Abstract
The immunogenicity and protective efficacy of formalin-inactivated influenza B/Memphis/1/93 virus vaccines propagated exclusively in Vero cells, MDCK cells, or embryonated chicken eggs (hereafter referred to as eggs) were investigated. Mammalian cell-grown viruses differ from the egg-grown variant at amino acid position 198 (Pro/Thr) in the hemagglutinin gene. The level of neuraminidase activity was highest in egg-grown virus, while MDCK and Vero cell-derived viruses possessed 70 and 90% less activity, respectively. After boosting, each of the vaccines induced high levels of hemagglutinin-inhibiting, neuraminidase-inhibiting, and neutralizing antibodies that provided complete protection from MDCK-grown virus challenge. Mammalian cell-derived virus vaccines induced serum antibodies that were more cross-reactive, while those induced by egg-grown virus vaccines were more specific to the homologous antigen. Enzyme-linked immunospot analysis indicated that cell-grown virus vaccines induced high frequencies of immunoglobulin G (IgG)-producing cells directed against both cell- and egg-grown virus antigens, whereas egg-grown virus vaccine induced higher frequencies of IgG- and IgM-producing cells reacting with homologous antigen and low levels of IgG-producing cells reactive with cell-grown viruses. These studies indicate that influenza B virus variants selected in different host systems can elicit different immune responses, but these alterations had no detectable influence on the protective efficacy of the vaccines with the immunization protocol used in this study.
Whole influenza virus vaccine induces antibodies against both hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins, and these antibodies play a major role in protection against challenge infection (5). Antibody against HA neutralizes viral infectivity and prevents infection. Although the immune response to HA overrides the NA immune responses in primed subjects through intravirionic antigenic competition (9, 10), antibody to NA exerts its effect at later stages of infection (11, 16).
Embryonated chicken eggs (hereafter referred to simply as eggs) have been an extremely useful substrate for the propagation of influenza viruses. However, it has been established that variant viruses are selected when human influenza virus is first passaged in eggs, while virus propagated exclusively in mammalian cell systems is structurally and antigenically identical to the natural virus (13, 24, 26). The amino acid substitutions in the HA of egg-grown influenza A and B viruses cluster around the receptor binding site (12, 23, 25), which is located at the distal tip of the HA molecule. Therefore, altered immune responses can be induced by influenza virus vaccines produced in different host systems, and the resulting small number of changes in the HA can influence the efficacy of the vaccine (35). Recent studies have indicated that viruses isolated and passaged in MDCK cells possess low NA activity compared to that of egg-grown isolates (3). However, the immunological consequences of these changes have not been elucidated.
Influenza A virus vaccines of the H1N1 and H3N2 subtypes that were propagated in MDCK cells were more protective in animal models than were their egg-grown counterparts (15, 35). A single amino acid change in the HA decreased the efficacy of inactivated egg-grown influenza A virus vaccines (17); reduced memory B-cell responsiveness at the challenge site accounted for this effect. The significance of host cell-associated mutations for influenza B viruses have been less well characterized. It was shown that egg-grown influenza B viruses lost a potential glycosylation site in the HA at amino acid positions 196 to 198, and this was associated with loss of both infectivity and virulence for human volunteers (21, 36). Vaccination of mice with recombinant vaccinia viruses expressing either egg- or MDCK cell-derived HA genes from influenza B/England/222/82 virus can protect against challenge with influenza B virus isolates exhibiting minor HA sequence differences (27, 28).
Continuous mammalian cell lines provide a realistic substitute for eggs in vaccine production. Of the cell lines that might meet production requirements, Vero and MDCK cells are the most promising. Vero cells are a suitable host cell system for the cultivation of infectious influenza A and B viruses (8). This cell line is certified for vaccine production and is currently used for the preparation of vaccines against poliomyelitis and rabies (20). MDCK cells are generally used to grow influenza viruses, and they have recently been used to produce safe and well-tolerated influenza virus subunit vaccines (22).
In the present study we compare the serum and B-cell responsiveness induced in BALB/c mice by influenza B/Memphis/1/93 virus vaccines propagated in mammalian cells and in eggs. The protective efficacy of vaccine preparations obtained from different host systems was determined against challenge with MDCK cell-grown virus.
Characteristics of influenza B/Memphis/1/93 virus variants grown in mammalian cells and chicken eggs.
Influenza B/Memphis/1/93 virus from the original clinical sample was cultured in Vero and MDCK cells in the presence of l-1-tosylamide-2-phenylethyl chloromethyl ketone-treated trypsin (1.0 μg/ml; Worthington Diagnostics, Freehold, N.J.) and in eggs. Nucleotide sequences were obtained by the dideoxynucleotide chain termination method (29) with the fmol DNA sequencing system (Promega, Madison, Wis.) and end-labeled primers. Sequence analysis of the HA1 regions of these variants revealed that those grown in mammalian cells maintained a potential carbohydrate attachment site at amino acid residues 196 through 198 (Asn-Lys-Thr). In contrast, the egg-grown counterpart had a substitution at position 198, yielding the sequence Asn-Lys-Pro and resulting in the loss of a glycosylation site. This change occurred at the distal tip of the HA molecule, which is homologous to antigenic site B of the influenza A virus HA molecule. This raises the possibility that these variant viruses may differ antigenically, but antigenic analysis with available polyclonal and monoclonal antibodies failed to demonstrate any differences (results not shown).
The NA activity of the concentrated viral preparations obtained in Vero and MDCK cells and eggs was quantitated by the method of Warner and O’Brien (33) with 1 mM 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4-MUAc) (Sigma Chemical Co., St. Louis, Mo.) as the substrate and by using the Warren method (34) with fetuin as the substrate. The NA activity is reported as enzymatic activity per milligram of total viral protein. To compare the viral enzymatic activities, we considered the NA activity of egg-grown virus as 100%. The NA activity of viruses propagated in MDCK cells was 56 to 70% lower than that of viruses grown in eggs, as determined with 4-MUAc and fetuin, respectively. The NA activity of viruses produced in Vero cells was reduced by 82 to 91% compared to that of the egg-grown variant, as determined with the two substrates.
Serum antibodies in mice immunized with influenza B/Memphis/1/93 virus vaccines.
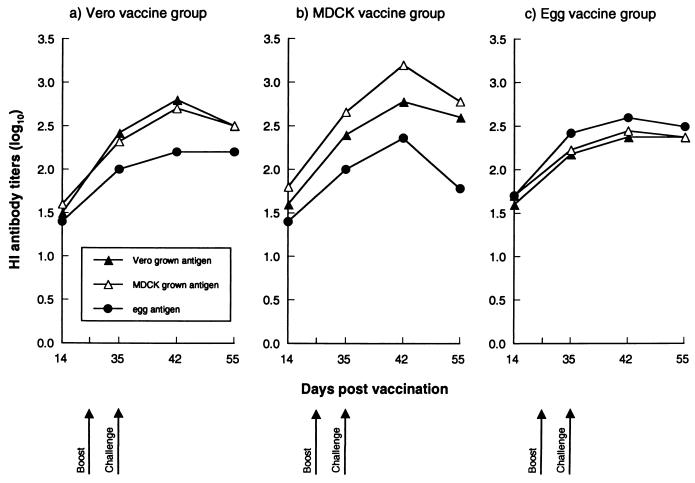
Inactivated whole-virus vaccine was prepared by treating purified virus preparations (40,000 hemagglutinating units/ml) with 0.025% formalin at 4°C for 3 days (14). The immunogenicity of influenza B virus vaccines grown in different host systems was determined in BALB/c mice (H-2d), which were immunized subcutaneously with a dose of whole-virus vaccine containing 5 μg of HA adsorbed to aluminum hydroxide adjuvant (Rehydragel LV; Reheis Inc., Berkeley Heights, N.J.) (2). Four weeks later, the mice received an intraperitoneal booster inoculation of the same dose of vaccine without the adjuvant. One week after receiving the boost, the mice were challenged intranasally with 200 50% mouse infectious doses of MDCK cell-grown virus. Blood samples were obtained from anesthetized mice on days 14, 35, 42, and 55 postvaccination (p.v.). The level of serum antibodies was examined by HA-inhibiting (HI), NA-inhibiting (NI), and neutralization assays. The titers of HI antibodies produced within the first 2 weeks p.v. did not differ between the experimental groups (Fig. 1). In all vaccine groups, booster vaccination (7 days postboost, i.e., day 35 p.v.) of the mice increased the titers of HI antibodies against all three antigens. One week after challenge with MDCK cell-grown virus, the mice primed with cell-grown virus vaccine produced higher levels of antibodies that were cross-reactive with cell-grown virus antigens. Although post-challenge antibodies induced by mice primed with egg-grown virus vaccine were cross-reactive with both egg-grown and cell-grown virus antigens, they were of lower magnitude than antibodies induced by cell-grown virus vaccines. The Vero cell-grown virus vaccine induced at least as high HI antibody response as did the egg-grown virus vaccine. The MDCK cell-derived virus vaccine induced HI antibody titers that were detectably higher than those of egg- or Vero cell-grown virus vaccine, probably due to the homologous challenge.
FIG. 1.
HI antibody titers in sera of mice immunized with inactivated cell- or egg-grown influenza B/Memphis/1/93 virus vaccines. BALB/c mice were immunized subcutaneously with the vaccines containing 5 μg of HA with aluminum hydroxide adjuvant and, four weeks later, with vaccine containing HA alone. One week postboost, the mice were challenged with 200 50% mouse infectious doses of MDCK cell-grown virus. The data points represent the means from five mice for each time point.
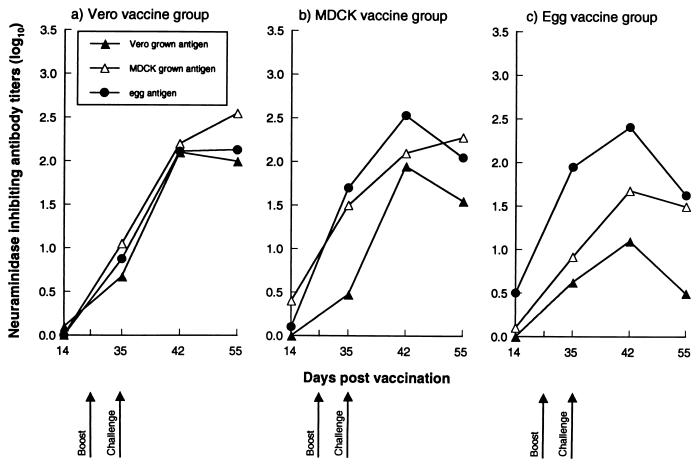
Titers of anti-NA antibodies produced in response to vaccination with influenza B/Memphis/1/93 virus isolated and grown in Vero cells, MDCK cells, or eggs are shown in Fig. 2. MDCK cell- and egg-derived virus vaccines induced moderate and comparable levels of prechallenge anti-NA antibodies, ranging from 1.5 to 2.0 log10 NI titer with homologous antigen. The challenge appeared to increase anti-NA antibody titers in all vaccine groups; this increase was most marked in the group of mice receiving Vero cell-derived virus vaccine. The levels of postchallenge antibodies obtained with Vero cell-grown virus vaccine were not distinguishable from those induced by egg-grown virus vaccine (2.1 versus 2.4 log10). However, with the small interval between boosting and challenge we cannot clearly discriminate between the responses induced by each. Anti-NA antibodies induced by Vero cell-grown virus vaccine cross-reacted with the egg- and MDCK cell-grown virus antigens to similar titers, while immunization of mice with the egg-grown virus vaccine resulted in the induction of antibodies that were less efficacious against mammalian cell-grown virus vaccines.
FIG. 2.
Serologic response of mice to immunization with inactivated cell- or egg-grown influenza B/Memphis/1/93 virus as measured by the NI test. The dilution of serum that inhibited 50% of virus NA activity was taken as the antibody titer.
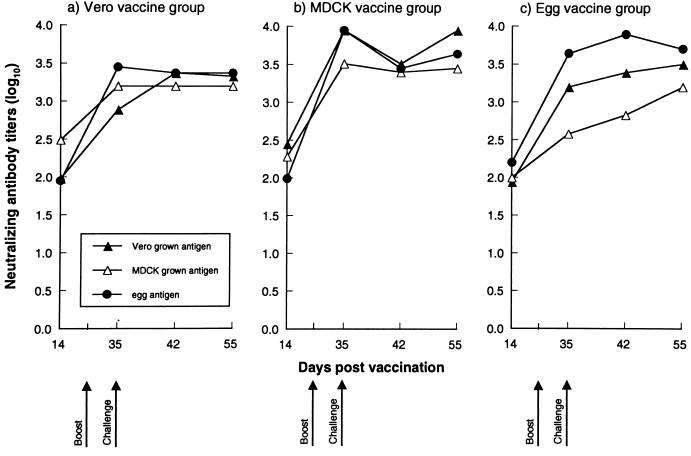
The levels of neutralizing antibodies induced by influenza B virus vaccines grown in different host systems are shown in Fig. 3. At 14 days p.v., they ranged from 1.8 to 2.5 log10 in all vaccine groups tested, and they increased after booster vaccination. The mammalian cell-grown virus vaccines induced cross-reactive antibodies, while those induced by egg-grown virus vaccine were specific for egg-grown virus and gave less neutralization of cell-grown viruses. After challenge there were no anamnestic neutralizing antibody responses, suggesting that the vaccine dose of 10 μg of HA had achieved titers similar to those in postchallenge animals.
FIG. 3.
Neutralizing antibody titers in sera of mice in response to immunization with inactivated cell- or egg-grown influenza B/Memphis/1/93 virus. The mean titer of antibody is expressed as the reciprocal of the highest dilution of serum that neutralized 100 50% tissue culture infective doses in virus-infected MDCK cells.
Thus, cell-derived influenza B virus vaccines are able to induce levels of anti-HA, anti-NA, and neutralizing antibodies comparable to those induced by egg-grown virus vaccines. It is important to emphasize that mammalian cell-derived virus vaccines induced more-cross-reactive serum antibodies, while those obtained in the egg-grown group were more specific to the homologous antigen.
B-cell response to glycoproteins of influenza B/Memphis/1/93 viruses.
B-cell responsiveness to purified surface glycoproteins was evaluated by using a modified enzyme-linked immunospot assay (6). An HA-NA-rich fraction was obtained by treating virus preparations with the nonionic detergent n-octyl β-thioglucopyranoside as described previously (9). Mice (5 per group) were anesthetized and exsanguinated, and the superficial cervical and mediastinal lymph nodes (cells from these lymph nodes were pooled) and spleens were collected, at 14 and 32 days p.v. The tissues were processed individually as previously described (1). Antibody-forming cells (AFCs) of the immunoglobulin M (IgM) and IgG isotypes were visualized by using alkaline phosphatase-conjugated goat anti-mouse Ig isotype-specific reagents (Southern Biotechnology Associates, Birmingham, Ala.) diluted in 5% bovine serum albumin and developed with 5-bromo-4-chloro-3-indolylphosphate (Sigma). The B-cell response was statistically analyzed by using the Kruskal-Wallis test to determine whether there was an overall difference in B-cell response among the three vaccine groups for a particular antigen. If such a difference was seen, a post hoc analysis was performed to compare the vaccine groups pairwise with the Wilcoxon-Mann-Whitney test.
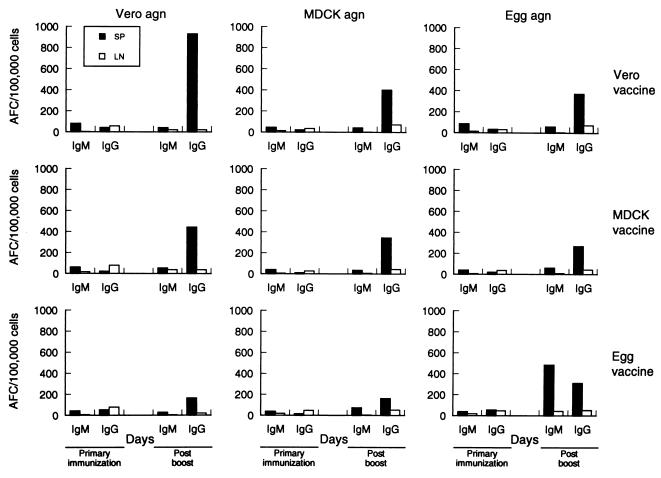
At 14 days p.v., all vaccine groups had low numbers of HA- and NA-specific AFCs (median, 12 IgM and 38 IgG AFCs per 105 cells) in the lymph nodes and moderate (median, 3 IgM and 31 IgG AFCs per 105 cells) numbers in the spleen. Although the number of IgG-producing AFCs in the lymph nodes increased after booster vaccination, there was no significant difference between the groups when assayed against glycoproteins, regardless of the host system in which the virus was propagated. However, the egg-grown virus vaccine group appeared to have a significantly higher number of IgM-producing AFCs (median = 42; P = 0.008) reacting with egg-grown virus antigen in comparison to those of the MDCK (median = 8) and Vero (median = 6) cell-grown virus vaccine groups. The cell-grown virus vaccines induced low numbers of IgM-producing cells when assayed against glycoproteins from all the host systems.
The number of IgG-producing AFCs did not differ significantly among the three groups in reaction to the egg-grown virus antigen. In mice immunized with Vero cell-grown virus vaccine, the number of IgG-producing cells increased significantly when assayed against homologous antigen (median = 899; P = 0.008) in comparison to egg-grown (median = 370) or MDCK cell-grown antigen (median = 360). In animals that were vaccinated with virus produced in MDCK cells (Fig. 4), the profiles of AFC induction were similar to those of mice that received Vero-grown virus vaccine. Although high numbers of AFCs of IgG isotype (median = 363) reacting with MDCK cell-grown virus antigen were induced in mice immunized with MDCK cell-grown virus vaccine in comparison to the numbers induced in mice immunized with egg-grown virus vaccine (median = 160), there was no significant difference in the antibody-forming cells induced by MDCK or Vero cell-grown vaccine against MDCK cell-grown virus antigen (P = 1.0), suggesting that there are similarities in the antigenicities of these two cell-grown virus vaccines.
FIG. 4.
B-cell responsiveness induced in mice by inactivated influenza B/Memphis/1/93 virus vaccines grown in mammalian cells or in eggs. Lymphoid tissues of immunized mice were collected on days 14 and 32 (5 days after boost) p.v. At each time point in the enzyme-linked immunospot assay, freshly isolated cells from each tissue (mediastinal lymph nodes and spleens) were sampled for Ig isotype determination against glycoprotein preparations derived from cell- or egg-grown B/Memphis/1/93 virus. The data shown are the means of five mice per vaccine group. agn, antigen; LN, lymph nodes; SP, spleen.
The profiles of the AFCs induced by the egg-grown virus (Fig. 4) differed from those stimulated by vaccines that were produced in mammalian cell lines. The predominant AFC response was directed against the egg-grown virus antigen and was of the IgM isotype. The egg-grown virus vaccine group also induced high numbers (median = 330) of IgG-producing AFCs per 105 cells reacting with homologous antigen. A significantly lower (P = 0.008) number of IgG-producing cells were induced in the egg-grown virus vaccine group against virus grown in MDCK (median = 160) or Vero (median = 163) cells compared to the number induced against egg-grown virus antigen (median = 330), suggesting that AFCs within this group were more specific for the homologous antigen.
Response of vaccinated mice to challenge with MDCK cell-grown virus.
To evaluate vaccine efficacy following challenge with MDCK cell-grown influenza B/Memphis/1/93 virus, 3 days postchallenge, the lungs of eight mice in each vaccine group were processed separately by the method described by Liang et al. (19). As exemplified by our inability to recover infectious virus from any vaccinated animal, all three vaccine preparations were considered to give complete protection against challenge. All of the control (unvaccinated) mice shed infectious virus (mean titer, 3.5 log10 50% tissue culture infective doses/ml).
The findings presented in this study indicate that influenza B viruses produced in Vero and MDCK cells are equally immunogenic and induce serum antibodies and B-cell responses against mammalian cell-propagated as well as egg-grown virus antigens. Mammalian cell-derived virus vaccines elicited more-cross-reactive serum antibodies, while egg-grown virus induced antibodies more specific to the homologous antigen. Despite their differing immunogenicities, comparative studies showed that all vaccines induced complete protection in mice against MDCK cell-grown virus challenge.
Our results regarding protective immunity are in agreement with a previous study, which suggested that vaccination with egg-derived influenza B viruses protected against variant viruses produced in mammalian cells (27). However, recombinant vaccinia virus-expressing construct could affect the type of immune response, presumably inducing both cellular and humoral immunity. In contrast to our results, it has been reported that inactivated influenza A virus vaccines containing single or multiple amino acid substitutions in HA due to growth in eggs have reduced capacity to protect against challenge with native virus (14, 17). The differences between these reports and the results of the present study may be due to differences in the type of influenza antigen used (influenza A or B) and in the location of the amino acids involved in the antigenic region.
There are several possible reasons for the reduced reactivity pattern of the serum antibodies and the B-cell responsiveness with egg-grown virus vaccine. The first possibility is that amino acid changes in one of the neutralization epitopes on the HA could influence the response. The influenza A antigenic site B (positions 174 to 209) is immunodominant in BALB/c mice for both antibody and helper T cells (4, 7, 30). Subtle conformational changes induced by sequences flanking epitopes have been shown to be sufficient to alter immunogenicity (32). Based on the similarities in the HA structures of influenza A and B viruses (18, 31), it is possible that substitution at amino acid residues 196 to 198 in egg-grown virus vaccine may have affected both the specificities and the levels of antibody-producing cells induced.
Secondly, a change in the glycosylation site could affect immune recognition by either uncovering or hiding the epitope. Because of the extensive reciprocity of B-cell and T-cell recognition of influenza virus HA, B cells can selectively process and present antigen to class II-restricted helper T cells, thereby defining the memory T- and B-cell responses. Changes in the glycosylation site could result in differential antigen processing, resulting in differences in the spectrum of peptides generated for the stimulation of helper T cells.
Thirdly, it is possible that the differences in immunogenicity may be due to differences in the NAs of the variant viruses. It is interesting to note that the egg-grown virus, with the highest NA activity, induced serum antibodies that reacted weakly with Vero cell-grown antigen, which has the lowest NA activity. There could be several reasons for this pattern of reactivity. (i) In the NI assay we used whole virus, not subunits of NA. The anti-HA antibodies in serum may have caused stearic hindrance of NA activity. (ii) The differences in the NA activities of Vero cell- and egg-grown viruses may be influenced by the glycosylation site at amino acid positions 196 to 198 in the HA. This effect would be indirect and might affect the affinity of binding of the HA to sialic acid and might require less NA for release. (iii) Vero cell-grown virus may require more antibodies to inhibit NA activity, even though it has the least NA activity. This possibility seems less likely, but if the NA on Vero cells differs in specificity, it may have some validity.
The present study raises an interesting question: would challenge of the egg-grown virus vaccine group with homologous virus generate memory AFC responsiveness and a serum antibody response equivalent to that seen with the MDCK cell-grown virus? Our earlier study (14) indirectly addressed this issue and showed that MDCK cell-grown virus vaccine provided better protection than did an egg-grown counterpart to challenge with both MDCK cell- and egg-grown virus. Overall, our results suggested that the close antigenic relatedness with prevalent natural viruses may be responsible for the greater immunogenicity of MDCK and Vero cell-grown virus candidate vaccines. These studies support the notion that mammalian cells (both Vero and MDCK cells) can provide a host cell system alternative to eggs for the preparation of influenza B virus vaccines. A dose-response study is needed to determine if the higher levels of cross-reactive antibody induced by mammalian cell-grown virus vaccine provides any advantage over egg-grown influenza virus vaccine.
Acknowledgments
We thank Mikhail Matrosovich for valuable consultation, Amy L. B. Frazier for scientific editing, Deepthi A. Jayawardene for statistical analysis, and members of our laboratories (Scott Krauss, Larisa V. Gubareva, and Darwyn Kobasa) for helpful discussions.
This work was supported by a research grant from Pasteur Merieux with core support from Cancer Center grant CA-21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Allan W, Tabi Z, Cleary A, Doherty P C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 2.Al-Shakhshir R, Regnier F, White J L, Hem S L. Effect of protein adsorption on the surface charge characteristics of aluminum-containing adjuvants. Vaccine. 1994;12:472–474. doi: 10.1016/0264-410x(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Aymard M, Gerentes L, Valette M, Million-Jolly J, Lina B, Kessler N, Douglas A, Cameron K, Hay A J. Options for the control of influenza III: Proceedings of the Third International Conference on Options for the Control of Influenza. Amsterdam, The Netherlands: Elsevier; 1996. Variation of neuraminidase activity of influenza A H3N2 viruses isolated in MDCK cells; pp. 485–490. [Google Scholar]
- 4.Caton A J, Brownlee G G, Yewdel J W, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 5.Couch R B, Douglas R G, Jr, Fedson D S, Kasel J A. Correlated studies of recombinant influenza virus vaccine. III. Protection against experimental influenza in man. J Infect Dis. 1971;124:473–480. doi: 10.1093/infdis/124.5.473. [DOI] [PubMed] [Google Scholar]
- 6.Czerkinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarknowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 7.Gerhard W, Haberman A M, Scherle P A, Taylor A H, Palladino G, Caton A J. Identification of eight determinants in the hemagglutinin molecule of influenza virus A/PR/8/34 (H1N1) which are recognized by class II-restricted T cells from BALB/c mice. J Virol. 1991;65:364–372. doi: 10.1128/jvi.65.1.364-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govorkova E A, Kaverin N V, Gubareva L V, Meignier B, Webster R G. Replication of influenza A viruses in a green monkey kidney continuous cell line (Vero) J Infect Dis. 1995;172:250–253. doi: 10.1093/infdis/172.1.250. [DOI] [PubMed] [Google Scholar]
- 9.Johansson B E, Bucher D J, Kilbourne E D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989;63:1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson B E, Kilbourne E D. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J Virol. 1993;67:5721–5723. doi: 10.1128/jvi.67.10.5721-5723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson B E, Kilbourne E D. Immunization with purified N1 and N2 influenza virus neuraminidase demonstrates cross-reactivity without antigenic competition. Proc Natl Acad Sci USA. 1994;19:2358–2361. doi: 10.1073/pnas.91.6.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz J M, Naeve C W, Webster R G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987;156:386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- 13.Katz J M, Wang M, Webster R G. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals identity with mammalian cell-grown virus. J Virol. 1990;64:1808–1811. doi: 10.1128/jvi.64.4.1808-1811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz J M, Webster R G. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J Infect Dis. 1989;160:191–198. doi: 10.1093/infdis/160.2.191. [DOI] [PubMed] [Google Scholar]
- 15.Katz J M, Webster R G. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J Gen Virol. 1992;73:1159–1165. doi: 10.1099/0022-1317-73-5-1159. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourne E D, Cerini C P, Khan M W, Mitchell J W, Jr, Ogra P O. Immunologic response to the influenza neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J Immunol. 1987;138:3010–3013. [PubMed] [Google Scholar]
- 17.Kodihalli S, Justewicz D, Gubareva L V, Webster R G. Selection of a single amino acid substitution in the hemagglutinin molecule by chicken eggs can render influenza A virus (H3) candidate vaccine ineffective. J Virol. 1995;69:4888–4897. doi: 10.1128/jvi.69.8.4888-4897.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krystal M, Elliott R M, Benz E W, Jr, Young J F, Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci USA. 1982;79:4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 20.Montagnon B J, Fanget B, Nicolas A J. The large-scale cultivation of Vero cells in microcarrier culture for virus vaccine production. Preliminary results for killed poliovirus vaccine. Dev Biol Stand. 1981;64:47–55. [PubMed] [Google Scholar]
- 21.Oxford J S, Schild G C, Corcoran T, Newman R, Major D, Robertson J, Bootman J, Higgins P, Al-Nakib W, Tyrrel D A J. A host-cell-selected variant of influenza B virus with a single nucleotide substitution in HA affecting a potential glycosylation site was attenuated in virulence for volunteers. Arch Virol. 1990;110:37–46. doi: 10.1007/BF01310701. [DOI] [PubMed] [Google Scholar]
- 22.Palache A M, Brands R, van Scharrenburg G J M. Immunogenicity and reactogenicity of influenza subunit vaccines produced in MDCK cells or fertilized chicken eggs. J Infect Dis. 1997;176:520–523. doi: 10.1086/514169. [DOI] [PubMed] [Google Scholar]
- 23.Robertson J S, Bootman J S, Newman R, Oxford J S, Daniels R S, Webster R G, Schild G C. Structural changes in the hemagglutinin which accompany egg adaptation of an influenza A (H1N1) virus. Virology. 1987;160:31–37. doi: 10.1016/0042-6822(87)90040-7. [DOI] [PubMed] [Google Scholar]
- 24.Robertson J S, Bootman J S, Nicolson C, Major D, Robertson E W, Wood J M. The hemagglutinin of influenza B virus present in clinical material is a single species identical to that of mammalian cell-grown virus. Virology. 1990;179:35–40. doi: 10.1016/0042-6822(90)90270-2. [DOI] [PubMed] [Google Scholar]
- 25.Robertson J S, Naeve C W, Webster R G, Bootman J S, Newman R, Schild G C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985;143:166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- 26.Robertson J S, Nicolson C, Bootman J S, Major D, Robertson E W, Wood J M. Sequence analysis of the haemagglutinin (HA) of influenza A (H1N1) viruses present in clinical material and comparison with the HA of laboratory-derived virus. J Gen Virol. 1991;72:2671–2677. doi: 10.1099/0022-1317-72-11-2671. [DOI] [PubMed] [Google Scholar]
- 27.Rota P A, Shaw M W, Kendal A P. Comparison of the immune response to variant influenza type B hemagglutinins expressed in vaccinia virus. Virology. 1987;161:269–275. doi: 10.1016/0042-6822(87)90118-8. [DOI] [PubMed] [Google Scholar]
- 28.Rota P A, Shaw M W, Kendal A P. Cross-protection against microvariants of influenza virus type B by vaccinia viruses expressing haemagglutinins from egg- or MDCK cell-derived subpopulations of influenza virus type B/England/222/82. J Gen Virol. 1989;70:1533–1537. doi: 10.1099/0022-1317-70-6-1533. [DOI] [PubMed] [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 31.Verhoeyen M, Van Rompuy L, Jou W M, Huylebroeck D, Fiers W. Complete nucleotide sequence of the influenza B/Singapore/222/79 virus hemagglutinin gene and comparison with B/Lee/40 hemagglutinin. Nucleic Acids Res. 1983;11:4703–4712. doi: 10.1093/nar/11.14.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Smith J A, Gefter M L, Perkins D L. Immunodominance: intermolecular competition between MHC class II molecules by covalently linked T cell epitopes. J Immunol. 1992;148:3034–3041. [PubMed] [Google Scholar]
- 33.Warner T G, O’Brien J S. Synthesis of 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 34.Warren L J. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 35.Wood J M, Oxford J S, Dunleavy U, Newman R W, Major D, Robertson J S. Influenza A (H1N1) vaccine efficacy in animal models is influenced by two amino-acid substitutions in the hemagglutinin molecule. Virology. 1989;171:214–221. doi: 10.1016/0042-6822(89)90528-x. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman M A, Cox R J, Oxford J S. Attenuation of virulence in influenza B viral infection of volunteers. J Infect Dis. 1994;28:41–48. doi: 10.1016/s0163-4453(94)94099-1. [DOI] [PubMed] [Google Scholar]