Abstract
High blood levels of low-density lipoprotein (LDL)-cholesterol (LDL-C) are associated with atherosclerosis, mainly by promoting foam cell accumulation in vessels. As cholesterol is an essential component of cell plasma membranes and a regulator of several signaling pathways, LDL-C excess may have wider cardiovascular toxicity. We examined, in untreated hypercholesterolemia (HC) patients, selected regardless of the cause of LDL-C accumulation, and in healthy participants (HP), the expression of the adenosine A2A receptor (A2AR), an anti-inflammatory and vasodilatory protein with cholesterol-dependent modulation, and Flotillin-1, protein marker of cholesterol-enriched plasma membrane domains. Blood cardiovascular risk and inflammatory biomarkers were measured. A2AR and Flotillin-1 expression in peripheral blood mononuclear cells (PBMC) was lower in patients compared to HP and negatively correlated to LDL-C blood levels. No other differences were observed between the two groups apart from transferrin and ferritin concentrations. A2AR and Flotillin-1 proteins levels were positively correlated in the whole study population. Incubation of HP PBMCs with LDL-C caused a similar reduction in A2AR and Flotillin-1 expression. We suggest that LDL-C affects A2AR expression by impacting cholesterol-enriched membrane microdomains. Our results provide new insights into the molecular mechanisms underlying cholesterol toxicity, and may have important clinical implication for assessment and treatment of cardiovascular risk in HC.
Keywords: hypercholesterolemia, LDL-C, cholesterol, adenosine receptor, lipid rafts, Flotillin-1, cardiovascular risk
1. Introduction
Systemic cholesterol excess, i.e., hypercholesterolemia (HC), defined by high blood levels of total-cholesterol (TC) and low-density lipoprotein (LDL) cholesterol (LDL-C), has been associated with atherosclerosis and coronary artery disease (CAD) due to promotion of the accumulation of pathological macrophage (foam cells) within arterial walls. However, because cholesterol is a component of all mammalian cell membranes, excess levels might have a wider impact on tissue and organ functionality and multiple mechanisms might explain cholesterol toxicity and the increased cardiovascular risk associated with hypercholesterolemia.
Atherosclerosis is the major cause of cardiovascular disease and stroke [1]. This slowly progressing pathology, characterized by activation of inflammation and endothelial dysfunction, is in large part initiated by LDL and triglycerides (TG)-rich lipoprotein accumulation in the intima of vessels. Lipids are oxidized and internalized by recruited macrophages, which then transform into foam cells, accumulate within the arterial walls, and initiate lesions. Lesion growth can reduce blood flow and may cause angina, particularly during exercise. Lesions may also be unstable, and their rupture can create a local clot that may completely obstruct blood flow and lead to a heart attack or a stroke. High LDL-C blood levels have been associated with an increased risk of lesion formation, while high-density lipoprotein cholesterol (HDL-C) levels are generally considered protective, because they are involved in the recycling and removal of excess cholesterol [2,3]. Hypercholesterolemia may be idiopathic, secondary to another disease or condition, multifactorial or genetic, such as in familial hypercholesterolemia (FH), which is mainly associated with mutations in genes encoding proteins involved in LDL transport (apolipoprotein B, apoB), or internalization [LDL receptor (LDLR) and Proprotein Convertase Subtilisin/Kexin Type 9 (PCKS9)] [4,5]. To date, the majority of hypercholesterolemia studies have focused on the link between LDL-C, foam cells and lesion formation. However, cholesterol is an essential molecule in mammalian metabolism. While it is a precursor for bile acid and steroid hormone biosynthesis, it is principally an indispensable component of all mammalian cells, particularly of the plasma membrane, where it constitutes 25% of total lipids. By modulating membrane physico-chemical properties, it controls membrane permeability, fluidity, and structural and functional organization [6,7,8]. Together with glycosphingolipids, cholesterol forms membrane micro-domains, known as lipid rafts. These microdomains are associated with specific proteins, including Flotillins, and regulate intracellular trafficking and signaling of several plasma membrane receptors [9,10,11]. Almost all mammalian cells can synthesize cholesterol and internalize LDL-C via LDLR. Therefore, perturbation in cholesterol homeostasis and LDL-C levels would be expected to have a greater impact on human metabolism [8].
Adenosine A2A receptor (A2AR) is a G-protein-coupled receptor expressed mainly in immune cells and platelets, as well as in vascular smooth muscle and endothelial cells [12,13,14,15]. It activates intracellular processes through G-proteins in response to adenosine binding. A2AR has a protective immunosuppressive function: it attenuates production of pro-inflammatory cytokines and promotes synthesis of anti-inflammatory molecules. A2AR activation also induces relaxation of vascular smooth muscle, coronary and peripheral artery vasodilation, and regulates cardiac rhythm [16,17,18,19,20]. Alterations in A2AR expression and function have been associated with CAD. A decrease in basal A2A receptor expression has been found in patients with CAD, or subjects with a positive stress test [21,22,23] and a reduced fractional flow reserve (FFR), which has led to the analysis of A2AR expression and function being proposed as a prognostic biomarker of CAD severity [21]. In recent years, a reciprocal link has been described between cholesterol metabolism and A2AR [24,25,26,27,28,29,30,31,32]. A2AR appears to modulate intracellular cholesterol metabolism at several stages: synthesis, efflux, influx, and intracellular transport [28,29,30,31,32,33]. On the other hand, specific cholesterol-binding sites have been identified in the structure of A2AR [24,25,26,27] and A2AR has been proposed associated with lipid rafts [33], suggesting that cholesterol is an important regulator of A2AR function. Therefore, we hypothesize that alterations in A2AR expression and function may be present in hypercholesterolemia.
We have previously shown, in a pilot study of a small cohort of FH patients, where LDL-C excess is genetically induced, that high LDL-C was linked to decreased A2AR expression in PBMCs [34]. In the present study, we analyzed the relationship between elevated blood LDL-C levels, intracellular cholesterol levels and A2AR expression in a larger cohort of healthy participants (HP) and HC patients, chosen regardless the origin of their LDL-C excess and without any hypolipidemic treatment, which are known to have pleiotropic effects independent of their cholesterol-lowering properties [35,36]. To investigate whether alterations in A2AR expression might be explained by alterations in membrane micro-domains, we examined the expression of the lipid raft marker Flotillin-1. Interestingly, we found significant differences between the two groups of individuals which we believe provide new insight into the cellular mechanisms of cardiovascular risk in hypercholesterolemia.
2. Materials and Methods
2.1. Study Population
This protocol was a prospective, comparative, single-center observational and experimental study. Patients were recruited from the Nutrition, Metabolic Diseases and Endocrinology Department of the Hospital “La Conception”, APHM, (Marseille, France) and met the following inclusion criteria: age (20–65 years), high TC (>199.9 mg/dL or >5.17 mM) and LDL-C (>160 mg/dL or >4.14 mM) blood levels, and no hypolipidemic treatment.
Healthy participants were recruited at the Service of Biochemistry, Biogenopôle, Hospital La Timone (Marseille, France) and met the following inclusion criteria: TC (100–200 mg/dL or 2.59–5.17 mM) and LDL-C (59.9–160 mg/dL or 1.55–4.14 mM) blood levels, no hypolipidemic treatment, lack of history of diabetes, hypertension, previously elevated cholesterol, or hypertriglyceridemia. The two groups were matched according to age, gender, and body mass index (BMI). The main difference between the two groups was in blood levels of LDL-C.
All biochemical parameters were analyzed by the Biochemistry Department, Medical Biology Laboratory (LBM), Hospital La Timone, APHM (Marseille, France). This study was conducted in accordance with the Declaration of Helsinki on experiments involving humans, and approved by the institutional Ethics Committee (CPP Sud-Méditerranée II, Marseille, France), 21 June 2021, number ID-RCB: 2021-A01196-35). All participants gave written informed consent.
2.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation
Blood samples were collected by venipuncture from the brachial vein into 8 mL tubes containing sodium citrate/Ficoll (BD Vacutainer CPT, Becton Dickinson, Franklin lakes, NJ, USA). PBMCs were prepared according to the manufacturer’s instructions. Briefly, blood samples were centrifuged for 30 min at 2000 RCF at 20 °C. PBMCs were then collected from the plasma/Ficoll interface and washed twice with phosphate-buffered saline (PBS). After cell counting, aliquots were used for SDS-PAGE and Western blot analysis and the remainder frozen in freezing medium containing 10% DMSO (C6164, Sigma®, Saint-Quentin-Fallavier Cedex, France) at −80 °C.
2.3. PBMCs Treatment with LDL-C Enriched Medium
PBMCs isolated from a healthy participant were incubated with or without a high LDL-C-enriched human serum, ref. 360-10, LeeBiosolutions, Maryland Heights, MO, USA) medium at a final LDL-C concentration of 309 mg/dL (or 8 mM), at 37 °C for 6, 24 and 48 h. Cells were then centrifuged and used for SDS-PAGE and Western blot analysis of A2AR and Flotillin-1 expression.
2.4. SDS-PAGE and Western Blot Analysis
A2AR and Flotillin-1 expression in PBMCs were determined by SDS-PAGE and Western blot analysis as previously described [37]. PBMC pellets were homogenized in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 7.4, 150 mM NaCl, 0.5 mM EDTA). Protein concentrations were measured using a BCA Protein Assay Kit (Novagen®, Gauteng, South Africa) according to the manufacturer’s instructions. For each sample, 10 µg of total proteins were precipitated by addition of nine volumes of 100% cold acetone and then incubated 2 h at 4 °C. After centrifugation for 10 min at 14,000 RCF supernatants were discarded, and pellets dried at room temperature. Total protein pellets were solubilized using Laëmmli buffer containing 5% ß-mercaptoethanol and TBS 1× and then heated 15 min at 65 °C. Samples were then submitted to standard 12% polyacrylamide gel electrophoresis under reducing conditions, before transfer to a polyvinylidene difluoride membrane. The membrane was incubated with Adonis, a home-made antibody (9 µM stock solution, diluted 1/3500 [37]), or human Flotillin-1 antibody (0.55 mg/mL stock solution, diluted 1/10,000, ab133497, Abcam®, Cambridge, UK). Blots were developed using alkaline phosphatase-labeled anti-mouse (A2179, Sigma®) and anti-rabbit secondary antibodies (A3137, Sigma®), diluted at 1/3500, using the colorimetric substrate BCIP/NBT (Sigma®). The upper part of each gel was stained using amido black solution (0.05% amido black in a solution of 10% methanol/10% acetic acid) to quantify the total amount of proteins loaded in each lane. For quantification, the intensity of the band corresponding to each protein (45 kDa for A2AR or 47 kDa for Flotillin-1) or of total proteins was measured by densitometry using Image J software (1.53k, Wayne Rasband and contributors, National Institutes of Health, Stapleton, NY, USA). Results were expressed in arbitrary units (A.U.) as the ratio between the intensity of adenosine A2A receptor or Flotillin-1 bands and the intensity of the stained total protein band. For each subject (patient or healthy donor), the A.U. value is the average of a minimum of n = 3 repeated Western blot experiments.
2.5. Statistical Analysis
Categorical parameters are expressed as counts (%) and continuous parameters as mean ± standard deviation (SD). When comparing patients to HP, the categorical parameters were analyzed by Χ2 or Fisher’s exact tests. Continuous variables were analyzed using t-tests with Welch’s correction when variance differed. Associations between continuous parameters were estimated using Pearson correlation coefficients (r). All tests were two-sided and statistical significance was defined as p ≤ 0.05 *; p ≤ 0.001 **; p ≤ 0.0001 ***. Statistical analyses were performed using IBM SPSS Statistics 27.0 (IBM Inc., New York, NY, USA).
3. Results
3.1. Hypercholesterolemic Patient and Healthy Subject Characteristics
The demographic and clinical characteristics of the participants are shown in Table 1. We recruited 37 HC patients and 31 HP. The two groups were similar in terms of age, gender, and body mass index (BMI): HC patients (70.96% women, 29.03% men; age 46.03 ± 15.43 years; BMI 23.89 ± 3.80 kg/m2) and HP (67.57% women, 32.43% men; age 40.71 ± 13.15 years; BMI 23.00 ± 3.73 kg/m2) (Table 1). As expected, mean TC levels were significantly higher in the HC patient group (8.06 ± 1.40 mM) than in the HP group (4.752 ± 0.642 mM) (p < 0.0001). A similar difference was observed for LDL-C levels: 5.90 ± 1.29 mM for HC patients vs. 2.683 ± 0.564 mM for HP (p < 0.0001). In line with the LDL-C variation, the two groups also had a significant difference in apoB concentration (1.51 ± 0.27 mM HC vs. 0.778 ± 0.144 mM HP, p < 0.0001). No significant difference was observed for HDL-cholesterol (HDL-C, p = 0.807) and TG (p = 0.095), indicating that the difference in TC concentration is associated with the difference in LDL-C concentrations. The two groups showed no significant differences regarding lipoprotéine A (Lp(a)) and homocysteine (Hcy) concentrations (p = 0.509 and p = 0.953, respectively), two factors that have been associated with cardiovascular risk [38]. Additionally, no significant difference was found for biomarkers of systemic inflammatory response, including C-reactive protein (CRP) (p = 0.251), haptoglobin (p = 0.291), orosomucoid (p = 0.361), prealbumin (p = 0.920) and albumin (p = 0.069). Significant differences were observed only in the case of transferrin and ferritin, two modulators of iron metabolism: transferrin levels were lower (2.27 ± 0.32 g/L vs. 2.62 ± 0.49 g/L, p < 0.001) and ferritin blood levels were higher (102.04 ± 110.45 µg/L and 57.81 ± 56.11 µg/L, p < 0.05) in HC patients compared to HP.
Table 1.
Biological characteristics of the healthy participants and patients’ groups.
| Healthy Participants (31) | Patients (37) | p | |
|---|---|---|---|
| Gender | 22 women; 9 men | 25 women; 12 men | 0.762 |
| Age | 40.71 ± 13.15 | 46.03 ± 15.43 | 0.135 |
| BMI (kg/m2) | 23.00 ± 3.73 | 23.89 ± 3.80 | 0.345 |
| TC (mM) | 4.75 ± 0.64 | 8.06 ± 1.40 | <0.0001 *** |
| HDL-C (mM) | 1.62 ± 0.42 | 1.60 ± 0.35 | 0.807 |
| LDL-C (mM) | 2.68 ± 0.56 | 5.90 ± 1.29 | <0.0001 *** |
| TG (mM) | 1.02 ± 0.48 | 1.24 ± 0.55 | 0.095 |
| apoB (mM) | 0.78 ± 0.14 | 1.51 ± 0.27 | <0.0001 *** |
| Lp(a) (g/L) | 0.24 ± 0.22 | 0.29 ± 0.32 | 0.509 |
| CRP (mg/L) | 1.37 ± 2.29 | 2.29 ± 3.87 | 0.251 |
| Hcy (µM) | 11.28 ± 3.72 | 11.34 ± 3.64 | 0.953 |
| Neutrophils (N) (109/L) | 3.87 ± 1.33 | 3.77 ± 1.82 | 0.803 |
| Lymphocytes (L) (109/L) | 2.06 ± 0.36 | 1.86 ± 0.61 | 0.180 |
| NLR (N/L) | 1.96 ± 0.71 | 2.22 ± 1.28 | 0.328 |
| Albumin (g/L) | 42.59 ± 2.61 | 43.97 ± 3.15 | 0.069 |
| Transferrin (g/L) | 2.62 ± 0.49 | 2.27 ± 0.32 | 0.001 ** |
| Iron (µM) | 18.53 ± 9.88 | 15.60 ± 5.00 | 0.168 |
| FCT (µM) | 65.48 ± 12.23 | 56.97 ± 8.09 | 0.002 * |
| TSC (%) | 27.30 ± 13.44 | 28.61 ± 7.70 | 0.656 |
| Ferritin (µg/L) | 57.81 ± 56.11 | 102.04 ± 110.45 | 0.045 * |
| Haptoglobin (g/L) | 1.04 ± 0.35 | 1.16 ± 0.50 | 0.291 |
| Orosomucoid (g/L) | 0.62 ± 0.17 | 0.67 ± 0.21 | 0.361 |
| Prealbumin (g/L) | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.920 |
Data are mean ± SD. Mean dates were compared between patients with hypercholesterolemia (HC) and healthy participants (HP). p ≤ 0.0001 ***, p ≤ 0.001 **, p ≤ 0.05 * are significant. BMI, body mass index, kg/m2; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; TG, triglycerides; apoB, Apoliprotein B; Lp(a), lipoprotein(a); CRP, C-reactive protein; Hcy, homocystein; NLR, neutrophils-lymphocytes ratio; FCT, iron fixation capacity of transferrin; TSC, transferrin saturation coefficient.
3.2. Analysis of A2A Receptor Expression in Hypercholesterolemic Patients and Healthy Participants
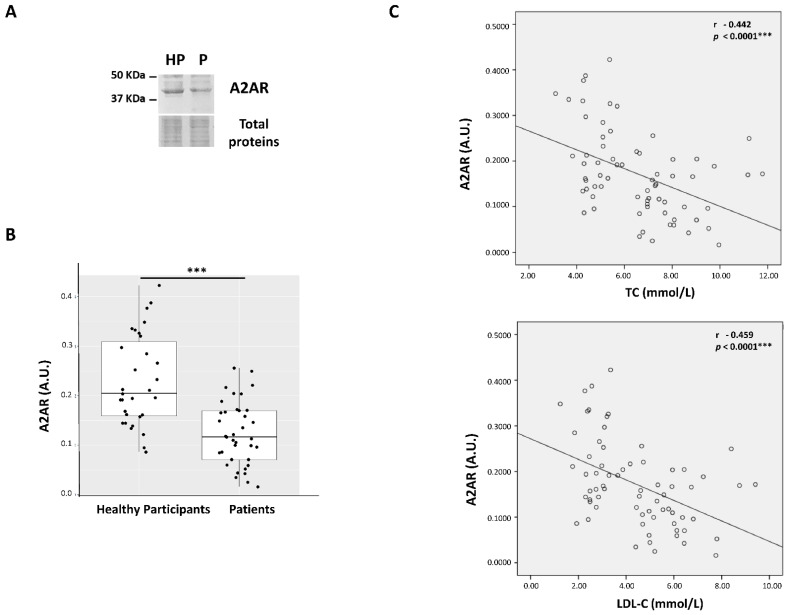
Expression of A2AR in PBMCs of hypercholesterolemia patients and HP was analyzed, quantified, and found to be significantly lower (46% lower) in HC patients (P) compared to HP [0.124 ± 0.064 arbitrary units (A.U.) vs. 0.229 ± 0.092 A.U., p < 0.0001] (Figure 1A,B). Additionally, we observed that A2AR expression was negatively correlated to both TC (r = −0.442, p = 0.001) and LDL-C (r = −0.459, p < 0.0001) blood levels (Figure 1C).
Figure 1.
A2A receptor expression is found to be lower in hypercholesterolemic patients compared to healthy participants and negatively correlates to TC or LDL-C blood levels. Representative images of the A2AR 45 kDa band visualized by Western blot in PBMCs of HP or hypercholesterolemic patients (P) (A). After Image J densitometry analysis, A2AR expression was expressed in arbitrary units (A.U.) as the ratio of the intensities between the A2AR 45k Da band and the stained total protein band. Each individual value represents the mean ± SD of three separate experiments. t-test was used for comparisons between groups (p < 0.0001 ***) (B). A2AR expression levels (in A.U.) of the entire group (hypercholesterolemic patients and HP) were correlated to TC or LDL-C blood levels. Pearson’s correlation coefficient and the coefficient of determination r2 were calculated, p ≤ 0.05 was significant (C).
3.3. Analysis of Flotillin-1 Expression in Hypercholesterolemic Patients and Healthy Participants
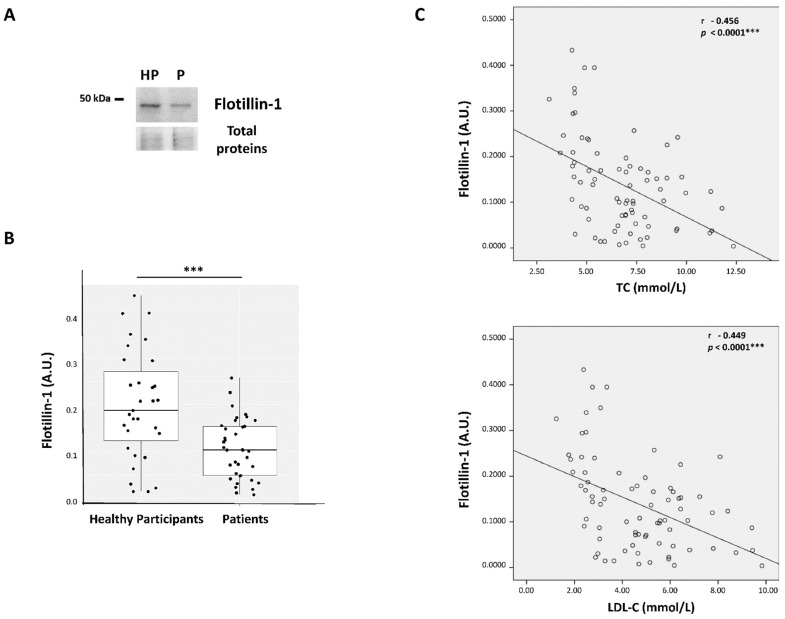
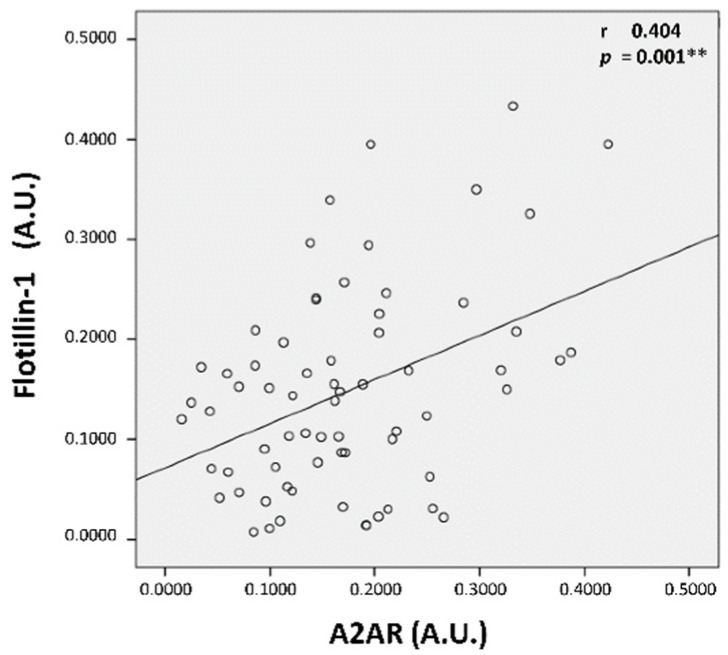
To investigate the possible mechanism of the decreased expression of A2AR, we analyzed, in parallel, the expression of Flotillin-1, a protein associated with lipid raft microdomains in the plasma membrane [39], whose localization is correlated with intracellular cholesterol distribution [40]. We found that Flotillin-1 expression was significantly lower (53% less) in HC patients compared to HP (0.105 ± 0.063 vs. 0.198 ± 0.117 A.U., p < 0.0001) (Figure 2A,B). Moreover, as seen for A2AR, a negative correlation was seen between Flotillin-1 expression and TC (r = −0.456, p < 0.0001) and LDL-C (Pearson r = −0.449, p < 0.0001) (Figure 2C). Interestingly, A2AR expression was also positively correlated with Flotillin-1 expression levels (r = 0.404, p = 0.001) (Figure 3).
Figure 2.
Flotillin-1 expression is found to be lower in hypercholesterolemic patients compared to healthy participants and negatively correlates to TC or LDL-C blood levels. Representative image of the Flotillin-1 47k Da band visualized by Western blot in PBMCs from HP or hypercholesterolemic patients (P) (A). After Image J densitometry analysis, Flotillin-1 expression was expressed in arbitrary units (A.U.) as the ratio of the intensities between the Flotillin-1 47 kDa band and the stained total protein band. Individual A.U. values represent the mean ± SD from three separate experiments. t-tests were used for comparisons between groups (p < 0.0001 ***) (B). Flotillin-1 expression levels (in A.U.) for the whole subject group (hypercholesterolemic patients and HP) were correlated to TC or LDL-C blood levels. Pearson’s correlation coefficient and the coefficient of determination r2 were calculated, p ≤ 0.05 was considered significant (C).
Figure 3.
Positive correlation between A2AR and Flotillin-1 expression in all individuals. A2AR and Flotillin-1 expression levels (in A.U.) were positively correlated for the whole participant group. Pearson’s r coefficient of correlation and the coefficient of determination r2 were used for the correlation study, p ≤ 0.001 considered significant (**).
3.4. Effect of LDL-C Supplementation on A2AR and Flotillin-1 Expression
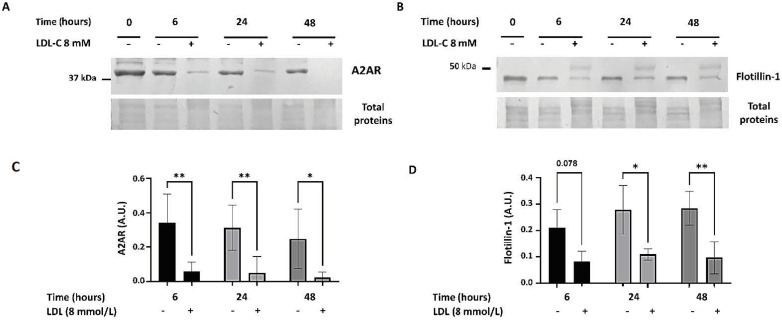
To investigate whether the effect of high LDL-C levels is a direct cellular response, we incubated healthy individual PBMCs with or without highly LDL-C-enriched (8 mM final concentration) medium. As shown in Figure 4, A2AR expression decreased rapidly and significantly over time in the presence of LDL-C compared to control (without LDL-C-enrichment) (Time 6 h: 0.058 ± 0.006 (+) vs. 0.344 ± 0.165 (−) A.U., p = 0.003; T24 h: 0.050 ± 0.094 (+) vs. 0.313 ± 0.132 (−) A.U., p = 0.006; T48 h: 0.023 ± 0.033 (+) vs. 0.249 ± 0.173 (−) A.U., p = 0.020; n = 3) (Figure 4A). Moreover, a decrease in the Flotillin-1 47 kDa band was also observed at these time points (T6 h: 0.082 ± 0.039 (+) vs. 0.210 ± 0.070 (−) A.U., p = 0.078; T24 h: 0.108 ± 0.021 (+) vs. 0.279 ± 0.092 (−) A.U., p = 0.017; T48 h: 0.097 ± 0.061 (+) vs. 0.285 ± 0.064 (−) A.U., p = 0.009).
Figure 4.
A2AR and Flotillin-1 expression decreases in PBMCs after incubation with LDL-C enriched (8 mM) medium. PBMCs (T0) were incubated with (+) or without (−) LDL-C enriched (8 mM final concentration) medium for 6, 24 and 48 h. Representative image of the A2AR 45 kDa band (A) and the Flotillin-1 47 kDa band (B) visualized by Western blot. After Western blotting and Image J densitometry analysis, A2AR and Flotillin-1 expression is shown in arbitrary units (A.U.) as the ratio of the intensities between the A2AR or Flotillin-1 band and the stained total protein band. Data are represented as mean ± SD from five separate experiments (for A2AR) and three experiments (Flotillin-1). A 2-way ANOVA test was performed, p ≤ 0.05 *; p ≤ 0.001 ** (C,D).
4. Discussion
In this study, we describe the specific association between high LDL-C blood levels and decreased adenosine A2A receptor expression in PBMCs of untreated hypercholesterolemia patients, together with the negative correlation between A2AR expression and TC and LDL-C blood levels. Furthermore, we report similar alterations and correlations for Flotillin-1, a protein marker of cholesterol-enriched membrane microdomains. Changes in A2AR as well as Flotillin-1 were found to be directly induced in healthy PBMCs by LDL-C enrichment of cell culture medium. Interestingly, A2AR was also positively correlated with Flotillin-1 expression in PBMCs.
Taken together, these data suggest that alterations in A2AR expression are associated and correlated with high blood levels of LDL-C and hypercholesterolemia, regardless of the cause of LDL-C excess. The decrease in Flotillin-1 expression in HC patients and its correlation to cholesterol levels suggest a perturbation of intracellular cholesterol levels and lipid raft organization in HC patients and may explain the tight correlation of A2AR expression with cholesterol levels in hypercholesterolemia.
In recent years, a reciprocal relationship has been described between cholesterol metabolism and A2A adenosine receptors. Cholesterol appears to be an important regulator of A2AR function since several X-ray crystallographic and simulation models have suggested the presence of up to three cholesterol interaction sites in the molecular structure of A2AR [10,41,42]. Cholesterol depletion in rat embryonic cortical neurons [43] and in erythrocytes [44] by cyclodextrin abolishes A2AR activation of cyclic adenosine 3′,5′-monophosphate (cAMP) synthesis by the agonist CGS21680. Gs proteins and adenylyl cyclase are also present in cholesterol-enriched microdomains (lipid rafts) in the plasma membrane in various cell types [6,45,46] and it has been proposed that A2AR associates with these domains [47]. Additionally, A2ARs appears to participate in the regulation of cholesterol metabolism at several stages: synthesis, efflux, influx, and intracellular transport [28,29,30,31,32]. Patients with dyslipidemia showed a reduction in myocardial blood flow and reduced coronary artery dilation upon intravenous administration of adenosine and, because A2AR is implicated in coronary artery dilation, it has been suggested that its expression or function may be impacted in dyslipidemia [48]. In the present study, our results confirm and extend our previous observations on Familial hypercholesterolemia patients [34] that a clear relationship at the systemic level exists between cholesterol metabolism and A2A adenosine receptor expression. Indeed, hypercholesterolemic patients, selected independently of the origin of LDL-C accumulation, show decreased expression of A2AR in PBMCs. No other difference was noted between the two groups in terms of age, gender, blood lipid markers (HDL-C or triglycerides), obesity (BMI), history of diabetes or hypertension, in terms of the principal risk factors for hypercholesterolemia. Interestingly, the higher LDL-C blood levels were, the lower A2AR expression was, and this downregulation might be directly induced by LDL-C, as suggested by our in vitro experiments on healthy PBMCs. Our results thus suggest that, regardless of the cause of LDL-C accumulation (primary or secondary), this excess is sufficient to lower A2AR expression.
To date, studies on hypercholesterolemia and atherosclerosis have mostly focused on mechanisms of LDL-C oxidation and internalization by recruited macrophages, which then transform into foam cells, accumulate within arterial walls, and initiate lesion formation [1]. Nonetheless, because cholesterol is an indispensable ubiquitous lipid, LDL-R is expressed in almost all mammalian cells and LDL-C internalization is tightly regulated. Thus, it could be expected that in a context of perturbations in cholesterol metabolism, i.e., LDL-C accumulation, every cell could potentially recognize and respond to this alteration. However, little is currently known regarding intracellular cholesterol levels and cholesterol distribution being modified in PBMCs or other cells exposed to high LDL-C concentrations and the mechanism underlying such modifications. Pfisterer et al. (2022) recently quantified LDL uptake and storage in cytoplasmic droplets in PBMCs of hypercholesterolemic patients [49]. They reported that increased circulating LDL is associated with low intracellular LDL-C uptake and low lipid storage in lipid droplets, which suggests that intracellular membrane cholesterol levels are also affected in PBMCs. To investigate whether, and how, in the presence of LDL-C excess, intracellular membrane cholesterol levels are altered and impact A2AR expression in our study, we evaluated the expression of Flotillin-1, a marker of cholesterol-enriched intracellular membrane domains. Flotillin-1 is essential for Niemann-Pick C1-like 1 (NPC1L1)-mediated cellular cholesterol uptake and, together with NPC1L1, forms cholesterol-enriched membrane microdomains in the plasma membrane [40]. Cholesterol recruitment into low-density membrane fractions is substantially reduced in cells with reduced levels of Flotillin-1. To our knowledge, our study is the first to report that Flotillin-1 expression is decreased in PBMCs from hypercholesterolemic patients, and this decrease is induced in healthy PBMCs after LDL-C supplementation. This downregulation might reflect an alteration in lipid rafts composition and/or function at the plasma membrane, which may destabilize A2AR causing its degradation. The significant positive correlation observed between Flotilin-1 and A2AR protein expression supports the hypothesis of A2AR localization to Flotillin 1-positive and cholesterol enriched membrane microdomains. Nonetheless, future experiments are needed to elucidate whether and how association to lipid rafts might modulate A2AR localization, stability, and function and the impact of LDL-C excess on such modulation.
The identification of altered Flotillin-1 and A2AR expression in hypercholesterolemia provides new insights into the mechanisms of LDL-C excess toxicity. Aside from the formation and accumulation of foam cells in the arterial wall, multiple additional mechanisms might explain the cardiovascular risk associated with high blood LDL-C levels:
-
(1)
A2AR is activated in response to hypoxia and ischemia and has a protective function, by attenuating the production of pro-inflammatory cytokines such as interleukins IL-2 and IL-4, TNF-α and INF-γ, and by promoting the synthesis of anti-inflammatory molecules such as interleukin IL-10 [50,51,52,53,54]. Analysis of A2AR expression and function in PBMCs are also similar to expression in the myocardium [55], coronary arteries, aorta [22] and iliac arteries [23], these tissue sites being the preferred location for cholesterol deposits. A2AR activation induces relaxation of vascular smooth muscle cells, resulting in coronary and peripheral vasodilation [16,20,56,57,58]. Therefore, decreased expression of A2AR in PBMCs could potentially decrease adenosine-induced vasodilation in response to ischemia and therefore prevent the compensatory mechanisms that are activated in response to obstruction of blood flow due to lesion formation.
-
(2)
Because Flotillin-1 is a ubiquitous component of cholesterol-enriched membrane domains [59,60] (REF, alteration in its expression might indicate a destabilization of these membrane domains, which might have a larger impact on the localization and function of other plasma membrane-associated proteins in PBMCs, as well as in other cell types exposed to high LDL levels). Lipid rafts are already known as crucial modulators of receptor-mediated signaling processes [61,62]. The results of this report highlight the relevance to broadly investigate the effect of LDL-C excess on lipid rafts organization and function and to screen on all signaling pathways that might be modified. This knowledge might contribute to understanding the molecular mechanisms underlining LDL-C toxicity and to find novel targets and therapeutic approaches against hypercholesterolemia, as for patients resistant to current hypolipidemic treatments.
Potential correlations of decreased expression of A2AR and Flotillin-1 with altered expression of common systemic inflammatory biomarkers or of other factors associated with cardiovascular risk, such as homocysteine [63,64] and lipoprotein(a) [38], were also looked for. We observed no difference between the HC patient and HP groups with the exception of two important biomarkers of iron metabolism: transferrin, a protein regulating iron transport, and ferritin the protein involved in iron storage. Nonetheless, serum transferrin and ferritin are acute phase factors. Lower transferrin (<2 g/L or 200 mg/dL) [65] and higher ferritin (>300 μg/L serum ferritin in men and >200 μg/L in women [66], blood concentrations are recognized as biomarkers in inflammation and might even contribute to atherosclerosis [67] and CVD [68]. Abnormal iron metabolism leads to ferroptosis, a regulated cell death that is characterized by the accumulation of lipid peroxidation products [69,70], which has been associated with cardiac inflammation and dysfunction [71,72,73]. While still in the normal range, we found significantly lower transferrin levels and higher ferritin levels in HC patients compared to healthy individuals (Table 1), which might indicate the beginning of an inflammatory processus. Interestingly, the iron exporter, ferroportin, in macrophages has been mostly detected in detergent-resistant membranes containing Flotillin-1 [74], suggesting a possible link. Further investigation will be required to understand whether in our cohort inflammation and dysfunction are in progress, i.e., quantification of pro-inflammatory and anti-inflammatory cytokines and of endothelial and cardiac dysfunction biomarkers [75]. Moreover, a long-term follow up might help to understand whether these variations over time will evolve and be associated with cardiovascular complications in our cohort. In such case, the identification of A2AR and Flotillin-1 downregulation and transferrin and ferritin alterations might represent a novel panel of very early predictive biomarkers of the cardiovascular risk in hypercholesterolemia. In addition, our study also presents new perspectives on the use of A2AR modulators to counteract the cardiovascular risk of LDL-C excess. In fact, caffeine, which induces A2AR overexpression [76], has been shown to protect against mortality in cardiovascular disease [77] and counteract the cardiovascular deleterious effects associated with HC [78].
5. Conclusions
In this study, we provide insights on novel molecular mechanisms that could explain LDL-C cell toxicity: a decreased expression of adenosine A2A receptor is likely associated with decreased anti-inflammatory responses and vessels vasodilation, and thus may contribute to increasing cardiovascular risk associated with hypercholesterolemia. Moreover, the decrease in the lipid raft-associated protein Flotillin-1 suggests a broader impact of LDL-C excess on the organization and functionality of cholesterol-enriched membrane domains, which may perturb other signaling pathways. A better understanding of these mechanisms and the discovery of novel key factors will contribute to improve therapeutic options for the treatment of hypercholesterolemia, particularly in case of resistance or development of pathological complications to the current hypolipidemic treatments. This study also highlights the possible employment of novel diagnostic biomarkers of inflammation and progression towards atherosclerosis and cardiovascular pathology in hypercholesterolemia. The evaluation of A2AR and Flotillin-1 protein expression, together with the quantification of transferrin and ferritin blood levels, in future might be integrated in the tools for cardiovascular risk assessment in HC patients.
Acknowledgments
We would like to thank B. Desnues (Aix-Marseille Université).
Abbreviations
| TC | total cholesterol |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol |
| HDL-C | high-density lipoprotein-cholesterol |
| TG | triglycerides |
| HC | hypercholesterolemia |
| HP | healthy participants |
| FH | familial hypercholesterolemia |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| PBMCs | peripheral blood mononuclear cells |
| A2AR | adenosine A2A receptor |
| CAD | coronary artery disease |
| A.U. | arbitrary units |
Author Contributions
Conceptualization, G.M. (Giovanna Mottola) and R.V.; methodology, G.M. (Giovanna Mottola) and R.V.; software, J.M.; validation, G.M. (Giovanna Mottola) and R.V.; formal analysis, M.M. (Marie Maraninchi) and J.M.; investigation, M.-C.C., G.M. (Giorgia Musto), H.C., J.D.-R. and M.M. (Marion Marlinge); resources, S.B., F.M. and R.V.; data curation, G.M. (Giovanna Mottola); writing—original draft preparation, G.M. (Giovanna Mottola); writing—review and editing, R.V., R.G., J.F. and N.L.; visualization, M.-C.C., M.M. (Marie Maraninchi), J.M. and G.M. (Giovanna Mottola); supervision, G.M. (Giovanna Mottola); project administration, G.M. (Giovanna Mottola) and R.V.; funding acquisition, R.G., N.L., G.M. (Giovanna Mottola) and R.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional Ethics Committee (CPP Sud-Méditerranée II, Marseille, France), 21 June 2021, number ID-RCB: 2021-A01196-35.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Aix-Marseille Université (AMU), Institut national de la santé et de la recherche médicale (INSERM) and Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Björkegren J.L.M., Lusis A.J. Atherosclerosis: Recent developments. Cell. 2022;185:1630–1645. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bersot T.P. Drug Therapy for Hypercholesterolemia and Dyslipidemia. In: Brunton L.L., Chabner B.A., Knollmann B.C., editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill Education; New York, NY, USA: 2015. [Google Scholar]
- 3.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Brandts J., Ray K.K. Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 2021;78:1831–1843. doi: 10.1016/j.jacc.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Di Taranto M.D., Fortunato G. Genetic Heterogeneity of Familial Hypercholesterolemia: Repercussions for Molecular Diagnosis. Int. J. Mol. Sci. 2023;24:3224. doi: 10.3390/ijms24043224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J., Yang H., Song B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 7.Grouleff J., Irudayam S.J., Skeby K.K., Schiøtt B. The influence of cholesterol on membrane protein structure, function, and dynamics studied by molecular dynamics simulations. Biochim. Biophys. Acta. 2015;1848:1783–1795. doi: 10.1016/j.bbamem.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Song Y., Liu J., Zhao K., Gao L., Zhao J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33:1911–1925. doi: 10.1016/j.cmet.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 10.Meister M., Tikkanen R. Endocytic Trafficking of Membrane-Bound Cargo: A Flotillin Point of View. Membranes. 2014;4:356–371. doi: 10.3390/membranes4030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouweneel A.B., Thomas M.J., Sorci-Thomas M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020;61:676–686. doi: 10.1194/jlr.TR119000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borea P.A., Gessi S., Merighi S., Vincenzi F., Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 13.Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 14.Fredholm B.B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 15.Palmer T.M., Stiles G.L. Structure-function analysis of inhibitory adenosine receptor regulation. Neuropharmacology. 1997;36:1141–1147. doi: 10.1016/S0028-3908(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 16.Shryock J.C., Belardinelli L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am. J. Cardiol. 1997;79:2–10. doi: 10.1016/S0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S., Fatteh N., El-Sherbiny N.M., Naime M., Ibrahim A.S., El-Sherbini A.M., El-Shafey S.A., Khan S., Fulzele S., Gonzales J., et al. Potential role of A2A adenosine receptor in traumatic optic neuropathy. J. Neuroimmunol. 2013;264:54–64. doi: 10.1016/j.jneuroim.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Vinapamula K.S., Pemmaraju S.V., Bhattaram S.K., Bitla A.R., Manohar S.M. Serum Adenosine Deaminase as Inflammatory Marker in Rheumatoid Arthritis. J. Clin. Diagn. Res. 2015;9:BC08–BC10. doi: 10.7860/JCDR/2015/14296.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutryb-Zajac B., Mateuszuk L., Zukowska P., Jasztal A., Zabielska M.A., Toczek M., Jablonska P., Zakrzewska A., Sitek B., Rogowski J., et al. Increased activity of vascular adenosine deaminase in atherosclerosis and therapeutic potential of its inhibition. Cardiovasc. Res. 2016;112:590–605. doi: 10.1093/cvr/cvw203. [DOI] [PubMed] [Google Scholar]
- 20.Belardinelli L., Shryock J.C., Snowdy S., Zhang Y., Monopoli A., Lozza G., Ongini E., Olsson R.A., Dennis D.M. The A2A adenosine receptor mediates coronary vasodilation. J. Pharmacol. Exp. Ther. 1998;284:1066–1073. [PubMed] [Google Scholar]
- 21.Sobey C.G. Potassium channel function in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2001;21:28–38. doi: 10.1161/01.ATV.21.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Gariboldi V., Vairo D., Guieu R., Marlingue M., Ravis E., Lagier D., Mari A., Thery E., Collart F., Gaudry M. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int. J. Cardiol. 2017;230:427–431. doi: 10.1016/j.ijcard.2016.12.089. [DOI] [PubMed] [Google Scholar]
- 23.Gaudry M., Marlinge M., Deharo P., Vairo D., Bottone S., Mottola G., Kipson N., Criado C., Mace P., Chefrour M., et al. Pharmacological profile of adenosine A2A receptors in patients with lower extremity peripheral artery disease and associated coronary artery disease: A pilot study. Int. J. Cardiol. 2019;285:121–127. doi: 10.1016/j.ijcard.2019.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.Y., Lyman E. Predictions for cholesterol interaction sites on the A2A adenosine receptor. J. Am. Chem. Soc. 2012;134:16512–16515. doi: 10.1021/ja307532d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGraw C., Yang L., Levental I., Lyman E., Robinson A.S. Membrane cholesterol depletion reduces downstream signaling activity of the adenosine A2A receptor. Biochim. Biophys. Acta Biomembr. 2019;1861:760–767. doi: 10.1016/j.bbamem.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovera S., Cuzzolin A., Kelm S., De Fabritiis G., Sands Z.A. Reconstruction of apo A2A receptor activation pathways reveal ligand-competent intermediates and state-dependent cholesterol hotspots. Sci. Rep. 2019;9:14199. doi: 10.1038/s41598-019-50752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guixà-González R., Albasanz J.L., Rodriguez-Espigares I., Pastor M., Sanz F., Martí-Solano M., Manna M., Martinez-Seara H., Hildebrand P.W., Martín M., et al. Membrane cholesterol access into a G-protein-coupled receptor. Nat. Commun. 2017;8:14505. doi: 10.1038/ncomms14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss A.B., Rahman M.M., Chan E.S., Montesinos M.C., Awadallah N.W., Cronstein B.N. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J. Leukoc. Biol. 2004;76:727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 29.Voloshyna I., Carsons S., Littlefield M.J., Rieger J.M., Figler R., Reiss A.B. Adenosine A2A receptor activation supports an atheroprotective cholesterol balance in human macrophages and endothelial cells. Biochim. Biophys. Acta. 2013;1831:407–416. doi: 10.1016/j.bbalip.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 30.De Nuccio C., Bernardo A., Ferrante A., Pepponi R., Martire A., Falchi M., Visentin S., Popoli P., Minghetti L. Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci. Rep. 2019;9:9782. doi: 10.1038/s41598-019-46268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visentin S., De Nuccio C., Bernardo A., Pepponi R., Ferrante A., Minghetti L., Popoli P. The Stimulation of Adenosine A 2A Receptors Ameliorates the Pathological Phenotype of Fibroblasts from Niemann-Pick Type C Patients. J. Neurosci. 2013;33:15388–15393. doi: 10.1523/JNEUROSCI.0558-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrante A., De Nuccio C., Pepponi R., Visentin S., Martire A., Bernardo A., Minghetti L., Popoli P. Stimulation of adenosine A2A receptors reduces intracellular cholesterol accumulation and rescues mitochondrial abnormalities in human neural cell models of Niemann-Pick C1. Neuropharmacology. 2016;103:155–162. doi: 10.1016/j.neuropharm.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Lasley R.D. Adenosine receptors and membrane microdomains. Biochim. Biophys. Acta. 2011;1808:1284–1289. doi: 10.1016/j.bbamem.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vairo D., Giacobbe C., Guiol C., Chaptal M.C., Di Taranto M.D., Bruzzese L., Ruf J., Guieu R., Fortunato G., Fenouillet E., et al. Correlation between low adenosine A2A receptor expression and hypercholesterolemia: A new component of the cardiovascular risk? Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2021;1866:158850. doi: 10.1016/j.bbalip.2020.158850. [DOI] [PubMed] [Google Scholar]
- 35.Jamialahmadi T., Abbasifard M., Reiner Ž., Rizzo M., Eid A.H., Sahebkar A. The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:5251. doi: 10.3390/jcm11175251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahedian-Azimi A., Beni F.H., Fras Z., Banach M., Mohammadi S.M., Jamialahmadi T., Sahebkar A. Effects of statins on the incidence and outcomes of acute kidney injury in critically ill patients: A systematic review and meta-analysis. Arch. Med. Sci. 2023;19:952–964. doi: 10.5114/aoms/159992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.By Y., Durand-Gorde J.M., Condo J., Lejeune P.J., Mallet B., Carayon P., Guieu R., Ruf J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009;46:400–405. doi: 10.1016/j.molimm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Tomic Naglic D., Manojlovic M., Pejakovic S., Stepanovic K., Prodanovic Simeunovic J. Lipoprotein(a): Role in atherosclerosis and new treatment options. Biomol. Biomed. 2023;23:575–583. doi: 10.17305/bb.2023.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J., Deyoung S.M., Zhang M., Dold L.H., Saltiel A.R. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:16125–16134. doi: 10.1074/jbc.M500940200. [DOI] [PubMed] [Google Scholar]
- 40.Ge L., Qi W., Wang L.J., Miao H.H., Qu Y.X., Li B.L., Song B.L. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA. 2011;108:551–556. doi: 10.1073/pnas.1014434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwan B.C., Kronenberg F., Beddhu S., Cheung A.K. Lipoprotein Metabolism and Lipid Management in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2007;18:1246–1261. doi: 10.1681/ASN.2006091006. [DOI] [PubMed] [Google Scholar]
- 42.Hussain M.M., Strickland D.K., Bakillah A. The mammalian low-density lipoprotein receptor family. Annu. Rev. Nutr. 1999;19:141–172. doi: 10.1146/annurev.nutr.19.1.141. [DOI] [PubMed] [Google Scholar]
- 43.Charalambous C., Gsandtner I., Keuerleber S., Milan-Lobo L., Kudlacek O., Freissmuth M., Zezula J. Restricted Collision Coupling of the A2A Receptor Revisited. J. Biol. Chem. 2008;283:9276–9288. doi: 10.1074/jbc.M706275200. [DOI] [PubMed] [Google Scholar]
- 44.O’Malley M.A., Helgeson M.E., Wagner N.J., Robinson A.S. The Morphology and Composition of Cholesterol-Rich Micellar Nanostructures Determine Transmembrane Protein (GPCR) Activity. Biophys. J. 2011;100:L11–L13. doi: 10.1016/j.bpj.2010.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlienger J.L. L’édifiante histoire du cholestérol: De la pierre de fiel au récepteur aux LDL. Médecine Des Mal. Métaboliques. 2012;6:97–103. doi: 10.1016/S1957-2557(12)70367-X. [DOI] [Google Scholar]
- 46.Benito-Vicente A., Uribe K.B., Jebari S., Galicia-Garcia U., Ostolaza H., Martin C. Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease. Int. J. Mol. Sci. 2018;19:3426. doi: 10.3390/ijms19113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mojsilovic-Petrovic J., Jeong G.B., Crocker A., Arneja A., David S., Russell D.S., Kalb R.G. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J. Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexanderson E., García-Rojas L., Jiménez M., Jácome R., Calleja R., Martínez A., Ochoa J.M., Meave A., Alexanderson G. Effect of ezetimibe–simvastatine over endothelial dysfunction in dyslipidemic patients: Assessment by 13N-ammonia positron emission tomography. J. Nucl. Cardiol. 2010;17:1015–1022. doi: 10.1007/s12350-010-9273-8. [DOI] [PubMed] [Google Scholar]
- 49.Pfisterer S.G., Brock I., Kanerva K., Hlushchenko I., Paavolainen L., Ripatti P., Islam M.M., Kyttälä A., Di Taranto M.D., Scotto di Frega A., et al. Multiparametric platform for profiling lipid trafficking in human leukocytes. Cell Rep. Methods. 2022;2:100166. doi: 10.1016/j.crmeth.2022.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sitkovsky M.V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C., Ohta A., Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 51.Naganuma M., Wiznerowicz E.B., Lappas C.M., Linden J., Worthington M.T., Ernst P.B. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J. Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 52.Lappas C.M., Rieger J.M., Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 53.Csóka B., Himer L., Selmeczy Z., Vizi E.S., Pacher P., Ledent C., Deitch E.A., Spolarics Z., Németh Z.H., Haskó G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romio M., Reinbeck B., Bongardt S., Hüls S., Burghoff S., Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am. J. Physiol. Cell Physiol. 2011;301:C530–C539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 55.Varani K., Laghi-Pasini F., Camurri A., Capecchi P.L., Maccherini M., Diciolla F., Ceccatelli L., Lazzerini P.E., Ulouglu C., Cattabeni F., et al. Changes of peripheral A2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2003;17:280–282. doi: 10.1096/fj.02-0543fje. [DOI] [PubMed] [Google Scholar]
- 56.Conti A., Monopoli A., Gamba M., Borea P.A., Ongini E. Effects of selective A1 and A2 adenosine receptor agonists on cardiovascular tissues. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993;348:108–112. doi: 10.1007/BF00168545. [DOI] [PubMed] [Google Scholar]
- 57.Iwamoto T., Umemura S., Toya Y., Uchibori T., Kogi K., Takagi N., Ishii M. Identification of Adenosine A2 Receptor-cAMP System in Human Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 1994;199:905–910. doi: 10.1006/bbrc.1994.1314. [DOI] [PubMed] [Google Scholar]
- 58.Monahan T.S., Sawmiller D.R., Fenton R.A., Dobson J.G., Jr. Adenosine A2a-receptor activation increases contractility in isolated perfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1472–H1481. doi: 10.1152/ajpheart.2000.279.4.H1472. [DOI] [PubMed] [Google Scholar]
- 59.Kwiatkowska K., Matveichuk O.V., Fronk J., Ciesielska A. Flotillins: At the Intersection of Protein S-Palmitoylation and Lipid-Mediated Signaling. Int. J. Mol. Sci. 2020;21:2283. doi: 10.3390/ijms21072283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhan Z., Ye M., Jin X. The roles of FLOT1 in human diseases (Review) Mol. Med. Rep. 2023;28:212. doi: 10.3892/mmr.2023.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capozzi A., Manganelli V., Riitano G., Caissutti D., Longo A., Garofalo T., Sorice M., Misasi R. Advances in the Pathophysiology of Thrombosis in Antiphospholipid Syndrome: Molecular Mechanisms and Signaling through Lipid Rafts. J. Clin. Med. 2023;12:891. doi: 10.3390/jcm12030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos A.L., Preta G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018;75:1909–1927. doi: 10.1007/s00018-018-2765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guieu R., Ruf J., Mottola G. Hyperhomocysteinemia and cardiovascular diseases. Ann. Biol. Clin. 2022;80:7–14. doi: 10.1684/abc.2021.1694. [DOI] [PubMed] [Google Scholar]
- 64.Paganelli F., Mottola G., Fromonot J., Marlinge M., Deharo P., Guieu R., Ruf J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021;22:1690. doi: 10.3390/ijms22041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Worwood M., May A.M., Bain B.J. Dacie and Lewis Practical Haematology. 12th ed. Elsevier Health Sciences; Amsterdam, The Netherlands: 2017. Iron Deficiency Anaemia and Iron Overload; pp. 165–186. [Google Scholar]
- 66.Valenti L., Corradini E., Adams L.A., Aigner E., Alqahtani S., Arrese M., Bardou-Jacquet E., Bugianesi E., Fernandez-Real J.M., Girelli D., et al. Consensus Statement on the definition and classification of metabolic hyperferritinaemia. Nat. Rev. Endocrinol. 2023;19:299–310. doi: 10.1038/s41574-023-00807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naito Y., Tsujino T., Masuyama T., Ishihara M.J. Crosstalk between Iron and Arteriosclerosis. J. Atheroscler. Thromb. 2022;29:308–314. doi: 10.5551/jat.RV17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed M.S., Jadhav A.B., Hassan A., Meng Q.H. Acute phase reactants as novel predictors of cardiovascular disease. ISRN Inflamm. 2012;2012:953461. doi: 10.5402/2012/953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han C., Liu Y., Dai R., Ismail N., Su W., Li B. Ferroptosis and Its Potential Role in Human Diseases. Front. Pharmacol. 2020;11:239. doi: 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oh B.M., Lee S.J., Park G.L., Hwang Y.S., Lim J., Park E.S., Lee K.H., Kim B.Y., Kwon Y.T., Cho H.J., et al. Erastin Inhibits Septic Shock and Inflammatory Gene Expression via Suppression of the NF-κB Pathway. J. Clin. Med. 2019;8:2210. doi: 10.3390/jcm8122210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C., Yuan W., Hu A., Lin J., Xia Z., Yang C.F., Li Y., Zhang Z. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol. Med. Rep. 2020;22:175–184. doi: 10.3892/mmr.2020.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Haehling S., Ebner N., Evertz R., Ponikowski P., Anker S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019;7:36–46. doi: 10.1016/j.jchf.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Auriac A., Willemetz A., Canonne-Hergaux F. Lipid raft-dependent endocytosis: A new route for hepcidin-mediated regulation of ferroportin in macrophages. Haematologica. 2010;95:1269–1277. doi: 10.3324/haematol.2009.019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chia P.Y., Teo A., Yeo T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020;7:542567. doi: 10.3389/fmed.2020.542567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varani K., Portaluppi F., Gessi S., Merighi S., Ongini E., Belardinelli L., Borea P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: Functional and biochemical aspects. Circulation. 2000;102:285–289. doi: 10.1161/01.CIR.102.3.285. [DOI] [PubMed] [Google Scholar]
- 77.Freedman N.D., Park Y., Abnet C.C., Hollenbeck A.R., Sinha R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendoza M.F., Sulague R.M., Posas-Mendoza T., Lavie C.J. Impact of Coffee Consumption on Cardiovascular Health. Ochsner J. 2023;23:152–158. doi: 10.31486/toj.22.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.