Abstract
Pseudorabies virus (PRV), a swine neurotropic alphaherpesvirus, is known to invade the central nervous system (CNS) of a variety of animal species through peripherally projecting axons, replicate in the parent neurons, and then pass transsynaptically to infect other neurons of a circuit. Studies of the human pathogen herpes simplex virus type 1 have reported differences in the direction of transport of two strains of this virus after direct injection into the primate motor cortex. In the present study we examined the direction of transport of virulent and attenuated strains of PRV, utilizing injections into the rat prefrontal cortex to evaluate specific movement of virus through CNS circuitry. The data demonstrate strain-dependent patterns of infection consistent with bidirectional (anterograde and retrograde) transport of virulent virus and unidirectional (retrograde) transport of attenuated PRV from the site of injection. The distribution of infected neurons and the extent of transsynaptic passage also suggest that a release defect in the attenuated strain reduces the apparent rate of viral transport through neuronal circuitry. Finally, injection of different concentrations of virus influenced the onset of replication within a neural circuit. Taken together, these data suggest that viral envelope glycoproteins and virus concentration at the site of injection are important determinants of the rate and direction of viral transport through a multisynaptic circuit in the CNS.
Considerable insight into the neurotropism of alphaherpesviruses has been gained from examination of the invasiveness and replication of different strains of virus in experimental animals. For example, analysis of the invasiveness of various strains of the swine pathogen pseudorabies virus (PRV) have demonstrated strain-dependent patterns of infection of the central nervous system (CNS) after peripheral inoculation. In the visual system, these differences are manifested by differential replication of attenuated strains of virus in functionally distinct circuits. After intravitreal injection, virulent PRV infects all retinorecipient regions of the brain in two temporally separated waves of infection, while isogenic strains that contain selective deletions of genes encoding the gI and gE envelope glycoproteins produce restricted infections of components of this circuitry involved in the regulation of circadian timing (10, 12, 17, 45). Similar findings have been reported in rat cardiac circuitry after injection of the same strains of PRV into the heart (40, 41), and a number of investigators have reported more restricted patterns of infection than that of the wild-type virus after identical injection of attenuated strains of PRV or other viruses into a variety of sites (1–3, 19, 23–25, 31, 32, 36, 37). A common theme that has emerged from these studies is that defects in one or more envelope glycoprotein genes can not only alter the invasiveness and/or replication of these viruses but also reduce virulence.
The ability of neurotropic alphaherpesviruses to pass transsynaptically has led to the increasing use of these viruses for analysis of neuronal circuitry (see references 9, 15, 26, 29, and 44 for recent reviews). Most investigations have introduced viruses into select populations of peripherally projecting neurons by inoculating their synaptic targets and then followed the progressive retrograde movement of the virus through multisynaptic circuits impinging upon these first-order neurons. Fewer studies have examined patterns of viral infection after direct injection of virus into the CNS (3, 20, 22, 28, 33–35, 46). One such study compared the patterns of infection produced by injection of two strains of herpes simplex virus type 1 into the motor strip of the primate cortex (46). These investigators reported that the McIntyre-B strain was transported transneuronally only in the retrograde direction, while identical injection of the H129 strain produced a pattern of infection consistent only with anterograde transneuronal passage. Further support for selective anterograde transneuronal infection by the H129 strain in the CNS has recently been reported in the murine visual system (42) and thalamocortical projection systems infected by tooth pulp inoculation (4). In the present study we sought to determine if strain-dependent patterns of infection could be achieved by injection of different strains of PRV into the rat prefrontal cortex (PFC).
(Early experiments included in this report were presented at a meeting of the Society for Neuroscience [16].)
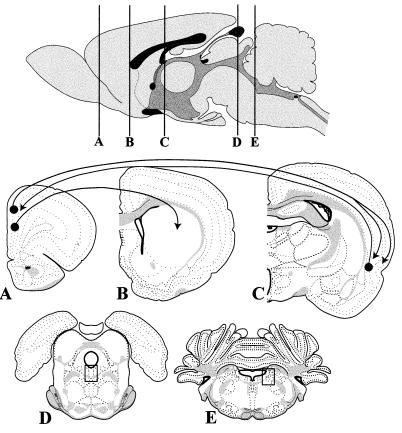
To address this question, we injected virulent or attenuated PRV into the PFC and analyzed the distribution of infected neurons throughout the brain at postinoculation intervals extending to 68 h (Table 1). The PFC was selected because it possesses a well-characterized circuitry in which the direction of viral transport from the site of injection can be unambiguously evaluated (Fig. 1). Neurons in this region project to the striatum but do not receive reciprocal projections from the same area (7, 18, 39). Therefore, the appearance of infected neurons in the striatum at short survival times provides direct evidence that the virus replicated in PFC neurons and was transported in an anterograde direction to produce a transsynaptic infection of striatal neurons. The PFC also shares reciprocal projections with a variety of regions, including the perirhinal cortex (Fig. 1). Neurons in lamina V of the perirhinal cortex are known to give rise to a dense projection to laminae I, II, and VI of the PFC (27), and therefore, this circuit provides an accurate measure of retrograde infection. However, the PFC also projects to the perirhinal cortex and elaborates an axonal arbor that terminates in both superficial and deep laminae (13). Thus, anterograde transsynaptic passage of virus would infect a more widely distributed group of perirhinal neurons than the restricted infection of lamina V that results from retrograde transport of virus from the PFC. In our experimental model, if the perirhinal cortex were being infected by bidirectional transport of virus, the need to replicate virus in the PFC prior to anterograde transport and the previous demonstration that retrograde transport of PRV is faster than anterograde transport (11) predict that retrograde infection of lamina V would precede the more widespread anterograde infection of other laminae in the perirhinal cortex. Therefore, analysis of the distribution of infected neurons in this region at various postinoculation intervals provides a further stringent test of the anterograde versus the retrograde route of viral infection.
TABLE 1.
Experimental groups
| Virus | n | Injection vol (nl) | PFU/injection | Survival (h) |
|---|---|---|---|---|
| PRV-Becker | 4 | 100 | 5.5 × 104 | 24–27 |
| 2 | 200 | 1.1 × 105 | 24–27 | |
| 2 | 100 | 5.5 × 104 | 43–48 | |
| 6 | 200 | 1.1 × 105 | 45–49 | |
| PRV-Bartha | 5 | 100 | 1.2 × 104 | 24 |
| 1 | 200 | 2.4 × 104 | 24 | |
| 5 | 100 | 1.2 × 104 | 43–48 | |
| 2 | 200 | 2.4 × 104 | 43–48 | |
| 2 | 100 | 1.2 × 104 | 68 | |
| 1 | 200 | 2.4 × 104 | 68 |
FIG. 1.
Organization of the brain circuitry that was the subject of this analysis. Virus was injected into the PFC at level A. In the majority of cases the cannula tract passed vertically through intermediate layers of the anterior cingulate and prelimbic cortex; in a few instances the cannula extended further ventrally into the infralimbic cortex. All of these regions contain neurons that give rise to a dense axonal projection to the striatum (level B). This monosynaptic projection is not reciprocated, creating an ideal means of determining if the virus is being transported in an anterograde direction to produce a transsynaptic infection. The perirhinal cortex is among a large group of structures that maintain reciprocal connections with the PFC (level C). Neurons in the PFC give rise to axons that terminate in both superficial and deep layers of the perirhinal cortex. Therefore, an anterograde transsynaptic infection would be characterized by a multilaminar distribution of PRV-immunoreactive neurons. In contrast, the reciprocal projection of the perirhinal cortex to the PFC arises predominantly from lamina V. Therefore, retrograde transport of virus from the PFC would produce a selective infection of this layer of the perirhinal cortex. The boxed areas in levels D and E illustrate the location of the raphe nucleus and locus coeruleus illustrated in Fig. 3. See the text for further details on the organization of this circuitry. The templates used in this figure were modified from reference 43.
We selected the Becker (PRV-Becker [6]) and Bartha (PRV-Bartha [5]) strains of PRV for this comparative analysis. PRV-Becker is a virulent wild-type isolate, while PRV-Bartha is an attenuated vaccine strain harboring a variety of mutations (29). One well-characterized mutation in PRV-Bartha is a deletion in the unique short region of the viral genome that eliminates genes encoding the gE and gI envelope glycoproteins. This strain also carries several mutations in the gC gene, including a signal sequence mutation that reduces the concentration of this glycoprotein in viral and host cell membranes. We have previously shown that PRV-Bartha only infects a subset of the visual pathways that are permissive to PRV-Becker replication (10, 12, 17, 45). In the present study we entertained the hypothesis that, after central injection, PRV-Bartha would produce a more restricted pattern of infection than that produced by identical injection of PRV-Becker and that this pattern would reflect the elimination of any infection produced by anterograde transport of the virus from the site of injection.
The experiments were conducted in the following manner. Adult male Sprague-Dawley albino rats, housed in a 12-12-h light-dark cycle, were anesthetized with ketamine (60 mg/kg of body weight) and xylazine (7 mg/kg) and placed in a stereotaxic frame (Stoelting Co., Wood Dale, Ill.) to secure the cranium. An incision was made in the scalp and a hole was drilled in the skull at the desired site of injection. The location of the injection site in the PFC was standardized between animals by using stereotaxic coordinates (AP = +2.5; ML = +0.25; DV = −4.0 from the skull, with the mouthpiece at −3.3) taken from reference 43. Virus (100 or 200 nl/animal [Table 1]) was injected through a 1-μl Hamilton syringe at a rate of 10 nl/min, and the needle was left in situ for 5 min following completion of the injection to reduce reflux of the inoculum up the cannula tract. Fourteen animals were injected with PRV-Becker and killed 24 to 49 h after injection. Sixteen animals were injected with PRV-Bartha and killed 24 to 68 h after injection. After the appropriate postinoculation interval, the animals were anesthetized and sacrificed by transcardiac perfusion fixation with buffered aldehyde solutions and procedures described previously (15). The brains were then removed, postfixed, cryoprotected, and sectioned serially in the coronal plane at 35 μm/section with a freezing microtome. Sections at a frequency of 210 μm (every sixth section) were processed for immunohistochemical localization of viral antigens with a rabbit polyclonal antiserum generated against acetone-inactivated PRV (11) and affinity-purified donkey anti-rabbit immunoglobulin G (Jackson ImmunoResearch, Inc.), using the avidin-biotin modification of the immunoperoxidase procedure (21) (reagents from Vector Laboratories). The processed sections were mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped. Specific details regarding the application of this procedure in our laboratory have been published previously (15). The material was analyzed and digitized with a Zeiss Axioplan microscope and a Simple32 image analysis system (C-Imaging Systems).
In all cases, the distribution of infected neurons was consistent with the established projections of the PFC and there was no evidence for nonsynaptic spread of virus within the time course analyzed. We focused upon the pattern of infection in the striatum and perirhinal cortex, but the conclusions are drawn from analysis of the full pattern of infection in each brain. Comparison of the infections produced by identical injection of PRV-Becker and PRV-Bartha revealed marked differences in the cellular distribution of viral antigens, as well as the resulting patterns of CNS infection. Additionally, injection of different concentrations of the same virus altered the time course of infection. Three general conclusions can be drawn from the data. First, injection of high concentrations of virus produced an earlier onset of viral replication than lower concentrations of the same strain. Second, neurons infected by PRV-Bartha exhibited a much more extensive intracellular distribution of viral antigens than those infected by PRV-Becker, even when PRV-Becker-infected animals, which survived a long time, were compared to PRV-Bartha-infected animals, which survived only a short time. Third, PRV-Becker produced a pattern of infection consistent with bidirectional (anterograde and retrograde) transport of virus from the injection site, whereas PRV-Bartha only replicated within neurons projecting to the injection site (retrograde transport). The evidence for strain-specific transport of PRV-Becker and PRV-Bartha will be discussed first, as this information is fundamental to the interpretation of the data supporting the other findings.
Direction of viral transport from the injection site.
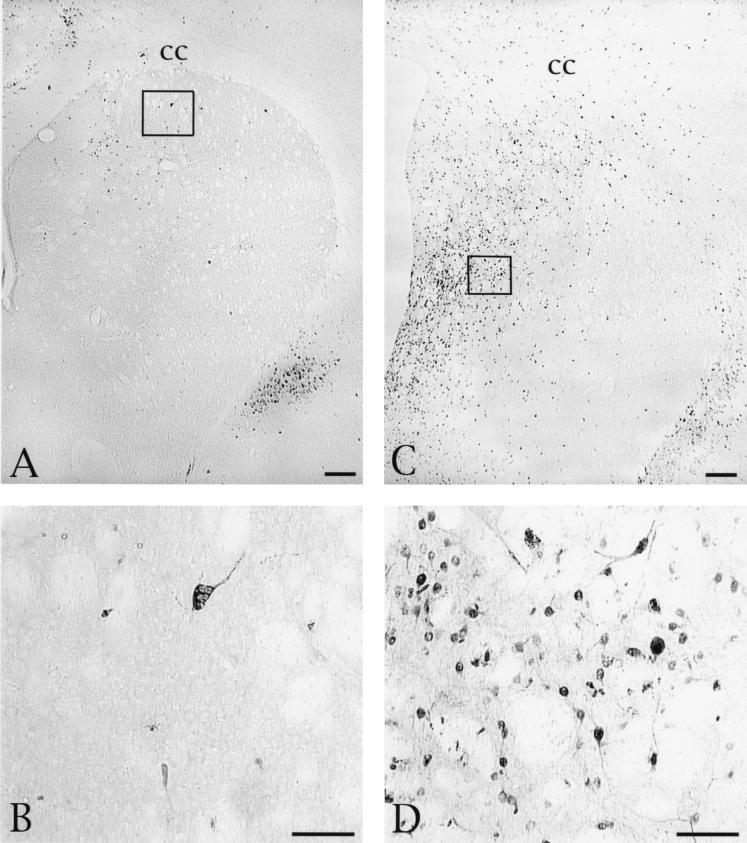
The striatum provided the most clear-cut test of directional specificity of viral transport. As noted previously, this region receives a direct projection from the PFC but does not give rise to a reciprocal projection. Furthermore, there is a differential distribution of PFC afferents in the striatum, located mostly in the rostral, dorsal, and centromedial zones (7, 18, 39), providing a further rigorous means of evaluation of the specificity of transsynaptic infection. PRV-Becker produced anterograde transsynaptic infection of neurons in the striatum, and the distribution of these cells was coextensive with the previously documented distribution of the PFC afferents in the striatum (Fig. 2). Scattered infected neurons in the rostral striatal quadrant were apparent as early as 24 h after injection of PRV-Becker into the PFC (Fig. 2A and B), and the number of infected cells increased progressively with longer survival (Fig. 2C and D). Neurons exhibiting morphology consistent with medium spiny projection neurons and the larger interneurons were present at all survival intervals. These neurons differ not only in size but also in neurochemical phenotype (interneurons are cholinergic, while the projection neurons contain the inhibitory neurotransmitter GABA). We did not characterize the neurochemical phenotypes of infected neurons in this study, but the morphological distinctions in the infected neurons (Fig. 2B and D) made it clear that both cell populations were involved. Minimal cytopathic changes were apparent in striatal neurons in the longest-surviving animals, but there was no evidence of necrotic spread of infection at survival intervals extending to 48 h (Fig. 2D).
FIG. 2.
Distribution and morphology of striatal neurons infected by injection of PRV-Becker into the striatum. (A) Distribution of infected neurons in an animal sacrificed 27 h after injection of virus into the PFC. Scattered neurons are distributed across the dorsal and medial extent of the striatum in a pattern consistent with the established termination of afferents arising in the anterior cingulate and prelimbic cortex. (B) The boxed area of panel A is shown at higher magnification, demonstrating that large interneurons and smaller projection neurons are both infected at this survival interval. (C) Photomicrograph of another animal that was sacrificed 47 h after injection of virus into the PFC. A larger number of infected neurons are present in this animal, but the distribution is similar to that seen in the animal which survived for a shorter time and is consistent with the known distribution of PFC projections to the striatum. (D) The boxed area in panel C is shown at higher magnification, revealing viral immunoreactivity in the soma and processes of striatal neurons. Bars, 100 μm. cc, corpus callosum.
The possibility that striatal neurons infected by PRV-Becker are second order to other neurons that became infected at earlier postinoculation intervals is precluded by temporal analysis of the appearance of viral immunoreactivity throughout the CNS. As noted above, infected striatal neurons became apparent within 24 h of injection of PRV-Becker into the PFC. This is the earliest time that viral antigen was detected in any region outside of the PFC. Taken together with the fact that the distribution of infected striatal neurons was coextensive with the previously established distribution of PFC axons in the striatum, these data strongly support the conclusion that the infection of striatal neurons by PRV-Becker occurred via anterograde transsynaptic passage of virus through the monosynaptic projection of the PFC to the striatum.
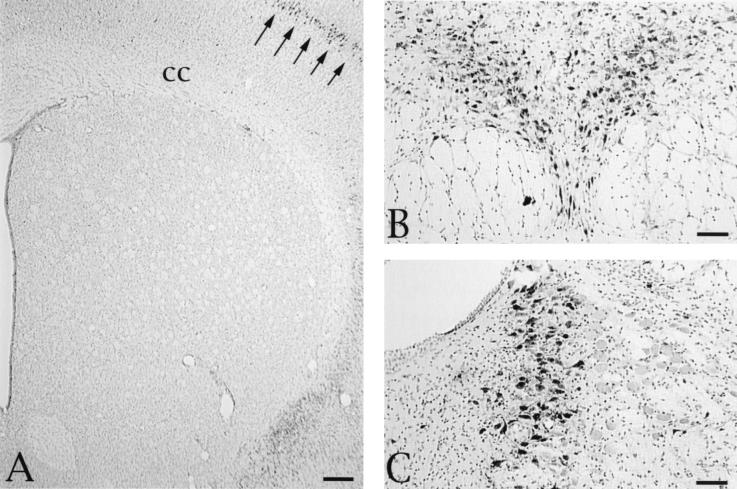
Animals injected with PRV-Bartha never exhibited infected striatal neurons at survival intervals extending to 68 h (Fig. 3A). This was true even though there was robust replication of virus throughout the cortical mantle and in other regions of the neuraxis much further from the site of injection than the striatum (Fig. 3). For example, neurons in the brain stem raphe nuclei and locus coeruleus were heavily infected as early as 43 h after injection of PRV-Bartha into the PFC (Fig. 3B). These areas are among a number of regions with monosynaptic connections with the PFC (e.g., mediodorsal and intralaminar thalamic nuclei and basolateral amygdaloid nucleus) that were infected. Since some of these regions also receive projections from the PFC, we cannot state definitively that the neuronal infections were due exclusively to retrograde passage of virus from the site of injection. However, the early appearance of virus in these regions certainly suggests that retrograde transport of virus played a prominent role. The absence of infection in the striatum could not be attributed to an inability of neurons in this region to replicate PRV-Bartha, since we have previously shown that striatal neurons can be infected with this virus by retrograde transsynaptic infection or by direct injection into the striatum (34, 35).
FIG. 3.
(A) Absence of anterograde transsynaptic infection of striatal neurons in an animal sacrificed 68 h following injection of 200 nl of PRV-Bartha into the PFC. Although infected neurons are clearly apparent in the overlying cortex (arrows), no PRV-immunoreactive cells are apparent in any region of the striatum. Other spatially distant regions with monosynaptic connections with the PFC also exhibited large numbers of infected neurons. Two of these areas in the brain stem are illustrated: dense viral immunoreactivity in the serotoninergic neurons of the dorsal raphe nucleus (B) and infected noradrenergic neurons in the locus coeruleus (C). These tissue sections were counterstained with cresyl violet to aid in the illustration of the cytoarchitecture in the regions of interest. Bars, 100 μm. cc, corpus callosum.
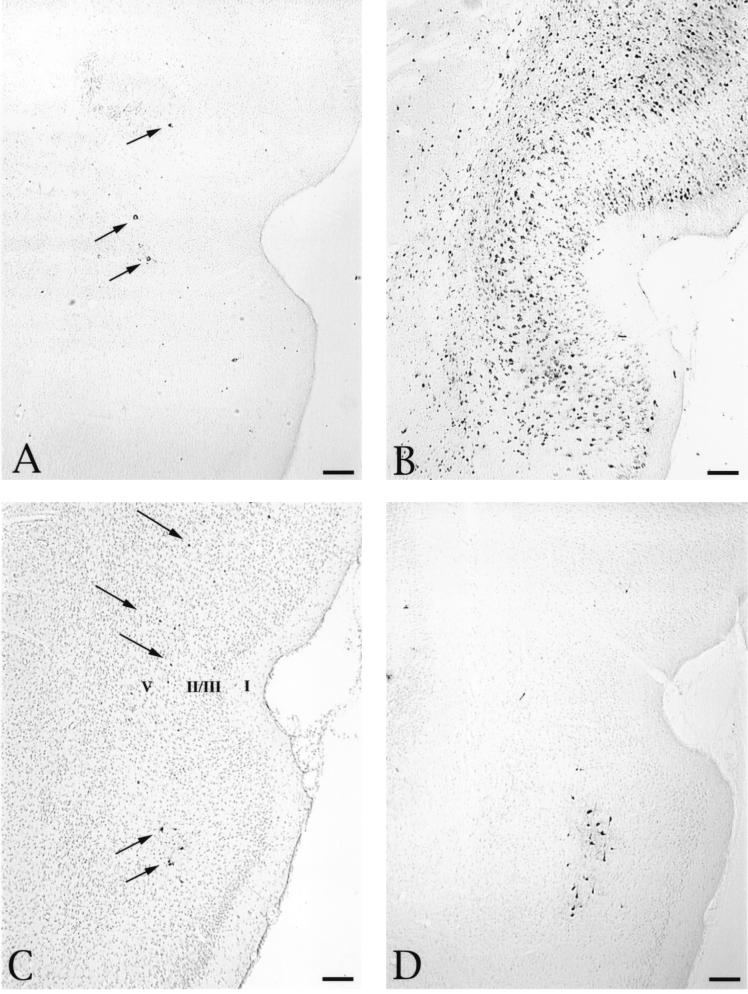
The pattern of infection in the perirhinal cortex also supports the conclusion that PRV-Becker infects neurons through both anterograde and retrograde transport while PRV-Bartha only infects via retrograde transport. As noted earlier, the perirhinal cortex has reciprocal connections with the PFC, but it is not organized by point-to-point connectivity. Projections to the PFC arise principally from neurons in lamina V of the perirhinal cortex (27), while the reciprocal projection ramifies throughout both superficial and deep laminae of the perirhinal cortex (13, 39). Therefore, selective infection of perirhinal neurons by retrograde transport of virus from the PFC would produce a restricted pattern of infection of layer V pyramidal neurons while bidirectional infection of perirhinal neurons via anterograde and retrograde pathways would be characterized by a wider distribution of infected neurons across cortical laminae. The differential distribution of infected perirhinal neurons after PFC injection of PRV-Becker and PRV-Bartha reflects this pattern (Fig. 4D). The earliest infection of the perirhinal cortex after injection of PRV-Becker and PRV-Bartha into the PFC was confined to lamina V, consistent with retrograde infection of perirhinal neurons (Fig. 4A, C, and D). Longer postinoculation intervals resulted in the appearance of progressively larger numbers of neurons across all cortical laminae. This is typified by the extensive distribution of infected neurons in the perirhinal cortex 48 h following injection of 200 nl of PRV-Becker into the PFC, as shown in Fig. 4B. A similar pattern of perirhinal cortex infection was observed 68 h after injection of PRV-Bartha (data not shown). Collectively, the pattern and temporal order of infection observed with each strain support the conclusion that PRV-Becker infected the perirhinal cortex by both retrograde and anterograde transsynaptic passage of virus, while PRV-Bartha only reached the perirhinal cortex by retrograde transport, either directly from the PFC or transsynaptically through other regions of cortex.
FIG. 4.
Distribution of infected neurons in the perirhinal cortex following injection of PRV-Becker or PRV-Bartha into the PFC. The distribution of infected neurons approximately 27 (A) and 48 (B) h after injection of 200 nl of PRV-Becker into the PFC is shown. At the shorter survival time, infected neurons are few and confined to lamina V of the perirhinal cortex (arrows). A substantially larger group of infected neurons are present in the perirhinal cortex 47 h after injection and extend through all cortical laminae. Scattered infection of layer V neurons is also observed 48 h after injection of 100 nl of either PRV-Becker (C) or PRV-Bartha (D) (arrows). The section illustrated in panel C was counterstained with cresyl violet to aid in the identification of cortical laminae, which are numbered according to the criteria defined by Swanson (43) to serve as points of orientation for defining the laminar disposition of infected neurons in the sections that were not counterstained. Bars, 200 μm.
Our data also demonstrated that PRV-Bartha had the capability of infecting neurons by retrograde transsynaptic passage of virus. Although our primary focus was upon first-order transport of virus, evidence in support of retrograde transsynaptic infection was found in essentially every system through which PRV-Bartha was transported in long-surviving animals. For example, retrograde transsynaptic movement of virus through corticocortical connections produced a robust infection throughout the neocortex (Fig. 3A). Similarly, temporally separated retrograde infection of the intralaminar thalamic nuclei followed by transsynaptic infection of neurons in the reticular thalamic nuclei is consistent with retrograde transsynaptic infection of this well-characterized dysynaptic circuit (data not shown).
Intracellular distribution of viral antigens.
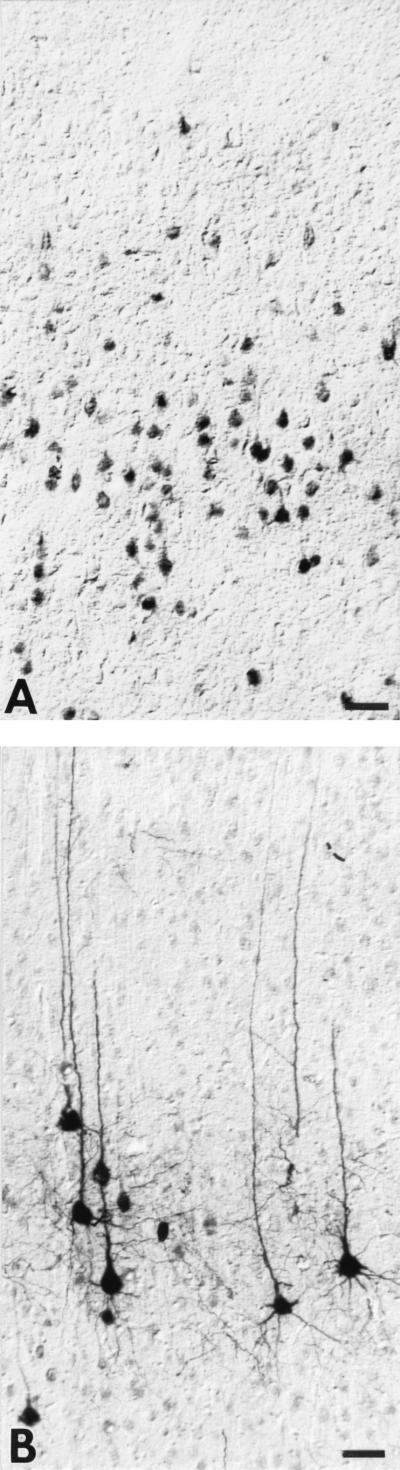
Comparison of PRV-Becker- and PRV-Bartha-infected neurons revealed a remarkable difference in the intracellular distribution of viral antigens. Even at long postinoculation intervals, viral immunoreactivity was confined to the cell soma and proximal dendrites of neurons infected with PRV-Becker. In contrast, viral immunoreactivity was widely distributed throughout the cells and dendritic processes of neurons at shorter postinoculation intervals following PRV-Bartha infection. This was particularly apparent in the polarized pyramidal cells of the perirhinal cortex (Fig. 5). These cells characteristically exhibit a triangular cell body that gives rise to extensive dendritic arbors. The apical dendritic processes of these cells are quite long and can extend to the surface of the brain even when the cell bodies are located in deep cortical laminae. Figure 5A illustrates a number of infected neurons in lamina V of the perirhinal cortex 45 h after injection of PRV-Becker into the PFC. Viral immunoreactivity, although dense, is confined to the cell body and proximal dendrites. Neurons from the same region 36 h after PRV-Bartha injection exhibit a dramatically different intracellular distribution of viral antigen. In spite of the shorter postinoculation survival, viral antigen is densely distributed throughout the processes of the infected cells and extends into the most distal processes of the apical dendrites (Fig. 5B).
FIG. 5.
Intracellular distribution of viral immunoreactivity in the perirhinal cortex 45 h after injection of PRV-Becker into the PFC (A) and 36 h after identical intracerebral injection of an equivalent concentration of PRV-Bartha (B). Viral antigens were identified with a rabbit polyclonal antibody generated against acetone-inactivated virus. The same dilution and period of incubation of tissue in the primary antibody was used in both cases, and the tissues were processed simultaneously. Note that immunoperoxidase reaction product is confined to the soma and primary dendrites of PRV-Becker-infected pyramidal neurons (A) whereas cells infected with PRV-Bartha exhibit a more extensive intracellular distribution of viral antigen, even though the animal was sacrificed 9 h earlier than the PRV-Becker-infected rat. Bars, 40 μm.
We are not certain which Bartha mutation accounts for the altered intracellular distribution of viral antigen. Prior studies have demonstrated that gene deletions and mutations present in PRV-Bartha adversely effect the efficiency of virus release in vitro (8, 30, 38, 47). In particular, deletion of the gE gene in the unique short region of the PRV genome is known to compromise release of infectious progeny from rabbit kidney cells (8, 30), and this defect is further compounded in mutants lacking both the gE and gC genes (38). PRV-Bartha contains a deletion in the unique short region of the viral genome that eliminates both the gE and gI genes, and the gC gene carries several mutations, including a signal sequence mutation that reduces the concentration of the mature glycoprotein in viral progeny and host cell membranes. It may be that a similar defect in the release of PRV-Bartha occurs in neurons in vivo, but this has not been directly examined. Nevertheless, it is clear from numerous investigations that if such a defect is expressed in neurons, it does not compromise the ability of the virus to pass transsynaptically and infect other neurons within a polysynaptic circuit (see references 14, 26, and 29 for recent reviews).
Effect of viral concentration.
A comparison of the progression of infection after injection of different volumes of the same strain of virus into the PFC demonstrated that there was an earlier onset of PRV replication in animals injected with higher concentrations of virus. This was clearly apparent in comparing the patterns of infection in the perirhinal cortex following injection of 100 or 200 nl of PRV-Becker into the PFC. As previously described, injection of 200 nl of virus led to an early infection of layer V pyramidal neurons (Fig. 4A) followed by an extensive infection of neurons across cortical laminae at 48 h (Fig. 4B). In contrast, injection of half that volume of virus led to a delay in the onset of infection such that the magnitude of infection observed 48 h after injection of 100 nl (Fig. 4C) was equivalent to that produced at 24 h after injection of 200 nl (Fig. 4A). The present data set does not allow us to determine whether this delay resulted from the reduction in viral concentration or the reduced volume. However, in other experiments involving intrastriatal injection of PRV-Bartha we have demonstrated that concentration rather than volume is the important variable that determines the onset of production of infectious progeny in a permissive neuron (35).
The substantial differences observed in the patterns and progression of infection after intracerebral injection of PRV-Becker and PRV-Bartha are consistent with the strain-dependent differences in viral replication observed in other studies. Although many studies have demonstrated retrograde transsynaptic infections of the CNS with a variety of alphaherpesvirus strains, only a few have reported anterograde transsynaptic infections (3, 4, 42, 46). Zemanick and colleagues (46) reported strain-specific transport of two strains of herpes simplex virus type 1 injected into the motor strip of the primate cortex, with the H129 strain moving selectively in the anterograde direction. Further evidence in support of anterograde transneuronal infection of H129 has recently been provided by Sun and coworkers (42), who demonstrated anterograde transneuronal infection of the lateral geniculate nucleus and striate cortex after intravitreal injection of this strain, and Barnett and colleagues reported anterograde transneuronal infection in the CNS after inoculation of the tooth pulp with this strain (4). Our data differ from these reports in that we observed bidirectional transport of PRV-Becker and unidirectional retrograde transport of PRV-Bartha rather than selective anterograde transneuronal passage of either strain. The mechanisms underlying the directional specificity of H129 have not been established, but it is likely that the differences noted in our analysis may be related to the well-characterized alterations in the viral genome that distinguish PRV-Bartha from PRV-Becker. Prior studies have demonstrated that the absence of envelope glycoprotein genes can profoundly alter the invasiveness and/or spread of PRV. For example, gB and gD have both been shown to be essential for entry of virus into neurons, but only gB is necessary for subsequent transsynaptic passage (1, 19, 31, 36). Prominent roles for the gE and gI glycoproteins have also been demonstrated. We have shown that mutants isogenic with PRV-Becker that lack gE, gI, or both of these gene products produce a restricted pattern of infection of a functionally distinct component of the visual circuitry (10, 12, 17, 45). These findings are similar to data demonstrating altered invasiveness of PRV through olfactory or trigeminal circuitry in pigs after intranasal inoculation (24, 31, 32) and support a role for these virally encoded gene products in anterograde transneuronal passage. We are currently testing gE and gI mutants in the PFC paradigm and predict that these studies will demonstrate that the absence of these genes eliminates the anterograde component of the infection.
In summary, we have demonstrated distinct differences in the patterns of infection produced by two strains of PRV, in terms of both the direction of transport and the intracellular distribution of viral antigens. Deletions in the viral genome documented for the strain with the more restricted phenotype, PRV-Bartha, suggest that these alterations may be due to the absence of one or more viral envelope glycoproteins in the unique short region of the viral genome.
Acknowledgments
We gratefully acknowledge the technical assistance of Jen-Shew Yen and Marlies Eldridge.
This work was supported by NIH RO1s MH53574 (J.P.C.), MH45507 (P.L.), and NINDS33506 (L.W.E.).
REFERENCES
- 1.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 3.Barnett E M, Cassell M D, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett E M, Evans G D, Sun N, Perlman S, Cassell M D. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci. 1995;15:2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartha A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allatorv Lapja. 1961;16:42–45. [Google Scholar]
- 6.Becker C H. Zur primaren Schadingung vegetativer Ganglien nach Infektion mit deme Herpes suis Virus bei verschiedenen Tierarten. Experientia. 1967;23:209–217. doi: 10.1007/BF02136291. [DOI] [PubMed] [Google Scholar]
- 7.Beckstead R M. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Porat T, DeMarchi J, Pendrys J, Veach R A, Kaplan A S. Proteins specified by the short unique region of the genome of pseudorabies virus play a role in the release of virions from certain cells. J Virol. 1986;57:191–196. doi: 10.1128/jvi.57.1.191-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P, Strick P L. Transneuronal mapping of neural circuits with alpha herpesviruses. In: Bolam J P, editor. Experimental neuroanatomy, a practical approach. Oxford, England: IRL Press; 1992. pp. 81–101. [Google Scholar]
- 10.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two alpha herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 13.Deacon T W, Eichenbaum H, Rosenberg P, Eckman K W. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- 14.Enquist L W. Infection of the mammalian nervous system by pseudorabies virus. Semin Virol. 1994;5:221–231. [Google Scholar]
- 15.Enquist L W, Card J P. Pseudorabies virus: a tool for tracing neuronal connections. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 333–348. [Google Scholar]
- 16.Enquist L W, Levitt P, Card J P. Connections of the adult rat prefrontal cortex revealed by intracerebral injection of a swine alpha herpesvirus. Soc Neurosci Abstr. 1993;19:589.11. [Google Scholar]
- 17.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerfen C R. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- 19.Heffner S, Kovács F, Klupp B G, Mettenleiter T C. Glyco-protein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoover J E, Strick P L. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 21.Hsu S M, Raine L, Fanger H. The use of avidin-biotin-peroxidase complex in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 22.Jasmin L, Burkey A R, Card J P, Basbaum A. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimman T G, de Wind N, Oei-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contribution of single genes within the unique short region of Aujeszky’s disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 24.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky’s disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 25.Lafay F, Coulon P, Astic L, Saucier D, Riche D, Holley A, Flamand A. Spread of the CVS strain of rabies virus and of the avirulent mutant AvO1 along the olfactory pathways of the mouse after intranasal inoculation. Virology. 1991;183:320–330. doi: 10.1016/0042-6822(91)90145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loewy A D. Pseudorabies virus: a transneuronal tracer for neuroanatomical studies. In: Kaplitt M G, Loewy A D, editors. Viral vectors. San Diego, Calif: Academic Press; 1995. pp. 349–366. [Google Scholar]
- 27.McIntyre D C, Kelly M E, Staines W A. Efferent projections of the anterior perirhinal cortex in the rat. J Comp Neurol. 1996;369:302–318. doi: 10.1002/(SICI)1096-9861(19960527)369:2<302::AID-CNE10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.McLean J H, Shipley M T, Bernstein D. Golgi-like transneuronal retrograde labeling with CNS injections of herpes simplex virus type 1. Brain Res Bull. 1989;22:867–881. doi: 10.1016/0361-9230(89)90032-4. [DOI] [PubMed] [Google Scholar]
- 29.Mettenleiter T C. Molecular properties of alphaherpesviruses used in transneuronal pathway tracing. In: Kaplitt M G, Loewy A D, editors. Viral vectors. San Diego, Calif: Academic Press; 1995. pp. 367–393. [Google Scholar]
- 30.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder W A M, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M A. Glycoprotein gE-negative virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 33.Norgren R, Lehman M N. Retrograde transneuronal transport of herpes simplex virus in the retina after injection in the superior colliculus, hypothalamus and optic chiasm. Brain Res. 1989;479:374–378. doi: 10.1016/0006-8993(89)91644-2. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell P O, Lavin A, Enquist L W, Grace A A, Card J P. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transsynaptic transport of pseudorabies virus. J Neurosci. 1997;17:2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Enquist L W, Moore R Y, Card J P. The effect of viral concentration upon invasiveness, replication, and transsynaptic passage of pseudorabies virus injected into striatum. Soc Neurosci Abstr. 1996;22:1730. [Google Scholar]
- 36.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pol J M A, Gielkens A L J, van Oirschot J T. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microb Pathog. 1989;7:361–371. doi: 10.1016/0882-4010(89)90039-9. [DOI] [PubMed] [Google Scholar]
- 38.Schreurs C, Mettenleiter T C, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988;62:2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sesack S R, Deutch A Y, Roth R H, Bunney B S. Togographical organization of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 40.Standish A, Enquist L W, Schwaber J S. Innervation of the heart and its central medullary origin defined by viral tracing. Science. 1994;263:232–234. doi: 10.1126/science.8284675. [DOI] [PubMed] [Google Scholar]
- 41.Standish A, Enquist L W, Escardo J A, Schwaber J S. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun N, Cassell M D, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson L W. Brain maps: structure of the rat brain. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1992. [Google Scholar]
- 44.Ugolini G. Transneuronal tracing with alpha-herpesviruses: a review of the methodology. In: Kaplitt M G, Loewy A D, editors. Viral vectors. San Diego, Calif: Academic Press; 1995. pp. 293–317. [Google Scholar]
- 45.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H-J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zemanick M C, Strick P L, Dix R D. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain dependent. Proc Natl Acad Sci USA. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zsak L, Mettenleiter T C, Sugg N, Ben-Porat T. Release of pseudorabies virus from infected cells is controlled by several viral functions and is modulated by cellular components. J Virol. 1989;63:5475–5477. doi: 10.1128/jvi.63.12.5475-5477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]