Abstract
Purple sweet potatoes (Ipomoea batatas (L.) genotype) in Southern Africa have a phytonutritional composition and antioxidant properties that can increase incomes and improve nutrition. This study compared the phytonutrient composition and antioxidant properties of four purple-colour sweet potato genotypes (local Purple-purple, ‘2019-1-1’, and USA genotypes, ‘08-21P’ and ‘16-283P’). These purple sweet potato genotypes were characterised by UPLC/QTOF/MS and 16 phenolic compounds were identified. Purple-purple (very dark purple) showed the highest concentration of cyanidins and peonidin derivatives. Chlorogenic acid derivatives were highest in the genotype ‘16-283P’. ‘Puple-purple’ and ‘16-283P’ displayed the strongest antioxidant power and scavenging activities. Diaffeoylquinic acid isomer 1 was identified as the marker candidate for distinguishing the four purple sweet potato genotypes. Southern Africa’s highest-protein sweet potato genotypes are Purple-purple (28.81 g/100 g) and ‘08-21 P’ (24.31 g/100 g). A 13.65 g portion of ‘2019-1-1′ would meet the Recommended Dietary Allowance (RDA) for iron for men, while 25.59 g would meet the RDA for children, and 30.72 g would meet the RDA for women. The sweet potato root of genotype ‘2019-1-1′ provides 31.43 g of Zn per day for children and 22.86 g for adults. The roots of local cultivar Purple-purple can be used as functional food ingredients.
Keywords: morphological traits, caffeoylquinic acid derivative, cyanidin glycosides, peonidin glycosides, ferric reducing antioxidant power
1. Introduction
Sweet potatoes are dicotyledonous plants (Ipomoea batatas (L.) Lam) of the Convolvulaceae family [1]. It is one of the most widely grown crops in the world and is considered a food security crop. Sweet potato is among several crops that have been used successfully for biofortification to reduce hidden hunger, specifically, a micronutrient deficiency aligned with a shortage of vitamin A, by breeding orange-fleshed cultivars rich in carotenoids [2]. Sweet potatoes are key staple food in South America, the Caribbean, Asia, and Africa and are a good source of calcium, iron, zinc, vitamins A and C, magnesium, phosphorus, and potassium [3]. The Food and Agriculture Organisation (FAO) stated that 109 nations produced sweet potatoes in 2019. China produced 46.6 million metric tons of sweet potatoes in 2022 [4]. The production of sweet potatoes increased by 1.5% in 2022 after two years of decline in Africa [5]. Malawi, Nigeria, Tanzania, Uganda, Ethiopia, Angola, Rwanda, Madagascar, Burundi, and Kenya are 10 of the world’s top 20 sweet potato-producing countries (Food and Agriculture Organization) [6]. Sweet potatoes are commonly cultivated for the consumption of their storage roots of various pleasant colours from cream/white, purple, and yellow to orange [7]. There are 131 million tons of sweet potatoes grown worldwide each year, and it ranks third in importance after potatoes [8]. The varieties of staple roots in Sub-Saharan Africa are white- or cream-fleshed, distinguished by having high starch content [9].
In South Africa, the Agricultural Research Council programme includes the breeding and commercialisation of sweet potatoes, developing cultivars with a tolerance to major diseases and a better yield and root quality traits, i.e., high levels of anthocyanins [10]. The South African Agricultural Research Council has released 33 genotypes thus far, with some, like ‘Ribbok’, ‘Bosbok’, and ‘Blesbok’, being commercialised. ‘Blesbok’, a cultivar with purple skin, is known for its high yield and low dry matter content [11,12]. Ndou and Monate have a high yield and high dry matter content, with cream flesh and cream skin [13]. Additionally, there are orange-fleshed cultivars with a high β-carotene content, such as Bophelo and Khumo. Among the more recent developments are breeding lines FS10-25 and FS10-21, which produce cream-fleshed storage roots with purple skin known for their excellent storability and wilt tolerance.
Recently, breeders have shown interest in purple-fleshed sweet potato cultivars, as highlighted by Parker et al. [14]. The main aim of breeding is to produce cultivars with high levels of anthocyanin and antioxidant capacity [15]. Purple sweet potatoes are rich in anthocyanins, starch, polysaccharides, caffeoylquinic acid derivatives, vitamins, and minerals [16]. Additionally, anthocyanins have antimutagenic, hepato-protective, antihypertensive, antihyperglycemic, antimicrobial, anti-inflammatory, and anti-obesity properties [17]. According to Ghasemzadeh et al. [18] and Dwiyanti et al. [19], compared to the anthocyanin found in red cabbage, elderberries, blueberries, and red corn, the pigment anthocyanin in purple sweet potatoes has a higher stability. Sweet potatoes with purple flesh are rich in cyanidin and peonidin glycosides, which acylate their sophorose through the presence of p-hydroxybenzoyl, caffeoyl, and feruloyl moieties [20]. However, the content of anthocyanin, caffeoylquinic acid derivatives, protein, fibre, Fe, Zn, and the antioxidant activity of purple varieties grown in South Africa are unknown. The objective of the present study was to investigate phytonutritional composition, including mineral elements Fe and Zn, total and individual phenolic compounds, and the antioxidant activities of the purple-colour flesh sweet potato roots found in the Southern African region—the Agricultural Research Council breeding lines ‘2019-1-1’ (purple skin with purple, cream-ring flesh), the locally collected genotype Purple-purple (dark purple skin with very dark purple flesh), and two imported cultivars from the USA (‘08-21P’ and ‘16-283P’).
2. Materials and Methods
2.1. Plant Material
The locally sourced genotype (Purple-purple), one Agricultural Research Council breeding line (‘2019-1-1′), and two imports from the USA (‘08-21P’ and ‘16-283P’) (Figure 1) were grown during the 2022 and 2023 growing seasons at the Roodeplaat campus (25°36’26” S, 28°33’40” E, altitude 1220 m above sea level) of the Agricultural Research Council–Vegetables, Industrial and Medicinal Plants (ARC-VIMP) located in northern Gauteng, South Africa.
Figure 1.
Shape and size of roots and distribution of anthocyanin in four purple-fleshed genotypes.
2.2. Total Marketable Yield and Morphological Assessment
After harvest, the total marketable yield was determined (Scale Model Alpha 2 LCD, Jiangsu, China) and the results were expressed as kg/20 plants. On the day of harvesting, ten storage roots from each genotype were chosen for morphological assessment.
Diameter (cm), length (cm), and cortex thickness (mm) were measured using a calliper. The shape, pigmentation, and distribution of pigmentation of the storage roots were recorded based on International Potato Centre (CIP) sweet potato descriptors (Huamari, 1992). The pigmentation and distribution of pigmentation of the storage roots were recorded as explained by Selokela et al. [21].
2.3. Colour Measurement
Skin and flesh colour were recorded from 10 replicate samples using a chromameter (Model CR-400 Minolta, Konica Minolta Sensing, Inc., Osaka, Japan) and expressed as CIELab* values. The chromameter was calibrated to a white calibration plate. L* (+100 = white, −100 = black), the Hunter colour scale’s tri-stimulus values (L: light, a (+a = red), b (b = blue, +b = yellow, a = green), chromaticity or C* (colour intensity) and hue angle or hº (0 = red, 90 = yellow, 180 = green, 270 = blue) of the flesh [22].
2.4. Chemical Analysis
A set of 50 storage roots of each genotype, devoid of deterioration or damage, was selected randomly and pooled, and from those, 10 subsamples were taken for chemical analysis. These were washed with tap water [23] and transported to the Fruit and Vegetable Laboratory at the Tshwane University of Technology, in Pretoria West. Thereafter, the roots were chopped and freeze-dried (VirTis Sp Scientific, Model # 2kBTES-55, Gardiner, NY, USA) at −47 to −53 °C for 72 h and ground into fine powder, then stored at 4 °C until use.
2.4.1. Chemicals
The chemicals and standards mentioned below were purchased from Lasec SA (Pty) Ltd. in Midrand, Gauteng, South Africa.
2.4.2. Total Phenolic Content (TPC)
The TPC was determined from purple-fleshed storage roots using the procedure explained by Hong et al. [24]. Each sample weighing 10 mg was extracted with 10 mL of 80% methanol using magnetic stirring (Edison, H4000-HSB, Sayreville NJ 08872, USA). The TPC was calculated using the standard curve generated with chlorogenic acid, which had concentrations ranging from 0–100 ppm, and the measurements were expressed in milligrams per gram of chlorogenic acid equivalent.
2.4.3. Ferric Reducing Antioxidant Power (FRAP)
The FRAP value was established according to the protocol described by Selokela et al. [21]. The absorbance was then measured at 593 nm using a spectrophotometer. A standard curve was created with standards ranging from 0 to 700 concentrations, and the results were expressed as mM TEAC/g. The equation for the standard curve was Y = 0.0005x + 0.1216, with an R2 value of 0.98.
2.4.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The DPPH scavenging ability was used to measure inhibiting activity with some minor changes, following the method described by Suárez et al. [25]. The inhibiting activity of the storage roots was measured by the decrease in absorbance in the methanol solution of DPPH. The IC₅₀ (mg/mL) was calculated using the concentration versus inhibition % graph.
2.4.5. 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-sulfonic Acid) Scavenging Activity
ABTS scavenging activity was carried out, following the method explained by Seke et al. [26] A measurement was made of the decline in absorbance at 734 nm. A graph of the percentage of inhibition versus the concentration was used to determine the IC50 (mg/mL).
2.5. Mineral Composition
Fe, Zn, and K were determined using the digest solution in an aliquot and (ICP-OES) inductively coupled plasma optical emission spectrophotometer [(Agilent 725 Series) Santa Clara, CA, USA] device. Mineral content was expressed on a dry weight basis [21].
2.6. Quantification of Phenolic Compounds Using UPLC-QTOF/MS
The identification and quantification of predominant phenolic acids and flavonoids were achieved using a UPLC-QTOF/MS system (Waters, Milford, MA, USA) equipped with a Quadrupole 120 time-of-flight (QTOF) mass spectrometer, following the method described by Managa et al. [27] without any modifications. The calibration curve set up using a chlorogenic acid standard was used to quantitatively and semi-quantitatively measure all identified compounds. The content of phenolic compounds in the study is expressed as mg/kg. Data were processed using TargetLynx software as previously reported [28]. The data generated by the UPLC-Q-TOF/MS and HPLC-DAD (individual anthocyanins) analysis were analysed using principal component analysis (PCA) and Projections to Latent Structures–Discriminant Analysis (PLS-DA) approaches to identify the differences between the phenolic profiles of the different genotypes of the sweet potatoes’ roots. The regression equation, and retention times’ limit of detection (LOD) and limit of quantification (LOQ), for anthocyanins and phenolic compounds are shown in Supplementary Table S1.
2.7. Proximate Composition
Analyses were conducted to determine protein, total dietary fibre, and fat, following the procedures outlined by the Association of Official Analytical Chemists [29].
2.8. Statistical Analysis
Variance was analysed on all data collected and means separation using Tukey’s truly significant difference (Tukey’s HSD). All the data collected in this study were analysed using statistical package design Gen stat 18.1. The data were obtained during the 2022 and 2023 growing seasons. The results of the two harvests were compared, and as the data did not differ, it was pooled together and subjected to analysis of variance (ANOVA) using GenStat 11.1.
3. Results and Discussion
3.1. Morphological Characteristics of Five Purple-Fleshed Genotypes
The storage roots of different coloured sweet potato genotypes differ greatly from one another in terms of their morphological characteristics (Table 1). Most of the roots were long, elliptical, obovate, ovate, or round. The size and quantity of sweet potato storage roots depend on the root system and other characteristics of the plant, which are influenced by the environmental conditions of the growing areas [30]. Additionally, several factors can affect sweet potato morphology, including the season, agricultural practices, and the characteristics of the plant itself [31]. The skin colour of the storage roots of ‘2019-1-1′, ‘08-21P’, ‘16-283P’, and Purple-purple ranged from light purple to dark purple. The flesh of ‘2019-1-1’ had purple inner rings surrounded by cream outer rings. The roots of ‘08-21P’ were violet and pink-cream, and the roots of ‘16-283P’ were dark purple with a slight ring. Only the roots of Purple-purple showed a very dark purple flesh colour (Figure 1). The surface defects of sweet potato roots ranged from being absent to long thick veins, severe cracks, and alligator-like skin.
Table 1.
Morphological characteristics of four genotypes of purple-fleshed sweet potato storage roots.
| Purple Sweet Potato Genotypes |
Skin Colour | Flesh Colour | Shape | Surface Defects |
|---|---|---|---|---|
| ‘08-21P’ | Light purple | Violet, pink-cream | Long elliptic | Absent |
| ‘2019-1-1′ | Purple | Purple, cream ring | Obovate–elliptic | Grooves, some cracks |
| Purple-purple | Dark purple | Very dark purple | Long elliptic | Long thick veins |
| ‘16-283P’ | Light purple | Dark purple, slight ring | Long elliptic | Absent |
Furthermore, the yield of these four genotypes varied significantly during the growing seasons (Table 2). Harvested yields for these four purple sweet potatoes were as follows: ‘08-21P’ produced 20.64 kg/20 plants; ‘2019-1-1’ (5.45 kg/20 plants); Purple-purple (15.48 kg/20 plants); and ‘16-283P’ (7.56 kg/20 plants). The ARC-developed genotype ‘2019-1-1’ showed lower yields than the locally found Purple-purple. The root lengths of these four purple sweet potatoes were 21.80 cm, 20.40 cm, 17.00 cm, and 16.85 cm in the ‘08-21P’, Purple-purple, ‘16-283P’, and ‘2019-1-1′ genotypes, respectively (Table 2). The diameter of the roots varied among the four genotypes. The highest root diameter was observed in ‘2019-1-1’ (5.48), followed by ‘08-21P’ (5.07), Purple-purple (4.26 cm), and ‘16-283P’ (4.18 cm). The Purple-purple (3.9 cm) and ‘08-21P’ (3.40 cm) genotypes showed the largest cortex diameters and were followed by ‘16-283P’ (2.80 cm) and ‘2019-1-1’ (1.16 cm) as shown in Table 2. Sweet potato storage roots are reported to vary in length and diameter [3;303]. The farmer’s choice of sweet potato variety depends on the independent variable weight, since it directly affects crop yield [32]. Furthermore, sweet potato morphology is crucial when screening new genotypes because consumers may reject roots with undesirable traits [32].
Table 2.
Yield, length, diameter, and cortex of the four different genotypes of purple sweet potato storage roots.
| Genotype | Yield (kg/20 Plants) S1 | Length (cm) | Diameter (cm) | Cortex (mm) |
|---|---|---|---|---|
| ‘08-21P’ | 20.64+ 1.90 a | 21.80 ± 2.88 a | 5.07 ± 1.28 a | 3.40 ± 1.17 ab |
| ‘2019-1-1′ | 5.45 ± 1.26 d | 16.85 ± 3.35 bc | 5.48 ± 1.17 a | 1.16 ± 0.31 c |
| Purple-purple | 15.48 ± 2.43 b | 20.40 ± 3.85 ab | 4.26 ± 0.83 b | 3.85 ± 1.03 a |
| ‘16-283P’ | 7.56 ± 1.72 c | 17.00 ± 3.63 bc | 4.18 ± 0.91 b | 2.80 ± 0.75 b |
| LSD | 2.81 | 7.19 | 2.19 | 1.65 |
Data present mean and standard deviation (n = 10) and Tukey’s HSD of four purple-fleshed sweet potato storage root genotypes. Significant variances are indicated by distinct letters within the same column at (p < 0.001).
3.2. Colour Properties
Food colour is a crucial quality parameter for sweet potatoes. Table 3 displays the colour attributes of four storage roots of purple-fleshed sweet potatoes. Purple-purple had the lowest L* value (deep purple) and exhibited a darker colour. Hence, the brightness or darkness of the flesh colour of the roots is determined by the L* value. Light purple flesh colours were seen with a higher L* value, while dark purple flesh colours were seen with a lower L* value [17]. It is also important to note that the proportion of lightness (L*) and colour coordinates a* and b* influence flesh colour. Meanwhile, a positive a* colour coordinate relates to a higher red-colour intensity, while a positive b* colour coordinate relates to a higher yellow intensity. The genotype ‘2019-1-1’ exhibited a higher L* value when compared to Purple-purple and the two genotypes from the USA. However, its a* colour coordinate was similar to that of ‘16-283P’ but lower than both ‘08-21P’ and Purple-purple. The chroma value of genotype ‘2019-1-1’ was higher than ‘08-21P’ but lower than ‘16-283P’ and similar to Purple-purple, indicating a purple and cream flesh colour. In addition, colour values are very important to breeders to breed new varieties (Nakagawa et al. [33]). Genotypes with a deep purple colour are appropriate as constituents for flour colourants and snacks [17]. Sweet potatoes are purple because of anthocyanins. Sweet potatoes contain peonidin and cyanidin, which are the most abundant anthocyanins. The flesh appears red-purple when peonidin levels exceed 1, while it appears purple-blue or grey when cyanidin levels dominate. The genotype ‘08-21P’ consistently had the highest value for the a* colour coordinate in both seasons, while the genotypes ‘2019-1-1′ and ‘16-283P’ had the lowest.
Table 3.
Flesh colour values of four genotypes of purple-fleshed sweet potato storage roots.
| Genotypes | L* | a* | b* |
|---|---|---|---|
| ‘08-21P’ | 45.58 ± 3.50 a | 30.58 ± 5.14 a | 5.57 ± 2.20 a |
| ‘2019-1-1′ | 42.69 ± 5.94 b | 8.64 ± 3.02 c | 5.18 ± 4.98 b |
| Purple-purple | 28.82 ± 2.74 d | 18.36 ± 3.18 b | 3.09 ± 0.51 c |
| ‘16-283P’ | 34.79 ± 3.39 c | 8.12 ± 1.11 c | 2.21 ± 2.64 cd |
| LSD | 4.4 | 3.79 | 3.3 |
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within the same column at (p < 0.001).
3.3. Total Phenolic Content
A comparison of the phenol content of the four genotypes of purple sweet potato roots is shown in Table 4. The ARC-developed genotype ‘2019 1-1’ (50.69 mg/g on dry weight (dw) basis) and USA genotype ‘16-283P’ (53.24 mg/g dw) showed the highest TPC. In contrast, Purple-purple (42.45 mg/g) and ‘08-21′P (43.54 mg/g dw) had the lowest TPC in the growing season. In five Korean purple sweet potato genotypes (‘Sinjami’, ‘Jami’, ‘Yeonjami’, ‘Danjami’, and ‘Borami’), the TPC ranged from 1.80 to 7.37 mg GAE/g DW [34]. In contrast to the authors, our values were much higher than those of all the Korean purple sweet potato genotypes. This is because they used gallic acid instead of chlorogenic acid, a phenolic compound found in sweet potatoes. The study by Franková et al. [35] found that flesh colour was a major factor influencing polyphenol levels in sweet potatoes. The purple genotype (414-purple) yielded 1.5 and 3.8 times higher polyphenol levels than the other genotypes such as ‘Beauregard’ and ‘O’Henry’ [35]. The results from our study, however, contradict this statement, because the TPC of the genotype ‘2019-1-1‘ (purple and cream) was higher than ‘16-283P’ (purple-with-orange-spots flesh); (D) ‘08-21P’ (purple-with-cream flesh); and (E) Purple-purple (dark purple flesh).
Table 4.
Total phenolic content of four genotypes of purple-fleshed sweet potato storage roots.
| Genotypes | Total Phenolic (CAE mg/g) DW |
|---|---|
| ‘08-21P’ | 43.54 ± 7.90 b |
| ‘2019-1-1′ | 50.69 ± 1.48 a |
| Purple-purple | 42.45 ± 5.31 b |
| ‘16-283P’ | 53.24 ± 3.86 a |
| LSD | 8.72 |
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within the same column at (p < 0.001). (CAE mg/g): chlorogenic acid equivalent; DW: dry weight.
3.4. UHPLC-QTOF-MS Identification and Characterisation of Phenolic Compounds
3.4.1. Chlorogenic Acid Derivatives and Flavonoids
The main phenolic compounds in sweet potatoes are chlorogenic acids [36]. In all four genotypes, chlorogenic acid isomers (3-O-Caffeoylquinic acid; 3CQA), neochlorogenic acid (5-O-caffeoylquinic acid; 5CQA), 1,3-dicaffeoylquinic acid (1,3-diCQA), dicaffeoylquinic acid isomer 1diCQA, dicaffeoylquinic acid isomer 2, diCQA, dicaffeoylquinic acid isomer 3diCQA, 3-O-caffeoyl-4-O-methylquinic acid, 3,5-dicaffeoylquinic methyl ester, and quercetin 3,4’-diglucoside were detected in this study (Table 5). The characterisation and MS spectra of these metabolites are described in Supplementary Figures S1, S3–S11. Out of the four purple sweet potato genotypes, the highest concentration of 3CQA isomer, all diCQA isomers, 3,5-dicaffeoylquinic methyl ester, and quercetin 3,4’-diglucoside was found in the ‘16-283P’ genotype. In contrast, the highest concentrations of 5CQA were found in the genotypes ‘16-283P’ and Purple-purple. In addition, genotypes ‘16-283P’ and ‘08-21P’ had the highest concentrations of diCQA isomer 2. Furthermore, the ‘08-21P’ genotype contained the highest concentration of 1,3-diCQA. On the other hand, the Purple-purple genotype had the highest concentrations of 3CQA isomer and 3-O-Caffeoyl-4-O-methylquinic acid compared to all the other three genotypes. Genotype ‘08-21P’ had the highest concentration of 1,3-diCQA. Overall, the ‘16-283P’ genotype had the largest proportion of chlorogenic acid derivatives and quercetin 3,4’-diglucoside compared to the other genotypes in this study. It has been observed that sweet potatoes grown in North Italy, specifically the ‘Beauregard’ genotype, have higher concentrations of 3-CQA (205.5 mg kg−1 DW) [37]. In Italy, white-fleshed and orange-fleshed sweet potato roots were found to have 3-CQA levels of 436 and 221–333 mg kg−1 DW, respectively [38]. On the other hand, a study conducted on four sweet potato genotypes from China showed that the concentration of chlorogenic acids varied between 300–730 mg kg−1 DW of 3-CQA, 260–480 mg kg−1 DW of 5-CQA, and 600–930 mg kg−1 DW of 4-CQA) [39]. It is worth noting that the concentration of chlorogenic acids in sweet potatoes is influenced by both the variety and the region of cultivation.
Table 5.
Identification and quantification of cinnamic acids and derivatives and flavonoid composition of purple-coloured sweet potato (Ipomoea batatas L.) genotypes found in Sub-Saharan Africa by UPLC–QTOF/MS.
| RT | [M-H]− (m/z) | MSE Fragments | Molecular Formula | Tentative Identification | Sweet Potato Genotypes (Roots) | |||
|---|---|---|---|---|---|---|---|---|
| ‘16-283P’ | ‘08-21P’ | ‘2019-1-1′ | Purple-purple | |||||
| Concentrations of cinnamic acids and derivatives in mg/kg | ||||||||
| 4.44 | 353.087 | 135.043 179.034 191.055 201.016 |
C16H18O9 | Chlorogenic acid 3CQA 3-O-Caffeoylquinic acid |
246.26 a ± 3.54 | 118.81 d ± 1.81 | 147.41 c ± 3.22 | 198.26 b ± 1.19 |
| 5.29 | 707.182 | 135.043 161.022 179.034 191.055 353.088 707.183 |
C16H18O9 | Chlorogenic acid 3CQA 3-O-Caffeoylquinic acid |
2201.27 b ± 2.02 | 450.30 c ± 2.25 | 670.03 c ± 2.37 | 3032.33 a ± 1.46 |
| 5.29 | 353.087 | 135.043 161.023 179.034 191.055 353.088 |
C16H18O9 | Chlorogenic acid 5CQA 5-O-Caffeoylquinic acid (Neochlorogenic acid) |
4354.93 a ± 1.66 | 2195.08 b ± 2.43 | 2178.12 b ± 2.60 | 4210.58 a ± 2.52 |
| 6.48 | 367.103 | 191.056 | C17H20O9 | 3-O-Caffeoyl-4-O-methylquinic acid (MCGA3) | 184.71 b ± 5.14 | 95.49 d ± 2.89 | 106.71 c ± 2.53 | 221.10 a ± 1.73 |
| 7.41 | 515.119 | 135.043 161.023 173.044 179.033 191.055 201.016 335.076 353.087 388.996 |
C25H24O12 | 1,3-Dicaffeoylquinic acid (1,3-diCQA) | 222.45 b ± 1.03 | 433.27 a ± 1.28 | 98.34 d ± 2.45 | 179.91 c ± 1.34 |
| 7.60 | 515.119 | 135.043 179.033 191.055 353.087 375.069 |
C25H24O12 | Dicaffeoylquinic acid isomer 1 diCQA |
10547.26 a ± 1.44 | 5420.71 b ± 1.98 | 3068.42 c ± 2.02 | 3467.92 c ± 3.07 |
| 8.04 | 515.119 | 173.044 191.054 353.088 375.070 |
C25H24O12 | Dicaffeoylquinic acid isomer 2 diCQA |
124.65 a ± 0.23 | 118.34 a ± 1.11 | 82.08 b ± 1.84 | 61.58 c ± 1.90 |
| 8.68 | 515.119 | 179.034 191.055 339.050 353.087 375.070 |
C25H24O12 | Dicaffeoylquinic acid isomer 3 diCQA |
18.08 a ± 1.10 | 11.48 b ± 0.88 | 7.19 c ± 0.20 | 6.33 c ± 0.49 |
| 8.89 | 529.136 | 367.104 375.167 397.146 519.331 |
C26H26O12 | 3,5-Dicaffeoylquinic methyl ester; (-)-3,5-Dicaffeoylquinic methyl ester 3,5-diCQA) |
22.13 a ± 1.01 | 14.76 b ± 0.86 | 6.66 c ± 0.32 | 9.43 c ± 3.44 |
| Concentrations of flavonoids in mg/kg | ||||||||
| 6.28 | 625.141 | 300.027 301.032 339.051 371.098 471.152 533.129 555.112 |
C27H30O17 | Quercetin 3,4′-diglucoside | 29.23 a ± 0.71 | 8.54 c ± 0.52 | 7.86 c ± 0.23 | 18.88 b ± 0.46 |
Means followed by the same letter within the row are not significantly different (p < 0.05); each of the samples was replicated three times, and the results are expressed as mean ± standard deviation.
3.4.2. Anthocyanins
Different anthocyanin compounds were quantified in the storage roots of all four purple-fleshed genotypes found in the Southern African region (Table 6). These metabolites’ characterisations and MS spectra are shown in Supplementary Figures S2, S12–S17. Three mono-acylated (cyanidin-caffeoyl-sophoroside-glucoside, peonidin feruloyl-sophoroside-glucoside, peonidin caffeoyl-sophoroside-glucoside) and three diacylated anthocyanins (cyanidin-caffeoyl-feruloyl-sophoroside-glucoside, peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside, peonidin caffeoyl-feruloyl-sophoroside-glucoside) were detected in purple sweet potato storage roots that are consumed in the Southern African region. The ratios of peonidin/cyanidin derivatives ranged from 2.16 to 6.72, indicating that peonidin-based anthocyanins were predominant in all the samples, with the 2019-1-1 sample showing the highest ratio (6.72), followed by Purple-purple pp (4.11); 16-283p (2.95) and 08-21p (2.16) were detected in purple sweet potato storage roots that are consumed in South Africa. The ratios of peonidin/cyanidin derivatives ranged from 2.16 to 6.72, indicating that peonidin-based anthocyanins were predominant in all the samples, with the 2019-1-1 sample showing the highest ratio (6.72), followed by Purple-purple pp (4.11), 16-283p (2.95) and 08-21p (2.16). Among the different purple genotypes, Purple-purple (very dark purple) contained the highest concentrations of cyanidin-caffeoyl-feruloyl-sophoroside-glucoside (230.90 mg/kg), peonidin feruloyl-sophoroside-glucoside (353.33 mg/kg), peonidin caffeoyl-sophoroside-glucoside (327.52 mg/kg), peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside (276.95 mg/kg), and peonidin caffeoyl-feruloyl-sophoroside-glucoside (185.25 mg/kg), while 16-283p contained cyanidin-caffeoyl-sophoroside-glucoside (123.12 mg/kg). Conversely, the ‘2019-1-1’ genotype did not exhibit peonidin caffeoyl-feruloyl-sophoroside- glucoside. In comparison to the other genotypes in this study, the cyanidin-caffeoyl-feruloyl-sophoriside-glucoside concentration in the ‘2019-1-1’ genotype was significantly lower than that in the other genotypes. On the other hand, both the 08-21p and 2019-1-1 genotypes showed significantly lower concentrations of cyanidin-caffeoyl-sophoroside-glucoside, peonidin feruloyl-sophoroside-glucoside, and peonidin caffeoyl-sophoroside-glucoside. According to Terahara et al. [40], six diacylated anthocyanins were detected in the storage roots of Ipomoea batatas cv. Yamagawamurasaki. The purple sweet potatoes P40 (USA genotype) contained 12 acylated anthocyanins [41]. Studies have shown that anthocyanin content varies within the different genotypes [41]. Based on the shade of colour and peonidin/cyanidin (pn/cy) ratio, sweet potato’s anthocyanin composition can be divided into two categories: cyanidins with blue domains (pn/cy >1.0) and peonidins with red domains (pn/cy > 1.0) [42]. Studies have shown that cyanidin anthocyanins have stronger antimutagenic, antioxidant, and antidepressant properties compared to peonidin anthocyanins [43,44]. The Purple-purple genotype has 20% cyanidin derivatives compared to its 80% peonidin derivatives. In contrast, 16-283p and Purple-purple have similar levels of cyanidin derivatives at 37%, while 08-21p has 22% and 2019-1-1 has 4%. Therefore, the genotype Purple-purple, with high cyanidin derivatives, would serve as a better food for physiological functions. It has been estimated that the daily intake of total anthocyanins should be between 3 and 215 mg [45]. After consumption, in 1.5 h, the plasma levels of anthocyanins were found to be from 0.5 to 1.0 µM [46]. According to Yi et al. [47], more hydroxyl groups and fewer OCH3 groups can decrease anthocyanin bioavailability. Until now, no study has reported the anthocyanin composition of the ‘2019-1-1 and Purple-purple genotypes. Sweet potatoes are ideal for commercial anthocyanin production because of their low cost, rapid growth cycle, and adaptability [48]. Moreover, due to their higher concentration of acyl groups, Purple-purple genotypes can withstand high temperatures and are UV stable, which makes them ideal for use as food additives.
Table 6.
Identification and quantification of anthocyanin composition of purple-coloured sweet potato (Ipomoea batatas L.) genotypes found in Sub-Saharan Africa by UPLC–QTOF/MS.
| RT | [M-H]− (m/z) | MSE Fragments | M-H Formula | Identified Compound | Sweet Potato Genotypes (Roots) | |||
|---|---|---|---|---|---|---|---|---|
| ‘16-283P’ | ‘08-21P’ | ‘2019-1-1′ | Purple-purple | |||||
| Concentrations in mg/kg | ||||||||
| 5.66 | 933.231 | 287.055 | C42H45O24 | Cyanidin-caffeoyl-sophoroside-glucoside | 123.12 a ± 1.13 | 18.17 c ± 0.30 | 11.54 c ± 0.75 | 47.25 b ± 1.48 |
| 7.00 | 1109.278 | 287.055 | C52H53O27 | Cyanidin-caffeoyl-feruloyl-sophoroside-glucoside | 159.22 b ± 2.79 | 151.45 b ± 3.48 | 17.91 c ± 0.52 | 230.90 a ± 1.10 |
| 6.47 | 961.263 | 301.071 | C44H49O24 | Peonidin feruloyl-sophoroside-glucoside | 301.27 b ± 1.5 | 160.03 c ±1.79 | 167.06 c ± 5.11 | 353.33 a ± 1.97 |
| 6.50 | 947.247 | 301.091 | C43H47O24 | Peonidin caffeoyl-sophoroside-glucoside | 203.34 b ± 1.46 | 28.42 c ± 1.73 | 12.71 c ± 0.95 | 327.52 a ± 1.69 |
| 6.90 | 1067.268 | 301.071 463.123 |
C50H51O26 | Peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside | 180.97 b ± 1.62 | 159.41 c ± 1.85 | 18.05 d ± 0.76 | 276.95 a ± 1.70 |
| 7.03 | 1123.293 | 301.071 463.124 |
C53H55O27 | Peonidin caffeoyl-feruloyl-sophoroside-glucoside | 147.9 b ± 1.75 | 19.13 c ± 0.35 | 0c | 185.25 a ± 1.16 |
Means followed by the same letter within the row are not significantly different (p < 0.05); each of the samples was replicated three times, and the results are expressed as mean ± standard deviation.
3.5. The Metabolomic and Chemometric Profiles of Five Purple Sweet Potatoes Available in the Sub-Saharan African Region
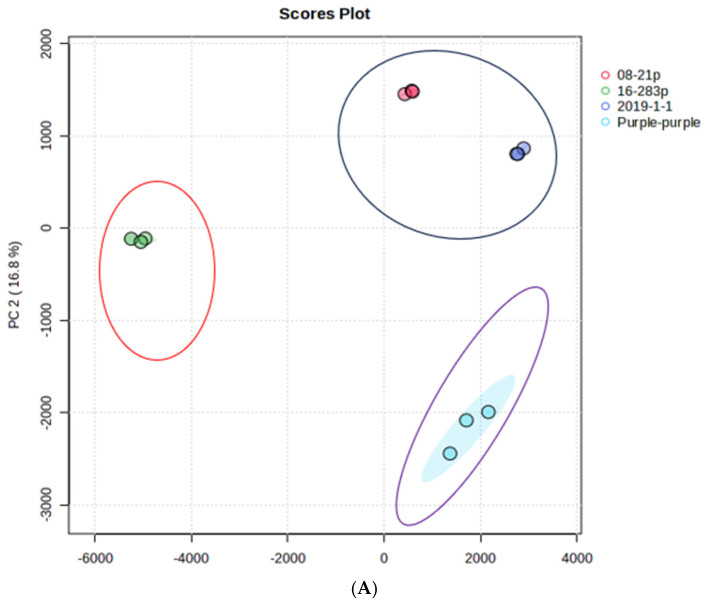
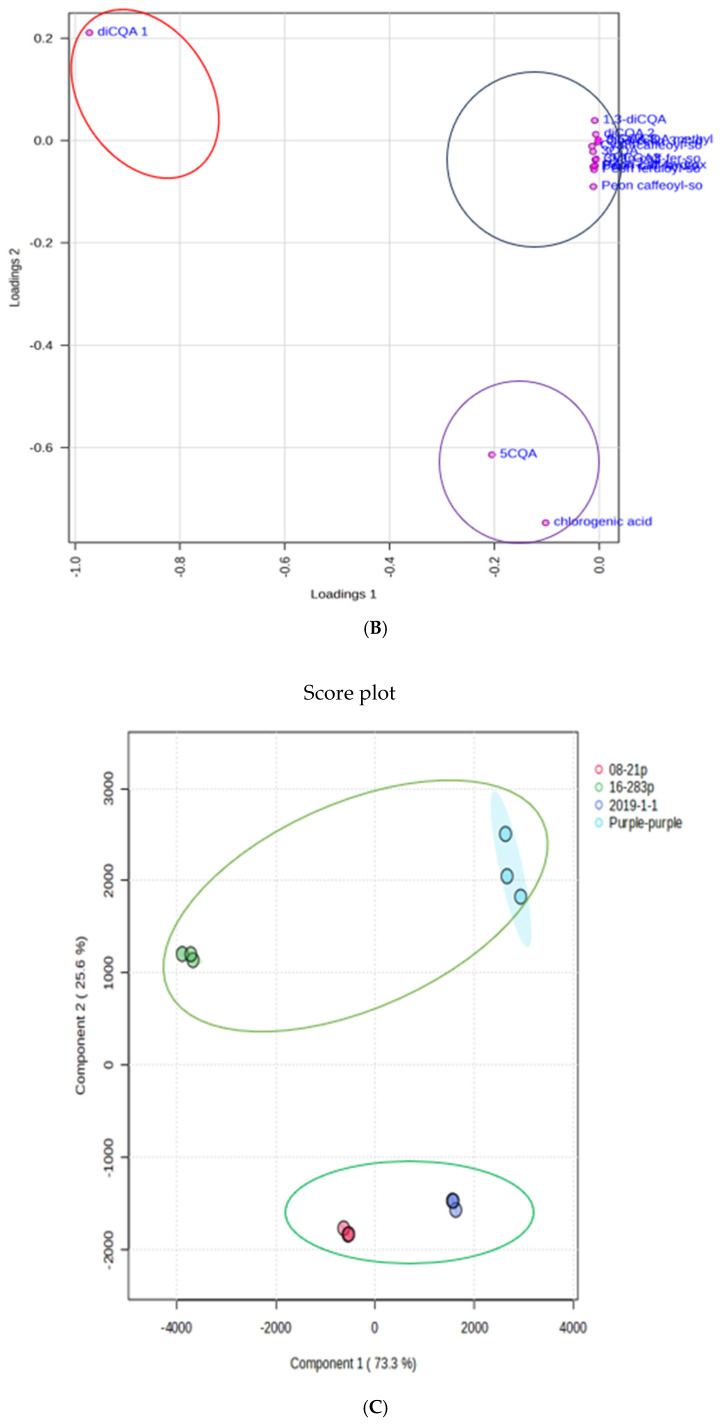
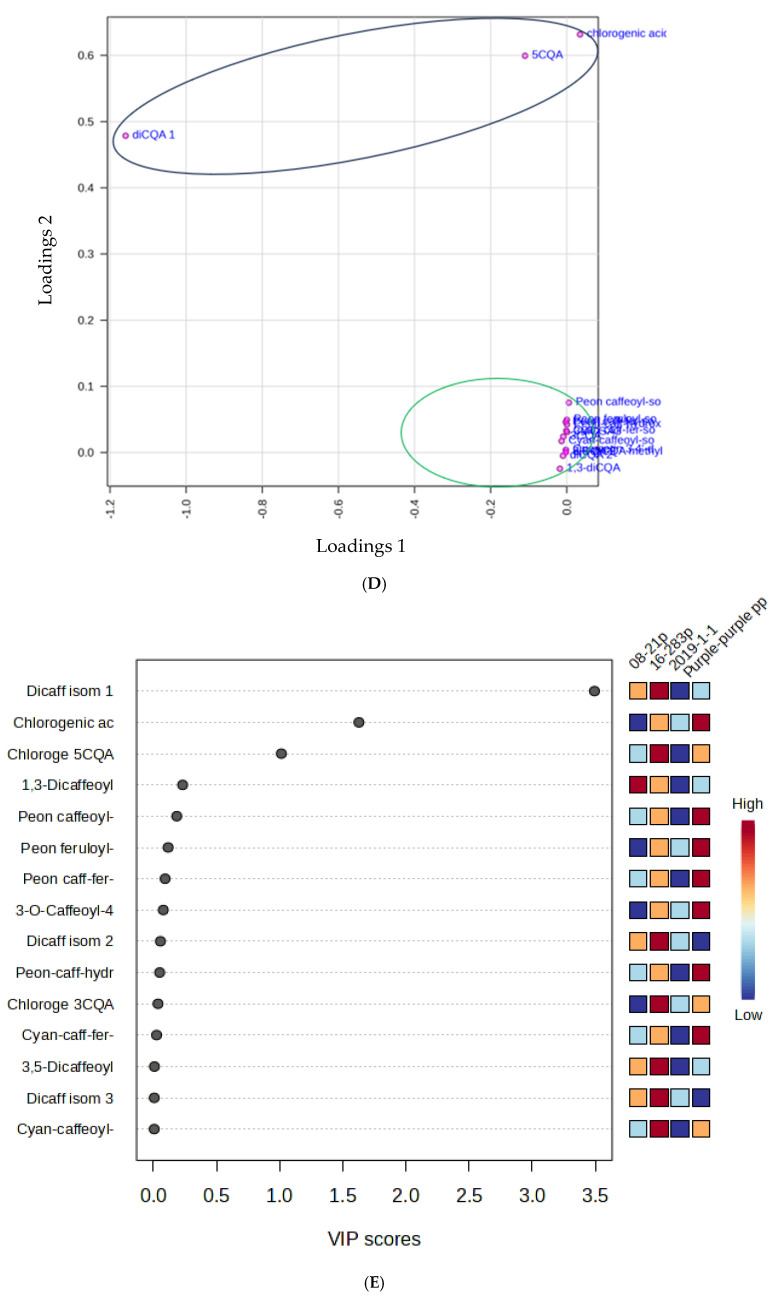
Using UPLC-Q-TOF/MS results, unsupervised PCA analysis showed which sweet potato genotypes have the most and least phenolic compounds. Two-dimensional scatter plots of PC1 versus PC2 explained 98.9% of the variance (82.1% and 16.8%, respectively) (Figure 2A). The metabolites of the four genotypes helped to separate the four genotypes’ purple sweet potatoes into three distinct clusters, with their corresponding loadings provided in Supplementary Table S2. The loading plot (Figure 2B) revealed that the larger the distance between its point and its original point, the more a compound contributes to the total variation. Thus, the compound dicaffeoylquinic acid isomer 1 (diCQA 1), which was loaded negatively (r= −0.97) on PC1 and was the most distant from the original point, helped to separate the 16-283p genotype from the rest. Chlorogenic acid (components coefficient r = −0.75) and 5CQA (r = −0.61) were loaded negatively on PC2 and separated the genotypes Purple-purple and 16-283 from the other two genotypes. PLS-DA was utilised in this study to classify different genotypes based on their 16 metabolites. In total, 98.9% of the variation in bioactive compounds can be explained by the first two principal components (PC1 73.3% and PC2 25.6%) (Figure 2A). Three major clusters were identified based on the PLS-DA plot. Figure 2B shows the loading of different phenolic metabolites on PC1 and PC2, and the loading of the compounds is given in Supplementary Table S3. The compound diCQA 1 was loaded positively (r = 0.5) on PC2 and was distant from the original point, while 5CQA (r= 6.0) and chlorogenic acid (r= 0.63) were also loaded positively on PC2 and were able to separate the Purple-purple and 16-283 genotypes from the others. In addition to generating more accurate predictions, PLS-DA produces more meaningful models [49]. The PLS-DA model showed a high prediction level (Q2 = 0.97) as well as a high goodness-of-fit level (R2 = 0.82).
Figure 2.
(A) An unsupervised PCA score plot of phenolic metabolites generated by UPLC-QTOF/MS analysis showing the separation of three clusters. (B) Loading of phenolic metabolites in the PCA score plot. (C) A PLS-DA score plot showing four sweet potato cultivars clustered into three groups. (D) PLS-DA score plots loaded with different phenolic compounds detected by UPLC-QTOF-MS showing two clustered groups, (E). Metabolites are assigned VIP scores in PLS-DA. Variable importance is determined by the score they receive from low to high. Each metabolite’s relative concentration is shown in the coloured boxes on the right. Low blue levels indicate low levels, while high red levels indicate high levels. (F) Heat map. In the map, the various phenolic compounds found in different sweet potato cultivars are coloured according to their concentration. The rows represent phenolic compounds, and the columns represent the sweet potato genotype. The colours red and blue indicate high and low levels, respectively.
A variable importance in projection (VIP) score was used to evaluate the contribution of each metabolite to the separation of groups (Figure 2E). VIP scores are determined by summing the weighted PLS regression coefficients and the squares of the PLS loadings. Only the top metabolites with the highest VIP scores are considered for interpreting the results [50]. Among the top six metabolites with VIP scores >1 are chlorogenic acid 5CQA, chlorogenic acid 3CQA, and dicaffeoylquinic acid isomer 1diCQA. Diaffeoylquinic acid isomer 1 diCQA allowed us to distinguish the two groups.
In addition to the analysis, a heat map was created based on metabolite concentrations in all samples. Each row of phenolic compound data was represented by a colour block, with red boxes representing higher levels and blue boxes representing lower levels. Figure 2F displays the 16 identified metabolites from the two groups. In addition, the heat map represents the composition of phenolic metabolites in the storage roots of the four genotypes of purple sweet potato. According to the heat map, the concentration of peonidin feruloyl-sophoroside- glucoside, 3-O-caffeoyl-4-O-methylquinic acid (MCGA3), peonidin caffeoyl-sophoroside-glucoside, peonidin-caffeoyl-feruloyl-sophoriside-glucoside, chlorogenic acid 3CQA, chlorogenic acid, 5 CQA, peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside, and cyanidin-caffeoyl-feruloyl-sophoroside-glucoside were remarkably higher in purple genotypes. In contrast, cyanidin-caffeoyl-sophoroside-glucoside, quercetin 3,4’-diglucoside, chlorogenic acid (3CQA), dicaffeoylquinic acid isomer 1 (diCQA), dicaffeoylquinic acid isomer 3 (diCQA), 3,5-dicaffeoylquinic methyl ester, and (-)-3,5-dicaffeoylquinic methyl ester ((3,5-diCQA)) were found at higher concentrations in genotype 16-283P. Conversely, the ‘2019-1-1′ genotype showed a lower concentration of all phenolic metabolites.
3.6. Antioxidant Activities
Based on a genotype comparison, the Purple-purple and ‘16-283P’ genotypes had the highest antioxidant activities (FRAP, ABTS, and DPPH) (Table 7). In general, sweet potato roots with a purple colour had a higher antioxidant activity, which confirmed Ji et al.’s [51] findings.
Table 7.
Antioxidant activities of four different genotypes of purple-fleshed sweet potato storage roots.
| Genotype | FRAP TEAC mM/g |
ABTS IC50 Value mg/g | DPPH IC50 Value mg/g |
|---|---|---|---|
| ‘08-21P’ | 16.03 ± 0.83 b | 1.68 ± 0.07 b | 1.84 ± 0.07 c |
| ‘2019-1-1′ | 14.56 ± 2.43 b | 1.99 ± 0.11 b | 2.31 ± 0.15 d |
| Purple-purple | 21.23 ± 1.51 a | 1.50 ± 0.09 a | 1.41 ± 0.01 b |
| ‘16-283P’ | 21.19 ± 1.80 a | 1.21 ± 0.02 a | 1.05 ± 0.02 a |
| LSD | 2.97 | 0.60 | 0.28 |
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within the same column at (p < 0.001).
The discrepancy in antioxidant activities and total phenolic content may be explained by the amount of rainfall that each harvest season received. In the presence of water stress, secondary metabolites can be produced to protect against oxidative stress [52]. As a result of accumulating polyphenols and acylated anthocyanins with high antioxidant activity from the very beginning of their growth, sweet potato roots are protected from biotic and abiotic stress [53].
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within the same column at (p < 0.001).
Figure 3A shows a significant correlation between individual anthocyanins and antioxidant power (FRAP). Cyanidin-caffeoyl-sophoroside-glucoside (r = 0.80, p < 0.05)) had the strongest correlation with the FRAP value, followed by peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside (r = 0.76, p < 0.05), peonidin caffeoyl-sophoroside- glucoside (r = 0.77, p < 0.05), cyanidin-caffeoyl-feruloyl-sophoroside-glucoside (r = 0.76, p < 0.05), chlorogenic acid (r = 0.75, p < 0.05), MCGA3 (r = 0.71, p < 0.05), peonidin feruloyl-sophoroside-glucoside (r = 0.69), 5CQA (r = 0.67), and 3CQA (r = 0.68). diCQA 1 (r = 0.86) had the highest correlation with ABTS scavenging activity (Figure 3B), followed by 3,5-dicaffeoylquinic methyl ester (r = 0.85, p < 0.05), cyanidin-caffeoyl-sophoroside- glucoside (r = 0.80, p < 0.05), diCQA 3 (r = 0.66, p < 0.05), diCQA 2 (r = 0.63, p < 0.05), 3CQA (r = 0.61, p < 0.05), 5CQA (r = 0.58, p < 0.05), 1,3-diCQA (r = 0.57, p < 0.05), peonidin feruloyl-sophoroside-glucoside (r = 0.51, p < 0.05), and peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside (r = 0.50, p < 0.05). Whereas, the DPPH scavenging activity correlated strongly (Figure 3 C) with 5CQA (r = 0.80, p < 0.05), peonidin feruloyl-sophoroside-glucoside (r = 0.77), peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside (r = 0.79, p < 0.05), diCQA 1 (r = 0.77, p < 0.05), peonidin caffeoyl-sophoroside-glucoside (r = 0.76, p < 0.05), 3CQA (r = 0.75, p < 0.05), cyanidin-caffeoyl-feruloyl-sophoroside-glucoside (r = 0.75, p < 0.05), 3,5-Dicaffeoylquinic methyl ester (r = 0.73, p < 0.05), peonidin feruloyl-sophoroside-glucoside (r = 0.59, p < 0.05), and MCGA3 (r = 0.57, p < 0.05).
Figure 3.
Correlation between FRAP, ABTS, DPPH and individual bioactive compounds in five genotypes of purple-fleshed sweet potato storage roots. (A): FRAP, (B): ABTS, (C): DPPH.
Caffeoylquinic acids (chlorogenic acids, neochlorogenic acids, cryptochlorogenic acids, and isochlorogenic acids) are known to possess great antioxidant properties as free radical scavengers by donating protons or electrons by their phenolic hydroxyl groups [54]. Liao et al. [55] found a strong correlation between total anthocyanin content and antioxidant activity. Specifically, the phenolic hydroxyl group in the anthocyanin molecule plays a crucial role as a scavenger of reactive oxygen radicals. Anthocyanins have a greater antioxidant activity compared to acylated anthocyanins because the phenolic hydroxyl group in the anthocyanin structure plays a significant role in scavenging reactive oxygen radicals [56]. On the other hand, Azima et al. [57] explained that anthocyanins have been found to exhibit antioxidant activity through two primary mechanisms: the hydrogen atom donor mechanism and single electron transfer. In the hydrogen atom donor mechanism, a free radical R• removes hydrogen from the antioxidant (Ao+), which converts it into a more stable product. On the other hand, antioxidants (Ao+) donate electrons to free radicals in single electron transfer mechanisms, which reduce oxidised intermediates into stable forms. Acylated anthocyanins were more important for antioxidant activity in ABTS+ and FRAP activities than non-acylated forms [55].
3.7. Protein, Fat, Dietary Fibre
Table 8 shows significant differences in protein, fat, and dietary fibre composition between coloured sweet potato genotypes. The protein content of the sweet potato genotypes varied significantly, with the genotype Purple-purple exhibiting the highest value (28.81 ± 1.34) and the genotype 16-283P exhibiting the least value (19.12 ± 0.62) in Table 8. In addition, the total protein values (on a dry basis) were higher than those documented in the literature for sweet potatoes. Cartier et al. [58] reported a protein content of 4.07 ± 0.21–6.20 ± 0.17 g/100 g and Rodrigues et al. [59] (5.82 ± 1.43 g/100 g). Proteins play a vital role in biological activities like development and repair, depending on their bioaccessibility after digestion [58]. According to the current study, Purple-purple (28.81 g/100 g) and ‘08-21 P’ (24.31 g/100 g) are the sweet potato genotypes with the highest protein content for human nutrition in Sub-Saharan Africa. Therefore, sweet potato storage roots should be promoted and encouraged to maintain a balanced diet. A 100 g portion of Purple-purple would contribute 17.7% of a 65 kg adult male’s recommended daily protein allowance based on 0.8 g per day multiplied by weight. The protein content of the sweet potato roots of genotypes Purple-purple and ‘08-21P’ showed a higher composition than the Ethiopian genotypes G1 (Ukrewe × Ejumula-10) (7.43%), G5 (Ukrewe × Ogansagan-5) (7.29%), G6 (Resisto × Ejumula-7) (6.99%), G8 (Resisto × PIPI-2) (7.08%), and G17 (Resisto × Ogansagen-16) (6.44%), and G18 (Resisto × Ogansagen-16) (7.84%) showed the highest protein content [60].
Table 8.
Protein, fat, and dietary fibre composition of four different, purple-fleshed sweet potato storage root genotypes (DW).
| Genotypes | Fat g/100 g | Protein g/100 g | Total Dietary Fibre g/100 g |
|---|---|---|---|
| ‘08-21 P’ | 0.75 ± 0.01 b | 24.31 ± 1.25 b | 5.35 ± 0.06 a |
| ‘2019-1-1′ | 0.83 ± 0.01 a | 21.44 ± 1.31 c | 3.81 ± 0.01 b |
| Purple-purple | 0.47 ± 0.02 c | 28.81 ± 1.34 a | 2.51 ± 0.03 c |
| ‘16-283P’ | 0.76 ± 0.10 b | 19.12 ± 0.62 d | 2.86 ± 0.12 c |
| LSD | 0.02 | 0.34 | 0.78 |
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within a same column at (p < 0.001).
The fat content of the storage roots was the highest in 2019-1-1 (0.83 ± 0.01 g/100 g), with genotype Purple-purple showing the lowest content of 0.47 ± 0.02 g/100 g (Table 8). On the contrary, Mitiku and Teka, [61] reported that sweet potatoes contained 1.25–1.52 g/100 g of fat and Hossain et al. [30] reported ranges of between 0.73 ± 0.05 and 2.41± 0.02 g/100 g, which were significantly higher than the results from our current study. In Brazil, a purple genotype has been reported with a lower fat content between 0.42 ± 0.04 g/100 g and 0.39 ± 0.03 g/100 g [59]. The differences can be attributed to climatic growing conditions, soil type, and location differences. Considering the recommended daily allowance for fats, a 100 g portion of genotype ‘2019-1-1′ on a fresh weight basis would contribute 1% to the daily allowance for a 65 kg adult male. Genotype ‘2019-1-1′, based on its high fat content, might be marketed as a food flavouring.
The dietary fibre content of sweet potato genotypes differed significantly, with the genotype Purple-purple having the lowest (2.51 ± 0.03 g/100 g) amount and genotype ‘08-21P’ having the highest amount (5.35 ± 0.06 g/100 g), suggesting differences in digestibility. In China [51], these values were higher, but in Brazil they were lower [32]. Soluble fibre, approximately half of the total, varied across genotypes, with ‘08-21P’ having the highest (2.48 ± 0.03 g/100 g) and ‘16-283P’ the lowest (0.45 g/100 g). The insoluble fibre was significantly higher, with ‘08-21P’ having the highest (2.87 ± 0.10 g/100 g) and ‘16-283P’ the lowest (1.95 ± 0.23 g/100 g). Soluble fibres affect digestive system transit time and glucose absorption [62], while insoluble fibres impact intestinal transit and faecal characteristics [62]. Incorporating a variety of fibre-rich foods into the diet is essential due to their distinct qualities.
3.8. Fe, Zn and K Content
Fe and Zn deficiencies are estimated to be 5 and 40% in Sub-Saharan Africa, respectively [63]. Sweet potato roots of the genotypes ‘2019-1-1’ and Purple-purple (Table 9) measured similar Fe levels as the Ethiopian genotypes G8 (2.55 mg per 100 g), G17 (2.16 mg per 100 g), and G19 (2.20 mg per 100 g). Iron (Fe) content ranged from 1.85 to 2.77 mg/100 g in purple sweet potato genotypes, higher than those in Benin (0.53 to 0.73 mg/100 g) [64] and orange-and-cream-flesh genotypes from South Africa (0.73 to 1.26 mg/100 g) [12]. The significant differences in Fe content may be attributed to genotype variation, as all samples were collected from the same experimental farm. Fe deficiency is linked to common micronutrient deficits such as anaemia, particularly in children, as well as vitamin A insufficiency. A 13.65 g portion of 2019-1-1 on a fresh weight basis would meet the Recommended Dietary Allowance (RDA) for iron for men, while 25.59 g for children and 30.72 g for women would meet their respective RDAs. The zinc (Zn) content ranged between 0.95 and 1.42 mg/100 g, with genotype ‘2019-1-1′ (1.42 ± 0.20 mg/100 g), ‘16-283P’ (1.32 ± 0.10 mg/100 g), and Purple-purple (1.3 ± 0.13 mg/100 g) (Table 9) exhibiting the highest composition. Nevertheless, Sanoussi et al. [64] reported a lower Zn content (0.27 mg/100 g). The trace element Zn is essential for protein synthesis, immunity, and gene expression [65]. The sweet potato root of genotype 2019-1-1 will meet the daily recommended allowance of Zn for children at 31.43 g and 22.86 g for adults. Potassium (K) content ranged from 13.3 to 22.2 mg/100 g, with cultivar 2019-1-1 having the highest K content at 22.20 mg/100 g. Hossain et al. [30] reported a range between 8.8 and 12.4 mg/100 g, which is lower than the genotypes studied here. Sanoussi et al. [64] and Senthilkumar et al. [66] reported higher levels (308.67 to 328.67 mg/100 g). K plays a role in controlling water balance, neurotransmission, and heart rate [64]. However, the 100 g sweet potato 2019-1-1 studied would contribute only 0.21% of the recommended daily allowance for adults and 0.24% of the recommended daily allowance for children.
Table 9.
Iron, zinc, and potassium composition of four different, purple-fleshed sweet potato storage root genotypes.
| K (mg/100 g) | Fe mg/100 g | Zn mg/100 g | |
|---|---|---|---|
| ‘08-21P’ | 17.2 ± 0.11 bc | 2.08 ± 0.05 d | 0.95 ± 0.09 c |
| ‘2019-1-1′ | 22.2 ± 0.06 a | 2.77 ± 0.06 a | 1.42 ± 0.20 a |
| Purple-purple | 16.9 ± 0.04 bc | 2.17 ± 0.08 c | 1.3 ± 0.13 a |
| ‘16-283P’ | 16.8 ± 0.11 c | 2.60 ± 0.13 b | 1.32 ± 0.10 a |
| CV% | 5.2 | 3.18 | 10.23 |
| LSD | 0.17 | 0.20 | 0.24 |
Data present mean and standard deviation (n = 3) and Tukey’s HSD. Significant variances are indicated by distinct letters within a same column at (p < 0.001). Key: Fe: Iron, K: Potassium, Zn: Zinc.
4. Conclusions
Identifying the genotypes of sweet potatoes with the highest concentration of nutritional and bioactive components, the current study determined the nutrition and bioactive components of purple sweet potato roots in the Southern African region. Additionally, purple sweet potato genotypes could provide genetic resources for producing biofortified purple-fleshed sweet potato genotypes to compensate for macronutrient Fe and Zn deficiencies. The study found that the phenolic compounds in the roots of four sweet potato genotypes were almost indistinguishable, but their compositions varied. A study identified the local purple sweet potato genotype Purple-purple as a potential source of antioxidants and protein, and ‘2019-1-1’ for the dietary minerals Fe and Zn. Furthermore, it would be ideal to recommend purple sweet potato genotypes available in the Southern African region for consumption. Moreover, these genotypes must be tested in different environments to ensure stable yields.
Acknowledgments
The authors thankfully acknowledge the SARChI Research Chair grant for the Phytochemical Food Network to Improve Nutritional Quality for Consumers, supported by the NRF (National Research Foundation).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13030338/s1, Figure S1: MS chromatogram of sweet potato roots with identified phenolic compounds. Figure S2: MS/MS chromatogram overlaid on the MS and UV chromatograms of sweet potato roots with identified anthocyanins. Figure S3: Illustrating the increase/decrease of phenolic acids in the different genotypes of sweet potato roots. Figure S4: MS spectrum of 3CQA overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S5: MS spectrum of 5CQA overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S6: MS spectrum of quercetin 3,4’-diglucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S7: MS spectrum of 3-O-caffeoyl-4-O-methylquinic acid overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S8: Showing the MS spectrum of 1,3-diCQA overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S9: MS spectrum of diCQA 1 overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S10: Showing the MS spectrum of 4,5-diCQA overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S11: Showing the MS spectrum of 3,5-dicaffeoylquinic methyl ester overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S12: MS spectrum of cyanidin-caffeoyl-sophoroside-glucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S13: MS spectrum of peonidin feruloyl-sophoroside-glucoside overlaid on its MS/MS spectrum adjacent to its chemical structure. Figure S14: MS spectrum of peonidin caffeoyl-sophoroside-glucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S15: MS spectrum of cyanidin-caffeoyl-feruloyl-sophoroside-glucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S16: MS spectrum of peonidin-caffeoyl-hydroxybenzoyl-sophoroside-glucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Figure S17: MS spectrum of peonidin caffeoyl-feruloyl-sophoroside-glucoside overlaid on its MS/MS and UV spectra adjacent to its chemical structure. Table S1: Regression equations, retention time, LOD, and LOQ for anthocyanins and phenolic compounds quantified using TargetLynx. Table S2: Principal component analysis (PCA) showing the loadings of phenolic metabolites on PC1 and PC2. Table S3: Partial least squares–discriminant analysis (PLS-DA) showing the loadings of phenolic metabolites on PC1 and PC2.
Author Contributions
A.N.; investigation, writing—original draft preparation. F.S.; methodology and visualisation. S.M.M.; methodology, software, validation, chemometric analysis. L.M.S.; conceptualisation, project support, cultivation, and supervision. D.S.; resources, conceptualisation, data curation, writing—review and editing, visualisation, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by NRF (National Research Foundation) grant number 98352 for the Phytochemical Food Network.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Behera S., Chauhan V.B.S., Pati K., Bansode V., Nedunchezhiyan M., Verma A.K., Naik S.K. Biology and biotechnological aspect of sweet potato (Ipomoea batatas L.): A commercially important tuber crop. Planta. 2022;256:40. doi: 10.1007/s00425-022-03938-8. [DOI] [PubMed] [Google Scholar]
- 2.Pereira G.S., Amankwaah V.A., Ketavi M., Oloka B.M., Nair A.G., da Mata A.P., Campos H. Compendium of Crop Genome Designing for Nutraceuticals. Springer Nature; Singapore: 2023. Sweet potato: Nutritional constituents and genetic composition; pp. 1–43. [DOI] [Google Scholar]
- 3.Hayati M., Anhar A. Morphological characteristics and yields of several sweet potato (Ipomoea batatas L.) tubers. IOP Conf. Ser. Earth Environ. Sci. 2020;425:012055. doi: 10.1088/1755-1315/425/1/012055. [DOI] [Google Scholar]
- 4.Textor C. Sweet Potatoes Production in China, 2012–2022. The Statistic Shows the Extent of Sweet Potato Production in China from 2012 to 2022. [(accessed on 27 February 2024)]. Available online: https://www.statista.com/statistics/697819/china-sweet-potatoes-production/#:~:text=The%20statistic%20shows%20the%20extent,potatoes%20were%20produced%20in%20China.
- 5.South Africa—Sweet Potato—Market Analysis, Forecast, Size, Trends and Insights Please mention the Source. [(accessed on 27 February 2024)]. Available online: https://www.indexbox.io/search/production-sweet-potato-south-africa/
- 6.FAOSTAT. Food and Agriculture Organization of the United Nations-Statistic Division, 2019. [(accessed on 9 February 2024)]. Available online: https://www.fao.org/faostat/en/#data.
- 7.Oke M.O., Workneh T.S. A review on sweet potato postharvest processing and preservation technology. Afr. J. Agric. Res. 2013;8:4990–5003. doi: 10.5897/AJAR2013.6841. [DOI] [Google Scholar]
- 8.Prakash P., Prabhat Kishore D., Jaganathan S.I., Sivakumar P.S. The Status, Performance and Impact of Sweet Potato Cultivation on Farming Communities of Odisha, India. AgEcon; St. Paul, MN, USA: 2018. [DOI] [Google Scholar]
- 9.Tumwegamire S., Kapinga R., Rubaihayo P.R., LaBonte D.R., Grüneberg W.J., Burgos G., Mwanga R.O. Evaluation of dry matter, protein, starch, sucrose, β-carotene, iron, zinc, calcium, and magnesium in East African sweetpotato [Ipomoea batatas (L.) Lam] germplasm. HortScience. 2011;46:348–357. doi: 10.21273/HORTSCI.46.3.348. [DOI] [Google Scholar]
- 10.Laurie S.M., Mulabisana J., Sutherland R., Sivakumar D., Pofu K., Mphela W.M., Bairu M.W. Seventy years of sweet potato [Ipomoea batatas L. Lam] research in South Africa. Crop Sci. 2023 doi: 10.1002/csc2.21097. [DOI] [Google Scholar]
- 11.Phahlane C.J., Laurie S.M., Shoko T., Manhivi V.E., Sivakumar D. Comparison of caffeoylquinic acids and functional properties of domestic sweet potato (Ipomoea batatas (L.) Lam.) storage roots with established overseas varieties. Foods. 2022;11:1329. doi: 10.3390/foods11091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurie S.M., Booyse M., Labuschagne M.T., Greyling M.M. Multienvironment performance of new orange-fleshed sweetpotato cultivars in South Africa. Crop Sci. 2015;55:1585–1595. doi: 10.2135/cropsci2014.09.0664. [DOI] [Google Scholar]
- 13.Laurie S.M., Tjale S.S., van den Berg A.A., Mtileni M.M., Labuschagne M.T. Agronomic performance of new cream to yellow-orange sweetpotato cultivars in diverse environments across South Africa. S. Afr. J. Plant Soil. 2015;32:147–155. doi: 10.1080/02571862.2015.1014436. [DOI] [Google Scholar]
- 14.Parker T., Leach K., Stoddard C.S., Roser L., Palkovic A., Williams T., Brummer E.C. Opportunities to Breed Diverse Sweetpotato Varieties for California Organic Production. Agriculture. 2023;13:2191. doi: 10.3390/agriculture13122191. [DOI] [Google Scholar]
- 15.Ginting E., Yulifianti R., Indriani F.C. Selected purple-fleshed sweet potato genotypes with high anthocyanin contents. IOP Conf. Ser. Earth Environ. Sci. 2020;456:012023. doi: 10.1088/1755-1315/456/1/012023. [DOI] [Google Scholar]
- 16.De Albuquerque T.M.R., Sampaio K.B., de Souza E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds—A review. Trends Food Sci. Technol. 2018;85:277–286. doi: 10.1016/j.tifs.2018.11.006. [DOI] [Google Scholar]
- 17.Laveriano-Santos E.P., López-Yerena A., Jaime-Rodríguez C., González-Coria J., Lamuela-Raventós R.M., Vallverdú-Queralt A., Pérez M. Sweet potato is not simply an abundant food crop: A comprehensive review of its phytochemical constituents, biological activities, and the effects of processing. Antioxidants. 2022;11:1648. doi: 10.3390/antiox11091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghasemzadeh A., Talei D., Jaafar H.Z., Juraimi A.S., Mohamed M.T.M., Puteh A., Halim M.R.A. Plant-growth regulators alter phytochemical constituents and pharmaceutical quality in Sweet potato (Ipomoea batatas L.) BMC Complement. Altern. Med. 2016;16:152. doi: 10.1186/s12906-016-1113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwiyanti G., Siswaningsih W., Febrianti A. Production of purple sweet potato (Ipomoea batatas L.) juice having high anthocyanin content and antioxidant activity. J. Phys. Conf. Ser. 2018;1013:012194. doi: 10.1088/1742-6596/1013/1/012194. [DOI] [Google Scholar]
- 20.Bennett A.A., Mahood E.H., Fan K., Moghe G.D. Untargeted metabolomics of purple and orange-fleshed sweet potatoes reveals a large structural diversity of anthocyanins and flavonoids. Sci. Rep. 2021;11:16408. doi: 10.1038/s41598-021-95901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selokela L.M., Laurie S.M., Sivakumar D. Impact of different postharvest thermal processes on changes in antioxidant constituents, activity and nutritional compounds in sweet potato with varying flesh colour. S. Afr. J. Bot. 2022;144:380–388. doi: 10.1016/j.sajb.2021.09.009. [DOI] [Google Scholar]
- 22.Michael Jackson D., Harrison H.F., Jarret R.L., Wadl P.A. Color analysis of storage roots from the USDA, ARS sweetpotato (Ipomoea batatas) germplasm collection. Gene Res. Crop Evol. 2018;65:1217–1236. doi: 10.1007/s10722-018-0609-6. [DOI] [Google Scholar]
- 23.Laurie S.M., Mphela W.M. Consumer acceptability of fried chips made from South African sweet potato cultivars. S. Afr. J. Plant Soil. 2022;39:290–298. doi: 10.1080/02571862.2022.2103192. [DOI] [Google Scholar]
- 24.Hong J., Mu T., Sun H., Richel A., Bleck C. Valorization of the green waste parts from sweet potato (Impoea batatas L.): Nutritional, phytochemical composition, and bioactivity evaluation. Food Sci. Nutr. 2020;8:4086–4097. doi: 10.1002/fsn3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suárez S., Mu T., Sun H., Añón M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020;23:178–188. doi: 10.1080/10942912.2020.1716796. [DOI] [Google Scholar]
- 26.Seke F., Manhivi V.E., Shoko T., Slabbert R.M., Sultanbawa Y., Sivakumar D. Effect of freeze drying and simulated gastrointestinal digestion on phenolic metabolites and antioxidant property of the Natal plum (Carissa macrocarpa) Foods. 2021;10:1420. doi: 10.3390/foods10061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Managa M.G., Sultanbawa Y., Sivakumar D. Effects of different drying methods on untargeted phenolic metabolites, and antioxidant activity in Chinese cabbage (Brassica rapa L. subsp. chinensis) and nightshade (Solanum retroflexum Dun.) Molecules. 2020;25:1326. doi: 10.3390/molecules25061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ndou A., Tinyani P.P., Slabbert R.M., Sultanbawa Y., Sivakumar D. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stages. Food Chem. 2019;293:499–510. doi: 10.1016/j.foodchem.2019.04.102. [DOI] [PubMed] [Google Scholar]
- 29.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis of the Association of Analytical Chemists International. Association of Official Analytical Chemists; Bray, Ireland: 2017. [Google Scholar]
- 30.Hossain M.M., Rahim M.A., Moutosi H.N., Das L. Evaluation of the growth, storage root yield, proximate composition, and mineral content of colored sweet potato genotypes. J. Agric. Food Res. 2022;8:100289. doi: 10.1016/j.jafr.2022.100289. [DOI] [Google Scholar]
- 31.Gajanayake B., Reddy K.R., Shankle M.W., Arancibia R.A., Villordon A.O. Quantifying storage root initiation, growth, and developmental responses of sweetpotato to early season temperature. Agron. J. 2014;106:1795–1804. doi: 10.2134/agronj14.0067. [DOI] [Google Scholar]
- 32.Leite C.E.C., Souza B.D.K.F., Manfio C.E., Wamser G.H., Alves D.P., de Francisco A. Sweet potato new varieties screening based on morphology, pulp color, proximal composition, and total dietary fiber content via factor analysis and principal component analysis. Front. Plant Sci. 2022;13:852709. doi: 10.3389/fpls.2022.852709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa S., Setoguchi Y., Ohmura R., Toshima S., Park H., Narasako Y., Kunitake H. Effects of cross combination on the total content and its composition of anthocyanins in sweetpotato (Ipomoea batatas L.) Sci. Hort. 2022;299:110999. doi: 10.1016/j.scienta.2022.110999. [DOI] [Google Scholar]
- 34.Im Y.R., Kim I., Lee J. Phenolic composition and antioxidant activity of purple sweet potato (Ipomoea batatas (L.) Lam.): Varietal comparisons and physical distribution. Antioxidants. 2021;10:462. doi: 10.3390/antiox10030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franková H., Musilová J., Árvay J., Harangozo L., Šnirc M., Vollmannová A., Jaško E. Variability of bioactive substances in potatoes (Solanum tuberosum L.) depending on variety and maturity. Agronomy. 2022;12:1454. doi: 10.3390/agronomy12061454. [DOI] [Google Scholar]
- 36.Terahara N., Shimizu T., Kato Y., Nakamura M., Maitani T., Yamaguchi M.A., Goda Y. Six diacylated anthocyanins from the storage roots of purple sweet potato, Ipomoea batatas. Biosci. Biotech. Biochem. 1991;63:1420–1424. doi: 10.1271/bbb.63.1420. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Su X., Lim S., Griffin J., Carey E., Katz B., Tomich J., Smith J.S., Wang W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015;186:90–96. doi: 10.1016/j.foodchem.2014.08.123. [DOI] [PubMed] [Google Scholar]
- 38.Montilla E.C., Hillebrand S., Butschbach D., Baldermann S., Watanabe N., Winterhalter P. Preparative isolation of anthocyanins from Japanese purple sweet potato (Ipomoea batatas L.) varieties by high-speed countercurrent chromatography, 2010. J. Agric. Food Chem. 2010;58:9899–9904. doi: 10.1021/jf101898j. [DOI] [PubMed] [Google Scholar]
- 39.Islam M.S., Yoshimoto M., Terahara N., Yamakawa O. Anthocyanin compositions in sweetpotato (Ipomoea batatas L.) leaves. Biosci. Biotech. Biochem. 2002;66:2483–2486. doi: 10.1271/bbb.66.2483. [DOI] [PubMed] [Google Scholar]
- 40.Kurnianingsih N., Ratnawati R., Nazwar T.A., Ali M., Fatchiyah F. Purple sweet potatoes from East Java of Indonesia revealed the macronutrient, anthocyanin compound and antidepressant activity candidate. Med. Arch. 2021;75:94. doi: 10.5455/medarh.2021.75.94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamora-Ros R., Knaze V., Lujan-Barroso L., Slimani N., Romieu I., Touillaud M., Gonzalez C.A. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011;106:1090–1099. doi: 10.1017/S0007114511001437. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Alonso M., Minihane A.M., Rimbach G., Rivas-Gonzalo J.C., de Pascual-Teresa S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma, 2009. J. Nutr. Biochem. 2009;20:521–529. doi: 10.1016/j.jnutbio.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Chen C.C., Lin C., Chen M.H., Chiang P.Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods. 2019;8:393. doi: 10.3390/foods8090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishiguro K., Yahara S., Yoshimoto M. Changes in Polyphenolic Content and Radical-Scavenging Activity of Sweetpotato (Ipomoea batatas L.) during Storage at Optimal and Low Temperatures. J. Agric. Food Chem. 2007;55:10773–10778. doi: 10.1021/jf072256v. [DOI] [PubMed] [Google Scholar]
- 45.Padda M.S., Picha D.H. Quantification of phenolic acids and antioxidant activity in sweetpotato genotypes. Sci. Hortic. 2008;119:17–20. doi: 10.1016/j.scienta.2008.07.008. [DOI] [Google Scholar]
- 46.Nicoletto C., Vianello F., Sambo P. Effect of different home-cooking methods on textural and nutritional properties of sweet potato genotypes grown in temperate climate conditions. J. Sci. Food Agric. 2018;98:574–581. doi: 10.1002/jsfa.8499. [DOI] [PubMed] [Google Scholar]
- 47.Yi W., Akoh C.C., Fischer J., Krewer G. Absorption of anthocyanins from blueberry extracts by caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006;54:5651–5658. doi: 10.1021/jf0531959. [DOI] [PubMed] [Google Scholar]
- 48.Makori S.I., Mu T.H., Sun H.N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Comm. 2020;15:1934578X20936931. doi: 10.1177/1934578X20936931. [DOI] [Google Scholar]
- 49.Lee L.C., Liong C.Y., Jemain A.A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst. 2018;143:3526–3539. doi: 10.1039/C8AN00599K. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho F.V., Santana L.F., da Silva V.D.A., Costa S.L., Zambotti-Villelae L., Colepicolo P., Ribeiro P.R. Combination of a multiplatform metabolite profiling approach and chemometrics as a powerful strategy to identify bioactive metabolites in Lepidium meyenii (Peruvian maca) Food Chem. 2021;364:130453. doi: 10.1016/j.foodchem.2021.130453. [DOI] [PubMed] [Google Scholar]
- 51.Ji H., Zhang H., Li H., Li Y. Analysis on the Nutrition Composition and Antioxidant Activity of Different Types of Sweet Potato Cultivars. Food Nutr. Sci. 2015;6:161–167. doi: 10.4236/fns.2015.61017. [DOI] [Google Scholar]
- 52.Rautenbach F., Faber M., Laurie S., Laurie R. Antioxidant capacity and antioxidant content in roots of 4 sweetpotato varieties, 2010. J. Food Sci. 2010;75:C400–C405. doi: 10.1111/j.1750-3841.2010.01631.x. [DOI] [PubMed] [Google Scholar]
- 53.Setoguchi Y., Nakagawa S., Ohmura R., Toshima S., Park H., Narasako Y., Kunitake H. Effect of Growth Stages on Anthocyanins and Polyphenols in the Root System of Sweet Potato. Plants. 2023;12:1907. doi: 10.3390/plants12091907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., XiaoHui Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharm. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 55.Liao M., Zou B., Chen J., Yao Z., Huang L., Luo Z., Wang Z. Effect of domestic cooking methods on the anthocyanins and antioxidant activity of deeply purple-fleshed sweetpotato GZ9. Heliyon. 2019;5:e01515. doi: 10.1016/j.heliyon.2019.e01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motohashi N., Sakagami H. Bioactive Heterocycles VI: Flavonoids and Anthocyanins in Plants, and Latest Bioactive Heterocycles I. Springer; Berlin/Heidelberg, Germany: 2008. Functionality of anthocyanins as alternative medicine; pp. 1–48. [DOI] [Google Scholar]
- 57.Siti Azima A.M., Noriham A., Manshoor N. Anthocyanin content in relation to the antioxidant activity and colour properties of Garcinia mangostana peel, Syzigium cumini and Clitoria ternatea extracts. Int. Food Res. J. 2014;21:2369–2375. [Google Scholar]
- 58.Cartier A., Woods J., Sismour E., Allen J., Ford E., Githinji L., Xu Y. Physiochemical, nutritional and antioxidant properties of fourteen Virginia-grown sweet potato varieties. J. Food Meas. Charact. 2017;11:1333–1341. doi: 10.1007/s11694-017-9511-8. [DOI] [Google Scholar]
- 59.Rodrigues N.D.R., Barbosa Junior J.L., Barbosa M.I.M.J. Determination of physico-chemical composition, nutritional facts and technological quality of organic orange and purple-fleshed sweet potatoes and its flours. Int. Food Res. J. 2016;23:74–98. doi: 10.1080/15428052.2022.2029658. [DOI] [Google Scholar]
- 60.Gurmu F., Shimelis H., Laing M., Mashilo J. Genotype-by-environment interaction analysis of nutritional composition in newly-developed sweetpotato clones. J. Food Comp. Analy. 2020;88:103426. doi: 10.1016/j.jfca.2020.103426. [DOI] [Google Scholar]
- 61.Mitiku D.H., Teka T.A. Nutrient and antinutrient composition of improved sweet potato [Ipomea batatas (L.) Lam] varieties grown in eastern Ethiopia. Nutr. Food Sci. 2017;47:369–380. doi: 10.1108/NFS-07-2016-0098. [DOI] [Google Scholar]
- 62.Gill S.K., Rossi M., Bajka B., Whelan K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 63.Mudgil D. Dietary Fiber for the Prevention of Cardiovascular Disease. Academic Press; Cambridge, MA, USA: 2017. The interaction between insoluble and soluble fiber; pp. 35–59. [DOI] [Google Scholar]
- 64.Sanoussi A.F., Adjatin A., Dansi A., Adebowale A., Sanni L.O., Sanni A. Mineral composition of ten elites’ sweet potato (Ipomoea Batatas L. Lam) landraces of Benin. Int. J. Curr. Microbiol. Appl. Sci. 2016;5:103–115. doi: 10.20546/ijcmas.2016.501.009. [DOI] [Google Scholar]
- 65.Li J., Cao D., Huang Y., Chen B., Chen Z., Wang R., Liu L. Zinc intakes and health outcomes: An umbrella review. Front. Nutr. 2022;9:798078. doi: 10.3389/fnut.2022.798078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senthilkumar R., Muragod P.P., Muruli N.V. Nutrient analysis of sweet potato and its health benefits. Ind. J. Pure Appl. Biosci. 2020;8:614–618. doi: 10.18782/2582-2845.7933. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.