Abstract
The human cdc2-related kinase PITALRE is the catalytic component of TAK, the Tat-associated kinase. Previously, we have proposed that TAK is a cellular factor that mediates Tat transactivation function. Here we demonstrate that transient overexpression of PITALRE specifically squelches Tat-1 activation of both a transfected and an integrated human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR), suggesting that PITALRE mediates Tat function as a multiprotein complex. A catalytic mutant of PITALRE, D167N, was found to be more efficient than wild-type PITALRE in squelching Tat transactivation. Neither wild-type PITALRE nor D167N was able to squelch transactivation of the human T-cell leukemia type 1 LTR by the Tax protein. Additionally, we show that artificial targeting of PITALRE to a nascent RNA element, in the absence of Tat, activated HIV-1 LTR expression. These results indicate that a PITALRE-containing complex mediates transactivation by Tat and suggest that Tat proteins function by localizing such a PITALRE-containing complex to the site of the transcribing provirus.
The proviral genome of human immunodeficiency virus type 1 (HIV-1) and HIV-2 is transcribed by the host cell RNA polymerase II (RNAPII) complex. The viral RNAs are subject to complex patterns of splicing that yield both messenger and genomic viral RNA products. Both HIV RNA synthesis and splicing, while dependent on host functions, are subject to regulation by viral functions. Autoregulation of HIV transcription is primarily mediated by the HIV Tat protein (2, 35), and Tat function is essential for efficient virus replication (7, 9). Tat interacts directly with the nascently transcribed transactivation response region (TAR) RNA structure at the 5′ end of all viral transcripts, and this interaction is a requisite for maximal Tat-mediated upregulation of viral transcription (reviewed in reference 16). Genetic experiments have identified an activation domain within Tat that can function independently of Tat’s RNA-binding domain to activate transcription (17, 33, 34, 36).
In the absence of Tat, the HIV promoter specifies primarily abortive transcription complexes, whereas in the presence of Tat, the quantity of full-length transcripts is greatly increased (18, 20, 26). These observations have suggested that the HIV-1 promoter assembles primarily transcription complexes which are subject to abortive elongation and that Tat acts by mediating an alteration of the initiated complex such that the processivity of the transcription complex is enhanced. It thus appears likely that Tat effects a discrete step during the RNAPII transcription cycle.
Genetic and biochemical data indicate the existence of one or more cellular cofactors that mediate the transcriptional effect of Tat proteins. It has been observed that the activation domains of the lentiviral Tat proteins of HIV and equine infectious anemia virus can squelch Tat transactivation, suggesting that a limiting cellular target exists (3, 22). Such a cofactor should interact specifically with the functional activation domains of Tat proteins and should enable Tat proteins to activate elongation of transcription. Studies in vitro with fractionated systems suggest that Tat-mediated transcription requires a cellular function which is not conferred by general transcription factors (37, 45). Based on biochemical observations, a number of cofactors that could constitute the cellular interface to Tat function have been proposed, including cellular general transcription factor TFIIH (11, 31), Tat-SF1 (46), and Tat-associated kinase (TAK) (14, 15, 42).
As Tat proteins act to stabilize RNAPII elongation, the biochemical events associated with RNAPII elongation have served as the basis for mechanistic analyses of Tat. Of significance in this regard are the findings that (i) elongation of RNAPII transcription can be specifically inhibited by the nucleoside analog 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) (10, 38), (ii) DRB inhibits Tat-mediated transcription (27); (iii) DRB can specifically inhibit the activities of some cellular kinases (44), and (iv) the hyperphosphorylation of RNAPII on its repetitive carboxyl-terminal domain (CTD) correlates with the transition from transcription initiation to elongation (6, 29, 32). These results have suggested that a DRB-sensitive CTD kinase is required for Tat function and, further, that such a kinase could be the direct cofactor mediating Tat function. Both TFIIH (43) and TAK (15) are sensitive to DRB and are able to hyperphosphorylate the CTD of RNAPII. Further, it has been observed that the CTD of RNAPII is required for Tat function in cells (4, 30, 42) and that purified Tat is able to enhance CTD phosphorylation in vitro by TFIIH and, perhaps, other kinases (11, 31). Thus, it seems probable that a Tat cofactor is a DRB-sensitive kinase(s) which can phosphorylate RNAPII on its CTD.
Recent experiments in this laboratory (41) indicate that the 42-kDa phosphorylated protein which copurifies with TAK activity is a previously identified cdc2-related protein kinase, PITALRE (13). PITALRE is ubiquitously expressed in human tissues, has no apparent cell cycle regulation, and associates with several additional cellular proteins in vivo (12, 13). Antibodies to PITALRE deplete HeLa cell nuclear extracts of TAK activity, indicating that PITALRE is an essential component of TAK. PITALRE-containing complexes can hyperphosphorylate the CTD, suggesting that PITALRE is the CTD kinase present in the TAK complex. Additionally, we have observed that TAK can be activated in peripheral blood lymphocytes and promonocytic cell lines, suggesting that regulation of TAK catalytic function is one mechanism underlying the sensitivity of HIV to its cellular environment (41). It is notable that PITALRE is expressed at high levels in lymphoid tissue relative to other human tissues (8).
To determine the role of TAK in Tat-mediated viral transcription, in this study, wild-type PITALRE or catalytically inactive mutant PITALRE was transiently overexpressed and the resultant effect on transactivation of the HIV-1 long terminal repeat (LTR) by Tat-1 was observed. Additionally, we determined whether PITALRE could activate the transcription of the HIV-1 LTR when targeted directly to a promoter-proximal RNA element. These studies have provided evidence that TAK mediates Tat’s transactivation function in vivo.
Transiently expressed wild-type PITALRE and catalytic mutant D167N specifically associate with Tat in vitro.
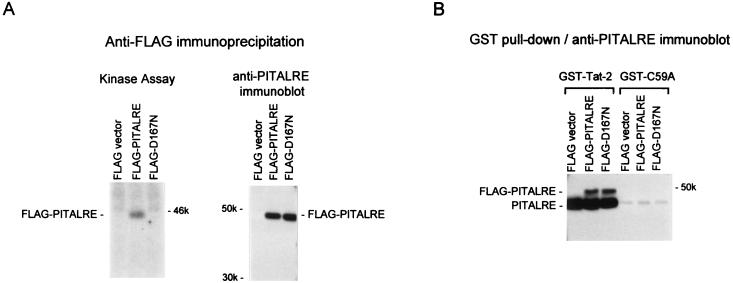
Plasmid expression vectors were constructed that express a FLAG epitope-tagged wild-type or mutant PITALRE. The mutant protein, termed D167N, contains a substitution of asparagine for aspartic acid at residue 167 which inactivates catalytic function (12). To verify that the plasmids express stable proteins and that the D167N mutation disrupts catalytic function, HeLa cells were transfected with FLAG vector, PITALRE, or D167N plasmid DNA. At 72 h posttransfection, lysates were prepared and immunoprecipitation was carried out with anti-FLAG antibodies, followed by in vitro kinase assays (Fig. 1A). The results demonstrated that a 46-kDa autophosphorylated product was present in PITALRE-transfected cells which was absent in D167N-transfected cells, confirming disruption of PITALRE catalysis by the D167N substitution. Immunoprecipitation of lysates with anti-FLAG antibodies followed by immunoblotting with PITALRE antibodies demonstrated equivalent expression levels for PITALRE and D167N (Fig. 1A).
FIG. 1.
Transiently expressed wild-type and catalytic mutant PITALRE proteins specifically associate with Tat in vitro. HeLa cells (30% confluent in 10-cm-diameter dishes) were transfected by Lipofectamine (Life Tech) with 3 μg of a CMV2-FLAG or CMV2-FLAG-PITALRE plasmid, and at 72 h posttransfection, 1-ml lysates were prepared as previously described (42). (A) Kinase assays and immunoblots. Lysates (500 μl) were immunoprecipitated with 5 μl of anti-FLAG monoclonal antibody of M2 (3 mg/ml; Kodak). For kinase assays, immunoprecipitates were bound to protein A-Sepharose (RepliGen), washed three times in EBC (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% Nonidet P-40, 5 mM dithiothreitol) plus 0.03% sodium dodecyl sulfate, and in vitro kinase assays were performed as previously described (15); 50% of the reactions were analyzed on a sodium dodecyl sulfate–9% polyacrylamide gel. For immunoblots, lysates (500 μl) were immunoprecipitated with 5 μl of anti-FLAG monoclonal antibody M2 and bound and washed as described above, and 50% of the immunoprecipitates were loaded onto a sodium dodecyl sulfate–8% polyacrylamide gel and then transferred to a nitrocellulose membrane; to detect PITALRE proteins, an enhanced-chemiluminescence procedure was used with a commercial PITALRE antiserum (Santa Cruz Biotechnology). (B) GST-Tat-2 in vitro binding assay. Lysates (500 μl) were incubated with 0.5 μg of GST-Tat2 or GST-C59A (Tat-2) attached to glutathionine-Sepharose beads as described previously (15). After incubations, the bead complexes were washed three times in EBC plus 0.03% sodium dodecyl sulfate and then loaded onto a sodium dodecyl sulfate–8% polyacrylamide gel. The gel was transferred to nitrocellulose and probed with an anti-PITALRE antibody as described above. The values beside the gels are molecular weights in thousands.
To determine whether the transiently expressed PITALRE proteins were capable of specific association with Tat proteins, in vitro glutathione S-transferase (GST) “pull-down” assays were performed with wild-type HIV-2 Tat (Tat-2) and the transactivation-defective C59A Tat-2 mutant protein. An immunoblot performed with anti-PITALRE antibodies indicated that endogenous HeLa PITALRE and both recombinant PITALRE and D167N associated with the GST fusion to wild-type but not mutant Tat-2 (Fig. 1B). We conclude from these experiments that the D167N mutant lacks a detectable catalytic function and, like wild-type PITALRE, is able to specifically associate in a TAK complex with Tat-2.
PITALRE association with Tat proteins could require that PITALRE first assemble into a complex with other cellular factors. Using baculovirus-expressed PITALRE and Escherichia coli-expressed PITALRE, we have been unable to observe a specific association between PITALRE and Tat proteins in vitro (unpublished observations). The ability of recombinant PITALRE isolated from cells to associate with Tat-2 in vitro, as shown in Fig. 1B, may require an additional human cellular component. Likely candidates for such an additional component(s) include the cyclin partner of PITALRE.
PITALRE overexpression in HeLa cells inhibits transactivation by Tat-1.
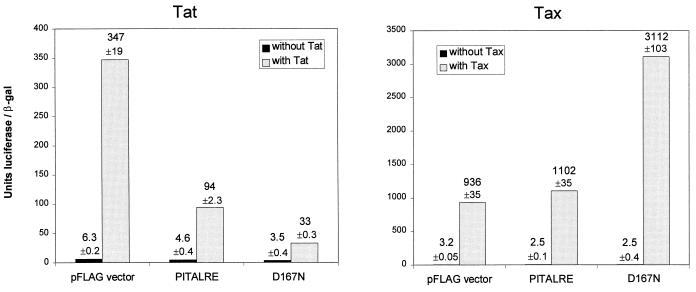
To examine the effects of overexpression of wild-type and catalytic mutant PITALRE proteins on Tat function in vivo, HeLa cells were cotransfected with an HIV-1 LTR luciferase reporter plasmid, a Tat-1 expression plasmid, and a plasmid expressing PITALRE or D167N (Fig. 2A). Measurement of luciferase activities 72 h posttransfection and normalization to β-galactosidase activities from an internal reference plasmid indicated that Tat-1 activated reporter expression 55-fold in the presence of the parental FLAG vector. PITALRE overexpression resulted in 2.7-fold inhibition of Tat transactivation function, and overexpression of D167N resulted in 5.8-fold inhibition of transactivation. Overexpression of the wild-type and D167N proteins had relatively little effect on basal-level expression of the HIV-1 LTR luciferase plasmid; in several independent experiments, the wild-type and D167N proteins reduced basal expression from 1.1-fold to 1.4-fold and from 1.3-fold to 1.9-fold, respectively (also see Fig. 3).
FIG. 2.
Effect of PITALRE overexpression on transactivation by HIV-1 Tat and HTLV-1 Tax. (A) PITALRE overexpression inhibits transactivation by Tat-1. HeLa cells (50% confluent in 6-cm-diameter dishes) were transfected with 15 ng of an HIV-1 LTR luciferase reporter plasmid (1), 1,500 ng of a simian virus 40 early promoter β-galactosidase (β-gal) reporter plasmid (pCH110; Pharmacia), 25 ng of CMV2-FLAG-Tat-1, and 500 ng of the CMV2-FLAG parental vector (Kodak) or a FLAG-PITALRE plasmid, as indicated. Transfections were performed with Lipofectamine (Life Tech) and a procedure suggested by the manufacturer. Luciferase assays were performed with the Luciferase Assay System (Promega), by using 5 or 20 μl of lysate plus 20 μl of substrate, and light units were measured by using a TD20-e luminometer (Turner). For β-galactosidase assays, 50-μl lysates were used in standard enzyme assays. The luciferase values shown were normalized to β-galactosidase expression and are averaged from duplicate samples. (B) PITALRE overexpression does not inhibit transactivation by Tax. HeLa cells (50% confluent in 6-cm-diameter dishes) were cotransfected with 15 ng of an HTLV-1 LTR luciferase reporter plasmid (obtained from M. Goff and S. Marriott, Baylor College of Medicine), 1,500 ng of a simian virus 40 early promoter β-galactosidase reporter plasmid, 25 ng of CMV-Tax (obtained from S. Marriott), and 500 ng of a CMV2-FLAG or FLAG-PITALRE plasmid as described above. Cells were harvested at 72 h, and luciferase and β-galactosidase assays were performed as described above. The values shown were averaged from duplicate samples.
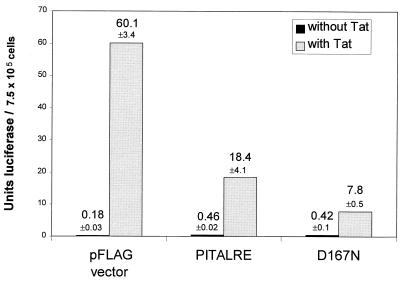
FIG. 3.
PITALRE overexpression inhibits Tat activation of an integrated HIV-1 LTR. 1G5 cells (3.0 × 106) were transfected with 1 μg of a CMV2-FLAG-Tat-1 and 10 μg of a CMV2-FLAG or FLAG-PITALRE plasmid by electroporation (Gene Pulser; Bio-Rad) at 250 V and 960 μF in 400 μl of serum-free RPMI medium. Cells were harvested at 24 h posttransfection, and luciferase assays were performed with the Luciferase Assay System (Promega), using 100 μl of lysate plus 100 μl of substrate, and light units were measured by using a TD20-e luminometer (Turner). The values shown were averaged from duplicate samples.
To analyze the specificity of inhibition by PITALRE overexpression, we examined the transactivation of the human T-cell leukemia virus type 1 (HTLV-1) LTR by its cognate Tax transactivator protein. HeLa cells were cotransfected with an HTLV-1 LTR luciferase reporter plasmid, a Tax expression plasmid, and PITALRE plasmids (Fig. 2B). Measurement of luciferase and β-galactosidase activities indicated 290-fold Tax activation in the presence of the FLAG parent vector, 433-fold activation upon PITALRE overexpression, and 1,260-fold activation upon overexpression of D167N. In multiple separate experiments, D167N reproducibly enhanced Tax transactivation 1.5- to 4.4-fold. The significance of the increase in Tax transactivation by overexpression of the D167N protein is unclear. However, our results imply that PITALRE function may repress the expression of some promoters such as the HTLV-1 LTR. Such a possibility has a precedent in the yeast SRB10 protein, which is a homolog of mammalian CDK8; SRB10 is a CTD kinase, and genetic evidence indicates that it can function as both an activator and a suppressor of transcription (19, 21, 24, 40).
Overexpression of the wild-type and D167N proteins did not significantly affect basal-level expression of the HTLV-1 LTR (Fig. 2). We conclude from these experiments that transactivation of the HTLV-1 LTR by Tax was not inhibited by PITALRE or PITALRE-D167N expression, indicating that PITALRE overexpression does not generally squelch activated transcription. It may be noteworthy that Tax function does not require the CTD of RNAPII (4).
In the experiment presented in Fig. 2, the Tat, Tax, and PITALRE proteins were expressed from the same cytomegalovirus (CMV) promoter; therefore, it is highly unlikely that the levels of inhibition of Tat transactivation by the wild-type and D167N PITALRE proteins can be explained by reduced Tat protein levels. These results indicate that both wild-type PITALRE and D167N, upon overexpression in HeLa cells, can specifically squelch Tat-mediated transactivation. The ability of wild-type overexpressed PITALRE to squelch Tat function suggests that TAK function requires one or more subunits in addition to PITALRE, such that overexpression of one subunit disrupts the stoichiometry of the functional complex. The observation that D167N squelches Tat function more efficiently than does wild-type PITALRE suggests that the catalytic function of PITALRE is important for TAK function and is consistent with our previously proposed model that the function of TAK during HIV transcription is to phosphorylate the CTD of RNAPII (15).
PITALRE overexpression inhibits transactivation of an integrated HIV-1 LTR.
To determine whether PITALRE overexpression could inhibit Tat activation of an integrated HIV-1 LTR, we examined Jurkat T-cell line 1G5, which contains an integrated HIV-1 LTR with luciferase as the reporter protein (1). 1G5 cells were cotransfected by electroporation with a Tat-1 expression plasmid and a PITALRE or D167N expression plasmid (Fig. 3). Measurement of Tat-induced luciferase activities at 24 h posttransfection yielded 60.1 luciferase U in the presence of the parental vector, 18.4 U upon wild-type PITALRE overexpression, and 7.8 U upon D167N overexpression. No inhibition of basal luciferase units by PITALRE or D167N was observed. Therefore, in 1G5 cells, PITALRE overexpression inhibited Tat-induced luciferase expression 3.3-fold, while D167N overexpression inhibited Tat-induced luciferase expression 7.7-fold.
The observed effect of PITALRE overexpression in 1G5 cells is consistent with results observed in HeLa cells. Both wild-type PITALRE and D167N squelch Tat-mediated transactivation, and D167N squelches more efficiently than wild-type PITALRE. These results obtained with 1G5 cells indicate, additionally, that PITALRE overexpression can squelch Tat-1-mediated transactivation of an integrated HIV-1 LTR.
PITALRE fused to Rev activates an HIV-1 LTR containing an RRE in place of TAR.
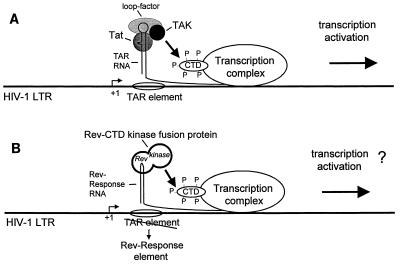
Our observation that Tat associates with TAK led us to suggest the model for the mechanism of action of Tat shown in Fig. 4A. This model proposes that by binding to TAR RNA, Tat is able to functionally recruit TAK to the RNAPII complex, leading to hyperphosphorylation of the CTD and a resultant activation of transcription (15). To test this model, we investigated whether gene expression directed by the HIV-1 LTR sequences can be activated in vivo by direct recruitment of PITALRE to a promoter-proximal RNA element. For these experiments, we utilized an HIV-1 LTR-chloramphenicol acetyltransferase (CAT) reporter plasmid containing the minimal HIV-1 Rev response element (RRE) in place of the TAR element (39). This reporter has been shown to be activated by fusions of the Rev protein to Tat or herpesvirus VP16, but not by the other transcriptional activators tested (23, 39).
FIG. 4.
(A) Model of mechanism of action of Tat. Tat is believed to bind along with a cellular factor (loop factor) directly to the TAR RNA element (5, 16). This model proposes that Tat recruits a cellular protein kinase, TAK, to the RNAPII holoenzyme (15). TAK is thereby positioned to phosphorylate the CTD of the large subunit of RNAPII, resulting in activated transcription. (B) Experimental strategy. To test this model, plasmids expressing fusions of cellular kinases to the HIV-1 Rev protein are cotransfected with an HIV-1 LTR reporter plasmid containing the minimal HIV-1 RRE in place of TAR. To minimize any posttranscriptional effect of Tat on the expression of CAT mRNAs from the reporter, a 5′ nontranslated leader sequence from poliovirus was inserted before the CAT coding sequence (39).
Plasmids for the expression of PITALRE-Rev or a control Cdk2-Rev fusion protein were constructed and used in cotransfection experiments. Measurement of CAT activities at 72 h posttransfection indicated that PITALRE-Rev expression activated the SLIIB-CAT reporter (wild-type RRE) 4.5-fold, while Cdk2-Rev expression did not activate the CAT reporter (Fig. 5). Reporter activation by PITALRE-Rev required targeting of the fusion protein to the SLIIB element, as a reporter bearing a mutated SLIIB element (ΔRRE) was not activated by PITALRE-Rev. In an additional experiment, we compared activation of the reporter plasmid by PITALRE-Rev and Tat-Rev fusion proteins (Fig. 5B). The Tat-Rev protein was considerably more active, resulting in 37-fold activation versus 4.8-fold activation by the PITALRE-Rev protein.
FIG. 5.
PITALRE activates reporter expression when targeted to an RNA element. (A) HeLa cells were transfected with 200 ng of a wild-type (wt) RRE SLIIB-CAT or a mutant RRE (ΔRRE) SLIIB-CAT reporter plasmid, 500 ng of a Rev fusion expression plasmid, and 500 ng of pCH110 (simian virus 40 early promoter driving β-galactosidase; Pharmacia). Cells were harvested at 72 h posttransfection, and CAT enzyme activities were measured by standard assays, which were quantified with a Molecular Dynamics PhosphorImager. (B) HeLa cells were transfected with the indicated plasmids as described for panel A. The Tat-Rev expression plasmid has been described previously (39).
The observation that PITALRE targeted to a nascent RNA element, in the absence of Tat, can activate HIV-1 LTR-driven gene expression is consistent with the suggestion that PITALRE can act as a transcription activator. The relatively low levels of activation by PITALRE-Rev may indicate that other factors, such as its cyclin partner or additional TAK components, are needed to reconstitute the total functional entity that is interfaced by Tat.
While this report was in preparation, we became aware of two recent studies describing the involvement of PITALRE in Tat transactivation (25, 47). Those studies further demonstrated that TAK is similar to human pTEFb, an activity first described in in vitro transcription studies with Drosophila extracts (28). Using an vitro transcription assay, both studies demonstrated that PITALRE is required for Tat transactivation in vitro. Additionally, one study also investigated the effect of transient overexpression of PITALRE on Tat transactivation in vivo (25); however, those data, in contrast to the results presented here, indicate activation of Tat function by overexpression of wild-type PITALRE. The reason for this discrepancy with our results is not apparent and may reflect the different experimental protocols used. However, our finding that overexpression of wild-type PITALRE can inhibit Tat function is consistent with the proposal that PITALRE functions in vivo as a multiprotein complex. In agreement with our results, in this recent study it was also observed that a catalytic mutant of PITALRE does inhibit Tat transactivation (25). Taken together, recent work by several laboratories has demonstrated that PITALRE does, indeed, mediate Tat transactivation. Studies are now possible to explore the details of the molecular mechanisms of Tat transactivation.
Acknowledgments
We gratefully acknowledge Susan Marriott, Mark Goff, Bryan Cullen, and John Belmont for useful reagents.
This work was supported by NIH grant AI35381 and Training Program T32 AI07483 for Basic Research and AIDS (M.O.G.).
ADDENDUM IN PROOF
The cyclin partner of PITALRE has recently been identified (P. Wei, M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones, Cell 92:451–462, 1998). PITALRE has consequently been termed CDK9 and its cyclin partner has been termed cyclin T.
REFERENCES
- 1.Aguilar-Cordova E, Chinen J, Donehower L, Lewis D E, Belmont J W. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retroviruses. 1994;10:295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 2.Arya S K, Gallo R C. Human immunodeficiency virus type 2 long terminal repeat: analysis of regulatory elements. Proc Natl Acad Sci USA. 1988;85:9753–9757. doi: 10.1073/pnas.85.24.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll R, Peterlin B M, Derse D. Inhibition of human immunodeficiency virus type 1 Tat activity by coexpression of heterologous trans activators. J Virol. 1992;66:2000–2007. doi: 10.1128/jvi.66.4.2000-2007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun R F, Jeang K T. Requirements for RNA polymerase II carboxyl-terminal domain for activated transcription of human retroviruses human T-cell lymphotropic virus I and HIV-1. J Biol Chem. 1996;271:27888–27894. doi: 10.1074/jbc.271.44.27888. [DOI] [PubMed] [Google Scholar]
- 5.Cullen, B. R. 1995. Regulation of HIV gene expression. AIDS 9(Suppl. A):S19–S32. [PubMed]
- 6.Dahmus M E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;217:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 7.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 8.De Luca A, Esposito V, Baldi A, Claudio P P, Fu Y, Caputi M, Pisano M M, Baldi F, Giordano A. CDC2-related kinase PITALRE phosphorylates pRb exclusively on serine and is widely expressed in human tissues. J Cell Physiol. 1997;172:265–273. doi: 10.1002/(SICI)1097-4652(199708)172:2<265::AID-JCP13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Fisher A G, Feinberg M B, Josephs S F, Harper M E, Marselle L M, Reyes G, Gonda M A, Aldovini A, Debouk C, Gallo R C, et al. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 1986;320:367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- 10.Fraser N W, Sehgal P B, Darnell J E. DRB-induced premature termination of late adenovirus transcription. Nature. 1978;272:590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez L F, Mavankal G, Neveu J M, Lanes W S, Ivanov D, Gaynor R B. Purification of a Tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garriga J, Mayol X, Grana X. The CDC2-related kinase PITALRE is the catalytic subunit of active multimeric protein complexes. Biochem J. 1996;319:293–298. doi: 10.1042/bj3190293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grana X, De Luca A, Sang N, Fu Y, Claudio P P, Rosenblatt J, Morgan D O, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann C H, Rice A P. Specific interaction of the human immunodeficiency virus Tat proteins with a cellular protein kinase. Virology. 1993;197:601–608. doi: 10.1006/viro.1993.1634. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 17.Kamine J, Subramanian T, Chinnadurai G. Sp1-dependent activation of a synthetic promoter by human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci USA. 1991;88:8510–8514. doi: 10.1073/pnas.88.19.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao S Y, Calman A F, Luciw P A, Peterlin B M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 19.Kuchin S, Yeghiayan P, Carlson M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc Natl Acad Sci USA. 1995;92:4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laspia M F, Rice A P, Mathews M B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989;59:283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- 21.Liao S M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 22.Madore S J, Cullen B R. Genetic analysis of the cofactor requirement for human immunodeficiency virus type 1 Tat function. J Virol. 1993;67:3703–3711. doi: 10.1128/jvi.67.7.3703-3711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madore S J, Cullen B R. Functional similarities between HIV-1 Tat and DNA sequence-specific transcriptional activators. Virology. 1995;206:1150–1154. doi: 10.1006/viro.1995.1041. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C W, Linn S, Reinberg D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature. 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 25.Mancebo H S Y, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D H, Flores O. P-TEFb kinase is required for HIV Tat transactivation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marciniak R A, Calnan B J, Frankel A D, Sharp P A. HIV-1 Tat protein trans-activates transcription in vitro. Cell. 1990;63:791–802. doi: 10.1016/0092-8674(90)90145-5. [DOI] [PubMed] [Google Scholar]
- 27.Marciniak R A, Sharp P A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991;10:4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall N F, Price D H. Control of formation of two distinct classes of RNA polymerase II elongation complexes. Mol Cell Biol. 1992;12:2078–2090. doi: 10.1128/mcb.12.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien T, Hardin S, Greenleaf A, Lis J T. Phosphorylation of RNA polymerase II C-terminal domain and transcriptional elongation. Nature. 1994;370:75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H, Sheline C T, Corden J L, Jones K A, Peterlin B M. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 32.Payne J M, Laybourn P J, Dahmus M E. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J Biol Chem. 1989;264:19621–19629. [PubMed] [Google Scholar]
- 33.Rhim H, Rice A P. Exon2 of HIV-2 Tat contributes to transactivation of the HIV-2 LTR by increasing binding affinity to HIV-2 TAR RNA. Nucleic Acids Res. 1994;22:4405–4413. doi: 10.1093/nar/22.21.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selby M J, Peterlin B M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990;62:769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 35.Sodroski J G, Patarca R, Rosen C A, Haseltine W A. Location of the trans-activating region on the genome of human T-cell lymphotropic virus III. Science. 1985;229:74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- 36.Southgate C D, Green M R. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 1991;5:2496–2507. doi: 10.1101/gad.5.12b.2496. [DOI] [PubMed] [Google Scholar]
- 37.Suñé C, García-Blanco M A. Transcriptional trans activation by human immunodeficiency virus type 1 Tat requires specific coactivators that are not basal factors. J Virol. 1995;69:3098–3107. doi: 10.1128/jvi.69.5.3098-3107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamm I, Kikuchi T. Early termination of heterogeneous nuclear RNA transcripts in mammalian cells: accentuation by 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole. Proc Natl Acad Sci USA. 1979;76:5750–5754. doi: 10.1073/pnas.76.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiley L S, Madore S J, Malim M H, Cullen B R. The VP16 transcription activation domain is functional when targeted to a promoter-proximal RNA sequence. Genes Dev. 1992;6:2077–2087. doi: 10.1101/gad.6.11.2077. [DOI] [PubMed] [Google Scholar]
- 40.Wahi M, Johnson A D. Identification of genes required for alpha 2 repression in Saccharomyces cerevisiae. Genetics. 1995;140:79–90. doi: 10.1093/genetics/140.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Gold M O, Ng Tang D, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yankulov K, Yamashita K, Roy R, Egly J M, Bentley D L. The transcriptional elongation inhibitor 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J Biol Chem. 1995;270:23922–23925. doi: 10.1074/jbc.270.41.23922. [DOI] [PubMed] [Google Scholar]
- 44.Zandomeni R, Zandomeni M C, Shugar D, Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986;261:3414–3419. [PubMed] [Google Scholar]
- 45.Zhou Q, Sharp P A. Novel mechanism and factor for regulation by HIV-1 Tat. EMBO J. 1995;14:321–328. doi: 10.1002/j.1460-2075.1995.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Q, Sharp P A. Tat-SF1: cofactor for stimulation of transcriptional elongation by HIV-1 Tat. Science. 1996;274:605–610. doi: 10.1126/science.274.5287.605. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N F, Marshall T A, Amendt B, Mathews M B, Price D H. Transcriptional elongation factor p-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]