Abstract
Vaccination is a key intervention to prevent and control cholera in conjunction with water, sanitation and hygiene activities. An oral cholera vaccine (OCV) stockpile was established by the World Health Organization (WHO) in 2013. We reviewed its use from July 2013 to all of 2018 in order to assess its role in cholera control. We computed information related to OCV deployments and campaigns conducted including setting, target population, timelines, delivery strategy, reported adverse events, coverage achieved, and costs.
In 2013–2018, a total of 83,509,941 OCV doses have been requested by 24 countries, of which 55,409,160 were approved and 36,066,010 eventually shipped in 83 deployments, resulting in 104 vaccination campaigns in 22 countries. OCVs had in general high uptake (mean administrative coverage 1st dose campaign at 90.3%; 2nd dose campaign at 88.2%; mean survey-estimated two-dose coverage at 69.9%, at least one dose at 84.6%) No serious adverse events were reported. Campaigns were organized quickly (five days median duration). In emergency settings, the longest delay was from the occurrence of the emergency to requesting OCV (median: 26 days). The mean cost of administering one dose of vaccine was 2.98 USD.
The OCV stockpile is an important public health resource. OCVs were generally well accepted by the population and their use demonstrated to be safe and feasible in all settings. OCV was an inexpensive intervention, although timing was a limiting factor for emergency use. The dynamic created by the establishment of the OCV stockpile has played a role in the increased use of the vaccine by setting in motion a virtuous cycle by which better monitoring and evaluation leads to better campaign organization, better cholera control, and more requests being generated. Further work is needed to improve timeliness of response and contextualize strategies for OCV delivery in the various settings.
Keywords: Oral Cholera Vaccine, Vaccination Campaigns, Global Task Force on Cholera Control, Cholera Elimination, End Cholera
1. Introduction
Vibrio cholerae (serotypes O1 and, to a much lesser extent, O139) is a highly transmissible bacterium, which can cause a rapidly dehydrating, watery diarrheal disease known as cholera [1]. In 2017, 34 countries reported a total of 1,227,391 cases including 5654 deaths to the World Health Organization (WHO), resulting in an overall case fatality rate (CFR) of 0.5% [2]. However, official figures are significantly underreported and the global cholera burden was estimated at 2.86 million cases (range: 1.3–4.0 m) and 95,000 deaths (range: 21,000–143,000) per year and a population at risk of approximately 1.3 billion in 69 endemic countries [3]. Cholera outbreaks are frequent and prolonged in endemic areas with recurrent seasonal patterns [4]. Outbreaks also occur in non-endemic areas, initiated by exogenous introduction of V. cholerae, often associated with complex emergencies that result in the breakdown of infrastructure or population displacement [5]. Although cholera affects any age group, children under five years of age are at higher risk of contracting cholera in endemic settings [6].
Successful control of cholera is directly related to improvements in hygiene and availability of clean drinking water as well as sanitary disposal of waste, disease detection and case management, as was seen with the curbing of the Latin American cholera epidemic in the 1990s [7]. It is therefore not surprising that cholera remains a persistent problem in many resource-limited settings where poverty, political instability, natural calamities, or security conditions make implementation of appropriate surveillance and control measures challenging [8]. In those contexts, vaccination can serve as a complementary strategy for cholera prevention and control, which can be implemented for short to medium term, while access to other primary prevention measures such as safe water, sanitation and hygiene (WASH) improves [9]. Oral cholera vaccines (OCVs) currently available in the global market include Dukoral (SBL Vaccin, Sweden), Shanchol (Shantha Biotechnics Ltd, India), and Euvichol (Eubiologics, South Korea) [10,11]. They have an average two-dose efficacy of 58% (95% confidence interval [CI], 42–69%) and effectiveness of 76% (95% CI, 62–85%) for at least 3 years [12], with one study showing efficacy for up to 5 years[13]. Although OCV currently used in mass campaigns are administered according to a two-dose regimen 14 days apart, a single dose provides short-term protection, with a pooled effectiveness of 69% (95% CI 35–85%) within the first year, which has important implications for outbreak management [12].
In 2013, WHO, with funding (i.e. vaccine costs and since 2016 operational costs for campaign implementation) from GAVI, the Vaccine Alliance, created an OCV stockpile to respond to emergency situations [14], including outbreaks and humanitarian crises [15-17]. The OCV stockpile is also used in non-emergency settings as one of the key strategies to contribute controlling cholera in endemic areas (i.e. “cholera hotspots”), [18,19]. Ideally, OCV should be used in conjunction with other preventative measures such as WASH interventions and social mobilization. The global stockpile only includes prequalified vaccines (i.e. meeting WHO recommendations in terms of quality, safety and efficacy) [20]. Prior to 2011, Dukoral was the only WHO prequalified OCV, but since it requires a buffer to be dissolved in potable water prior to administration, its use was not ideal for mass vaccination campaigns [11]. Shanchol (derived from Dukoral thanks to a successful technology transfer agreement, prequalified in 2011) and Euvichol (result of a similar technology transfer and prequalified in 2015) are modified versions of Dukoral, which do not require a buffer, making them more suitable for use during mass vaccination campaigns [10]. These two more recently prequalified OCVs are the vaccines available through the stockpile for public health purposes for all individuals above one year of age living in the targeted areas; whereas Dukoral is predominantly used for travelers. In all settings, a series of criteria should be considered to guide the decision to vaccinate, namely the risk of cholera among the targeted populations and the risk of geographic spread; and the programmatic capacity to cover as many person as possible who are eligible to receive the vaccine and living in the targeted area (e.g. those aged ≥1 year) [9]. Because of constraints on global supply and availability, OCV doses for emergency use are released from the stockpile after review of the requests by the International Coordinating Group (ICG), composed of UNICEF, Médecins Sans Frontières (MSF), the International Federation of Red Cross and Red Crescent Societies (IFRC), and WHO of applications by countries (i.e. primarily Ministries of Health, often with the support of partner institutions) [21]. Since 2016, a mechanism to access OCV in non-emergency settings to contribute controlling endemic cholera in hotspots was established under the OCV Working Group (WG) of the Global Task Force on Cholera Control (GTFCC), a WHO collaborative mechanism between institutions active in cholera control [22]. Non-emergency requests are assessed by the GTFCC OCV WG based on the risk of cholera and contextual criteria (e.g. vulnerabilities which make cholera a recurrent problem), and are conditional on the country’s capacity and commitment for long-term cholera control/elimination. Multisectoral plans integrating the use of OCV with other interventions (most notably WASH improvement plans) are required to be appended to GTFCC requests and are assessed also by WASH specialists within the GTFCC. In general ICG requests result in one vaccination campaign conducted in urgency for a specific emergency; while GTFCC requests tend to be larger and are intended for systematic OCV use as part of a multisectoral National Cholera Elimination or Control Plan (NCP) targeting a country’s cholera hotspots with multiple campaigns which can be rolled out in phases for more than one year [23]. Once approved, shipment of vaccines to requesting countries is handled by UNICEF Supply Division. A minimum quantity (set at two million doses for 2018, and revised yearly based on global supply) is always reserved for emergency use, and the remaining doses are allocated to non-emergency situations depending on availability at any given time [24].
To draw lessons from OCV use in different contexts and improve its implementation, we reviewed information related to OCV deployments and related campaigns conducted since the creation of the stockpile.
2. Methods
2.1. Study design
We obtained information on OCV doses requested, approved, and shipped globally from July 2013 (date of stockpile creation) until all of 2018 by the ICG or GTFCC secretariats. We obtained information on population targeted, interval from request to delivery (especially with regards to outbreak response), delivery strategy used, adverse events recorded, coverage achieved, and costs incurred from reports of vaccination campaigns submitted by requestors following campaign implementation. We supplemented this information with published reports by using the search terms “cholera vaccination” and “cholera vaccination campaign” on PubMed. We excluded use of OCV from the stockpile related to research studies.
2.2. Definitions and measures
OCV doses were requested from the stockpile using standard forms available on the WHO Cholera webpages, either through the GTFCC OCV WG or the ICG. One of these groups either approved or did not approve the request. Doses shipped (i.e. deployments) were the ones eventually deployed to requesting countries, prioritized based on vaccines availability in the stockpile, and whether requests were made in emergency (i.e. outbreak response to curb spread of cholera or humanitarian crisis to prevent a cholera outbreak in a vulnerable setting) or non-emergency (i.e. to contribute controlling endemic cholera in hotspots) settings. A deployment may have resulted in more than one campaign (e.g. doses shipped to cover multiple areas at different times), while a campaign may be the result of more than one deployment (e.g. when doses for the first round and for the second round are shipped separately). The target population was defined as the number of individuals eligible for the vaccine (i.e. above one year of age) who are members of the circumscribed population (e.g. a geographic area) to whom OCV was to be offered. This figure was generally an estimation based on existing administrative population figures, or a more precise figure based on a study census. Administrative coverage was defined as the proportion of the target population who received one dose of the vaccine, during the vaccination round, by dividing the number of doses administered per round (i.e. 1st dose campaign or 2nd dose campaign) by the target population. The estimated vaccine coverage, assessed during population surveys conducted in the close follow-up of the vaccine campaigns, was defined as the percentage of the target population who received at least one or two doses of the vaccine. Adverse events following immunization (AEFI) were defined as reported medical incidents that take place after vaccination and cause concern. An AEFI was considered serious if it resulted in death, was life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity, was a congenital anomaly/birth defect, or required intervention to prevent permanent impairment or damage. To allow comparison of the expenses for vaccination across various campaigns, the costing lines were categorized: cost of vaccines, international shipment, and operational costs. The delivery cost per fully immunized person was calculated using the total local expenses incurred (excluding vaccine, international shipment and technical support costs) as the numerator and the number of fully immunized persons as the denominator.
Key dates of OCV campaign events were taken from various sources including outbreak situation reports (sitreps), campaign reports, and ICG / GTFCC secretariats’ records. Major milestones included in the timeline for OCV vaccination were as follows: (1) planning events (from the date of the first laboratory confirmation of cholera case to the dates of the official decision to use OCV and of the request; (2) administrative events (from receipt of OCV request, to approval and eventual arrival of shipment to the country); and (3) vaccine implementation events (for both rounds of vaccination, when applicable). For emergency vaccination, time to partial protection was defined as the interval from laboratory confirmation of cholera or the occurrence of the humanitarian crisis to seven days after start of the first round, in days. Time to full protection was defined as the interval from laboratory confirmation to seven days after end of the second round, in days. We obtained data on OCV use before the creation of the stockpile (pre-2013) starting from 1997 from a previous publication [25].
3. Results
3.1. Deployments
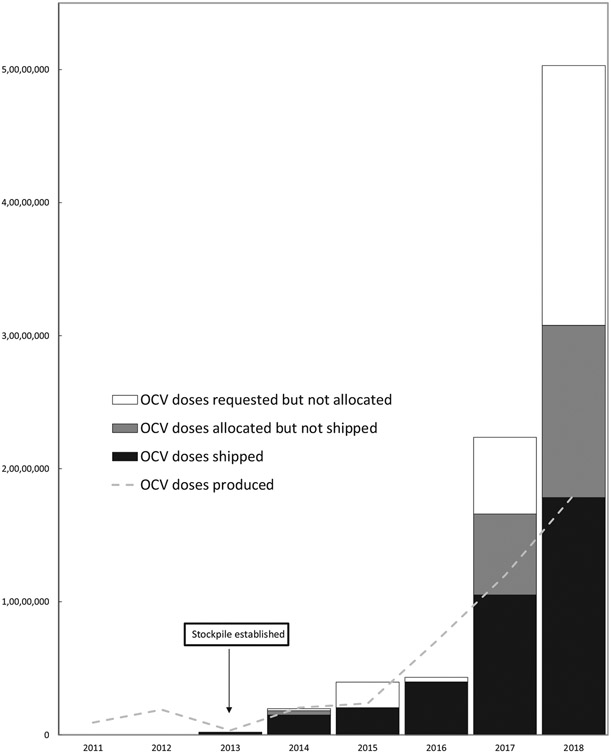
Since its creation in 2013 up until all of 2017, 83,509,941 OCV doses have been requested from the stockpile by ministries of health and partners from 24 countries, of which 55,409,160 (66.3%) doses were approved (either by the ICG or the GTFCC OCV WG) and 36,066,010 (43.2% of requested and 65.1% of approved) shipped within 83 deployments to 22 countries. In three instances countries (in Chad in 2014, in Yemen in 2017 and in DRC in 2018), emergency requests were approved by the ICG, but later canceled by requestors. The number of doses shipped has roughly doubled each year, increasing from approximately 200,000 in 2013, to 1.5 million in 2014, 2 million in 2015, 4 million in 2016, 10.5 million in 2017, and 17.8 in 2018. The proportion of requested doses approved was 100% in 2013, 92.0% in 2014, 54.6%, in 2015, 93.0% in 2016, 73.6% in 2017, and 60.4% in 2018. Virtually all doses approved in 2013–2016 were shipped. However, of the 16.0 million doses approved in 2017, only 10.0 million (62.2%) were shipped, while in 2018 17.5 million of the 30.4 million approved (57.4%) were shipped. In total 19.3 million of the 55.4 million doses approved (34.9%), remained to be sent due to insufficient supply (Fig. 1).
Fig. 1.
Global oral cholera vaccine use, demand, and production, 1997–2018. Legend to Fig. 1: in 2018 large multi-stage GTFCC requests were submitted (by DRC, Haiti, Nigeria, Somalia, Sudan, Uganda, and Yemen) for a total of 38.1 million doses, some of which were still in process at the time of writing. If approved these requests result in multiple shipments that may take place across multiple years in function of vaccine availability.
Thirteen requests for a total of 3.4 million doses, were not approved. They were all processed through the ICG framework. Five were for outbreak response (three were not approved because the epidemics were considered “too mature” for OCV to have an impact, one because the risk of spread was assessed to be low, and one because the vaccination strategy and the target group represented only by children were not considered appropriate), six for humanitarian crisis (mostly because the risk of cholera was deemed to be low and not immediate; in fact two of these requests were advised to resubmit through the GTFCC as part of plans for controlling endemic cholera), and two for endemic use (one came in 2015 when the GTFCC framework was not yet established and was not approved since priority was given to emergency requests and the other in 2016, which was redirected as part of a larger request to the GTFCC framework and eventually approved).
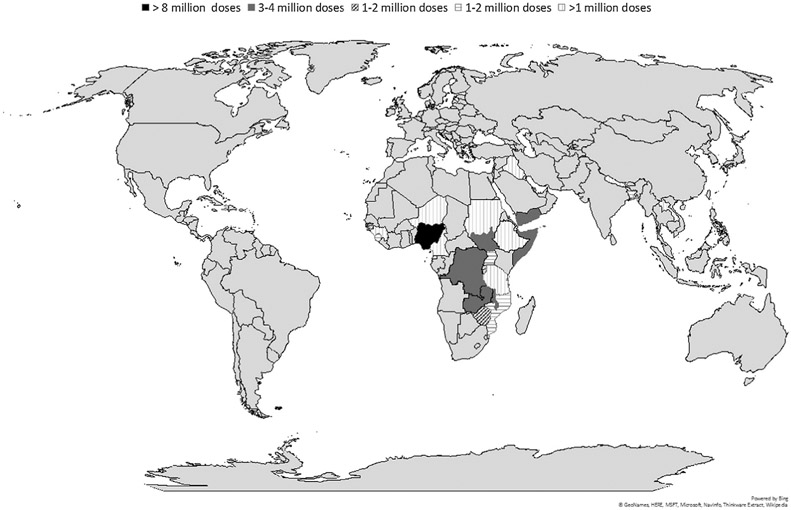
The majority (73.5%; 61/83) of deployments were in the African Region. The three countries receiving the most doses were Nigeria (8.1 million), South Sudan (3.7 million), and Zambia (3.6 million) (Fig. 2). In 2015, 200,000 doses were shipped to Bangladesh for a clinical study and were therefore excluded from the statistics presented in this paper. The number of countries using OCV from the stockpile has increased over the years from one (Haiti) in 2013, to six in 2014, seven in 2015, eight in 2016, nine in 2017, and 11 in 2018.
Fig. 2.
Countries (n = 22) receiving OCV from the stockpile, 2013–2018.
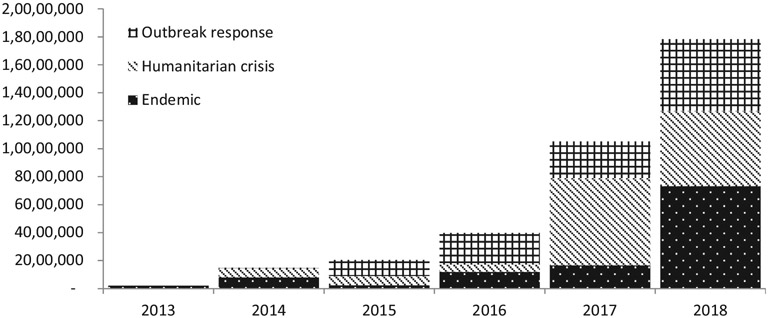
Approximately 24.7 million doses (68.4%) were shipped to emergency settings, of which 13.5 million (54.3%) were deployed during humanitarian crises and 11.2 million (45.5%) for outbreak responses; while 11.4 million (31.6%) were shipped for the purpose of contributing to controlling endemic cholera in hotspots (Figs. 2 and 3).
Fig. 3.
Oral cholera vaccine doses shipped by setting and by year since the creation of the stockpile, 2013–2018.
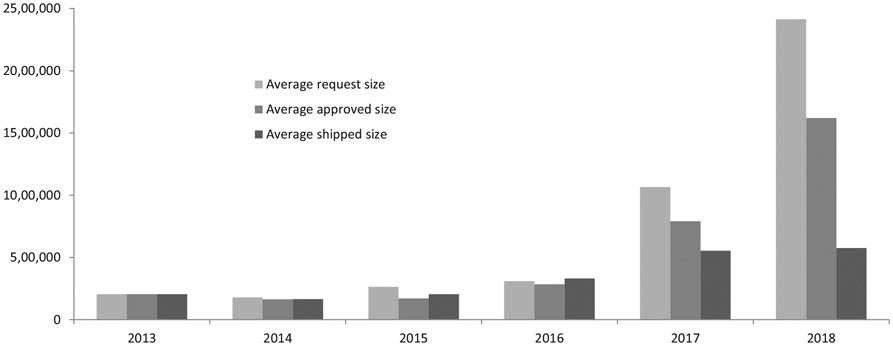
For each request, the average number of doses requested was 1.0 million, with an increasing trend from 0.20 million in 2013 (one request), 0.18 in 2014 (11 requests), 0.26 million in 2015 (15 requests), 0.31 in 2016 (14 requests), 1.07 million in 2017 (28 requests) to 2.4 million in 2018 (41 requests). This resulted in 0.71 million doses approved on average and 0.44 million doses shipped on average per request, with a comparable increasing trend from 2014 to 2018. The average proportion of doses approved out of requested from 2013 to 2018 was 100.0%, 92.0%, 64.5%, 91.8%, 74.2%, 67.1% (Fig. 4).
Fig. 4.
Average number of oral cholera vaccine doses requested from stockpile per request, approved, and shipped, by year, 2013–2018.
3.2. Campaigns
The 83 deployments resulted in 104 campaigns. Not all campaigns had reported the results at the time of writing, but so far 19,300,891 people were targeted during a first round, of which 17,417,707 (90.3%) were vaccinated; while 14,840,677 people were targeted with a second dose, of which 13,282,965 (88.2%) were vaccinated.
The average number of campaigns conducted per country was 4.7, with South Sudan conducting 37 campaigns, Malawi 14, Haiti 13, Nigeria 10, and the rest of the countries between one and three campaigns.
The most common vaccine delivery strategy was fixed post, followed by a combination of mobile and fixed strategies; on two campaigns, both targeting the fishermen communities living on Lake Chilwa in Malawi, self-administration was piloted for the second dose [26-28] (Table 1). All campaigns were planned according to a two-dose vaccination schedule, except one campaign in Juba, South Sudan, in 2015, where a single dose approach was used for outbreak response [29].
Table 1.
Strategies of vaccine delivery used by oral cholera vaccination campaigns from the stockpile (n = 104), 2013–2018.
| Strategy | Campaigns | Percentage |
|---|---|---|
| Fixed | 35 | 33.7 |
| Mixed (Fixed; Mobile) | 41 | 39.4 |
| Not specified | 15 | 14.4 |
| Mobile | 9 | 8.7 |
| Mixed (Fixed; Community-based; Self administration) | 2 | 1.9 |
| Mixed (Fixed; Mobile; Road Side) | 2 | 1.9 |
The median duration of a campaign was five days for both rounds. The interval between first and second round ranged from 5 to 234 days with a median of 16 days. The campaigns with the longest interval between rounds were in Lusaka, Zambia, in 2016 with 234 days, in Kalemie, DRC, in 2013–2014 (note that doses requested from the stockpile were for the second round only) with 215 days, and in Sud and Grande Anse, Haiti in 2016 with 189 days. Haiti and Zambia administered a first dose to cover the highest population number in an emergency setting and delivered the second dose when more vaccine became available, several months later. The long delay in Kalemie was due to insecurity resulting in operational and access constraints.
Administrative vaccination coverage of the first round ranged from 45.0% to 128.3%, with an average of 92.1%; while for the second round the range was 42.7–140%% and the average 88.2% (Table 2). Vaccination coverage surveys were documented following at least 31 campaigns. The estimated two-dose coverage ranged from 27.5 to 95.3%, with an average of 69.9%; while the estimated coverage with at least one dose ranged from 67.0 to 98.7%, with an average of 84.6% (Table 2).
Table 2.
Oral cholera vaccination coverage (administrative and estimated) for campaigns by context and strategy of vaccine delivery used, from stockpile, 2013–2018.
| Average of admin coverage first round |
Average of admin coverage second round |
Average estimated coverage at least one dose* |
Average estimated coverage two doses* |
|
|---|---|---|---|---|
| Context | ||||
| Endemic | 93.6% | 92.4% | 85.1% | 69.3% |
| Humanitarian crisis | 82.9% | 82.0% | 73.2% | 68.4% |
| Outbreak | 98.8% | 93.0% | 86.3% | 73.6% |
| Strategy | ||||
| Fixed | 86.5% | 81.2% | 74.5% | 62.0% |
| Mixed (Fixed; Community-based; Self administration) | 117.9% | 77.9% | 87.4% | 72.4% |
| Mixed (Fixed; Mobile) | 94.9% | 94.3% | 87.4% | 76.6% |
| Mixed (Fixed; Mobile; Road Side) | 98.3% | 98.5% | 89.5% | 85.1% |
| Mobile | 89.3% | 88.5% | 74.4% | 50.4% |
| Overall | 90.3% | 88.2% | 84.6% | 69.9% |
Based on 31 OCV campaigns reporting coverage survey results.
The most common reason for non-vaccination was absence during the campaign (e.g. conflict with working hours); other reasons included lack of awareness regarding the occurrence of the vaccination campaign, being too busy to get the vaccine or believing that the vaccine was unsafe or ineffective [17,26,30,31]. Another reason cited for non-vaccination during door-to-door campaigns was that the teams were not visiting the respondents’ residential structures [17]. Vaccine shortage was documented at least once [26]. One study conducted in South Sudan pointed to heightened fears of insecurity and disease, contributing to the community’s perception of cholera as a serious illness and increased trust in United Nations and NGOs providing the vaccine to IDPs, as reasons for accepting OCV [30].
No serious AEFI was reported in any of the campaigns. Minor AEFIs were reported, including mainly minor gastrointestinal symptoms, such as nausea, abdominal pain, vomiting, diarrhea and fever [16,17,26,32]. There was one documented occurrence of rash following vaccination [17]. One study followed a cohort of pregnant women after the campaign in Nsanje, Malawi, in 2015 [33] and found no increased risk of complications in vaccine recipients [30].
Data on costs were available from six campaigns from 2013 to 2016 [34,35]. The cost of vaccine was constant at 1.85USD per dose. The cost of international shipment ranged from 0.07 to 0.35 USD per dose, with a mean of 0.16 USD. The operational costs of vaccination in the field ranged from 0.41 to 2 USD per dose with a mean of 0.93 USD per dose. In total, the cost of administering one dose of vaccine ranged from 2.33 USD to 3.97 USD, with a mean of 2.98 USD.
In emergency situations, the median time from the event (i.e. first laboratory confirmation of cholera or occurrence of humanitarian emergency) to the receipt of the official OCV request was 26 days (range 12–206 days). It took a median of five days (range 0–180 days) from receipt to request approval. This includes the time required for countries to provide additional information, when requested. The median time from approval to arrival of vaccines in the country was 13 days (range, 4–24 days) and the median time from arrival to the start of vaccination was 15 days (range, −2 to 87 days – the negative count indicates that the country may start to vaccinate with doses available from previous campaigns). In total, the sum of median times from the occurrence of the event to one week after the end of the first round (time required for immunity to develop) was 66 days.
4. Discussion
Since the OCV stockpile creation in 2013, with 104 campaigns conducted in 24 countries using more than 36 million doses, the stockpile allowed for a significant increase in OCV use in cholera-affected countries. OCV was well accepted (as indicated by generally high coverage) by the population and its use demonstrated to be safe and feasible for both emergency response and endemic cholera control. In general, vaccination was an inexpensive and timely intervention, although timeliness was a limiting factor in case of emergency campaigns conducted for outbreak response. The experience described so far demonstrates that countries are increasingly integrating OCV use in their cholera control strategies generating further demand, which results in significant growth in vaccine supply.
The first explanation of the increased OCV use since the creation of the stockpile is the availability of prequalified vaccines. From 2011 by the end of 2018, through all of 2018, 44 million doses of OCV had been produced by the two manufacturers, from approximately two million doses per year between 2013 and 2014, to seven million in 2016, 12 million in 2017 and 18 million in 2018. This is is in stark contrast compared to the previous 15 years (1997–2012) with 13 campaigns implemented and 1.4 million doses used [11,23]. Another likely reason for increased demand of OCV globally is its use by more and more countries adding to the shared experience and knowledge gained and stimulating the global community to consider this tool.
Although the use of OCV in non-emergency situations (i.e. endemic use) has been steadily increasing due to increased supply, most requests, so far, have been for use in emergencies. Hence, not surprisingly the African Region, which is currently the most affected by cholera outbreaks, remains the region with the highest number of OCV deployments. OCV use in the Eastern Mediterranean Region has mostly been related to emergency situations as well, in the context of the humanitarian crises, whereas its use in the American region was localized in Haiti, mostly for endemic cholera control. Finally, and perhaps counterintuitively, the region with the lowest OCV use was South East Asia. Cholera outbreaks tend to go unreported in this region [36], making it less likely for them to request OCV in emergency. With increased availability of OCV for non-emergency use, this situation could change soon, and larger requests may be expected globally. In fact, starting from 2017 several countries (DRC, Haiti, Malawi, Nigeria, South Sudan, Uganda, Yemen, and Zambia) submitted large requests to the GTFCC OCV WG and received approval to conduct multiple campaigns with the administration of millions of doses over longer periods of time, as part of multisectoral NCPs. However, while vaccine production has increased, allowing for these larger requests, OCV supply remains still constrained, resulting in the gap observed since 2017 between the average numbers of doses approved and the number of doses ultimately shipped.
The campaigns using stockpiled OCV have confirmed that OCVs are safe, as seen in the clinical trials [37-42] and in the early vaccination campaigns conducted before the establishment of the stockpile [43-51]. One observational cohort study in pregnant women was conducted following the deployment of OCV in Malawi in 2015 [33], and has contributed further evidence of the safety of OCV in pregnant women [52], previously only measured in retrospective studies [53-55], leading to the WHO recommendation to include this group in the target population [9]. Furthermore, the generally high coverage observed during all the campaigns confirms that OCV is an intervention which is well accepted [16,30,56], with some exceptions related to the relative lower coverage sometimes observed in adult males, who are not traditionally the target for vaccination and are more likely to be at work during vaccination; and some decrease in coverage during second rounds due probably to the misunderstanding by the population that the vaccine is given with only one dose [47,57,58,49].
Although there was significant fluctuation in delivery costs depending on the settings, OCV was confirmed to be generally a low-cost intervention, in line with pre-existing studies [59-61]. However, documenting costs is only the first step in economic analysis and more analysis of cost-effectiveness data is needed [62-64].
This review also demonstrates that OCV is an easy intervention which can even be self-administered, resulting in a reduction of the delivery costs [26], the main limitation being the cold chain requirements [43,48,57,58,65]. OCVs have demonstrated good heat stability [66,67]. While Shanchol has already been approved for use in a controlled temperature chain (CTC), efforts are ongoing to grant label variation to allow for its at temperatures of up to 40 °C for all pre-qualified OCVs, similar to the meningitis A and human papillomavirus vaccines [68,69]. This will facilitate considerably vaccination campaigns in the field. In addition since 2018 Euvichol is now presented in plastic tubes instead of glass vials which has further facilitated delivery in the field and administration.
Although OCV use so far has been timely at the delivery stage with campaigns often lasting less than one week, achieving good timeliness is challenging for emergency use. A median delay of 3 months between the occurrence of an emergency and the start of the first round is unsatisfactory. Late outbreak confirmation, due to poor laboratory capacity or reluctance of countries to report cholera, further increased this delay. One other factor to consider was the delay between first dose and second dose. Supply constraints also play a role in delaying campaigns especially when there are not enough doses in stock to allow translating into campaigns all approved campaigns, making further prioritization necessary.
From a response point of view, the experiences with OCV demonstrated that two OCV doses provide protection against cholera for at least three years, and that one dose provides at least short-term protection [12]. However, although OCV also seems to provide herd protection [70-75], evidence with regards to its impact in reducing the cholera risk at a population level and changing the course of outbreaks is mostly theoretical [76-79], with only a few controlled studies being reported [74,80-82], and could be coincidental.
Another challenge which is directly connected to the impact of the vaccination, is the implementation of OCV simultaneously with other preventive interventions, especially the strengthening of WASH [19]. This can be explained in part because of the difficulties in rapidly improving access to WASH in settings where OCV is implemented, especially in emergencies [16,17,82-84]. Current efforts driven by the GTFCC include the promotion of OCV use within integrated multisectoral cholera control plans [85-87] as an essential requirement for accessing OCV for endemic use.
This review is subject to a number of limitations. First, general information on OCV campaigns was not standardized or recorded systematically in a way that could be easily analyzed. This applies less to the data on requests, which are handled by a central location since both ICG and GTFCC secretariats are housed within WHO headquarters. However, it affects campaign data, the quality of which is dependent on the local context and on the implementing agency’s reporting capacity. Systems to allow systematic and timely reporting (campaign implementation reporting, costs, coverage survey results, other monitoring and evaluation activities, etc.) should be put in place as a requirement to access OCV. A second limitation relates to the availability of systematic data on cholera disease in the countries requesting and using OCV. Although this limitation doesn’t directly affect the quality of OCV utilization data, having reliable and systematic surveillance data allows to better plan OCV campaigns (and all other cholera control interventions) and evaluate their performance.
In conclusion, since the creation of the stockpile, increased availability and demand from countries have contributed to a cycle of increased supply and increased use. The stockpile has also confirmed its role as resource for operational research to inform vaccination strategies locally and globally. This is reflected not only in the increased number of countries using OCV each year, but also in the average size of approved requests, which went from a few hundred thousand doses in 2013 to an average number of doses approved of more than one million per request; demonstrating that the increased availability results in a larger use by countries, motivating other countries to also do the same. On a less positive note, the demand is always exceeding the demand and countries with approved requests are often asked to split their approved requests into smaller shipments and often delay non-urgent campaigns to when supply will allow doses to be shipped.
Further efforts should be directed to ensure that the increased demand, when technically appropriate and realistic, is met with increased supply, especially if the vaccine is expected to be used more and more to control endemic cholera and thus contribute to cholera elimination as laid out by “Ending Cholera: A Global Roadmap to 2030”, the global cholera elimination strategy launched by the GTFCC in 2017. This issue will become even more urgent if countries in the SEARO region will start using OCV systematically like the AFRO region. It is also important to ensure that that OCV is not used alone, but as part of comprehensive package of multisectoral interventions, including provision of adequate, affordable and sustainable safe water supply and sanitation to vulnerable groups, active social mobilization, and reinforcement of surveillance and case management, coordinated at the highest political level within an NCP. Additionally, effort should be allocated to the improvement of the timeliness of response, delivery costs, and more globally in designing innovative and effective strategies for OCV delivery in the different contexts (e.g. balancing “reactive use” in emergencies as quickly as possible with more strategically planned “endemic use” in hotspots). To achieve this, adequate monitoring capacity should be in place to continuously document and refine OCV’s role for global cholera control every time that it is used. In this sense, the momentum generated by OCV campaigns and the mobilization of operational costs should be capitalized to reinforce health systems in general.
Acknowledgements
We are grateful to countries and partners for requesting and using OCV. We wish to thank Gavi for the support with the stockpile and for providing the operational costs, and the European Union/ECHO for covering implementation costs and monitoring and evaluation activities of selected campaigns, and the Bill and Melinda Gates Foundation for supporting the GTFCC. A special acknowledgement goes to Malika Bouhenia (WHO) for her revision of the manuscript.
Appendix
1 Members of the Global Task Force on Cholera Control (GTFCC) Oral Cholera Vaccine (OCV) Working Group: • Agence de Médecine Préventive: Philippe Cavailler, Martin Mengel; • The Bill and Melinda Gates Foundation: Helen Matzger, Tina Lorenson; • Dipika Sur (independent); • Epicentre: Francisco Luquero, Rebecca Grais; • Gavi: Melissa Ko, Adam Soble; • GHESKIO: Vanessa Rouzier, Jean William Pape; • Harvard University: Caroline Buckee; • icddr,b: Firdausi Qadri; • International Federation of Red Cross and Red Crescent Societies (IFRC): Frank Mahoney; • International Medical Corps (IMC): Jill John Kall; • International Rescue Committee: Justine Landegger, Michelle Gayer; • International Vaccine Institute: Julia Lynch; • Johns Hopkins University: Andrew S. Azman, David Sack; • Médecins Sans Frontières: Myriam Henkens, Iza Ciglenecki; • National Institutes of Health: Robert Hall; • Massachusetts General Hospital Center for Global Health: Louise C. Ivers; • Save the Children: Emma Diggle; • Swiss Tropical and Public Health Institute: Mitchell Weiss; • Task Force for Global Health: Alan Hinman; • UNHCR: Kahindo Maina; UNICEF: Imran Mirza (Program Division), Heather Papowitz (Program Division), Guillermo Gimeno (Supply Division), Monica Ramos (WASH); • University of Maryland: Myron M. Levine; • US Centers for Disease Control and Prevention: Kashmira Date,Nandini Sreenivasan* (Disclaimer: Kashmira Date and Nandini Sreenivasan are employees of the US Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.)
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lippi D, Gotuzzo E, Caini S. Cholera. Microbiol Spectr 2016;4:4. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Cholera, 2017. Wkly Epidemiol Rec 2018;93(38):489–500. [Google Scholar]
- [3].Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 2015;9(6):e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet 2017. [DOI] [PubMed] [Google Scholar]
- [5].Toole MJ, Waldman RJ. The public health aspects of complex emergencies and refugee situations. Annu Rev Public Health 1997;18:283–312. [DOI] [PubMed] [Google Scholar]
- [6].Alberti KP, Guthmann JP, Fermon F, Nargaye KD, Grais RF. Use of Lot Quality Assurance Sampling (LQAS) to estimate vaccination coverage helps guide future vaccination efforts. Trans R Soc Trop Med Hyg 2008;102(3):251–4. [DOI] [PubMed] [Google Scholar]
- [7].Koo D, Traverso H, Libel M, Drasbek C, Tauxe R, Brandling-Bennett D. Epidemic cholera in Latin America, 1991–1993: implications of case definitions used for public health surveillance. Bull Pan Am Health Organ 1996;30(2):134–43. [PubMed] [Google Scholar]
- [8].World Health Organization. New momentum in prevention, control and elimination of cholera in Africa: priority actions identified by affected countries. Wkly Epidemiol Rec 2016;91(24):305–14. [PubMed] [Google Scholar]
- [9].World Health Organization. Cholera vaccines: WHO position paper - August 2017. Wkly Epidemiol Rec 2017;92(34):477–98. [PubMed] [Google Scholar]
- [10].Desai SN, Pezzoli L, Martin S, et al. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. Lancet Glob Health 2016;4(4):e223–4. [DOI] [PubMed] [Google Scholar]
- [11].Desai SN, Pezzoli L, Alberti KP, et al. Achievements and challenges for the use of killed oral cholera vaccines in the global stockpile era. Hum Vaccin Immunother 2017;13(3):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bi Q, Ferreras E, Pezzoli L, et al. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bhattacharya SK, Sur D, Ali M, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2013;13(12):1050–6. [DOI] [PubMed] [Google Scholar]
- [14].Yen C, Hyde TB, Costa AJ, et al. The development of global vaccine stockpiles. Lancet Infect Dis 2015;15(3):340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lam E, McCarthy A, Brennan M. Vaccine-preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Hum Vaccin Immunother 2015;11(11):2627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Msyamboza KP, M’bang’ombe M, Hausi H, et al. Feasibility and acceptability of oral cholera vaccine mass vaccination campaign in response to an outbreak and floods in Malawi. Pan Afr Med J 2016;23:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lam E, Al-Tamimi W, Russell SP, et al. Oral cholera vaccine coverage during an outbreak and humanitarian crisis, Iraq, 2015. Emerg Infect Dis 2017;23(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Deen J, Von Seidlein L, Luquero FJ, et al. The scenario approach for countries considering the addition of oral cholera vaccination in cholera preparedness and control plans. Lancet Infect Dis 2016;16(1):125–9. [DOI] [PubMed] [Google Scholar]
- [19].Clemens J, Holmgren J. When, how, and where can oral cholera vaccines be used to interrupt cholera outbreaks? Curr Top Microbiol Immunol 2014;379:231–58. [DOI] [PubMed] [Google Scholar]
- [20].Dellepiane N, Wood D. Twenty-five years of the WHO vaccines prequalification programme (1987–2012): lessons learned and future perspectives. Vaccine 2015;33(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abubakar A, Azman AS, Rumunu J, et al. The first use of the global oral cholera vaccine emergency stockpile: lessons from south Sudan. PLoS Med 2015;12(11):e1001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Munier A, Njanpop-Lafourcade BM, Sauvageot D, et al. The African cholera surveillance network (Africhol) consortium meeting, 10–11 June 2015, Lome, Togo. BMC Proc 2017;11(Suppl 1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].M’bangombe M, Pezzoli L, Reeder B, et al. Oral cholera vaccine in cholera prevention and control, Malawi. Bull World Health Organ 2018;96(6):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].World Health Organization. Deployments from the oral cholera vaccine stockpile, 2013–2017. Wkly Epidemiol Rec 2017;92(32):437–42. [PubMed] [Google Scholar]
- [25].Martin S, Lopez AL, Bellos A, et al. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ 2014;92(12):881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sauvageot D, Saussier C, Gobeze A, et al. Oral cholera vaccine coverage in hard-to-reach fishermen communities after two mass Campaigns, Malawi, 2016. Vaccine 2017;35(38):5194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heyerdahl LW, Ngwira B, Demolis R, et al. Innovative vaccine delivery strategies in response to a cholera outbreak in the challenging context of Lake Chilwa. A rapid qualitative assessment. Vaccine 2018;36(44):6491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grandesso F, Kasambara W, Page AL, et al. Effectiveness of oral cholera vaccine in preventing cholera among fishermen in Lake Chilwa, Malawi: a case-control study. Vaccine 2019;37(28):3668–76. [DOI] [PubMed] [Google Scholar]
- [29].Azman AS, Parker LA, Rumunu J, et al. Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study. Lancet Glob Health 2016;4(11):e856–63. [DOI] [PubMed] [Google Scholar]
- [30].Peprah D, Palmer JJ, Rubin GJ, et al. Perceptions of oral cholera vaccine and reasons for full, partial and non-acceptance during a humanitarian crisis in South Sudan. Vaccine 2016;34(33):3823–7. [DOI] [PubMed] [Google Scholar]
- [31].Parker LA, Rumunu J, Jamet C, et al. Neighborhood-targeted and case-triggered use of a single dose of oral cholera vaccine in an urban setting: feasibility and vaccine coverage. PLoS Negl Trop Dis 2017;11(6):e0005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].World Health Organization. Oral cholera vaccine campaign among internally displaced persons in South Sudan. Wkly Epidemiol Rec 2014;89(20):214–20. [PubMed] [Google Scholar]
- [33].Ali M, Nelson A, Luquero FJ, et al. Safety of a killed oral cholera vaccine (Shanchol) in pregnant women in Malawi: an observational cohort study. Lancet Infect Dis 2017;17(5):538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Routh JA, Sreenivasan N, Adhikari BB, et al. Cost evaluation of a government-conducted oral cholera vaccination campaign-Haiti, 2013. Am J Trop Med Hyg 2017;97(4_Suppl):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Poncin M, Zulu G, Voute C, et al. Implementation research: reactive mass vaccination with single-dose oral cholera vaccine, Zambia. Bull World Health Organ 2018;96(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].World Health Organization. Cholera, 2016. Wkly Epidemiol Rec 2017;92(36):521–30. [PubMed] [Google Scholar]
- [37].Anh DD, Canh DG, Lopez AL, et al. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 2007;25(6):1149–55. [DOI] [PubMed] [Google Scholar]
- [38].Desai SN, Akalu Z, Teshome S, et al. A randomized, placebo-controlled trial evaluating safety and immunogenicity of the killed, bivalent, whole-cell oral cholera vaccine in Ethiopia. Am J Trop Med Hyg 2015;93(3):527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Qadri F, Ali M, Chowdhury F, et al. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet 2015;386(10001):1362–71. [DOI] [PubMed] [Google Scholar]
- [40].Kanungo S, Sen B, Ramamurthy T, et al. Safety and immunogenicity of a live oral recombinant cholera vaccine VA1. 4: a randomized, placebo controlled trial in healthy adults in a cholera endemic area in Kolkata, India. PLoS ONE 2014;9(7):e99381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baik YO, Choi SK, Kim JW, et al. Safety and immunogenicity assessment of an oral cholera vaccine through phase I clinical trial in Korea. J Korean Med Sci 2014;29(4):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sur D, Lopez AL, Kanungo S, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 2009;374(9702):1694–702. [DOI] [PubMed] [Google Scholar]
- [43].Luquero FJ, Grout L, Ciglenecki I, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med 2014;370(22):2111–20. [DOI] [PubMed] [Google Scholar]
- [44].Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352(8):757–67. 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- [45].Ciglenecki I, Sakoba K, Luquero FJ, et al. Feasibility of mass vaccination campaign with oral cholera vaccines in response to an outbreak in Guinea. PLoS Med 2013;10(9):e1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Anh DD, Lopez AL, Thiem VD, et al. Use of oral cholera vaccines in an outbreak in Vietnam: a case control study. PLoS Negl Trop Dis 2011;5(1):e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Luquero FJ, Grout L, Ciglenecki I, et al. First outbreak response using an oral cholera vaccine in Africa: vaccine coverage, acceptability and surveillance of adverse events, Guinea, 2012. PLoS Negl Trop Dis 2013;7(10):e2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Porta MI, Lenglet A, De WS, et al. Feasibility of a preventive mass vaccination campaign with two doses of oral cholera vaccine during a humanitarian emergency in South Sudan. Trans R Soc Trop Med Hyg 2014;108(12):810–5. [DOI] [PubMed] [Google Scholar]
- [49].Tohme RA, Francois J, Wannemuehler K, et al. Oral cholera vaccine coverage, barriers to vaccination, and adverse events following vaccination, Haiti, 2013. Emerg Infect Dis 2015;21(6):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Phares CR, Date K, Travers P, et al. Mass vaccination with a two-dose oral cholera vaccine in a long-standing refugee camp, Thailand. Vaccine 2016;34(1):128–33. [DOI] [PubMed] [Google Scholar]
- [51].Scobie HM, Phares CR, Wannemuehler KA, et al. Use of oral cholera vaccine and knowledge, attitudes, and practices regarding safe water, sanitation and hygiene in a long-standing refugee camp, Thailand, 2012–2014. PLoS Negl Trop Dis 2016;10(12):e0005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moro PL, Sukumaran L. Cholera vaccination: pregnant women excluded no more. Lancet Infect Dis 2017;17(5):469–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grout L, Martinez-Pino I, Ciglenecki I, et al. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine in Guinea: a retrospective cohort study. PLoS Negl Trop Dis 2015. Dec;9(12):e0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hashim R, Khatib AM, Enwere G, et al. Safety of the recombinant cholera toxin B subunit, killed whole-cell (rBS-WC) oral cholera vaccine in pregnancy. PLoS Negl Trop Dis 2012;6(7):e1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khan AI, Ali M, Chowdhury F, et al. Safety of the oral cholera vaccine in pregnancy: retrospective findings from a subgroup following mass vaccination campaign in Dhaka, Bangladesh. Vaccine 2017;35(11):1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sundaram N, Schaetti C, Merten S, et al. Sociocultural determinants of anticipated oral cholera vaccine acceptance in three African settings: a meta-analytic approach. BMC Public Health 2016;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kar SK, Sah B, Patnaik B, et al. Mass vaccination with a new, less expensive oral cholera vaccine using public health infrastructure in India: the Odisha model. PLoS Negl Trop Dis 2014;8(2):e2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hsiao A, Desai SN, Mogasale V, Excler JL, Digilio L. Lessons learnt from 12 oral cholera vaccine campaigns in resource-poor settings. Bull World Health Organ 2017;95(4):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mogasale V, Ramani E, Wee H, Kim JH. Oral cholera vaccination delivery cost in low- and middle-income countries: an analysis based on systematic review. PLoS Negl Trop Dis 2016;10(12):e0005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mogasale V, Kar SK, Kim JH, et al. An estimation of private household costs to receive free oral cholera vaccine in Odisha, India. PLoS Negl Trop Dis 2015;9(9):e0004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sarker AR, Islam Z, Khan IA, et al. Estimating the cost of cholera-vaccine delivery from the societal point of view: a case of introduction of cholera vaccine in Bangladesh. Vaccine 2015. Sep 11;33(38):4916–21. [DOI] [PubMed] [Google Scholar]
- [62].Troeger C, Sack DA, Chao DL. Evaluation of targeted mass cholera vaccination strategies in Bangladesh: a demonstration of a new cost-effectiveness calculator. Am J Trop Med Hyg 2014;91(6):1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schaetti C, Weiss MG, Ali SM, et al. Costs of illness due to cholera, costs of immunization and cost-effectiveness of an oral cholera mass vaccination campaign in Zanzibar. PLoS Negl Trop Dis 2012;6(10):e1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Naficy A, Rao MR, Paquet C, Antona D, Sorkin A, Clemens JD. Treatment and vaccination strategies to control cholera in sub-Saharan refugee settings: a cost-effectiveness analysis. JAMA 1998;279(7):521–5. [DOI] [PubMed] [Google Scholar]
- [65].Ivers LC, Teng JE, Lascher J, et al. Use of oral cholera vaccine in Haiti: a rural demonstration project. Am J Trop Med Hyg 2013;89(4):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Saha A, Khan A, Salma U, et al. The oral cholera vaccine Shanchol when stored at elevated temperatures maintains the safety and immunogenicity profile in Bangladeshi participants. Vaccine 2016;34(13):1551–8. [DOI] [PubMed] [Google Scholar]
- [67].Saha A, Rosewell A, Hayen A, MacIntyre CR, Qadri F. Improving immunization approaches to cholera. Expert Rev Vaccines 2017;16(3):235–48. [DOI] [PubMed] [Google Scholar]
- [68].Kahn AL, Kristensen D, Rao R. Extending supply chains and improving immunization coverage and equity through controlled temperature chain use of vaccines. Vaccine 2017;35(17):2214–6. [DOI] [PubMed] [Google Scholar]
- [69].Zipursky S, Djingarey MH, Lodjo JC, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine 2014;32(13):1431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Root ED, Giebultowicz S, Ali M, Yunus M, Emch M. The role of vaccine coverage within social networks in cholera vaccine efficacy. PLoS ONE 2011;6(7):e22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ali M, Emch M, Von SL, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 2005;366(9479):44–9. [DOI] [PubMed] [Google Scholar]
- [72].Ali M, Emch M, Yunus M, et al. Vaccine Protection of Bangladeshi infants and young children against cholera: implications for vaccine deployment and person-to-person transmission. Pediatr Infect Dis J 2008;27(1):33–7. [DOI] [PubMed] [Google Scholar]
- [73].Emch M, Ali M, Park JK, Yunus M, Sack DA, Clemens JD. Relationship between neighbourhood-level killed oral cholera vaccine coverage and protective efficacy: evidence for herd immunity. Int J Epidemiol 2006;35(4):1044–50. [DOI] [PubMed] [Google Scholar]
- [74].Khatib AM, Ali M, Von SL, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis 2012;12(11):837–44. [DOI] [PubMed] [Google Scholar]
- [75].Ali M, Debes AK, Luquero FJ, et al. Potential for controlling cholera using a ring vaccination strategy: re-analysis of data from a cluster-randomized clinical trial. PLoS Med 2016;13(9):e1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Azman AS, Luquero FJ, Rodrigues A, et al. Urban cholera transmission hotspots and their implications for reactive vaccination: evidence from Bissau city, Guinea bissau. PLoS Negl Trop Dis 2012;6(11):e1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack DA, Lessler J. The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: a modeling study. PLoS Med 2015;12(8):e1001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kirpich A, Weppelmann TA, Yang Y, Morris JG Jr, Longini IM Jr. Controlling cholera in the Ouest Department of Haiti using oral vaccines. PLoS Negl Trop Dis 2017;11(4):e0005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chao DL, Halloran ME, Longini IM Jr. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci U S A 2011;108(17):7081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Azman AS, Rumunu J, Abubakar A, et al. Population-level effect of cholera vaccine on displaced populations, south Sudan, 2014. Emerg Infect Dis 2016;22(6):1067–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ali M, Sur D, You YA, et al. Herd protection by a bivalent killed whole-cell oral cholera vaccine in the slums of Kolkata. India. Clin Infect Dis 2013;56(8):1123–31. [DOI] [PubMed] [Google Scholar]
- [82].Severe K, Rouzier V, Anglade SB, et al. Effectiveness of oral cholera vaccine in Haiti: 37-month follow-up. Am J Trop Med Hyg 2016;94(5):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ciglenecki I, Azman AS, Rumunu J, Cabrol JC, Luquero FJ. Vaccination against cholera in Juba - Authors’ reply. Lancet Infect Dis 2017;17(5):480–1. [DOI] [PubMed] [Google Scholar]
- [84].Ivers LC, Hilaire IJ, Teng JE, et al. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. Lancet Glob Health 2015;3(3):e162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rouzier V, Severe K, Juste MA, et al. Cholera vaccination in urban Haiti. Am J Trop Med Hyg 2013;89(4):671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ivers LC, Farmer PE, Pape WJ. Oral cholera vaccine and integrated cholera control in Haiti. Lancet 2012;379(9831):2026–8. [DOI] [PubMed] [Google Scholar]
- [87].Mengel MA. Cholera in Africa: new momentum in fighting an old problem. Trans R Soc Trop Med Hyg 2014;108(7):391–2. [DOI] [PubMed] [Google Scholar]