Abstract
The nectar spur is an important feature of pollination and ecological adaptation in flowering plants, and it is a key innovation to promote species diversity in certain plant lineages. The development mechanism of spurs varies among different plant taxa. As one of the largest angiosperm genera, we have little understanding of the mechanism of spur development in Impatiens. Here, we investigated the initiation and growth process of spurs of Impatiens uliginosa based on histology and hormone levels, and the roles of AUXIN BINDING PROTEIN (ABP) and extensin (EXT) in spur development were explored. Our results indicate that the spur development of I. uliginosa is composed of cell division and anisotropic cell elongation. Imbalances in spur proximal-distal cell division lead to the formation of curved structures. Endogenous hormones, such as auxin and cytokinins, were enriched at different developmental stages of spurs. IuABP knockdown led to an increase in spur curves and distortion of morphology. IuEXT knockdown resulted in reduced spur length and loss of curve and inner epidermal papillae structures. This study provides new insights into the mechanism of spur development in core eudicots.
Introduction
The floral organs of angiosperms play an important role in reproduction, and their various evolutionary characteristics have clear purposes and adaptive significance; they attract pollinators with their bright colors, fragrant odors, and abundant nectar rewards. The nectar spur, a typical evolutionary floral feature, is a tubular structure extending from petals that have undergone several independent evolutions in angiosperms [1, 2]. Nectar spurs have extensive species diversity in morphology, color, and internal structure [3–5], and play a crucial role in pollination by secreting and storing nectar [6]. In some plant lineages, such as Aquilegia and Linaria, the evolution of spur length leads to the specialization of pollinators, promoting reproductive isolation and improving pollination efficiency [2, 7–9]. In other plant lineages, such as the Impatiens, the pollination system was proven to be generalization rather than specialization [10], and spurs tend to improve pollination efficiency and ecological adaptability through the evolution of curvature [11, 12]. Plant lineages with spurs have an amazing species diversity and faster speciation rate [2, 13]. Therefore, spurs are considered a key innovation [14, 15]. A study on spur development is helpful in understanding the species diversity and evolutionary mechanism of plant lineages.
Spur development consists of cell division and/or cell expansion [16]. In Aquilegia and Centranthus ruber, this process is divided into two stages. First, cell division at the base of the petal forms the initial spur, after which cell division decreases significantly. The spur attains its final length primarily through anisotropic cell elongation, as the initial spur is only a small fraction of the eventual length [17–19]. The diversity of spur length in Aquilegia is primarily influenced by anisotropic cell elongation. However, the study of Aquilegia rockii has revealed that differences in spur length within the species are due to variations in cell numbers [20]. In Pelargonium and Linaria, the difference in spur length cannot be explained by the difference in cell expansion. The differences in spur growth rates caused by changes in cell division and cell number are more important factors, indicating that spur development varies among different species [21, 22].
There is no general molecular mechanism that regulates the formation and development of spurs in angiosperm. Early studies suggest that the KNOTTED 1-like homeobox (KNOX) gene is expressed in the early stages of spur development in Linaria and Dactylorhiza fuchsii, regulating spur morphogenesis by promoting cell division and maintaining indeterminate growth [16, 23, 24]. A recent study suggests that the differential expression of CYCLIN-D3–3 (CCD33) and LONELY GUY 1 (LOG1) genes related to cell division and cell cycle on the dorso-ventral side of petals early in spur development may play an important role in the spur formation in Linaria [25]. In Aquilegia, the C2H2 zinc-finger transcription factor POPOVICH (POP) is a key gene controlling spur’s presence or absence [26]. TEOSINTE BRANCHED/CYCLOIDEA/PCF 4 (TCP4) sculpts three-dimensional forms of spurs from a two-dimensional primordium by controlling local cell division. In contrast, no KNOX gene expression was detected in the transcriptome of early spurs and in in situ hybridization in a broader development stage [27]. Studies on Tropaeolum showed that both TCP and KNOX genes may be involved in spur development and play a crucial role in extreme adaxial-abaxial asymmetry [28]. Furthermore, coregulated gene groups that mediate hormone synthesis or response also participate in spur development. AUXIN RESPONSE FACTOR ARF6 and ARF8 regulate spur cell elongation and nectar development in Aquilegia [29]. The brassinosteroid pathway gene BRI1-EMS-SUPRESSOR1/BRASSINAZOLE-RESISTANT1 (BEH) regulates the anisotropic elongation and division of spur cells, and the exogenous application of brassinosteroid increased the petal spur length of Aquilegia [30].
The genetic mechanism of spur development may vary among different plant lineages. Little is known about spur development in Impatiens at present. In previous studies, we divided spur development into three stages based on the length and morphology of the spur in Impatiens uliginosa. The early stage lasts about 7 days, with a straight spur reaching 5–8 mm. In the middle stage, the spur develops a curved structure and elongates to 25–28 mm in 4 days. At the anthesis stage, the length and shape of the spur remain stable. We conducted a preliminary exploration of spur development in Impatiens through transcriptome sequencing of spurs at these three developmental stages [31]. The results showed that the enrichment function of differentially expressed genes (DEGs) changed with spur development. For instance, in the early stage, ‘regulation of cell cycle’ and ‘auxin-activated signaling pathway’ are some of the most significantly enriched items, while in the middle stage, ‘cell wall organization’ is more prominent. The ‘hormone-mediated signaling pathway’ is the most significant during spur development. These results may reveal the transition between cell division and differentiation, and the key role of hormone regulation.
Candidate genes related to spur development were identified using transcriptome data. Among them, our focus was on the gene AUXIN BINDING PROTEIN (ABP), enriched in the auxin-activated signaling pathway, and exhibiting significant differential expression across various tissues and developmental stages. In Arabidopsis and tobacco, ABP expression is positively correlated with cell size [32–34]. ABP-mediated cellular processes (division or expansion) are influenced by auxin concentrations [35]. We hypothesize that ABP may regulate the division and elongation of spur cells by mediating the auxin signaling pathway. In addition, the extensin (EXT) gene has also attracted our interest, as it has extremely high expression levels in spurs and exhibits significant differences from the limb [31]. Extensins (EXTs) belong to the hydroxyproline-rich glycoprotein (HRGP) superfamily and are important structural proteins in plant cell walls [36, 37]. EXTs affect root hair elongation and lateral root development by regulating the assembly and structure of cell walls in Arabidopsis, and this regulation is influenced by auxin induction [38, 39]. Knocking out EXT can cause abnormal cell wall morphology, resulting in germination-defective seedlings with defective root, shoot, and hypocotyl [40, 41].
Here, we studied the spur development process of I. uliginosa based on morphology, histocytology, and physiology; the role of IuABP and IuEXT in spur development was also explored through virus-induced gene silencing (VIGS) technology. The results revealed the cellular and molecular basis of the spur development in I. uliginosa.
Results
Structural characteristics of spur during morphogenesis
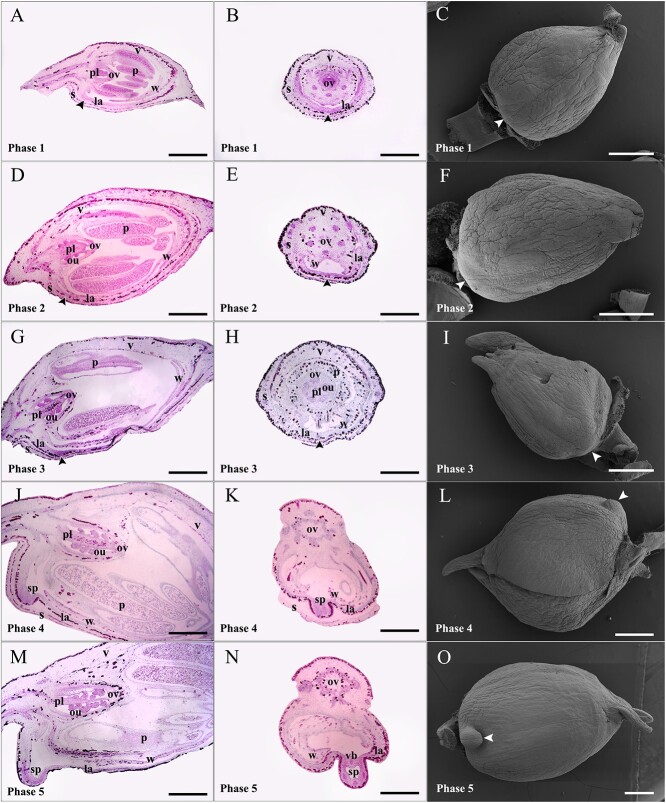
To understand spur development and structural features during morphogenesis, we dissected flower buds of I. uliginosa across five phases. In phase 1, the sepal, vexillum, wing, labellum, pollen, ovary, and placenta had developed, but ovules were not visible. The spur’s growth point was flat without signs of differentiation (Fig. 1A–C). Phase 2 showed the presence of ovules, yet the spur remained undifferentiated (Fig. 1D–F). During phase 3, a clear deep staining appeared at the spur’s growth point, with a slight protrusion visible under scanning electron microscopy (SEM) (Fig. 1G–I). In phase 4, the labellum thickened, and the spur protruded, forming a shallow cavity (Fig. 1J–L). In phase 5, the spur extended further, forming a distinct cavity, and four vascular bundles were observed in the transverse section. The deep staining on the spur was striking, creating a notable contrast with the limb. It is worth noting that the I. uliginosa spur initially grows upward along the ventral midline of the bud, not downward (Fig. 1M–O).
Figure 1.
Early spur development of Impatiens uliginosa. Paraffin sectioning (longitudinal and transverse sections) was performed on the buds of five consecutive phases to observe the internal structure of spurs, and SEM was used to observe their external morphology. (A–C) Longitudinal section (A), transverse section (B), and SEM (C) of flower buds in phase 1; spurs and ovules have not developed. (D–F) Longitudinal section (D), transverse section (E), and SEM (F) of flower buds in phase 2; ovules can be observed, and spurs are still undeveloped. (G–I) Longitudinal section (G), transverse section (H), and SEM (I) of flower buds in phase 3; spurs are ready to develop, with deep staining (G) and eminence (I) at the growth point. (J–L) Longitudinal section (J), transverse section (K), and SEM (L) of flower buds in phase 4; the primordial spurs extend from the labellum. (M–O) Longitudinal section (M), transverse section (N), and SEM (O) of flower buds in phase 5; spurs extend to about 0.4 mm, forming a distinct intracavity and vascular bundles and growing toward the tip of the bud. Arrows indicate spurs or their growth points. la, labellum; ou, ovule; ov, ovary; p, pollen; pl, placenta; s, sepal; sp, spur; v, vexillum; vb, vascular bundle; w, wing. Bars = 500 μm.
The spur development of I. uliginosa consists of cell division and anisotropic cell elongation
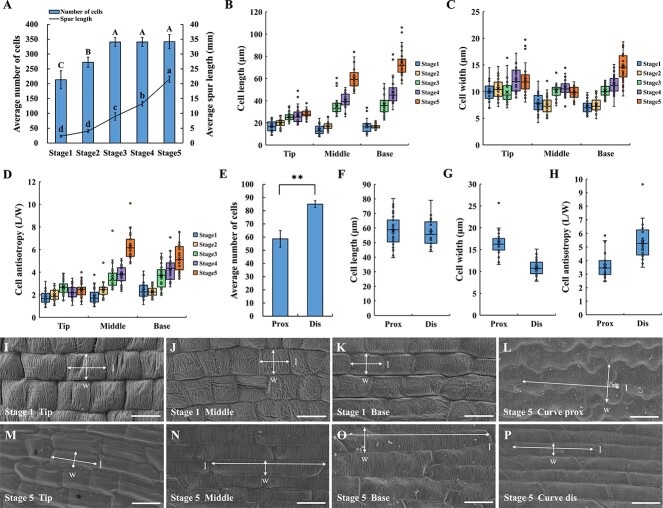
To understand the spur development process in I. uliginosa, the number, length, and width of epidermal cells were measured at five different developmental stages of spurs. From stage 1 to stage 3, there was a continuous and significant increase in average cell number, rising from 213 to 341, while the average spur length increased by about 7 mm. However, from stage 3 to stage 5, the average number of cells remained almost constant (changing from 341 to 342), while the average spur length increased by about 13 mm. These changes in cell number and spur length suggest that cell division primarily occurs in the early stage, with cell division ceasing after entering the middle stage, making cell number no longer a contributing factor for increased spur length (Fig. 2A; Table S1, see online supplementary material). Epidermal cells of the spur exhibit significant elongation with development (Fig. 2I–K and M–O). The length, width, and anisotropy of cells at the spur tip maintain a gentle growth rate. Although there is little difference in cell length between the middle and base in the first two stages, significant increases occur after stage 2, particularly with the maximum increase in cell length from stage 4 to stage 5. While there is no significant difference in cell length between the three sites during stage 1, as spur development progresses, cells closer to the base exhibit greater elongation (Fig. 2B–D; Table S1, see online supplementary material). Consequently, spur development, once in the middle stage, is primarily driven by the anisotropic cell elongation, with the increase in spur length mainly attributed to cell elongation in the middle and base.
Figure 2.
The variation in cell number and cell morphology during spur development. (A) Spur length and cell number at five developmental stages, counting cells from the base to the tip of the proximal outer epidermis. (B–D) Cell length (B), width (C), and anisotropy (D) at five developmental stages, measured at the tip, middle, and base of the spur. (E–H) Cell number (E), length (F), width (G), and anisotropy (H) on the proximal (prox) and distal (dis) sides of the spur curve in stage 5, with epidermal cells counted and measured within a 5.2 mm range of the curved part. (I–K) Cell morphology at the tip (I), middle (J), and base (K) of the spur in stage 1. (L) Morphology of cells on the proximal (prox) side of the spur curve in stage 5. (M–O) Cell morphology at the tip (M), middle (N), and base (O) of the spur in stage 5. (P) Morphology of cells on the distal (dis) side of the spur curve in stage 5. Error bars represent ± SD in (A) and (E) (n = 3). Different letters in (A) indicate statistically significant differences (Tukey’s test: α = 0.05). Asterisks in (E) indicate a significant difference relative to the proximal side (t test: **P < 0.01). Data for (B–D) and (F–H) are from three biological replicates, with 10 replicates taken for each biological replicate. Horizontal and vertical lines in (I–P) indicate cell length (l) and width (w), respectively. Bars = 10 μm in (I–K). Bars = 20 μm in (L–P).
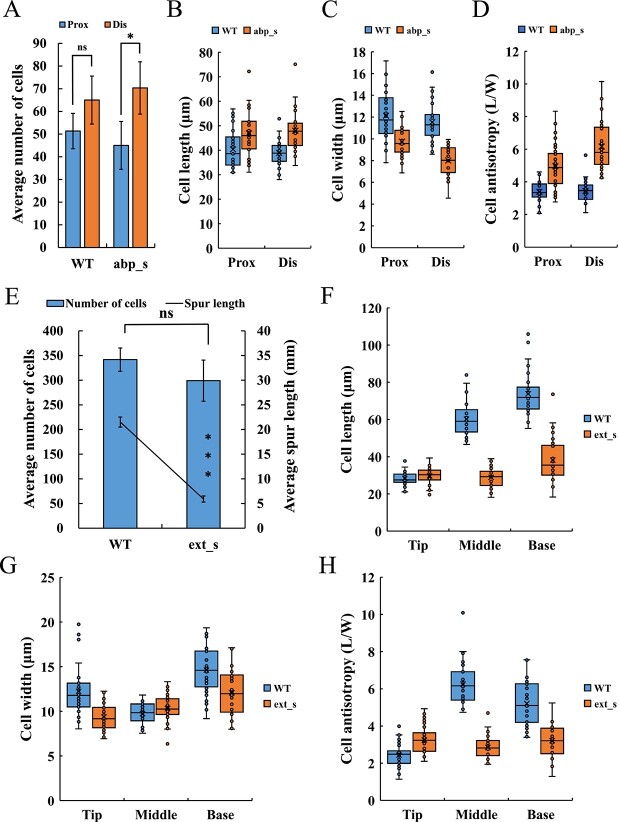
The essence of the spur curve lies in the length difference between the proximal and distal sides. To confirm whether this difference is caused by differences in cell number or length, the number, length, and width of cells on the proximal and distal sides of the spur curve at the anthesis stage were measured. Results showed that within the range of spur curve, the average number of cells in the distal epidermis was significantly greater than that in the proximal epidermis, with a difference of about 26 cells (Fig. 2E). Although the anisotropy of cells on both sides was significantly different due to differences in cell width, their cell length was extremely close (Fig. 2F–H). Therefore, it is speculated that the formation of the spur curve is due to the difference in cell number caused by cell division on the proximal and distal sides. In addition, the cells on both sides showed distinct morphological characteristics, with the proximal cells being wider and exhibiting wavy cell boundaries, while the distal cells being narrower and exhibiting a more regular oblong-like shape (Fig. 2L and P).
Endogenous hormones change dynamically at different stages of spur development
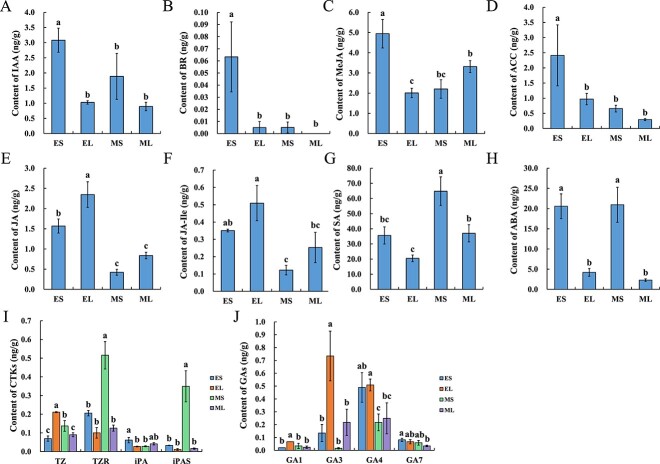
The GO and KEGG enrichment analysis of the transcriptome of I. uliginosa spur showed that ‘hormone-mediated signaling pathway’ and ‘Plant hormone signal transduction’ pathway were significantly enriched throughout the spur development [31]. To further explore the role of endogenous hormones in spur development, the targeted metabolites of 10 hormones in the early (5 to 8 mm) and middle (13 to 16 mm) spur and limb were detected by liquid chromatography-mass spectrometry (LC–MS) [42]. Hormone levels had significant changes in different tissue and stages. Auxin (IAA), brassinolide (BR), methyl jasmonate (MeJA), and aminocyclopropane carboxylic acid (ACC) were all significantly enriched in the early spur and showed no significant difference in the other three samples (Fig. 3A–D). The content of jasmonic acid (JA) and jasmonic acid-isoleucine (JA-Ile) decreased from the early to middle stages, and the content in spurs was always lower than that in the limb (Fig. 3E and F). Salicylic acid (SA) content significantly increased, and it was higher in the spurs than in the limb (Fig. 3G). The abscisic acid (ABA) content was stable at different stages, but the content in the spur was significantly higher than that in the limb (Fig. 3H).
Figure 3.
The endogenous hormone content of spurs at different developmental stages and tissues. LC-MS was used to determine the hormone content in the early and middle spur and limb tissues. Each sample contained three biological replicates, and each replicate contained over 100 flower tissues. (A) IAA. (B) BR. (C) MeJA. (D) ACC. (E) JA. (F) JA-Ile. (G) SA. (H) ABA. (I) CTKs. (J) GAs. EL, early limb; ES, early spur; ML, middle limb; MS, middle spur. Error bars represent ± SD (N = 3). Different letters indicate statistically significant differences (Tukey’s test: α = 0.05).
The content levels of trans-Zeatin riboside (TZR) and isopentenyl adenosine (iPAS) were the highest among the four cytokinins (CTKs); both had the highest content in the middle spur, while there was no significant difference between the other three samples. Trans-zeatin (TZ) increased in spurs, and the early limb had the highest content. Isopentenyl adenine (iPA) and IAA had the same change trend, and these were significantly enriched in the early spurs (Fig. 3I). Both Gibberellin A1 (GA1) and GA3 had the highest content in the early limb, and there was no significant difference between the other three samples. There was no significant difference in GA4 content between the spur and limb at the same stage, but a significant decline from the early to middle stage was observed. GA7 showed no significant differences in different tissues and stages (Fig. 3J).
Identification of IuABP and IuEXT
Annotation analysis and NCBI BLAST were utilized to identify the ABP and EXT homologs from the transcriptome, denoted as IuABP and IuEXT, respectively [31]. IuABP comprises 208 amino acid residues encoded by 573 base pairs, featuring a Cupin domain at positions 64–612 bp. Currently, there is no comprehensive research available on ABP gene family members. In maize, five ABP gene family members (ABP1, ABP2, ABP3, ABP4, ABP5) have been identified, but only ABP1 and ABP4 are present in the NCBI database [43, 44]. The Arabidopsis genome contains only one ABP1 gene [33, 45]. Therefore, to further confirm the orthology of IuABP, all available members of the ABP gene family were considered to construct a phylogenetic tree with 36 ABP genes. These genes were divided into two distinct clades; the first clade included ABP1, ABP4, ABP-T85, and ABP-T92, while the second clade contained ABP19a, ABP19b, and ABP20. The ABP genes of eight monocotyledonous plants were not independent of all dicotyledonous plants but rather clustered in the first clade, indicating that the ABP genes may be conserved in angiosperms. IuABP and Impatiens glandulifera ABP19a were clustered on a strongly supported branch, suggesting that IuABP is an orthology of ABP19a (Fig. S1, see online supplementary material).
The total length of IuEXT is 1617 bp, encoding 538 amino acids. There are many repeated SPPPPPP motifs in its sequence, with a signal peptide at the N-terminal. Based on these characteristics, it was concluded that IuEXT belongs to classical extensin [46, 47]. To further confirm the orthology of IuEXT, a phylogenetic tree was constructed for 34 classical EXT genes using two monocotyledon species as an outgroup. IuEXT and I. glandulifera EXT2 were clustered on a strongly supported branch, suggesting that IuEXT is an orthology of EXT2 (Fig. S2).
Silencing of IuABP leads to an increase in curved structures and the distortion of spur morphology
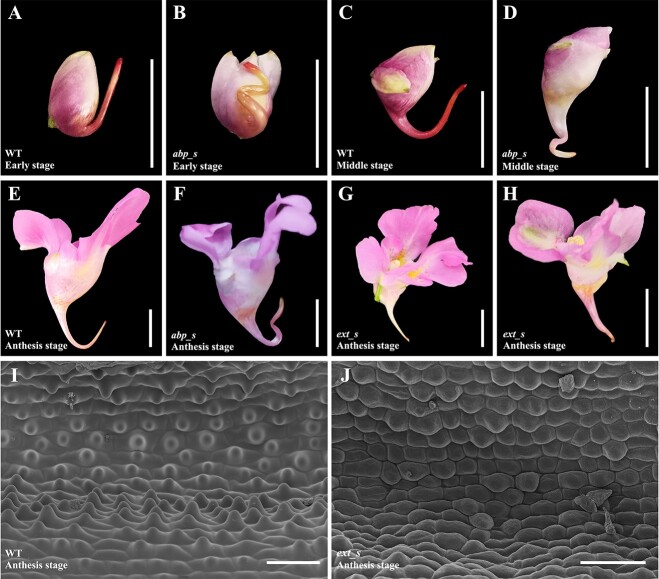
A total of 67 flowers from three plants treated with TRV2-IuABP displayed phenotypic changes. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis revealed that the expression level of IuABP in TRV2-IuABP silenced (abp_s) spurs was 13.84% of the wild-type (WT) (Fig. S3A). In WT I. uliginosa, the spur forms only one curve in the middle stage, while the abp_s spurs exhibited two or three twisted curves in the early stages, causing the entire spur to no longer lie in the same plane. This multicurved morphology was most evident in the early stage, and it persisted until the anthesis stage, although it lessened somewhat with spur development and extension (Fig. 4A–F). We measured the length and curve angle of 25 abp_s spurs. The results showed that the length of abp_s spurs was not significantly different from that of the WT (Fig. S3C, see online supplementary material), but the curve angles were slightly larger than the WT. As spur development progressed, the angles of the curves tended to increase (Fig. S3D, see online supplementary material).
Figure 4.
IuABP and IuEXT silencing led to phenotypic changes in spurs. (A) WT flower in the early stage; the curve was not yet formed. (B) abp_s flower in the early stage with 2–3 twisted curves. (C) WT flower in the middle stage, which only had one curve. (D) abp_s flower in the middle stage. (E) WT flower during anthesis. (F) abp_s flower during anthesis. (G–H) Strongly ext_s flowers during anthesis, with shortened spur and loss of curve, some individuals exhibited a distortion of the entire flower (H). (I) Mature WT spurs had distinct papillae on the inner epidermis. (J) The inner epidermis of mature ext_s spurs; most cells had no papillae. Bars = 10 mm in (A–H). Bars =100 μm in (I–J).
A statistical analysis of the number, length, width, and anisotropy of cells on the proximal and distal sides of abp_s spurs’ curves was performed. Results showed that the cell number on the proximal side was significantly less than that on the distal side, while there was no significant difference in cell length between these two sides. Proximal cells had less cellular anisotropy due to their wider cell width, but this did not affect the spur length on both sides (Fig. 5A–D;, Fig. S4G and H, see online supplementary material). Therefore, the origin of the curved structure of abp_s spurs is consistent with that of WT, which was mainly due to differences in cell number between the two sides. Compared with the WT spurs at the same stage, the cells on both sides of the abp_s spurs’ curves have a more significant numerical difference, as well as longer length and smaller width, which leads to significantly higher cell anisotropy than the WT (Fig. 5A–D; Fig. S4E–H, see online supplementary material). In addition, the parenchyma cells at the curved part of abp_s spurs showed a local, discontinuous compressed morphology due to irregular distortion (Fig. S4A–D, see online supplementary material).
Figure 5.
Cell number, length, width, and anisotropy of silenced spurs. (A–D) Number (A), length (B), width (C), and anisotropy (D) of the proximal (prox) and distal (dis) cells of abp_s spurs’ curve compared with those of WT. Epidermal cells within 1.3 mm range of the curved part were counted and measured. (E–H) Cell number (E), length (F), width (G), and anisotropy (H) of ext_s spurs compared with those of WT. Cells were counted along a single line from the base to the tip of the proximal outer epidermis, and the tip, middle, and base of the spur were measured. Error bars in (A) and (E) represent ± SD (n = 3). Asterisks in (A) and (E) indicate a significant difference (t test: *P < 0.05; ns, not significant). The data for (B–D) and (F–H) came from three biological replicates, with 10 replicates taken for each biological replicate.
IuEXT silencing led to a reduction in spur length and disappearance of curve and papillae
Thirty-nine flowers from five plants treated with TRV2-IuEXT showed phenotypic changes. The expression level of IuEXT in TRV2-IuEXT silenced (ext_s) spurs was 14.15% of that of WT (Fig. S3B). Notably, ext_s spurs displayed a significant reduction in length. We measured the final length of 24 ext_s spurs and compared it with that of WT. The average length of ext_s spurs was approximately 10.2 mm, which was about 11.8 mm shorter than that of WT (approximately 22 mm) (Fig. S3C, see online supplementary material). In cases of strong silencing, spur lengths were only 5–6 mm. These spurs often lost their curve, straightened, and some individuals exhibited flower shrinkage and distortion (Fig. 4G and H). Furthermore, strongly silenced mature spurs were dissected and compared with WT. In mature WT spurs, the inner epidermal cells displayed prominent papillae, especially those closer to the base (Fig. 4I; Fig. S5A and C, see online supplementary material). However, the inner epidermis of the ext_s spur was relatively smooth and flat, with significantly reduced cell volume compared to WT. The cells were densely arranged, and no prominent papillae were observed. Only a few cells exhibited a slight degree of eminence (Fig. 4J; Fig. S5B and D, see online supplementary material). This suggests that the significant downregulation of IuEXT inhibited papillae development and may impact nectar secretion.
To further explore the mechanism of IuEXT in regulating spur length, the cell number, length, and width of WT and strongly ext_s spurs were measured. Results showed that, although the length of ext_s spurs was significantly shorter than that of WT, the cell number was reduced by only about 42 (Fig. 5E). The cell length of the WT spur at the anthesis stage ranged from 28.4 to 73.8 μm (from the tip to the base). Using this standard, the spur length contributed by the reduced cells was in the range of 1.2 to 3.1 mm, which is much smaller than the reduction in length observed in the silenced spur compared to the WT (15.6 mm). Hence, the decrease in cell number is unlikely to be the primary factor in the shortening of spur length. In terms of cell morphology, the cells at the tips of the ext_s spurs displayed narrower widths and greater anisotropy compared to the WT, with no significant difference in their length. However, the length and anisotropy of the cells in the middle and base of the ext_s spurs significantly decreased (Fig. 5F–H; Fig. S4I–N, see online supplementary material). Therefore, the shortening of the ext_s spur was primarily caused by reduced cell elongation in the middle and base of the spur.
Discussion
Spur morphogenesis and development in I. uliginosa depend on cell division and anisotropic cell elongation
The observation of the anatomical structure of I. uliginosa flower buds at various developmental stages revealed deep staining in the spur’s growth point and the primary spur, significantly contrasting with the limb. This indicates a substantial cell accumulation, signifying an active cell division in this region. Just before spur differentiation, a vigorous cell division process commences at the growth point to prepare for spur formation. This localized cell division drives the extension of the spur from the labellum and remains concentrated on the primary spur until it reaches about 9 mm, accounting for 30%–40% of the final length. Cell division on the spur ceases at the 9-mm stage and transitions to cell elongation. At this point, the spur length from the curve to the base (approximately 2.8 mm) is much less than the length from the curve to the tip (about 8.0 mm). By the 22-mm stage (anthesis), the length from the curve to the base (around 10.5 mm) increases significantly compared to the 9 mm stage, while the length from the curve to the tip (about 9.4 mm) shows minimal growth (Fig. S6A and B, see online supplementary material). This variation in spur length aligns with the finding that spur elongation after the middle stage is primarily driven by cell elongation from the middle to the base. It is noteworthy that during the transition from the 4-mm (early stage) to the 9-mm stage (middle stage), both cell division and cell elongation coexist. A key event during this period is the formation of the curve. It is postulated that the gene expression controlling curve formation also initiates the process of cell elongation. Once the curve is formed, cell division comes to a complete halt.
The development process of I. uliginosa spur is very similar to that of Aquilegia and C. ruber, consisting of cell division and anisotropic cell elongation, with cell elongation driving most of the spur growth [17–19]. This process is consistent with previous transcriptome analyses on the functional enrichment of DEGs at different stages [31]. It is worth noting that the spur of I. uliginosa experienced two proximal-distal (dorso-ventral) cell division imbalances during development, and this specific spatiotemporal differential cell division constructed the final V shape of the spur. The first time occurs during the early stage of spur differentiation, where the difference in cell number caused by imbalanced division results in a greater dorsal length of the spur, leading to the growth of the primary spur toward the top of the bud (Fig. S6C, see online supplementary material). The second time occurs during the transition from the early stage to the middle stage, where the difference in proximal-distal cell division leads to curve formation, which is similar to that of Aquilegia brevistyla [48]. The two sides of the spur curve were subjected to different mechanical stresses, with the proximal side being compressed and the distal side being stretched, resulting in different cell morphology (Fig. 2L and P).
Hormones affect the spur development
Previous studies provided evidence on the significant enrichment of genes related to the spur development of I. uliginosa in hormone-mediated signaling pathway, such as Indol-3-acetic acid (IuIAA), SMALL AUXIN UP RNA (IuSAUR), IuARF, and IuABP, which showed high expression at the early stage [31]. Influenced by coregulatory gene groups, hormone content changes in different stages and tissues. Auxin usually concentrates on vigorous growth parts and organ formation regions [49, 50], regulating cellular processes, such as cell division, elongation, and differentiation, and affecting the final plant structure [51]. In this study, IAA distribution and concentration showed significant spatial–temporal differences and were associated with cellular processes of spur development. Therefore, it is presumed that auxin and its induced response play a crucial role in spur development. To confirm this hypothesis, exogenous IAA was applied to the outside of the initial spur (approximately 1–2 mm). Results showed that spurs applied with 0.5–10 mM IAA had a faster elongation rate and longer final length (unpublished data). In contrast with Aquilegia, IAA application did not cause morphological distortion in I. uliginosa due to over and/or uncoordinated lamina tissue proliferation [29], but it only promoted length and growth rate. In addition, hormones, such as GA4 and JAS, have similar concentration distribution patterns in spurs to auxin, which may form an interactive regulatory network with auxin [52–54]. It is speculated that these hormones not only promote cell division and vigorous early spur growth by maintaining a certain concentration level, but also regulate spur development through mutual response reactions and coregulated downstream genes.
The total CTK content was relatively low in the early stage, and it increased significantly in the middle stage. It seems to be contrary to the process of cell division in the early stage and cell elongation in the middle stage. One possible explanation for this is that the ratio of CTKs to IAA in the spur is relatively low in the early stage and relatively high in the middle stage. Changes in this ratio promote different biological activities, such as inducing callus to form roots when the ratio is low and promoting aboveground formation when the ratio is high [55–57]. It is speculated that during spur development, the growth activities in different stages are regulated by adjusting the ratio of CTKs to IAA, which promotes cell division at a lower ratio in the early stage and cell elongation at a higher ratio in the middle stage. Another possibility is that the CTK types detected were limited and cannot fully reflect the content level in spur. Further validation and supplementary experiments are needed in the future.
Transcriptome analysis showed that DEGs were significantly enriched in the ‘plant-pathogen interaction’ pathway. As the two hormones with the highest levels, SA and ABA may be related to stress resistance during spur development [58, 59]. The increase in SA content in the middle stage may be for flowering preparation [60]. The high level of ABA content in spurs may be related to the enrichment of auxin, and their content maintained a proper balance through interaction and regulation [61, 62]. The ethylene produced by IAA and ABA metabolism led to an increased ACC content, which explains the enrichment of ACC in the early spurs. The GA1, GA3, and GA7 content in the spur was stable and less than that in the limb, while the content of BR in the early spur is extremely low (0.06 ng/g); the content in other tissues approaches zero. It is speculated that these hormones have very limited effects on spur development, contrasting the effect that BR promotes spur development in Aquilegia [27, 30].
IuABP and IuEXT are involved in the regulation of spur morphology and development by affecting cell division and anisotropic elongation
IuABP is an orthology of ABP19a. Little is known about the function of ABP19 in plant development, but a study found that ABP19 can specifically bind to auxin in Prunus persica, and its activity or expression is regulated by auxin concentration [63]. Similarly, ABP1 mediates cellular processes regulated by auxin and is affected by its concentration. At higher auxin concentrations, ABP1 regulates cell growth and division, while at lower concentrations, it regulates cell expansion [35, 64]. During the development of I. uliginosa’s spurs, the correlation between auxin concentration and cellular processes aligns with the auxin response mediated by ABP, suggesting that endogenous hormones may regulate corresponding developmental processes by adjusting their content. As a crucial component in the auxin signaling pathway, IuABP likely plays a role not only in regulating cell division or elongation processes at various developmental stages but also in coordinating the transition between them [65]. A significant event during this transition is spur curve formation.
Silencing IuABP resulted in notable changes in spur morphology, characterized by an increase in the number of curves and a distortion in orientation. The cells on both sides of the abp_s spurs’ curves exhibit more significant numerical differences. We hypothesize that downregulating IuABP releases a signal resembling the decrease in auxin concentration in the WT spur, disrupting the spur’s normal growth and development pattern. This disruption leads to the premature and additional occurrence of the unbalanced event of proximal-distal cell division, which should have occurred only once during the middle stage. This regulation of cell division might be achieved by influencing cell cycle genes, as previously confirmed in studies of Arabidopsis [32, 66]. However, the coordination of cell programs seems to be important for I. uliginosa spur development. Downregulation of IuABP also leads to a greater degree of anisotropic elongation of cells on the curve that appears earlier. As we previously assumed, the formation of curves may initiate the process of cell elongation. Furthermore, the local inhibition of ABP1 activity in tobacco shoot apical meristem causes cells to exhibit an irregular division pattern [32], which may explain why the curves of abp_s spurs form multi-angle distortion. The existing data provide us with the idea of the increasing number of abp_s spurs, but it is still insufficient to explain the cause. We will conduct more targeted additional experiments to explore the mechanism.
EXT, which belongs to the superfamily of plant cell wall proteins, affects cell wall morphology by adjusting its structure [67, 68]. Spur growth and development require active cell wall remodeling [25, 69]. The most significant phenotype caused by knocking down IuEXT is spur shortening. The length of strongly silenced spurs was only 5–6 mm, equivalent to the length of WT spurs at the early stage, indicating that cell elongation was almost completely contained. The significant decrease in cell elongation from the central to basal regions of ext_s spurs indicates that IuEXT positively regulates cell elongation in I. uliginosa, which is in contrast to the negative correlation between EXT ortholog and cell elongation in Allium cepa, Oryza sativa, and Arabidopsis thaliana [70–72]. These results indicate that EXT has a conservative and redundant function in regulating cell elongation. However, there may be differences in regulation patterns among different species due to the absence of fixed conserved domains in the EXT gene family [47].
Shortened ext_s spurs also lose their curves, suggesting that the proximal-distal cell division imbalance occurring during the middle stage is substantially reduced or eliminated. However, we suggest that IuEXT regulation on cell division affecting curves may not be direct; the failure of cell program conversion was due to anisotropic elongation inhibition, so the downstream genes controlling curve formation cannot be successfully started, showing an opposite effect on abp_s spurs. In addition, papilla formation can lead to changes in cell wall structure and morphology. Extensin is an important component of the primary plant cell wall, and the knockdown of IuEXT may affect the reconfiguration of cell walls, leading to abnormal papilla development. Trichomes (or papillae, hair) within spurs usually produce nectar and are thought to increase the total surface area for nectar secretion and reabsorption [25, 73, 74]. We hypothesize that the abnormal development of the inner epidermal papillae caused by IuEXT silencing may affect the nectar secretion of spurs, which will be confirmed by further nectar content and composition detection.
Conclusion
In this study, we investigated spur development in I. uliginosa based on histomorphology and hormone levels, and the functions of IuABP and IuEXT were verified to explore the mechanism of spur development. Our results suggest that spurs reach their final length and morphology through cell division in the early stage and anisotropic elongation in the middle stage. Endogenous hormones regulate the developmental process of spurs by changing their concentrations. The expression of IuABP will affect the morphology, formation time, and number of spur curves. IuEXT regulates spur length by affecting the anisotropic elongation of spur cells, and it participates in the regulation of the development of the epidermal papillae within spurs.
Materials and methods
Plant materials and growth conditions
Seeds were collected from a wild I. uliginosa population in Laoyuhe National Wetland Park in Kunming and cultivated in a greenhouse of Southwest Forestry University. Growth conditions were maintained at 18°C–25°C, with 11–13 h of daylight and 40%–60% humidity.
Scanning electron microscopy and histology
Tissues were fixed in FAA (50% ethanol, glacial acetic acid, and 38% formaldehyde at 18:1:1 ratio). The samples were dehydrated in an ethanol series, dried using an EMS (Hatfield, Pennsylvania, USA) 850 CO2 critical point dryer, and imaged using a ZEISS (Oberkochen, Germany) Sigma 300 SEM. Histological samples were dehydrated in an ethanol series, became transparent with xylene, waxed, and embedded. The tissues were sectioned with a thickness of 8 μm, stained with 0.01% Safranin O and 50% Fast Green [75], and imaged using a Leica (Wetzlar, Germany) DM750 optical microscope.
Observation of the internal structure of flower buds and mature spurs
Flower buds of I. uliginosa were obtained from 10 WT plants and divided into five phases according to their size. The spurs cannot be observed with the naked eye for phases 1–3 buds. The spur length of the buds in phases 4 and 5 was 0.3 mm and 0.5 mm, respectively. The buds were observed by SEM and paraffin section. Mature spurs were obtained from WT and strongly ext_s plants, and the inner epidermal structure was observed by SEM after dissection. Due to the large volume of mature spurs, continuous photos were taken and merged through Photoshop.
Cell counts and measurements
For WT plants, spurs at 2 mm (stage 1, early stage), 4 mm (stage 2, early stage), 9 mm (stage 3, middle stage), 13 mm (stage 4, middle stage), and 22 mm (stage 5, anthesis stage) were selected (Figs S7A). Three biological replicates (spur) were selected from 2 or 3 plants at each stage. For abp_s plants, spurs at the 9-mm stage were selected because the twisting curves were most obvious. For ext_s plants, spurs at the anthesis stage were selected. Three biological replicates (spur) were selected from two or three silenced plants. Longitudinal paraffin sections of spurs were made, and complete images were obtained using a CaseViewer for cell counts; a measurement tool was used to obtain the spur length data. The epidermal cells on the proximal side of WT and ext_s spurs were counted from the base to the tip. The proximal-distal epidermal cells at the curved part at the 9 mm stage (WT and abp_s spurs, about 1.3-mm long range, 90° angle) and the anthesis stage (WT spurs, about 5.2-mm long range, 30° angle) were counted (Fig. S7B, see online supplementary material) [17, 19]. In addition, SEM was used to randomly select two to three visual fields of the base, middle, and tip of WT and ext_s spurs, as well as the proximal and distal sides of the curved part of abp_s spurs (9 mm stage) and WT spurs (9 mm stage and the anthesis stage). Ten cells were randomly selected from these two to three fields for measurement (Fig S7B and C, see online supplementary material). ImageJ was used to measure the maximum cell length and width [19]. Cell anisotropy was calculated based on the ratio of cell length to width [22].
Hormone content determination
The labellum was removed from WT flowers with spur lengths of 5–8 mm (early stage) and 13–16 mm (middle stage). The spur and limb tissues were frozen separately with liquid nitrogen (see Fig. S7C, see online supplementary material). We collected mixed samples from over 100 plants, each sample with three biological replicates, each weighing at least 100 mg. After crushing, the samples were extracted and purified. The extracts were then analysed using a Vanquish UPLC-Orbitrap-MS system (ThermoFisher, Waltham, MA, USA). HRMS data were recorded on a Q Exactive hybrid Q-Orbitrap mass spectrometer (ThermoFisher, Waltham, MA, USA) using SIM MS acquisition methods, and data were processed using TraceFinder [76, 77].
Isolation and identification of candidate genes
Sequences and annotation information of IuABP (TRINITY_DN6970_c0_g1) and IuEXT (TRINITY_DN9685_c0_g1) were obtained from previous transcriptome data [31]. Total RNA was extracted from labella using an Omega (Norcross, Georgia, USA) E.Z.N.A Plant RNA Kit. cDNA was obtained by reverse transcription using Transgen (Beijing, China) EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix, which was used as a template to amplify the cDNA sequences of IuABP and IuEXT. Amplified fragments were purified and cloned into a TaKaRa (Dalian, China) pMDTM19-T Vector for sequencing. Primers used for gene isolation are listed in Table S2 (see online supplementary material). To confirm the orthology of IuABP and IuEXT, coding sequences of homologous genes from other species were obtained through BLAST search on GenBank. Among them, the 20 members of classical extensin in Arabidopsis were identified by Showalter et al. [67]. Phylogenetic analysis was performed in MEGA-X using the maximum-likelihood method (Figs S1 and S2, see online supplementary material) [78]. All DNA sequences were aligned using Clustalw [79], and 1000 bootstrap replicates were performed.
Virus-induced gene silencing
A 309-bp fragment of IuABP and a 165-bp fragment of IuEXT were amplified using primers that incorporated XbaI and BamHI sites at the 5′ and 3′ ends, respectively (Table S2, see online supplementary material). The fragments were introduced into a tobacco rattle virus 2 (TRV2) vector to generate TRV2-IuABP and TRV2-IuEXT constructs. These constructs were transformed into GV3101 Agrobacterium cells by the freeze–thaw method [80, 81]. Then, 75 flower branches from 15 plants were treated with a TRV2-IuABP construct, 110 flower branches from 22 plants were treated with a TRV2-IuEXT construct, while 56 flower branches from 10 plants were treated with a TRV2 construct (empty vector). Each flower branch contains 8–30 flower buds. Flowers with silencing phenotypes were photographed, and their spur lengths were measured. The spurs were frozen at −80°C for subsequent RNA expression analysis or fixed in FAA solution for histological anatomy and SEM analysis.
Quantitative real-time polymerase chain reaction
qRT-PCR experiments were conducted to investigate the silencing efficiency of VIGS experiments. Total RNA was extracted from WT, TRV2 (empty vector), IuABP-silenced, and IuEXT-silenced tissues; then, it was reverse transcribed. The obtained cDNA was diluted 10 times and used as templates. qRT-PCR was performed on Roche (Rotkreuz, Switzerland) LightCycler 480II Real-Time Quantitative PCR Detection System using Hieff (Shanghai, China) qPCR SYBR Green Master Mix. Relative gene expression values were calculated using a comparative CT (2−ΔΔCT) method [82]. IuActin was used as an internal control. The primers used are listed in Table S2 (see online supplementary material). There were three biological replicates per sample, each with three technical replicates.
Accession numbers
Sequence data from this article can be found in the GenBank under accession numbers IuABP (ON803510.1) and IuEXT (OR036915).
Supplementary Material
Acknowledgements
This work was supported by National Natural Science Foundation of China (32060364 to M.J.H. and 32060366 to H.Q.H.); Major Science and Technology Projects in Yunnan Province (202102AE090052 to H.Q.H. and M.J.H.); Doctoral Tutor Team for Genetic Improvement and High-efficient Propagation of Landscape Plants in Yunnan Province (503210103 to H.Q.H. and M.J.H.).
Author contributions
Y.L. and H.Q.H. conceived and designed this study. Y.L. carried out the SEM experiment, VIGS experiment, data analysis, and manuscript writing. W.L.H. carried out the sample collection, phylogenetic analyses, qRT-PCR experiment and manuscript revision. X.Y.L. carried out hormone measurement experiments. Y.D.Z. carried out histology experiments. D.C.M. and C.M.W. assisted in the VIGS experiment. M.J.H. and H.Q.H. provided guidance on all the experiments and manuscripts revision. All authors have reviewed and approved the final submission.
Data availability
The data underlying this article are available in the article and its online supplementary material.
Conflict of interest
The authors declare that they have no competing interests.
Supplementary data
Supplementary data is available at Horticulture Research online.
Contributor Information
Yang Li, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China; School of Art and Design, Lanzhou Jiaotong University, Lanzhou 730070, China.
Wu-lue Huang, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
Xin-yi Li, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
Ying-duo Zhang, Department of Biodiversity Conservation, Department of Life Science, Southwest Forestry University, Kunming 650224, China.
Dan-chen Meng, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
Chun-mei Wei, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
Mei-juan Huang, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
Hai-quan Huang, College of Landscape Architecture and Horticulture Sciences, Southwest Research Center for Engineering Technology of Landscape Architecture (State Forestry and Grassland Administration), Yunnan Engineering Research Center for Functional Flower Resources and Industrialization, Research and Development Center of Landscape Plants and Horticulture Flowers, Southwest Forestry University, Kunming, Yunnan, 650224, China.
References
- 1. Hodges SA, Arnold ML. Spurring plant diversification: are floral nectar spurs a key innovation? Proc Biol Sci. 1995;262:343–8 [Google Scholar]
- 2. Fernández-Mazuecos M, Blanco-Pastor JL, Juan A. et al. Macroevolutionary dynamics of nectar spurs, a key evolutionary innovation. New Phytol. 2019;222:1123–38 [DOI] [PubMed] [Google Scholar]
- 3. Davies KL, Stpiczyńska M. Micromorphology of the labellum and floral spur of Cryptocentrum Benth. and Sepalosaccus Schltr. (Maxillariinae: Orchidaceae). Ann Bot. 2007;100:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–9 [DOI] [PubMed] [Google Scholar]
- 5. Jabbour F, Renner SS. Spurs in a spur: perianth evolution in the Delphinieae (Ranunculaceae). Int J Plant Sci. 2012;173:1036–54 [Google Scholar]
- 6. Martins DJ, Johnson SD. Hawkmoth pollination of aerangoid orchids in Kenya, with special reference to nectar sugar concentration gradients in the floral spurs. Am J Bot. 2007;94:650–9 [DOI] [PubMed] [Google Scholar]
- 7. Darwin C. The Various Contrivances by which Orchids Are Fertilized by Insects. Chicago: University of Chicago Press; 1862: [Google Scholar]
- 8. Wasserthal LT. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Bot Acta. 1997;110:343–59 [Google Scholar]
- 9. Hodges SA, Whittall JB. One-sided evolution or two? A reply to Ennos. Heredity. 2008;100:541–2 [DOI] [PubMed] [Google Scholar]
- 10. Vlašánková A, Padyšáková E, Bartoš M. et al. The nectar spur is not only a simple specialization for long-proboscid pollinators. New Phytol. 2017;215:1574–81 [DOI] [PubMed] [Google Scholar]
- 11. Young HJ. Selection on spur shape in Impatiens capensis. Oecologia. 2008;156:535–43 [DOI] [PubMed] [Google Scholar]
- 12. Bartoš M, Janeček S. Pollinator-induced twisting of flowers sidesteps floral architecture constraints. Curr Biol. 2014;24:R793–5 [DOI] [PubMed] [Google Scholar]
- 13. Hodges SA, Fulton M, Yang JY. et al. Verne Grant and evolutionary studies of Aquilegia. New Phytol. 2004;161:113–20 [Google Scholar]
- 14. Hodges SA, Arnold ML. Columbines: a geographically widespread species flock. Proc Natl Acad Sci U S A. 1994;91:5129–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodges SA. Floral nectar spurs and diversification. Int J Plant Sci. 1997;158:S81–8 [Google Scholar]
- 16. Box MS, Dodsworth S, Rudall PJ. et al. Characterization of Linaria KNOX genes suggests a role in petal-spur development. Plant J. 2011;68:703–14 [DOI] [PubMed] [Google Scholar]
- 17. Puzey JR, Gerbode SJ, Hodges SA. et al. Evolution of spur-length diversity in Aquilegia petals is achieved solely through cell-shape anisotropy. Proc Biol Sci. 2012;279:1640–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma B, Yant L, Hodges SA. et al. Understanding the development and evolution of novel floral form in Aquilegia. Curr Opin Plant Biol. 2014;17:22–7 [DOI] [PubMed] [Google Scholar]
- 19. Mack JLK, Davis AR. The relationship between cell division and elongation during development of the nectar-yielding petal spur in Centranthus ruber (Valerianaceae). Ann Bot. 2015;115:641–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou ZL, Duan YW, Luo Y. et al. Cell number explains the intraspecific spur-length variation in an Aquilegia species. Plant Divers. 2019;41:307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai T, Diggle PK, Frye HA. et al. Contrasting lengths of Pelargonium floral nectar tubes result from late differences in rate and duration of growth. Ann Bot. 2018;121:549–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cullen E, Fernández-Mazuecos M, Glover BJ. Evolution of nectar spur length in a clade of Linaria reflects changes in cell division rather than in cell expansion. Ann Bot. 2018;122:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Golz JF, Keck EJ, Hudson A. Spontaneous mutations in KNOX genes give rise to a novel floral structure in Antirrhinum. Curr Biol. 2002;12:515–22 [DOI] [PubMed] [Google Scholar]
- 24. Box MS, Dodsworth S, Rudall PJ. et al. Flower-specific KNOX phenotype in the orchid Dactylorhiza fuchsii. J Exp Bot. 2012;63:4811–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cullen E, Wang Q, Glover BJ. How do you build a nectar spur? A transcriptomic comparison of nectar spur development in Linaria vulgaris and gibba development in Antirrhinum majus. Front Plant Sci. 2023;14:1190373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ballerini ES, Min Y, Edwards MB. et al. POPOVICH, encoding a C2H2 zinc-finger transcription factor, plays a central role in the development of a key innovation, floral nectar spurs, in Aquilegia. Proc Natl Acad Sci U S A. 2020;117:22552–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yant L, Collani S, Puzey J. et al. Molecular basis for three-dimensional elaboration of the Aquilegia petal spur. Proc Biol Sci. 2015;282:20142778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez-Salazar S, González F, Alzate JF. et al. Molecular framework underlying floral bilateral symmetry and nectar spur development in Tropaeolum, an atypical member of the Brassicales. Am J Bot. 2021;108:1315–30 [DOI] [PubMed] [Google Scholar]
- 29. Zhang R, Min Y, Holappa LD. et al. A role for the auxin response factors ARF6 and ARF8 homologs in petal spur elongation and nectary maturation in Aquilegia. New Phytol. 2020;227:1392–405 [DOI] [PubMed] [Google Scholar]
- 30. Conway SJ, Walcher-Chevillet CL, Barbour KS. et al. Brassinosteroids regulate petal spur length in Aquilegia by controlling cell elongation. Ann Bot. 2021;128:931–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Wei CM, Li XY. et al. De novo transcriptome sequencing of Impatiens uliginosa and the analysis of candidate genes related to spur development. BMC Plant Biol. 2022;22:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braun N, Wyrzykowska J, Muller P. et al. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell. 2008;20:2746–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen JG, Ullah H, Young JC. et al. ABPl is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001;15:902–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones AM, Im KH, Savka MA. et al. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science. 1998;282:1114–7 [DOI] [PubMed] [Google Scholar]
- 35. Chen JG, Shimomura S, Sitbon F. et al. The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J. 2001;28:607–17 [DOI] [PubMed] [Google Scholar]
- 36. Kieliszewski MJ, Lamport DT. Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 1994;5:157–72 [DOI] [PubMed] [Google Scholar]
- 37. Liu X, Wolfe R, Welch LR. et al. Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS One. 2016;11:e0150177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewis DR, Olex AL, Lundy SR. et al. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell. 2013;25:3329–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Velasquez SM, Ricardi MM, Dorosz JG. et al. O-glycosylated cell wall proteins are essential in root hair growth. Science. 2011;332:1401–3 [DOI] [PubMed] [Google Scholar]
- 40. Hall Q, Cannon MC. The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell. 2002;14:1161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cannon MC, Terneus K, Hall Q. et al. Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci U S A. 2008;105:2226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Q, Cai WJ, Yu L. et al. Comprehensive profiling of phytohormones in honey by sequential liquid-liquid extraction coupled with liquid chromatography-mass spectrometry. J Agric Food Chem. 2017;65:575–85 [DOI] [PubMed] [Google Scholar]
- 43. Hesse T, Feldwisch J, Balshüsemann D. et al. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989;8:2453–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwob E, Choi SY, Simmons C. et al. Molecular analysis of three maize 22 kDa auxin-binding protein genes — transient promoter expression and regulatory regions. Plant J. 1993;4:423–32 [DOI] [PubMed] [Google Scholar]
- 45. Palme K, Hesse T, Campos N. et al. Molecular analysis of an auxin binding protein gene located on chromosome 4 of Arabidopsis. Plant Cell. 1992;4:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Held MA, Tan L, Kamyab A. et al. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J Biol Chem. 2004;279:55474–82 [DOI] [PubMed] [Google Scholar]
- 47. Ding Q, Yang X, Pi Y. et al. Genome-wide identification and expression analysis of extensin genes in tomato. Genomics. 2020;112:4348–60 [DOI] [PubMed] [Google Scholar]
- 48. Edwards MB, Ballerini ES, Kramer EM. Complex developmental and transcriptional dynamics underlie pollinator-driven evolutionary transitions in nectar spur morphology in Aquilegia (columbine). Am J Bot. 2022;109:1360–81 [DOI] [PubMed] [Google Scholar]
- 49. Benková E, Michniewicz M, Sauer M. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602 [DOI] [PubMed] [Google Scholar]
- 50. Reinhardt D, Pesce ER, Stieger P. et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–60 [DOI] [PubMed] [Google Scholar]
- 51. Tao LZ, Cheung AY, Wu HM. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell. 2002;14:2745–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ozga JA, Yu J, Reinecke DM. Pollination-, development-, and auxin-specific regulation of gibberellin 3beta-hydroxylase gene expression in pea fruit and seeds. Plant Physiol. 2003;131:1137–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He H, Yamamuro C. Interplays between auxin and GA signaling coordinate early fruit development. Hortic Res. 2022;9:uhab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagpal P, Ellis CM, Weber H. et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–18 [DOI] [PubMed] [Google Scholar]
- 55. Hobbie L, Estelle M. Genetic approaches to auxin action. Plant Cell Environ. 1994;17:525–40 [DOI] [PubMed] [Google Scholar]
- 56. Coenen C, Lomax TL. Auxin-cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci. 1997;2:351–6 [DOI] [PubMed] [Google Scholar]
- 57. Wu W, Du K, Kang X. et al. The diverse roles of cytokinins in regulating leaf development. Hortic Res. 2021;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–2 [DOI] [PubMed] [Google Scholar]
- 59. Chen K, Li GJ, Bressan RA. et al. Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol. 2020;62:25–54 [DOI] [PubMed] [Google Scholar]
- 60. Koo YM, Heo AY, Choi HW. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J. 2020;36:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abel S, Nguyen MD, Chow W. et al. ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem. 1995;270:19093–9 [DOI] [PubMed] [Google Scholar]
- 62. Stepanova AN, Robertson-Hoyt J, Yun J. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–91 [DOI] [PubMed] [Google Scholar]
- 63. Ohmiya A. Characterization of ABP19/20, sequence homologues of germin-like protein in Prunus persica L. Plant Sci. 2002;163:683–9 [Google Scholar]
- 64. Bauly JM, Sealy IM, Macdonald H. et al. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol. 2000;124:1229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Davies PJ. Plant Hormones and their Role in Plant Growth and Development. Dordrecht: Kluwer Academic Publishers; 1987: [Google Scholar]
- 66. David KM, Couch D, Braun N. et al. The auxin-binding protein1 is essential for the control of cell cycle. Plant J. 2007;50:197–206 [DOI] [PubMed] [Google Scholar]
- 67. Showalter AM, Keppler B, Lichtenberg J. et al. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010;153:485–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lamport DT, Miller DH. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971;48:454–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Monniaux M, Hay A. Cells, walls, and endless forms. Curr Opin Plant Biol. 2016;34:114–21 [DOI] [PubMed] [Google Scholar]
- 70. De Tullio MC, Paciolla C, Dalla Vecchia F. et al. Changes in onion root development induced by the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta. 1999;209:424–34 [DOI] [PubMed] [Google Scholar]
- 71. Roberts K, Shirsat AH. Increased extensin levels in Arabidopsis affect inflorescence stem thickening and height. J Exp Bot. 2006;57:537–45 [DOI] [PubMed] [Google Scholar]
- 72. Fan C, Li Y, Hu Z. et al. Ectopic expression of a novel OsExtensin-like gene consistently enhances plant lodging resistance by regulating cell elongation and cell wall thickening in rice. Plant Biotechnol J. 2018;16:254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stpiczyńska M, Davies KL, Kamińska M. Comparative anatomy of the nectary spur in selected species of Aeridinae (Orchidaceae). Ann Bot. 2011;107:327–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Davies KL, Stpiczyńska M. The Anatomical Basis of floral, Food-Reward Production in Orchidaceae. London: Global Science Books; 2008: [Google Scholar]
- 75. Ruzin SE. Plant Microtechnique and Microscopy. New York: Oxford University Press; 1999: [Google Scholar]
- 76. Balcke GU, Handrick V, Bergau N. et al. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods. 2012;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Glauser G, Grund B, Gassner AL. et al. Validation of the mass-extraction-window for quantitative methods using liquid chromatography high resolution mass spectrometry. Anal Chem. 2016;88:3264–71 [DOI] [PubMed] [Google Scholar]
- 78. Tamura K, Peterson D, Peterson N. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Larkin MA, Blackshields G, Brown NP. et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8 [DOI] [PubMed] [Google Scholar]
- 80. Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–86 [DOI] [PubMed] [Google Scholar]
- 81. Tian J, Pei H, Zhang S. et al. TRV-GFP: a modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. J Exp Bot. 2014;65:311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.