Abstract
The induction kinetics of the transcriptional activities of interferon regulatory factor 1 (IRF-1), interleukin-1β-converting enzyme (ICE), and CPP32 by respiratory syncytial virus (RSV) infection of human type II alveolar epithelial cells (A549 cells) were analyzed semiquantitatively by reverse transcriptase PCR. The appearance of ICE and CPP32 protein in cell lysate was examined by Western blotting analysis. The induction of apoptosis by RSV infection was examined by the appearance of DNA fragmentation detected by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling. RSV moderately enhanced IRF-1 mRNA as early as 4 h after infection, and this enhancement lasted several hours. Following induction of the IRF-1 gene, ICE gene expression increased significantly, and an increase of ICE protein was observed in the RSV-infected cell lysate. These increments were observed in cells treated with live RSV but not in cells treated with inactivated RSV or control antigen. However, no infection-specific increase of CPP32 gene expression or the protein was observed. No nucleosomal fragmentation was observed in RSV-infected cells during the whole course of infection, despite the appearance of extensive cytopathic change and cell death. These observations suggest that RSV infection of human alveolar epithelial cells induces the ICE gene and its protein as a result of increased IRF-1 induction but that the increased ICE was insufficient to cause apoptosis in the RSV-infected cells. ICE might not be able to activate CPP32, which is thought to be the more important protease for apoptosis.
Respiratory syncytial virus (RSV) infection in neonates and young infants often causes life-threatening acute bronchiolitis (1, 2). The peculiar tropism of RSV for the bronchiolar epithelium and the fragile anatomy of the infants’ bronchioles are possible factors in the pathogenesis of acute bronchiolitis (12). However, the precise mechanisms of respiratory epithelial cell death after RSV infection remain unclear.
Recently, two major morphologically and biochemically distinct modes of cell death have been described, apoptosis, or programmed cell death, and necrosis (4, 14, 36). In human viral infections, such as human immunodeficiency virus type 1 (9, 29) and influenza (11, 25), apoptosis is thought to be the major mode of cell death due to the virus infection. The induction of apoptosis in virus-infected cells is now considered to be the major mechanism for viral clearance by the mammalian immune system (3). However, the participation of apoptosis in other human respiratory viral infections, including RSV, has not yet been fully investigated.
Tumor suppressor interferon regulatory factor 1 (IRF-1) plays an essential role in apoptosis (26, 28) and is a transcriptional activator of the interleukin-1β-converting enzyme (ICE) gene (26, 27). ICE is the first mammalian homolog of the Caenorhabditis elegans cell death gene, ced-3, and ICE and ICE-related protease have been implicated in apoptosis (26, 27). Furthermore, another member of the ced3/ICE family, CPP32/yama, is now thought to be the more important and dominant protease that directly cleaves the death substrate poly(ADP-ribose) polymerase (5, 10, 31). The existence of a protease cascade is now postulated; activated TX, an ICE family member, cleaves pro-ICE, and activated ICE cleaves pro-CPP32 to make the active CPP32 form (5, 6, 19).
However, the precise kinetics and role of IRF-1, ICE, and CPP32 gene expression in virus-induced apoptosis in human cells has not yet been fully investigated. In this study, we examined the occurrence of apoptosis in human type II alveolar epithelial cells (A549 cells) infected by RSV. The kinetics of the transcriptional activities of the IRF-1, ICE, and CPP32 genes and the appearance of these proteins in RSV-infected cells were analyzed, and the appearance of DNA fragmentation was investigated.
A549 cells, which were thought to be susceptible to RSV infection, were used for these studies (21). A549 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum (FCS). For the experiments, cells were detached from the plastic by incubation in trypsin and EDTA for 5 min and then seeded (105 cells) to 24-well semimicroplates (Nunc, Roskilde, Denmark) on the 2 days before infection. The cells were generally 90% confluent by the 2nd day after seeding.
RSV strain Long (prototype RSV group A strain) grown in HEp-2 cells was used for infection. The stock virus titer was 107 PFU/ml. Virus was made replication deficient (inactive) by treatment in hot water (55°C) for 20 min. Uninfected HEp-2 cell culture fluid was processed similarly for use in a mock infection. RSV was diluted in DMEM supplemented with 2% FCS to a multiplicity of infection of 1.0. The medium was removed from the A549 cells and replaced with DMEM and 2% FCS at the desired multiplicity of infection of RSV for 1 h at 37°C. The same volume of inactivated virus preparations and HEp-2 cell culture fluid was used in a similar fashion for controls.
To examine the expression of mRNA for IRF-1, ICE, and CPP32, A549 cells were harvested at 0 h (preinfection) and at 4, 7, and 10 h after RSV exposure; A549 cells were washed with phosphate-buffered saline and treated with 0.8 ml of RNAzol B (Biotecx Laboratories, Houston, Tex.) for RNA extraction.
Total cellular RNA was isolated from A549 cells with or without RSV infection with RNAzol B and tested for IRF-1, ICE, or CPP32 mRNA by specific reverse transcriptase (RT) PCR as described previously (17). As an internal control, the activity of β-actin mRNA was also determined. Fifty nanograms of the total RNA was used for RT PCR. For cDNA synthesis, 40 μl of an RNA solution (50 ng) and a random hexamer at 150 pmol/3 μl (Takara, Kyoto, Japan) was heated at 70°C for 10 min and cooled rapidly. After the addition of a solution of 17 μl of 5× first-strand buffer (250 mM Tris-HCl, 375 mM KCl, 15 mM MgCl2), 9 μl of 0.1 mM dithiothreitol (GIBCO BRL), 17 μl of 2.5 mM (each) deoxynucleoside triphosphate (Takara), and 200 U of Maloney murine leukemia virus RT (GIBCO BRL), the mixture was stored at 37°C for 1 h. Sequences of the PCR primer pairs are as follows: for β-actin, CCTTCCTGGGCATGGAGTCCTG and GGAGCAATGATCTTGATCTTC; for IRF-1, AAGCATGCTGCCAAGCATGGCTGG and ATCAGGCAGAGTGGAGCTGCT; for ICE, GCTATTAAGAAAGCCCA and TCAGTGGTGGGCATCTG; and for CPP32, AGCACTGGAATGACATCTCGGT and CAGCATGGCACAAAGCGAC. These were described previously (15, 31, 32). PCR primers and specific probes for ICE were chosen from the nucleotide sequence for ICE p10 (32). The PCR mixture contained 50 ng of cDNA, 10 μl of PCR buffer (500 mM KCl, 10 mM Tris, 1% Triton X-100), 8 μl of 2.5 mM each deoxynucleoside triphosphate (Takara), 6 μl of 25 mM MgCl2, 100 pM 5′ and 3′ primers, and distilled water for a total volume of 100 μl. After being denatured at 94°C for 10 min and cooled to 80°C, the mixture was seeded with 2.5 μl of thermostable Taq polymerase (Promega, Madison, Wis.). Twenty-four cycles of amplification for β-actin, 24 for IRF-1, 31 for ICE, and 24 for CPP32 were carried out with a DNA thermal cycler (Perkin-Elmer, Norwalk, Conn.). Each cycle consisted of warming at 95°C for 35 s, 55°C for 2 min, and 72°C for 2 min. Finally, the preparations were incubated at 72°C for 15 min. Ten-microliter samples of the RT PCR products were analyzed by electrophoresis on a 2% agarose gel, and the amplified products were visualized by UV fluorescence after staining with ethidium bromide. The UV fluorescence signals of specific PCR products in agarose gels were quantified with a FluorImager SI (Molecular Dynamics, Sunnyvale, Calif.). The specificity of each PCR product was confirmed by determining its predicted size on agarose gels and by Southern blot analysis as described previously (22) (data not shown). Sequences of the specific oligonucleotide probes are as follows: for β-actin, AAAGACCTGTACGCCAACA; for IRF-1, AAGGCCAACTTTCGCTGTGCC; for ICE, ATAGAGAAGGATTTTATCGC; and for CPP32, GCCATCCTTTGAATTTCGCC (15, 31, 32).
As a positive control for mRNA for β-actin, IRF-1, ICE, and CPP32, total RNA from normal adult peripheral blood mononuclear cells which were incubated with 10 μg of concanavalin A per ml for 3 h was used.
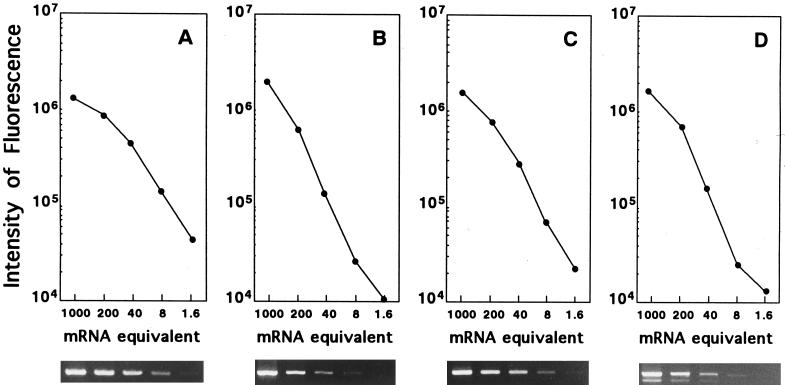
To quantify relative levels of mRNA, a standard curve was obtained by titration (1/5 dilution) of first strands obtained from 250 ng of RNA from the positive controls described above (Fig. 1). Relative differences in mRNA expression in the test samples were obtained by determination of the standard curves run for the same number of cycles as the unknown samples. The intensity of fluorescence of DNA amplified from first strands obtained from 250 ng of RNA was defined arbitrarily as 1,000 mRNA equivalents.
FIG. 1.
Ethidium bromide-stained gels and standard curves of β-actin (A), IRF-1 (B), ICE mRNA (C), and CPP32 mRNA (D) generated by RT PCR with control RNA. Two hundred fifty nanograms of total RNA was reverse transcribed, and fivefold dilutions of the first strand were amplified by PCR. The intensity of fluorescence of DNA amplified from the first strand obtained from 250 ng of total RNA was defined arbitrarily as 1,000 mRNA equivalents.
The level of β-actin in each unknown sample was determined from the actin standard curve, and the levels generally varied less than 30% when the first strand was obtained from the same amount of total RNA (data not shown).
The production of ICE and CPP32 protein in RSV-infected cell lysate was investigated 12, 18, and 24 h after treatment by Western blot analysis (33). Briefly, RSV-infected and control A549 cells were dissolved in RIPA buffer (0.05 M Tris [pH 7.2], 0.15 M NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.05% sodium dodecyl sulfate) and centrifuged. Ten micrograms of each sample was mixed with sample buffer (0.06 M Tris [pH 6.8], 2% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 10% glycerol), heated at 95°C for 5 min, and electrophoresed on a sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis gel. The separated protein was transferred to polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) in transfer buffer (0.1 M Tris, 0.1 M glycine, 20% methanol). The membrane was blocked with a solution of 0.02 M Tris (pH 7.6), 0.137 M NaCl, 0.1% Tween 20, and 5% skim milk and then treated with anti-ICE p20 rabbit antibody (Upstate Biotechnology, Lake Placid, N.Y.) or anti-CPP32 mouse antibody (Transduction Laboratories, Lexington, Ky.) and incubated for 1 h at room temperature. After being washed, the membrane was treated with horseradish peroxidase-conjugated goat anti-rabbit or -mouse immunoglobulin G for 1 h at room temperature. The membrane was treated with enhanced chemiluminescence Western blotting detection reagents (Amersham, Buckinghamshire, United Kingdom). The positive signals in the membrane were detected by X-ray film.
DNA cleavage into oligonucleosomal-length DNA fragments in RSV-infected cells was checked by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (8) by using the in situ cell death detection kit with fluorescein (Boehringer, Mannheim, Germany) and flow cytometry according to the manufacturer’s instructions. Monolayer cells in 24-well semimicroplates were rinsed twice with phosphate-buffered saline and trypsinized preinfection and 36 and 48 h after RSV infection. For positive control of DNA fragmentation, A549 cells treated with DNase I (GIBCO BRL) (10 μg/ml for 10 min) were employed (8).
Multiple foci of typical syncytia could be detected by light microscopy 18 h after infection. Approximately 30% (18 h) and 60% (36 h) of A549 cells were positive for viral antigen as determined by immunofluorescent antibody staining with rabbit antibodies to the Long strain of RSV (DAKOPATTS, Copenhagen, Denmark). Around 2 × 105 PFU of live virus per ml were detected in the culture medium 48 h after infection. At that time, extensive cytopathic change, cell death, and detachment of cells from the plastic surface were evident. Cell viability was checked by the trypan blue dye exclusion test. Around 20, 50, and 75% of cells in RSV-treated wells were positive for this test or detached from the plastic surface as a result of cytopathic effect at 24, 36, and 48 h after RSV infection, respectively.
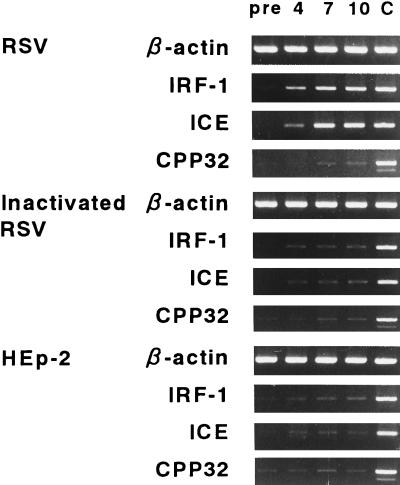
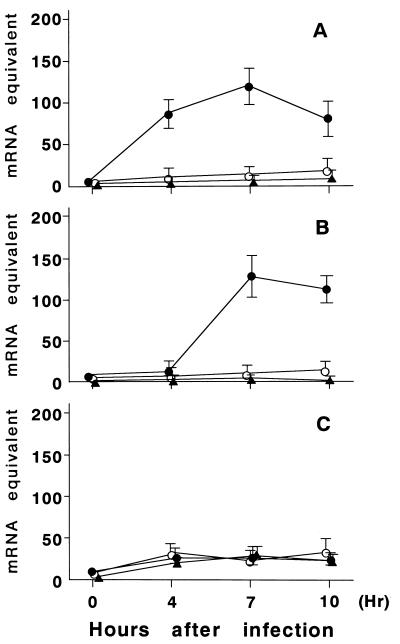
The expression of mRNA for IRF-1, ICE, and CPP32 in A549 cells was determined at 0 h (preinfection) and at 4, 7, and 10 h after RSV exposure (Fig. 2). The cells exposed by inactivated RSV or HEp-2 cells were analyzed similarly. RSV infection resulted in a significant increase in IRF-1 gene expression as early as 4 h after infection that lasted several hours (Fig. 3). Following induction of the IRF-1 gene, ICE gene expression increased over 100 times compared to preinfection levels about 7 h after infection. This increase continued at least 10 h after infection. These increments were observed in cells treated by live RSV but not in cells treated by inactivated RSV or in HEp-2 control cells. A slight increase of CPP32 mRNA expression was observed 4 h after RSV infection; this increase was thought to be nonspecific, because a similar induction of the CPP32 gene was observed in cells treated by inactivated RSV or HEp-2 control cells.
FIG. 2.
Expression of IRF-1, ICE, and CPP32 genes in A549 cells exposed to RSV determined by RT PCR analysis. mRNA expression was assessed before treatment (pre) and at 4, 7, and 10 h after treatment. Lane C, positive control.
FIG. 3.
Semiquantitative analysis of IRF-1 (A), ICE (B), and CPP32 (C) gene expression in A549 cells treated with live RSV (•), inactivated RSV (○), or HEp-2 control cells (▴). mRNA expression was assessed before treatment (pre) and at 4, 7, and 10 h after treatment with standard curves shown in Fig. 1. Error bars represent standard deviations of three experiments.
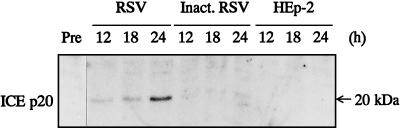
ICE and CPP32 protein levels were analyzed with Western blots. No ICE protein expression was detected in the lysate of pretreated or inactivated virus- or HEp-2 control cell-treated cells. However, in the RSV-infected cells, a 20-kDa (p20) ICE protein was detected 12 h after infection and accumulated during the next 12 h of incubation (Fig. 4).
FIG. 4.
Western blot analysis of ICE p20 protein expression. RSV-infected, inactivated (Inact.) RSV-treated, or HEp-2 cell-treated A549 cells were lysed after the indicated times (h) of treatment, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred, and developed with anti-human ICE p20 antibodies.
On the other hand, slight amounts of CPP32 protein were detected in the pretreated cells. However, no infection-specific increase of this protein was observed 24 h after infection (data not shown).
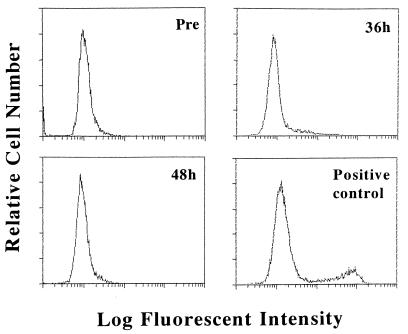
Flow cytometric analysis by TUNEL showed that no apoptotic cells, which have fragmented DNA, were detected in RSV-infected cells throughout infection (Fig. 5).
FIG. 5.
Flow cytometric analysis of apoptotic cells with fragmented DNA in RSV-infected cells. A549 cells in a semimicroplate were trypsinized pretreatment (pre) and at 36 and 48 h after RSV infection, labeled by TUNEL, and analyzed by flow cytometry. As a positive control, DNase I-treated A549 cells were used; around 20% of these cells were shown to be positive.
Several mechanisms must be analyzed to clarify the pathogenesis of RSV bronchiolitis, in which RSV causes cytopathic damage of infected cells. In this study, the well-differentiated human alveolar epithelial cell line A549 was used to investigate apoptosis in RSV-infected cells. RSV infected A549 cells effectively, and moderate amounts of infectious virus could be recovered from infected cells. While RSV infection did not induce apoptosis, it was confirmed for the first time that RSV infection in human cells enhances IRF-1 and ICE gene expression and the production of the 20-kDa (p20) ICE protein, a constituent of the active ICE that is processed proteolytically from an inactive 45-kDa precursor (6, 32). Inactivated RSV did not induce these transcriptional activities, suggesting that the replicative activity of RSV is essential for these events.
IRF-1 was first identified as a nuclear factor that specifically binds to the upstream regulatory region of the IFN-β gene, while IRF-1 gene expression was induced by Newcastle disease virus in mouse L929 cells (18). IRF-1-dependent upregulation of ICE in mitogen-stimulated or IFN-γ-treated cells is, by itself, insufficient to trigger apoptosis (26, 27). Additional stimuli, such as γ-irradiation or antitumor agents, which may damage cellular DNA, are needed for apoptosis (26, 27). In our study, RSV-induced ICE gene expression was not sufficient to induce apoptosis in A549 cells, although overexpression of ICE may bring about increased susceptibility to apoptosis in infected cells.
The lack of apoptosis in RSV-infected cells may be explained by the role of CPP32, the dominant protease for apoptosis (5, 10, 31). There was no increase in either CPP32 mRNA or protein level, despite the enhanced ICE mRNA and protein level in the RSV-infected cells. However, it is unlikely that the constant level of CPP32 in RSV-infected cells explains the absence of apoptosis, because in Fas-mediated apoptosis in mice and human cells, CPP32 cleaves poly(ADP-ribose) polymerase, the death substrate, without a change in CPP32 mRNA and protein levels (5, 10). Although RSV infection of A549 cells induced overexpression of the ICE gene, this increased ICE may be insufficient to continue the protease cascade in which activated ICE is believed to cleave pro-CPP32 to active CPP32 (31).
In contrast, influenza virus infection induces apparent apoptosis in tissue culture cells (11, 25). Enhancement of ICE and CPP32 protease activities and Fas gene and Fas antigen expression accompany this influenza virus-induced apoptosis (23, 24, 25). In our study, infection-specific induction of the Fas gene was not observed in RSV-infected A549 cells (data not shown). This may be another reason why RSV infection by itself does not induce apparent apoptosis.
Recently, it was reported that the transcriptional activator nuclear factor kappa B (NF-κB) induces protective proteins which can help cells resist apoptosis (34, 35). Apoptotic stimuli such as radiation, daunorubicin, or tumor necrosis factor alpha (TNF-α) also lead to the inhibition of apoptosis through the activation of NF-κB (34, 35). RSV infection of A549 cells is known to increase NF-κB activity (7, 21). Therefore, RSV infection of A549 cells might be speculated to induce apoptosis gene and protein, but the cells are protected from apoptosis by NF-κB, which is also activated by RSV infection.
Upon activation, both Fas and TNF have been shown to induce apoptosis either by their respective ligands or by cross-linking with an agonist antibody (13, 30, 37). Many respiratory viruses, including RSV, induce TNF-α at their infection sites (16, 20). Possibly, TNF-α produced by RSV infection induces apoptosis on the RSV-infected respiratory epithelium, which already has increased susceptibility to apoptosis in an autocrine or paracrine form. The precise mechanism and participation of apoptosis in virus-induced cell damage during acute bronchiolitis caused by RSV or other respiratory viruses remain to be elucidated.
REFERENCES
- 1.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, editors. Fields virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 1313–1351. [Google Scholar]
- 2.Denny F W, Clynde W A. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 3.Duke R C. Apoptosis in cell-mediated immunity. In: Tomei L D, Cope F O, editors. Apoptosis: the molecular basis of cell death. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 209–226. [Google Scholar]
- 4.Duvall E, Wyllie A H. Death and the cell. Immunol Today. 1986;7:115–119. doi: 10.1016/0167-5699(86)90152-0. [DOI] [PubMed] [Google Scholar]
- 5.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 6.Faucheu C, Diu A, Chan A W E, Blanchet A M, Miossec C, Herve F, Collard-Dutilleul V, Gu Y, Aldape R A, Lippke J A, Rocher C, Su M M S, Livingston D J, Hercend T, Lalanne J L. A novel human protease similar to the interleukin-1β converting enzyme induces apoptosis in transfected cells. EMBO J. 1995;14:1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedler M A, Wernke-Dollries K, Stark J M. Inhibition of viral replication reverses respiratory syncytial virus-induced NF-κB activation and interleukin-8 gene expression in A549 cells. J Virol. 1996;70:9079–9082. doi: 10.1128/jvi.70.12.9079-9082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gougeon M, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Involvement of CPP32/Yama(-like) proteases in Fas-mediated apoptosis. Cancer Res. 1996;56:1713–1718. [PubMed] [Google Scholar]
- 11.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg J C, Williams J, Richardson J B, Macklem P T, Thurlbeck W M. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282:1283–1287. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- 13.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptides encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 14.Kerr J F R, Harmon B V. Definition and incidence of apoptosis: a historical perspective. In: Tomei L D, Cope F O, editors. Apoptosis: the molecular basis of cell death. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 5–29. [Google Scholar]
- 15.Maruyama M, Fujita T, Taniguchi T. Sequence of a cDNA coding for human IRF-1. Nucleic Acids Res. 1989;17:3292. doi: 10.1093/nar/17.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda K, Tsutsumi H, Okamoto Y, Chiba S. Development of interleukin 6 and tumor necrosis factor alpha activity in nasopharyngeal secretion of infants and children during infection with respiratory syncytial virus. Clin Diagn Lab Immunol. 1996;2:322–324. doi: 10.1128/cdli.2.3.322-324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda K, Tsutsumi H, Sone S, Yoto Y, Oya K, Okamoto Y, Ogra P L, Chiba S. Characteristics of IL-6 and TNF-α production by respiratory syncytial virus-infected macrophages in the neonate. J Med Virol. 1996;48:199–203. doi: 10.1002/(SICI)1096-9071(199602)48:2<199::AID-JMV13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-β gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 20.Noah T L, Henderson F W, Wortman I A, Devlin R B, Handy J, Koren H S, Becker S. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 21.Patel J A, Kunimoto M, Sim T C, Garofalo R, Elliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra P L, Schmalstieg F. IL-1α mediates enhanced expression of ICAM-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi R, Tsutsumi H, Osaki M, Sone S, Imai S, Chiba S. Respiratory syncytial virus infection of neonatal monocytes stimulates synthesis of interferon regulatory factor 1 and interleukin-1β (IL-1β)-converting enzyme and secretion of IL-1β. J Virol. 1998;72:837–840. doi: 10.1128/jvi.72.1.837-840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takizawa T. Induction of apoptosis by influenza virus infection. Virus. 1997;47:69–76. doi: 10.2222/jsv.47.69. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 24.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–296. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 26.Tamura T, Ishihara M, Lamphier M S, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak T W, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;376:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 27.Tamura T, Ueda S, Yoshida M, Matsuzaki M, Mohri H, Okubo T. Interferon-γ induces ICE gene expression and enhances cellular susceptibility to apoptosis in the U937 leukemia cell line. Biochem Biophys Res Commun. 1996;229:21–26. doi: 10.1006/bbrc.1996.1752. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier M S, Aizawa S, Mak T W, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 29.Terai C, Kornbluth R S, Pauza C D, Richman D D, Carson D A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 31.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beilder D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 32.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, Elliston K O, Ayala J M, Casano F J, Chin J, Ding G J F, Egger L A, Gaffney E P, Limjuco G, Palyha O C, Raju S M, Rolando A M, Salley J P, Yamin T T, Lee T D, Shively J E, MacCross M, Mumford R A, Schmidt J A, Tocci M J. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi H, Flanagan T D, Ogura P L. Monoclonal antibodies to the large glycoproteins of respiratory syncytial virus: possible evidence for functional antigenic sites. J Gen Virol. 1987;68:2161–2167. doi: 10.1099/0022-1317-68-8-2161. [DOI] [PubMed] [Google Scholar]
- 34.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 35.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 36.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 37.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]