Abstract
The proline-rich SH3-binding (SH3B) motif of the tyrosine kinase-interacting protein (Tip) of herpesvirus saimiri (HVS) is required for binding to the cellular Src family kinase Lck. We constructed a mutant form of HVS in which prolines in the SH3B motif of Tip were altered to alanines. This mutant form of Tip was incapable of binding to Lck. The mutant virus, HVS/Tip mSH3B, retained its ability to immortalize common marmoset lymphocytes in culture. In fact, common marmoset lymphocytes immortalized by the HVS/Tip mSH3B mutant displayed increased expression of HLA-DR lymphocyte activation marker, an altered pattern of tyrosine phosphorylation, increased expression of the tyrosine kinase Lyn, and a shift in electrophoretic mobility of Lck compared to cells immortalized by wild-type HVS. Experimental infection of common marmosets resulted in fulminant lymphoma with both HVS/Tip mSH3B and wild-type HVS. However, HVS/Tip mSH3B produced greater infiltration of affected organs by proliferating lymphoid cells compared to wild-type HVS. These results demonstrate that Tip binding to Lck is not necessary for transformation and that abrogation of Tip binding to Lck alters the characteristics of transformed cells and the severity of the pathologic lesions.
Herpesvirus saimiri (HVS) infection is endemic and nonpathogenic in its natural host, squirrel monkeys (Saimiri sciureus) (10, 14). The same virus induces rapidly fatal T-cell lymphomas, leukemias, and lymphosarcomas in several other species of New World primates (15, 18). HVS is a member of the gamma subfamily of herpesviruses (Gammaherpesvirinae), which also includes Epstein-Barr virus, herpesvirus ateles, and herpesvirus sylvilagus. These latter viruses are also capable of inducing lymphoproliferative disorders in natural or experimental hosts. HVS, a gamma-2 herpesvirus (rhadinovirus [34]), is related to Kaposi’s sarcoma-associated herpesvirus (also called human herpesvirus 8), which is the only known human representative of the gamma-2 herpesvirus subgroup (7). While Kaposi’s sarcoma-associated herpesvirus and HVS both contain homologs of known cellular genes that may influence their oncogenicity (1, 35), only novel open reading frames at the left end of the HVS genome have been definitively implicated in disease induction (8, 9, 11, 21, 23, 32). Sequence divergence at the left end of the viral genome defines three viral subgroups (A, B, and C) of HVS that differ with respect to oncogenic potential (8, 10, 27). Strains from subgroups A and C are highly oncogenic and are able to immortalize peripheral blood lymphocytes of common marmosets in vitro to interleukin 2 (IL-2)-independent growth. Subgroup C strains are further capable of immortalizing lymphocytes of human and rhesus monkey peripheral blood into continuously proliferating T-cell lines (2–4, 6, 31).
Tyrosine kinase-interacting protein (Tip) is expressed together with saimiri transforming protein (STP-C488) from a bicistronic transcription unit at the left end of the genome of HVS subgroup C strain 488 (HVS C488) (4, 5). Although Tip expression alone is insufficient for oncogenic transformation of rodent fibroblasts (21), studies with a recombinant HVS C488 containing a deletion in Tip have demonstrated that Tip is required for oncogenic transformation (12). Tip has been shown to associate with the tyrosine kinase Lck (5, 26), a member of the Src kinase family. Tip is efficiently phosphorylated on tyrosine residues by purified Lck in several cell-free assay systems (5, 19, 20). Lck-binding elements of Tip have been defined by mutational analysis. Regions related to the carboxyl terminus of Src-related kinase homology domain and an SH3-binding (SH3B) domain that are separated by a spacer sequence were sufficient to form a stable complex with Lck in vitro and in vivo (19). Tip also interacts with a novel cellular protein called Tap (Tip-associated protein) (40). Coexpression of Tip and Tap in Jurkat T cells upregulated surface expression of adhesion molecules and activated NF-κB transcription factor activity (40). The specificity of Tip binding to both Lck and Tap suggests a contributory role of each to the viral life cycle. Whether Tip binding to Tap and Lck makes distinct or related contributions to the virus remains to be defined.
Expression of Tip in Jurkat T cells dramatically suppressed cellular tyrosine phosphorylation and expression of lymphocyte surface antigens (20). Expression of Tip also blocked the induction of tyrosine phosphorylation by anti-CD3 stimulation. Similarly, expression of Tip suppressed transformation by oncogenic F505 Lck in fibroblasts (20). Additionally, mutation of tyrosine 114 of Tip significantly increased Lck-binding activity (16). This mutant exhibited a dramatic increase in the suppression of cellular tyrosine phosphorylation and surface expression of lymphocyte antigens compared with wild-type (wt) Tip (16). Therefore, Tip appears to act at an early stage of the T-cell signal transduction cascade by associating with Lck and downregulating Lck-mediated activation. Similar signal blocking has been associated with the LMP2A product of Epstein-Barr virus (28–30).
While the results cited above indicate downregulation of Lck-mediated signal transduction by Tip, upregulation of Lck signaling has been suggested as a function of Tip in a different experimental setting (25, 38). Since a mutant form of Tip altered in its SH3-binding domain (mSH3B) no longer associates with Lck but retains its Tap-binding ability (40), we introduced this mutated tip gene into the viral genome in order to study the effects of this mutation on the properties of the virus. In this study, we demonstrate that recombinant HVS/Tip mSH3B is fully capable of immortalizing primary lymphocytes in vitro and inducing lymphomas in vivo. Furthermore, altered cellular signal transduction and increased lymphocyte infiltration of affected organs in vivo are associated with transformation by HVS/Tip mSH3B. These results support a role for Tip in negatively regulating T-cell signal transduction via its interaction with Lck.
MATERIALS AND METHODS
Cell culture, virus propagation, and in vitro immortalization assays.
HVS C488 was propagated in low-passage (<30 passages) owl monkey kidney (OMK 637) cells in minimal essential medium supplemented with penicillin, streptomycin, l-glutamine, and 10% (vol/vol) heat-inactivated fetal bovine serum (GIBCO BRL, Grand Island, N.Y.).
Primary peripheral blood mononuclear cells (PBMCs) from common marmosets (Callithrix jacchus) were purified from 3-ml heparinized blood specimens, using lymphocyte separation medium (Organon Teknika Corp., Malvern, Pa.). Washed cells were resuspended and cultured in RPMI 1640 medium supplemented with 20% (vol/vol) heat-inactivated fetal bovine serum and 5 μg of β-mercaptoethanol per ml. HVS infection of marmoset PBMCs was performed in 12-well tissue culture plates by methods described previously (11). Immortalization of primary lymphocytes to IL-2-independent growth was typically established within a month after infection. Uninfected common marmoset PBMCs were also cultivated by stimulation with 1 μg of phytohemagglutinin per ml for 48 h followed by culture in RPMI 1640 with 20% fetal bovine serum and 10% IL-2.
Construction of mSH3B Tip mutant plasmids.
Generation of the mSH3B mutation in Tip by oligonucleotide-directed mutagenesis was described previously (19). A 3.6-kb clone, pNEB-C488-PX, containing Tip, STP-C488, and HVS U RNAs that was used previously (4, 13) provided a subcloning vector with adequate flanking sequence to facilitate recombination during cotransfection. Digestion of this vector with BamHI and HpaI permitted insertion of the corresponding Tip fragment containing the mSH3 mutation.
Transfections and isolation of HVS recombinants.
Intact HVS virion DNA for transfections was isolated as previously described (13). Virion DNA was derived from a mutant in which a 442-bp deletion in the tip gene was replaced with a reporter expression cassette containing the secreted engineered alkaline phosphatase (SEAP) gene driven from the simian virus 40 (SV40) early promoter (13). Cotransfection of linearized plasmid and mutant virion DNA for production of recombinant virus was performed as described previously (13). The Tip mSH3B plasmid was linearized with FspI and cotransfected along with the virion DNA into subconfluent OMK cell monolayers, using Ca2+ phosphate precipitation. Viruses that had lost SEAP expression by recombination were isolated by repeated limiting-dilution infections of OMK cell monolayers in 48-well tissue culture plates (Fig. 1). SEAP production in individual wells showing cytopathic effect was assessed with the Phospha-Light chemiluminescent assay (Tropix, Bedford, Mass.) performed in opaque 96-well microtiter plates by using a MicroBeta scintillation counter (Wallac, Gaithersburg, Md.). Presence of the mSH3B mutation was assessed by PCR to amplify DNA fragment from nucleotides 454 to 1224 of the published sequence (4). Amplified DNA was cloned into the TA cloning vector (Invitrogen, San Diego, Calif.). Both strands of five independent clones were subsequently sequenced by using an ABI PRISM 377 automatic DNA sequencer.
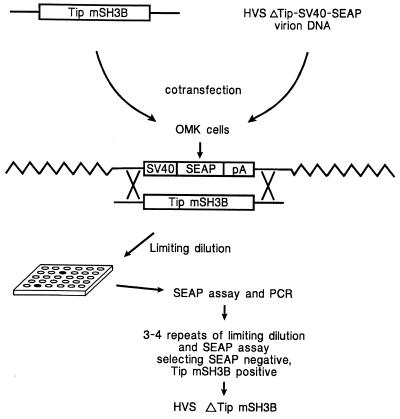
FIG. 1.
Schematic diagram to construct HVS/Tip mSH3B recombinant. Linearized plasmid DNA containing Tip mSH3B was cotransfected with HVSΔTip-SV40-SEAP virion DNA. Recombinant SEAP-negative virus in infected OMK cells was isolated by limiting dilution. The presence of Tip mSH3B was determined by PCR and DNA sequence analysis.
Experimental infection of common marmosets.
Oncogenicity of the HVS recombinants was assessed by experimental infection of common marmosets (C. jacchus). Six marmosets were injected intramuscularly with 105 50% tissue culture infective doses of virus in a volume of 1 ml. Two marmosets received HVS/Tip mSH3B, two were injected with wt HVS, and two were received with HVSΔTip-SV40-SEAP. Sera and blood cell pellets were collected and frozen at −70°C weekly. Animals were euthanized when they became moribund and received complete necropsies. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Antibodies.
The purified recombinant glutathione S-transferase-Tip protein was used to generate polyclonal anti-Tip antibody (20). Antibodies to Lck, Syk, Zap70, Hck, Yes, Hck, TRAF1, TRAF2, TRAF3, p50, p65, and phosphotyrosine were purchased commercially from Upstate Biotechnology Inc. (Lake Placid, N.Y.) and Santa Cruz Biotech (Santa Cruz, Calif.).
Immunoprecipitation and immunoblotting.
Cells were harvested and lysed with lysis buffer (0.15 M NaCl, 0.5% Nonidet P-40, 50 mM HEPES buffer [pH 8.0]) containing 1 mM Na2 VO3, 1 mM NaF, and protease inhibitors (leupeptin, aprotinin, phenylmethylsulfonyl fluoride, and bestatin). Precleared lysates were incubated for 4 h at 4°C with anti-Lck or anti-Tip antibody bound to protein A/G-agarose beads. Washed immune complexes were used for in vitro kinase assay. For protein immunoblots, polypeptides in cell lysates corresponding to 105 cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane filter. Immunoblot detection was performed with a 1:200 or 1:3,000 dilution of primary antibody with an enhanced chemiluminescence (ECL) system (ECL kit; Amersham, Chicago, Ill.).
In vitro kinase assays.
For in vitro protein kinase assays, complexes prepared as described above were washed once more with kinase buffer (10 mM MgCl2, 1 mM dithiothreitol, 10 μM unlabeled ATP, 20 mM Tris [pH 7.0]), resuspended with 10 μl of the same buffer containing 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN) for 15 min at room temperature, and separated by SDS-PAGE.
Fluorescence-activated cell sorting (FACS) analysis of immortalized lymphocytes.
A total of 5 × 105 cells were washed with RPMI 1640 medium containing 10% fetal bovine serum and incubated with fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated monoclonal antibody for 30 min at room temperature. After washing, each sample was fixed with 1% formalin solution and cytofluorographic analysis of cell populations was performed with a FACScan (Becton Dickson and Co., Mountainview, Calif.). Antibodies for CD2 (RPA-2.10), CD4 (94b1), CD8 (21Thy2.3), CD11a (HI111), CD25 (2A3), CD38 (HIT2), CD45 (2D1), CD49d (9F10), CD56 (MY31), CD95 (DX2), HLA-A,B,C (G46-2.6), and HLA-DR (G46-6) were purchased from PharMingen (San Diego, Calif.) or Becton Dickinson (San Jose, Calif.).
Luciferase and SEAP assays.
Approximately 107 cells were electroporated at 960 μF and 200 V. All transfections included pGKβgal, which expresses β-galactosidase from a phosphoglucokinase promoter, together with 3X-κB-luc, Oct1-SEAP, NFAT-SEAP, or pLN-11-luc/pLN-V-luc which have a functional lyn promoter as described previously (36). At 24 or 48 h after transfection, cells were washed once in phosphate-buffered saline and lysed in 200 μl of reporter lysis buffer (Promega, Madison, Wis.). Assays for luciferase or alkaline phosphatase activity were performed with an Luminometer, using luciferase assay reagent (Promega) or with the Phospha-Light chemiluminescent assay (Tropix). Values were normalized by β-galactosidase activity.
RESULTS
Isolation of HVS/Tip mSH3B recombinant.
A recently described procedure (13) was used to isolate a recombinant HVS with point mutations in the SH3B region of Tip in which proline residues at positions 175, 177, 178, 180, and 183 were replaced with alanine. Plasmid clones containing these mutations were described in a previous study (19). Virion DNA for transfection was derived from a virus in which Tip sequences were replaced by a SEAP reporter expression cassette. The 442-bp deletion in Tip of this virus has been shown to render the virus nontransforming in culture and nononcogenic in common marmosets (12). After cotransfection of virion DNA and linearized plasmid containing the mSH3B mutation in Tip, limiting-dilution purification of SEAP-negative virus was performed to isolate recombinant HVS/Tip mSH3B as shown schematically in Fig. 1. Since the virion DNA that was used for transfection was purified from HVSΔTip-SV40-SEAP virion DNA, not from wt HVS, the possibility of contamination with wt HVS is virtually excluded. To confirm the correct genetic structure of the recombinant virus, virion DNA from HVS/Tip mSH3B was used for PCR and sequence analysis. Five of five plasmid clones derived from virion DNA of this recombinant virus were shown to contain the presence of the appropriate mutations in the SH3-binding domain of Tip and the absence of undesired aberrant mutations or wt Tip sequence.
In vitro immortalization of common marmoset T lymphocytes with recombinant HVS/Tip mSH3B.
In vitro immortalization of primary T lymphocytes of common marmosets was attempted with recombinant HVS/Tip mSH3B. wt HVS and HVSΔTip-SV40-SEAP were used for controls in these assays. wt HVS, HVSΔTip-SV40-SEAP, and HVS/Tip mSH3B at equivalent titers were separately added to individually purified, unstimulated, common marmoset PBMC. As with wt HVS, the recombinant HVS/Tip mSH3B also uniformly transformed the primary T lymphocytes of common marmosets to IL-2-independent growth within about 1 month after infection (Table 1). No obvious difference in transforming efficiency between wt HVS and HVS/Tip mSH3B was detected in this assay. By contrast, none of the primary lymphocyte culture was immortalized by infection with HVSΔTip-SV40-SEAP (Table 1).
TABLE 1.
In vitro and in vivo oncogenicity of HVS/Tip mSH3B
| Virus | No. with characteristic/no. tested
|
Survival (days) | ||
|---|---|---|---|---|
| In vitro immortalizationa | Virus recoveryb | In vivo lymphoma | ||
| None | 0/5 | |||
| HVS C488 | 23/24 | 2/2 | 2/2 | 19 and 20 |
| HVSΔTip | 0/9 | 2/2 | 0/2 | >370 |
| HVSΔTip-SV40-SEAP | 0/9 | 2/2 | 0/2 | >370 |
| HVS/Tip mSH3B | 12/12 | 2/2 | 2/2 | 20 and 21 |
Primary T-lymphocyte immortalization.
Virus was recovered from PBMCs of an infected marmoset as described previously (12).
We measured surface expression of lymphocyte antigens on common marmoset T cells transformed by wt HVS and by HVS/Tip mSH3B by FACS analysis. The results of these analysis showed that common marmoset T cells transformed by both viruses were CD2+, CD4−, CD8+, and CD56+ cells which were likely derived from natural killer cells as described previously (22). These results indicated that HVS/Tip mSH3B was fully capable of immortalizing common marmoset lymphocytes to continuous growth.
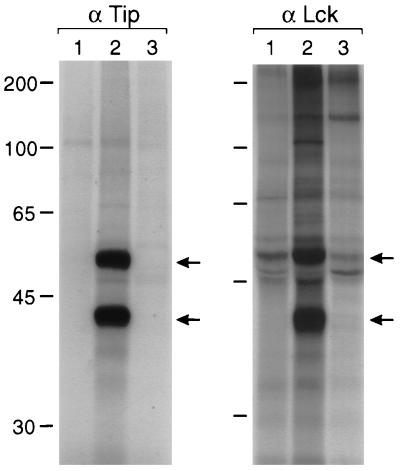
We performed experiments to demonstrate that HVS/Tip mSH3B mutant virus was actually responsible for the transformation. Virus from common marmoset T lymphocytes transformed by HVS/Tip mSH3B was isolated by cocultivation with OMK cells. One microliter of isolated virus was directly used for PCR to amplify the Tip gene. DNA sequencing of five independent clones confirmed that the amplified fragment contained the Tip mSH3B mutation and the absence of wt Tip sequence. In addition, in vitro kinase assay with Tip or Lck immune complexes showed that association of Tip with Lck was detected in wt HVS-transformed T cells but not in HVS/Tip mSH3B-transformed T cells (Fig. 2). When immunoblot assay with an anti-Tip antibody was used to examine expression of wt Tip and Tip mSH3B mutant in transformed T cells, however, we were unable to detect the Tip expression with any of these transformed T cells. This was likely caused by the low expression of Tip in HVS.
FIG. 2.
Absence of interaction of Tip mSH3B with Lck in transformed T cells. A total of 2 × 107 cells were the source of extracts for immunoprecipitations with anti-Tip (α Tip) and anti-Lck (α Lck) antibodies. Each immune complex was subjected to in vitro kinase assay with [γ-32P]ATP. Lane 1, IL-2-stimulated common marmoset PBMCs; lane 2, wt HVS-transformed common marmoset T cells; lane 3, HVS/Tip mSH3B-transformed common marmoset T cells. Top arrows indicate Lck; bottom arrows indicate Tip; sizes are indicated in kilodaltons.
FACS analysis of HVS/Tip mSH3B transformed T cells.
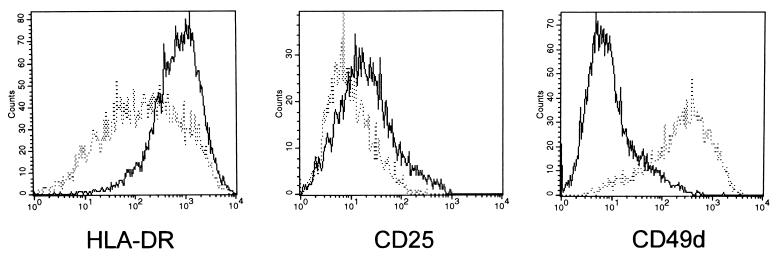
While wt HVS and HVS/Tip mSH3B immortalized primary common marmoset T cells with similar efficiencies, a slight morphological change was noticed in HVS/Tip mSH3B-transformed common marmoset T cells. Thus, we examined the level of surface expression of lymphocyte antigens in these cells. The mean fluorescence of HLA-DR surface expression, which has been shown to be associated with the activation of T cells (33), was approximately 8- to 10-fold higher on HVS/Tip mSH3B-transformed T cells than on wt HVS-transformed T cells (Fig. 3). Additionally, HVS/Tip mSH3B-transformed T cells repeatedly showed slightly increased surface expression of CD25 compared with wt HVS-transformed T cells (Fig. 3). In contrast, CD49d, which is a ligand for V-CAM, was dramatically downregulated in HVS/Tip mSH3B-transformed T cells compared to wt HVS-transformed T cells (Fig. 3). Several other surface markers, including CD2, CD8, CD11a, CD38, CD45, CD56, CD95, and major histocompatibility complex class I, were found to be similarly expressed on cells immortalized by both viruses. Also, results for an additional common marmoset cell line independently transformed by HVS/Tip mSH3B were essentially the same as those described above (data not shown).
FIG. 3.
Alteration of surface expression of lymphocyte antigens in HVS/Tip mSH3B-transformed T cells. A total of 5 × 105 cells were washed with RPMI 1640 medium containing 10% fetal calf serum and incubated with fluorescein isothiocyanate-conjugated monoclonal antibody or phycoerythrin-conjugated monoclonal antibody for 30 min at room temperature. After washing, cytofluorometric analysis of cell populations was performed with a FACScan. Dotted and solid lines indicate wt HVS-transformed common marmoset T cells and HVS/Tip mSH3B-transformed T cells, respectively.
Alteration of cellular signal transduction in T cells transformed by HVS/Tip mSH3B.
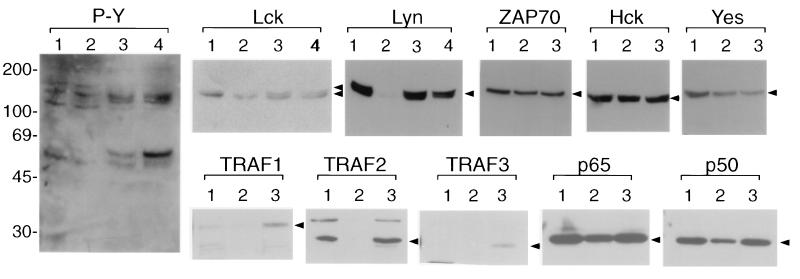
To investigate further the altered phenotype of HVS/Tip mSH3B-transformed T cells, we examined cellular tyrosine phosphorylation by immunoblot with antiphosphotyrosine antibody. We used IL-2-stimulated common marmoset PBMCs, common marmoset T cells transformed by wt HVS, and common marmoset T cells transformed by HVS/Tip mSH3B for these assays. Immunoblotting with antiphosphotyrosine antibody showed that wt HVS-transformed common marmoset T cells had a level of tyrosine phosphorylation similar to that of IL-2-stimulated common marmoset PBMCs (Fig. 4). In contrast, HVS/Tip mSH3B-transformed common marmoset T cells showed an altered pattern of tyrosine phosphorylation (Fig. 4). Specifically, proteins of 55 and 100 to 110 kDa were highly tyrosine phosphorylated in HVS/Tip mSH3B-transformed common marmoset T cells compared to IL-2-stimulated common marmoset PBMCs and wt HVS-transformed common marmoset T cells (Fig. 4). To investigate whether the expression of tyrosine kinases contributed to the alteration of cellular tyrosine phosphorylation, we examined the level of tyrosine kinase expression in immortalized common marmoset T cells by immunoblot assays. These assays showed that all of the tested cells contained similar levels of tyrosine kinases expression with the exception of Lyn (Fig. 4). While Lyn was readily detected in IL-2-stimulated marmoset PBMCs, it was only weakly detected in wt HVS-transformed T cells (Fig. 4). However, the level of Lyn expression was dramatically higher in HVS/Tip mSH3B-transformed T cells than in wt HVS-transformed T cells (Fig. 4). Additionally, Lck was present as a doublet (p56 and p60) only in HVS/Tip mSH3B-transformed common marmoset T cells (Fig. 4). Various T-cell stimuli have been shown to induce a shift in the electrophoretic mobility of Lck from p56 to p60 which was induced by phosphorylation at the serine 59 residue (17). These results demonstrate altered cellular signal transduction in HVS/Tip mSH3B-transformed cells based on the distinct pattern of cellular tyrosine phosphorylation, increased Lyn expression, and the shift in electrophoretic mobility of Lck.
FIG. 4.
Alteration of intracellular signal-transducing activity in HVS/Tip mSH3B-transformed common marmoset T cells. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and reacted with antiphosphotyrosine antibody (P-Y) or antibodies against cellular proteins as indicated at the top. Reactivity was detected by ECL. Lane 1, IL-2-stimulated common marmoset PBMCs; lane 2, wt HVS-transformed common marmoset T cells; lanes 3 and 4, independently established HVS/Tip mSH3B-transformed common marmoset T cells. Arrowheads indicate individual proteins; sizes are indicated in kilodaltons.
Activation of lyn promoter and NF-κB activity in HVS/Tip mSH3B-transformed common marmoset T cells.
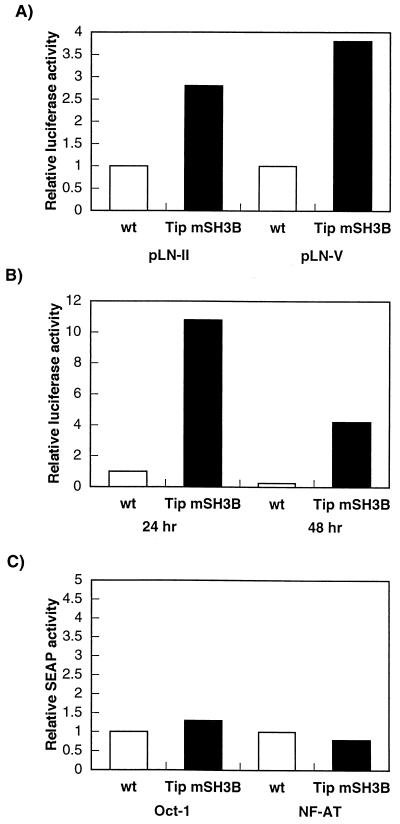
Lyn is expressed at highest levels in macrophages, platelets, and B lymphocytes and at very low levels in T lymphocytes (39). Since the level of Lyn expression was drastically increased in HVS/Tip mSH3B-transformed T cells relative to wt HVS-transformed T cells, we measured lyn promoter activity by using reporter constructs. wt HVS-transformed T cells and HVS/Tip mSH3B-transformed T cells were transfected with the pLN-11 or pLN-V reporter construct, which contains a functional lyn promoter as described previously (36), and control β-galactosidase plasmid pGKβgal. Lyn promoter activity in HVS/Tip mSH3B-transformed T cells was approximately three to fourfold higher than that in wt HVS-transformed T cells (Fig. 5A).
FIG. 5.
(A) Activation of lyn promoter activity in HVS/Tip mSH3B-transformed T cells. (B) Activation of NF-κB activity in HVS/Tip mSH3B-transformed T cells. (C) Oct-1 and NF-AT activity in HVS/Tip mSH3B-transformed T cells. Cells were transfected with 30 μg of pLN-11, pLN-V, NF-κB, Oct-1, or NF-AT reporter plasmid along with plasmid pGKβgal; 24 or 48 h after transfection, cell lysates and culture media were used for luciferase, alkaline phosphatase, and β-galactosidase assays. Luciferase and alkaline phosphatase activities were determined and normalized on the basis of β-galactosidase activities. Fold activation represents normalized luciferase activity relative to that of the wt HVS-transformed cells at 24 h after transfection. Values represent averages of three experiments.
We also measured NF-κB activity in these cells by using a reporter construct. wt HVS-transformed T cells and HVS/Tip mSH3B-transformed T cells were transfected with NF-κB-driven reporter plasmid 3X-κB-luc and control β-galactosidase plasmid pGKβgal. NF-κB activity in HVS/Tip mSH3B-transormed T cells was approximately 10-fold higher than that in wt HVS-transformed T cells (Fig. 5B). Additional HVS/Tip mSH3B-transformed T cells showed a level of activation of NF-κB similar to that of wt HVS-transformed T cells (data not shown). However, NF-AT and Oct-1 transcriptional activity was not altered in these cell lines under the same conditions (Fig. 5C). Finally, we examined expression of cellular molecules involved in the NF-κB signaling pathway by immunoblot assays. While TRAF1, TRAF2, and TRAF3 were poorly expressed in wt HVS-transformed T cells, they were highly expressed in IL-2-stimulated PBMCs and HVS/Tip mSH3B-transformed cells (Fig. 4). By contrast, the p50 and p65 subunits of the NF-κB transcription factor were similarly expressed in all these cells (Fig. 4). Also, Bcl-2, Bax, IκB, and cellular serine/threonine kinases were found to be similarly expressed in these cells (data not shown). Thus, these results demonstrate that HVS/Tip mSH3B-transformed T cells showed an increased activity of the lyn promoter and of transcriptional factor NF-κB compared to wt HVS-transformed T cells. Additionally, some of the cellular NF-κB signaling molecules showed higher expression in HVS/Tip mSH3B-transformed T cells than in wt HVS-transformed T cells.
Enhanced pathogenic activity of HVS/Tip mSH3B recombinant in infected common marmosets.
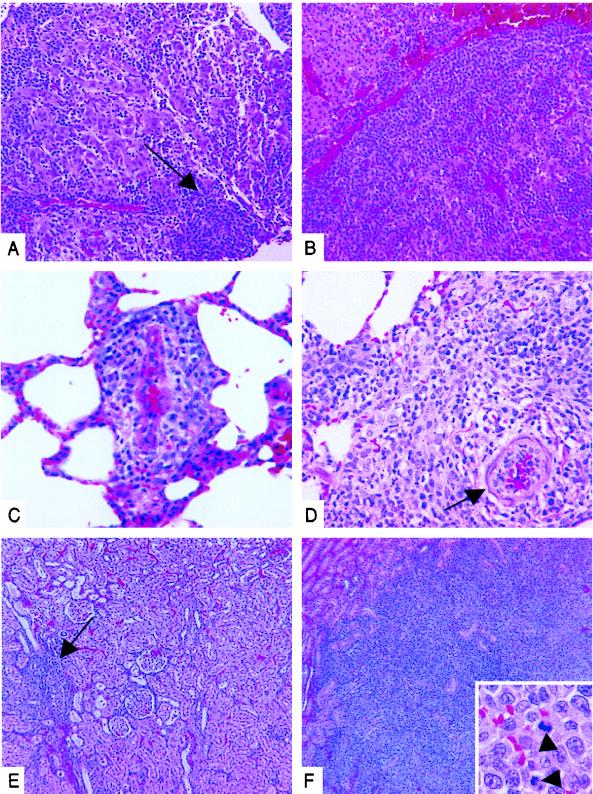
Experimental infection of common marmosets with HVS containing a deletion in Tip has shown that Tip is required for lymphoma induction in vivo (12). Common marmosets infected with wt HVS or with HVS/Tip mSH3B progressed to fatal lymphoproliferative disease with roughly equivalent time courses. Animals infected with wt HVS became moribund and were sacrificed on days 19 and 20 postinfection; animals infected with HVS/Tip mSH3B became moribund and were sacrificed on days 20 and 21 postinfection (Table 1). Necropsy of each animal revealed multicentric lymphoma consistent with wt HVS-induced pathology as previously described (8). Necropsy findings indicated more extensive lymphoid infiltrates in marmosets infected with HVS/Tip mSH3B than in those infected with the wt HVS (Fig. 6). Cellular infiltrates with the immature lymphoblast/reticulum cell morphology described previously were observed in all cases, and eosinophils were frequently admixed. Comparisons of adrenal gland (Fig. 6A and B), lung (Fig. 6C and D), and kidney (Fig. 6E and F) tissues are shown. Mild to moderate lymphoid infiltrates were found in the adrenal medullar and lung tissues in animals infected with wt HVS (Fig. 5A and C), while much more extensive infiltrates were found in the same tissues in animals infected with HVS/Tip mSH3B (Fig. 6B and D). Kidney tissue showed the most severe effects of infection with HVS/Tip mSH3B. While small lymphoid infiltrates were observed in the renal cortex of wt HVS-infected animals (Fig. 6E), much more extensive infiltrate composed of pleomorphic lymphoid cells showing many mitotic events effaced much of the renal cortex in HVS/Tip mSH3B-infected animal (Fig. 6F).
FIG. 6.
Comparative histopathology of common marmosets infected with wt HVS or HVS/Tip mSH3B. Necropsy tissue samples from common marmoset infected with wt HVS (A, C, and E) or with HVS/Tip mSH3B (B, D, and F) are shown. Tissues are adrenal gland (A and B), lung (C and D), and kidney (E and F). (A) A mild to moderate lymphoid infiltrates in adrenal gland as indicated by the arrow. (B) A extensive infiltrate effaced the medulla and extended partially into the cortex (upper left). (C) Neoplastic lymphoid cells surrounded a pulmonary vessel and mildly expanded adjacent alveolar septa. (D) The lymphoid infiltrate formed a mass extending from a pulmonary vessel. (E) Occasional small lymphoid infiltrate in the renal cortex as indicated by the arrow. (F) The extensive infiltrate in the renal cortex; arrowheads in inset indicate mitoses. Magnifications: ×124 (A and B), ×250 (C and D), ×50 (E and F), and ×750 (inset of panel F).
DISCUSSION
In this study, we analyzed the properties of a Tip mSH3B mutant in the context of virus infection of lymphocytes in culture and of experimental infection in an animal model. Our results demonstrate that an interaction between Lck and Tip of HVS is not required for immortalization of primary T cells in culture and for lymphoma induction in common marmosets. This report also demonstrates for the first time the introduction of specific point mutations into HVS.
As first reported by Biesinger et al. (5, 19) and confirmed by others (25), Tip associates with Lck. This interaction requires regions of Tip that include an SH3-binding motif and sequences with homology to the carboxyl terminus of Src-related kinases (19). While all have regarded Tip-Lck association as likely to be important in the biology of HVS infection, different viewpoints regarding whether the interaction results in activation or downregulation of Lck-mediated signaling have been expressed (5, 16, 20, 25, 38). Our findings are most consistent with the notion that Tip association blocks Lck-mediated signaling. It is, however, clearly demonstrated here that interaction of Tip with Lck is not essential for transforming activity of HVS. Interaction of Tip with Lck, a major T-cell tyrosine kinase, has been considered of possible importance in the T-cell specificity of HVS persistent infection and transformation (5, 25, 38). Results presented here do not support such a role for Tip association with Lck.
Mutation of the SH3-binding motif of Tip results in an altered phenotype in immortalized marmoset T lymphocytes in vitro and altered pathogenicity in vivo. While this phenotype appears to be a result of the loss of a blockade to Lck-mediated signaling, it is also possible that mSH3B mutation changes the dynamics of Tip interaction with other cellular partners. Recently, we described an additional cellular partner, Tap (40). Tip-Tap interaction occurs independently of the Tip-Lck interaction. Tip-Tap association results in NF-κB activation and increased cellular aggregation in cotransfected Jurkat T cells, which resembles the changes observed in HVS-transformed lymphocytes (40). However, surface expression of CD49d, an α4 integrin subunit, was found to be downregulated in HVS/Tip mSH3B-transformed T cells compared to wt HVS-transformed T cells. The specific role of downregulation of CD49d surface expression in HVS/Tip mSH3B-transformed T cells is unknown. Nevertheless, the precise mechanism of altered cellular signal transduction and enhanced pathogenicity of HVS/Tip mSH3B needs to be characterized.
The importance of Lck-mediated signaling in regulation of T-cell activation is well established (37). The specific interaction of a viral gene product such as Tip with Lck is unlikely to be trivial to the natural history of the virus. Precedent for decreased Lck-mediated signaling during virus-induced T-cell oncogenesis has also been reported for human T-cell leukemia virus type 1 (HTLV-1) (24). However, HTLV-1 appears to achieve the downregulation of Lck-mediated signaling by suppressing Lck expression rather than by blocking its function. Additionally, a B-cell major tyrosine kinase, Lyn, has been shown to be preferentially expressed in HTLV-1-transformed T cells, while it is expressed very little in normal T lymphocytes (36). Tax protein encoded by HTLV-1 has been shown to induce the Lyn promoter activity specifically through the Oct-1-binding motif (36). Since Oct-1 activity was not induced in recombinant HVS/Tip mSH3B-transformed T cells, transcriptional factors other than Oct-1 are involved for the activation of Lyn expression in these cells. In contrast, an increased in vitro kinase activity of Lyn, Fyn, Yes, and Src kinase activity has been detected in HVS-transformed human T-cell clones, while the level of expression of these tyrosine kinases has not been examined (38). Nonetheless, downregulation of Lck-mediated signal transduction and enhanced Lyn expression may be common features with virus-induced transformation of T lymphocytes.
Our results demonstrate unambiguously that Tip binding to Lck is not required for oncogenic transformation by HVS. Since a Tip gene is required for oncogenic transformation, some other activity or activities of Tip must be critical for this cell growth transformation. Binding to the cellular factor called Tap (40) is a leading candidate for this critical activity. Study of additional viral point mutations should be able to provide evidence for or against this hypothesis. Despite the fact that Tip binding to Lck is not critical for oncogenic transformation, binding to Lck is likely to contribute in some way to the viral life cycle. It could, for example, facilitate cell growth transformation in a noncritical fashion, or it could benefit the virus in some way entirely unrelated to cell growth transformation. Further studies of SH3B and other mutants by using New World primates models will be needed to understand the contribution of Tip binding to Lck.
ACKNOWLEDGMENTS
We thank G. Crabtree and T. Yamamoto for providing the reporter plasmids and J. Newton and A. Hampson for preparing the manuscript.
This work was supported by Public Health Service grants CA31363 and RR00168.
REFERENCES
- 1.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittman S, Craxton M, Coleman H, Fleckenstein B, Honness R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berend K R, Jung J U, Boyle T J, DiMaio J M, Mungal S A, Desrosiers R C, Lyerly H K. Phenotypic and functional consequences of herpesvirus saimiri infection of human CD8+ cytotoxic T lymphocytes. J Virol. 1993;67:6317–6321. doi: 10.1128/jvi.67.10.6317-6321.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesinger B, Trimble J J, Desrosiers R C, Fleckenstein B. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 5.Biesinger B, Tsygankov A Y, Fickenscher H, Emmrich R, Fleckenstein B, Bolen J B, Broker B M. The product of the herpesvirus saimiri ORF1 (Tip) interacts with T cell specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 6.Bröker B M, Tsygankov A Y, Müller-Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by Herpesvirus saimiri. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 7.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 8.Desrosiers R C, Bakker A, Kamine J, Falk L A, Hunt R D, King N W. A region of the Herpesvirus saimiri genome required for oncogenicity. Science. 1985;228:184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C, Burghoff R L, Bakker A, Kamine J. Construction of replication-competent herpesvirus saimiri deletion mutants. J Virol. 1984;49:343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desrosiers R C, Falk L A. Herpesvirus saimiri strain variability. J Virol. 1982;43:352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desrosiers R C, Silva D, Waldron L M, Letvin N L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986;57:701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duboise S M, Guo J, Desrosiers R C, Jung J U. Use of virion DNA as a cloning vector for the construction of mutant and recombinant herpesviruses. Proc Natl Acad Sci USA. 1996;93:11389–11394. doi: 10.1073/pnas.93.21.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk L, Wolfe L, Deinhardt F. Isolation of herpesvirus saimiri from blood of squirrel monkeys (saimiri sciureus) J Natl Cancer Inst. 1972;48:1499–1505. [PubMed] [Google Scholar]
- 15.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. New York, N.Y: Plenum Publishing Corporation; 1982. pp. 253–332. [Google Scholar]
- 16.Guo J, Duboise S M, Lee H, Li M, Choi J-K, Rosenzweig M, Jung J U. Enhanced downregulation of Lck-mediated signal transduction by a Y114 mutation of herpesvirus saimiri Tip. J Virol. 1997;71:7092–7096. doi: 10.1128/jvi.71.9.7092-7096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joung I, Kim T, Stolz L A, Payne G, Winkler D G, Walsh C T, Strominger J L, Shin J. Modification of Ser59 in the unique N-terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc Natl Acad Sci USA. 1995;92:5778–5782. doi: 10.1073/pnas.92.13.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung J U, Desrosiers R C. Herpesvirus saimiri and ateles. In: Webster R, Granoff A, editors. Encyclopedia of virology. Philadelphia, Pa: Saunders Scientific Publications, Inc.; 1994. pp. 614–622. [Google Scholar]
- 19.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of lck-binding elements in Tip of Herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 20.Jung J U, Lang S M, Jun T, Roberts T M, Veillette A, Desrosiers R C. Downregulation of Lck-mediated signal transduction by Tip of herpesvirus saimiri. J Virol. 1995;69:7814–7822. doi: 10.1128/jvi.69.12.7814-7822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyotaki M, Desrosiers R C, Letvin N L. Herpesvirus saimiri strain 11 immortalizes a restricted marmoset T8 lymphocyte subpopulation in vitro. J Exp Med. 1986;164:926–931. doi: 10.1084/jem.164.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koomey J M, Mulder C, Burghoff R L, Fleckenstein B, Desrosiers R C. Deletion of DNA sequences in a nononcogenic variant of herpesvirus saimiri. J Virol. 1984;50:662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemasson I, Robert-Hebmann V, Hamaia S, Duc Dodon M, Gazzolo L, DeVaux C. Transrepression of lck gene expression by human T-cell leukemia virus type 1-encoded p40tax. J Virol. 1997;71:1975–1983. doi: 10.1128/jvi.71.3.1975-1983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund T, Medveczky M, Medveczky P. Herpesvirus saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J Virol. 1997;71:378–382. doi: 10.1128/jvi.71.1.378-382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund T, Medveczky M M, Neame P J, Medveczky P G. A herpesvirus saimiri membrane protein required for interleukin-2 independence forms a stable complex with p56lck. J Virol. 1996;70:600–606. doi: 10.1128/jvi.70.1.600-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medveczky P, Szomolayi E, Desrosiers R C, Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984;52:938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller C L, Burkhard A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 29.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller C L, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittrücker H-W, Müller-Fleckenstein I, Fleckenstein B, Fleishcher B. CD2-mediated autocrine growth of herpes virus saimiri-transformed human T lymphocytes. J Exp Med. 1995;176:900–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy S C S, Trimble J J, Desrosiers R C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989;63:3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawelec G, Ziegler A, Wernet P. Dissection of human allostimulatory determinants with cloned T cells: stimulation inhibition by monoclonal antibodies TÜ 22, 34, 36, 37, 39, 43, and 58 against distinct human MHC class II molecules. Hum Immunol. 1985;12:165–176. doi: 10.1016/0198-8859(85)90333-7. [DOI] [PubMed] [Google Scholar]
- 34.Roizmann B, Desrosiers R C, Fleckenstein B, Lopez C, Minson A C, Studdert M J. The family Herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 35.Russo J J, Bohenzxy R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi’s sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchiumi F, Semba K, Yamanashi Y, Fujisawa J-I, Yoshida M, Inoue K, Toyoshima K, Yamamoto T. Characterization of the promoter region of the src family gene lyn and its trans activation by human T-cell leukemia virus type I-encoded p40tax. Mol Cell Biol. 1992;12:3784–3795. doi: 10.1128/mcb.12.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 38.Wiese N, Tsygankov A Y, Klauenberg U, Bolen J B, Fleischer B, Broker B M. Selective activation of T cell kinase p56lck by herpesvirus saimiri protein tip. J Biol Chem. 1997;271:847–852. doi: 10.1074/jbc.271.2.847. [DOI] [PubMed] [Google Scholar]
- 39.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase lyn. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 40.Yoon D-W, Lee H, Seol W, DeMaria M, Rosenzweig M, Jung J U. Tap; a novel cellular protein that interacts with tip of Herpesvirus saimiri and induces lymphocyte aggregation. Immunity. 1997;6:571–582. doi: 10.1016/s1074-7613(00)80345-3. [DOI] [PubMed] [Google Scholar]