Abstract
Objectives:
The objective of this study was to compare the biomechanical properties of locked and nonlocked diaphyseal fixation in a model of distal femur fractures using osteoporotic and nonosteoporotic human cadaveric bone.
Methods:
A supracondylar osteotomy was created to mimic a fracture (OTA/AO 33A3) in osteoporotic (n = 4) and nonosteoporotic (n = 5) cadaveric distal femurs. The left and right femurs of each pair were instrumented with a distal femoral locking plate and randomly assigned to have diaphyseal fixation with either locked or nonlocked screws. The construct was cyclically axially loaded, and construct stiffness and load to failure were evaluated.
Results:
In osteoporotic bone, locked constructs were more stiff than nonlocked constructs (mean 143 vs. 98 N/mm when all time points combined, P < 0.001). However, in nonosteoporotic bone, locked constructs were less stiff than nonlocked constructs (mean 155 N/mm vs. 185 N/mm when all time points combined, P < 0.001). In osteoporotic bone, the average load to failure was greater in the locked group than in the nonlocked group (mean 1159 vs. 991 N, P = 0.01). In nonosteoporotic bone, the average load to failure was greater for the nonlocked group (mean 1348 N vs. 1214 N, P = 0.02). Bone mineral density was highly correlated with maximal load to failure (R2 = 0.92, P = 0.001) and stiffness (R2 = 0.78, P = 0.002) in nonlocked constructs but not in locked constructs.
Conclusions:
Contrary to popular belief, locked plating constructs are not necessarily stiffer than nonlocked constructs. In healthy nonosteoporotic bone, locked diaphyseal fixation does not provide a stiffer construct than nonlocked fixation. Bone quality has a profound influence on the stiffness of nonlocked (but not locked) constructs in distal femur fractures.
Keywords: biomechanics, distal femur fracture, load to failure, mechanical testing, locking fixation
1. Introduction
Modern plate fixation of fractures relies on the surgeon to create a biomechanical environment optimal for healing.1–5 In general, simple fractures are amenable to anatomic reduction and compression of fracture fragments through lag screws and/or dynamic compression techniques.6 Absolute stability with relatively stiff constructs is beneficial in this scenario to promote primary bone healing.6 Comminuted fractures, on the other hand, are generally amenable to bridge plating.7 These fractures require relative, rather than absolute, stability to promote secondary bone healing.7 The surgeon controls construct stiffness through the choice of plate, number of used screws, screw location, and screw type (locked or nonlocked), among other factors.8,9 It is generally believed that locked screws provide a stiffer construct than nonlocked screws, yet there is a paucity of biomechanical data to support this assumption.10–12
Traditional (nonlocking) plate–screw constructs rely on compression between the plate and bone for stability and are subject to loosening during cyclic loading.13 Stable fixation can be problematic in osteoporotic bone as nonlocked screws can result in either insufficient tightening of screws or stripping. In either case, construct stability may be compromised due to insufficient compression of plate to bone. This is especially true with cyclic loading, as nonlocked screws gradually loosen and result in less stability over time. Locking screw technology does not rely on compression between the plate and the bone, and therefore, issues of insufficient tightening and stripping are obviated. In contrast to nonlocked plating in which individual screws fail sequentially, locked screws must fail in unison and therefore are generally thought to have a greater resistance to failure.14 These qualities make locked fixation beneficial in periarticular and osteoporotic areas where bone quality is relatively poor.13 Still, many plate systems provide locking options throughout the entire plate, including diaphyseal regions where bone quality may be adequate for nonlocking screws. For instance, most plates used in distal femur fractures offer locking options in both the (distal) metaphyseal and proximal (diaphyseal) segments. Although locking fixation has been nearly universally accepted for use in the articular segment, its advantage in the proximal segment is unclear.15 In fact, the use of locking screws in the diaphysis has been implicated in contributing to nonunion of various fractures but is enticing, especially in situations where a bridge plate construct is used as a load-bearing construct.5,16–18 In addition to the obvious biomechanical implications of locked versus nonlocked screws, there are economic issues as locked screws are substantially more costly than their nonlocked analogs.9,13,19 Thus, it would be useful to determine the precise biomechanical differences between locked and nonlocked diaphyseal fixation to understand how construct stiffness is influenced by screw type.
The purpose of this study was to directly compare locked and nonlocked diaphyseal fixation in osteoporotic and nonosteoporotic bone in a cadaveric model of a distal femoral fracture. Specifically, differences in construct stiffness and load to failure were studied in a cyclic loading model. Our null hypothesis was that locked screws would provide no biomechanical differences compared with nonlocked screws regardless of bone quality.
2. Materials and Methods
2.1. Cadaveric Specimens
The distal femoral metaphyses of human cadaveric specimens were DEXA scanned to determine bone mineral density. Specimens with bone mineral density (BMD) > 0.8 g/cm2, which correlates with a T-score at the hip and proximal femur of −2, were considered nonosteoporotic and those with BMD < 0.8 g/cm2 were considered osteoporotic.20 Five matched pairs of nonosteoporotic human cadaveric femurs (BMD 0.86–1.19 g/cm2) and 4 matched pairs of osteoporotic femurs (BMD 0.28–0.66 g/cm2) were used for testing.
2.2. Constructs
Each femur was instrumented using a contoured periarticular plate that provided for insertion of either locked or nonlocked screws in every hole (PERI-LOC, Smith & Nephew, Memphis, TN). The left and right femurs of each pair were then randomly assigned into 1 of 2 groups, based on the diaphyseal fixation method: locked or nonlocked. The locked groups had diaphyseal fixation with four 4.5-mm locked screws, whereas the nonlocked groups had fixation with four 4.5-mm nonlocked screws. Locked and nonlocked screws were identical other than the head of the screw: They had the same major diameter, minor diameter, and thread pitch. In both groups, diaphyseal fixation was in the 2 closest and 2 farthest holes from the fracture with 1 hole between left empty (Fig. 1). Distal fixation in both groups consisted of five 5.7-mm locking screws after clamping plate to bone. All screws were inserted to a torque of 3.96 Nm using a manual torque limiter. All plates were fixed in a consistent manner by the same investigator (WMR). After instrumentation, extra-articular distal femoral osteotomies were created at 6 and 9 cm proximal to the distal femoral joint line, thus producing a 3-cm gap simulating a comminuted axially unstable metaphyseal fracture (OTA/AO 33A3).
Figure 1.
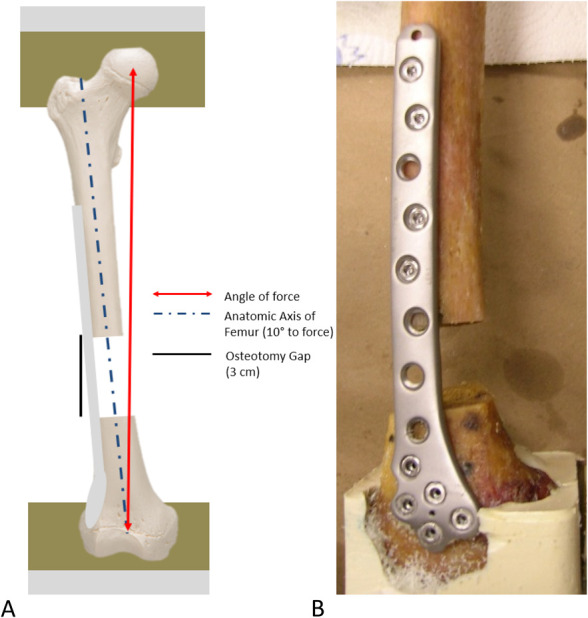
A, Diagram of setup with specimen mounted for biomechanical testing. The distal end of each femur was positioned and potted in the supporting potting fixture at a 10-degree angle from the vertical axis in the coronal plane to align the mechanical axis of the femur with the loading axis of the bone–plate construct. Special attention was taken to prevent potting material from constraining the plate and screws during the distal potting. Universal joints were used for proximal and distal attachment. B, Supracondylar distal femur fracture status postfixation with a distal femoral locking plate. Distal fixation in all groups consisted of five 5.7-mm locking screws. Proximal fixation consisted of 4 locked or nonlocked 4.5-mm screws. Proximal screw configuration consisted of 2 adjacent screws near the fracture and 2 screws at the end of the plate separated by a single empty screw hole.
2.3. Loading
The proximal end of each femur was then potted 1-cm deep in a loading fixture using Fast Cast (Environmental Technology, Fields Landing, CA). The distal end of each femur was positioned and potted in the supporting potting fixture at a 10-degree angle from the vertical axis in the coronal plane to align the mechanical axis of the femur with the loading axis of the bone–plate construct. Special attention was taken to prevent potting material from constraining the plate and screws during the distal potting. The loading fixture was then secured to the upper and lower platen of a servohydraulic load frame through universal joints (MTS, Eden Prairie, MN). All specimens were subjected to a cyclic axial compressive load of 500 N with a varus moment to simulate approximate human bodyweight (approximately 112 lbs). Loading was begun by subjecting each femur to an axial cyclic load of 50/500 N for 100 cycles at 2 Hz to remove any slack from the system. Subsequently, an axial cyclic load of 50/500 N was applied to each femur for 500,000 cycles at 2 Hz. Samples were kept moist throughout testing. Construct stiffness was evaluated at 11 time points, baseline, and then subsequently every 50,000 cycles by pausing cyclical loading and applying a ramp load. After completion of 500,000 cycles, specimens were ramp loaded until failure at a rate of 100 N/s.
2.4. Data Acquisition and Analysis
All data are shown as means with 95% confidence intervals. The stiffness of the constructs was evaluated by acquiring the slopes of the force–displacement curves from ramp loading. Load to failure was defined as the peak load that resulted in failure of the construct. Failure was detected by measuring a sharp drop-off in force on the load versus displacement curve. Statistical analysis was performed using SPSS Statistics 22.0 software (IBM, Armonk, NY). The normality of the acquired data was proven using a Shapiro–Wilk test before performing any parametric testing. A 3-way repeated measures analysis of variance (ANOVA) was performed to determine the effect of osteoporotic status, locked fixation, and cycle number on construct stiffness. Cycle number was used as a within-subject factor, while osteoporotic status and locked status were used as between-subject factors. A 2-way ANOVA was performed to determine the effect of osteoporotic status and locked fixation on load to failure. Subgroup analyses (eg, locked osteoporotic vs. unlocked osteoporotic) were performed with a post hoc Tukey test on variables in which significant effects were found by ANOVA. Partial eta squared () was calculated to evaluate the magnitude of effect of a given variable. A Pearson correlation coefficient was calculated between stiffness and applied load until failure versus bone mineral density in both locked and unlocked groups. Statistical significance was declared as P < 0.05.
3. Results
3.1. Stiffness
Osteoporotic status was found to have a significant effect on construct stiffness when averaged across all time points (P < 0.001, = 0.719). In addition, there was a significant interaction effect seen between osteoporotic status and the use of locked fixation (P = 0.001, = 0.591) on construct stiffness. Locked fixation in and of itself did not have a significant effect on construct stiffness when examining all samples together (P = 0.441). The number of loading cycles did not have a significant effect on the stiffness of the construct (P = 0.189). For construct stiffness, no significant interaction was found between cycle and osteoporotic status (P = 0.448); cycle and locked fixation (P = 0.143); or the combination of cycle, osteoporotic status, and locked fixation together (P = 0.136).
In nonosteoporotic bone, the average stiffness of locked constructs (155 N/mm, 95% CI: 138–172) was 16% less than nonlocked constructs (185 N/mm, 95% CI: 166–204, P < 0.001).
In osteoporotic bone, the average stiffness of locked constructs (143 N/mm, 95% CI: 124–162) was 46% greater than nonlocked constructs (98 N/mm, 95% CI: 76–120, P < 0.001). The results are presented in Table 1 and Figure 2A.
TABLE 1.
Stiffness of Nonosteoporotic and Osteoporotic Samples Used During Cyclical Loading
| Cycle Count | Stiffness (N/mm) | Mean | SD | 95% CI | ||||||
| Nonosteoporotic bone | Nonlocked fixation | 1L | 2R | 3L | 4R | 5R | ||||
| 0 | 158 | 187 | 212 | 211 | 233 | 200 | 29 | 944–1248 | ||
| 50,000 | 147 | 280 | 218 | 198 | 198 | 208 | 48 | 932–1474 | ||
| 100,000 | 144 | 172 | 267 | 203 | 353 | 228 | 84 | 901–1341 | ||
| 150,000 | 153 | 253 | 232 | 179 | 196 | 202 | 40 | 750–1582 | ||
| 200,000 | 182 | 239 | 177 | 185 | 194 | 195 | 25 | 966–1270 | ||
| 250,000 | 134 | 172 | 207 | 172 | 180 | 173 | 26 | 719–1235 | ||
| 300,000 | 139 | 152 | 163 | 191 | 186 | 166 | 22 | 756–1085 | ||
| 350,000 | 226 | 171 | 250 | 167 | 177 | 198 | 37 | 977–1349 | ||
| 400,000 | 205 | 142 | 148 | 219 | 0 | 178 | 39 | 695–1343 | ||
| 450,000 | 198 | 155 | 150 | 156 | 169 | 165 | 19 | 798–1082 | ||
| 500,000 | 195 | 159 | 132 | 154 | 136 | 155 | 25 | 747–1080 | ||
| Locked fixation | 1R | 2L | 3R | 4L | 5L | |||||
| 0 | 205 | 147 | 154 | 205 | 189 | 180 | 28 | 890–1162 | ||
| 50,000 | 198 | 191 | 139 | 165 | 285 | 195 | 55 | 874–1359 | ||
| 100,000 | 187 | 136 | 139 | 147 | 211 | 164 | 33 | 739–1133 | ||
| 150,000 | 134 | 139 | 134 | 177 | 216 | 160 | 36 | 541–1286 | ||
| 200,000 | 129 | 132 | 141 | 167 | 176 | 149 | 21 | 714–985 | ||
| 250,000 | 134 | 152 | 135 | 138 | 149 | 142 | 8 | 578–1039 | ||
| 300,000 | 130 | 129 | 143 | 177 | 144 | 144 | 19 | 678–972 | ||
| 350,000 | 130 | 140 | 133 | 123 | 160 | 137 | 14 | 617–950 | ||
| 400,000 | 126 | 124 | 127 | 145 | 136 | 132 | 9 | 462–1042 | ||
| 450,000 | 162 | 137 | 189 | 127 | 140 | 151 | 2 | 735–989 | ||
| 500,000 | 113 | 168 | 192 | 115 | 175 | 153 | 36 | 722–1021 | ||
| Osteoporotic bone | Nonlocked fixation | 6L | 7L | 8R | 9L | |||||
| 0 | 83 | 128 | 142 | 120 | 118 | 25 | 495–846 | |||
| 50,000 | 70 | 123 | 97 | 137 | 107 | 29 | 239–865 | |||
| 100,000 | 68 | 123 | 93 | 97 | 95 | 23 | 287–794 | |||
| 150,000 | 66 | 118 | 110 | 131 | 106 | 28 | 78–1039 | |||
| 200,000 | 65 | 113 | 139 | 90 | 102 | 32 | 428–779 | |||
| 250,000 | 63 | 106 | 98 | 125 | 98 | 26 | 209–805 | |||
| 300,000 | 68 | 164 | 84 | 75 | 98 | 44 | 412–791 | |||
| 350,000 | 100 | 107 | 82 | 67 | 89 | 18 | 334–763 | |||
| 400,000 | 62 | 162 | 82 | 65 | 93 | 47 | 209–958 | |||
| 450,000 | 79 | 103 | 110 | 80 | 93 | 16 | 392–721 | |||
| 500,000 | 60 | 105 | 77 | 81 | 22 | 268–652 | ||||
| Locked fixation | 6R | 7R | 8L | 9R | ||||||
| 0 | 156 | 139 | 141 | 136 | 143 | 9 | 665–970 | |||
| 50,000 | 137 | 137 | 126 | 130 | 132 | 5 | 485–1027 | |||
| 100,000 | 130 | 146 | 160 | 131 | 142 | 15 | 590–1029 | |||
| 150,000 | 128 | 359 | 123 | 130 | 185 | 116 | 640–1473 | |||
| 200,000 | 125 | 131 | 122 | 127 | 126 | 4 | 569–873 | |||
| 250,000 | 272 | 121 | 122 | 125 | 160 | 74 | 655–1170 | |||
| 300,000 | 127 | 113 | 117 | 126 | 121 | 7 | 525–854 | |||
| 350,000 | 199 | 120 | 118 | 125 | 140 | 39 | 616–988 | |||
| 400,000 | 140 | 298 | 119 | 122 | 170 | 86 | 646–1294 | |||
| 450,000 | 118 | 169 | 115 | 129 | 133 | 25 | 615–899 | |||
| 500,000 | 132 | 112 | 111 | 122 | 119 | 10 | 513–846 | |||
Figure 2.
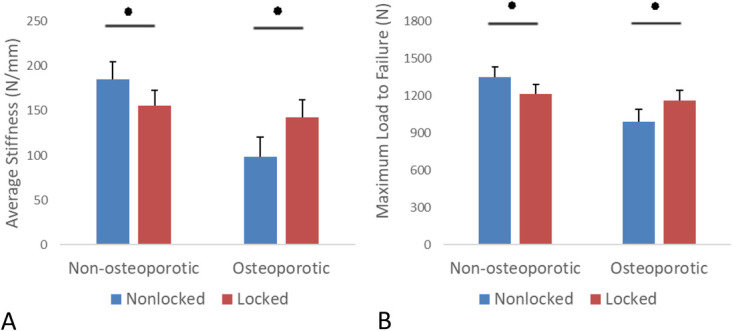
Average stiffness (A) and maximal load to failure (B) of nonosteoporotic and osteoporotic samples. Error bars represent 95% confidence interval. *P < 0.05.
3.2. Load to Failure
Osteoporotic status was found to have a significant effect on load to failure (P < 0.001, = 0.70). There was a significant interaction effect between osteoporotic status and the use of locked fixation (P = 0.002, = 0.56) on load to failure.
In nonosteoporotic bone, after completing 500,000 cycles, the average load to failure was greater for the nonlocked group (1348 N, 95% CI: 1263–1432) than the locked group (1214 N, 95% CI: 1139–1290, P = 0.02). The load to failure for each specimen is presented in Table 2. The nonlocked side in pair #2 exceeded the capacity of the load cell (1334 N). The observed mode of failure was plate deformation in all specimens.
TABLE 2.
Maximum Load for Load to Failure Test in Nonosteoporotic and Osteoporotic Groups
| Type of Proximal Fixation | Load to Failure (N) | Mean | SD | 95% CI | ||||
| Nonosteoporotic bone | 1 | 2 | 3 | 4 | 5 | |||
| Nonlocked | 1310 | 1324 | 1447 | 1313 | 1348 | 66 | 1263–1432 | |
| Locked | 1226 | 1324 | 1214 | 1060 | 1245 | 1214 | 96 | 1139–1290 |
| Osteoporotic bone | 6 | 7 | 8 | 9 | ||||
| Nonlocked | 941 | 1061 | 971 | 991 | 62 | 894–1090 | ||
| Locked | 1200 | 1063 | 1213 | 1163 | 1159 | 83 | 1076–1245 | |
The nonlocked sample in sample #2 exceeded the load capacity of the cell. The nonlocked sample in pair #9 was destroyed before maximal load testing could take place.
In osteoporotic bone, after completing 500,000 cycles, the average load to failure was greater in the locked group (1159 N, 95% CI: 1076–1245) than in the nonlocked group (991 N, 95% CI: 894–1090, P = 0.01). For the nonlocked side in pair #4, a technical error caused a pretesting fracture at the proximal margin of the plate. Failure modes were plate deformation, bone cracking in diaphyseal fixation region, and bone deformation in the region close to the proximal fixation. The results are presented in Table 2 and Figure 2B.
3.3. BMD Versus Stiffness and Load to Failure
DEXA revealed a BMD range of 0.48 to 1.19 g/cm2. Within the locked group, BMD did not correlate with average stiffness over all cycles (rho = 0.188, R2 = 0.035, P = 0.627). For the nonlocked group, BMD was highly correlated with stiffness (rho = 0.885, R2 = 0.783, P = 0.002). The intersection of these 2 lines occurred at a BMD of 0.81 g/cm2. The results are shown in Figure 3A.
Figure 3.
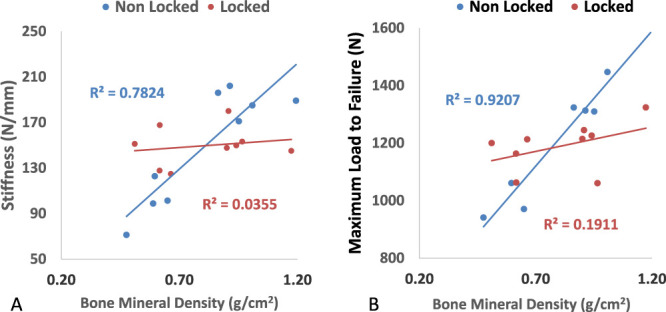
Average stiffness (A) and maximum load to failure (B) versus bone mineral density in nonlocked and locked groups. R-squared calculated from Pearson rho.
Within the locked group, BMD did not correlate with the load to failure (rho = 0.437, R2 = 0.19, P = 0.240). For the nonlocked group, BMD was highly correlated with the load to failure (rho = 0.960, R2 = 0.92, P = 0.001). The intersection of these 2 lines occurred at a BMD of 0.77 g/cm2. The results are shown in Figure 3B.
4. Discussion
The high rate of complications of distal femur fractures after open reduction internal fixation (ORIF) has spurred interest in the examination of surgeon-controlled variables that affect the rate of union.17,21,22 Stiffness has been identified as an important factor for timely healing of distal femur fractures even as the ideal stiffness for the prevention of nonunion is unknown.5,17,21,23,24 Our study found that bone quality had a significant interaction with the presence or absence of locked fixation on construct stiffness during cyclic loading. Contrary to popular belief, constructs with locked diaphyseal screws were significantly less stiff and had a decreased load to failure than constructs with nonlocked diaphyseal screws in nonosteoporotic bone. By contrast, locked constructs were stiffer and had a higher load to failure than nonlocked constructs in osteoporotic bone. Bone mineral density was found to be very highly correlated with both stiffness and load to failure in nonlocked samples and had no effect on either stiffness or load to failure in the locked group. Our findings indicate that nonlocking diaphyseal fixation is highly dependent on bone quality. Based on the mechanism of function of these locked and nonlocked screws, it is likely that in samples with higher bone densities, nonlocked constructs benefitted from higher friction between the plate and bone which overcame the benefits of locked fixation. This effect was not achieved in samples with poor bone quality, and therefore, the bone density independent strength of locked fixation was superior in this group.
Several other authors have used in vitro models to examine the effect of numerous variables on construct stiffness and load to failure in distal femur fractures after ORIF. Weaver et al used a synthetic femoral analog created from a short fiber epoxy shell to examine the effects of several plate length, screw type, working length, and plate material on stiffness. In this study, the use of bicortical nonlocking screws in the diaphysis decreased overall construct stiffness by 18% compared with locking screws (808 N/mm vs. 995 N/mm).24 Kandemir et al examined the use of hybrid (locked and nonlocked) and completely locked fixation in the diaphysis of a synthetic distal femur fracture model and detected no difference between these 2 groups. Our study found that the effect of locked fixation on stiffness was highly dependent on the quality of bone in which the fixation was used. Given that synthetic samples were used in both these studies, direct comparison of these results with our findings is difficult as the corresponding bone qualities of synthetic and cadaveric femurs are unclear. Cui et al examined axial/torsional stiffness and axial load to failure in an osteoporotic cadaveric distal femur fracture model which underwent ORIF with various combinations of locked and nonlocked screws in the diaphysis. They found no significant difference between locked and nonlocked constructs regarding axial stiffness. Conversely, they noted an increased maximum load to failure and torsional stiffness in the groups in which the distal most diaphyseal screw was locked, regardless of whether the rest of the diaphyseal screws were all locked or all nonlocked. Interestingly, their average T-score for all groups in this study was approximately −2. In our study, a T-score of −2 corresponded to the bone quality where the stiffness was equal between the locked and nonlocked groups, and these findings are consistent with those found in our study. Conversely, they found that locked fixation increased load to failure (1511 vs. 916 N), which differs from our results which expected no difference at this bone density. Explanations for this difference in results could include that this study tested all biomechanical parameters at very low cycle numbers (<1000) and so were measuring initial rather than final stiffness and maximal load to failure. Gardner et al compared the biomechanical behavior of a locked compression plate with a dynamic compression plate construct in a cadaveric radius model with an average bone mineral density of just above the margin generally used for being considered osteoporotic (0.39 gm/cm2) in this location. Their findings were similar to those of our study in that locked plates demonstrated improved resistance to failure but their model did not show any differences in stiffness between locked and nonlocked groups.12 A separate group examined 4 bridge plating configurations applied to a machine polyurethane bone model of osteoporotic femoral diaphysis.25 They found less than 10% differences in axial stiffness between 3 different configurations of locking screws and a conventional nonlocked plate.
Although several studies have examine the effects of surgeon and patient-controlled variables on stiffness and load to failure, the ideal values for these parameters remain largely unknown.24 In vivo studies have suggested that reported failures with locking plate constructs which are deemed too stiff for adequate interfragmentary motion and therefore callus production but these studies do not provide precise values to guide the clinician.17,26,27 This can be seen as a weakness of our study as although we report the effects of locking and nonlocking fixation in the diaphysis in osteoporotic and nonosteoporotic bone, it is unclear which patients would benefit from which mode of fixation to avoid nonunion. Our study has other limitations. The model we used focused on a single mode of axial loading to measure stiffness and failure strength. While simple and clinically relevant, this approach may miss the effects of torsional or more extreme bending forces on fixation. These forces have been found to affect the fixation parameters of locked and unlocked screws differently.25,28 In addition, more complex methods of detecting three-dimensional fracture site motion as estimated by finite element analysis have recently been shown to be better predictors of callus formation rather than simple axial stiffness.29 We examined the effects of stiffness over the course of 500,000 cycles which simulates the over the course of a year assuming approximately 10,000 cycles per week.30,31 Although this sets a high bar for our fixation constructs to clear, it discounts the effect of progressive fracture healing with time which would facilitate load sharing of the implant over a high number of cycles and largely represents situations in which there is a significant bone defect with little to no healing present which continues to rely on a load-bearing construct for stability longer than the expected 6–12 weeks required for fracture healing. In addition, our sample size was fairly low with only 4–5 samples per group, and so differences in construct stiffness could not be detected at individual time points. For maximal load to failure, 2 samples were lost and so there were only 4 samples were used in the nonosteoporotic nonlocked group and only 3 in the osteoporotic nonlocked group. Finally, our study used a model which placed all locking screws in the metaphyseal (distal bone) and then tested all locking or all nonlocking screws proximally (in the diaphysis). We did not examine combinations of nonlocking and locking screws for diaphyseal fixation which have been found to improve fixation parameters in other studies.25,32
5. Conclusion
Our results suggest that the relative effect of locked screws versus nonlocked screws placed in the diaphysis of a distal femur fracture on construct stiffness depends on the bone quality present at the site of insertion. Locked constructs are not necessarily stiffer than similar nonlocked constructs. Thus, surgeons considering locked screws at this location should be aware of the bone quality in this region and desired stiffness of their construct. These results provide a scientific basis to guide surgeons when deciding when locked screws are indicated for fracture fixation.
Footnotes
The plates and screws used in this study were provided by Smith and Nephew plc. Y.Z., Z.W., M.A., J.H., B.J., and J.C. were employed by Smith and Nephew plc during the execution of this study. A.D. or his immediate family or any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this manuscript.
W.R. and P.T.III received royalties for sale of intellectual property from Smith and Nephew. Y.Z., Z.W., M.A., J.H., B.J., and J.C. were employed by Smith and Nephew plc during the execution of this study. A.D. or his immediate family or any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this manuscript.
Contributor Information
William M. Ricci, Email: ricciw@hss.edu.
Aleksey Dvorzhinskiy, Email: advorzh@gmail.com.
Yanming Zheng, Email: Yanming.Zheng@smith-nephew.com.
Zakiyyah Walker, Email: zakiyyahwalker@yahoo.com.
Mary Anthony, Email: mekanthony@gmail.com.
Jeffrey Holbrook, Email: jeffrey.a.holbrook@medtronic.com.
Bob Jones, Email: bob.jones@smith-nephew.com.
Jacob Cartner, Email: jcartne@hotmail.com.
Paul Tornetta, III, Email: ptornetta@gmail.com.
References
- 1.Uhthoff HK, Finnegan MA. The role of rigidity in fracture fixation—an overview. Arch Orthop Trauma Surg. 1984;102:163–166. [DOI] [PubMed] [Google Scholar]
- 2.Bottlang M, Schemitsch CE, Nauth A, et al. Biomechanical concepts for fracture fixation. J Orthop Trauma. 2015;29(suppl 12):S28–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epari DR, Kassi JP, Schell H, et al. Timely fracture-healing requires optimization of axial fixation stability. J Bone Joint Surg Am. 2007;89:1575–1585. [DOI] [PubMed] [Google Scholar]
- 4.Perren SM. Evolution of the internal fixation of long bone fractures: the scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84:1093–1110. [DOI] [PubMed] [Google Scholar]
- 5.Bottlang M, Doornink J, Lujan TJ, et al. Effects of construct stiffness on healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(suppl 2):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris BL, Lang G, Russell TAT, et al. Absolute versus relative fracture fixation: impact on fracture healing. J Orthop Trauma 2018;32(suppl 1):S12–S16. [DOI] [PubMed] [Google Scholar]
- 7.Heitemeyer U, Kemper F, Hierholzer G, et al. Severely comminuted femoral shaft fractures: treatment by bridging-plate osteosynthesis. Arch Orthop Trauma Surg. 1987;106:327–330. [DOI] [PubMed] [Google Scholar]
- 8.Freeman AL, Tornetta P, Schmidt A, et al. How much do locked screws add to the fixation of “hybrid” plate constructs in osteoporotic bone? J Orthop Trauma 2010;24:163–169. [DOI] [PubMed] [Google Scholar]
- 9.Haidukewych GJ, Ricci W. Locked plating in orthopaedic trauma: a clinical update. J Am Acad Orthop Surg. 2008;16:347–355. [DOI] [PubMed] [Google Scholar]
- 10.Egol KA, Kubiak EN, Fulkerson E, et al. Biomechanics of locked plates and screws. J Orthop Trauma 2004;18:488–493. [DOI] [PubMed] [Google Scholar]
- 11.Fulkerson E, Egol KA, Kubiak EN, et al. Fixation of diaphyseal fractures with a segmental defect: a biomechanical comparison of locked and conventional plating techniques. J Trauma. 2006;60:830–835. [DOI] [PubMed] [Google Scholar]
- 12.Gardner MJ, Brophy RH, Campbell D, et al. The mechanical behavior of locking compression plates compared with dynamic compression plates in a cadaver radius model. J Orthop Trauma 2005;19:597–603. [DOI] [PubMed] [Google Scholar]
- 13.Haidukewych GJ. Innovations in locking plate technology. J Am Acad Orthop Surg. 2004;12:205–212. [DOI] [PubMed] [Google Scholar]
- 14.Ricci WM. Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Wolters Kluwer Health; 2015. [Google Scholar]
- 15.Strauss EJ, Schwarzkopf R, Kummer F, et al. The current status of locked plating: the good, the bad, and the ugly. J Orthop Trauma 2008;22:479–486. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann MF, Jones CB, Sietsema DL, et al. Clinical outcomes of locked plating of distal femoral fractures in a retrospective cohort. J Orthop Surg Res. 2013;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lujan TJ, Henderson CE, Madey SM, et al. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24:156–162. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CE, Kuhl LL, Fitzpatrick DC, et al. Locking plates for distal femur fractures: is there a problem with fracture healing? J Orthop Trauma 2011;25(suppl 1):S8–S14. [DOI] [PubMed] [Google Scholar]
- 19.Gardner MJ, Helfet DL, Lorich DG. Has locked plating completely replaced conventional plating? Am J Orthop. 2004;33:439–446. [PubMed] [Google Scholar]
- 20.Manisali M, Özaksoy D, Yilmaz E, et al. Bone mineral density reference values in the normal female and male population of Izmir, Turkey. Eur Radiol. 2003;13:157–162. [DOI] [PubMed] [Google Scholar]
- 21.Ricci WM, Streubel PN, Morshed S, et al. Risk factors for failure of locked plate fixation of distal femur fractures: an analysis of 335 cases. J Orthop Trauma 2014;28:83–89. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez EK, Boulton C, Weaver MJ, et al. Predictive factors of distal femoral fracture nonunion after lateral locked plating: a retrospective multicenter case-control study of 283 fractures. Injury. 2014;45:554–559. [DOI] [PubMed] [Google Scholar]
- 23.Streubel PN, Gardner MJ, Morshed S, et al. Are extreme distal periprosthetic supracondylar fractures of the femur too distal to fix using a lateral locked plate? J Bone Joint Surg Br. 2010;92:527–534. [DOI] [PubMed] [Google Scholar]
- 24.Weaver MJ, Chaus GW, Masoudi A, et al. The effect of surgeon-controlled variables on construct stiffness in lateral locked plating of distal femoral fractures. BMC Musculoskelet Disord. 2021;22:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzpatrick DC, Doornink J, Madey SM, et al. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallier HA, Hennessey TA, Sontich JK, et al. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88:846–853. [DOI] [PubMed] [Google Scholar]
- 27.Henderson CE, Lujan TJ, Kuhl LL, et al. 2010 mid-America Orthopaedic Association Physician in Training Award: healing complications are common after locked plating for distal femur fractures. Clin Orthop Relat Res. 2011;469:1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doornink J, Fitzpatrick DC, Boldhaus S, et al. Effects of hybrid plating with locked and nonlocked screws on the strength of locked plating constructs in the osteoporotic diaphysis. J Trauma. 2010;69:411–417. [DOI] [PubMed] [Google Scholar]
- 29.Elkins J, Marsh JL, Lujan T, et al. Motion predicts clinical callus formation: construct-specific finite element analysis of supracondylar femoral fractures. J Bone Joint Surg Am. 2016;98:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmeier KL, Hofmann GO, Mückley T. Choosing a proper working length can improve the lifespan of locked plates. A biomechanical study. Clin Biomech. 2011;26:405–409. [DOI] [PubMed] [Google Scholar]
- 31.Heiney JP, Barnett MD, Vrabec GA, et al. Distal femoral fixation: a biomechanical comparison of trigen retrograde intramedullary (I.M.) nail, dynamic condylar screw (DCS), and locking compression plate (LCP) condylar plate. J Trauma. 2009;66:443–449. [DOI] [PubMed] [Google Scholar]
- 32.Bottlang M, Doornink J, Byrd GD, et al. A nonlocking end screw can decrease fracture risk caused by locked plating in the osteoporotic diaphysis. J Bone Joint Surg Am. 2009;91:620–627. [DOI] [PubMed] [Google Scholar]


