Abstract
The effect of glucagon on the establishment of hepadnavirus infection was studied in vitro with the duck hepatitis B virus (DHBV) model. The presence of the peptide hormone throughout infection or starting up to 8 h after virus uptake resulted in a dose-dependent reduction in the levels of intra- and extracellular viral gene products and of secreted virions. Treatment with forskolin or dibutyryl-cyclic AMP, two drugs that also stimulate the cyclic AMP (cAMP) signal transduction pathway, resulted in comparable inhibition, suggesting that the inhibitor effect is related to changes in the activity of protein kinase A. In persistently infected hepatocytes, only a slight, but continuous, decrease in viral replication was observed upon prolonged drug treatment. Time course analysis, including detection of DHBV covalently closed circular (ccc) DNA templates, revealed that glucagon acts late during the establishment of infection, at a time when the virus is already internalized, but before detectable ccc DNA accumulation in the nucleus. These data suggest that nuclear import (and reimport) of DHBV DNA genomes from cytosolic capsids is subject to cAMP-mediated regulation by cellular factors responding to changes in the state of the host cell.
Hepadnaviruses are a family of small, enveloped DNA viruses which cause acute and chronic liver infections in their respective hosts. With the human hepatitis B virus (HBV) being the prototype because of its medical importance, this virus family also comprises HBVs that infect woodchucks and ducks, which are presently being used as valuable animal models (16). A narrow host range and a distinct organ tropism are characteristics of these viruses, as is a pararetroviral replication strategy which involves a circular DNA genome and protein-primed reverse transcription of a linear RNA pregenome. In recent years, much progress has been made in defining, on the molecular level, the mechanisms of intracellular genome replication by use of genetic and biochemical analyses with transfected hepatoma cells (for recent reviews, see references 15 and 17). In contrast, very little is known about the early steps of HBV infection, since there is no cell culture system that allows controlled infection studies in vitro. Although the latter are possible with the duck HBV (DHBV) animal model, with which detailed studies have created a general outline of the hepadnavirus entry pathway and the steps thereafter, many details as well as some basic elements are still missing.
Thus, even for DHBV, there is only limited knowledge of the initial steps of virus uptake, which is generally assumed to include receptor binding (12), endocytosis (14), capsid release by membrane fusion, and nuclear import of the genome (11). The steps following nuclear repair synthesis of the open circular viral DNA genome into covalently closed circular (ccc) DNA templates are better understood. Early during the infection cycle, the DNA in mature capsids is reimported into the nucleus, resulting in amplification of the copy number of ccc DNA. Late during infection, the production of the large viral envelope protein (L-protein) inhibits this amplification by redirecting viral nucleocapsids into enveloped virus particles, which are exported from the cell (27, 28). A regulation mechanism based on the titration of cytoplasmic capsids by the membrane-bound L-protein may not be the only way in which hepadnaviruses control intracellular genome amplification via nuclear import (27). Other, more subtle regulatory mechanisms resulting in changes in the state of the cell are suggested by several, rather unrelated observations. There is evidence for human HBV (8) and DHBV (21) that the L-protein may act as a transcriptional transactivator through phosphorylation of cytosolic pre-S domains, which in DHBV was found to vary with stress-induced signal transduction (22). In addition, abnormally high levels of viral ccc DNA were observed in aging hepatocyte cultures, although levels of DHBV L-protein remained constant (5, 28). Finally, in HBV-transgenic mice, no ccc DNA synthesis takes place, although L-protein levels are normal, and furthermore, genome replication is highly sensitive to cytokine-mediated inhibition (6).
Here we have examined a regulatory effect on DHBV infection after disturbing the state of the host cell by treatment with glucagon, a peptide hormone that causes transient and long-term changes in hepatocytes. In vertebrates, glucagon is known to play a key role in the regulation of blood glucose concentration. Secreted from pancreatic islet cells in response to a low glucose level, it binds to a transmembrane receptor on the hepatocyte, its primary target, to activate a G-protein-coupled intracellular adenylate cyclase, which in turn results in a quick increase in the intracellular cyclic AMP (cAMP) level and thereby activates protein kinase A (PKA). Activation of PKA can lead to short-time effects dependent on direct phosphorylation of cytoplasmic proteins, e.g., the key enzymes of the glycogen-synthesizing pathway. In addition, glucagon is known to cause increases in the concentrations of intracellular calcium and inositol phosphates (26, 31), which could result in the activation of protein kinase C and/or Ca-calmodulin dependent kinase.
In this study, we demonstrate that glucagon treatment induces dramatic changes in the susceptibility of duck hepatocytes to DHBV infection. Our data indicate that this effect is related to cAMP-mediated down regulation of PKA activity. They furthermore suggest that interference with virus infection may occur at the level of nuclear import of viral genomes from the cytosol.
MATERIALS AND METHODS
Cell culture.
Primary duck hepatocytes were prepared and cultured essentially as described previously (20). Briefly, 2- or 3-week-old ducklings were starved for 24 h. Hepatocytes were isolated by two-step collagenase perfusion and seeded at a density of approximately 106 cells per well of a six-well plate (Costar). Cells were maintained at 37°C in 5% CO2 in William’s medium E (GIBCO) supplemented with gentamicin (50 μg/ml), l-glutamine (2.25 mM), glucose (0.06%), HEPES (pH 7.4) (23 mM), hydrocortisone (4.8 μg/ml), inosine (1 μg/ml), penicillin (50 IU/ml), streptomycin (50 μg/ml), and dimethyl sulfoxide (1.7%). Unless otherwise stated, cells were used for infection studies on the first or second day after plating. Medium and drugs were renewed every 24 or 48 h throughout the experiment.
DHBV infection.
Duck sera containing approximately 1010 to 1011 DNA genome equivalents per ml were obtained from 4- to 6-week-old DHBV-positive ducks, divided into aliquots, and stored at −70°C. This virus stock was diluted prior to inoculation into prewarmed (room temperature) culture medium, resulting in a final concentration of between 1 × 108 and 5 × 108 viral genomes per ml. To enhance infection, the virus and hepatocytes were incubated overnight at 20 to 23°C in 1 ml of culture medium per well (multiplicity of infection [MOI], 100 to 500, based on DHBV DNA genome equivalents), allowing binding but not penetration of the virus. Virus uptake was started by incubating the cells at 37°C for 6 h. Thereafter, external virus was inactivated by incubation with pH 2.2 buffer (50 mM glycine-HCl, 150 mM NaCl) for 45 s, followed by two washes with phosphate-buffered saline (PBS) (140 nM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4) and further incubation with culture medium. Unless otherwise stated, drugs were added to the culture medium at the following concentrations: 250 nM glucagon (Sigma Aldrich), 1 mM dibutyryl-cAMP (Sigma Aldrich), 25 μM forskolin (ICN Biomedicals), and 500 nM des-His1,[Glu9]-glucagonamid (Sigma Aldrich).
Assays detecting DHBV infection.
The infection efficiency was examined by immunofluorescence microscopy of fixed cells, enhanced chemiluminescence (ECL)-Western blotting of lysates, DNA dot blotting of lysates and supernatants, immunodot blotting of supernatants, and over-gap PCR of lysates.
(i) Immunofluorescence microscopy.
After removal of the supernatant, the cells were washed several times with PBS and fixed in 4% paraformaldehyde. Infected cells were detected with a mixture of antisera recognizing the DHBV core protein (D087 polyclonal rabbit serum [24]) and L-protein (D084 polyclonal rabbit serum [23]), followed by fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (Dianova).
(ii) ECL-Western blotting.
After removal of the culture medium, the cells were washed with PBS and lysed by the addition of 500 μl of protein sample buffer (200 mM Tris-HCl [pH 8.8], 10% sucrose, 5 mM EDTA, 0.1% bromphenol blue, 3% sodium dodecyl sulfate [SDS], 2% β-mercaptoethanol) per well. Lysates (5% of a well) were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by Western blotting. For detection of viral proteins, we used antisera recognizing the DHBV core protein or the pre-S domain of the L-protein, followed by peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dianova) and detection with an ECL system (Amersham Buchler).
(iii) DNA dot blotting.
For detection of intracellular viral DNA, part of the lysate prepared for protein determination was digested with proteinase K (200 μg/ml)–100 mM NaCl–25 mM EDTA for 3 h at 37°C. After phenol-chloroform extraction, aliquots corresponding to 3% of a well or, in the case of in vivo-infected cells, 0.2% of a well were diluted in 100 μl of PBS and used for DNA dot blotting. After denaturation, renaturation, and hybridization with a random-primed DHBV probe, filters were exposed on X-ray films or quantitated with a Molecular Dynamics PhosphorImager. Secreted virus present in 1 ml of cell culture medium (50% of a well) was pelleted by ultracentrifugation (TLA45 rotor, 44,000 rpm, 1 h 20°C), resuspended in 100 μl of PBS, and directly used for DNA dot blotting.
(iv) DHBeAg immunodot blotting.
Supernatants remaining after pelleting of virus particles were used for the determination of secreted duck hepatitis e antigen (DHBeAg). Medium corresponding to one fourth of a well was applied to a nitrocellulose filter with a dot blot apparatus. Filters were dried and blocked by incubation in 2% bovine serum albumin/TBST (TBST is 10 mM Tris-Cl [pH 8], 150 mM NaCl, and 0.2% Tween 20) for 1 h at room temperature. After overnight incubation with D087 (an antiserum recognizing duck hepatitis B core antigen and DHBeAg), DHBeAg was quantitated by binding of 35S-labeled protein A (0.04 μCi/ml; Amersham Buchler) and exposure on a Molecular Dynamics PhosphorImager.
(v) Over-gap PCR.
Over-gap PCR was performed as outlined by Köck and Schlicht (13). Briefly, DHBV-infected and uninfected hepatocytes were lysed 2 days postinfection by the addition of 0.5 ml of lysis buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.3], 15 mM MgCl2, 0.01% gelatin, 0.45% Nonidet P-40) and incubated with 50 μg of proteinase K for 2 h at 56°C. After proteinase inactivation (10 min, 95°C), a 5-μl aliquot was analyzed in a 100-μl PCR. PCR products in 10-μl aliquots were separated on a 1% agarose gel and analyzed by Southern blotting.
RESULTS
Treatment of primary duck hepatocytes with glucagon inhibits DHBV infection.
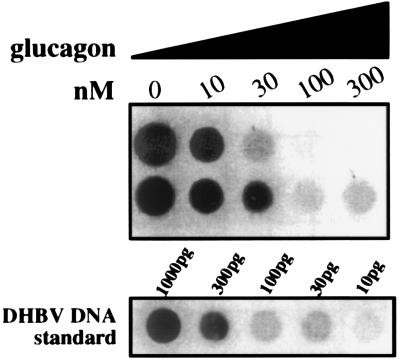
In initial experiments, we observed fortuitously that productive DHBV infection of cultured primary duck hepatocytes was substantially reduced by the peptide hormone glucagon. Follow-up experiments (Fig. 1) demonstrated that the inhibition was dose dependent, 10 or 300 nM glucagon resulting 10 days postinfection in a 10- or 100-fold reduction, respectively, of the virus titer in the culture medium (as determined by quantitative DNA dot blot analysis). At this late time point, the extent of inhibition probably reflects a cumulative effect on several rounds of virus replication. In the studies to follow, we therefore used a modified protocol to analyze predominantly primary infection and in which, furthermore, the fraction of infected DHBV cells was enhanced by overnight preadsorbtion of virus at a low temperature (20). Virus uptake was initiated by shifting the culture to 37°C and was terminated 6 h later by a short exposure to pH 2.2 to inactivate surface-exposed virus particles (20). Parallel cultures, infected or uninfected and with or without glucagon, were maintained at 37°C and, after 3, 4, or 5 days, investigated for the presence of viral proteins and DNA genomes in cell lysates or secreted into the culture medium.
FIG. 1.
Glucagon inhibits DHBV infection of primary hepatocytes in a concentration-dependent manner. Duplicate cultures of hepatocytes were infected in the presence of glucagon at the concentrations indicated. The culture medium was changed daily until day 6, and virus secreted between days 6 and 10 was quantitated in duplicate by DNA dot blotting as described in Materials and Methods.
The results of such an analysis by Western blotting and quantitative DNA dot blotting (Fig. 2A) show that levels of intracellular DHBV core protein and viral DNA as well as secreted DHBV virions had increased progressively from day 3 to day 5 in the untreated control cultures, indicating that productive DHBV infection had been established. The addition of glucagon resulted in a greater-than-10-fold decrease in viral DNA levels and also in a marked reduction in the core protein signal, thus confirming the initial observations. The results of this biochemical analysis were complemented by the results of immunostaining to detect DHBV-positive cells in duplicate wells by use of an antibody mixture detecting the large envelope protein and the core protein. In this assay, the number of DHBV-positive cells detected at day 4 was reduced about 10-fold in the glucagon-treated cultures (Fig. 2B).
FIG. 2.
Interference of glucagon with DHBV infection is related to activation of the cAMP signalling pathway. Hepatocytes were infected and maintained in the presence of 250 nM glucagon or pulses of 25 μM forskolin. Media were changed daily, and cells were analyzed for viral proteins and virus genomes 3, 4, and 5 days (d) postinfection (pI). (A) Production of DHBV core protein analyzed by SDS-PAGE and ECL-Western blotting. Lower panels show DNA dot blots quantitating intracellular (intracell.) viral DNA accumulated until the day of lysis or virions secreted (extracell.) during the last 24-h time interval. (B) Immunofluorescence assay of glucagon-treated cells performed on day 4 postinfection with a mixture of antisera recognizing DHBV core protein and L-protein.
To exclude the possibility that the observed inhibitory effect was due to unspecified contamination in the glucagon used, we tested preparations from different sources, including glucagon synthesized chemically, produced in yeast cells, or isolated from bovine or porcine pancreas; we did not detect any differences in their inhibitory potential (7). There was also no variation in their ability to raise intracellular cAMP levels in primary duck hepatocytes (about 200-fold after 1 h). In another control, des-His1,[Glu9]-glucagonamid, a glucagon analog which binds to the glucagon receptor without triggering signal transduction (29, 30), was found to be unable to inhibit DHBV infection. Taken together, these data indicate that the inhibition of DHBV replication occurs intracellularly after bona fide glucagon signal transduction, rather than by competition for a cell surface binding protein.
Drugs that raise the intracellular cAMP level inhibit DHBV infection.
In the liver, the major response to glucagon is cAMP-mediated activation of PKA, which in turn regulates by phosphorylation the activity of key enzymes in glycogen synthesis and breakdown. If the observed glucagon effect were due to such cAMP-mediated signal transduction, other drugs that increase the intracellular cAMP level should also impair DHBV infection. To test this hypothesis, we analyzed the effects of forskolin, a diterpenoid which is isolated from the plant Coleus forskolii and which directly binds to and rapidly activates adenylate cyclase (4, 10, 34). As this drug caused cytotoxic effects in the 5-day protocol used with glucagon, forskolin was added only during virus binding and up to 1 day after the termination of virus uptake, i.e., altogether for a maximum of 40 h. The effects exerted by such a pulsed forskolin treatment were comparable to those observed after the continuous presence of glucagon, in that forskolin also induced a dramatic reduction in the quantities of all DHBV gene products examined, i.e., of intracellular viral DNA and core protein, of secreted virions (as shown in Fig. 2A), and of secreted DHBeAg or intracellular L-protein (7). In addition, the number of DHBV-positive cells detected by immunofluorescence at day 4 was decreased to levels similar to those observed after glucagon treatment (Fig. 2B) (7). Essentially very similar results were also observed with dibutyryl-cAMP (see below and Fig. 4). This latter membrane-permeating cAMP analog can functionally substitute for cAMP and therefore directly raise its apparent intracellular level (18). Taken together, this comprehensive set of inhibition data strongly supports the notion that stimulation of the cAMP-mediated signal transduction pathway and the eventual resulting changes in PKA activity are the common denominator in the capacity of several pharmacologically similar but structurally very different drugs to effectively inhibit DHBV infection of primary duck hepatocytes.
FIG. 4.
Activation of the cAMP signalling cascade interferes with a step late in the establishment of infection. (A) Experimental outline. Glucagon (250 nM) or dibutyryl-cAMP (1 mM) was present in the culture medium throughout the times indicated by the lines. Forskolin (25 μM) was added in pulses as shown by the heavy bars. (B) On day 5, cells were lysed and 1/20 of a well was analyzed by SDS-PAGE followed by ECL-Western blotting detecting DHBV core protein or L-protein. Note that the band above the L-protein band is not influenced by the drug treatment and is due to cross-reactivity of the antiserum with a cellular protein. The lanes are labeled according to the experimental outline shown in panel A. d, day; p.i., postinfection; di-but cAMP, dibutyryl-cAMP.
Glucagon does not interfere with ongoing DHBV replication.
To better characterize the mechanism of glucagon action upon DHBV replication, we examined its inhibitory effect on persistent DHBV infection in duck hepatocytes isolated from congenitally infected ducklings. As shown in Fig. 3, continuous treatment with glucagon reduced only slightly the amount of intracellular core protein or L-protein or DHBeAg secretion. Similarly, pulses of forskolin or treatment with β-isoproterenol, a β-adrenergic agonist also resulting in an elevated cAMP level, did not result in any significant changes in the amount of core protein or L-protein in cell lysates (7). When viral DNA products were analyzed, a minor but significant gradual reduction in intracellular viral DNA and in extracellular virion DNA levels was observed at late time points; values for intracellular DHBV DNA, quantitated in a PhosphorImager, were 90, 70, 65, and 50% relative to the untreated control when glucagon treatment was continued for 4, 8, 12, or 16 days, respectively. Thus, drugs raising the intracellular cAMP level have a much smaller effect on already-established DHBV replication than when they are added prior to infection (as shown in Fig. 2). This result indicates that cAMP-mediated signal transduction does not significantly interfere with the basic steps of ongoing DHBV replication (such as transcription, translation, or reverse transcription and viral DNA synthesis) but that it negatively influences a regulatory process that is important not only for the initial establishment of DHBV infection but also for the long-term maintenance of persistent infection.
FIG. 3.
Glucagon (Gluc) treatment does not interfere with ongoing DHBV replication. Primary duck hepatocytes were isolated from the liver of a congenitally DHBV-infected duck. After 4 days in culture, the medium was supplemented with 250 nM glucagon and changed every second day. Cells and culture medium from parallel wells were analyzed for the production of viral proteins and viral DNA on days 4, 8, 12, and 16 after the beginning of drug treatment. First and second rows, ECL-Western blotting detecting DHBV core protein or L-protein; third and fourth rows, DNA dot blotting detecting intracellular (intracell.) DNA from the 1/500 well or extracellular (extracell.) virus DNA in half of the culture medium; fifth row, immunodot blotting detecting DHBeAg secreted within 48 h.
Glucagon acts late during the establishment of DHBV infection.
To better define the time window during which drugs activating the cAMP signalling cascade interfere with the establishment of DHBV infection, we performed a series of infection experiments, essentially following the protocol of Fig. 2 but (as outlined in Fig. 4A) starting drug treatment with glucagon, forskolin, or dibutyryl-cAMP at a variety of time points before and after virus uptake. All infections were terminated at day 5, and cell lysates and culture medium were analyzed for intracellular and extracellular viral gene products. Figure 4B shows Western blots detecting DHBV core protein or L-protein in samples from the complete set of time points for glucagon-treated cells (Fig. 4B, lanes a to i); only relevant values are shown for cells treated with dibutyryl-cAMP (Fig. 4B, di-but cAMP, lanes a, c, h, and i) or forskolin (Fig. 4B, forskolin, lanes a, c, g, and h). As expected, the data obtained confirmed the potential of glucagon (and of the other two cAMP-enhancing drugs) to inhibit productive DHBV infection when present from virus binding onward (Fig. 4B, lane a). Such treatment was equally effective even when the drug was present from −30 to −18 h, i.e., when it had already been removed before the start of virus binding (7). In contrast, reduced levels of inhibition were observed with each of the three drugs when added at relatively late time points, such as 24 or 48 h after the termination of DHBV internalization (Fig. 4B, lanes h and i). Analysis of time points in between these extremes confirmed that effective drug action did not directly correlate with virus uptake. Strong inhibition was observed regardless of whether glucagon treatment was begun before or immediately after the termination of virus internalization, as defined by the pH 2.2 inactivation step (Fig. 4B, lane b or c, respectively), and also when glucagon treatment was begun at 2, 4, 6, or 8 h after the inactivation of free virus particles, i.e., 8 to 14 h after the uptake of virus had been initiated (Fig. 4B, lanes d, e, f, and g). These results clearly indicate that inhibition of infection occurs at a step following the internalization of prebound virus and define the time window for a loss of sensitivity to cAMP-mediated inhibition of DHBV infection as lying between 8 and 24 h after the initiation of virus entry at 37°C.
These conclusions were confirmed by the determination of the fraction of hepatocytes productively infected by counting DHBV-positive cells by an immunofluorescence assay performed as shown in Fig. 2 (7). Further experiments with this latter technique (7) demonstrated that significant inhibition of DHBV infection depended on prolonged glucagon treatment, such as that used in the experiment shown in Fig. 4. No significant inhibition was observed after 2-h pulses of glucagon treatment at various times before or during virus binding or after a 6-h pulse during virus internalization; both results indicated that the initial rapid increase in cAMP levels following glucagon stimulation was not sufficient to cause the inhibitory effect observed after prolonged drug treatment.
Similar limits for the latest time of inhibition were observed with the other two cAMP-enhancing drugs investigated, again demonstrating that their mode of action on DHBV replication was comparable to that of glucagon. With dibutyryl-cAMP, levels of markers indicating productive DHBV infection were reduced when the drug was present before or starting with virus uptake (Fig. 4B, di-but cAMP, lanes a and c), maximal inhibition still being detected at 8 h postinfection and significant inhibition still being seen as late as 24 h postinfection (Fig. 4B, di-but cAMP, lane h). With forskolin, given in 24-h pulses (Fig. 4A, heavy bars), the latest time for drug addition to be effective was again found to lie between 8 and 24 h after the termination of infection by inactivation of residual cell surface-attached virus (Fig. 4B, forskolin, lanes a, c, and g).
Glucagon interferes with the establishment of DHBV infection at a step before ccc DNA formation and/or amplification.
A crucial step early in hepadnavirus infection is the conversion of the incoming viral DNA genome from open-circle DNA to ccc DNA, which then serves as a template for viral gene expression and genome replication. By introducing the sensitive technique of over-gap PCR, Köck and Schlicht detected an initial increase in the number of nuclear ccc DNA molecules between 9 and 20 h after the initiation of virus uptake and also showed that this process most likely reflects the formation of ccc DNA from infecting virus genomes (13). As this time window correlated favorably with the latest times allowing cAMP-mediated inhibition of DHBV infection, we examined whether glucagon treatment was inhibitory to ccc DNA formation. As expected, ccc DNA was barely detectable with the over-gap PCR technique when glucagon was added starting with virus binding or as late as the inactivation of noninternalized virions (Fig. 5, lanes a to c). ccc DNA signals were increased when glucagon was added between 2, 4, or 8 h after virus inactivation, i.e., 8 to 14 h after uptake had been initiated (Fig. 4 and Fig. 5, lanes d to g), while signals were comparable in strength to those of the untreated control when glucagon treatment was begun 24 h after virus inactivation (Fig. 5, lane h). This time window suggests that glucagon treatment blocks DHBV infection at a step before the formation of ccc DNA from the infecting virus DNA genome or by interference with steps involved in amplification of the nuclear ccc DNA pool.
FIG. 5.
Glucagon treatment interferes with ccc DNA formation. DHBV infection of primary duck hepatocytes and glucagon treatment were initiated as outlined in Fig. 4A, and lanes are labeled accordingly. Cell lysates prepared on day 2 postinfection were examined by over-gap PCR for the formation of ccc DNA (see Materials and Methods).
DISCUSSION
In this report, we demonstrate that glucagon-induced intracellular changes in cultured hepatocytes negatively influence a regulatory event that appears to be of critical importance for the establishment of hepadnavirus infection. Taking advantage of the DHBV system, which allows in vitro studies, we obtained convincing experimental evidence that these down-regulating changes are cAMP mediated, as demonstrated by the substitution of glucagon with forskolin, a drug which rapidly activates adenylate cyclase, or with dibutyryl-cAMP, a membrane-permeating cAMP analog. Therefore, the inhibition observed most likely does not involve the activation of protein kinase C by glucagon-induced increases in the intracellular Ca2+ concentration or in the amount of inositol phosphates (26, 31) but is a consequence of changes in the activity of PKA, which directly or indirectly modulates the function(s) of viral or cellular proteins that are important in an early step in virus replication. Although this modulation may initially involve the up regulation of PKA activity, desensitization of the cAMP signalling cascade and reduced PKA activity (9, 19) as a result of prolonged stimulation seem to be more likely, since the inhibitory effect was not achieved by short glucagon pulses and prolonged drug treatment was effective even when terminated before virus binding. Furthermore, attempts to counteract the presumed glucagon-induced PKA activation by the specifically PKA-inhibiting drug H89 (2) did not result in the expected relief (data not shown). Thus, the down regulation of PKA activity appears to be the most probable cause for the inhibition of DHBV infection.
Our findings also provide some insight into the step in DHBV infection that is down regulated by these cAMP-mediated cellular changes. The time window determined clearly indicates that inhibition of infection occurred at a step after the internalization of virus particles but before the appearance of significant amounts of ccc DNA in the cell nucleus (Fig. 5); this result is in agreement with the time course of primary ccc DNA formation (13). The particular step in DHBV infection that is affected cannot be identified with certainty, as the fate of incoming virus DNA genomes cannot be analyzed separately from that of progeny genomes arising from intracellular genome replication. Nevertheless, taking into account current knowledge of the events required early in hepadnavirus infection, a reasonable hypothesis explaining our data is that glucagon treatment does not affect any of the poorly defined steps preceding the appearance of naked nucleocapsids in the cytosolic compartment, such as receptor binding, potential vesicular transport, and fusion of the viral envelope with an intracellular membrane. Support for this view, which includes the intrinsic assumption that there is no distinction between whether cytosolic capsids arise from infecting virions or are products of established intracellular hepadnavirus replication, comes from two experimental observations. (i) Intermediate levels of inhibition were observed when glucagon was added at times coinciding with the intracellular amplification of the nuclear ccc DNA pool, thereby causing a delay in reaching steady-state levels of nuclear viral DNA templates and gene expression (Fig. 4 and 5). (ii) Given the limited half-life of nuclear DHBV ccc DNA (3), the inhibition of genome reimport also explains the gradual reductions in the levels of intracellular virus DNA and of virus secretion that we observed during prolonged glucagon treatment of cultures of congenitally DHBV-infected hepatocytes (Fig. 3).
Obvious possibilities for the molecular mechanism(s) that may affect the nuclear import of the virus DNA genome are changes in the phosphorylation state of viral proteins or of cellular interaction partners. Viral candidates are the capsid protein (core protein) and the DNA polymerase/reverse transcriptase (P-protein), which both carry nuclear localization signals as well as target sequences for PKA and for other cellular protein kinases (1, 32, 33). Changes in the phosphorylation state of the capsid protein may correlate with functional changes essential for capsid transport to the nuclear pore complex or may facilitate the capsid destabilization presumably required for the release of the DNA genome. An argument against this hypothesis is the lack of glucagon-induced changes in the multiband electrophoretic pattern indicative of the complex mixture of differentially phosphorylated core protein species, as presented in the Western blots in Fig. 2, 3, and 4; however, relevant changes not apparent by unidimensional PAGE could have escaped detection. Another attractive hypothesis is that glucagon treatment may impair a potential role of the P-protein in the nuclear import of the covalently linked DNA genome, a function suggested by a study analyzing the requirements for the nuclear import of woodchuck hepatitis B virus DNA genomes in a cell-free system (11).
Further experiments with mutations inactivating phosphorylation target sites or with better synchronization of infection by use of conditional mutants (25) should allow these and other options to be tested experimentally. However, whatever the mechanism might be, our present findings add, already at the present level of analysis, to those of other reports suggesting that hepadnavirus infection may be subject to modulation reflecting changes in the state of the host cell. This is not a surprising idea for a very small viral DNA genome establishing a persistent infection.
Considering the influence of the state of the cell, it is of note that about 10% of the cultured hepatocytes were not subject to cAMP-mediated down regulation of DHBV infection. As judged from the intensity of the immunostaining at early times (Fig. 2B), this subpopulation produced viral proteins as rapidly and as abundantly as untreated control cells. This result suggests that they were infected at a particular state (possibly in the cell cycle) at which they were refractory to glucagon inhibition and/or particularly susceptible to the nuclear import of infecting viral genomes and/or expansion of the pool of ccc DNA templates. A better understanding of this functional heterogeneity of avian hepatocyte cultures with respect to hepadnavirus infection may also provide a basis for improving the low levels of susceptibility to HBV infection of human primary hepatocytes. An understanding of the effects of glucagon treatment may also lead to the definition of new targets for an antiviral therapy potentially complementing conventional drugs interfering with virus DNA synthesis.
ACKNOWLEDGMENTS
We thank Frank Fehler for contributing to the initial phase of this work, Bärbel Glass for preparing primary duck hepatocytes and help in analysis, and Ulrike Protzer-Knolle, Uta Klöcker, Ira Swameye, and Klaus Breiner for discussion and helpful comments.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 229) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Bartenschlager R, Weber M, Schaller H. In vitro phosphorylation of the hepatitis B virus P gene product: a general method for radiolabelling of proteins. In: Adolph K W, editor. Methods in molecular genetics. Vol. 4. New York, N.Y: Academic Press, Inc.; 1994. pp. 391–401. [Google Scholar]
- 2.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 3.Civitico G M, Locarnini S A. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology. 1994;203:81–89. doi: 10.1006/viro.1994.1457. [DOI] [PubMed] [Google Scholar]
- 4.de Souza N J, Dohadwalla A N, Reden J. Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev. 1983;3:201–219. doi: 10.1002/med.2610030205. [DOI] [PubMed] [Google Scholar]
- 5.Galle P R, Schlicht H J, Kuhn C, Schaller H. Replication of duck hepatitis B virus in primary hepatocytes and its dependence on the state of differentiation of the host cell. Hepatology. 1989;10:459–465. doi: 10.1002/hep.1840100410. [DOI] [PubMed] [Google Scholar]
- 6.Giuidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 7.Hild M. Zelluläre Funktionen während der frühen und späten Schritte im Infektionszyklus des Enten Hepatitis B Virus. Ph.D. thesis. Heidelberg, Germany: University of Heidelberg; 1997. [Google Scholar]
- 8.Hildt E. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology. 1996;225:235–239. doi: 10.1006/viro.1996.0594. [DOI] [PubMed] [Google Scholar]
- 9.Houge G, Vintermyr O K, Doskeland S O. The expression of cAMP-dependent protein kinase subunits in primary rat hepatocyte cultures. Cyclic AMP down-regulates its own effector system by decreasing the amount of catalytic subunit and increasing the mRNAs for the inhibitory (R) subunits of cAMP-dependent protein kinase. Mol Endocrinol. 1990;4:481–488. doi: 10.1210/mend-4-3-481. [DOI] [PubMed] [Google Scholar]
- 10.Huang R D, Smith M F, Zahler W L. Inhibition of forskolin-activated adenylate cyclase by ethanol and other solvents. J Cyclic Nucleotide Res. 1982;8:385–394. [PubMed] [Google Scholar]
- 11.Kann M, Bischof A, Gerlich W H. In vitro model for the nuclear transport of the hepadnavirus genome. J Virol. 1997;71:1310–1316. doi: 10.1128/jvi.71.2.1310-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingmüller U, Schaller H. Hepadnavirus infection requires the interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67:7414–7422. doi: 10.1128/jvi.67.12.7414-7422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köck J, Schlicht H-J. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J Virol. 1993;67:4867–4874. doi: 10.1128/jvi.67.8.4867-4874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köck J, Borst E-M, Schlicht H J. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 1996;70:5827–5831. doi: 10.1128/jvi.70.9.5827-5831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason W S, Seeger C, editors. Hepadnaviruses. Molecular biology and pathogenesis. Current topics in microbiology and immunology. Vol. 168. Berlin, Germany: Springer-Verlag KG; 1991. [Google Scholar]
- 16.McLachlan A, editor. Molecular biology of the hepatitis B virus. Boca Raton, Fla: CRC Press, Inc.; 1991. [Google Scholar]
- 17.Nassal M, Schaller H. Hepatitis B virus replication—an update. J Viral Hepatitis. 1996;3:217–226. doi: 10.1111/j.1365-2893.1996.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 18.Posternak T, Weimann G. The preparation of acylated derivatives of cyclic nucleotides. Methods Enzymol. 1974;38:399–409. doi: 10.1016/0076-6879(74)38057-3. [DOI] [PubMed] [Google Scholar]
- 19.Premont R T, Iyengar R. Glucagon-induced desensitization of adenylyl cyclase in primary cultures of chick hepatocytes. J Biol Chem. 1988;263:16087–16095. [PubMed] [Google Scholar]
- 20.Rigg R J, Schaller H. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J Virol. 1992;66:2829–2836. doi: 10.1128/jvi.66.5.2829-2836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothmann, K., E. Hildt, and H. Schaller. 1997. Unpublished data.
- 22.Rothmann, K., M. Schnölzer, G. Radziwill, and H. Schaller. 1997. Unpublished data. [DOI] [PMC free article] [PubMed]
- 23.Schlicht H J, Kuhn C, Guhr B, Mattaliano R J, Schaller H. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J Virol. 1987;61:2280–2285. doi: 10.1128/jvi.61.7.2280-2285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlicht H J, Salfeld J, Schaller H. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J Virol. 1987;61:3701–3709. doi: 10.1128/jvi.61.12.3701-3709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeger C, Leber E H, Wiens L K, Hu J. Mutagenesis of a hepatitis B virus reverse transcriptase yields temperature-sensitive virus. Virology. 1996;222:430–439. doi: 10.1006/viro.1996.0440. [DOI] [PubMed] [Google Scholar]
- 26.Sistare F D, Picking R A, Haynes R C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985;260:12744–12747. [PubMed] [Google Scholar]
- 27.Summers J, Smith P M, Horwich A L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuttleman J S, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 29.Unson C G, Andreu D, Gurzenda E M, Merrifield R B. Synthetic peptide antagonists of glucagon. Proc Natl Acad Sci USA. 1987;84:4083–4087. doi: 10.1073/pnas.84.12.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unson C G, Macdonald D, Ray K, Durrah T L, Merrifield R B. Position 9 replacement analogs of glucagon uncouple biological activity and receptor binding. J Biol Chem. 1991;266:2763–2766. [PubMed] [Google Scholar]
- 31.Wakelam M, Murphy G, Hruby V, Houslay M D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- 32.Weber M. Das P-Protein des Enten Hepatitis B Virus: Untersuchungen zur Struktur und Funktion in der Hepadnaviralen Replikation. Ph.D. thesis. Heidelberg, Germany: University of Heidelberg; 1994. [Google Scholar]
- 33.Yu M, Summers J. Phosphorylation of the duck hepatitis B virus capsid protein associated with conformational changes in the C terminus. J Virol. 1994;68:2965–2969. doi: 10.1128/jvi.68.5.2965-2969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Liu Y, Ruoho A E, Hurley J H. Structure of the adenylyl cyclase catalytic core. Nature. 1997;386:247–253. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]