Abstract
Chemokines are cytokines with chemoattractant capacities that exert their physiological functions through the binding of chemokine receptors. Thus, chemokine and receptor complexes exert important roles in regulating development and homeostasis during routine immune surveillance and inflammation. Compared to mammals, the physiology and structure of chemokine receptors in fish have not been systematically studied. Furthermore, the salmonid-specific whole genome duplication has significantly increased the number of functional paralogs of chemokine receptors. In this context, in the current study, trout exhibited 17 cxcr genes, including 12 newly identified and 5 previously identified receptors. Interestingly, gene expression of brain cxcr1 and cxcr4, kidney cxcr3 and cxcr4, and spleen cxcr3, cxcr4, and cxcr5 subtypes were altered by bacterial infection, whereas brain cxcr1, kidney cxcr1 and cxcr7, and liver cxcr2, cxcr3, and cxcr4 subtypes were changed in response to environmental changes. Based on protein structures predicted by ColabFold, the conserved amino acids in binding pockets between trout CXCR4.1 subtypes and human CXCR4 were also analyzed. Our study is valuable from a comparative point of view, providing new insights into the identification and physiology of salmonid chemokine receptors.
Keywords: rainbow trout, chemokine receptors, bacterial infection, environmental changes
1. Introduction
Chemokines are small (8–15 kDa) proteins belonging to the cytokine family [1]. Chemokines bind to G protein-coupled receptors (GPCRs), and complexes of chemokine and receptor regulate cell movement and activation [2]. Based on the number and position of highly conserved N-terminal cysteines, chemokines are divided into four groups: CXC, CC, C, and CX3C (C indicates cysteine, and X/X3 indicates one or three non-cysteine amino acids) [1,2]. For example, CCL2 represents a chemokine ligand of the CC subfamily, number 2, and CCR2 represents the receptor of CCL2 [2,3]. It is well known that the physiological function of chemokines is to modulate cell migration, which gives them their name (from ‘chemotactic cytokines’) [4]. Chemokines play an important role in regulating cellular migration during routine immune surveillance, inflammation, and development [2]. Based on their physiological functions, chemokines can also be divided into two groups: inflammatory and homeostatic chemokines [5]. Inflammatory chemokines are induced directly by inflammatory stimuli or related cells [4,5]. Homeostatic chemokines, on the other hand, are involved in cell migration, organogenesis, and development, and they are constitutively expressed in discrete tissues or cells [4,5].
Chemokine receptors have also been divided into four groups: CXC, CC, C, and CX3C chemokine receptors, which are consistent with the four groups of chemokines [4]. Although a large number of chemokines have been identified, the number of chemokine receptors is lower [2,6,7]. For example, the human chemokine superfamily currently contains ~46 chemokines, and these chemokines bind to 18 chemokine receptors (six CXCRs, ten CCRs, one XCR, one CX3CR) [4]. Members of the chemokine superfamily (including ligands and receptors) have been identified in chicken, zebrafish, shark, and jawless fish [4].
Compared to mammals, teleosts exhibit increased gene copies of many immune genes, including chemokines, as a result of the teleost-specific whole genome duplication (which is also referred to as the third round of genome duplication (3R)) [8,9]. Hence, in 1998, the first teleost chemokine gene was identified in salmonids, and since then, a great number of chemokine orthologues, with a great complexity in physiology, have been identified in teleost, possibly also because chemokines are thought to evolve faster than other genes associated with immunomodulation [10,11,12]. On the other hand, previous studies in model animals showed that the CC chemokine receptor family contains at least 17 members in zebrafish (Danio rerio) and 10 members in medaka (Oryzias latipes) [13,14]. In aquacultured fish, 23 CC and 8 CXC chemokine receptors have been identified in channel catfish (Ictalurus punctatus) and 19 CC and 8 CXC chemokine receptors in orange-spotted grouper (Epinephelus coioides) [15,16]. Considering that chemokine receptors also influence how chemokines regulate immune development, homeostasis, and competence, the identification of the complete repertoire of chemokine receptors in teleost and the assessment of their functions will provide insights into the functionality of chemokines from a comparative point of view, thus contributing to boosting the immune response of fish for a sustainable development of aquaculture.
Rainbow trout (Oncorhynchus mykiss) belongs to the salmonid family and is one of the most studied teleost species, having been extensively used as a model in diverse research fields, including ecology, physiology, toxicology, immunology, and microbiology [17,18,19]. Rainbow trout is also an economically important aquacultured species with a global production of ~1,000,000 tons (FAO, 2022). In the trout industry, diseases caused by pathogen infection are of major ecological and commercial relevance to aquaculture. On the other hand, aquaculture and other anthropogenic activities might result in short-term and long-term changes in the natural aquatic environment. These environmental changes could provoke an important impact on fish physiology, including the immune system, thus having consequences on their well-being and disease resistance [20,21]. Interestingly, an additional round of whole genome duplication occurred in salmonid ancestors (which is referred to as the fourth round of genome duplication (4R) or salmonid-specific whole genome duplication) [18,22,23,24,25]. Duplicated copies of functional genes have been retained after this additional salmonid-specific whole genome duplication when compared to species that have only experienced the teleost-specific whole genome duplication [26,27,28]. These paralogs exhibit differences in sequence, transcription, and function [22,28,29,30].
In this context, the first aim of this study was to identify the complete repertoire of full-length CXC chemokine receptor genes in rainbow trout by exploiting the whole genomic data. We investigated the basal expressions of CXC chemokine receptors, as well as how they were transcriptionally regulated in response to Vibrio anguillarum and Aeromonas salmonicida infection. Vibrio anguillarum and Aeromonas salmonicida are two major pathogens that cause severe fatal diseases and considerable economic losses in cultured rainbow trout [31,32,33]. Environmental changes also impact fish immune systems by deregulating chemokine signaling in teleost [34,35], with triploid trout exerting different biochemistry and physiology when compared to diploid trout [36,37]. Therefore, we also investigated the transcriptional profiles of these CXC chemokine receptors in responses to environment changes in rainbow trout.
2. Materials and Methods
2.1. Ethics Statement
Our experiments were approved by the Institutional Review Board at Ocean University of China (permit number: 20141201) and performed in accordance with the U.K. Animal Scientific Procedures (Act, 1986) and associated guidelines, the EU Directive 2010/63/EU for animal experiments and the National Institutes of Health Guide for the Care and Use of Laboratory Animals use of laboratory animals (NIH Publications No. 8023, revised 1978). This study did not involve endangered or protected animals.
2.2. Genome-Wide Identification and Sequence Analyses
To identify the CXCR genes of rainbow trout, we searched the whole genome of rainbow trout obtained from NCBI (http://www.ncbi.nlm.nih.gov/, accessed on 24 January 2024) and performed tblastn analysis using all available CXCR sequences in the genome databases of human (Homo sapiens), mouse (Mus musculus), zebrafish (Danio rerio), Atlantic salmon (Salmo salar), fugu (Takifugu rubripes), Northern pike (Esox lucius), and channel catfish (Ictalurus punctatus) available in the NCBI (http://www.ncbi.nlm.nih.gov/), Ensembl (http://www.ensembl.org, accessed on 24 January 2024), and Uniport (http://www.uniprot.org/, accessed on 24 January 2024) as queries with e-values of 1 × 10−5. To remove redundant sequences, we used ClustalW for multiple alignments. Tandem arrangement genes were identified by their locations in the reference genome. The coding sequences were predicted using ORF (opening reading frames) finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html, accessed on 24 January 2024), which were further validated by BLASTP against NCBI nonredundant (nr) protein database. In addition, we used the online ProtParam tool to characterize the molecular weight (MW) and theoretical isoelectric point (pI).
Based on the amino acid sequences of CXC chemokine receptors of humans, mice, zebrafish, Atlantic salmon, medaka, fugu, Northern pike, and channel catfish, a phylogenetic analysis was conducted with MEGA 7, using the neighbor-joining method, with a set of 1000 bootstrap replicates [38].
2.3. Gene Structure, Conserved Domains, and Motif Analysis of the CXCR
Gene exon–intron structures were analyzed using the Gene Structure Display Server (GSDS2.0) by comparing the codon sequences and genomic sequences of the 17 CXCR members. The transmembrane (TM) domains were predicted by the TMHMM Server v. 2.0 (http://services.healthtech.dtu.dk/service.php?TMHMM-2.0, accessed on 24 January 2024), comparing the results of previous studies in human and zebrafish GPCRs. Motif analyses were performed with Multiple EM for Motif Elicitation (MEME, version 4.11.4), with the limitation of ten motifs and optimum widths of motifs of 6–50 amino acids [39].
2.4. Expression Analysis Using Available RNA-Seq Datasets
Using our available RNA-Seq datasets, we analyzed the cxcr expression levels of rainbow trout in response to bacterial infection (phenotype/timeline-specific expressions). The RNA-Seq datasets were retrieved from our previous studies described above:
Brain, kidney, and spleen samples from rainbow trout challenged with Vibrio anguillarum (SRA ID: PRJNA667799 [40,41,42]). Brain, kidney, and spleen samples were collected from control, asymptomatic, and symptomatic rainbow trout after V. anguillarum challenge, and 27 libraries of RNA-Seq samples were used (3 phenotypes × 3 tissues × 3 replicates [40,42]).
Brain and kidney samples from rainbow trout were challenged with Aeromonas salmonicida ([43]). Brain and kidney samples were collected from control and infected rainbow trout, and the RNA-Seq dataset included 12 libraries (2 (control vs. infection) × 2 tissues × 3 replicates [43]).
Brain, kidney, and liver samples from rainbow trout with environmental salinity changes ([44]). Diploid and triploid trout were classified into diploid trout in freshwater (DF), diploid trout in saltwater (DS, at salinity of 15 parts-per-thousand (ppt)), triploid trout in freshwater (TF), and triploid trout in saltwater (TS, at salinity of 15 ppt). Brain, liver, and kidney samples were collected from DS, TS, and TF. Twenty-seven libraries of RNA-Seq samples were used (3 groups (TF, DF, DS) × 3 tissues × 3 replicates [44]).
Liver samples from rainbow trout cultured in different stocking densities (unpublished data and count data are shown in Supplementary Materials). Rainbow trout were cultured in saltwater with initial densities at 9.15 kg/m3 (low density (LD)), 13.65 kg/m3 (moderate density (MD)), and 27.31 kg/m3 (high density (HD)) for 84 days. The final densities were 22.00 (LD), 32.05 (MD), and 52.24 (HD) kg/m3, respectively. Liver samples were collected from LD, MD, and HD on day 84.
2.5. Structural Analysis of Trout CXCR4.1 Subtypes
ColabFold (ColabFold v1.5.5) was used to predict the protein structures by combining MMseqs2 with AlphaFold2 or RoseTTAFold [45]. Compared to the ORF sequences, we showed amino acid sequences associated with TM, extracellular (ECL), and intracellular (ICL) loops with high confidence values. The amino acid sequences for structure prediction are shown in Supplementary Materials Text S1. The human CXCR4 (PDB ID: 4RWS) was used as a template. Comparison of the domains between trout and human CXCR4 and the cartoon, stick, and sphere structures of the proteins were generated by PyMOL software (PyMOL-2.5.4) [46,47].
2.6. Statistical Analysis
The RNA-Seq data (counts) were normalized with the Bioconductor DESeq2 Package [48,49]. In order to obtain the belt data (Poisson) distribution for further statistical analysis, data of RNA-Seq were normalized by log transformation [50]. After that, the normalized data were analyzed by an online R software Package (https://omicsforum.ca/, accessed on 24 January 2024) for multivariate analyses [51,52]. Based on previous studies in the fishery and biomedical studies [53,54], we evaluated the whole profile of the cxcr expressions by performing the heatmap, principal components analysis (PCA), correlation coefficients, and variable importance in projection (VIP). Gene expression analyses were performed with GraphPad Prism 8.0. The results were evaluated by one-way analysis of variance (ANOVA) followed by a Tukey multiple range test, with p < 0.05 set to assign significant differences. Student’s t-test was used for comparisons between two groups, with significance established when p < 0.05. Results were presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Identification and Annotation of cxcr Genes in Rainbow Trout
In our study, a total of 17 cxcr genes (12 newly identified and 5 previously identified receptors) were identified in the rainbow trout, with the predicted protein sequences ranging from 309 to 461 amino acids, the molecular weights ranging from 33.92 to 50.85 kDa, and the pIs ranging between 5.88 and 9.24 (Table 1). Based on the sequence information of identified receptors in humans, mice, and zebrafish, on sequence similarities among the trout receptors, and on the conserved seven transmembrane domains and DRY motif, the 17 cxcr genes were divided into seven families. Chromosomal locations of cxcr genes were also studied. In brief, the trout cxcr genes were distributed in eight different chromosomes (Chr2, 3, 8, 16, 18, 22, 24, and 28), including three genes on Chr2, five genes on Chr3, and four genes on Chr22. Copy numbers of the cxcr genes in rainbow trout were compared with those of human, mouse, chicken, zebrafish, and several teleost species (Table 2). Expanded copies of cxcr1, cxcr2, cxcr3, cxcr4, and cxcr7 genes were identified in rainbow trout (Table 2).
Table 1.
Summary of 17 cxcr genes in rainbow trout.
| Gene Name | Gene ID | Chromosome | Position (bp) | Protein Length (aa) | MW (kDa) | pI | Derived from | Chemokines [10,55,56,57] |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| cxcr1.1 | LOC100135914 | Chr18 | 3,898,505–3,899,881 | 359 | 39.98 | 9.2 | AF260964.1 | CXCL8 | [55,58] |
| cxcr1.2 | LOC110501285 | Chr22 | 5,736,707–5,742,123 | 362 | 40.1 | 8.31 | Newly Identified | CXCL8 | |
| cxcr2.1 | LOC110520605 | Chr3 | 78,906,276–78,908,301 | 362 | 40.3 | 8.94 | HG794530.1 | CXCL8 | [55,59] |
| cxcr2.2 | LOC110501383 | Chr22 | 11,509,532–11,512,348 | 362 | 40.07 | 8.78 | Newly Identified | CXCL8 | |
| cxcr3.1a | LOC110537629 | Chr2 | 7,072,125–7,076,959 | 371 | 41.7 | 6.08 | Newly Identified | CXCL9, 10, 11 | |
| cxcr3.1b | LOC110514317 | Chr2 | 102–3242 | 373 | 41.94 | 5.88 | Newly Identified | CXCL9, 10, 11 | |
| cxcr3b | LOC100136126 | Chr3 | 16,782,015–16,785,624 | 374 | 42.19 | 8.17 | AJ888881.1 | CXCL9, 10, 11 | [10,55,56,59] |
| cxcr3 | LOC110537622 | Chr2 | 7,038,779–7,047,349 | 382 | 42.37 | 9.1 | Newly Identified | CXCL9, 10, 11 | |
| cxcr3a | LOC100136649 | Chr3 | 16,764,415–16,768,463 | 380 | 42.27 | 9.24 | AJ888878.1 | CXCL9, 10, 11 | [10,55,56,59] |
| cxcr4.1a | LOC110520024 | Chr3 | 48,667,827–48,669,883 | 362 | 40.57 | 8.74 | AJ001039.1 | CXCL12 | [55,60] |
| cxcr4.1b | LOC110501543 | Chr22 | 18,785,356–18,787,371 | 357 | 39.99 | 8.86 | Newly Identified | CXCL12 | |
| cxcr4.2a | LOC110530627 | Chr8 | 70,897,183–70,922,718 | 373 | 40.82 | 8.58 | Newly Identified | CXCL12 | |
| cxcr4.2b | LOC110516585 | Chr28 | 1208–2756 | 373 | 41.08 | 8.54 | Newly Identified | CXCL12 | |
| cxcr5 | LOC110503290 | Chr24 | 38,512,905–38,516,651 | 309 | 33.92 | 5.96 | Newly Identified | CXCL13 | |
| cxcr6 | LOC110492888 | Chr16 | 21,961,814–21,965,417 | 461 | 50.85 | 8.68 | Newly Identified | ||
| cxcr7.1a | LOC110520437 | Chr3 | 70,094,498–70,106,981 | 375 | 42.01 | 6.94 | Newly Identified | CXCL11, 12 | |
| cxcr7.1b | LOC110501640 | Chr22 | 25,651,985–25,660,915 | 378 | 42.22 | 6.66 | Newly Identified | CXCL11, 12 |
Table 2.
Comparison of cxcr gene copies among mammals and teleosts.
| Name | Human | Mouse | Chicken | Frog | Zebrafish | Channel Catfish | Atlantic Salmon | Fugu | Northern Pike | Rainbow Trout |
|---|---|---|---|---|---|---|---|---|---|---|
| cxcr1 | 1 (~14%) | 1 (~14%) | 1 (~33%) | 0 | 1 (10%) | 1 (~11%) | 2 (~11%) | 2 (25%) | 2 (20%) | 2 (~11%) |
| cxcr2 | 1 (~14%) | 1 (~14%) | 0 | 0 | 1 (10%) | 1 (~11%) | 2 (~11%) | 1 (12.5%) | 1 (10%) | 2 (~11%) |
| cxcr3 | 1 (~14%) | 1 (~14%) | 0 | 1 (25%) | 3 (30%) | 3 (~33%) | 3 (~17%) | 1 (12.5%) | 2 (20%) | 5 (~29%) |
| cxcr4 | 1 (~14%) | 1 (~14%) | 1 (~33%) | 1 (25%) | 2 (20%) | 2 (~22%) | 4 (~23%) | 2 (25%) | 2 (20%) | 4 (~23%) |
| cxcr5 | 1 (~14%) | 1 (~14%) | 1 (~33%) | 1 (25%) | 1 (10%) | 1 (~11%) | 1 (~5%) | 1 (12.5%) | 1 (10%) | 1 (~5%) |
| cxcr6 | 1 (~14%) | 1 (~14%) | 0 | 0 | 0 | 0 | 1 (~5%) | 0 | 1 (10%) | 1 (~5%) |
| cxcr7 | 1 (~14%) | 1 (~14%) | 0 | 1 (25%) | 2 (20%) | 1 (~11%) | 4 (~23%) | 1 (12.5%) | 1 (10%) | 2 (~11%) |
| Total | 7 | 7 | 3 | 4 | 10 | 9 | 17 | 8 | 10 | 17 |
3.2. Phylogenetic Analysis and Gene Structure Analyses
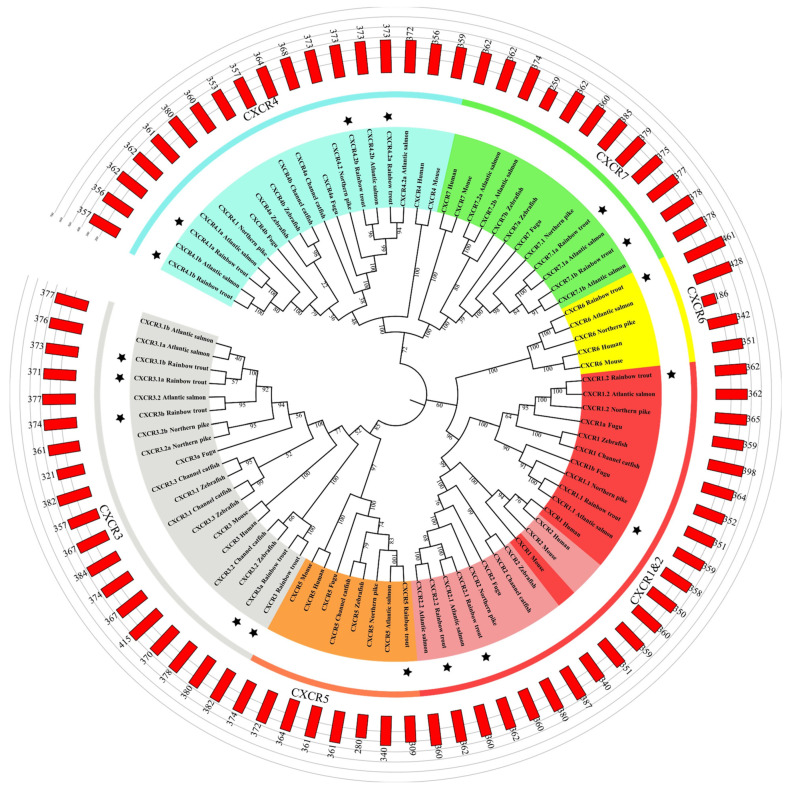
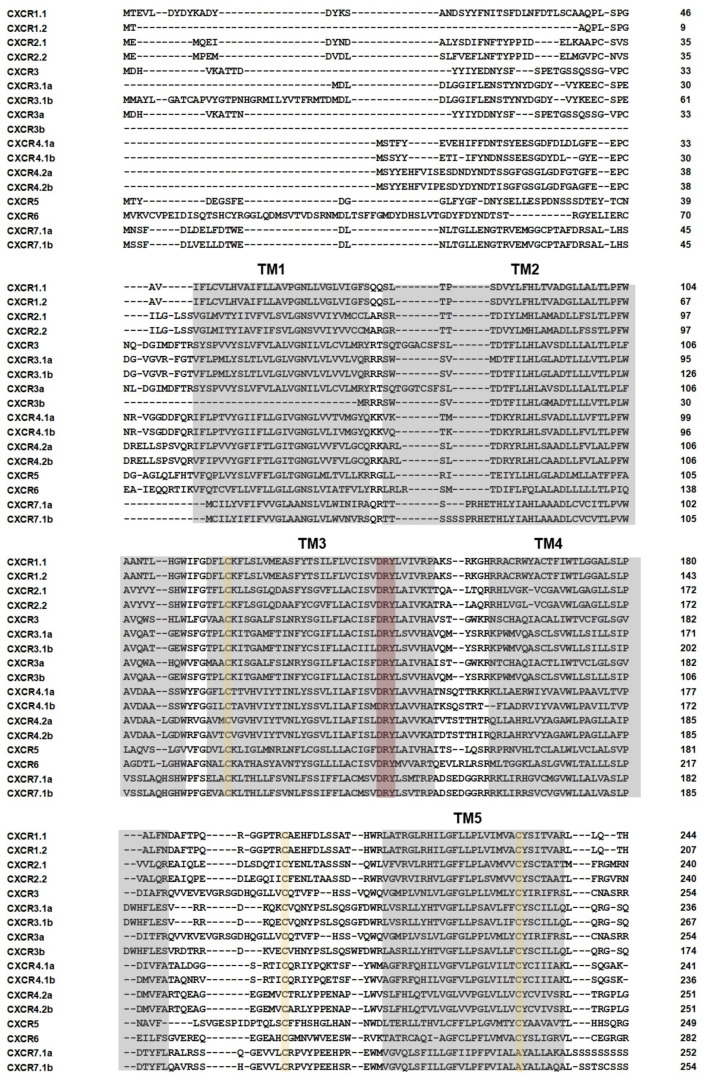
Amino acid sequences of CXCR in rainbow trout and other species were used to construct a phylogenetic tree. The phylogenetic tree exerted a total of six subgroups, including the CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, and CXCR7 subgroups (Figure 1). All these CXCR proteins contained seven transmembrane domains, which are typically observed in GPCRs (Figure 2).
Figure 1.
Phylogenetic tree of CXCRs. The CXCR sequences were obtained from rainbow trout, Atlantic salmon, zebrafish, human, mouse, Northern pike, Channel catfish, and fugu. The number of nodes shows the bootstrapping values, and the black stars indicate trout CXCRs.
Figure 2.
Alignment of CXCR proteins. Residues of transmembrane domains (TMs), DRY motif (a highly conserved motif in family A GPCRs), and (semi)conserved cysteines are shaded in grey, red, and orange. TMs and (semi)conserved cysteines are defined from previous studies of salmon and teleost CXCRs [61,62]. The highly conserved NPxxY motif in family A GPCRs is observed in TM 7.
3.3. Transcriptional Profiles of cxcr in Trout after Bacterial Infection
3.3.1. V. anguillarum
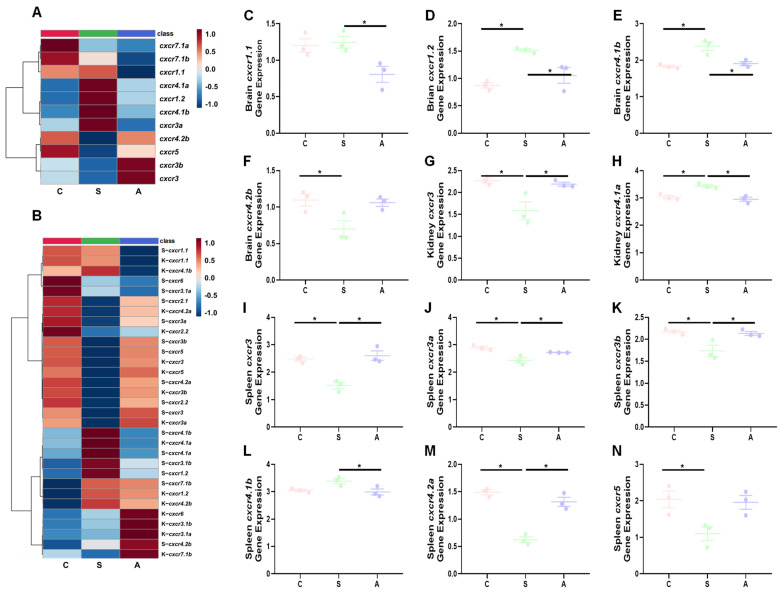
The heatmap showed the overall expression profiles of cxcr genes in the brain (Figure 3A), spleen, and kidney (Figure 3B). Brain cxcr1 and cxcr4, kidney cxcr3 and cxcr4, and spleen cxcr3, cxcr4, and cxcr5 subtypes were significantly altered by V. anguillarum infection (Figure 3C–N).
Figure 3.
Transcriptional profiles of cxcr in trout after V. anguillarum infection. (A,B): the heatmap of cxcr transcriptional profiles ((A): brain; (B): kidney and spleen). (C–N): expression of the representative genes (with significant differences among groups). Asterisks indicate significant differences (one-way analysis ANOVA followed by Tukey’s multiple comparison test with p < 0.05). Abbreviations: K—kidney; S—spleen; C—control trout; A—asymptomatic trout; S—symptomatic trout.
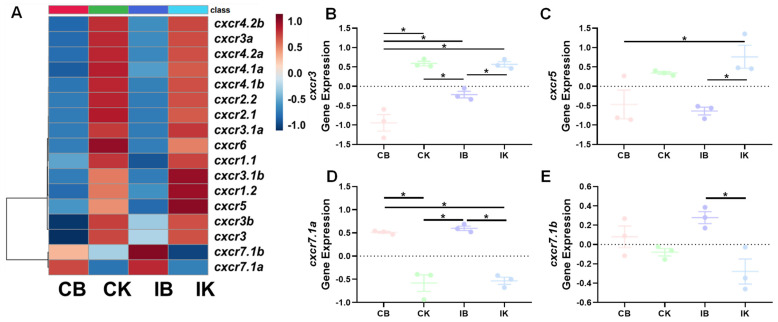
3.3.2. A. salmonicida
The heatmap indicated the overall expression profiles of cxcr genes in the brain and kidney between control and infected trout (Figure 4A). Expressions of representative genes (cxcr3, cxcr5, cxcr7.1a, and cxcr7.1b) are also shown (Figure 4B–E).
Figure 4.
Transcriptional profiles of cxcr in trout after A. salmonicida infection. (A): the heatmap of cxcr transcriptional profiles. (B–E): expression of the representative genes (with significant differences among groups). Asterisks indicate significant differences (one-way analysis ANOVA followed by Tukey’s multiple comparison test with p < 0.05). Abbreviations: CB—control trout brain tissue; CK—control trout kidney tissue; IB—infected trout brain tissue; IK—infected trout kidney tissue.
3.4. Transcriptional Profiles of cxcr in Trout in Response to Salinity Change and High Stocking Density
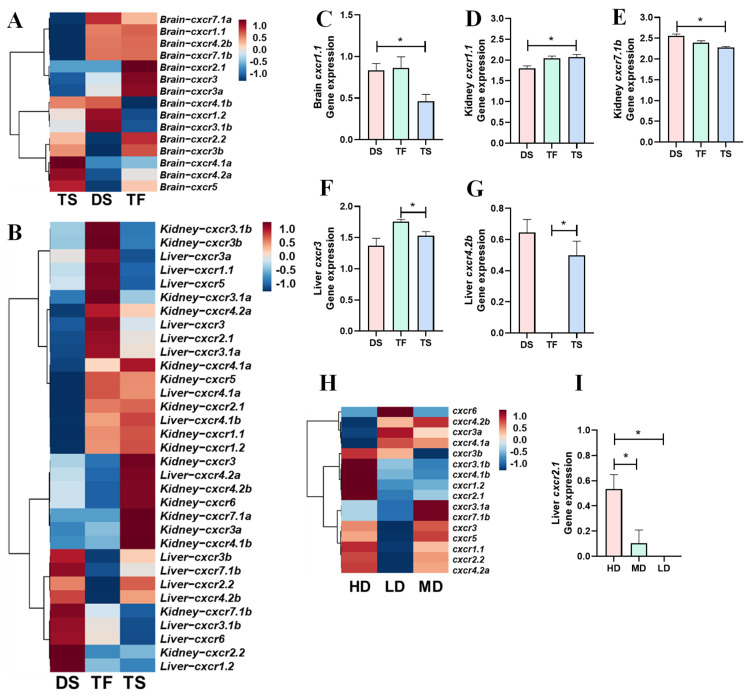
The overall transcriptional profiles of trout cxcr in the brain, kidney, and liver are shown in heatmaps (Figure 5A,B). Compared to DS, TS showed a significantly down-regulated expression of brain cxcr1.1, up-regulated expression of kidney cxcr1.1, and down-regulated expression of kidney cxcr7.1b (Figure 5C–E). Compared to TF, TS showed a significantly down-regulated expression of liver cxcr3 and up-regulated expression of liver cxcr4.2b (Figure 5F,G). The overall hepatic cxcr expressions were clustered in a heatmap (Figure 5H). High stocking density significantly increased cxcr2.1 expressions when comparing HD to MD and LD (Figure 5I).
Figure 5.
Transcriptional profiles of cxcr in trout after salinity and density changes. (A,B): principal component analysis (PCA) of cxcr transcriptional profiles in the brain (A) and liver and kidney (B). The separated PCA plots indicate specific cxcr transcriptions. (A,B): the heatmap of cxcr transcriptional profiles in the brain (A) and liver and kidney (B). (C–G): expression of the representative genes (with significant differences) between DS and TS or TF and TS (Student’s t-test was used for comparisons between two groups with p < 0.05). (H): the heatmap of cxcr transcriptional profiles in the liver. (I): expression of liver cxcr1.2 among different stocking densities. Asterisks indicate significant differences (one-way analysis ANOVA followed by Tukey’s multiple comparison test with p < 0.05). Abbreviations: DS—diploid trout in saltwater; TF—triploid trout in freshwater; TS—triploid trout in saltwater.
3.5. Structure Prediction of CXCR4.1a and CXCR4.1b
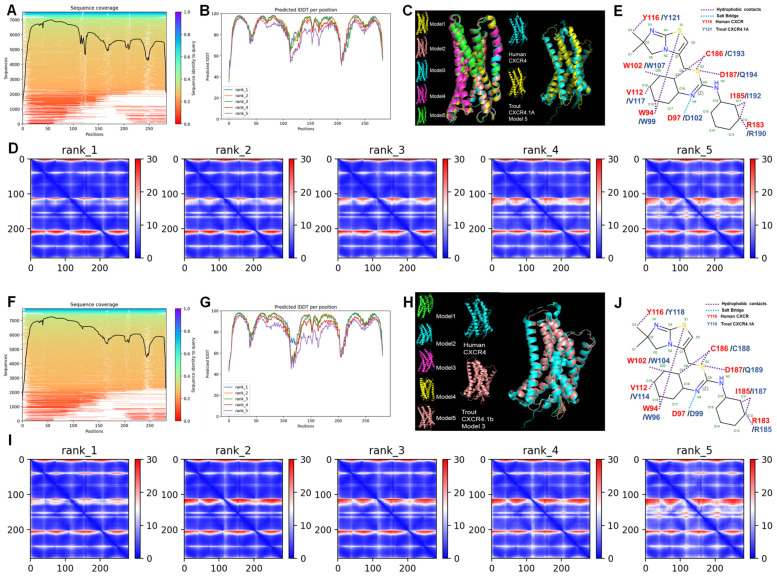
Sequence coverage of trout CXCR4.1a and CXCR4.1b residues are shown in Figure 6A,F. For trout CXCR4.1a, the pLDDT score of model 5, model 3, model 4, model 1, and model 2 were 88.9, 88.8, 87.2, 85.5, and 82.6, respectively (Figure 6B, cartoon representation in Figure 6C). The pLDDT scores of model 3, model 4, model 5, model 1, and model 2 of trout CXCR4.1b were 88.8, 88.4, 87.2, 84.6, and 80.1, respectively (Figure 6G, cartoon representation in Figure 6H). Uncertainty of the predicted distance between two residues is color-coded from blue (0 Å) to red (30 Å, Figure 6D,I). Trout CXCR4.1a (CXCR4.1b) showed conserved IT1t (a small molecule antagonist) binding sites of W99 (W96), D102 (D99), W107 (W104), V117 (V114), Y121 (Y118), C193 (C188), R190 (R185), and I192 (I187) to human CXCR4 with exception of D187. Human CXCR4 D187 was replaced by Q194 in trout CXCR4.1a and Q189 in trout CXCR4.1b (Figure 6E,J).
Figure 6.
Comparison analyses of trout CXCR4.1a and CXCR4.1b based on human CXCR4 crystal structure. (A): sequence coverage of the trout CXCR4.1a residues. (B): predicted local distance difference test (LDDT) score per residue for five models (model 5 = 88.9; model 3 = 88.8; model 4 = 87.2; model 1 = 85.5; model 2 = 82.6). (C): cartoon model of the structure of human CXCR4 and five predicted structures of trout CXCR4.1a. (D): prediction aligned error (PAE) score for five models. (E): schematic representation of interactions between human CXCR4/trout CXCR4.1a and IT1t. Red amino acids show human CXCR4 residues, and blue amino acids show trout CXCR4 residues. (F): sequence coverage of the trout CXCR4.1b residues. (G): prediction dictated local distance difference test (LDDT) score per residues for five models (model 3 = 88.8; model 4 = 88.4; model 5 = 87.2; model 1 = 84.6; model 2 = 80.1). (H): cartoon model of the structure of human CXCR4 and five predicted structures of trout CXCR4.1b. (I): prediction aligned error (PAE) score for five models. (J): schematic representation of interactions between human CXCR4/trout CXCR4.1b and IT1t. Red amino acids show human CXCR4 residues, and blue amino acids show trout CXCR4 residues.
4. Discussion
4.1. Characterization of cxcr Genes
Whole genome duplication occurred in teleost ancestors, resulting in increased paralogs of cxcr genes. Hence, previous studies have identified eight cxcr genes in a typical 3R teleost species, such as channel catfish [15]. Atlantic salmon is an important 4R salmonid species, and 19 cxcr genes have been identified in Atlantic salmon [61]. In the current study, a total of 17 cxcr genes were identified in rainbow trout based on available genomic information and our RNA-seq datasets (Figure 1 and Figure 2), being consistent with previous studies that showed an expansion of chemokine systems in teleosts [62,63]. Thus, the faster evolvement of chemokines and the fish-specific whole genome duplication have resulted in the expansion of both teleost chemokine and receptor genes [10,14,64,65,66]. In this study, we showed duplications of trout cxcr1, cxcr2, cxcr3, cxcr4, and cxcr7 due to whole genome duplication and lineage-specific tandem gene duplications (Figure 1 and Figure 2). For example, cxcr3 and cxcr3.1a were localized quite close to each other on chromosome 2, and a large cluster of cxcr genes were located on chromosome 3 and 22, all of this suggesting a rapid evolution through tandem duplications [67], suggesting that gene duplication of CXC chemokines and receptors might acts as a predominant evolutionary mechanism for environment adaptation in fish [68]. Compared to 3R teleosts, the 4R salmonid species exerted more cxcr paralogs, which is consistent with previous studies showing that genes (such as igf and igfbp genes, for example) involved in immunomodulation were further expanded in salmonid species [26,27].
The phylogenetic analysis showed explicit annotations of trout CXCR proteins, with most trout CXCR proteins clustered with their teleost counterparts (Figure 1). The seven transmembrane domains, a conserved and typical structure of GPCRs [69], were observed in all trout CXCR members, as well as a DRY motif in TM3 and an NPxxY motif in TM7, revealing sequence and structure similarities between trout CXCRs and mammalian family A GPCRs.
4.2. Physiological Functions of cxcr Genes
The chemokine system plays an important role in modulating the development of the immune system homeostasis during routine immune surveillance and inflammation [2,6,67]. Recent studies reported the involvement of chemokine systems in regulating immunomodulation in teleosts, including catfish, trout, croaker, and bream [15,59,70,71,72,73]. However, most of these studies focused on studying CXCR-regulated immunomodulation in peripheral immune tissues rather than in the central nervous system. Therefore, we evaluated cxcr transcription levels in both brain and peripheral tissues in trout in response to V. anguillarum or A. salmonicida infections.
In humans and rodents, CXCR1 has been reported to be widely expressed in the brain and to play an important role in modulating neuroinflammation [74,75]. In teleosts, CXCR1 regulates immune defense against pathogen infections [59,76,77]. For example, peripheral cxcr1 was up-regulated by viral and bacterial infections in trout [59]. Two cxcr1 subtypes have been identified in some teleost species as a consequence of the additional WGD [61,77], which is consistent with our results. In Asian swamp eel (Monopterus albus), a previous study showed different gene expressions between cxcr1.1 and cxcr1.2 after pathogen infection [77]. In this study, brain cxcr1.1 and cxcr1.2 showed different transcriptional regulation in response to bacterial infections (Figure 3). A recent study showed cxcrs exhibited tissue-specific and time-dependent regulation of transcription in the head kidney, liver, and gill after A. salmonicida infection in turbot (Scophthalmus maximus) and black rockfish (Sebastes schlegelii) [68,78]. These results suggest that cxcr1 subtypes might be differently involved in the response to disease in both brain and peripheral tissues.
Biomedical studies indicate that CXCR3 is involved in directing lymphocytes into inflammation areas and regulating the inflammatory state of both the CNS and peripheral tissues [79,80]. A previous study in grass carp (Ctenopharyngodon idella) showed that cxcr3 is widely expressed in the brain and protects the brain from pathogen infection [81]. We observed that brain cxcr3 was significantly up-regulated by A. salmonicida infection (Figure 4). Consistently, transcriptional profiles of cxcr3 subtypes were significantly altered by a bacterial and viral infection, LPS or polyI:C stimulation in teleost species, including rainbow trout, turbot, largemouth bass (Micropterus salmoides), and black rockfish [57,68,78,82]. In rainbow trout, cxcr3 subtypes were differently induced by inflammatory stimulants and cytokines in head kidney cells and macrophages [59,76,83]. Our results also showed that cxcr3 exhibited tissue-specific and subtype-dependent transcriptional regulation in peripheral tissues in response to pathogen infection. Our results further supported the involvement of CXCR3 in the neuro-immune network in fish [81]. Human studies showed that CXCR3A and CXCR3B exert opposite functions in regulating cell growth, with the “Survival” and “Death” signals derived from CXCR3A and CXCR3B, respectively [84,85]. In this study, we observed up-regulated cxcr3 subtypes in asymptomatic trout compared to symptomatic trout (Figure 3). Our results suggested trout cxcr3 subtypes might be functional orthologs of human CXCR3A. Further studies should investigate the regulatory mechanism(s) of the cxcr3-regulated “Survival” signals.
CXCR4 is one of the most well-studied chemokine receptors due to its important role in regulating the development of the immune system and also immunomodulation. In biomedical studies, CXCR4 serves as the therapeutic target of cancer metastasis and HIV-1 infection [86,87,88,89]. There is growing evidence that CXCR4 is involved in infection defense, neuron pathophysiology, and response to stress in teleosts ([90,91], Reviewed in [76]). For example, rainbow trout, grouper, and channel catfish showed up-regulated cxcr4 after viral and bacterial infections [15,82,90]. CXCR4 was also widely expressed in the CNS, where it seems to be involved in neuron pathology [76,90]. Our results showed brain cxcr4.1b and cxcr4.2b expression were significantly altered due to V. anguillarum infection, in correlation with a previous study that showed that nervous necrosis virus infection led to significantly upregulated cxcr4 expression in orange-spotted grouper [90]. Alterations of environmental nitrate also induce cxcr4b expression in Wuchang bream (Megalobrama amblycephala) [91]. Likewise, our results showed that both bacterial infection and environmental changes altered the expression of cxcr4 subtypes (Figure 3 and Figure 5). CXCR5 serves as the homeostatic regulator for immune responses and neuron regeneration ([92,93], reviewed in [76]). In fish, cxcr5 was shown to be highly expressed in lymphoid tissues, including the kidney and spleen in grass carp [92]. Consistent with previous studies showing cxcr5 expression is modulated by a range of immune stimulants and pathogen infection [68,92], in this study, symptomatic trout also showed down-regulated spleen cxcr5 in response to V. anguillarum infection (Figure 4).
Meanwhile, it is important to compare the extent of changes between chemokine receptors to the overall statistical pattern of changes in the RNA-Seq data and other genes that are not directly related to immunomodulation. In this study, we selected per1b (period circadian clock 1b). The per1b is widely expressed in both brain and peripheral tissues in humans (https://www.genecards.org/, accessed on 24 January 2024) and rainbow trout. The per1b gene regulates circadian rhythms of locomotion, metabolism, and behavior. In the brain, average fold-changes of all up-regulated genes between groups were ~1.59 (C/S) and 1.48 (C/A), and all down-regulated genes between groups were ~0.68 (C/S) and 0.75 (C/A). The fold-changes of per1b gene expression between groups were ~1.51 (C/S) and 0.94 (C/A), while fold-changes of cxcr1.2 gene expression between groups were ~0.21 (C/S) and 0.57 (C/A) (Figure 3). In the kidney, average fold-changes of all up-regulated genes between groups were ~2.51 (C/S) and 1.76 (C/A), and all down-regulated genes between groups were ~0.59 (C/S) and 0.63 (C/A). The fold-changes of per1b gene expression between groups were ~0.84 (C/S) and 0.82 (C/A), while fold-changes of cxcr3 gene expression between groups were ~3.97 (C/S) and 1.21 (C/A) (Figure 3). In the spleen, average fold-changes of all up-regulated genes between groups were ~4.35 (C/S) and 2.71 (C/A), and all down-regulated genes between groups were ~0.45 (C/S) and 0.60 (C/A). The fold-changes of per1b gene expression between groups were ~0.95 (C/S) and 1.39 (C/A), while fold-changes of cxcr3 gene expression between groups were ~8.89 (C/S) and 0.64 (C/A) (Figure 3). These results suggested that infection or the fish response to infection are directly provoking alterations in trout cxcr transcription levels, especially between groups of control trout and symptomatic trout.
Finally, we showed that the amino acids of the binding pocket of IT1t (a small molecule) in trout CXCR4.1a/b were conserved to those of human CXCR4 [94] (Figure 6). Compared to endogenous ligands, these small molecule ligands (drugs) are stable and orally bioavailable [95]. Therefore, small molecular ligands of human chemokine receptors could be used in the future as immunomodulators targeting fish CXCRs in the aquaculture industry.
5. Conclusions
In this study, we have identified 17 cxcr genes in rainbow trout with duplicated copies of cxcr1, cxcr2, cxcr3, cxcr4, and cxcr7. Gene expression analyses showed trout cxcr genes exhibited conserved functions with human orthologs. Transcription levels of cxcr genes were altered by bacterial infection and environmental changes, suggesting a pleiotropic role in regulating homeostasis and immune response. Trout CXCR4.1a(b) showed conserved residues for the binding pocket of IT1t (a small molecule ligand) with human CXCR4. Our results contribute to a better understanding of the immune role of CXCRs and their potential ligands in an important teleost species. This information could be used in the future to modulate immune responses to infectious diseases and adaptation to environmental changes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biom14030337/s1, Text S1: Input amino acids sequences of CXCR4.1a–b, Figure S1. Prediction aligned error (PAE) score for five models with CXCR4.1a ORF sequences. Figure S2. Prediction aligned error (PAE) score for five models with CXCR4.1a amino acid sequences associated with the transmembrane domain (TM), extracellular (ECL), and intra-cellular (ICL) loops. Table S1. Count of liver cxcr genes in trout at HD, LD, and MD.
Author Contributions
Conceptualization, Z.-S.H., J.-F.L. and H.-S.W.; methodology, Z.-S.H., M.-Q.L., X.-D.Y., H.-K.Z., C.Z., Y.-R.X., Q.Y. and H.-S.W.; validation, Z.-S.H., H.-K.Z., M.-Q.L., K.-W.X. and Z.L.; formal analysis, Z.-S.H., H.-K.Z., K.-W.X., Z.L. and P.P.; data curation, Z.-S.H., H.-K.Z., K.-W.X. and Z.L.; writing—original draft preparation, Z.-S.H., J.-F.L., P.P., C.T. and H.-S.W.; writing–review and editing, Z.-S.H., J.-F.L., P.P., C.T. and H.-S.W.; supervision, Z.-S.H. and H.-S.W.; project administration, Z.-S.H. and H.-S.W.; funding acquisition, Z.-S.H. and H.-S.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Animal studies were approved by the Institutional Review Board at Ocean University of China (permit number: 20141201).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Natural Science Foundation of Shandong Province (ZR2023QC196), China Postdoctoral Science Foundation (2023M743332), Development Plan of Youth innovation team in colleges and universities in Shandong Province (2023KJ031), Qingdao Postdoctoral Science Foundation (QDBSH20230102021), Foundation of Guangxi Academy of Aquatic Sciences (GXKEYLA-2023-01-21) and Support Foundation of Ocean University of China.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Koelink P.J., Overbeek S.A., Braber S., de Kruijf P., Folkerts G., Smit M.J., Kraneveld A.D. Targeting chemokine receptors in chronic inflammatory diseases: An extensive review. Pharmacol. Ther. 2012;133:1–18. doi: 10.1016/j.pharmthera.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Allen S.J., Crown S.E., Handel T.M. Chemokine: Receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 3.Bacon K., Baggiolini M., Broxmeyer H., Horuk R., Lindley I., Mantovani A., Maysushima K., Murphy P., Nomiyama H., Oppenheim J. Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21:48–49. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A., Yoshie O., Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotnik A., Burkhardt A.M., Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik A., Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 7.Yoshie O., Imai T., Nomiyama H. Advances in Immunology. Elsevier; Amsterdam, The Netherlands: 2001. Chemokines in immunity; pp. 57–110. [DOI] [PubMed] [Google Scholar]
- 8.Van de Peer Y., Mizrachi E., Marchal K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017;18:411. doi: 10.1038/nrg.2017.26. [DOI] [PubMed] [Google Scholar]
- 9.Jaillon O., Aury J.-M., Brunet F., Petit J.-L., Stange-Thomann N., Mauceli E., Bouneau L., Fischer C., Ozouf-Costaz C., Bernot A., et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 10.Valdés N., Cortés M., Barraza F., Reyes-López F.E., Imarai M. CXCL9-11 chemokines and CXCR3 receptor in teleost fish species. Fish Shellfish. Immunol. Rep. 2022;3:100068. doi: 10.1016/j.fsirep.2022.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon B., Shum B., Adams E.J., Magor K., Hedrick R.P., Muir D.G., Parham P. CK-1, a putative chemokine of rainbow trout (Oncorhynchus mykiss) Immunol. Rev. 1998;166:341–348. doi: 10.1111/j.1600-065X.1998.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Peatman E., Liu Z. Evolution of CC chemokines in teleost fish: A case study in gene duplication and implications for immune diversity. Immunogenetics. 2007;59:613–623. doi: 10.1007/s00251-007-0228-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Chang M., Wu S., Nie P. Characterization of C–C chemokine receptor subfamily in teleost fish. Mol. Immunol. 2009;46:498–504. doi: 10.1016/j.molimm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Nomiyama H., Osada N., Yoshie O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev. Comp. Immunol. 2011;35:705–715. doi: 10.1016/j.dci.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q., Yang Y., Li C., Zeng Q., Zhou T., Li N., Liu Y., Liu S., Liu Z. The CC and CXC chemokine receptors in channel catfish (Ictalurus punctatus) and their involvement in disease and hypoxia responses. Dev. Comp. Immunol. 2017;77:241–251. doi: 10.1016/j.dci.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Leu J.-H., Tsai C.-H., Tsai J.-M., Yang C.-H., Hsueh C.-Y., Chou H.-Y. Identification and expression analysis of 19 CC chemokine genes in orange-spotted grouper (Epinephelus coioides) Dev. Comp. Immunol. 2019;97:1–10. doi: 10.1016/j.dci.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Thorgaard G.H., Bailey G.S., Williams D., Buhler D.R., Kaattari S.L., Ristow S.S., Hansen J.D., Winton J.R., Bartholomew J.L., Nagler J.J., et al. Status and opportunities for genomics research with rainbow trout. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002;133:609–646. doi: 10.1016/S1096-4959(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 18.Berthelot C., Brunet F., Chalopin D., Juanchich A., Bernard M., Noël B., Bento P., Silva C.D., Labadie K., Alberti A., et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdés N., Gonzalez A., Garcia V., Tello M. Analysis of the microbiome of rainbow trout (Oncorhynchus mykiss) exposed to the pathogen Flavobacterium psychrophilum 10094. Microbiol. Resour. Announc. 2020;9:e01562-19. doi: 10.1128/MRA.01562-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden T.J. Modulation of the immune system of fish by their environment. Fish Shellfish. Immunol. 2008;25:373–383. doi: 10.1016/j.fsi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Makrinos D.L., Bowden T.J. Natural environmental impacts on teleost immune function. Fish Shellfish. Immunol. 2016;53:50–57. doi: 10.1016/j.fsi.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Macqueen D.J., Garcia de la serrana D., Johnston I.A. Evolution of ancient functions in the vertebrate insulin-like growth factor system uncovered by study of duplicated salmonid fish genomes. Mol. Biol. Evol. 2013;30:1060–1076. doi: 10.1093/molbev/mst017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allendorf F.W., Thorgaard G.H. Evolutionary Genetics of Fishes. Plenum Press; New York, NY, USA: 1984. Tetraploidy and the evolution of salmonid fishes; pp. 1–53. [Google Scholar]
- 24.Lien S., Koop B.F., Sandve S.R., Miller J.R., Kent M.P., Nome T., Hvidsten T.R., Leong J.S., Minkley D.R., Zimin A., et al. The Atlantic salmon genome provides insights into rediploidization. Nature. 2016;533:200–205. doi: 10.1038/nature17164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macqueen D.J., Johnston I.A. A well-constrained estimate for the timing of the salmonid whole genome duplication reveals major decoupling from species diversification. Proc. R. Soc. B Biol. Sci. 2014;281:20132881. doi: 10.1098/rspb.2013.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzaid A., Martin S.A.M., Macqueen D.J. The complete salmonid IGF-IR gene repertoire and its transcriptional response to disease. Sci. Rep. 2016;6:34806. doi: 10.1038/srep34806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alzaid A., Castro R., Wang T., Secombes C.J., Boudinot P., Macqueen D.J., Martin S.A.M. Cross talk between growth and immunity: Coupling of the IGF axis to conserved cytokine pathways in rainbow trout. Endocrinology. 2016;157:1942–1955. doi: 10.1210/en.2015-2024. [DOI] [PubMed] [Google Scholar]
- 28.Sequeida A., Castillo A., Cordero N., Wong V., Montero R., Vergara C., Valenzuela B., Vargas D., Valdés N., Morales J., et al. The Atlantic salmon interleukin 4/13 receptor family: Structure, tissue distribution and modulation of gene expression. Fish Shellfish. Immunol. 2020;98:773–787. doi: 10.1016/j.fsi.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Conant G.C., Wolfe K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 30.Taylor J.S., Raes J. Duplication and divergence: The evolution of new genes and old ideas. Annu. Rev. Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. [DOI] [PubMed] [Google Scholar]
- 31.Rebl A., Korytář T., Köbis J.M., Verleih M., Krasnov A., Jaros J., Kühn C., Köllner B., Goldammer T. Transcriptome profiling reveals insight into distinct immune responses to Aeromonas salmonicida in gill of two rainbow trout strains. Mar. Biotechnol. 2014;16:333–348. doi: 10.1007/s10126-013-9552-x. [DOI] [PubMed] [Google Scholar]
- 32.Frans I., Michiels C.W., Bossier P., Willems K.A., Lievens B., Rediers H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011;34:643–661. doi: 10.1111/j.1365-2761.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 33.Valdes N., Espinoza C., Sanhueza L., Gonzalez A., Corsini G., Tello M. Draft genome sequence of the Chilean isolate Aeromonas salmonicida strain CBA100. FEMS Microbiol. Lett. 2015;362:fnu062. doi: 10.1093/femsle/fnu062. [DOI] [PubMed] [Google Scholar]
- 34.Sun P., Bao P., Tang B. Transcriptome analysis and discovery of genes involved in immune pathways in large yellow croaker (Larimichthys crocea) under high stocking density stress. Fish Shellfish. Immunol. 2017;68:332–340. doi: 10.1016/j.fsi.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 35.San L., Liu B., Liu B., Guo H., Guo L., Zhang N., Zhu K., Jiang S., Zhang D. Transcriptome analysis of gills provides insights into translation changes under hypoxic stress and reoxygenation in golden pompano, Trachinotus ovatus (Linnaeus 1758) Front. Mar. Sci. 2021;8:763622. doi: 10.3389/fmars.2021.763622. [DOI] [Google Scholar]
- 36.de Fonseka R., Fjelldal P.G., Sambraus F., Nilsen T.O., Remø S.C., Stien L.H., Reinardy H.C., Madaro A., Hansen T.J., Fraser T.W.K. Triploidy leads to a mismatch of smoltification biomarkers in the gill and differences in the optimal salinity for post-smolt growth in Atlantic salmon. Aquaculture. 2022;546:737350. doi: 10.1016/j.aquaculture.2021.737350. [DOI] [Google Scholar]
- 37.Galbreath P.F., Thorgaard G.H. Saltwater performance of all-female triploid Atlantic salmon. Aquaculture. 1995;138:77–85. doi: 10.1016/0044-8486(95)01082-3. [DOI] [Google Scholar]
- 38.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37((Suppl. 2)):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou Z.-S., Xin Y.-R., Zeng C., Zhao H.-K., Tian Y., Li J.-F., Wen H.-S. GHRH-SST-GH-IGF Axis Regulates Crosstalk between Growth and Immunity in Rainbow Trout (Oncorhynchus mykiss) Infected with Vibrio anguillarum. Fish Shellfish. Immunol. 2020;106:887–897. doi: 10.1016/j.fsi.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Zeng C., Hou Z.-S., Zhao H.-K., Xin Y.-R., Liu M.-Q., Yang X.-D., Wen H.-S., Li J.-F. Identification and characterization of caspases genes in rainbow trout (Oncorhynchus mykiss) and their expression profiles after Aeromonas salmonicida and Vibrio anguillarum infection. Dev. Comp. Immunol. 2021;118:103987. doi: 10.1016/j.dci.2020.103987. [DOI] [PubMed] [Google Scholar]
- 42.Hou Z.-S., Xin Y.-R., Yang X.-D., Zeng C., Zhao H.-K., Liu M.-Q., Zhang M.-Z., Daniel J.G., Li J.-F., Wen H.-S. Transcriptional profiles of genes related to stress and immune response in Rainbow trout (Oncorhynchus mykiss) symptomatically or asymptomatically infected with Vibrio anguillarum. Front. Immunol. 2021;12:967. doi: 10.3389/fimmu.2021.639489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M., Yang X., Zeng C., Zhao H., Li J., Hou Z., Wen H. Transcriptional Signatures of Immune, Neural, and Endocrine Functions in the Brain and Kidney of Rainbow Trout (Oncorhynchus mykiss) in Response to Aeromonas salmonicida Infection. Int. J. Mol. Sci. 2022;23:1340. doi: 10.3390/ijms23031340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang K., Yang Q., Liu M., Yang X., Li J., Hou Z., Wen H. Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss) Int. J. Mol. Sci. 2022;23:8691. doi: 10.3390/ijms23158691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirdita M., Schütze K., Moriwaki Y., Heo L., Ovchinnikov S., Steinegger M. ColabFold: Making protein folding accessible to all. Nat. Methods. 2022;19:679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeLano W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002;40:82–92. [Google Scholar]
- 47.Yuan S., Chan H.S., Filipek S., Vogel H. PyMOL and Inkscape bridge the data and the data visualization. Structure. 2016;24:2041–2042. doi: 10.1016/j.str.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love M., Anders S., Huber W. Beginner’s guide to using the DESeq2 package. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson K.A., Krishnan A. Robust normalization and transformation techniques for constructing gene coexpression networks from RNA-seq data. Genome Biol. 2022;23:1. doi: 10.1186/s13059-021-02568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang Z., Chong J., Li S., Xia J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites. 2020;10:186. doi: 10.3390/metabo10050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chong J., Wishart D.S., Xia J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019;68:e86. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- 53.Mouton A.J., Ma Y., Rivera Gonzalez O.J., Daseke M.J., II, Flynn E.R., Freeman T.C., Garrett M.R., DeLeon-Pennell K.Y., Lindsey M.L. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res. Cardiol. 2019;114:6. doi: 10.1007/s00395-019-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H., Soufan O., Xia J., Tang R., Li L., Li D. Transcriptome and physiological analysis reveal alterations in muscle metabolisms and immune responses of grass carp (Ctenopharyngodon idellus) cultured at different stocking densities. Aquaculture. 2019;503:186–197. doi: 10.1016/j.aquaculture.2019.01.003. [DOI] [Google Scholar]
- 55.Bird S., Tafalla C. Teleost chemokines and their receptors. Biology. 2015;4:756–784. doi: 10.3390/biology4040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H., Liu F. Advances in chemokines of teleost fish species. Aquac. Fish. 2023;9:115–125. doi: 10.1016/j.aaf.2023.01.008. [DOI] [Google Scholar]
- 57.Qi Z., Xu Y., Dong B., Pi X., Zhang Q., Wang D., Wang Z. Molecular characterization, structural and expression analysis of twelve CXC chemokines and eight CXC chemokine receptors in largemouth bass (Micropterus salmoides) Dev. Comp. Immunol. 2023;143:104673. doi: 10.1016/j.dci.2023.104673. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Thorgaard G.H., Ristow S.S. Molecular cloning and genomic structure of an interleukin-8 receptor-like gene from homozygous clones of rainbow trout (Oncorhynchus mykiss) Fish Shellfish. Immunol. 2002;13:251–258. doi: 10.1006/fsim.2001.0399. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q., Li R., Monte M.M., Jiang Y., Nie P., Holland J.W., Secombes C.J., Wang T. Sequence and expression analysis of rainbow trout CXCR2, CXCR3a and CXCR3b aids interpretation of lineage-specific conversion, loss and expansion of these receptors during vertebrate evolution. Dev. Comp. Immunol. 2014;45:201–213. doi: 10.1016/j.dci.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniels G.D., Zou J., Charlemagne J., Partula S., Cunningham C., Secombes C.J. Cloning of two chemokine receptor homologs (CXC-R4 and CC-R7) in rainbow trout Oncorhynchus mykiss. J. Leukoc. Biol. 1999;65:684–690. doi: 10.1002/jlb.65.5.684. [DOI] [PubMed] [Google Scholar]
- 61.Grimholt U., Hauge H., Hauge A.G., Leong J., Koop B.F. Chemokine receptors in Atlantic salmon. Dev. Comp. Immunol. 2015;49:79–95. doi: 10.1016/j.dci.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Alejo A., Tafalla C. Chemokines in teleost fish species. Dev. Comp. Immunol. 2011;35:1215–1222. doi: 10.1016/j.dci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Nomiyama H., Hieshima K., Osada N., Kato-Unoki Y., Otsuka-Ono K., Takegawa S., Izawa T., Yoshizawa A., Kikuchi Y., Tanase S., et al. Extensive expansion and diversification of the chemokine gene family in zebrafish: Identification of a novel chemokine subfamily CX. BMC Genom. 2008;9:222. doi: 10.1186/1471-2164-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeVries M.E., Kelvin A.A., Xu L., Ran L., Robinson J., Kelvin D.J. Defining the origins and evolution of the chemokine/chemokine receptor system. J. Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 65.Nomiyama H., Osada N., Yoshie O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010;21:253–262. doi: 10.1016/j.cytogfr.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Chen J., Xu Q., Wang T., Collet B., Corripio-Miyar Y., Bird S., Xie P., Nie P., Secombes C.J., Zou J. Phylogenetic analysis of vertebrate CXC chemokines reveals novel lineage specific groups in teleost fish. Dev. Comp. Immunol. 2013;41:137–152. doi: 10.1016/j.dci.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Zlotnik A., Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Zhang P., Gao C., Cao M., Yang N., Li X., Li C., Fu Q. CXC chemokines and their receptors in black rockfish (Sebastes schlegelii): Characterization, evolution analyses, and expression pattern after Aeromonas salmonicida infection. Int. J. Biol. Macromol. 2021;186:109–124. doi: 10.1016/j.ijbiomac.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Lefkowitz R.J. Seven transmembrane receptors: Something old, something new. Acta Physiol. 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 70.Umasuthan N., Wan Q., Revathy K.S., Whang I., Noh J.K., Kim S., Park M.-A., Lee J. Molecular aspects, genomic arrangement and immune responsive mRNA expression profiles of two CXC chemokine receptor homologs (CXCR1 and CXCR2) from rock bream, Oplegnathus fasciatus. Fish Shellfish. Immunol. 2014;40:304–318. doi: 10.1016/j.fsi.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Liu X., Kang L., Liu W., Lou B., Wu C., Jiang L. Molecular characterization and expression analysis of the large yellow croaker (Larimichthys crocea) chemokine receptors CXCR2, CXCR3, and CXCR4 after bacterial and poly I: C challenge. Fish Shellfish. Immunol. 2017;70:228–239. doi: 10.1016/j.fsi.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 72.Xu T., Zhu Z., Sun Y., Ren L., Wang R. Characterization and expression of the CXCR1 and CXCR4 in miiuy croaker and evolutionary analysis shows the strong positive selection pressures imposed in mammal CXCR1. Dev. Comp. Immunol. 2014;44:133–144. doi: 10.1016/j.dci.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Fu Q., Yang Y., Li C., Zeng Q., Zhou T., Li N., Liu Y., Li Y., Wang X., Liu S., et al. The chemokinome superfamily: II. The 64 CC chemokines in channel catfish and their involvement in disease and hypoxia responses. Dev. Comp. Immunol. 2017;73:97–108. doi: 10.1016/j.dci.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Danik M., Puma C., Quirion R., Williams S. Widely expressed transcripts for chemokine receptor CXCR1 in identified glutamatergic, γ-aminobutyric acidergic, and cholinergic neurons and astrocytes of the rat brain: A single-cell reverse transcription-multiplex polymerase chain reaction study. J. Neurosci. Res. 2003;74:286–295. doi: 10.1002/jnr.10744. [DOI] [PubMed] [Google Scholar]
- 75.Puma C., Danik M., Quirion R., Ramon F., Williams S. The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 2001;78:960–971. doi: 10.1046/j.1471-4159.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 76.Zou J., Redmond A.K., Qi Z., Dooley H., Secombes C.J. The CXC chemokine receptors of fish: Insights into CXCR evolution in the vertebrates. Gen. Comp. Endocrinol. 2015;215:117–131. doi: 10.1016/j.ygcen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Gao W., Li S., Xu Q., Zhu D., Zhang Q., Luo K., Zhang W. Molecular characterization and expression analysis of Asian swamp eel (Monopterus albus) CXC chemokine receptor (CXCR) 1a, CXCR1b, CXCR2, CXCR3a, CXCR3b, and CXCR4 after bacteria and poly I: C challenge. Fish Shellfish. Immunol. 2019;84:572–586. doi: 10.1016/j.fsi.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 78.Zhao S., Li Y., Cao M., Yang N., Hu J., Xue T., Li C., Fu Q. The CC and CXC chemokine receptors in turbot (Scophthalmus maximus L.) and their response to Aeromonas salmonicida infection. Dev. Comp. Immunol. 2021;123:104155. doi: 10.1016/j.dci.2021.104155. [DOI] [PubMed] [Google Scholar]
- 79.Goldberg S.H., Van Der Meer P., Hesselgesser J., Jaffer S., Kolson D.L., Albright A.V., González-Scarano F., Lavi E. CXCR3 expression in human central nervous system diseases. Neuropathol. Appl. Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 80.Soto H., Wang W., Strieter R.M., Copeland N.G., Gilbert D.J., Jenkins N.A., Hedrick J., Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc. Natl. Acad. Sci. USA. 1998;95:8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang M.X., Sun B.J., Nie P. The first non-mammalian CXCR3 in a teleost fish: Gene and expression in blood cells and central nervous system in the grass carp (Ctenopharyngodon idella) Mol. Immunol. 2007;44:1123–1134. doi: 10.1016/j.molimm.2006.07.280. [DOI] [PubMed] [Google Scholar]
- 82.Aquilino C., Castro R., Fischer U., Tafalla C. Transcriptomic responses in rainbow trout gills upon infection with viral hemorrhagic septicemia virus (VHSV) Dev. Comp. Immunol. 2014;44:12–20. doi: 10.1016/j.dci.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Wang T., Hanington P.C., Belosevic M., Secombes C.J. Two macrophage colony-stimulating factor genes exist in fish that differ in gene organization and are differentially expressed. J. Immunol. 2008;181:3310–3322. doi: 10.4049/jimmunol.181.5.3310. [DOI] [PubMed] [Google Scholar]
- 84.Ehlert J.E., Addison C.A., Burdick M.D., Kunkel S.L., Strieter R.M. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J. Immunol. 2004;173:6234–6240. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- 85.Wu Q., Dhir R., Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol. Cancer. 2012;11:1–16. doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Müller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 87.Teicher B.A., Fricker S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 88.Oberlin E., Amara A., Bachelerie F., Bessia C., Virelizier J.-L., Arenzana-Seisdedos F., Schwartz O., Heard J.-M., Clark-Lewis I., Legler D.F., et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 89.Feng Y., Broder C.C., Kennedy P.E., Berger E.A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 90.Lin C.-Y., Chen Y.-M., Hsu H.-H., Shiu C.-T., Kuo H.-C., Chen T.-Y. Grouper (Epinephelus coioides) CXCR4 is expressed in response to pathogens infection and early stage of development. Dev. Comp. Immunol. 2012;36:112–120. doi: 10.1016/j.dci.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., Wei X.L., Chen L.P., Chen N., Li Y.H., Wang W.M., Wang H.L. Sequence analysis and expression differentiation of chemokine receptor CXCR4b among three populations of Megalobrama amblycephala. Dev. Comp. Immunol. 2013;40:195–201. doi: 10.1016/j.dci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Xu Q.Q., Chang M.X., Sun R.H., Xiao F.S., Nie P. The first non-mammalian CXCR5 in a teleost fish: Molecular cloning and expression analysis in grass carp (Ctenopharyngodon idella) BMC Immunol. 2010;11:25. doi: 10.1186/1471-2172-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kizil C., Dudczig S., Kyritsis N., Machate A., Blaesche J., Kroehne V., Brand M. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 2012;7:27. doi: 10.1186/1749-8104-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu B., Chien E.Y.T., Mol C.D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F.C., et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skerlj R.T., Bridger G.J., Kaller A., McEachern E.J., Crawford J.B., Zhou Y., Atsma B., Langille J., Nan S., Veale D., et al. Discovery of Novel Small Molecule Orally Bioavailable C− X− C Chemokine Receptor 4 Antagonists That Are Potent Inhibitors of T-Tropic (X4) HIV-1 Replication. J. Med. Chem. 2010;53:3376–3388. doi: 10.1021/jm100073m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.