875. doi: 10.3389/fneur.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gaddam S.S., Buell T., Robertson C.S. Systemic manifestations of traumatic brain injury. Handb. Clin. Neurol. 2015;127:205–218. doi: 10.1016/B978-0-444-52892-6.00014-3. [DOI] [PubMed] [Google Scholar]
- 232.Taraskina A., Ignatyeva O., Lisovaya D., Ivanov M., Ivanova L., Golovicheva V., Baydakova G., Silachev D., Popkov V., Ivanets T., et al. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells. 2022;11:1409. doi: 10.3390/cells11091409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhu C.S., Grandhi R., Patterson T.T., Nicholson S.E. A Review of Traumatic Brain Injury and the Gut Microbiome: Insights into Novel Mechanisms of Secondary Brain Injury and Promising Targets for Neuroprotection. Brain Sci. 2018;8:113. doi: 10.3390/brainsci8060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Celorrio M., Abellanas M.A., Rhodes J., Goodwin V., Moritz J., Vadivelu S., Wang L., Rodgers R., Xiao S., Anabayan I., et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021;9:40. doi: 10.1186/s40478-021-01137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ullah H., Arbab S., Tian Y., Liu C.-Q., Chen Y., Li Q., Khan M.I.U., Hassan I.U., Li K. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023;17:1225875. doi: 10.3389/fnins.2023.1225875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dinan T.G., Cryan J.F. Microbes, Immunity, and Behavior: Psychoneuroimmunology Meets the Microbiome. Neuropsychopharmacology. 2017;42:178–192. doi: 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Naliyadhara N., Kumar A., Gangwar S.K., Devanarayanan T.N., Hegde M., Alqahtani M.S., Abbas M., Sethi G., Kunnumakkara A. Interplay of dietary antioxidants and gut microbiome in human health: What has been learnt thus far? J. Funct. Foods. 2023;100:105365. doi: 10.1016/j.jff.2022.105365. [DOI] [Google Scholar]
- 238.Riaz Rajoka M.S., Thirumdas R., Mehwish H.M., Umair M., Khurshid M., Hayat H.F., Phimolsiripol Y., Pallarés N., Martí-Quijal F.J., Barba F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants. 2021;10:1563. doi: 10.3390/antiox10101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q.-H., Sperandio M., Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang X., Qi Y., Zheng H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants. 2022;11:1212. doi: 10.3390/antiox11061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Clark A., Mach N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017;8:319. doi: 10.3389/fphys.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]