Abstract
The rubella virus (RUB) nonstructural (NS) protease is a papain-like cysteine protease (PCP) located in the NS-protein open reading frame (NSP-ORF) that cleaves the NSP-ORF translation product at a single site to produce two products, P150 (the N-terminal product) and P90 (the C-terminal product). The RUB NS protease was found not to function following translation in vitro in a standard rabbit reticulocyte lysate system, although all of the other viral PCPs do so. However, in the presence of divalent cations such as Zn2+, Cd2+, and Co2+, the RUB NS protease functioned efficiently, indicating that these cations are required either as direct cofactors in catalytic activity or for correct acquisition of three-dimensional conformation of the protease. Since other viral and cell PCPs do not require cations for activity and the RUB NS protease contains a putative zinc binding motif, the latter possibility is more likely. Previous in vivo expression studies of the RUB NS protease failed to demonstrate trans cleavage activity (J.-P. Chen et al., J. Virol. 70:4707–4713, 1996). To study whether trans cleavage could be detected in vitro, a protease catalytic site mutant and a mutant in which the C-terminal 31 amino acids of P90 were deleted were independently introduced into plasmid constructs that express the complete NSP-ORF. Cotranslation of these mutants in vitro yielded both the native and the mutated forms of P90, indicating that the protease present in the mutated construct cleaved the catalytic-site mutant precursor. Thus, RUB NS protease can function in trans.
Rubella virus (RUB) is the sole member of the Rubivirus genus of the family Togaviridae; the other togavirus genus is the genus Alphavirus, of which Sindbis virus (SIN) is the type member. RUB has a positive-polarity, single-stranded RNA genome of 9,762 nucleotides that contains two long open reading frames (ORFs): the 5′ proximal nonstructural-protein ORF (NSP-ORF) (2,206 codons) encodes nonstructural (NS) proteins involved in viral RNA replication, and the 3′ proximal ORF (1,063 codons) encodes the virion proteins (reviewed in reference 10). Following translation of the NSP-ORF from the genomic RNA, a papain-like cysteine protease (PCP) within the NSP-ORF sequences cleaves the precursor (P200) into two mature products, P150 (150 kDa) and P90 (90 kDa), which are N- and C-terminal within the ORF, respectively. The catalytic dyad of the NS protease was mapped to C1152 and H1273 by deletion mapping and mutagenesis of amino acid residues predicted by computer alignment, and the cleavage site was shown to be between G1301 and G1302 by direct sequencing of cleavage products (5, 16) (the amino acid numbering has been updated to take into account corrections in the RUB genomic sequence [17]).
All of the other viral PCPs have been found to be active following in vitro translation in rabbit reticulocyte lysates, and in vitro mapping and activity studies of all of these enzymes have been done (1–4, 6, 7, 12, 14, 15, 19, 20); some of these enzymes also exhibit activity when expressed in Escherichia coli (4, 14). However, when lysates were programmed with genome-length, SP6 RNA polymerase transcripts synthesized from Robo12 template, a RUB genomic cDNA plasmid construct (23), processing was not detectable (Fig. 1A) (in vitro translation was as described in reference 13). Processing was also not detectable following in vitro translation of transcripts from pTM3/nsRUB, a plasmid construct in which the NSP-ORF sequences are downstream from a T7 RNA polymerase promoter and an encephalomyocarditis virus internal ribosome entry site (16) (data not shown). In contrast, in lysates programmed with genome-length, SP6 RNA polymerase transcripts from Toto1101, a SIN cDNA construct (18), processing was readily apparent. A time course is shown in Fig. 1B. Interestingly, although the G-plus-C content of the RUB genome is almost 70% compared to roughly 50% for the SIN genome, the rates of translation of the NSP-ORFs of these viruses were comparable. Attempts were made to stimulate processing of the RUB NSP-ORF product by incubating the in vitro translation reactions at 30 or 37°C for as long as 24 h and by doing the translation in the presence of pancreatic microsomes (22), but to no avail.
FIG. 1.
In vitro translation of RUB and SIN genomic transcripts. Capped genomic transcripts of Robo12 and Toto1101 (provided by C. Rice) were translated in rabbit reticulocyte lysates in the presence of [35S]methionine and resolved on sodium dodecyl sulfate-polyacrylamide gels as previously described (13). (A) Toto1101 (lanes 1 to 4) or Robo12 (lanes 5 to 8) transcripts at concentrations of 50 (lanes 1 and 5), 25 (lanes 2 and 6), 12.5 (lanes 3 and 7), or 6.25 μg/ml (lanes 4 and 8) were translated for 90 min at 30°C. (B) Toto1101 or Robo12 transcripts (17 μg/ml) were translated at 30°C, and aliquots were removed at 0 (lane 1), 10 (lane 2), 20 (lane 3), 30 (lane 4), 40 (lane 5), 50 (lane 6), 60 (lane 7), 70 (lane 8), 80 (lane 9), or 90 (lane 10) min. In both panels, the RUB NSP-ORF precursor (P200) and the SIN NSP-ORF precursors and products are indicated. For production of figures, autoradiographs were scanned with an Agfa Arcus II flatbed scanner operating with Fotolook version 2.05 with Adobe Photoshop 2.5 software, and prints were made with a Tektronics Phaser 440 dye sublimation printer with a monochrome ribbon.
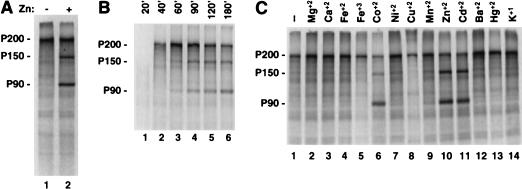
Recently, it was reported that in vitro activity of the NS3 protease of hepatitis C virus required the presence of zinc ions (21). Although the hepatitis C virus NS3 protease is a chymotrypsin-like serine protease, not a PCP, examination of the RUB NSP-ORF sequence revealed two potential metal-binding motifs (8). One of these motifs is located within the protease domain between the catalytic dyad of Cys1152 and His1273. This motif begins at Cys1174 and has the sequence Cys-X2-Cys-X11-His-X13-His. Similar cysteine-histidine-rich regions are not found in the NS polyproteins of alphaviruses. The presence of this motif suggested that zinc or other divalent cations might play a role in RUB NS protease activity. To investigate this possibility, in vitro translation was carried out with a cell-free coupled transcription-translation system (TNT System, Promega Biotec, Madison, Wis.) programmed with pTM3/nsRUB DNA in the absence or presence of ZnCl2. As shown in Fig. 2A, in the presence of Zn ion, processing of the P200 precursor into P150 and P90 was clearly apparent. A time course, shown in Fig. 2B, demonstrated that processing occurred after completion of translation of the P200 precursor, as had been shown previously for RUB-infected cells (9). Translation of the RUB NSP-ORF in the absence of Zn2+, followed by the addition of Zn2+ and further incubation for 3 h, resulted in no detectable processing (data not shown), suggesting that Zn2+ is necessary at the time of protein synthesis and folding and is not effective when added posttranslationally. Translations were also carried out in the presence of other metal ions. As shown in Fig. 2C, processing was detected in the presence of Zn2+, Cd2+, and Co2+.
FIG. 2.
In vitro translation of pTM1/nsRUB. PTM1/nsRUB DNA (1 μg) was used to program a TNT Coupled Reticulocyte Lysate Transcription/Translation System reaction in the presence of Trans 35S-Label (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.) according to the manufacturer’s instructions, and the resulting proteins were resolved on sodium dodecyl sulfate–10% polyacrylamide gels. (A) Transcription-translation reactions were carried out for 120 min at 30°C in the absence (lane 1) or presence (lane 2) of 200 μM ZnCl2. (B) Transcription-translation reactions were carried out at 30°C in the presence of 100 μM ZnCl2, and aliquots were removed at 20 (lane 1), 40 (lane 2), 60 (lane 3), 90 (lane 4), 120 (lane 5), and 180 (lane 6) min. (C) Transcription-translation reactions were carried out for 180 min at 30°C in the absence of cations (lane 1) or in the presence of MgCl2 (lane 2), CaCl2 (lane 3), FeCl2 (lane 4), FeCl3 (lane 5), CoCl2 (lane 6), NiCl2 (lane 7), CuCl2 (lane 8), MnCl2 (lane 9), ZnCl2 (lane 10), CdCl2 (lane 11), BaCl2 (lane 12), HgCl2 (lane 13), or KCl (lane 14), each at 100 μM.
To our knowledge, none of the other PCPs, either viral or cellular, have a requirement for divalent cations. Based on the results of this study, we cannot conclusively decide between the possibility that the RUB NS protease is a true metalloprotease in which the metal ion participates directly in catalytic activity and the possibility that the divalent cations assist in directing the correct folding of the NSP precursor required for protease activity. Since the RUB NS protease appears to contain the Cys-His catalytic dyad that suffices for activity in other PCPs, it seems more likely that by binding to the metal binding motif, the metal ion functions in the conformation of the protease as well as of possibly the entire NS precursor. However, further analysis is necessary to determine the roles played by divalent cations.
The advent of a working in vitro system allowed us to examine whether the RUB protease is active in trans as well as in cis. Previous in vivo experiments were performed with SIN (dsSIN) vectors that expressed only the region of the NSP-ORF containing the protease and its cleavage site (dsSIN-NSPro1 and -NSPro2 expressed residues 827 to 1508 and 1006 to 1508 of the NSP-ORF, respectively), and with these constructs only cis cleavage activity could be detected (5). To examine whether trans cleavage could be detected when constructs containing the complete NSP-ORF were used, we initially employed one of the strategies we had used with the NSPro constructs. In vitro transcription-translation reactions were coprogrammed in the presence of Zn ions with two mutant constructs: pTM1/nsRUB*, in which the catalytic C1152 was changed to G, and pTM1/nsRUB:G1301V (pTM1/nsRUB-GV), in which the amino acid at the P1 position of the cleavage site, G1301, was changed to V. Both mutations completely abrogate cleavage; however, the functional protease in pTM1/nsRUB-GV could cleave the unmutated cleavage site in pTM1/nsRUB* if the protease cleaves in trans. Cleavage in trans was not detectable (data not shown). Additional in vivo experiments were carried out with Vero cells transfected with pTM3/nsRUB plasmids and coinfected with vvTF7-3, a vaccinia virus that expresses T7 RNA polymerase; again, however, trans cleavage could not be detected.
Considering the proximity of the catalytic site to the cleavage site (the two are 28 amino acids apart), we reasoned that the G1301 V mutation might be severe enough to disrupt the conformation of the protease and thus employed a different strategy to detect trans cleavage. A C-terminal deletion of 31 amino acids was made in pTM1/nsRUB which would result in the production of a shorter P90 product. Figure 3A shows in vitro translation of this construct, pTM1/nsRUB-S, and the production of a shorter P90 (P90-S), which was clearly differentiable from nonmutated P90. When pTM1/nsRUB-S and pTM1/nsRUB* were added to the same in vitro transcription-translation reaction, both P90 and P90-S products were detected (Fig. 3A, lane 4), indicating that trans cleavage of the pTM1/nsRUB* template by the pTM1/nsRUB-S enzyme had occurred. Additionally, it was found that if separate transcription-translation reactions were performed, with pTM1/nsRUB* in the presence of radiolabel and pTM1/nsRUB or pTM1/nsRUB-S in the absence of radiolabel, followed by treatment of the reaction with both RNase and cycloheximide to stop translation, and if the labeled and unlabeled reaction mixtures were then combined and incubated, cleavage of the pTM1/nsRUB* could be detected (Fig. 3B). A construct which only expressed P150 (pTM1/P150) could also cleave the pTM1/nsRUB* template, indicating that the protease is active in the P150 context. To ascertain that the protease can function in cis, a dilution experiment was performed as shown in Fig. 3C. Transcription-translation reactions were programmed with dilutions of pTM1/nsRUB DNA ranging from 1:30 to 1:800. The 1:30, 1:60, and 1:100 dilutions produced roughly equal amounts of products, indicating that far less than the recommended amount of template DNA (1 μg) is needed for an optimal reaction. At the 1:200 and 1:400 dilutions, proportionately less translation occurred; all three products were visible and appeared to be present in roughly the same proportions, consistent with cis cleavage. Translation products could not be clearly discerned with the 1:800 dilution.
FIG. 3.
In vitro cotranslation of NSP-ORF expression constructs to detect trans cleavage. Transcription-translation was carried out with the TNT Coupled Reticulocyte Lysate System in the presence of Trans 35S-Label according to the manufacturer’s instructions, and the resulting proteins were resolved on sodium dodecyl sulfate–10% polyacrylamide gels. (A) Lysates were programmed with pTM1/nsRUB (NSP, lane 1), pTM1/nsRUB* (NSP*, a protease catalytic site mutant, lane 2), pTM1/nsRUB-S (NSP-S, a deletion mutant lacking the C-terminal 31 amino acids, lane 3), or pTM1/nsRUB* plus pTM1/nsRUB-S (lane 4). The positions of migration of the RUB NSPs as well as of the truncated P90 product (P90-S) are indicated. (B) Reactions programmed with pTM1/nsRUB* were carried out in the presence of radiolabel for 120 min. Reactions programmed with pTM1/P150 (a construct containing the P150 sequences), pTM1/nsRUB-S, or pTM1/nsRUB were carried out in the absence of radiolabel. RNase and cycloheximide were then added to final concentrations of 1 and 0.6 mg/ml, respectively, to stop translation. Aliquots of the pTM1/nsRUB* reaction then were mixed with aliquots of the cold, unlabeled pTM1/P150 reaction mixture (P150, lane 2), the pTM1/nsRUB-S reaction mixture (NSP-S, lane 3), or the pTM1/nsRUB reaction mixture (NSP) and incubated for another 120 min at 30°C. An aliquot of the pTM1/nsRUB* reaction mixture was also incubated alone (lane 1). (C) Transcription-translation reactions in the presence of radiolabel were programmed with the following dilutions of the standard amount of pTM1/nsRUB DNA: 1:30 (lane 1), 1:60 (lane 2), 1:100 (lane 3), 1:200 (lane 4), 1:400 (lane 5), or 1:800 (lane 6).
Gorbalenya et al. (11) classified the viral PCPs into two groups. Main PCPs, including the alphavirus nsP2 protease (12), the foot-and-mouth disease virus leader protease (14), and the coronavirus PLP-1 (2), cleave both in cis and in trans at multiple sites within the polyprotein precursor in which they are located. These sites are not close to the catalytic site of the enzyme. The other group, made up of the leader PCPs, cleaves only in cis at a single site very close downstream (25 to 40 residues) from the catalytic His residue. While the cleavage sites of all viral PCPs are generally between two residues with short side groups, the cleavage sites of leader PCPs uniformly have the configuration Gly-Gly. Leader PCPs include the arterivirus nsP1 proteases (the nsP1 protease of equine arteritis virus [20] and the PCP-α and -β proteases of lactate dehydrogenase virus and porcine reproductive and respiratory syndrome virus [7], the potyvirus HC-Pro [4], the p29 and p48 proteases of the hypovirulence-associated viruses [6, 19], and the PCP of beet yellows closterovirus [1]). Leader PCPs are generally located within short leader proteins at the amino termini of long ORFs and remove the leader from the rest of the translation product of the ORF. We had previously classified the RUB NS protease as a leader protease (5); however, on the basis of its ability to cleave in trans, it should be reclassified as a main PCP. However, its activity differs from that of the other main PCPs in that it only cleaves at a single site, and the spacing of its catalytic and cleavage sites is similar to those of the leader PCPs. Two additional viral PCPs have been described for turnip yellow mosaic virus (atymovirus) (3) and blueberry scorch carlavirus (15), which also do not fit neatly into the leader-main classification scheme. These enzymes appear to cleave exclusively in cis at a single site in the middle of a large polyprotein precursor; however, their cleavage sites are roughly 400 amino acids downstream from the catalytic His residue. Thus, the viral PCP family is more complicated than was previously thought.
On the basis of the presence of several amino acid motifs identified in the NS proteins of most plus-sense RNA viruses by computer-assisted alignments, the differing order of these motifs in the NSP-ORFs of RUB and the alphaviruses, and the differing relatedness of these motifs in RUB and the alphaviruses with respect to other plus-sense RNA viruses, we have proposed that the NSP-ORFs of these viruses evolved independently, rather than diverging from a single source (5, 10). However, the NS proteases of these viruses are the only motifs that have been functionally characterized for both of these genera. This study showed that the RUB and alphavirus NS proteases share the ability to cleave in trans (previously we thought that they differed in this regard), although the cleavage patterns of their NS proteins differ. This could be due to evolution of the cleavage sites after the two genera diverged. However, these proteases also differ in their requirements for divalent cations. Resolving whether this difference could also have evolved following divergence of these genera or whether it is indicative of independent evolution (possibly by acquisition from different sources) requires further analysis of the structures and functions of these enzymes.
Acknowledgments
We thank Ann Kaminski and Lee Marr Ganaway for help and advice.
This research was supported by grants from NIH (AI 21389 and AI 00923) and the Wellcome Trust. Part of the research described in this report was done while T.K.F. was on sabbatical leave in R.J.J.’s laboratory.
REFERENCES
- 1.Agranovsky A A, Koonin E V, Boyko V P, Maiss E, Frotschl R, Lunina N A, Atabekov J G. Beet yellows closterovirus: complete genome structure and identification of a leader papain-like thiol protease. Virology. 1994;198:311–324. doi: 10.1006/viro.1994.1034. [DOI] [PubMed] [Google Scholar]
- 2.Bonilla P J, Hughes S A, Weiss S R. Characterization of a second cleavage site and demonstration of activity in trans by the papain-like proteinase of the murine coronavirus mouse hepatitis virus A59. J Virol. 1997;71:900–909. doi: 10.1128/jvi.71.2.900-909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bransom K L, Dreher T W. Identification of the essential cysteine and histidine residues of the turnip yellow mosaic virus protease. Virology. 1994;198:148–154. doi: 10.1006/viro.1994.1017. [DOI] [PubMed] [Google Scholar]
- 4.Carrington J C, Freed D D, Sanders T C. Autocatalytic processing of the potyvirus helper component proteinase in Escherichia coli and in vitro. J Virol. 1989;63:4459–4463. doi: 10.1128/jvi.63.10.4459-4463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J-P, Strauss J H, Strauss E G, Frey T K. Characterization of the rubella virus nonstructural protease and its cleavage site. J Virol. 1996;70:4707–4713. doi: 10.1128/jvi.70.7.4707-4713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi G H, Pawlyk D M, Nuss D L. The autocatalytic protease p29 encoded by a hypovirulence-associated virus of the chestnut blight fungus resembles the potyvirus-encoded protease HC-Pro. Virology. 1991;183:747–752. doi: 10.1016/0042-6822(91)91004-z. [DOI] [PubMed] [Google Scholar]
- 7.Den Boon J A, Faaberg K S, Meulenberg J J M, Wassenaar A L M, Plagemann P G W, Gorbalenya A E, Snijder E J. Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papainlike cysteine proteases. J Virol. 1995;69:4500–4505. doi: 10.1128/jvi.69.7.4500-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez G. Determination and analysis of the sequence of the genome RNA of rubella virus. Ph.D. dissertation. Atlanta: Georgia State University; 1991. [Google Scholar]
- 9.Forng R-Y, Frey T K. Identification of the rubella virus nonstructural proteins. Virology. 1995;206:843–853. doi: 10.1006/viro.1995.1007. [DOI] [PubMed] [Google Scholar]
- 10.Frey T K. Molecular biology of rubella virus. Adv Virus Res. 1994;44:69–160. doi: 10.1016/S0065-3527(08)60328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbalenya A E, Koonin E V, Lai M M-C. Putative papain-related thiol proteases of positive-strand RNA viruses. FEBS Lett. 1991;288:201–205. doi: 10.1016/0014-5793(91)81034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy W R, Strauss J H. Processing the nonstructural polyproteins of Sindbis virus: nonstructural protease is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989;62:998–1007. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson R J, Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- 14.Kirchweger R, Ziegler E, Lamphear B J, Waters D, Liebig H-D, Sommergruber W, Sobrino F, Hohenadl C, Blaas D, Rhoads R E, Skern T. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4γ. J Virol. 1994;68:5677–5684. doi: 10.1128/jvi.68.9.5677-5684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence D M, Rozanov M N, Hillman B I. Autocatalytic processing of the 223 kDa protein of blueberry scorch carlavirus by a papain-like protease. Virology. 1995;207:127–135. doi: 10.1006/viro.1995.1058. [DOI] [PubMed] [Google Scholar]
- 16.Marr L D, Wang C-Y, Frey T K. Expression of the rubella virus nonstructural protein ORF and demonstration of proteolytic processing. Virology. 1994;198:586–592. doi: 10.1006/viro.1994.1070. [DOI] [PubMed] [Google Scholar]
- 17.Pugachev K V, Abernathy E S, Frey T K. Genomic sequence of the RA27/3 vaccine strain of rubella virus. Arch Virol. 1997;142:1165–1180. doi: 10.1007/s007050050150. [DOI] [PubMed] [Google Scholar]
- 18.Rice C M, Levis R, Strauss J H, Huang H V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapira R, Nuss D L. Gene expression by a hypovirulence-associated virus of the chestnut blight fungus involves 2 papain-like proteinase activities: essential residues and cleavage site requirements for P48 autoproteolysis. J Biol Chem. 1991;266:19419–19425. [PubMed] [Google Scholar]
- 20.Snijder E J, Wassenaar A L M, Spaan W J M. Proteolytic processing of the replicase ORF1a protein of equine arteritis virus. J Virol. 1994;68:5755–5764. doi: 10.1128/jvi.68.9.5755-5764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stempniak M, Hostomoska Z, Nodes B R, Hostomsky Z. The NS3 protease domain of hepatitis C virus is a zinc-containing enzyme. J Virol. 1997;71:2881–2886. doi: 10.1128/jvi.71.4.2881-2886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tibbles K W, Brierley I, Cavanagh D, Brown T D K. Characterization in vitro of an autocatalytic processing activity associated with the predicted 3C-like protease domain of the coronavirus avian infectious bronchitis virus. J Virol. 1996;70:1923–1930. doi: 10.1128/jvi.70.3.1923-1930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C-Y, Dominguez G, Frey T K. Construction of rubella virus genome-length cDNA clones and synthesis of infectious RNA transcripts. J Virol. 1994;68:3550–3557. doi: 10.1128/jvi.68.6.3550-3557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]