Abstract
Theiler’s virus, a murine picornavirus, infects the central nervous systems of C57BL/6 mice and is cleared after approximately 10 days by a process which requires CD8+ cytotoxic T cells. We used perforin-deficient C57BL/6 mice to test the role of this protein in viral clearance. Perforin-deficient mice died from viral encephalomyelitis between days 12 and 18 postinoculation. They had high levels of viral RNA in their central nervous systems until the time of death. In contrast, viral RNA had disappeared by day 11 postinoculation in wild-type C57BL/6 mice. Cytotoxic T cells can kill infected cells by two main mechanisms: the secretion of the pore-forming protein perforin or the interaction of the Fas ligand with the apoptosis-inducing Fas molecule on the target cell. Our results demonstrate that clearance of Theiler’s virus from the central nervous system in C57BL/6 mice is perforin dependent.
Theiler’s virus is a murine picornavirus that may persist in the central nervous system (CNS) and cause a demyelinating disease studied as a model for multiple sclerosis. Intracerebral inoculation with Theiler’s virus is followed by a biphasic neurological disease (24). The first phase is an acute encephalitis that takes place during the first 2 weeks of infection. At this stage the virus is found in the gray matter of the brain, mainly in neurons. Some strains of mice, such as the C57BL/6 strain, are able to clear the virus at this point, whereas other strains remain persistently infected for their lifetimes. In this case, the virus persists in the white matter of the spinal cord, mainly in macrophages, but also in astrocytes and oligodendrocytes (1, 25, 30, 33). Persistence is associated with perivascular infiltration of mononuclear cells, diffuse parenchymal inflammation, and myelin destruction with conservation of axons (7, 8).
The D region of the major histocompatibility complex (MHC) has a major effect in resistance to persistent infection (4), as shown by the facts that susceptible FVB mice become resistant when they are transgenic for the H-2Db gene (2) and that resistant H-2b mice become susceptible when the H-2Db gene is inactivated by homologous recombination (2a). A class-I-restricted, virus-specific cytotoxic response mediated by CD8+ T cells has been documented in infected mice (10, 23, 31). This response is important for resistance, since H-2b mice deficient in MHC class-I molecules are unable to clear the virus (11, 32). Furthermore, the virus-specific cytotoxic T-lymphocyte (CTL) response of resistant C57BL/6 mice, which is H-2Db restricted and directed at an immunodominant epitope, is particularly fast and intense (9, 10).
Perforin is a glycoprotein whose expression is mainly confined to CD8+ T cells and NK cells (13). Upon cell-cell contact, perforin is released onto the target cell, causing permeabilization of the membrane, which leads to the death of the cell. The use of mice in which the perforin gene has been inactivated by homologous recombination (perforin-deficient mice) has demonstrated a crucial role of perforin in CTL- and NK-mediated cytolysis (18, 21, 26, 35). For example, perforin-dependent cytotoxicity was shown to be crucial for the control of acute infection by lymphocytic choriomeningitis virus (LCMV) (18). On the other hand, protection against vaccinia virus, vesicular stomatitis virus (VSV), and Semliki Forest virus (SFV) was found to be perforin independent (19). Since LCMV is a noncytopathic virus whereas the other three are cytopathic viruses, it has been suggested that noncytopathic viruses might be controlled by specific lytic mechanisms which depend on cell contact, whereas cytopathic viruses might be efficiently controlled by soluble cytokines or neutralizing antibodies which prevent the infection of neighboring cells. In this study, we used perforin-deficient C57BL/6 mice to examine the role of perforin-dependent cytotoxicity during Theiler’s virus infection.
C57BL/6 mice were obtained from Janvier, Le Genest-St. Isle, France. Perforin-deficient mice with an inbred C57BL/6 background were obtained from the Institut für Labortierkunde, University of Zurich, Zurich, Switzerland (18). Four-week-old female mice were inoculated intracranially with 104 PFU of plaque-purified Theiler’s virus (DA strain). To measure the amount of viral RNA in the CNS, mice were perfused with phosphate-buffered saline (PBS) and the brain or spinal cord was immediately removed. Total RNA was extracted by the procedure of Chomczynski and Sacchi (6) and was quantified by spectrophotometry. For each mouse, a series of fivefold dilutions of total RNA, starting from 10 μg, were dotted on Hybond C-extra filters (Amersham) according to the manufacturer’s recommendations. The dot blots were hybridized overnight in 0.5 M sodium phosphate (pH 7.4)–7% sodium dodecyl sulfate (SDS) at 65°C with 106 cpm of a virus-specific, random-primed [32P]cDNA probe (specific activity, between 107 and 108 cpm/μg). The filters were washed three times for 15 min each time at 65°C in 40 mM sodium phosphate (pH 7.4)–1% SDS and exposed for 5 h in a phosphorimager or overnight at −80°C against an X-ray film. For each sample, the highest dilution which gave a positive hybridization signal after a standard exposure time was used as a measure of viral RNA content. Mice were also studied for CNS histopathology as described elsewhere (3). Mice were perfused with PBS, followed by 4% paraformaldehyde in PBS. The brain and spinal cord were dissected, cut into tissue blocks, and embedded in paraffin. Serial coronal sections of brain and longitudinal sections of the entire spinal cord were prepared. Viral antigens were detected by immunocytochemistry using a primary rabbit anti-capsid polyclonal serum, a secondary biotinylated goat anti-rabbit immunoglobulin, and the ABC peroxidase detection system (Vector). The sections were counterstained with hematoxylin.
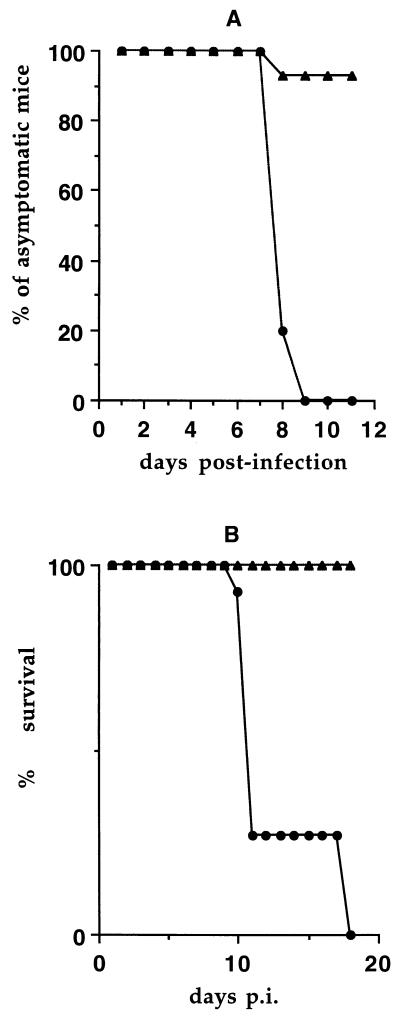
Wild-type C57BL/6 mice survived the infection, usually without showing neurological symptoms (Fig. 1), and cleared it after approximately 10 days (see Fig. 2). To evaluate the role of perforin-dependent immune responses in this resistance, 11 perforin-deficient mice with an inbred C57BL/6 background were inoculated and observed daily for clinical symptoms and mortality. As shown in Fig. 1, all these mice showed neurological signs of acute encephalomyelitis and paralysis between days 8 and 10 postinfection (p.i.). Eight of 11 mice (73%) died by day 11 p.i. Three survived until day 18 p.i., although they were moribund by this time. These three mice were sacrificed and used for histological studies.
FIG. 1.
Clinical symptoms and mortality in infected perforin-deficient (•) and C57BL/6 wild-type (▴) mice. Mice were infected intracerebrally with 104 PFU of the DA strain of Theiler’s virus and were observed daily for signs of encephalomyelitis (A) and survival (B).
FIG. 2.
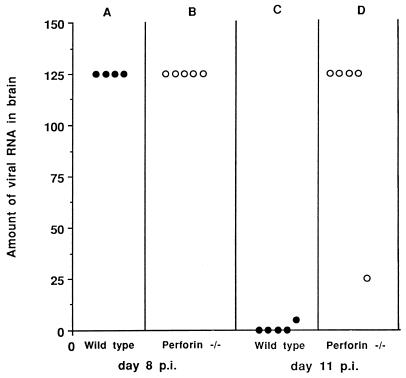
Amounts of viral RNA present in the brains of C57BL/6 wild-type and perforin-deficient (perforin −/−) mice 8 and 11 days after inoculation with 104 PFU of Theiler’s virus. Viral RNA was quantitated with a dot blot assay as described in the text. The ordinate shows, for each mouse, the highest dilution of the RNA solution which gave a positive hybridization signal. Each symbol represents one mouse.
Viral RNA was measured in the brains of five perforin-deficient mice with a dot blot assay. Wild-type C57BL/6 mice were studied in parallel as controls. Mice were inoculated, then killed at day 8 p.i., the time when the largest amount of virus is usually found in the brains of wild-type mice. As shown in Fig. 2, perforin-deficient mice and control C57BL/6 mice had similar amounts of viral RNA at that time (Fig. 2A and B). In another experiment, five more perforin-deficient mice were infected and studied at day 11 p.i., just before death. At this time point, a delay in viral clearance should be readily detected. As shown in Fig. 2, the levels of viral RNA in the brains of four of five perforin-deficient mice were comparable to those found at day 8 p.i. (Fig. 2B and D), whereas viral RNA was already undetectable in four of five wild-type mice and was present only in marginal amounts in the fifth (Fig. 2C).
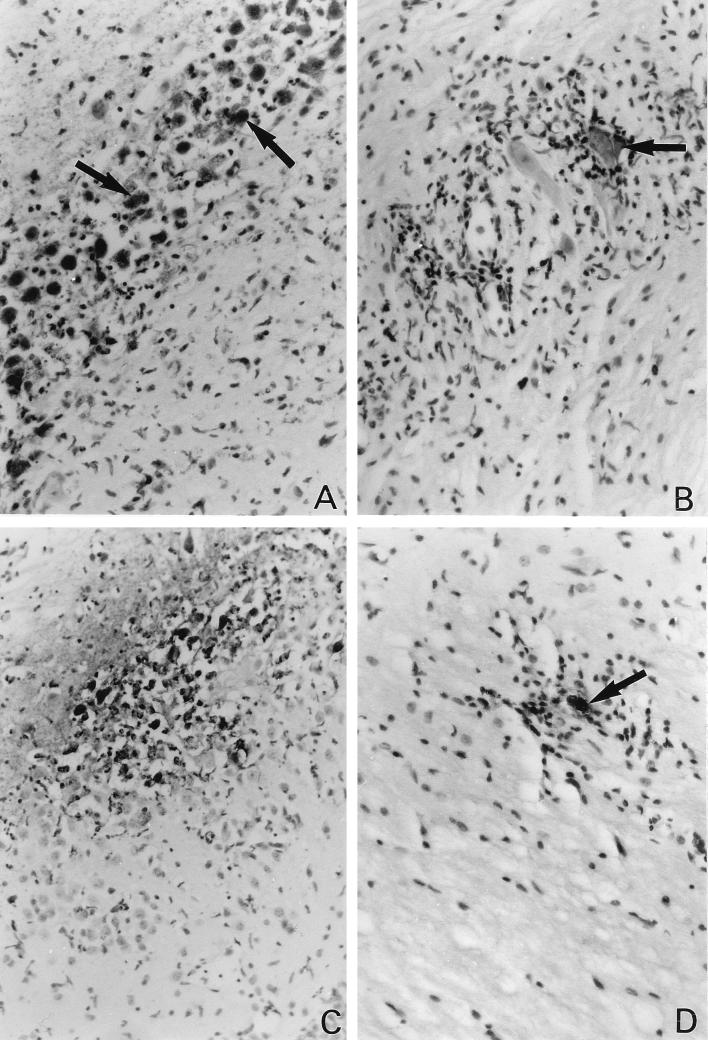
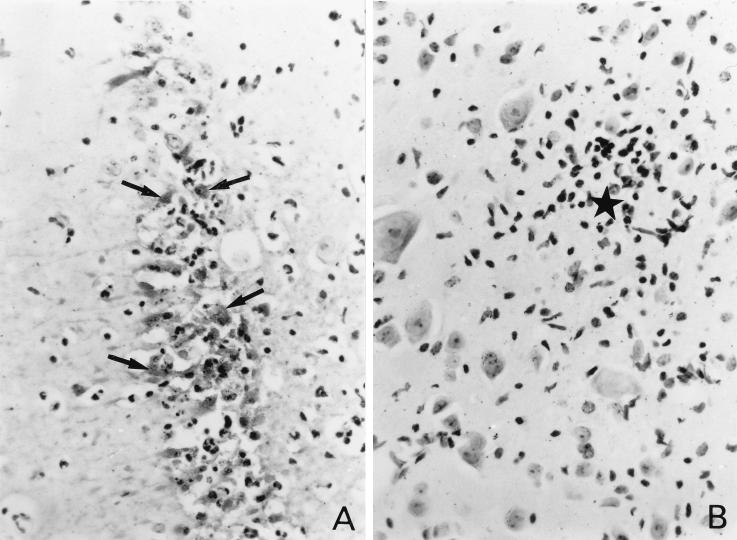
Histological studies were performed on sections of the brains and spinal cords of perforin-deficient mice and control mice at days 8, 11, and 18 p.i. Viral antigens were detected by immunohistochemistry. Three perforin-deficient mice and three wild-type mice were analyzed at day 8 p.i. A large number of infected cells were found in the gray matter of the brain, particularly in the hippocampus, for both perforin-deficient (Fig. 3A) and wild-type (Fig. 4A) mice. The majority of infected cells were neurons, as determined by morphological criteria. Many neurons containing viral antigens, and surrounded by intense inflammation, were also found in the gray matter of the spinal cord in perforin-deficient mice (Fig. 3B). Interestingly, no viral antigen was found in the spinal cord in control C57BL/6 mice at the same time p.i., although foci of mild inflammation were observed on occasion in the gray matter (Fig. 4B), suggesting the presence of residual infiltration after clearance of an infected cell. Two perforin-deficient mice were studied at day 11 p.i. Viral antigens were still present in the gray matter of the brain (Fig. 3C) and spinal cord in these mice, in association with inflammatory infiltrates. Viral antigens were more prominent in the spinal cord at this time than on day 8 p.i. In several instances, inflammation and viral antigens were also observed in the white matter of the spinal cord (Fig. 3D). In the three mice that survived until day 18 p.i., cells with viral antigens were detected in the gray matter of the brain and in both the gray and the white matter of the spinal cord (data not shown). Infected cells were associated with inflammatory infiltrates in the white matter of the spinal cord and elsewhere. No viral antigens were detected in control C57BL/6 mice at this time p.i. (data not shown). All the infected control and perforin-deficient mice described above were bled at the time of sacrifice and checked for the production of antiviral antibodies by an enzyme-linked immunosorbent assay. Perforin-deficient mice mounted antibody responses comparable to those of wild-type mice (data not shown).
FIG. 3.
(A) Viral antigens, inflammation, and tissue destruction in the brain (hippocampus) of a perforin-deficient mouse on day 8 p.i. Arrows point to examples of neurons containing viral antigens. Final magnification, ×427. (B) Viral antigens in a large motoneuron of the spinal cord (arrow) and intense inflammation in a perforin-deficient mouse on day 8 p.i. Final magnification, ×427. (C) Large amount of viral antigens, tissue destruction, and inflammation in the hippocampus of a perforin-deficient mouse on day 11 p.i. Final magnification, ×267. (D) Infected cell (arrow) and inflammation in the white matter of a perforin-deficient mouse on day 18 p.i. Final magnification, ×427.
FIG. 4.
(A) Viral antigens, inflammation, and tissue destruction in the hippocampus of a control C57BL/6 mouse on day 8 p.i. Arrows point to neuron cell bodies containing viral antigens. (B) An inflammatory infiltrate (star) in the gray matter of the spinal cord of a control C57BL/6 mouse on day 8 p.i. No viral antigen is present. Final magnification for both panels, ×427.
Previous studies have established that CD8+ T cells are essential for the clearance of Theiler’s virus infection (2, 11, 32). The present work, which addresses the question of the mechanism of viral clearance, indicates that the acute CNS infection is resolved mainly by a perforin-dependent mechanism. Indeed, perforin-deficient mice showed clinical and histological signs of acute encephalomyelitis and had high levels of viral RNA in their CNSs until the time of death. In contrast, control C57BL/6 mice remained asymptomatic, cleared the infection by day 11 to 12 p.i., and survived. Theiler’s virus infection and LCMV infection (18) of C57BL/6 mice are the only acute primary viral infections described so far in which perforin-dependent cytotoxicity is crucially involved in clearing the agent.
Although Theiler’s virus is cleared from the CNS in C57BL/6 mice, mainly by an efficient H-2Db-restricted CTL response which uses the perforin pathway, it is able to persist in susceptible mouse strains as well as in class-I-deficient H-2b mice (11, 32). Therefore it would have been important to determine if the virus could persist in perforin-deficient C57BL/6 mice. Unfortunately, because of the high mortality rate observed for these mice, in spite of a normal antibody response, the question could not be addressed directly. However, whereas in C57BL/6 mice the virus reaches maximum titer around day 8 p.i. and is cleared by day 11 to 12 p.i., in perforin-deficient C57BL/6 mice it remained at high levels until at least day 18 p.i., the last time at which they could be examined. In susceptible strains of mice, Theiler’s virus persists only in the white matter of the spinal cord. In resistant C57BL/6 mice, on the other hand, viral antigens are never observed in the white matter of the spinal cord. Although long-term persistence could not be demonstrated in perforin-deficient C57BL/6 mice because of mortality, it was interesting to observe viral antigens and inflammation in the white matter of the spinal cord at days 11 and 18 p.i. in these mice (Fig. 3D). At present, the cause of death for the perforin-deficient mice is unclear. It is probably not due simply to increased viral replication, since the same amount of virus was observed in class-I-deficient mice, although the latter survived (data not shown).
CD8+ T cells can control viral infections not only by killing infected cells but also by delivering cytokines, in particular gamma interferon (IFN-γ), to their targets. We have shown that IFN-γ has a major role in protecting white matter from persistent infection by Theiler’s virus (12). Our present data show, on the other hand, that a perforin-dependent mechanism is important in clearing the early infection of gray matter. The main viral targets at this stage are neurons, a cell type which does not normally express class-I molecules and which therefore should escape detection by CD8+ T cells. It has been shown, however, that neurons in culture, when treated with IFN-γ, may express class-I molecules if they are not electrically active, as could be the case as the result of an acute viral infection (27). Furthermore, the expression of class-I molecules on infected neurons has recently been documented in the brains of C57BL/6 mice infected with Theiler’s virus (28). CTLs may not be the only effector cells responsible for viral clearance in C57BL/6 mice. There are data suggesting that NK cells are important in limiting early gray-matter infection (29). However, nude mice, which have functional NK cells (14, 15) and lack CD8+ T cells, are unable to clear the infection; as a result, they die (34).
Based on the study of several viruses such as LCMV, VSV, vaccinia virus, and SFV, it has been hypothesized that noncytopathic viruses are controlled mainly by specific lytic mechanisms, whereas cytopathic viruses are controlled by cytokines and neutralizing antibodies (17). Whether Theiler’s virus should be considered a cytopathic or a noncytopathic virus in vivo is an open question. In vitro, the virus is lytic for fibroblasts of murine origin but viral replication is restricted in other cell types, and the cells survive when infected at a low multiplicity (20). In some macrophage cell lines, viral replication depends upon the state of differentiation and activation, can be highly restricted, and may not produce cytopathology (16). Also, a small fraction of macrophages isolated from mouse brain can be infected in vitro, without significant cytolysis (22). In vivo, viral replication seems to be restricted in the majority of infected cells during the persistent stage of the infection (5). These cells are mainly macrophages and to a lesser extent glial cells. Restricted replication may allow the virus to remain in those cells in the absence of cytopathicity. At that point a perforin-dependent cytotoxic mechanism might be required to eradicate the infection in genetically resistant strains of mice.
Clearing an acute infection of the CNS gray matter, such as that observed with the DA strain of Theiler’s virus in C57BL/6 mice, may require the cooperation of several defense mechanisms. The use of knockout mice has already shown the major roles of IFN-α/β (12) and of class-I-restricted CTLs (13, 14). The present work shows that these CTLs achieve their goal by making use of the perforin molecule.
Acknowledgments
This work was supported by grants from the Centre National de la Recherche Scientifique, the Institut Pasteur Fondation, the National Multiple Sclerosis Society, the Association pour la Recherche sur la Sclérose en Plaques, and the EC Human Capital and Mobility program (contract no. CHRX-CT94-0670).
We thank Mireille Gau for assistance with preparing the manuscript.
REFERENCES
- 1.Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay A, Brahic M, Bureau J F. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler’s virus. J Virol. 1994;68:4049–4052. doi: 10.1128/jvi.68.6.4049-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Azoulay-Cayla, A. Personal communication.
- 3.Brahic M, Ozden S. Simultaneous detection of cellular RNA and proteins. In: Wilkinson R G, editor. In situ hybridization—a practical approach. Practical Approach series no. 109. Oxford, United Kingdom: IRL Press; 1992. pp. 85–104. [Google Scholar]
- 4.Bureau J F, Montagutelli X, Lefebvre S, Guénet J-L, Pla M, Brahic M. The interaction of two groups of murine genes determines the persistence of Theiler’s virus in the central nervous system. J Virol. 1992;66:4698–4704. doi: 10.1128/jvi.66.8.4698-4704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cash E, Chamorro M, Brahic M. Theiler’s virus RNA and protein synthesis in the central nervous system of demyelinating mice. Virology. 1985;144:290–294. doi: 10.1016/0042-6822(85)90327-7. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Dal Canto M C, Lipton H L. Multiple sclerosis: animal model of human disease. Theiler’s virus infection in mice. Am J Pathol. 1977;88:497–500. [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels J B, Pappenheimer A M, Richardson S. Observations on encephalomyelitis of mice (DA strain) J Exp Med. 1952;96:22–24. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dethlefs S, Brahic M, Larsson-Sciard E-L. An early, abundant cytotoxic T-lymphocyte response against Theiler’s virus is critical for preventing viral persistence. J Virol. 1997;71:8875–8878. doi: 10.1128/jvi.71.11.8875-8878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dethlefs S, Escriou N, Brahic M, van der Werf S, Larsson-Sciard E-L. Theiler’s virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J Virol. 1997;71:5361–5365. doi: 10.1128/jvi.71.7.5361-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiette L, Aubert C, Brahic M, Pena Rossi C. Theiler’s virus infection of β2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiette L, Aubert C, Müller U, Huang S, Aguet M, Brahic M, Bureau J F. Theiler’s virus infection of 129Sv mice that lack the interferon α/β or interferon γ receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Sanz J A, Plaetinck G, Velotti F, Masson D, Tschopp J, MacDonald H R, Nabholz M. Perforin is present only in normal activated Lyt2+ T lymphocytes and not in L3T4+ cells, but the serine protease granzyme A is made by both subsets. EMBO J. 1987;6:933–938. doi: 10.1002/j.1460-2075.1987.tb04841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habu S, Fukui H, Shimamura K, Kasai M, Nagai Y, Okumura K, Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981;127:34–38. [PubMed] [Google Scholar]
- 15.Herberman R B, Holden H T. Natural cell mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- 16.Jelachich M L, Bandyopadhyay P, Blum K, Lipton H L. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 17.Kägi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 18.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 19.Kägi D, Seiler P, Pavlovic J, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick D R, Lipton H L. Predominant binding of Theiler’s viruses to a 34-kilodalton receptor protein on susceptible cell lines. J Virol. 1991;65:5244–5249. doi: 10.1128/jvi.65.10.5244-5249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, Shinkai Y, Alt F W, Matsuzawa A, Yonehara S, Takayama H. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 22.Levy M, Aubert C, Brahic M. Theiler’s virus replication in brain macrophages cultured in vitro. J Virol. 1992;66:3188–3193. doi: 10.1128/jvi.66.5.3188-3193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsley M D, Thiemann R, Rodriguez M. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler’s virus. J Virol. 1991;65:6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton H L. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann H, Schmidt H, Cavalié A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-γ and tumor necrosis factor (TNF)-α. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njenga M K, Pease L R, Wettstein P, Mak T, Rodriguez M. Interferon α/β mediates early virus-induced expression of H-2D and H-2K in the central nervous system. Lab Investig. 1997;77:71–84. [PubMed] [Google Scholar]
- 29.Paya C V, Patick A K, Leibson P J, Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler’s virus. J Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- 30.Pena Rossi C, Delcroix M, Huitinga I, McAllister A, van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler’s virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pena Rossi C, McAllister A, Fiette L, Brahic M. Theiler’s virus infection induces a specific cytotoxic T lymphocyte response. Cell Immunol. 1991;138:341–348. doi: 10.1016/0008-8749(91)90158-8. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez M, Dunkel A J, Thiemann R L, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler’s virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J Immunol. 1993;151:255–276. [PubMed] [Google Scholar]
- 33.Rodriguez M, Leibowitz J L, Lampert P W. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 34.Roos R P, Wollmann R. DA strain of Theiler’s murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984;15:494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- 35.Walsh C M, Matloubian M, Liu C-C, Ueda R, Kurahara C G, Christensen J L, Huang M T F, Young J D-E, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]