Abstract
Antimicrobial Stewardship Programs (ASP) were introduced in healthcare as a public health priority to promote appropriate prescribing of antimicrobials, to reduce adverse events related to antimicrobials, as well as to control the escalating challenges of antimicrobial resistance. To deliver aimed outcome objectives, ASPs involve multiple connected implementation process measures. A systematic review was conducted to evaluate both concepts of ASPs. Guided by PRISMA frames, published systematic reviews (SR) focusing on ASPs restricted to secondary and tertiary healthcare were evaluated over the past 10 years involving all age groups. Out of 265 identified SR studies, 63 met the inclusion criteria. The majority were conducted in Europe and North America, with limited studies from other regions. In the reviewed studies, all age groups were examined, although they were conducted mainly on adults when compared to children and infants. Both process and outcomes measures of ASPs were examined equally and simultaneously through 25 different concepts, dominated by efficacy, antimicrobial resistance, and economic impact, while information technology as well as role of pharmacy and behavioral factors were equally examined. The main broad conclusions from the review were that, across the globe, ASPs demonstrated effectiveness, proved efficacy, and confirmed efficiency, while focused evaluation advocated that developed countries should target medium- and small-sized hospitals while developing countries should continue rolling ASPs across healthcare facilities. Additionally, the future of ASPs should focus on embracing evolving information technology to bridge the gaps in knowledge, skills, and attitude, as well as to enhance appropriate decision making.
Keywords: Antimicrobial Stewardship Programs (ASP/AMSP), antimicrobial consumption, antimicrobial resistance
1. Introduction
The primary goals of public health (PH) are to improve population wellbeing and outcomes through promotion of health, prevention of diseases, and facilitation of fair access to healthcare [1]. Fundamental to the concept of PH is the provision of safe and quality care which is equitable and cost-effective [2]. To achieve such targets, efforts should be directed towards reliable mechanisms for the evaluation of healthcare program interventions [3]. Historically, aims were directed towards efficacy and outcomes; however, it has been argued that achieving the intended objectives does not always equate to delivering quality care, since, according to Linford et al., outcomes measures are “blunt instruments for judging performance” [4].To bridge that gap, quality experts recommend the provision of appropriate evaluation methods to facilitate the delivery of aimed objectives [4]. Therefore, programs evaluation is of utmost importance primarily to appraise effectiveness and desired outcomes, as well as to address challenges [5]. According to the Centers for Disease Control and Prevention (CDC) of the USA, the process is defined as: “a systematic method for collecting, analyzing, and using data to examine the effectiveness and efficiency of programs and, importantly, to contribute to continuous program improvement” [6].
Alarmed by concerns regarding inappropriate over-prescribing of antimicrobials in healthcare, which is directly linked to adverse events in patients as well as being indirectly associated with escalating antimicrobial resistance (AMR), at the turn of the 21th century, Western healthcare authorities across the Atlantic introduced the concept of Antimicrobial Stewardship Programs (ASPs) following a justifiable appeal from experts in the field [7,8]. According to the CDC, at inpatient hospital settings, more than 50% of prescribed antimicrobials are not consistent with the recommended practice, while the majority of common infections are over-treated [6]. Comparably, the National Institute for Healthcare and Excellence (NICE) in the UK defines the process as: “the organizational or healthcare-system-wide approach to promote and monitor the judicious use of antimicrobials to preserve their future effectiveness” [9]. The program entails the collaboration of a wide range of healthcare professionals led by infection and antimicrobial specialists with the prime aim to oversee appropriate and optimal prescribing of antimicrobials through the provision of guidance and awareness, in addition to monitoring of outcomes [10].
Almost twenty-five years following their implementation, ASPs became widely accepted and subsequently embraced at national and international levels. They were eventually promoted by the WHO to the point of being hailed as one of the major 21st century public health interventions [11,12,13]. Nevertheless, because of differences in healthcare settings and population diversity, there have been uncertainties regarding conclusive and generalizable evidence to answer raised questions about topics such as efficacy and outcomes [13,14]. For example, there are implementation challenges because of local epidemiology or healthcare settings [15,16], conflicting differences for optimal program elements [14,17], the key role of antimicrobial pharmacists [18], the paucity of reporting of microbiological outcomes [19], and challenges in surveillance processes [14]. Similarly, program outcomes such as targeted mortality has been conflicting with reports citing lower rates, while others show no differences [20,21]. Conversely, despite multiple studies concluding that ASPs can reduce AMR [22,23,24], others were not conclusive [20,25]. Equally, the economic impact of the program is confounded by difficulties in adopting consensus models [11,26]. Lastly and importantly, although the program has major components, some studies have looked at the additional value of information technology (IT), reinforcing the benefits of auxiliary concepts, such as the use of smart applications, that merit further evaluation [27,28].
For all these reasons, it is prudent to have an umbrella overview to evaluate ASPs’ process measures, impacts, and outcomes, as well as assess any specific service improvement concepts. Distinctively, although other systematic reviews, meta-analyses, and overviews have examined different concepts of ASPs repeatedly, they only focused on specific aspects of the program rather than overall comprehensive evaluation [25,29,30].
2. Methods and Search Strategies
A systematic literature review was conducted, guided by the framework of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [31]. We included only peer-reviewed primary systematic reviews (SRs) in ASPs that reported any implementation process, impact, or outcome measures specifically at inpatient hospital settings encompassing secondary and tertiary healthcare settings, with no age restrictions. Five major databases were searched: OVID-Medline, PubMed, Embase, Cochrane, and Google Scholar. The search was conducted and updated up to 28 August 2022 and was restricted to the last 10 years, focusing on humans with no language restrictions. Studies that were conducted at primary, ambulatory, or long-term facilities were excluded, together with studies that focused on singular countries or pathogens. Similarly, studies with projected titles and methods focused mainly on reviews or scoping reviews with non-congruent systematic methodology were excluded. Since the COVID-19 pandemic is still unfolding, related data on the subjects were excluded. Guided by inclusion and exclusion criteria, two separate primary investigators screened titles, abstracts, and methods and agreed on a final output when selected studies were read in depth, including ranking for critical appraisal.
3. Search Outcomes
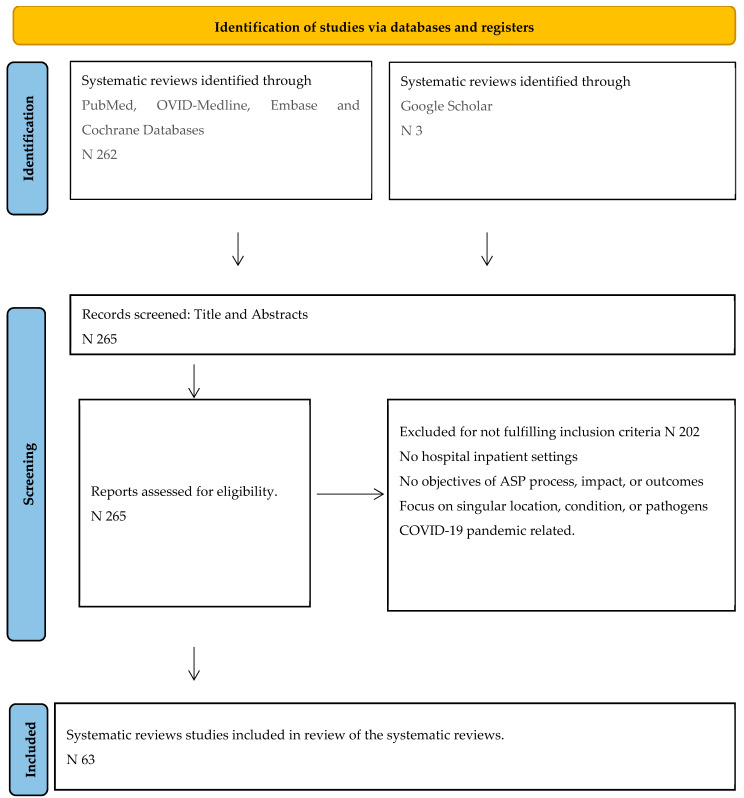
The search outcome is depicted as recommended in PRISMA Figure 1, and details of search protocols are provided in Supplementary Material. Out of 265 identified SRs, 202 studies were excluded, leaving the remaining 63 studies for final analysis.
Figure 1.
PRISMA flow diagram showing search steps of identification, screening, and final inclusion.
4. Synthesis of Evidence
Since studies were heterogenous in their concepts and methods, examining wide aspects of the program, thematic categorization of studies was performed to produce subclassifications according to the published reporting period, the origin of affiliated institutions, the global or regional focus of the studies, the studied population, and, more importantly, objective concepts. The six recognized WHO geographical criteria were used to group regional countries accordingly [32]. Since the concepts of effectiveness (adopting correct program approaches), efficacy (obtaining aimed outcomes), and efficiency (obtaining outcomes through cost effectiveness) are at the heart of healthcare, they were adopted in categorizing the concepts of ASPs [33]. Likewise, elements of process measures (implementation facets such as the role of antimicrobials guidelines) and elements of outcome measures such as antimicrobial consumption metrics were similarly examined. Additionally, historic concepts of ASPs such as closely linked AMR and quality indicators such as safety outcomes, represented by reducing adverse events, were similarly adopted, as well as other program aspects such as behavioral interventions, microbiological outcomes, and the roles of pharmacy and IT (Table 1 and Table 2). From each examined theme, specific conclusions were extracted together with relevant recommendations.
5. Critical Appraisal for Quality of Evidence
Critical appraisal of the examined SRs was conducted by two investigators using the updated online version of A Measurement Tool to Assess Systematic Reviews (AMSTAR-2) [34]. Studies were ranked from high to critically low depending on the overall non-numerical assessment. It must be emphasized that, since the concepts of ASPs are complex, with clear heterogeneity that is frequently not tested through desired evidence-based practice, including randomized trials or meta-analysis with no rigorous safeguarding for biases, it was not surprising that the stringent AMSTAR-2 critical appraisal criteria rated most SRs as low-quality, with few exceptions Table 1 [20,35].
Table 1.
Systematic review studies classified according to first author, year of publication, publishing institution according to the WHO regional classification, number of evaluated studies, primary studied concepts, examined process and outcome measures as well as critical appraisal of quality of evidence.
| Regions. | Systematic Reviews/Year | Approach | Primary Concept | Studies | Process Measures | Outcome Measures | * AMSTAR-2 Quality | Ref. |
|---|---|---|---|---|---|---|---|---|
| Europe (EUR) | Baur 2017 | Global | Adverse Events | 32 | No | Yes | CL | [36] |
| Chatzopoulou 2020 | Global | Antimicrobial Resistance | 15 | Yes | Yes | CL | [37] | |
| Chatzopoulou 2022 | Global | Antimicrobial Resistance | 29 | Yes | Yes | CL | [38] | |
| Corafa 2022 | Global | Critical Care | 13 | Yes | Yes | CL | [39] | |
| Davey 2013 | Global | Effectiveness | 89 | Yes | Yes | L | [20] | |
| Davey 2015 | Global | Behavior | 116 | Yes | No | CL | [40] | |
| Davey 2017 | Global | Safety and efficacy | 221 | Yes | Yes | H | [20] | |
| Donà 2020 | Global | Efficacy | 113 | No | Yes | CL | [41] | |
| Dik 2015 | Global | Economic impact | 95 | Yes | No | CL | [26] | |
| Helou 2020 | Global | Information Technology | 13 | No | Yes | CL | [28] | |
| Huebner 2019 | Global | Economic Impact | 16 | Yes | No | CL | [42] | |
| Kallen 2017 | Global | Quality Indicators | 14 | No | Yes | CL | [43] | |
| Lau 2022 | Global | Microbiological outcomes | 117 | Yes | Yes | CL | [19] | |
| Mas-Morey 2018 | Global | Role of Pharmacy | 28 | Yes | No | CL | [44] | |
| Micallef 2017 | Global | Information Technology | 14 | Yes | Yes | CL | [45] | |
| Monmaturapoj 2021 | Global | Role of Pharmacy | 52 | Yes | Yes | CL | [46] | |
| Monnier 2018 | Global | Quality Indicators | 70 | No | Yes | CL | [47] | |
| Nathwani 2019 | Global | Economic outcomes | 164 | Yes | Yes | CL | [48] | |
| O’Riordan 2021 | Regional | Quality Indicators | 16 | Yes | Yes | CL | [49] | |
| Porter 2021 | Global | Behavioral Factors | 14 | Yes | No | CL | [50] | |
| Pouly 2022 | Global | Behavioral Factors | 124 | Yes | Yes | CL | [51] | |
| Rajar 2021 | Global | Safety | 12 | Yes | No | H | [35] | |
| Rawson 2017 | Global | Information Technology | 58 | Yes | No | CL | [52] | |
| Rzewuska 2020 | Global | Implementation | 145 | No | Yes | CL | [53] | |
| Schuts 2016 | Global | Efficacy | 15 | Yes | Yes | CL | [54] | |
| Schuts 2021 | Global | Antimicrobial Resistance | 145 | Yes | No | L | [25] | |
| Schweitzer 2019 | Global | Quality of studies | 825 | Yes | No | CL | [55] | |
| Stanic 2018 | Global | Metrics | 168 | Yes | Yes | CL | [56] | |
| Tacconelli 2016 | Global | Surveillance | 78 | Yes | Yes | CL | [14] | |
| Van Dijck 2018 | Global | MLIC * | 27 | Yes | No | CL | [57] | |
| Warreman 2019 | Global | Behavioral Factors | 9 | Yes | No | CL | [58] | |
| Americas (AMR) | Araujo Silva 2018 | Global | Effectiveness | 9 | Yes | Yes | L | [59] |
| Bertollo 2018 | Global | Antimicrobial Resistance | 26 | No | Yes | CL | [60] | |
| Daniels 2021 | Global | Discharge medications. | 6 | Yes | No | CL | [61] | |
| Feazel 2014 | Global | Adverse Events | 78 | No | Yes | L | [62] | |
| Karanika 2016 | Global | Economic Impact | 26 | No | Yes | CL | [29] | |
| Kooda 2022 | Global | Role of Pharmacy | 24 | Yes | Yes | L | [18] | |
| Lindsay 2019 | Global | Critical care | 11 | No | Yes | CL | [21] | |
| Losier 2017 | Global | Emergency Department | 43 | Yes | No | CL | [17] | |
| Murray 2021 | Global | Antimicrobial Resistance | 29 | No | Yes | CL | [63] | |
| Rennert-May 2017 | Global | Guidelines | 5 | Yes | No | CL | [64] | |
| Rittmann 2019 | Global | Information Technology | 45 | Yes | Yes | CL | [65] | |
| Smith 2015 | Global | Efficacy | 9 | No | Yes | CL | [66] | |
| Wade 2021 | Regional | HCAIs ** | 21 | Yes | Yes | CL | [67] | |
| Wagner 2014 | Regional | Efficacy | 37 | Yes | Yes | CL | [68] | |
| West Pacific Region (WPR) | Abo 2020 | Global | Efficacy | 34 | Yes | Yes | L | [69] |
| Baysari 2016 | Global | Information Technology | 45 | Yes | No | CL | [27] | |
| Honda 2017 | Regional | Safety and efficacy | 46 | No | Yes | CL | [16] | |
| Lee 2018 | Regional | Safety and efficacy | 77 | No | Yes | CL | [70] | |
| Lim 2020 | Global | National Interventions | 34 | Yes | No | CL | [71] | |
| Roman 2018 | Global | Role of Pharmacy | 15 | Yes | No | CL | [72] | |
| Siachalinga 2022 | Global | Efficacy | 28 | Yes | No | L | [73] | |
| Tabah 2016 | Regional | Critical Care | 14 | Yes | Yes | CL | [74] | |
| East Mediterranean (EMRO) | Ababneh 2021 | Regional | Implementation | 20 | Yes | Yes | CL | [15] |
| Atamna 2021 | Global | Antimicrobial Resistance | 63 | Yes | Yes | CL | [75] | |
| Bitterman 2016 | Global | Antimicrobial consumption | 80 | Yes | No | CL | [76] | |
| Garwan 2022 | Global | Antimicrobial Switch | 36 | Yes | Yes | CL | [77] | |
| Hashad 2020 | Regional | Effectiveness | 17 | Yes | No | CL | [78] | |
| Keikha 22 | Global | Antimicrobial Resistance | 17 | No | Yes | L | [79] | |
| Nasr 2017 | Regional | Behavioral factors | 20 | Yes | No | CL | [80] | |
| Southeast Asia (SEAR) | Ibrahim 2017 | Global | Economic Impact | 5 | No | Yes | CL | [81] |
| Teerawattanapong 2017 | Global | Antimicrobial Resistance | 42 | Yes | Yes | CL | [82] | |
| Africa (AF) | Akpan 2020 | Regional | Implementation | 13 | Yes | Yes | CL | [83] |
* AMSTAR-2: Measurement Tool to Assess Systematic Reviews, evaluation tool to review the methodological quality of systematic reviews: H; high-quality review, M; moderate, L; low, CL; critically low. * MLIC: middle- and low-income countries. ** HCAIs: healthcare-associated infections.
6. Results
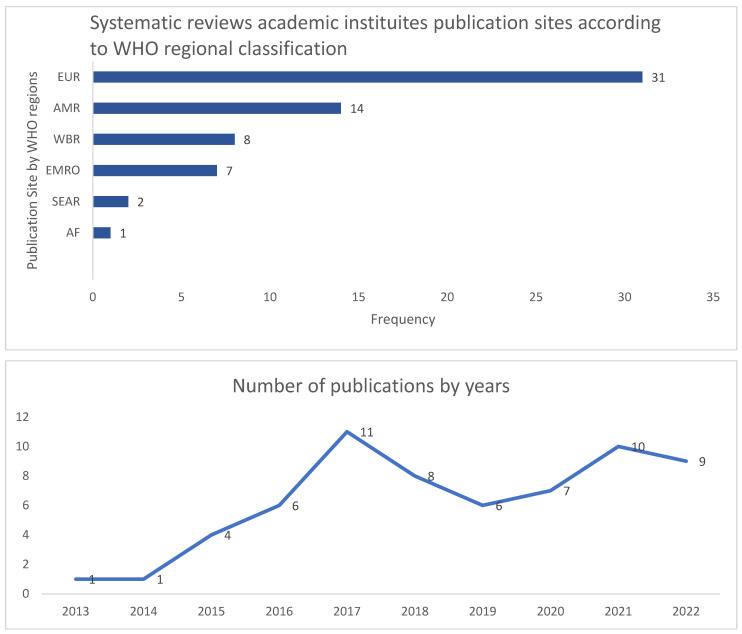
Out of 63 selected systematic reviews, the majority were conducted in the European region (EUR: 31) and regions of the Americas (AMR: 14) at a total of 71% (46/63), while the remaining regions constituted 29% (18/63), detailed as: Western Pacific region (WPR:8), Eastern Mediterranean Region (EMRO: 7), Southeast Asian Region (SEAR: 2), and African Region (AF:1), as outlined in Figure 2. Most of the SRs (84%) focused on global objectives, while only 16% reported regional data. Predominant SRs (51/63) included all age groups, while adults were specified in 6, children in 5, and infants in only 1 study. The ASPs were evaluated through 25 different concepts, dominated by efficacy and safety (9) and AMR as outcomes (8), while economic impact was evaluated in (5) studies, the role of information technology in (5), as well as the role of pharmacy (4) and behavioral factors (4), as outlined in Table 2. Out of the 63 SRs, 22 (35%) solely examined the implementation process, while 17 (27%) focused on outcome measures, and the two concepts were examined in 38%.
Table 2.
Primary examined concepts for the systematic reviews.
| Primary Focus | Frequency | Percentage |
|---|---|---|
| Antimicrobial Resistance | 8 | 12.7 |
| Efficacy | 6 | 9.52 |
| Behaviour | 5 | 7.94 |
| Information technology | 5 | 7.94 |
| Economic impact | 4 | 6.35 |
| Quality | 4 | 6.35 |
| Role of Pharmacy | 4 | 6.35 |
| Critical Care | 3 | 4.76 |
| Effectiveness | 3 | 4.76 |
| Efficacy and Safety | 3 | 4.76 |
| Implementation | 3 | 4.76 |
| Adverse Events | 2 | 3.17 |
| Antimicrobial Switch | 1 | 1.59 |
| Antimicrobial consumption | 1 | 1.59 |
| Clinical and economic outcomes | 1 | 1.59 |
| Discharge medications | 1 | 1.59 |
| Emergency department | 1 | 1.59 |
| Guidelines | 1 | 1.59 |
| Healthcare Associated infections | 1 | 1.59 |
| Metrics | 1 | 1.59 |
| Microbiological outcomes | 1 | 1.59 |
| Middle- and low-income countries | 1 | 1.59 |
| National interventions | 1 | 1.59 |
| Safety | 1 | 1.59 |
| Surveillance | 1 | 1.59 |
| Total | 63 | 100% |
7. Overview of Evaluation Process
Examining the spectrum of global studies, most SRs focused on Western populations, with a paucity of data from developing countries (Figure 2). In their large and highly rated SR focusing on efficacy and safety, the study of Davey et al. encompassed 221 articles, including 58 randomized controlled trials and 163 studies of other kinds: Most of the studies were from North America (96) and Europe (87), while the remaining were from Asia (19), South America (8), Australia (8), and East Asia (3) [20] (Table 1 and Figure 2).
Figure 2.
Systematic review publication sites according to the WHO regional classifications and cumulative records of publication year. EUR: European region, AMR: Region of Americas, WPR: Western Pacific Region, EMRO: Eastern Mediterranean Region, SEAR: Southeast Asian Region, and AF: African Region.
8. Studied Population
The ASP systematic reviews identified the studied populations as adults, children, and infants mainly from high income Western countries [20,41,66]. Most of the data from the SRs were extracted from adult populations, while data from the pediatric cohorts were scarce, especially regarding the implementation measures (Table 2) [19,66]. Despite the limited available data from the pediatric population, the ASPs managed to implement objectives without compromising safety, even at pediatric intensive care units (PICUs), with limited data regarding healthcare-associated infections and antimicrobial resistance [66].
9. Healthcare Settings
Although the majority of secondary and tertiary care facilities in developed countries are small- and medium-sized hospitals (200–500 beds), the majority of data were extracted from studies conducted at large-sized hospitals (>500 beds), where antimicrobial consumption might be similar, but the implementation barriers are more pronounced [11,84]. Conversely, despite the highlighted benefits of the program in different regions across the globe, there are equally outlined implementation and outcome challenges in developing regions, leading to strong demands to strengthen fundamental aspects of the program [12,15,16,83]. Therefore, to improve global delivery of ASP objectives, healthcare services in developed countries should focus their attention towards small- and medium-sized hospitals, while developing countries should cement ASPs’ core elements.
10. Process Implementation Interventions
The main examined interventions during program implementation include empirical therapy according to guidelines, timely de-escalation therapy, switching from intravenous to oral, therapeutic drug monitoring, and preauthorization through restricted antimicrobial lists. From multiple SRs, there is evidence of successful implementation of these interventions aligned with outcomes [16,20,25,29]. When comparing enablement objectives with or without ASP interventions, there were no observed mortality differences [20]. Efficacy without compromising safety has been similarly observed in vulnerable populations at critical care units, in both adult and pediatric settings, through effective program interventions such as prescribing audits, feedback, education, and persuasion [20,21,39].
11. Epidemiology and Surveillance
Although the importance of surveillance and epidemiological reports in guiding ASPs has been clearly overstated, from multiple SRs, challenges are evident in both developed and developing countries with variable representation [14,15,16]. Moreover, although ASPs have been implemented with anticipations to curb AMR as an outcome, SR studies have described challenges in accurate reporting in healthcare affecting accuracy [25,60]. Additionally, there is a paucity of studies that report accurate microbiological results linked to ASPs, which is the hallmark for monitoring AMR [19]. To bridge such gaps, it is advocated to conduct proper AMR surveillance methods in all healthcare facilities that should guide the ASP implementation process, and not vice versa [14,85].
12. Efficacy and Safety
Since the program’s concepts are based upon restricting antimicrobials as well as reducing the duration of therapy, there have been some concerns that it might impact patients’ safety. From multiple SRs, ASPs have proven to be efficacious in reducing antimicrobial consumption and inappropriate prescribing without compromising patients’ safety, for both adult and pediatric populations [20,41,48,59]. The feared prime concerns were understandably directed towards critical care, where the highest levels of hospital antimicrobial consumption are usually reported, combined with the critical nature of the patients’ cohort. Nevertheless, SRs in such important aspects affirmed safety and efficacy, although the evidence is more pronounced for adult when compared to pediatric populations because of limited data [21,39,59,74]. However, the picture is not fully clear, since there is a counterargument that restricting antimicrobials at critical care settings might be protective through limiting unfavorable adverse events or might be confounded by the plausible practice of reducing interventions in less critical patients [74].
13. Influencing Prescribing Behavior
The foremost definition of ASPs accentuates “appropriate and judicious prescribing of antimicrobials”, which entails focusing efforts to influence the behaviors and attitudes concerning antimicrobial prescribing directed towards healthcare professionals [20]. Determinant factors imported from behavioral studies, such as psychosocial theory of planned behavior, affect how developed cognition influences decision making and behavior [58,86]. In ASPs, the main positive attitude determinants that influence prescribing behavior have been identified as education and training, as well as audit and feedback aimed to alter conceived prescribing cultures [20]. Despite the conceivable association between the influence of cognitive behavioral factors and previous experience on prescribing cultures, some SRs have pointed towards gaps in targeting outlined attitudes, particularly the lack of qualitative studies, to address raised barriers. For such reasons, exploring prescribing attitudes and practices, particularly behavior change techniques, is encouraged in both developed and developing countries [20,50,58].
14. Quantifying Metrics
Appropriate quantifying metrics (QM) must be adopted to closely monitor consumption as well as to estimate associated costs. As the concept of ASPs was introduced without defining relevant QMs, subsequent mounting challenges were to evaluate existing ones as well as trying to develop new methods [87]. Amongst the existing QMs were the Defined Daily Dose (DDD), which was developed in the late 1970s and standardized through patients’ total hospital days, depicted per patient days (DDD-100 or 1000 patients days) [76]. In their SR regarding QMs, Stanic Benic et al. identified 12 common measurements that include the commonly used DDDs, Days of Therapy (DOT), as well as the total antimicrobial cost. The recommended practice is a combined metric approach using at least two of the prime measures [56]. Although there have been many cited limitations for using QMs such as DDDs because of variations in relation to geographical locations or hospital settings, the role of weight adjustments, and adjusted doses in renal impairment as well as combined therapy, it has been widely adopted across the globe in the adult population, as well as being recommended by the WHO [88]. Because of multiple factors dominated by patients standard weight, in the pediatric population, metric challenges have been identified with no optimal recommended measures, although DOT is generally preferred when compared to DDDs [89].
15. Role of Infection Prevention and Control
Although not examined as a primary specific concept of the program in recent SRs, mutiple studies have covered the alliance of Infection Prevention and Control (IPC) programs with the parallel and closely related ASPs, frequently featured as influencing both the process and outcome measures of each other [90]. Since the main objective of IPCPs is to limit the spread of infection across healthcare facilities, including communicable diseases as well as multidrug-resistant organims (MDROs), it is not surprising that failing in that domain will ultimately affect ASPs through subsequent secondary increases in antimicrobial prescribing and, hence, consumption. Likewise, the successful implementation of ASPs can directly facilitate the aims and objectives of IPCPs. These plausible observations of utmost synergy are supported by multiple infection societies and organizations that promote both concepts [91]. In their SR study, Buar et al. demonstrated that ASPs can reduce Clostridium difficile infections (CDIs) by almost 50%, thus positively impacting on the delivery of IPCPs [36]. Furthermore, one of the main outcome measures of ASPs is reducing rates of AMR consequences such as the acquisition of secondary MDROs infections [79,92]. Hence, poor implementation of IPC measures can directly lead to the ultimate failure of ASP outcomes. Correspondingly, in their recommendations to optimize the reporting of epidemiological studies for antimicrobial resistance to improve the implementation of ASPs, Tacconelli et al. advocate for the optimal implementation of IPC measures to limit the spread of MDROs across healthcare facilities [14]. Similarly, in their high-ranking SR, Davey et al. identified IPCPs as one of the fundamental interventions for the successful implementation of ASPs [20]. Furthermore, the cornerstone role of IPC in ASP has also been similarly oulined for the pediatric population [59]. Although the interface of ASPs and IPCPs is closely related with difficult-to-measure outcomes, we advocate the expansion of research on this vital aspect to guide effective interventions.
16. Role of Pharmacy
Few SRs have examined the roles of antimicrobial pharmacists during the implementation of ASPs, demonstrating effective interventions in the form of education-based interventions, compliance with guidelines, and reductions in the duration of antimicrobial therapy, calling for further empowerment [46]. Similarly, the role of pharmacists has been emphasized in the emergency department as well as in small- and medium-sized hospitals, where pharmacists play a major role in ensuring appropriate prescribing and guarding against misuse [18,44]. Uniquely, Daniels and Weber looked at the role of hospital-based pharmacists in verifying hospital discharge antimicrobials, highlighting positive outcomes in validating antibiotic choice, duration, frequency, and directed therapy in line with ASP objectives [61]. While reviewing the role of pharmacy in ASPs, it must be emphasized that the recommendations from the WHO since 2017 have been to safeguard against the development of AMR and help to encourage the successful implementation of ASPs by adopting specific prioritization of antimicrobials. The concept advocates for classifying antimicrobials into three categories: essentials, with lower potential barriers for AMRs (Access); critically important, with potential impacts on resistance (Watch); and last-resort antimicrobials to combat the challenges of MDROs (Reserve). These are combined in the acronym AWaRe [93]. Although the benefits of the recommended classification have been advocated globally, the need for raising awareness about the concept has been emphasized more for developing countries, particularly when facing antimicrobial cost constraints [94].
17. Evaluation of Outcome Measures
17.1. Interface of ASP and Its Impact on Antimicrobial Resistance
The global and secondary care burden of antimicrobial resistance on morbidity, mortality, and cost of management has been previously outlined in major studies [95,96]. Despite the plausible link between consumption of antimicrobials and the development of AMR, the causal relationship has proved to be difficult to establish. In their SR encompassing 32 studies and almost 9 million reviewed reported cases, Baur et al. reported that ASPs reduce hospital infections and colonization with MDROs and CDI by a factor of almost 50%. A similar outcome of reduction in infections caused by MDROs was shared by Karanika et al. who added that reduced antimicrobial consumption was not associated with observed adverse outcomes [29,36]. This view is contradicted by Tacconelli et al., who highlighted flaws with epidemiological reports, while Chatzopoulou et al. pointed that the plausible projected hypothesis is supported by poor evidence, in addition to Bertollo et al. citing the paucity of randomized controlled trials and reliance on observational and quasi-experimental studies [14,37,60]. The lack of conclusive evidence to support direct correlation has been affirmed by a wider meta-analysis conducted by Schuts et al. [25].
When trying to delve into potential explanations for the lack of supporting evidence despite the coherent assumption, there are major confounding factors which are impossible to control. For example, potential factors that might directly affect the propagation of AMR include the local environment and various healthcare settings and the demographics of the affected populations, including susceptibilities to infections, either from acquired or genetic factors or colonization with resistant strains; previous exposure to antimicrobials; dominance of certain resistant clones; and access to critical care, including the use of invasive devices as well as local practices of infection control and prevention [14,97,98]. Hence, to bridge the obvious gap between the implementation of ASPs and the reduction in AMRs in healthcare settings, it is conceivable to advocate for reliable and functional epidemiological reporting and surveillance systems, which must be continuously improved and developed [14].
17.2. Economic Impact and Cost Effectiveness
Although it was not declared amongst the main goals of ASPs at its inception, it was soon realized that there are observed secondary benefits based upon economic savings [8]. From reciprocated SRs, the economic benefit of the ASPs is evident mainly through three main aspects: direct saving from restricting more expensive broad-spectrum antimicrobials, reduction in the duration of therapy, and reducing patients’ length of hospital stay [11,26,29,42]. Nevertheless, there were conclusive remarks that there are non-uniform agreements for calculating economic parameters, the quality of extracted data, and the reported heterogeneity of studies in the pediatric population [55,66,81].
17.3. Quality Assessment and Development of ASPs
In healthcare, quality has been defined as “the systemic process to improve healthcare delivery”, which is integral to the provision of continuously developed ASPs [99]. In their SR, Schweitzer et al. concluded that the overall studies in ASPs are of low quality and have not improved over time, and additionally, they expressed concerns regarding the lack of microbiological and clinical data as an outcome, calling to improve studies’ methodologies [55]. To converge towards a consensus on quality indicators for ASPs, about 50 QIs have been outlined to serve towards future standardized measures [47]. Challenges regarding quality in reporting and surveillance have been outlined, calling for standardized methods in both developed and developing countries [14,16].
18. Role of Modern Information Technology
Since the ASPs heavily rely on updated knowledge, accurate prescribing, and applied and retrieved data, it is plausible to explore the role of information technology (IT) towards improvements in the quality of the program [100]. The concept has been explored by Baysari et al., who identified computerized decision support system (CDSSs), computerized antimicrobial approval systems, and surveillance methods, concluding that there are positive outcomes confounded by the absence of comparative studies [27]. Although the modern role of CDSSs has become more evident in ASPs, Rawson et al. expressed concerns that it was not designed properly to guide antimicrobial prescribers, calling for better models [52]. Furthermore, Micallef et al. advocated for the secondary use of information technology and hospital electronic systems to support the delivery of effective ASPs [45]. Lastly, although not supported by much updated evidence, specific ASP smartphone applications to improve knowledge, skills, and attitudes towards antimicrobial prescribing have been demonstrated as a promising platform that is worth exploring [28].
19. Limitations
One of the fundamental limitations of the evaluation of the SRs regarding ASPs is that the prime concept is relatively new in different parts of the world, with less than 25 years of experience with continuously evolving standards. From the review, most of the data have been obtained from established Western healthcare facilities, with limited comparators from global developing countries. Additionally, from multiple SRs, extracted evidence has mainly been obtained from observational studies rather than the gold standards of randomized controlled or interventional trials with no comparators, dwarfing generated evidence and conclusions. Even upon review of studies examining shared concepts such as AMR or economic burden, there is clearly highlighted heterogeneity in the studies, objectives, and methodology, and no consensus in agreed-upon measurements, making combining evidence synthesis a daunting task. These limitations are undoubtedly reflected in the quality of evidence extracted from SRs examined through the AMSTAR critical appraisal tool, which demonstrated uniformly low-quality standards. Furthermore, in reviewing the concept of ASPs, there are multiple co-dependent confounding factors that can affect implantations and outcome processes. For example, facilities related to infection prevention and control program practices can directly impact both implantation processes, such as increased antimicrobial consumption, and outcomes by spreading AMR. Additionally, local population characteristics, healthcare settings, resources, and facilities, as well as professional practice culture, have direct effects on ASPs.
20. Summary and Conclusions
This review of the SRs evaluating the implementation processes, impacts, and outcomes of ASPs has delineated that the concepts are strongly cemented in Western healthcare facilities but are evolving in developing countries. The studied population mainly consists of adults from Western countries, with limited data from children and infants. The process of implementation of effectiveness consists of multiple connected concepts ranging from guidelines and restricted antimicrobials to the roles of IT and pharmacists to influence prescribers’ behaviors. For safety and efficacy, objective interventions were met in all evaluating studies including vulnerable populations such as children, as well as patients under critical care. As for efficiency, evaluation of the economic impact of ASPs as a secondary outcome demonstrated proven benefits, mainly through reducing the expenditure of antimicrobial therapy as well as reducing the length of patients’ hospital stays. Although one of the fundamental aims of the ASPs is to reduce the mounting scale of AMR, the evaluated evidence does not conclusively support the objective opening of the gates for conducting further research in this field with better-designed studies. Similarly, there is a lack of consensus regarding quality indicators that set the objectives for the program standards for future evaluation. Correspondingly, with the advances in terms of reliance on IT in healthcare, there are promising, but not sufficiently explored, opportunities to expand that aspect for the better delivery of ASPs’ aimed objectives.
Acknowledgments
We would like to acknowledge the support of Qatar National Library (QNL) towards the support of the academic publication.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13030253/s1, Supplement S1: Outlines of the review protocol.
Author Contributions
H.A.H. conceived the systematic review ideas and developed the search protocol, as well as drafted the initial manuscript. H.A.H., S.A.B. and F.E. examined and reviewed search outcomes, including critical appraisal to assess the quality of included studies. J.D. performed statistical analysis. F.A. and C.M. provide the needed supervision for the overall project. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study utilized available online resources using no identifiable patients records so no patients consent, or IRB approval was needed.
Data Availability Statement
All utilized data are available online from academic search engines.
Conflicts of Interest
The HMC team consisted of an ID physician (Hamad Abdel Hadi), a microbiologist (Faiha Eltayeb), and two biostatisticians/epidemiologists (Sara Al Balushi and Joanne Daghfal) who were part of the review process, including critical appraisal. There are no conflicts of interest from all authors in relation to the project as declared.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bloland P., Simone P., Burkholder B., Slutsker L., De Cock K.M. The role of public health institutions in global health system strengthening efforts: The US CDC’s perspective. PLoS Med. 2012;9:e1001199. doi: 10.1371/journal.pmed.1001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayberry R.M., Nicewander D.A., Qin H., Ballard D.J. Improving quality and reducing inequities: A challenge in achieving best care. Proceedings (Bayl. Univ. Med. Cent.) 2006;19:103–118. doi: 10.1080/08998280.2006.11928138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacouture A., Breton E., Guichard A., Ridde V. The concept of mechanism from a realist approach: A scoping review to facilitate its operationalization in public health program evaluation. Implement. Sci. 2015;10:153. doi: 10.1186/s13012-015-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilford R.J., Brown C.A., Nicholl J. Use of process measures to monitor the quality of clinical practice. BMJ. 2007;335:648–650. doi: 10.1136/bmj.39317.641296.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon-Woods M., McNicol S., Martin G. Ten challenges in improving quality in healthcare: Lessons from the Health Foundation’s programme evaluations and relevant literature. BMJ Qual. Saf. 2012;21:876–884. doi: 10.1136/bmjqs-2011-000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC Program Evaluation 2022. [(accessed on 20 February 2024)];2022 Available online: https://www.cdc.gov/evaluation/index.htm.
- 7.Dyar O.J., Huttner B., Schouten J., Pulcini C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017;23:793–798. doi: 10.1016/j.cmi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 8.McGowan J.E., Gerding D.N. Does antibiotic restriction prevent resistance? New Horiz. 1996;4:370–376. [PubMed] [Google Scholar]
- 9.NICE Guidelines: Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use. 2015. [(accessed on 20 February 2024)]. Available online: https://www.nice.org.uk/guidance/ng15/resources/antimicrobial-stewardship-systems-and-processes-for-effective-antimicrobial-medicine-use-pdf-1837273110469.
- 10.Mendelson M., Morris A.M., Thursky K., Pulcini C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020;26:447–453. doi: 10.1016/j.cmi.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control. 2019;8:35. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce J., Apisarnthanarak A., Schellack N., Cornistein W., Maani A.A., Adnan S., Stevens M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int. J. Infect. Dis. 2020;96:621–629. doi: 10.1016/j.ijid.2020.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kraker M.E.A., Abbas M., Huttner B., Harbarth S. Good epidemiological practice: A narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin. Microbiol. Infect. 2017;23:819–825. doi: 10.1016/j.cmi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Tacconelli E., Cataldo M.A., Paul M., Leibovici L., Kluytmans J., Schröder W., Foschi F., De Angelis G., De Waure C., Cadeddu C., et al. STROBE-AMS: Recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open. 2016;6:e010134. doi: 10.1136/bmjopen-2015-010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ababneh M.A., Nasser S.A., Rababa’h A.M. A systematic review of Antimicrobial Stewardship Program implementation in Middle Eastern countries. Int. J. Infect. Dis. 2021;105:746–752. doi: 10.1016/j.ijid.2021.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Honda H., Ohmagari N., Tokuda Y., Mattar C., Warren D.K. Antimicrobial Stewardship in Inpatient Settings in the Asia Pacific Region: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017;64((Suppl. 2)):S119–S126. doi: 10.1093/cid/cix017. [DOI] [PubMed] [Google Scholar]
- 17.Losier M., Ramsey T.D., Wilby K.J., Black E.K. A Systematic Review of Antimicrobial Stewardship Interventions in the Emergency Department. Ann. Pharmacother. 2017;51:774–790. doi: 10.1177/1060028017709820. [DOI] [PubMed] [Google Scholar]
- 18.Kooda K., Canterbury E., Bellolio F. Impact of Pharmacist-Led Antimicrobial Stewardship on Appropriate Antibiotic Prescribing in the Emergency Department: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2022;79:374–387. doi: 10.1016/j.annemergmed.2021.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Lau T.M.M., Daniel R., Hughes K., Wootton M., Hood K., Gillespie D. A systematic review investigating the use of microbiology outcome measures in randomized controlled trials evaluating antimicrobial stewardship interventions published between 2011 and 2021. JAC Antimicrob. Resist. 2022;4:dlac013. doi: 10.1093/jacamr/dlac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey P., Brown E., Charani E., Fenelon L., Gould I.M., Holmes A., Ramsay C.R., Wiffen P.J., Wilcox M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2013:Cd003543. doi: 10.1002/14651858.CD003543.pub4. Erratum in Cochrane Database Syst. Rev. 2017, 2, Cd003543. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay P.J., Rohailla S., Taggart L.R., Lightfoot D., Havey T., Daneman N., Lowe C., Muller M.P. Antimicrobial Stewardship and Intensive Care Unit Mortality: A Systematic Review. Clin. Infect. Dis. 2019;68:748–756. doi: 10.1093/cid/ciy550. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara D., Sato K., Miyazaki M., Kamada M., Moriwaki N., Nakano T., Shiotsuka S., Tokushige C., Toh H., Kamimura H., et al. The impact of earlier intervention by an antimicrobial stewardship team for specific antimicrobials in a single weekly intervention. Int. J. Infect. Dis. 2018;77:34–39. doi: 10.1016/j.ijid.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Tandan M., Thapa P., Maharjan P., Bhandari B. Impact of antimicrobial stewardship program on antimicrobial-resistance and prescribing in nursing homes: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2022;29:74–87. doi: 10.1016/j.jgar.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Yusef D., Hayajneh W.A., Bani Issa A., Haddad R., Al-Azzam S., Lattyak E.A., Lattyak W.J., Gould I., Conway B.R., Bond S., et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2021;76:516–523. doi: 10.1093/jac/dkaa464. [DOI] [PubMed] [Google Scholar]
- 25.Schuts E.C., Boyd A., Muller A.E., Mouton J.W., Prins J.M. The Effect of Antibiotic Restriction Programs on Prevalence of Antimicrobial Resistance: A Systematic Review and Meta-Analysis. Open Forum Infect Dis. 2021;8:ofab070. doi: 10.1093/ofid/ofab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dik J.W., Vemer P., Friedrich A.W., Hendrix R., Lo-Ten-Foe J.R., Sinha B., Postma M.J. Financial evaluations of antibiotic stewardship programs-a systematic review. Front. Microbiol. 2015;6:317. doi: 10.3389/fmicb.2015.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baysari M.T., Lehnbom E.C., Li L., Hargreaves A., Day R.O., Westbrook J.I. The effectiveness of information technology to improve antimicrobial prescribing in hospitals: A systematic review and meta-analysis. Int. J. Med. Inform. 2016;92:15–34. doi: 10.1016/j.ijmedinf.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Helou R.I., Foudraine D.E., Catho G., Peyravi Latif A., Verkaik N.J., Verbon A. Use of stewardship smartphone applications by physicians and prescribing of antimicrobials in hospitals: A systematic review. PLoS ONE. 2020;15:e0239751. doi: 10.1371/journal.pone.0239751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karanika S., Paudel S., Grigoras C., Kalbasi A., Mylonakis E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016;60:4840–4852. doi: 10.1128/AAC.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dort B.A., Penm J., Ritchie A., Baysari M.T. The impact of digital interventions on antimicrobial stewardship in hospitals: A qualitative synthesis of systematic reviews. J. Antimicrob. Chemother. 2022;77:1828–1837. doi: 10.1093/jac/dkac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO WHO Regions 2023. [(accessed on 20 February 2024)]; Available online: https://www.who.int/about/who-we-are/regional-offices.
- 33.Burches E. Efficacy, Effectiveness and Efficiency in the Health Care: The Need for an Agreement to Clarify its Meaning. Int. Arch. Public. Health Community Med. 2022;4:1–3. doi: 10.23937/2643-4512/1710035. [DOI] [Google Scholar]
- 34.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajar P., Saugstad O.D., Berild D., Dutta A., Greisen G., Lausten-Thomsen U., Mande S.S., Nangia S., Petersen F.C., Dahle U.R., et al. Antibiotic Stewardship in Premature Infants: A Systematic Review. Neonatology. 2020;117:673–686. doi: 10.1159/000511710. [DOI] [PubMed] [Google Scholar]
- 36.Baur D., Gladstone B.P., Burkert F., Carrara E., Foschi F., Döbele S., Tacconelli E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017;17:990–1001. doi: 10.1016/S1473-3099(17)30325-0. [DOI] [PubMed] [Google Scholar]
- 37.Chatzopoulou M., Reynolds L. Role of antimicrobial restrictions in bacterial resistance control: A systematic literature review. J. Hosp. Infect. 2020;104:125–136. doi: 10.1016/j.jhin.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Chatzopoulou M., Reynolds L. Systematic review of the effects of antimicrobial cycling on bacterial resistance rates within hospital settings. Br. J. Clin. Pharmacol. 2022;88:897–910. doi: 10.1111/bcp.15042. [DOI] [PubMed] [Google Scholar]
- 39.Chorafa E., Komatsiouli V., Iosifidis E., Kourti M., Sdougka M., Roilides E. Antimicrobial Stewardship Programs in PICU settings: A Systematic Review. Pediatr. Crit. Care Med. 2022;24:e20–e27. doi: 10.1097/PCC.0000000000003069. [DOI] [PubMed] [Google Scholar]
- 40.Davey P., Peden C., Charani E., Marwick C., Michie S. Time for action-Improving the design and reporting of behaviour change interventions for antimicrobial stewardship in hospitals: Early findings from a systematic review. Int. J. Antimicrob. Agents. 2015;45:203–212. doi: 10.1016/j.ijantimicag.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Donà D., Barbieri E., Daverio M., Lundin R., Giaquinto C., Zaoutis T., Sharland M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control. 2020;9:3. doi: 10.1186/s13756-019-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huebner C., Flessa S., Huebner N.O. The economic impact of antimicrobial stewardship programmes in hospitals: A systematic literature review. J. Hosp. Infect. 2019;102:369–376. doi: 10.1016/j.jhin.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Kallen M.C., Prins J.M. A Systematic Review of Quality Indicators for Appropriate Antibiotic Use in Hospitalized Adult Patients. Infect. Dis. Rep. 2017;9:6821. doi: 10.4081/idr.2017.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mas-Morey P., Valle M. A systematic review of inpatient antimicrobial stewardship programmes involving clinical pharmacists in small-to-medium-sized hospitals. Eur. J. Hosp. Pharm. 2018;25:e69–e73. doi: 10.1136/ejhpharm-2017-001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Micallef C., Chaudhry N.T., Holmes A.H., Hopkins S., Benn J., Franklin B.D. Secondary use of data from hospital electronic prescribing and pharmacy systems to support the quality and safety of antimicrobial use: A systematic review. J. Antimicrob. Chemother. 2017;72:1880–1885. doi: 10.1093/jac/dkx082. [DOI] [PubMed] [Google Scholar]
- 46.Monmaturapoj T., Scott J., Smith P., Abutheraa N., Watson M.C. Pharmacist-led education-based antimicrobial stewardship interventions and their effect on antimicrobial use in hospital inpatients: A systematic review and narrative synthesis. J. Hosp. Infect. 2021;115:93–116. doi: 10.1016/j.jhin.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Monnier A.A., Schouten J., Le Maréchal M., Tebano G., Pulcini C., Stanic Benic M., Vlahović-Palĉevski V., Milanič R., Adriaenssens N., Versporten A., et al. Quality indicators for responsible antibiotic use in the inpatient setting: A systematic review followed by an international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018;73((Suppl. 6)):vi30–vi39. doi: 10.1093/jac/dky116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathwani D., Sneddon J., Malcolm W., Wiuff C., Patton A., Hurding S., Eastaway A., Seaton R.A., Watson E., Gillies E., et al. Scottish Antimicrobial Prescribing Group (SAPG): Development and impact of the Scottish National Antimicrobial Stewardship Programme. Int. J. Antimicrob. Agents. 2011;38:16–26. doi: 10.1016/j.ijantimicag.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 49.O’Riordan F., Shiely F., Byrne S., Fleming A. Quality indicators for hospital antimicrobial stewardship programmes: A systematic review. J. Antimicrob. Chemother. 2021;76:1406–1419. doi: 10.1093/jac/dkab034. [DOI] [PubMed] [Google Scholar]
- 50.Porter G.J., Owens S., Breckons M. A systematic review of qualitative literature on antimicrobial stewardship in Sub-Saharan Africa. Glob. Health Res. Policy. 2021;6:31. doi: 10.1186/s41256-021-00216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pouly E., Coppry M., Rogues A.M., Dumartin C. Systematic review of factors promoting behaviour change toward antibiotic use in hospitals. Clin. Microbiol. Infect. 2022;28:911–919. doi: 10.1016/j.cmi.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Rawson T.M., Moore L.S.P., Hernandez B., Charani E., Castro-Sanchez E., Herrero P., Hayhoe B., Hope W., Georgiou P., Holmes A.H. A systematic review of clinical decision support systems for antimicrobial management: Are we failing to investigate these interventions appropriately? Clin. Microbiol. Infect. 2017;23:524–532. doi: 10.1016/j.cmi.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Rzewuska M., Duncan E.M., Francis J.J., Morris A.M., Suh K.N., Davey P.G., Grimshaw J.M., Ramsay C.R. Barriers and Facilitators to Implementation of Antibiotic Stewardship Programmes in Hospitals in Developed Countries: Insights from Transnational Studies. Front. Sociol. 2020;5:41. doi: 10.3389/fsoc.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuts E.C., Hulscher M., Mouton J.W., Verduin C.M., Stuart J., Overdiek H., van der Linden P.D., Natsch S., Hertogh C.M.P.M., Wolfs T.F.W., et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016;16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 55.Schweitzer V.A., van Heijl I., van Werkhoven C.H., Islam J., Hendriks-Spoor K.D., Bielicki J., Bonte M.J., Walker A.S., Llewelyn M.J., Harbarth S., et al. The quality of studies evaluating antimicrobial stewardship interventions: A systematic review. Clin. Microbiol. Infect. 2019;25:555–561. doi: 10.1016/j.cmi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Stanic Benic M., Milanic R., Monnier A.A., Gyssens I.C., Adriaenssens N., Versporten A., Zanichelli V., Huttner B., Tebano G., Hulscher M.E., et al. Metrics for quantifying antibiotic use in the hospital setting: Results from a systematic review and international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018;73((Suppl. 6)):vi50–vi58. doi: 10.1093/jac/dky118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Dijck C., Vlieghe E., Cox J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bull. World Health Organ. 2018;96:266–280. doi: 10.2471/BLT.17.203448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warreman E.B., Lambregts M.M.C., Wouters R.H.P., Visser L.G., Staats H., van Dijk E., De Boer M.G.J. Determinants of in-hospital antibiotic prescription behaviour: A systematic review and formation of a comprehensive framework. Clin. Microbiol. Infect. 2019;25:538–545. doi: 10.1016/j.cmi.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Araujo da Silva A.R., Albernaz de Almeida Dias D.C., Marques A.F., Biscaia di Biase C., Murni I.K., Dramowski A., Sharland M., Huebner J., Zingg W. Role of antimicrobial stewardship programmes in children: A systematic review. J. Hosp. Infect. 2018;99:117–123. doi: 10.1016/j.jhin.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Bertollo L.G., Lutkemeyer D.S., Levin A.S. Are antimicrobial stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am. J. Infect. Control. 2018;46:824–836. doi: 10.1016/j.ajic.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Daniels L.M., Weber D.J. Interventions to improve antibiotic prescribing at hospital discharge: A systematic review. Infect. Control Hosp. Epidemiol. 2021;42:96–99. doi: 10.1017/ice.2020.367. [DOI] [PubMed] [Google Scholar]
- 62.Feazel L.M., Malhotra A., Perencevich E.N., Kaboli P., Diekema D.J., Schweizer M.L. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2014;69:1748–1754. doi: 10.1093/jac/dku046. [DOI] [PubMed] [Google Scholar]
- 63.Murray M.T., Beauchemin M.P., Neu N., Larson E.L. Prior antibiotic use and acquisition of multidrug-resistant organisms in hospitalized children: A systematic review. Infect. Control Hosp. Epidemiol. 2019;40:1107–1115. doi: 10.1017/ice.2019.215. [DOI] [PubMed] [Google Scholar]
- 64.Rennert-May E., Chew D.S., Conly J., Guirguis M., Slobodan J., Fryters S., Bresee L. Clinical practice guidelines for creating an acute care hospital-based antimicrobial stewardship program: A systematic review. Am. J. Infect. Control. 2019;47:979–993. doi: 10.1016/j.ajic.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Rittmann B., Stevens M.P. Clinical Decision Support Systems and Their Role in Antibiotic Stewardship: A Systematic Review. Curr. Infect. Dis. Rep. 2019;21:29. doi: 10.1007/s11908-019-0683-8. [DOI] [PubMed] [Google Scholar]
- 66.Smith M.J., Gerber J.S., Hersh A.L. Inpatient Antimicrobial Stewardship in Pediatrics: A Systematic Review. J. Pediatr. Infect. Dis. Soc. 2015;4:e127–e135. doi: 10.1093/jpids/piu141. [DOI] [PubMed] [Google Scholar]
- 67.Wade T., Heneghan C., Roberts N., Curtis D., Williams V., Onakpoya I. Healthcare-associated infections and the prescribing of antibiotics in hospitalized patients of the Caribbean Community (CARICOM) states: A mixed-methods systematic review. J. Hosp. Infect. 2021;110:122–132. doi: 10.1016/j.jhin.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Wagner B., Filice G.A., Drekonja D., Greer N., MacDonald R., Rutks I., Butler M., Wilt T.J. Antimicrobial stewardship programs in inpatient hospital settings: A systematic review. Infect. Control Hosp. Epidemiol. 2014;35:1209–1228. doi: 10.1086/678057. [DOI] [PubMed] [Google Scholar]
- 69.Abo Y.N., Freyne B., Kululanga D., Bryant P.A. The Impact of Antimicrobial Stewardship in Children in Low- and Middle-income Countries: A Systematic Review. Pediatr. Infect. Dis. J. 2022;41:S10–S17. doi: 10.1097/INF.0000000000003317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee C.F., Cowling B.J., Feng S., Aso H., Wu P., Fukuda K., Seto W.H. Impact of antibiotic stewardship programmes in Asia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018;73:844–851. doi: 10.1093/jac/dkx492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim J.M., Singh S.R., Duong M.C., Legido-Quigley H., Hsu L.Y., Tam C.C. Impact of national interventions to promote responsible antibiotic use: A systematic review. J. Antimicrob. Chemother. 2020;75:14–29. doi: 10.1093/jac/dkz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roman C., Edwards G., Dooley M., Mitra B. Roles of the emergency medicine pharmacist: A systematic review. Am. J. Health Syst. Pharm. 2018;75:796–806. doi: 10.2146/ajhp170321. [DOI] [PubMed] [Google Scholar]
- 73.Siachalinga L., Mufwambi W., Lee I.H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022;129:124–143. doi: 10.1016/j.jhin.2022.07.031. [DOI] [PubMed] [Google Scholar]
- 74.Tabah A., Cotta M.O., Garnacho-Montero J., Schouten J., Roberts J.A., Lipman J., Tacey M., Timsit J.-F., Leone M., Zahar J.R., et al. A Systematic Review of the Definitions, Determinants, and Clinical Outcomes of Antimicrobial De-escalation in the Intensive Care Unit. Clin. Infect. Dis. 2016;62:1009–1017. doi: 10.1093/cid/civ1199. [DOI] [PubMed] [Google Scholar]
- 75.Atamna-Mawassi H., Huberman-Samuel M., Hershcovitz S., Karny-Epstein N., Kola A., Cortés L.E.L., Leibovici L., Yahav D. Interventions to reduce infections caused by multidrug resistant Enterobacteriaceae (MDR-E): A systematic review and meta-analysis. J. Infect. 2021;83:156–166. doi: 10.1016/j.jinf.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Bitterman R., Hussein K., Leibovici L., Carmeli Y., Paul M. Systematic review of antibiotic consumption in acute care hospitals. Clin. Microbiol. Infect. 2016;22:561.e7–561.e19. doi: 10.1016/j.cmi.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 77.Garwan Y.M., Alsalloum M.A., Thabit A.K., Jose J., Eljaaly K. Effectiveness of antimicrobial stewardship interventions on early switch from intravenous-to-oral antimicrobials in hospitalized adults: A systematic review. Am. J. Infect. Control. 2022;51:89–98. doi: 10.1016/j.ajic.2022.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Hashad N., Perumal D., Stewart D., Tonna A.P. Mapping hospital antimicrobial stewardship programmes in the Gulf Cooperation Council states against international standards: A systematic review. J. Hosp. Infect. 2020;106:404–418. doi: 10.1016/j.jhin.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Keikha M., Kamali H., Ghazvini K., Karbalaei M. Conceptual framework of antibiotic stewardship programs in reducing ESBL-producing Enterobacteriaceae: A systematic review and meta-analysis. J. Chemother. 2022;34:483–491. doi: 10.1080/1120009X.2022.2085473. [DOI] [PubMed] [Google Scholar]
- 80.Nasr Z., Paravattil B., Wilby K.J. The impact of antimicrobial stewardship strategies on antibiotic appropriateness and prescribing behaviours in selected countries in the Middle East: A systematic review. East. Mediterr. Health J. 2017;23:430–440. doi: 10.26719/2017.23.6.430. [DOI] [PubMed] [Google Scholar]
- 81.Ibrahim N.H., Maruan K., Mohd Khairy H.A., Hong Y.H., Dali A.F., Neoh C.F. Economic Evaluations on Antimicrobial Stewardship Programme: A Systematic Review. J. Pharm. Pharm. Sci. 2017;20:397–406. doi: 10.18433/J3NW7G. [DOI] [PubMed] [Google Scholar]
- 82.Teerawattanapong N., Kengkla K., Dilokthornsakul P., Saokaew S., Apisarnthanarak A., Chaiyakunapruk N. Prevention and Control of Multidrug-Resistant Gram-Negative Bacteria in Adult Intensive Care Units: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017;64((Suppl. 2)):S51–S60. doi: 10.1093/cid/cix112. [DOI] [PubMed] [Google Scholar]
- 83.Akpan M.R., Isemin N.U., Udoh A.E., Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020;22:317–324. doi: 10.1016/j.jgar.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Stenehjem E., Hyun D.Y., Septimus E., Yu K.C., Meyer M., Raj D., Srinivasan A. Antibiotic Stewardship in Small Hospitals: Barriers and Potential Solutions. Clin. Infect. Dis. 2017;65:691–696. doi: 10.1093/cid/cix407. [DOI] [PubMed] [Google Scholar]
- 85.Pezzani M.D., Mazzaferri F., Compri M., Galia L., Mutters N.T., Kahlmeter G., Zaoutis T.E., Schwaber M.J., Rodríguez-Baño J., Harbarth S., et al. Linking antimicrobial resistance surveillance to antibiotic policy in healthcare settings: The COMBACTE-Magnet EPI-Net COACH project. J. Antimicrob. Chemother. 2020;75((Suppl. 2)):ii2–ii19. doi: 10.1093/jac/dkaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rich A., Medisauskaite A., Potts H.W.W., Griffin A. A theory-based study of doctors’ intentions to engage in professional behaviours. BMC Med. Educ. 2020;20:44. doi: 10.1186/s12909-020-1961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brotherton A.L. Metrics of Antimicrobial Stewardship Programs. Med. Clin. N. Am. 2018;102:965–976. doi: 10.1016/j.mcna.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Zanichelli V., Monnier A.A., Gyssens I.C., Adriaenssens N., Versporten A., Pulcini C., Le Maréchal M., Tebano G., Vlahović-Palčevski V., Benić M.S., et al. Variation in antibiotic use among and within different settings: A systematic review. J. Antimicrob. Chemother. 2018;73((Suppl. 6)):vi17–vi29. doi: 10.1093/jac/dky115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fortin É., Fontela P.S., Manges A.R., Platt R.W., Buckeridge D.L., Quach C. Measuring antimicrobial use in hospitalized patients: A systematic review of available measures applicable to paediatrics. J. Antimicrob. Chemother. 2014;69:1447–1456. doi: 10.1093/jac/dku003. [DOI] [PubMed] [Google Scholar]
- 90.Taplitz R.A., Ritter M.L., Torriani F.J. Infection Prevention and Control, and Antimicrobial Stewardship. Infect. Dis. 2017:54–61.e1. doi: 10.1016/B978-0-7020-6285-8.00006-X. [DOI] [Google Scholar]
- 91.Manning M.L., Septimus E.J., Ashley E.S.D., Cosgrove S.E., Fakih M.G., Schweon S.J., Myers F.E., Moody J.A. Antimicrobial stewardship and infection prevention-leveraging the synergy: A position paper update. Am. J. Infect. Control. 2018;46:364–368. doi: 10.1016/j.ajic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Okeah B.O., Morrison V., Huws J.C. Antimicrobial stewardship and infection prevention interventions targeting healthcare-associated Clostridioides difficile and carbapenem-resistant Klebsiella pneumoniae infections: A scoping review. BMJ Open. 2021;11:e051983. doi: 10.1136/bmjopen-2021-051983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zanichelli V., Sharland M., Cappello B., Moja L., Getahun H., Pessoa-Silva C., Sati H., van Weezenbeek C., Balkhy H., Simão M., et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023;101:290–296. doi: 10.2471/BLT.22.288614. [DOI] [Google Scholar]
- 94.Fuller W., Kapona O., Aboderin A.O., Adeyemo A.T., Olatunbosun O.I., Gahimbare L., Ahmed Y.A. Education and Awareness on Antimicrobial Resistance in the WHO African Region: A Systematic Review. Antibiotics. 2023;12:1613. doi: 10.3390/antibiotics12111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naylor N.R., Atun R., Zhu N., Kulasabanathan K., Silva S., Chatterjee A., Knight G.M., Robotham J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control. 2018;7:58. doi: 10.1186/s13756-018-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chatterjee A., Modarai M., Naylor N.R., Boyd S.E., Atun R., Barlow J., Holmes A.H., Johnson A., Robotham J.V. Quantifying drivers of antibiotic resistance in humans: A systematic review. Lancet Infect. Dis. 2018;18:e368–e378. doi: 10.1016/S1473-3099(18)30296-2. [DOI] [PubMed] [Google Scholar]
- 98.Chen Q., Li D., Beiersmann C., Neuhann F., Moazen B., Lu G., Müller O. Risk factors for antibiotic resistance development in healthcare settings in China: A systematic review. Epidemiol. Infect. 2021;149:e141. doi: 10.1017/S0950268821001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McGregor J.C., Fitzpatrick M.A., Suda K.J. Expanding Antimicrobial Stewardship Through Quality Improvement. JAMA Netw. Open. 2021;4:e211072. doi: 10.1001/jamanetworkopen.2021.1072. [DOI] [PubMed] [Google Scholar]
- 100.Bremmer D.N., Trienski T.L., Walsh T.L., Moffa M.A. Role of Technology in Antimicrobial Stewardship. Med. Clin. N. Am. 2018;102:955–963. doi: 10.1016/j.mcna.2018.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All utilized data are available online from academic search engines.