Abstract
Background
Hepatopulmonary syndrome affects 10–30% of patients with cirrhosis and portal hypertension. We evaluated the serum angiogenic profile of hepatopulmonary syndrome and assessed the clinical impact of hepatopulmonary syndrome in patients evaluated for liver transplantation.
Methods
The Pulmonary Vascular Complications of Liver Disease 2 study was a multicentre, prospective cohort study of adults undergoing their first liver transplantation evaluation. Hepatopulmonary syndrome was defined as an alveolar–arterial oxygen gradient ⩾15 mmHg (⩾20 mmHg if age >64 years), positive contrast-enhanced transthoracic echocardiography and absence of lung disease.
Results
We included 85 patients with hepatopulmonary syndrome and 146 patients without hepatopulmonary syndrome. Patients with hepatopulmonary syndrome had more complications of portal hypertension and slightly higher Model for End-Stage Liver Disease-Na score compared to those without hepatopulmonary syndrome (median (interquartile range) 15 (12–19) versus 14 (10–17), p=0.006). Hepatopulmonary syndrome patients had significantly lower 6-min walk distance and worse functional class. Hepatopulmonary syndrome patients had higher circulating angiopoietin 2, Tie2, tenascin C, tyrosine protein kinase Kit (c-Kit), vascular cell adhesion molecule 1 and von Willebrand factor levels, and lower E-selectin levels. Patients with hepatopulmonary syndrome had an increased risk of death (hazard ratio 1.80, 95% CI 1.03–3.16, p=0.04), which persisted despite adjustment for covariates (hazard ratio 1.79, 95% CI 1.02–3.15, p=0.04). This association did not vary based on levels of oxygenation, reflecting the severity of hepatopulmonary syndrome.
Conclusion
Hepatopulmonary syndrome was associated with a profile of abnormal systemic angiogenesis, worse exercise and functional capacity, and an overall increased risk of death.
Shareable abstract (@ERSpublications)
The presence of hepatopulmonary syndrome with even mild oxygenation abnormalities was associated with shorter survival in candidates evaluated for liver transplantation and was characterised by higher levels of pro-angiogenic biomarkers https://bit.ly/3EAihft
Introduction
Hepatopulmonary syndrome (HPS) occurs when intrapulmonary vascular dilation and pulmonary arteriovenous malformations lead to abnormal systemic oxygenation in the setting of liver disease or portal hypertension [1]. This syndrome has been found in 10–30% of patients with cirrhosis being evaluated for liver transplantation [2–4]. We and others have shown that HPS is associated with a doubling in the risk of death even after accounting for liver transplantation, which is curative [3, 5, 6].
The mechanism of HPS is currently unknown. Prior studies of patients and the experimental model of HPS have suggested that dysregulated angiogenesis plays an important role in this manifestation of advanced liver disease. Increased circulating levels of haematopoietic progenitor cells/monocytes were found in the common bile duct ligation rat model [7–9]. Genetic variants of the gene that codes for von Willebrand factor (vWF) and higher vWF levels as well as the precursor of endostatin were associated with HPS in a prior study [10]. Anti-angiogenic interventions (e.g. endostatin and angiostatin expression and sorafenib) improved gas exchange and shunting in the HPS experimental model [11]. While a small phase II randomised double-blind placebo-controlled trial of sorafenib showed a reduction in vascular endothelial growth factor (VEGF) receptor-2, sorafenib did not affect gas exchange, exercise capacity or quality of life in patients with HPS [12].
Some have questioned the importance of the impact of HPS, especially when it presents subclinically without significant hypoxaemia. We previously published a prospective multicentre cohort of liver transplantation candidates in which most HPS was characterised by mild reductions in arterial oxygen tension () and oxygen saturation in arterial blood and infrequent severe hypoxaemia [3]. Even so, HPS had a clinically significant negative impact on functional status, health-related quality of life and survival. However, there are no prospective, multicentre human studies of the angiogenic milieu of HPS and the impact of HPS on other clinical end-points with systematic evaluation of heart and lung function and adjustment for confounders.
Therefore, we evaluated the clinical characteristics, angiogenic biomarker profile, exercise capacity and risk of hospitalisation and mortality in patients with HPS compared to those without HPS with advanced liver disease being evaluated for liver transplantation.
Methods
Study design and study sample
The Pulmonary Vascular Complications of Liver Disease (PVCLD2) study enrolled a cohort of 454 patients evaluated for liver transplantation at centres in the USA between 2013 and 2017 (supplementary figure E1). The only inclusion criterion was the presence of portal hypertension with or without intrinsic liver disease. We excluded patients with active infection or recent (<2 weeks) gastrointestinal bleeding and patients who had undergone prior liver or lung transplantation. The study was approved by the institutional review board of each centre.
The study sample for this analysis was drawn from enrolled patients undergoing their first liver transplantation evaluation at the University of Pennsylvania, Mayo Clinic and the University of Texas at Houston. We excluded patients thought to have portopulmonary hypertension.
HPS was defined as 1) alveolar–arterial oxygen tension difference (or if age was >64 years), 2) late passage of contrast on contrast-enhanced transthoracic echocardiography (positive contrast-enhanced transthoracic echocardiography), 3) absence of a significant obstructive and restrictive ventilatory defect on spirometry and 4) absence of intracardiac shunting [1, 3].
The main analysis excluded patients with obstructive or restrictive ventilatory defects (defined below), missing testing and those with intracardiac shunting from the control group. A sensitivity analysis for outcomes included all other patients undergoing their first liver transplantation evaluation without portopulmonary hypertension who did not meet diagnostic criteria for HPS.
Data collection and variables
Informed consent was obtained from eligible patients, who were then scheduled for research assessment, which included medical history, anthropometrics, physical examination, phlebotomy, pulse oximetry, arterial blood gas sampling, spirometry, 6-min walk testing and contrast echocardiography. All study visits and study procedures were conducted in the outpatient setting. Patients were asked to avoid smoking before the research assessment.
Phlebotomy was performed after overnight fasting except water. Serum and plasma were banked at −80°C, while samples for flow cytometry were processed within 24 h. All samples were shipped and assays performed at the University of Vermont Laboratory for Clinical Biochemistry Research except for vWF multimer studies, which were performed at the University of Pennsylvania.
Clinical data were collected from formal interviews on the date of study procedures and from the medical record. Pulse oximetry was performed using a standard professional grade oximeter after the study participant maintained an upright seated posture for 5 min and was then repositioned supine for 5 min. Patients underwent a physical examination. Clinical laboratory results obtained closest to the date of the study visit were recorded. The Model for End-Stage Liver Disease (MELD-Na) score was calculated using the following formulae: MELD=10×((0.957×ln(creatinine))+(0.378×ln(bilirubin))+(1.12×ln(international normalised ratio)))+0.643 and MELD-Na=MELD+(1.32×(137−Na))−(0.033×MELD×(137−Na)) [13, 14].
Radial artery blood gas sampling was performed on ambient air in a seated position after 10 min of rest. The samples were processed in a blood gas analyser after a one-point calibration. The was calculated using the following formula: where was the inspiratory oxygen fraction, was the barometric pressure measured on the date (and in the city) of the study visit, was the arterial carbon dioxide tension and R was assumed to be 0.8 [15].
Pre-bronchodilator spirometry was performed according to American Thoracic Society and European Respiratory Society recommendations [16]. Obstructive ventilatory defect was defined as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)<0.70 with FEV1<80% predicted, and restrictive ventilatory defect was defined as FVC<70% predicted. A minimum of three efforts with no acceptability errors and at least two with repeatability per standards (FVC within 150 mL of largest, FEV1 within 150 mL of largest and peak flow within 15% of largest) were required. Testing was continued until the above criteria were met, a total of eight tests were performed or the patient was unable to continue testing. Sex-, age- and race-specific equations were used to determine per cent predicted based on spirometric reference values derived from the National Health and Nutrition Evaluation Survey III [17]. The 6-min walk test was performed according to American Thoracic Society guidelines [18].
Contrast-enhanced transthoracic echocardiography was performed by injecting agitated saline via a peripheral vein during transthoracic echocardiographic imaging. The apical four-chamber view was the preferred window for image acquisition, although the parasternal long axis, modified or para-apical four-chamber view, or subcostal views were utilised if the four-chamber view was suboptimal or unavailable. At least 10 continuous cardiac cycles were captured, beginning immediately prior to contrast injection so that cardiac cycles could be accurately assessed to determine the delay from the injection of agitated saline to visualisation of contrast entering the left heart. Identification of microbubbles in either the left atrium or left ventricle after three or more cardiac cycles was considered to indicate the presence of intrapulmonary vascular dilatation [19]. Patients with evidence of immediate (less than three cycles) opacification of the left atrium or left ventricle were presumed to have an intracardiac shunt. A Doppler flow signal across the atrial septum was presumed to indicate a patent foramen ovale, also considered to be an intracardiac shunt. Post-Valsalva images were not utilised for study purposes. The Echocardiography Core Laboratory at the Mayo Clinic evaluated all contrast echocardiograms performed at individual study sites and echocardiographers interpreted the studies offline while blinded to all clinical information.
World Health Organization functional class
Assessment of symptoms and World Health Organization functional class was performed at baseline. The World Health Organization functional classification is modified from the New York Heart Association functional classification, with Class I defined as no symptoms, Class II as symptoms with more than usual activity, Class III as symptoms with less than usual activity and Class IV as symptoms at rest.
Patients were contacted by the research team every 6 months until 2017. Dates of hospitalisation, liver transplantation and death were obtained from the patients, medical records and the patients’ physicians. Patients who were alive at the end of follow-up were censored at May 2017.
Laboratory assays
All assays were performed in bulk at the conclusion of the study except for flow cytometry. We used the MILLIPLEX Human Angiogenesis Panel 2 (#HANG2MAG-12K), which is a bead-based Luminex multiplex assay, to measure plasma angiostatin, soluble tyrosine protein kinase Kit (c-Kit), soluble E-selectin, soluble epithelial growth factor receptor, tenascin C, soluble Tie2, soluble VEGF receptors-1, −2 and −3, platelet-derived growth factor AB/BB and platelet endothelial cell adhesion molecule-1 (MilliporeSigma, Burlington, MA, USA). We used the MILLIPLEX Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay (#HCYTOMAG-60K) to measure plasma fractalkine and VEGFA (MilliporeSigma). We measured plasma angiopoietin 2 using the Meso Scale Discovery Human Angiopoietin-2 Kit (#K151KCD, Mesa Scale Diagnostics, Rockville, MD, USA). We measured plasma vascular cell adhesion molecule 1 (#DVC00) and plasma endostatin (#DNST0) using ELISAs (R&D Systems, Minneapolis, MN, USA). vWF antigen was assessed using an immunoturbidimetric method from Stago (cat #00518).
Details of vWF multimer studies and flow cytometry are provided in the supplementary material. Because flow cytometry occurred in “real time” throughout the cohort, we used the standard flow cytometric classifications at the time of cohort initiation, which have generally remained similar [20]. We focused on haematopoietic progenitor cells defined by CD34+, CD34+CD133+ and CD34+CD133+KDR+, which were CD45dim (expressed as % of peripheral blood mononuclear cells) and intermediate class (M2) monocytes and Tie2-expressing M2 monocytes (CD14+CD16+ and CD14+CD16+Tie2+), both expressed as percentage of CD14+ cells.
Statistical analysis
Continuous data were summarised using mean±SD or median (interquartile range (IQR)), as appropriate. Categorical variables were summarised with n (%). We compared HPS to non-HPS patients using unpaired t-tests, Wilcoxon rank sum tests, chi-squared tests and Fisher’s exact tests, as appropriate. We used bivariate and multivariate linear regression to analyse the association between HPS status and the 6-min walk distance and biomarkers of angiogenesis.
We analysed the association of HPS with the risk of hospitalisation using relevant models for recurrent event analysis including gamma frailty, Andersen–Gill, Prentice–Williams–Peterson gap-time and total-time, and multistate models [21]. Survival was assessed using the Kaplan–Meier estimator and Cox proportional hazards models and expressed with a hazard ratio (HR) in bivariate and multivariate analyses. We included age and MELD-Na as covariates in the multivariable models. We analysed overall survival (including time before and after liver transplantation) as the primary analysis, because ultimately that is what is of clinical importance to patients and clinicians. We performed sensitivity analyses with censoring at liver transplantation, considering liver transplantation a competing risk for death [22], and adjusting for liver transplantation as a time-varying covariate. We also performed multistate modelling [23]. We calculated E-values for the main survival analyses [24]. We used laboratory parameters that were correlated with MELD-Na and a random forest imputation algorithm to impute missing MELD-Na scores (2%) for the adjusted analyses [25]. Owing to the independent hypotheses investigated, there was no correction for multiple comparisons. All analyses used R version 3.6.1 (www.r-project.com).
Results
A total of 454 patients were enrolled in the cohort (supplementary figure E1). 43 were excluded for presumed portopulmonary hypertension, leaving 411. Of these, 26 were excluded for lack of arterial blood gas or pulmonary function testing, 40 were excluded for obstructive (and 65 for restrictive) ventilatory defects and 49 had evidence of patent foramina ovalia. Of the remaining 231, 85 (37%, 95% CI 31– 43%) met criteria for HPS. Of the full cohort without a possible diagnosis of portopulmonary hypertension (n=411), at least 21% (95% CI 17–25%) had HPS. There were no substantive differences between the final study sample (n=231) and those new patients without possible portopulmonary hypertension who were excluded (n=180) (supplementary table E1).
There were 85 patients with HPS and 146 without HPS (table 1). Patients with HPS were slightly younger and more likely to be female. HPS patients were more likely to be non-Hispanic white than non-HPS patients, but had similar educational attainment and household income. A high proportion of patients in both groups had liver disease attributable to alcohol use and/or hepatitis C infection. The median MELD-Na score was one point higher in patients with HPS compared to those without HPS (15, IQR 12–19, versus 14, 10–17; p=0.006). Patients with HPS were more likely to have a history of ascites, varices and encephalopathy but were less likely to have a history of hepatocellular carcinoma. Smoking and alcohol use were similar between the groups.
TABLE 1.
Demographics, liver disease characteristics and past medical history
| Variable | Total participants, n | HPS (n=85) | No HPS (n=146) | p-value# |
|---|---|---|---|---|
|
| ||||
| Age (years), mean±SD | 231 | 55.2±9.4 | 57.6±8.9 | 0.06 |
| Female | 231 | 33 (38.8) | 40 (27.4) | 0.07 |
| Race/ethnicity | 231 | 0.02 | ||
| Non-Hispanic white | 70 (82.4) | 95 (65.1) | ||
| Hispanic white | 12 (14.1) | 29 (19.9) | ||
| Non-Hispanic black | 2 (2.4) | 16 (11.0) | ||
| Other | 1 (1.2) | 6 (4.1) | ||
| Born in the USA/Puerto Rico | 230 | 74 (88.1) | 135 (92.5) | 0.27 |
| Language spoken in the household | 230 | 1.0 | ||
| English | 78 (92.9) | 133 (91.1) | ||
| Spanish | 1 (1.2) | 3 (2.1) | ||
| Other | 5 (6.0) | 10 (6.8) | ||
| Education | 230 | 0.51 | ||
| No schooling or Grades 1–11 | 11 (13.1) | 24 (16.4) | ||
| High school or GED degree | 26 (31.0) | 42 (28.8) | ||
| Some college education or technical/vocational certificate | 18 (21.4) | 25 (17.1) | ||
| Associate or Bachelor’s degree | 26 (31.0) | 42 (28.8) | ||
| Professional or Graduate degree | 3 (3.6) | 13 (8.9) | ||
| Family income for past 12 months (USD) | 230 | 0.33 | ||
| ⩽19999 | 18 (21.4) | 39 (26.7) | ||
| 20000–49999 | 22 (26.2) | 31 (21.2) | ||
| 50000–99999 | 17 (20.2) | 28 (19.2) | ||
| ⩾100000 | 14 (16.7) | 35 (24.0) | ||
| Unknown | 13 (15.5) | 13 (8.9) | ||
| Aetiology of liver disease | ||||
| Alcohol | 231 | 34 (40.0) | 48 (32.9) | 0.28 |
| Hepatitis C infection | 231 | 37 (43.5) | 62 (42.5) | 0.88 |
| Autoimmune hepatitis | 231 | 4 (4.7) | 6 (4.1) | 1.0 |
| Non-alcoholic fatty liver disease | 231 | 20 (23.5) | 33 (22.6) | 0.87 |
| Hepatitis B infection | 231 | 1 (1.2) | 6 (4.1) | 0.43 |
| Primary sclerosing cholangitis | 231 | 4 (4.7) | 8 (5.5) | 1.0 |
| Primary biliary cholangitis | 231 | 9 (10.6) | 6 (4.1) | 0.05 |
| Cryptogenic cirrhosis | 231 | 3 (3.5) | 11 (7.5) | 0.22 |
| Other | 231 | 6 (7.1) | 5 (3.4) | 0.22 |
| MELD-Na score, median (IQR) | 227 | 15.0 (12.0–19.0) | 14.0 (10.0–17.0) | 0.006 |
| History of liver disease complications | ||||
| Ascites | 231 | 64 (75.3) | 91 (62.3) | 0.04 |
| Varices | 231 | 64 (75.3) | 93 (63.7) | 0.07 |
| Variceal bleeding | 231 | 29 (34.1) | 42 (28.8) | 0.40 |
| Encephalopathy | 231 | 55 (64.7) | 73 (50.0) | 0.03 |
| Multiple paracenteses | 231 | 31 (36.5) | 42 (28.8) | 0.23 |
| Spontaneous bacterial peritonitis | 231 | 4 (4.7) | 8 (5.5) | 1.0 |
| Hepatocellular carcinoma | 231 | 22 (25.9) | 58 (39.7) | 0.03 |
| Hepatic hydrothorax | 231 | 10 (11.8) | 13 (8.9) | 0.48 |
| Transjugular intrahepatic porto-systemic shunt | 231 | 11 (12.9) | 8 (5.5) | 0.05 |
| Past medical history | ||||
| Chronic obstructive pulmonary disease | 231 | 5 (5.9) | 7 (4.8) | 0.76 |
| Chronic bronchitis | 231 | 7 (8.2) | 7 (4.8) | 0.30 |
| Asthma | 231 | 12 (14.1) | 7 (4.8) | 0.01 |
| Venous thromboembolism | 231 | 5 (5.9) | 5 (3.4) | 0.50 |
| Diabetes mellitus | 231 | 22 (25.9) | 63 (43.2) | 0.009 |
| Hypertension | 231 | 28 (32.9) | 82 (56.2) | 0.001 |
| Hypercholesterolaemia | 231 | 16 (18.8) | 28 (19.2) | 0.95 |
| Congestive heart failure | 231 | 3 (3.5) | 8 (5.5) | 0.75 |
| Smoked at least 100 cigarettes in lifetime | 230 | 53 (63.1) | 82 (56.2) | 0.30 |
| Pack-years for ever-smokers, median (IQR) | 106 | 22 (6–35) | 11 (4–27) | 0.12 |
| Smoked in the last 30 days | 231 | 13 (15.3) | 16 (11.0) | 0.34 |
| Consumed alcohol | 230 | 76 (90.5) | 138 (94.5) | 0.25 |
| Duration of alcohol consumption (years), median (IQR) | 214 | 30 (20–37) | 32 (22–40) | 0.12 |
| Current alcohol use | 231 | 5 (5.9) | 12 (8.2) | 0.51 |
| Medications | ||||
| β-blockers | 230 | 44 (51.8) | 72 (49.7) | 0.76 |
| Spontaneous bacterial peritonitis prophylaxis/antibiotics | 230 | 42 (49.4) | 61 (42.1) | 0.28 |
| Bile acid resins | 230 | 14 (16.5) | 13 (9.0) | 0.09 |
| Midodrine | 230 | 0 (0.0) | 2 (1.4) | 0.53 |
Data are presented as n (%), unless otherwise indicated. HPS: hepatopulmonary syndrome; GED: General Educational Development; MELD: Model for End-Stage Liver Disease; IQR: interquartile range.
: Pearson’s chi-squared test, Fisher’s exact test, two-sample t-test, Wilcoxon rank sum test, as appropriate.
Dyspnoea was more common and functional class was significantly worse in patients with HPS (table 2); cyanosis and jaundice were more common in HPS. Other physical examination findings such as clubbing and asterixis appeared to be more common in patients with HPS although ascites, spider angiomata and an increased degree of encephalopathy were not.
TABLE 2.
Symptoms, signs, physical findings and laboratory evaluation
| Variable | Total participants, n | HPS (n=85) | No HPS (n=146) | p-value# |
|---|---|---|---|---|
|
| ||||
| Symptoms | ||||
| Dyspnoea | 231 | 34 (40.0) | 33 (22.6) | 0.005 |
| Chest pain | 231 | 8 (9.4) | 7 (4.8) | 0.17 |
| Orthopnoea | 230 | 1 (1.2) | 4 (2.7) | 0.65 |
| Palpitations | 231 | 5 (5.9) | 8 (5.5) | 1.0 |
| Syncope | 231 | 2 (2.4) | 2 (1.4) | 0.63 |
| Platypnoea | 230 | 2 (2.4) | 2 (1.4) | 0.62 |
| WHO functional class | 231 | <0.001 | ||
| I | 16 (18.8) | 64 (43.8) | ||
| II | 47 (55.3) | 57 (39.0) | ||
| III | 22 (25.9) | 25 (17.1) | ||
| IV | 0 (0.0) | 0 (0.0) | ||
| Signs | ||||
| Cyanosis | 231 | 7 (8.2) | 1 (0.7) | 0.004 |
| Jaundice | 231 | 43 (50.6) | 34 (23.3) | <0.001 |
| Lower extremity oedema | 231 | 47 (55.3) | 65 (44.5) | 0.11 |
| Clubbing | 231 | 11 (12.9) | 6 (4.1) | 0.01 |
| Spider angiomata | 231 | 3 (3.5) | 6 (4.1) | 1.0 |
| Asterixis | 230 | 36 (42.4) | 43 (29.7) | 0.05 |
| Ascites | 231 | 0.47 | ||
| Absent | 45 (52.9) | 89 (61.0) | ||
| Mild-moderate | 32 (37.6) | 47 (32.2) | ||
| Severe | 8 (9.4) | 10 (6.8) | ||
| Encephalopathy | 231 | 0.27 | ||
| Absent | 67 (78.8) | 127 (87.0) | ||
| Mild (I–II) | 17 (20.0) | 18 (12.3) | ||
| Severe (III–VI) | 1 (1.2) | 1 (0.7) | ||
| Physical examination, mean±SD | ||||
| Body mass index (kg·m−2) | 231 | 31±7 | 30±7 | 0.22 |
| Waist–hip ratio | 218 | 1.0±0.1 | 1.0±0.1 | 0.67 |
| Pulse (beats per min) | 231 | 74±14 | 72±13 | 0.26 |
| Respiratory rate (breaths per min) | 230 | 15±3 | 16±3 | 0.17 |
| Systolic blood pressure (mmHg) | 231 | 121±16 | 124±18 | 0.26 |
| Diastolic blood pressure (mmHg) | 231 | 66±9 | 70±11 | 0.006 |
| Oxygen saturation (%) | 231 | 96±4 | 98±2 | <0.001 |
| Orthodeoxia ¶ | 227 | 10 (12) | 7 (5) | 0.04 |
| Laboratory results, median (IQR) | ||||
| Blood urea nitrogen (mg·dL−1) | 209 | 14 (10–20) | 15 (11–21) | 0.19 |
| Creatinine (mg·dL−1) | 230 | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | 0.06 |
| Haemoglobin (g·dL−1) | 231 | 11.8 (10.4–13.7) | 12.4 (10.9–13.6) | 0.52 |
| Platelet count (109 per L) | 229 | 86 (62–109) | 92 (66–136) | 0.10 |
| International normalised ratio | 228 | 1.4 (1.2–1.6) | 1.3 (1.1–1.5) | 0.002 |
| Alanine aminotransferase (U·L−1) | 230 | 38 (28–64) | 46 (27–72) | 0.33 |
| Aspartate aminotransferase (U·L−1) | 230 | 64 (41–98) | 58 (36–92) | 0.17 |
| Total bilirubin (mg·dL−1) | 230 | 2.4 (1.5–3.7) | 1.5 (0.8–2.8) | <0.001 |
| Direct bilirubin (mg·dL−1) | 226 | 0.9 (0.6–1.6) | 0.6 (0.2–1.1) | <0.001 |
| Alkaline phosphatase (U·L−1) | 230 | 149 (93–220) | 148 (112–194) | 0.98 |
| Total protein (g·dL−1) | 230 | 6.8 (6.4–7.3) | 7.2 (6.6–7.6) | 0.004 |
| Albumin (g·dL−1) | 230 | 3.0 (2.6–3.4) | 3.2 (2.8–3.7) | 0.01 |
| Pulmonary function testing, mean±SD | ||||
| FVC (% pred) | 231 | 88±10 | 91±12 | 0.15 |
| FEV1 (% pred) | 231 | 89±11 | 89±12 | 0.68 |
| FEV1/FVC | 231 | 0.77±0.05 | 0.78±0.06 | 0.26 |
| Arterial blood gas, mean±SD | ||||
| pH | 231 | 7.45±0.04 | 7.44±0.04 | 0.17 |
| (mmHg) | 231 | 33±5 | 35±5 | 0.001 |
| (mmHg) | 231 | 78±13 | 92±14 | <0.001 |
| Alveolar–arterial oxygen gradient (mmHg), median (IQR) | 231 | 26 (20–37) | 12 (7–19) | <0.001 |
Data are presented as n (%), unless otherwise indicated. HPS: hepatopulmonary syndrome; WHO: World Health Organization; IQR: interquartile range; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; : arterial carbon dioxide tension; : arterial oxygen tension.
: Pearson’s chi-squared test, Fisher’s exact test, two-sample t-test, Wilcoxon rank sum test, as appropriate;
: increase of ⩾3% in oxygen saturation from pulse oximetry from sitting to supine position.
Patients with HPS had a mean oxygen saturation of 96% by pulse oximetry while sitting which was on average only 2% lower than the oxygen saturation of liver disease patients without HPS (table 2). While orthodeoxia (decrease in oxygen saturation by pulse oximetry ⩾3% from supine to seated position) was significantly more common in HPS only 12% of patients with HPS demonstrated this. Lung function between the groups was similar and and were significantly different between the groups (table 2). Only seven HPS patients (8%) had a or oxygen saturation by pulse oximetry <90% on ambient air. Abdominal imaging demonstrated ascites in 50 patients with HPS (60.2%) and 67 patients without HPS (46.5%) (p=0.05). Six patients in each group had portal vein thrombosis (p=0.36).
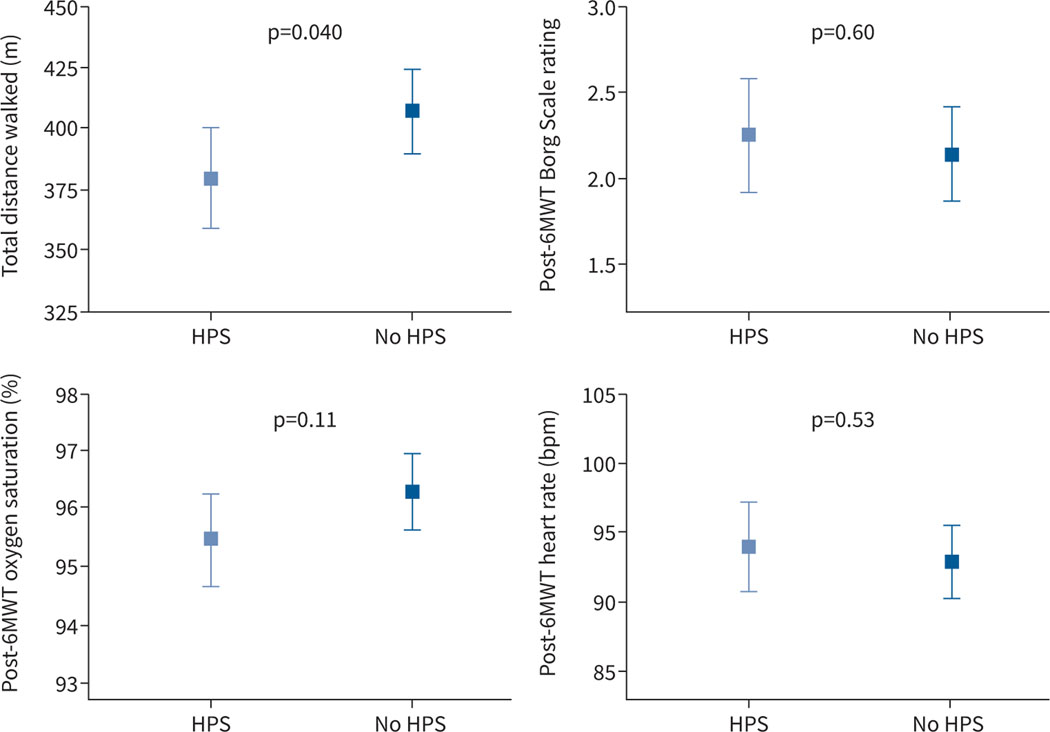
Patients with HPS had a 29 m (95% CI 3–56 m) shorter 6-min walk distance compared to liver disease controls with adjustment for age, sex and MELD-Na (p=0.04, n=197) (figure 1). There were no significant differences in oxygen saturation, heart rate or Borg score at the end of the walk.
FIGURE 1.
Least square means and 95% confidence intervals of 6-min walk test (6MWT) parameters. All values are adjusted for age, sex, Model for End-Stage Liver Disease (MELD)-Na and baseline values (other than distance). HPS: hepatopulmonary syndrome.
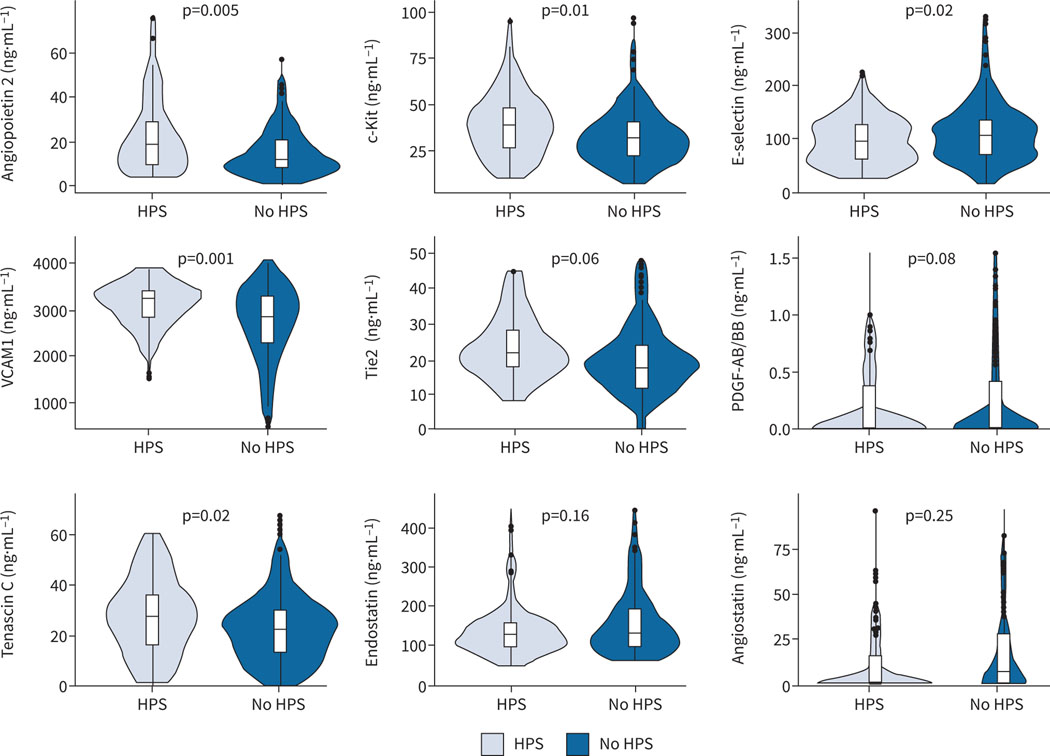
We performed a panel of blood biomarkers of angiogenesis with adjustment for age and MELD-Na score (figure 2 and supplementary table E2). Several pro-angiogenic biomarkers were significantly higher in patients with HPS compared to liver disease controls including angiopoietin 2, c-Kit, vascular cell adhesion molecule 1 and tenascin C. Tie2 and platelet-derived growth factor showed a nonsignificant increase in HPS patients compared to liver disease controls. Endostatin and angiostatin (both anti-angiogenic molecules) showed a nonsignificant reduction in HPS. We did not find differences in VEGF1 or VEGF receptors or other protein or flow cytometry biomarkers (supplementary table E2). There was no association between angiogenesis biomarkers and or after adjustment for MELD-Na in patients with HPS (data not shown).
FIGURE 2.
Violin plots of selected angiogenesis biomarkers. Boxes are interquartile ranges with median. Whiskers are observations within 1.5×interquartile range. Plots of platelet-derived growth factor (PDGF)-AB/BB, endostatin and angiostatin only show data that are within 1.5×interquartile range. p-values are from multivariable linear regression models adjusted for age and Model for End-Stage Liver Disease-Na score. HPS: hepatopulmonary syndrome; C-Kit: tyrosine protein kinase Kit; VCAM1: vascular cell adhesion molecule 1; Tie2: TEK tyrosine kinase, endothelial.
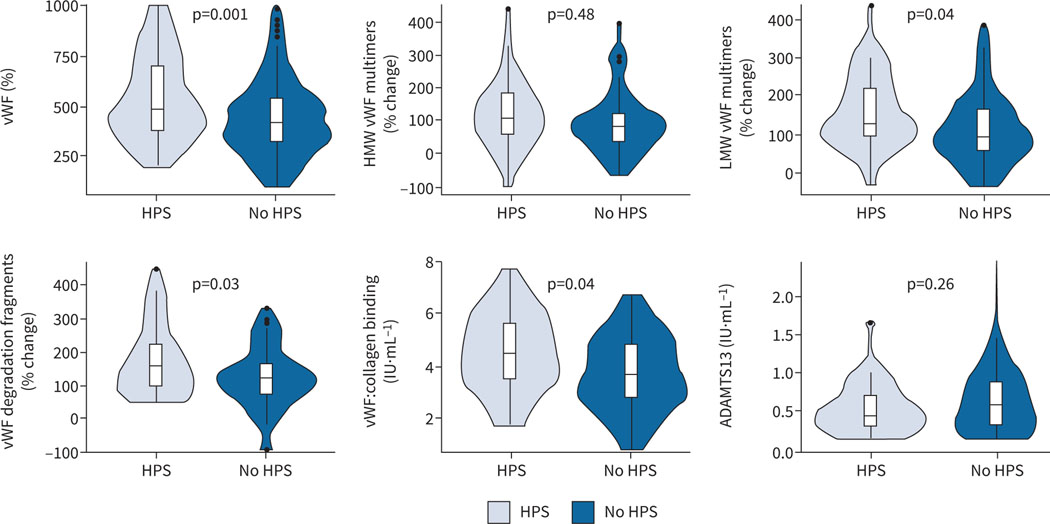
vWF antigen levels were significantly higher in patients with HPS compared to liver disease controls (figure 3 and supplementary table E2). Based on this finding, we performed analyses of circulating vWF multimer size in 40 randomly selected patients with HPS and 60 liver disease controls. HPS patients had significantly higher circulating levels of low-molecular-weight vWF multimers and vWF degradation fragments compared to liver disease controls after adjustment for age and MELD-Na. These findings were accompanied by significantly elevated levels of vWF clotting function in HPS (vWF:collagen binding). Levels of vWF antigen were strongly associated with angiopoietin 2 levels (r=0.50, p<0.001).
FIGURE 3.
Violin plots of von Willebrand factor (vWF) biomarkers. Boxes are interquartile ranges with median. Whiskers are observations within 1.5×interquartile range. p-values are from multivariable linear regression models adjusted for age and Model for End-Stage Liver Disease-Na score. HPS: hepatopulmonary syndrome; HMW: high molecular weight; LMW: low molecular weight; ADAMTS13: ADAM metallopeptidase with thrombospondin type 1 motif 13.
The median follow-up time in the cohort was 2 years (IQR 1.2–2.8 years), and there were 461.1 person-years of follow-up. 92 patients underwent liver transplantation (∼40% in each group), and there was no difference in the time to liver transplantation (supplementary figure E2). 13% of patients were not censored as alive or dead by the end of follow-up.
There were 421 hospitalisations (89 per 100 person-years); the median number of hospitalisations per patient was one (IQR 0–3). Liver transplantation was not considered as a hospitalisation. Supplementary figure E3 shows that the mean cumulative function plots of hospitalisations were similar for patients with HPS and liver disease controls, and supplementary figure E4 shows the number of hospitalisations in the groups. HPS was not associated with the risk of recurrent hospitalisation with adjustment for age, sex and MELD-Na using the gamma frailty model (HR 1.06, 95% CI 0.77–1.46, p=0.70). Sensitivity analyses with adjustment for transplantation, examining transplantation-free hospitalisation, and using Andersen–Gill, Prentice–Williams–Peterson gap-time and total-time, and multistate models still showed no difference in hospitalisation (data not shown).
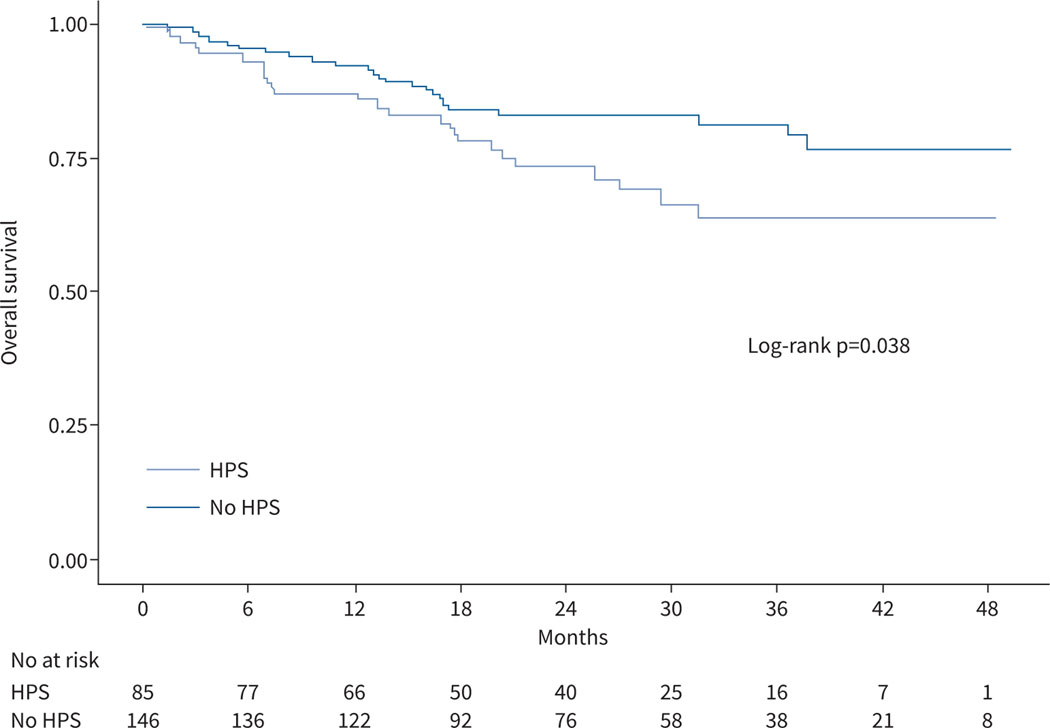
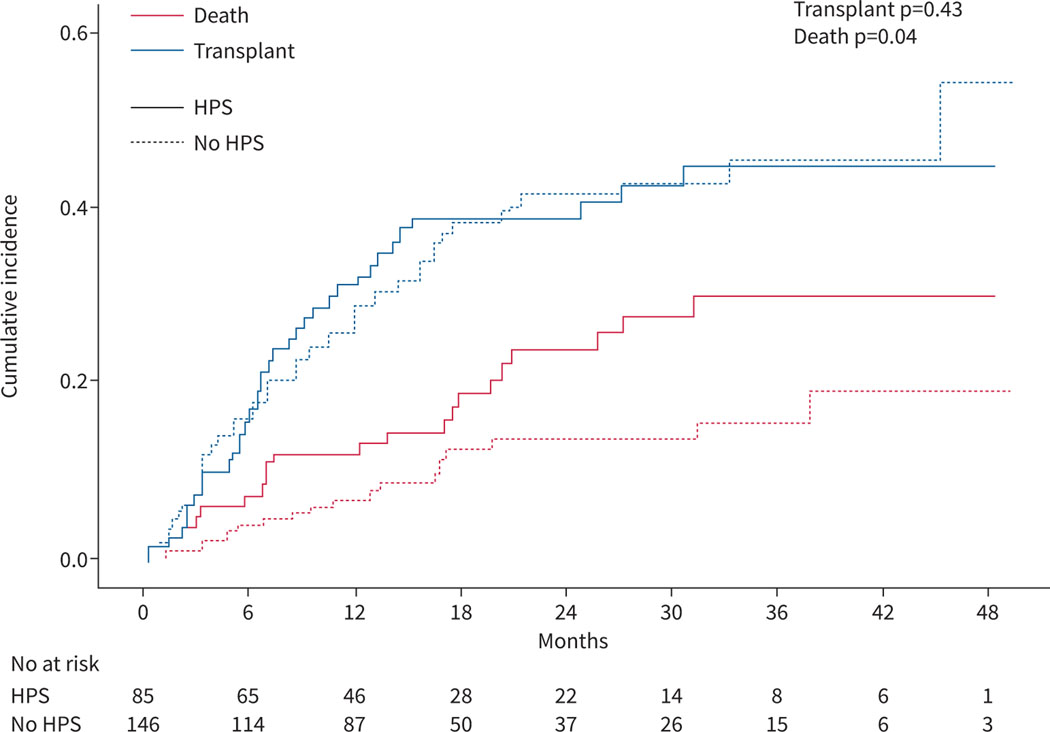
Patients with HPS had worse survival than liver disease controls (figure 4, log rank test p=0.04). 24 HPS patients (28%) and 25 liver disease controls (17%) died during follow-up. Patients with HPS had a lower probability of being alive than liver disease controls at 1 year (87% versus 92%), 2 years (73% versus 83%) and 3 years (63% versus 81%). Causes of death are shown in supplementary table E3. Cox proportional hazards models showed that patients with HPS had an 80% increase in the risk of death in bivariate (HR 1.80, 95% CI 1.03–3.16, p=0.04) and multivariate (HR 1.79, 95% CI 1.02–3.15, p=0.04) analyses adjusted for age and MELD-Na (table 3). A model with adjustment for liver transplantation as a time-varying covariate showed similar results (table 3). When transplantation was considered as a competing risk using the Fine–Gray model, the sub-distributional HR of HPS versus liver disease controls for death was 1.91 (95% CI 1.06–3.45, p=0.03) after adjustment for age and MELD-Na (figure 5). A multistate model (supplementary figure E5) suggested that HPS was associated with an increased risk of death in patients without liver transplantation (HR 1.84, 95% CI 0.99–3.44, p=0.05) but not with the chances of receiving liver transplantation (HR 1.03, 95% CI 0.67–1.58, p=0.89) or the risk of death after liver transplantation (HR 0.99, 95% CI 0.24–4.15, p=0.99).
FIGURE 4.
Kaplan–Meier curves comparing patients with hepatopulmonary syndrome (HPS) and liver disease controls.
TABLE 3.
Models for the risk of death
| Models | HR (95% CI) | p-value | aHR (95% CI) | p-value# | E-value (for the limit of the CI) |
|---|---|---|---|---|---|
|
| |||||
| Overall survival | 1.80 (1.03–3.16) | 0.04 | 1.79 (1.02–3.15) | 0.04 | 2.35 (1.13) |
| Overall survival with transplant as a time-varying covariate | 1.78 (1.02–3.13) | 0.04 | 1.71 (0.97–3.00) | 0.06 | 2.25 (1.00) |
| Transplant-free survival | 1.99 (1.07–3.71) | 0.03 | 1.84 (0.99–3.44) | 0.05 | 2.42 (1.00) |
| Survival with transplant as competing risk (Fine–Gray model) ¶ | 1.91 (1.06–3.45) | 0.03 | 1.87 (1.04–3.35) | 0.04 | 2.45 (1.20) |
| Multistate model + | |||||
| Transition from evaluation to liver transplant | 1.11 (0.73–1.69) | 0.62 | 1.03 (0.67–1.58) | 0.89 | |
| Transition from evaluation to death without liver transplant | 1.99 (1.07–3.71) | 0.03 | 1.84 (0.99–3.44) | 0.05 | 2.42 (1.00) |
| Transition from liver transplant to death | 0.94 (0.23–3.80) | 0.93 | 0.99 (0.24–4.15) | 0.99 | |
HR: hazard ratio; aHR: adjusted hazard ratio.
: adjusted for age and Model for End-Stage Liver Disease-Na score;
: sub-distributional HR for mortality;
: schema for multistate model is shown in supplementary figure S3.
FIGURE 5.
Predicted cumulative incidences of death and liver transplantation for patients with hepatopulmonary syndrome (HPS) and liver disease controls.
The association of HPS with overall survival was partially attenuated after adjustment for angiopoietin 2 (28% attenuated, HPS versus no HPS HR=1.30, 95% CI 0.70–2.41, p=0.40) or vWF levels (21% attenuated, HPS versus no HPS HR=1.42, 95% CI 0.77–2.61, p=0.30). This suggests that these biomarkers were in the causal pathway or were confounders of the association of HPS with outcomes. Other biomarkers did not have qualitatively important impacts on the effect estimate. After excluding patients with hepatocellular carcinoma, findings were generally consistent with the main results albeit nonsignificant in some cases with the smaller sample size and lower power.
The differences in overall risk of death for HPS versus liver disease controls did not differ based on (p for interaction=0.30) or (p for interaction=0.40), suggesting that the relationship between HPS and worse outcomes was not dependent on the severity of HPS. We also compared the survival of patients with HPS with all others in the prospective cohort without portopulmonary hypertension, including those with restrictive or obstructive lung diseases, patent foramina ovalia, or missing data who were excluded from the primary analyses. Many of these excluded patients likely had HPS coexisting with their underlying exclusion. For example, of the 105 patients excluded for restrictive or obstructive lung disease, 49 (47%) had a positive contrast-enhanced transthoracic echo and 38 (36%) had abnormal and a positive contrast-enhanced transthoracic echo. Even with including these patients in the “control” group, HPS still appeared to be associated with a higher risk of death (HR 1.56, 95% CI 0.97–2.50, p=0.07) with a weaker effect estimate, as expected with the likely presence of undiagnosable HPS in the “control” group.
Discussion
We have shown that HPS was a common complication in patients who were referred for evaluation for liver transplantation. In contrast to some prior studies, patients with HPS had more severe liver disease and complications of portal hypertension. Despite only mild abnormalities in oxygenation of the blood, with only 8% being clinically hypoxaemic, HPS patients had more respiratory symptoms, worse functional class and lower 6-min walk distance after adjustment for severity of liver disease, which is a novel finding. HPS patients were characterised by a profile of dysregulated angiogenic peptides compared to liver disease controls even after accounting for differences in the severity of liver disease, which has not been demonstrated previously. Patients with HPS had a risk of hospitalisation that was similar to that of liver disease controls; however, HPS patients had a significantly increased risk of death overall regardless of the degree of abnormal oxygenation. Some of this increased risk was accounted for by higher levels of angiopoietin 2 and vWF in patients with HPS, representing the first possible biological mechanisms for how HPS affects outcome in patients. This study used sophisticated research-grade prospective heart and lung phenotyping and adjusted for confounders in multivariate analyses (notably severity of liver disease), distinguishing our results from those of other studies.
We found that 37% of our patients without other potential causes of oxygen abnormalities and ⩾21% of the entire cohort had HPS based on established diagnostic criteria. A prior study (which recruited candidates for liver transplantation 10 years before the current study) did not show differences in severity of liver disease between patients with HPS and those without HPS [3]. This may be due to the evolving characteristics of patients being evaluated for liver transplantation over time or spectrum bias. For example, almost one quarter of patients in the current sample had non-alcoholic fatty liver disease compared to only 11% in the prior study, and approximately one third of the current sample had hepatocellular carcinoma compared to only 9% in the prior study. These differences may reflect not only changes in the general population (e.g. increased obesity) and aetiologies of advanced liver disease but also newer exception point policies that prioritise certain patients for transplantation (e.g. with hepatocellular carcinoma) in the USA, thereby influencing the composition of referrals for evaluation. The higher severity of liver disease in patients with HPS in the current study (reflected by higher MELD-Na score and more complications of portal hypertension) could either be a cause or consequence of HPS and has been seen in other recent cohorts [2, 4, 26]. One study of patients listed for liver transplantation actually showed lower laboratory MELD scores in those with HPS listed for liver transplantation (although this study’s focus on HPS with exception scores could introduce selection bias) [27]. More severe liver disease could lead to HPS as one of the sequelae or the presence of HPS could causally contribute to more liver disease complications; angiogenesis or other pathobiological processes could cause both worse liver disease and HPS.
There were small differences in oxygen saturation in HPS and non-HPS patients, and only 8% of HPS patients were hypoxaemic [3]. Even so, patients with HPS had both clinically and statistically significantly shorter distance walked in 6 min compared to other patients with liver disease. This difference in exercise capacity substantiates the greater symptoms reported by patients with HPS and the worse functional class assigned by clinicians [28]. Worse liver disease did not explain the difference in exercise capacity because this was independent of MELD-Na. Other systemic processes may cause worse symptoms and exercise capacity in HPS.
Studies in experimental models of HPS have demonstrated increased lung angiogenesis [11]. We showed higher levels of several important circulating biomarkers of angiogenesis, including angiopoietin 2, c-Kit and possibly Tie2, in HPS even after adjustment for the differences in severity of liver disease. Higher angiopoietin 2 (which signals via Tie2) has been linked to increased pathological angiogenesis in several studies of patients with liver disease [29–31]. Tenascin C is an extracellular matrix protein that has been linked to vascular remodelling in the lung. Both angiostatin and endostatin tended to be lower in HPS, which parallels their roles as anti-angiogenic molecules preventing HPS in the experimental model [11]. Vascular cell adhesion molecule 1 was higher in HPS as in one prior study, suggesting that leukocyte adhesion to the endothelium may play a role [32]. We have previously demonstrated lower bone morphogenetic protein 9 and 10 levels (linked to increased angiogenesis) in a small sample of HPS patients from this cohort [33].
HPS patients had significantly higher levels of vWF antigen, low-molecular-weight vWF multimers and vWF degradation fragments. Levels of vWF multimers and/or vWF degradation fragments may alter angiopoietin signalling, a potent destabiliser of blood vessels [34–36]; vWF levels were significantly associated with angiopoietin 2 levels in our study [34–38]. Indeed, abnormalities in vWF metabolism are associated with angiodysplasia and arteriovascular malformations in multiple diseases [39–46]. In infants with single ventricle anatomy and a superior-cavopulmonary (Glenn) circulation, abnormalities in vWF and angiopoietin 2 play a role in the development of pulmonary angiodysplasia and arteriovascular malformations also without increased VEGF [47]. High shear stress from continuous-flow left ventricular assist devices increases enzymatic degradation of large vWF multimers into vWF degradation fragments [37], which may alter circulating levels of angiopoietin 2 [48] and cause mucosal arteriovascular malformations [37, 38]. Patients with HPS may more commonly have varices, which may relate to these biomarker findings.
There was no association between the presence of HPS and the risk of hospitalisation. Even so, we found a significant association between HPS and a higher overall risk of death with similar findings when adjusting for liver transplantation, analysing transplantation-free survival and with liver transplantation as a competing risk. The major population accounting for this finding appeared to be patients who did not receive liver transplantation; HPS does not generally pose a significant increase in risk after liver transplantation in recent studies [2, 26, 27, 49]. When comparing patients with HPS to the rest of the liver transplantation candidates in our cohort (including patients with restrictive and obstructive lung diseases, patent foramina ovalia, etc.), there was still a 56% increase in risk of death overall (95% CI −3% to 250%), a conservative estimate considering that the comparison group likely included up to one third of patients with HPS (undiagnosable due to the other comorbid conditions).
Prior studies of survival have been single centre, without multivariate analyses, and without careful phenotyping of the presence of HPS (or the exclusion of patients with other reasons for abnormal oxygenation) [4–6, 50]. Our prior prospective multicentre cohort study showed that HPS was independently associated with an increased risk of death even after adjusting for MELD score and liver transplantation [3]. More recently, two single-centre cohort studies showed that HPS was associated with an increased risk of death, but not after multivariate adjustment [4, 26]. In a prior study of patients listed for liver transplantation in the United Network for Organ Sharing, we found that patients with HPS actually had better overall survival than patients without HPS, which was attributable to HPS patients receiving higher priority for liver transplantation with MELD exception [27].
The association of HPS with worse outcomes in liver disease has vexed some owing to the very mild subclinical abnormalities in oxygenation in most HPS patients. However, we have now replicated this finding in two distinct multicentre prospective cohort studies with similar effect estimates. The role of angiogenesis (which causes other well-known sequelae of portal hypertension) may address the question of how HPS affects survival, although findings from a recent clinical trial of sorafenib to target angiogenesis in patient with HPS were null [12].
The only therapy that reverses HPS is liver transplantation. The fact that HPS is associated with worse survival in patients with liver disease, particularly in those patients who do not receive liver transplantation, underscores the need to develop effective medical therapies. These findings may further justify screening for HPS using arterial blood gases and contrast-enhanced transthoracic echocardiography in all candidates for liver transplantation [51] and argue for consideration of carefully phenotyped HPS (irrespective of the ) in the prioritisation for liver transplantation.
There are several limitations to this study. First, we only included patients being considered for liver transplantation in the USA, which is a select population. Differences in the makeup of the liver transplantation populations and allocation policies in other countries warrant similar studies of HPS in those populations. Second, we excluded some patients from the main analyses in order to create “clean” phenotypes. However, survival analyses including all patients still suggested an increased risk of death for patients with HPS. Third, it is possible that unmeasured or imprecisely measured variables could have confounded the findings, quantified by the E-values. Hypothesis-generating analyses suggested that angiopoietin 2, vWF and other angiogenic biomarkers could explain how HPS reduces survival. We did not correct for multiple comparisons owing to the multiple hypotheses investigated, therefore type 1 error is possible. Finally, although this was a multicentre study and conducted over several years, the sample size was relatively small. Still, this cohort remains one of the largest (and only) prospective multicentre studies of HPS with extensive protocolised lung and heart phenotyping to our knowledge.
In summary, HPS is common in patients being evaluated for liver transplantation and is associated with worse functional status, exercise capacity and overall survival. Understanding the mechanisms of the adverse effects of HPS and developing effective medical therapies merit high priority to improve the outcomes of patients with advanced liver disease.
Supplementary Material
Support statement:
This work was supported by grants NIH R01 HL113988, K24 HL103844, K23 DK090209 and K23-HL141584. Funding information for this article has been deposited with the Crossref Funder Registry.
Footnotes
Conflict of interest: None declared.
References
- 1.Rodriguez-Roisin R, Krowka MJ, Herve P, et al. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24: 861–880. [DOI] [PubMed] [Google Scholar]
- 2.Al-Harbi A, Abdullah K, Al-Abdulkareem A, et al. Prevalence, severity, and prognostic effect of hepatopulmonary syndrome in liver transplant candidates. Ann Transplant 2016; 21: 180–184. [DOI] [PubMed] [Google Scholar]
- 3.Fallon MB, Krowka MJ, Brown RS, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008; 135: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younis I, Sarwar S, Butt Z, et al. Clinical characteristics, predictors, and survival among patients with hepatopulmonary syndrome. Ann Hepatol 2015; 14: 354–360. [PubMed] [Google Scholar]
- 5.Schenk P, Schoniger-Hekele M, Fuhrmann V, et al. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology 2003; 125: 1042–1052. [DOI] [PubMed] [Google Scholar]
- 6.Swanson K, Wiesner R, Krowka M. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 2005; 41: 1122–1129. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Zhang J, Yang W, et al. Role of splenic reservoir monocytes in pulmonary vascular monocyte accumulation in experimental hepatopulmonary syndrome. J Gastroenterol Hepatol 2016; 31: 1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Zhang J, Hu B, et al. The role of receptor tyrosine kinase activation in cholangiocytes and pulmonary vascular endothelium in experimental hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 2014; 306: G72–G80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Yang W, Luo B, et al. The role of CX3CL1/CX3CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome. J Hepatol 2012; 57: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts KE, Kawut SM, Krowka MJ, et al. Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology 2010; 139: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Luo B, Tang L, et al. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology 2009; 136: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawut SM, Ellenberg SS, Krowka MJ, et al. Sorafenib in hepatopulmonary syndrome: a randomized, double-blind, placebo-controlled trial. Liver Transpl 2019; 25: 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 14.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008; 359: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crapo RO, Jensen RL, Hegewald M, et al. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med 1999; 160: 1525–1531. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S population. Am J Respir Crit Care Med 1999; 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 19.Abrams GA, Jaffe CC, Hoffer PB, et al. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology 1995; 109: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 20.Rose JA, Erzurum S, Asosingh K. Biology and flow cytometry of proangiogenic hematopoietic progenitors cells. Cytometry A 2015; 87: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hougaard P. Frailty models for survival data. Lifetime Data Anal 1995; 1: 255–273. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 23.Meira-Machado L, de Una-Alvarez J, Cadarso-Suarez C, et al. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res 2009; 18: 195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019; 321: 602–603. [DOI] [PubMed] [Google Scholar]
- 25.Stekhoven DJ, Buhlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 2012; 28: 112–118. [DOI] [PubMed] [Google Scholar]
- 26.Pascasio JM, Grilo I, Lopez-Pardo FJ, et al. Prevalence and severity of hepatopulmonary syndrome and its influence on survival in cirrhotic patients evaluated for liver transplantation. Am J Transplant 2014; 14: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg DS, Krok K, Batra S, et al. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology 2014; 146: 1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faustini-Pereira JL, Homercher-Galant L, Garcia E, et al. Exercise capacity of cirrhotic patients with hepatopulmonary syndrome. Ann Hepatol 2015; 14: 361–368. [PubMed] [Google Scholar]
- 29.Lefere S, Van de Velde F, Hoorens A, et al. Angiopoietin 2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology 2019; 69: 1087–1104. [DOI] [PubMed] [Google Scholar]
- 30.Osawa Y, Yoshio S, Aoki Y, et al. Blood angiopoietin 2 predicts liver angiogenesis and fibrosis in hepatitis C patients. BMC Gastroenterol 2021; 21: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholz A, Rehm VA, Rieke S, et al. Angiopoietin 2 serum levels are elevated in patients with liver cirrhosis and hepatocellular carcinoma. Am J Gastroenterol 2007; 102: 2471–2481. [DOI] [PubMed] [Google Scholar]
- 32.Raevens S, Coulon S, Van Steenkiste C, et al. Role of angiogenic factors/cell adhesion markers in serum of cirrhotic patients with hepatopulmonary syndrome. Liver Int 2015; 35: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 33.Rochon ER, Krowka MJ, Bartolome S, et al. BMP9/10 in pulmonary vascular complications of liver disease. Am J Respir Crit Care Med 2020; 201: 1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randi AM, Laffan MA, Starke RD. Von Willebrand factor, angiodysplasia and angiogenesis. Mediterr J Hematol Infect Dis 2013; 5: e2013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randi AM, Smith KE, Castaman G. von Willebrand factor regulation of blood vessel formation. Blood 2018; 132: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011; 117: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartoli CR, Restle DJ, Zhang DM, et al. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: mechanical demolition and enzymatic cleavage. J Thorac Cardiovasc Surg 2015; 149: 281–289. [DOI] [PubMed] [Google Scholar]
- 38.Bartoli CR, Zhang DM, Hennessy-Strahs S, et al. Clinical and in vitro evidence that left ventricular assist device-induced von Willebrand factor degradation alters angiogenesis. Circ Heart Fail 2018; 11: e004638. [DOI] [PubMed] [Google Scholar]
- 39.Castaman G, Federici AB, Tosetto A, et al. Different bleeding risk in type 2A and 2M von Willebrand disease: a 2-year prospective study in 107 patients. J Thromb Haemost 2012; 10: 632–638. [DOI] [PubMed] [Google Scholar]
- 40.Heilmann C, Trummer G, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients on long-term support with HeartMate II. Eur J Cardiothorac Surg 2017; 51: 587–590. [DOI] [PubMed] [Google Scholar]
- 41.Jehangir A, Pathak R, Ukaigwe A, et al. Association of aortic valve disease with intestinal angioectasia: data from the Nationwide Inpatient Sample. Eur J Gastroenterol Hepatol 2018; 30: 438–441. [DOI] [PubMed] [Google Scholar]
- 42.Kang J, Hennessy-Strahs S, Kwiatkowski P, et al. Continuous-flow LVAD support causes a distinct form of intestinal angiodysplasia. Circ Res 2017; 121: 963–969. [DOI] [PubMed] [Google Scholar]
- 43.Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med 2003; 349: 343–349. [DOI] [PubMed] [Google Scholar]
- 44.Godino C, Lauretta L, Pavon AG, et al. Heyde’s syndrome incidence and outcome in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol 2013; 61: 687–689. [DOI] [PubMed] [Google Scholar]
- 45.Loscalzo J. From clinical observation to mechanism—Heyde’s syndrome. N Engl J Med 2012; 367: 1954–1956. [DOI] [PubMed] [Google Scholar]
- 46.Warkentin TE, Moore JC, Anand SS, et al. Gastrointestinal bleeding, angiodysplasia, cardiovascular disease, and acquired von Willebrand syndrome. Transfus Med Rev 2003; 17: 272–286. [DOI] [PubMed] [Google Scholar]
- 47.Bartoli CR, Hennessy-Strahs S, Dowling RD, et al. Abnormalities in the von Willebrand-angiopoietin axis contribute to dysregulated angiogenesis and angiodysplasia in children with a Glenn circulation. JACC Basic Transl Sci 2021; 6: 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabit CE, Chen P, Kim GH, et al. Elevated angiopoietin 2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation 2016; 134: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raevens S, Rogiers X, Geerts A, et al. Outcome of liver transplantation for hepatopulmonary syndrome: a Eurotransplant experience. Eur Respir J 2019; 53: 1801096. [DOI] [PubMed] [Google Scholar]
- 50.Sulieman B, Voigt M, Katz D, et al. Data driven policy for points allocation in hepatopulmonary syndrome. Hepatology 2005; 42: 347A. [Google Scholar]
- 51.Forde KA, Fallon MB, Krowka MJ, et al. Pulse oximetry is insensitive for detection of hepatopulmonary syndrome in patients evaluated for liver transplantation. Hepatology 2019; 69: 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.