Abstract
In addition to eleven glycoproteins, the herpes simplex virus type 2 (HSV-2) genome encodes several proteins with potential membrane-spanning segments but no asparagine-linked carbohydrates. One of these is UL45. Fractionation of infected cells showed that HSV-2 UL45 is an integral membrane protein, and analysis of UL45 mutants with potential glycosylation sites showed that it has a type II membrane orientation, the first HSV protein known to have this orientation. Furthermore, it is detectable in infected cells at a time similar to that when glycoproteins gB and gD are detected, consistent with a role in cell-cell fusion, which has previously been found for HSV-1 UL45.
The genomes of herpes simplex virus types 1 and 2 (HSV-1 and -2) encode at least 11 glycoproteins, of which 10 have transmembrane anchor regions. Several additional open reading frames, such as UL45, encode proteins that have potential membrane-spanning segments but that lack consensus sites for the addition of asparagine-linked carbohydrates (N-CHO) (9). The HSV-1 UL45 protein (UL45-1) has 172 residues and a molecular mass of 18 kDa (11). It is not required for growth of virus in tissue culture (11) but is required for cell-cell fusion caused by a syncytial variant of HSV-1 (6). HSV-2 UL45 (UL45-2) is also a 172-residue protein, with 75% sequence identity to UL45-1. It was first identified as a 20-kDa protein by in vitro translation of transcripts from HSV-2-infected cells (10) and more recently was shown to cross-react weakly with an antibody raised against UL45-1 (12). To further characterize UL45-2, we have raised an antibody against a glutathione S-transferase (GST)–UL45-2 fusion protein and have made a UL45-2 expression vector; these reagents were used to study the time course of UL45-2 synthesis in infected cells, its association with membranes, its orientation in the membrane, and its cellular location.
A UL45-2 expression vector (pAC265) was derived from pGR44 (a gift from Gary Hayward), which contains the BamHI A fragment from HSV-2 strain 333. A 1.0-kb SmaI fragment containing the UL45-2 open reading frame was subcloned from pGR44 into pCMV3 (1), next to the human cytomegalovirus immediate-early promoter, to produce pAC265. A plasmid carrying a gene expressing a GST–UL45-2 fusion protein was then derived from pAC265 by amplification of the coding sequence for residues 52 to 121 with Pfu DNA polymerase and primers 5′-TGGATCCTCGTGGGGACTCTCG-3′ and 5′-TGGATCCGTTCACCCACCAGTAACC-3′ (BamHI sites underlined); after digestion with BamHI, this fragment was cloned into the BamHI site of pGEX-4T-3 (Pharmacia), in frame with the GST gene, to produce pAC270. Residues 52 to 121 were chosen in order to avoid hydrophobic sequences that might cause the GST–UL45-2 fusion protein to aggregate.
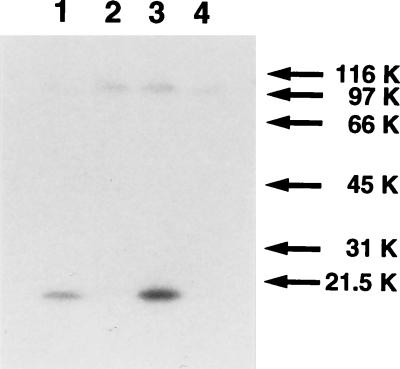
GST–UL45-2 was expressed in Escherichia coli containing plasmid pAC270, purified from a bacterial lysate with glutathione-Sepharose beads, and used to immunize rabbits (Cocalico Biologicals, Inc., Reamstown, Pa.). The rabbits received an initial inoculation of 100 μg, followed by three 50-μg booster injections. Two sera, LSU32 and LSU33, were obtained, and preliminary Western blots showed that both reacted with GST, with GST–UL45-2, and with many proteins in uninfected CV1 and COS7 cells (data not shown). To reduce this background, anti-GST antibodies were removed from LSU32 by passage through a column of GST cross-linked to glutathione-Sepharose beads by dimethyl pimelimidate-HCl (2). Dot blot analysis showed that LSU32 then reacted with GST–UL45-2 but not with GST. To test its reactivity with bona fide UL45-2, cytoplasmic extracts were prepared from CV1 cells infected with HSV-2 and from COS7 cells transfected with pAC265, using Nonidet P-40 (NP-40) and sodium deoxycholate detergents for cell lysis (7). The extracts were electrophoresed on a sodium dodecyl sulfate (SDS)-polyacrylamide gel (5), transferred to nitrocellulose, and probed with LSU32 followed by 125I-protein A (Fig. 1). A band of 20 kDa was present in the infected extract (lane 1) and the transfected extract (lane 3), but not in mock-infected (lane 2) or mock-transfected (lane 4) extracts, confirming the reactivity of LSU32 with UL45-2. A second band, of approximately 100 kDa, was found in all extracts and therefore represents a cellular protein with which LSU32 cross-reacts.
FIG. 1.
Recognition of UL45-2 by LSU32 antiserum. CV1 cells were infected with HSV-2 strain 333 at an MOI of 1 PFU per cell (lane 1) or mock infected (lane 2). COS7 cells were transfected with pAC265 by the calcium phosphate procedure (lane 3) or mock transfected (lane 4). Cytoplasmic extracts were prepared at 18 h p.i. or 40 h posttransfection, electrophoresed on a 12.5% denaturing polyacrylamide gel, transferred to nitrocellulose, and probed with LSU32 and then 125I-protein A. Molecular weights (in thousands [K]) are noted at the right.
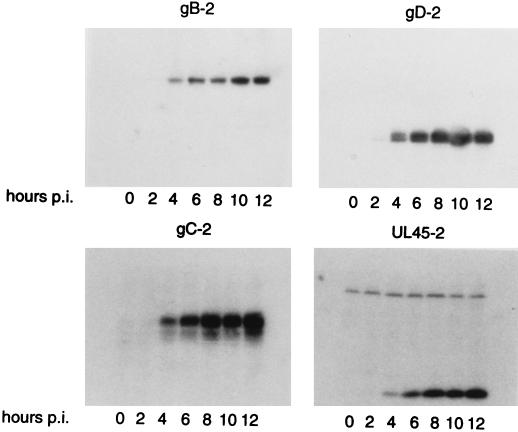
UL45-1 has been shown to be required for cell-cell fusion (6); however, the protein was not detected in infected cells until 9 h postinfection (p.i.) (12) whereas fusion has been observed as early as 5 h p.i. (8). Many factors may be responsible for this difference, such as the use of different cell types, virus strains, and multiplicities of infection (MOI). Therefore, the time course of UL45-2 synthesis in infected cells was compared to that of the viral glycoproteins gB-2 and gD-2, which by analogy with their HSV-1 counterparts are probably essential for cell-cell fusion, and also to that of gC-2. Cytoplasmic extracts of CV1 cells infected with HSV-2 at an MOI of 1 PFU per cell were prepared at 0 to 12 h p.i., and Western blots of SDS-polyacrylamide gels were probed with the polyclonal antibodies LSU32 (UL45-2), R90 (gB-2), R46 (gC-2), and R7 (gD-2) (Fig. 2). UL45-2 was first detected at 4 h p.i., as was gC-2; gB-2 and gD-2 were easily detectable at 4 h and barely detectable at 2 h. The appearance of UL45-2 at a time comparable to the time of appearance of these glycoproteins is consistent with a role in membrane fusion.
FIG. 2.
Time course of UL45-2 synthesis, compared to those of gB-2, gC-2, and gD-2. CV1 cells were infected with HSV-2 at an MOI of 1 PFU per cell. Cytoplasmic extracts were prepared at 2-h intervals from 0 to 12 h p.i., electrophoresed on denaturing polyacrylamide gels, transferred to nitrocellulose, and probed with LSU32 (UL45-2), R90 (gB-2), R46 (gC-2), or R7 (gD-2) and then with 125I-protein A.
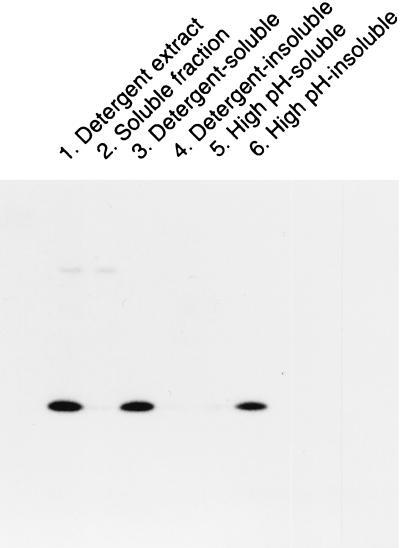
Kyte and Doolittle hydropathy plots of the UL45-1 and UL45-2 amino acid sequences reveal a potential membrane-spanning sequence between residues 24 and 48. Solubilization of UL45-1 from HSV-1 virions with NP-40 detergent provides further evidence for its association with membranes (12). To determine if UL45-2 is a membrane protein, CV1 cells were infected with HSV-2 at an MOI of 1 PFU per cell and fractionated at 18 h p.i. The cells were scraped into phosphate-buffered saline, pelleted by low-speed centrifugation, and resuspended in 10 mM Tris-HCl (pH 7.5)–10 mM NaCl–3 mM MgCl2 (reticulocyte standard buffer [RSB]). After cell lysis by one freeze-thaw cycle and sonication, the lysate was centrifuged at 70,000 × g for 30 min at 4°C to produce a pellet and a soluble fraction. The pellet was resuspended either in 0.1 M Na2CO3 (pH 11.0) (to solubilize peripheral membrane proteins) or in RSB containing NP-40 and sodium deoxycholate (to solubilize peripheral and integral membrane proteins) and then centrifuged again at 70,000 × g for 30 min. The supernatants contained high-pH-soluble or detergent-soluble proteins, respectively. The pellets containing high-pH-insoluble or detergent-insoluble proteins were resuspended in SDS-polyacrylamide gel electrophoresis sample buffer. As a control, a cytoplasmic extract of unfractionated cells was prepared by detergent lysis. All fractions were electrophoresed, transferred to nitrocellulose, and probed with LSU32 (Fig. 3). As before, UL45-2 was present in the cytoplasmic extract (lane 1); it was also present in the detergent-soluble fraction (lane 3) and the high-pH-insoluble fraction (lane 6) but not in the soluble fraction (lane 2) or detergent-insoluble (lane 4) and high-pH-soluble (lane 5) fractions. UL45-2 is therefore an integral membrane protein.
FIG. 3.
Membrane association of UL45-2. CV1 cells were infected with HSV-2 for 18 h and then fractionated as described in the text. All fractions were electrophoresed on a denaturing gel, transferred to nitrocellulose, and probed with LSU32 and then with 125I-protein A.
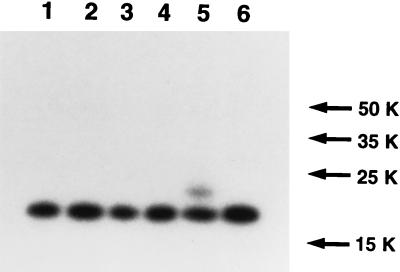
UL45-1 and -2 have only one region sufficiently hydrophobic to span a membrane, so this region presumably acts as a signal sequence and as a membrane anchor. Since neither protein has a consensus site for the addition of N-CHO, their orientation in the membrane was not obvious. The approach taken to determine the orientation of UL45-2 was one that has frequently been used to study membrane topology, i.e., the creation of potential glycosylation sites on either side of the transmembrane anchor (3, 4). Mutagenesis to create consensus N-CHO sites was performed by the QuikChange procedure (Stratagene) with Pfu DNA polymerase and using plasmid pAC265 as the template. The primers for changing residue 4 from Arg to Asn were 5′-GTCCCGAAGCATTGAAAGCCATAGCAC-3′ and 5′-GTGCTATGGCTTTCAATGCTTCGGGAC-3′. The primers for changing residue 111 from Glu to Asn were 5′-AACCCGACGATCTGTTCGCCTGGACGCGTG-3′ and 5′-CACGCGTCCAGGCGAACAGATCGTCGGGTT-3′. The amplification conditions were one cycle of 98°C for 1 min followed by 16 cycles of 98°C for 1 min, 70°C for 1 min 20 s, and 75°C for 12 min. The reaction mix was then incubated with DpnI for 90 min to nick template DNA, followed by transformation of XL1-Blue E. coli. Colonies carrying genes with the desired mutations were identified by DNA sequencing. Plasmid pMM336 carries a gene that encodes a protein with a consensus N-CHO site at residue 4 (R4N); plasmid pMM337 carries a gene that encodes a protein with an N-CHO site at residue 111 (E111N). COS7 cells were transfected with pAC265, pMM336, or pMM337, and cytoplasmic extracts were prepared 40 h later. Each extract was subsequently incubated for 2 h at 37°C in the presence or absence of a mixed preparation of endoglycosidase F and N-glycosidase F (endo F; Boehringer Mannheim) and electrophoresed on an SDS-polyacrylamide gel. A Western blot was probed with LSU32 (Fig. 4). The R4N mutant (lanes 3 and 4) was identical to the wild type (lanes 1 and 2) and therefore was not glycosylated. In contrast, the E111N mutant (lane 5) had an additional band of lower mobility than that of nonglycosylated UL45-2. Incubation of the extract with endo F resulted in the disappearance of this band (lane 6), confirming that it represents glycosylated UL45-2. Even though the majority of the mutant protein was not glycosylated, presumably because glycosylation at this site is inefficient, the observation that glycosylation occurred on the C-terminal side of the membrane anchor but not on the N-terminal side indicates that the C-terminal side is present in the lumen of the endoplasmic reticulum (ER), defining UL45-2 as a type II membrane protein.
FIG. 4.
Membrane orientation of UL45-2. COS7 cells were transfected with pAC265 (wild-type UL45-2, lanes 1 and 2), pMM336 (R4N mutant, lanes 3 and 4), or pMM337 (E111N mutant, lanes 5 and 6). Cytoplasmic extracts were prepared at 40 h posttransfection, incubated for 2 h at 37°C in the presence (lanes 2, 4, and 6) or absence (lanes 1, 3, and 5) of endo F, electrophoresed on a denaturing gel, transferred to nitrocellulose, and probed with LSU32 and then with 125I-protein A. Molecular weights (in thousands [K]) are noted at the right.
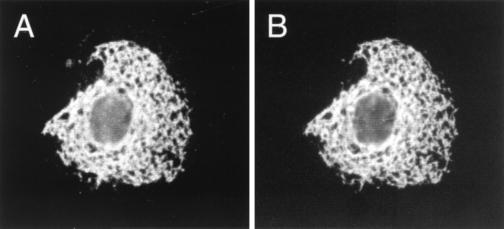
The cellular location of UL45-2 was examined by immunofluorescence analysis of COS7 cells transfected with plasmid pAC265. The cells were fixed with 3% paraformaldehyde–2% sucrose in phosphate-buffered saline; for staining of internal antigens, they were subsequently permeabilized by incubation in Dulbecco modified Eagle medium (DMEM)–5% fetal bovine serum (FBS) containing 0.5% Triton X-100 detergent and 10% sucrose. Incubation with the primary antibody (LSU32) was for 30 min at room temperature in DMEM–5% FBS. Incubation with the secondary antibody (goat anti-rabbit immunoglobulin G [IgG]–fluorescein isothiocyanate [FITC] [Southern Biotechnologies]) was for 30 min at room temperature in DMEM–1% FBS. UL45-2 was detected within the cell but not at the cell surface (data not shown). Given its type II membrane orientation, it should have been detected by LSU32 if it was present at the cell surface. The intracellular location was further investigated by cotransfection of COS7 cells with pAC265 and pXM-BiP-Tag (7a), a plasmid that expresses an epitope-tagged form of the ER-resident protein BiP. After fixation and permeabilization, the cells were stained with LSU32 and a mouse monoclonal antibody that recognizes the epitope tag (12CA5; Boehringer Mannheim), followed by goat anti-rabbit IgG-FITC and goat anti-mouse IgG-tetramethyl rhodamine isothiocyanate (TRITC). As shown in Fig. 5, the patterns of staining for UL45-2 (Fig. 5A) and BiP (Fig. 5B) were very similar, indicating that UL45-2 is largely if not entirely contained within the ER in transfected cells. Clearly, it would be interesting to know if UL45-2 is also retained within the ER in infected cells. Unfortunately, after blocking the viral Fc receptor with an excess of nonspecific rabbit IgG, we have not been able to detect UL45-2 in infected cells using biotinylated LSU32 and then streptavidin-FITC.
FIG. 5.
Immunofluorescence analysis of UL45-2’s location. COS7 cells cotransfected with pAC265 and pXM-BiP-Tag were fixed, permeabilized, and then stained with LSU32 (rabbit anti-UL45-2) and 12CA5 (mouse anti-BiP-Tag), followed by goat anti-rabbit IgG-FITC and anti-mouse IgG-TRITC. (A) Photographed for FITC; (B) photographed for TRITC.
In summary, the work described in this paper is a characterization of important properties of the HSV-2 UL45 protein. A polyclonal antibody against UL45-2 recognized the protein in infected cells and in cells transfected with an expression plasmid. UL45-2 was shown to be an integral membrane protein and was synthesized sufficiently early during infection to be involved in cell-cell fusion. Furthermore, it was found to have a type II membrane orientation, the first HSV membrane protein known to have this orientation. In transfected cells, UL45-2 is retained within the ER, but in infected cells, its location is not yet known. Future studies will address the issues of whether UL45-2 is required for HSV-2 entry into cells or for cell-cell fusion in various syncytial backgrounds and, if so, how it participates in these processes.
Acknowledgments
This investigation was supported by grant LEQSF-RD-A-13 to M.I.M. We also acknowledge the support of the LSUMC Center for Excellence in Cancer Research, Treatment and Education and the LSUMC Center for Excellence in Arthritis and Rheumatology.
We are grateful to Gary Cohen and Roz Eisenberg for their generous gifts of polyclonal antibodies R7, R46, and R90 and to Gary Hayward for his gift of plasmid pGR44.
REFERENCES
- 1.Andersson S, Davis D N, Dahlback H, Jornvall H, Russell D W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 2.Bar-Peled M, Raikhel N V. A method for isolation and purification of specific antibodies to a protein fused to the GST. Anal Biochem. 1996;241:140–142. doi: 10.1006/abio.1996.0390. [DOI] [PubMed] [Google Scholar]
- 3.Boyd D. Use of gene fusions to determine membrane protein topology. In: White S H, editor. Membrane protein structure: experimental approaches. Oxford, United Kingdom: Oxford University Press; 1994. pp. 144–163. [Google Scholar]
- 4.Chavez R A, Hall Z A. The transmembrane topology of the amino terminus of the alpha subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1991;266:15532–15538. [PubMed] [Google Scholar]
- 5.Cohen G H, Isola V J, Kuhns J, Berman P W, Eisenberg R J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60:157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haanes E J, Nelson C M, Soule C L, Goodman J L. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J Virol. 1994;68:5825–5834. doi: 10.1128/jvi.68.9.5825-5834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muggeridge M I, Isola V J, Byrn R A, Tucker T J, Minson A C, Glorioso J C, Cohen G H, Eisenberg R J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988;62:3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Murray P J, Watowich S S, Lodish H F, Young R A, Hilton D J. Epitope tagging of the human endoplasmic reticulum HSP70 protein, BiP, to facilitate analysis of BiP-substrate interactions. Anal Biochem. 1995;229:170–179. doi: 10.1006/abio.1995.1399. [DOI] [PubMed] [Google Scholar]
- 8.Person S, Knowles R W, Read G S, Warner S C, Bond V C. Kinetics of cell fusion induced by a syncytia-producing mutant of herpes simplex virus type 1. J Virol. 1976;17:183–190. doi: 10.1128/jvi.17.1.183-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 10.Swain M A, Peet R W, Galloway D A. Characterization of the gene encoding herpes simplex virus type 2 glycoprotein C and comparison with the type 1 counterpart. J Virol. 1985;53:561–569. doi: 10.1128/jvi.53.2.561-569.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visalli R J, Brandt C R. The HSV-1 UL45 gene product is not required for growth in Vero cells. Virology. 1991;185:419–423. doi: 10.1016/0042-6822(91)90790-i. [DOI] [PubMed] [Google Scholar]
- 12.Visalli R J, Brandt C R. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29:167–178. doi: 10.1016/0168-1702(93)90057-t. [DOI] [PubMed] [Google Scholar]