Abstract
Chronic myeloid leukemia (CML) is a type of leukemia whose main genetic marker is the reciprocal translocation that leads to the production of the BCR::ABL1 oncoprotein. The expression of some genes may interfere with the progression and development of leukemias. MicroRNAs are small non-coding RNAs that have the potential to alter the expression of some genes and may be correlated with some types of leukemia and could be used as biomarkers in the diagnosis and prognosis of patients. Therefore, this project carried out an analysis of microRNA-type plasma biomarkers in patients with chronic myeloid leukemia at unique points, including follow-up analysis of patients from the Erasto Gaertner Hospital. 35 microRNAs were analyzed in different cohorts. Inside those groups, 70 samples were analyzed at unique points and 11 patients in a follow-up analysis. Statistically different results were found for microRNA-7-5p, which was found to be upregulated in patients with high expression of the BCR::ABL1 transcript when compared to healthy controls. This microRNA also had evidence of behavior related to BCR::ABL1 when analyzed in follow-up, but strong evidence was not found. In this way, this work obtained results that may lead to manifestations of a relationship between miR-7-5p and chronic myeloid leukemia, and evaluations of possible microRNAs that are not related to this pathology.
Keywords: chronic myeloid leukemia, microRNA, imatinib, BCR::ABL1, biomarkers
1. Introduction
Chronic myeloid leukemia is a malignant myeloproliferative disease, mainly caused by a reciprocal translocation between chromosomes 9 and 22 (9; 22) (q34; q11), resulting in the Philadelphia chromosome (Ph), and the corresponding BCR::ABL1 fusion gene, which is the main biomarker of this pathology [1,2].
The resulting transcriptional fusion encodes the oncoprotein BCR::ABL1, which is a constitutively active tyrosine kinase, whose activity stimulates leukemogenesis [3]. Therefore, treatment is carried out with tyrosine kinase inhibitors (TKIs), and despite this being a highly efficient treatment, approximately 13% of patients develop resistance to this class of compounds [4].
Patient monitoring through molecular quantification of the BCR::ABL1 transcript is highly relevant for evaluating the prognosis of patients with CML. Treatment failure is related to mechanisms of resistance to TKIs, the origin of which may be a mutation in the coding region of the kinase domain of the BCR::ABL1 tyrosine kinase. Several mutations in this domain have already been described, such as T315I, V299L, G250E, F317L, Y253H, E255K/V, F359V/C/I, and L248V [5] among others. Although CML has BCR::ABL1 as both a diagnostic and monitoring marker, the search for other plasma biomarkers are important for the prognosis of the disease. Among the biomolecules that can be explored with this potential are non-coding RNAs (ncRNAs), which can help in the identification of altered or modulated targets in different phases of the disease; this can be extremely relevant in clinical use, as they can be related to resistance to the chemotherapy drugs used.
MicroRNAs make up a broad and well-studied class of ncRNAs, being correlated with many signaling pathways [6] playing a role in the regulation of gene expression, RNA maturation, protein synthesis, and can also have their activity regulated at a post-transcriptional level [7,8,9].
Considering the presented information, this study aimed to evaluate the dynamics of microRNA expression in samples from patients with CML, correlating the results with the stage of the disease and BCR::ABL1 transcript quantification to help in the disease follow-up and prognosis.
2. Results
2.1. Epidemiological Description
The epidemiological data of participants recruited for single-point analyses (patients with a single sample collected) are described in Table 1.
Table 1.
Epidemiological data of patients recruited for single-point analyses and p value related to Kruskall–Wallis analysis showing the significances of differences between the ages of the respective groups. High: 0.1% or higher percentage of BCR::ABL1 transcript; ND: Non Detected BCR::ABL1 transcript; HC: Health Control group. ns: non-significant.
| Group | BCR::ABL1 | Sex | Treatment Time in Months (Mean ± Deviation) | Age (Mean ± Deviation) | Age Differences between Groups (p Value and Significance) |
|||
|---|---|---|---|---|---|---|---|---|
| M | F | High vs. ND | High vs. HC | ND vs. HC | ||||
| 1 | High | 18 | 12 | 15.3 ± 29.4 | 50.4 ± 20.7 | 0.1291 | >0.9999 | 0.1606 |
| 2 | ND | 10 | 10 | 54 ± 23.6 | 62 ± 13.4 | ns | ns | ns |
| 3 | HC | 10 | 10 | - | 51.8 ± 6.9 | - | - | - |
2.2. Single-Point Plasma Analysis
In the first stage, eight microRNAs were evaluated: miR-17-5p [10,11,12,13], miR-23a-3p [14,15,16], miR-93-5p [17,18], miR-130a-3p [19], miR-142-5p [20,21], miR-148b-3p [22,23], miR-199a-3p [24,25], and miR-331-5p [26]. These microRNAs have been chosen based on oncology potential found in the literature. Some of them are related to hematological malignancies and others are related to other types of cancer, in addition to the exogenous normalizer cel-miR-39-3p. For statistical analysis, the samples were subdivided into three sample groups: samples with a value higher than 0.1% of BCR::ABL1 transcript, samples with undetectable BCR::ABL1, and samples from healthy individuals (Table 2). No significant differences were observed in the expression of these eight miRNAs between the three sample groups (considering a p value of <0.05).
Table 2.
p value related to Kruskall–Wallis analysis showing the general rank p value and the p value for multiple comparisons. High: 0.1% or higher percentage of BCR::ABL1 transcript; ND: Non Detected BCR::ABL1 transcript; HC: Health Control group.
| miRNA | General Rank | Multiple Comparisons (p Value) | Mean ± Deviation | ||||
|---|---|---|---|---|---|---|---|
| High vs. ND | High vs. HC | ND vs. HC | High | ND | HC | ||
| miR-93-5p | 0.9979 | >0.9999 | >0.9999 | >0.9999 | 12.1 ± 37.1 | 1.8 ± 2.2 | 5.6 ± 18.8 |
| miR-23a-3p | 0.8934 | >0.9999 | >0.9999 | >0.9999 | 9.3 ± 28.1 | 1.1 ± 1.6 | 1.2 ± 2.6 |
| miR-199a-3p | 0.9534 | >0.9999 | >0.9999 | >0.9999 | 12.2 ± 38.6 | 1.9 ± 2.6 | 3.1 ± 8.05 |
| miR-17-5p | 0.9005 | >0.9999 | >0.9999 | >0.9999 | 1.5 ± 3.5 | 0.4 ± 0.8 | 0.6 ± 1.7 |
| miR-148b-3p | 0.5818 | >0.9999 | 0.9747 | >0.9999 | 19.8 ± 54.4 | 4.5 ± 7.9 | 10.3 ± 35.5 |
| miR-142-5p | 0.5077 | >0.9999 | >0.9999 | >0.9999 | 1.9 ± 1.0 | 12.1 ± 15.9 | 21.3 ± 51.0 |
| miR-130a-3p | 0.8636 | >0.9999 | >0.9999 | >0.9999 | 248.3 ± 653.1 | 60.9 ± 136.7 | 122.3 ± 410.9 |
| miR-331-5p | 0.5376 | >0.9999 | >0.9999 | 0.7997 | 3.7 ± 1.3 | 2.4 ± 5.7 | 5.1 ± 2.0 |
In the second stage, 27 additional microRNAs were evaluated, including 10 samples with a high percentage of BCR::ABL1 transcript (0.1% or more) and 10 samples from the healthy control group. The miRNAs evaluated were: miR-7-5p, miR-19b-3p, miR-20a-5p, miR-21-5p, miR-25-3p, miR-27b-3p, miR-29a-3p, miR-29b-3p, miR-92a-1-5p, miR-103a-3p, miR-106b-5p, miR-122-5p, miR-125b-5p, miR-130a-5p, miR-150-5p, miR-155-5p, miR-186-5p, miR-192-5p, miR-193b-3p, miR-205-5p, miR-214-5p, miR-221-3p, miR-221-5p, miR-361-5p, miR-451, miR-486-5p, and miR-494-3p (Table 3).
Table 3.
p value related to Mann–Whitney analysis showing the general rank p value and significance of statistical difference between samples with High percentages of BCR::ABL1 transcript (1% or higher percentage of BCR::ABL1 transcript) and Health controls. s: significant; ns: non-significant.
| miRNA | General Rank (p Value) | Mean ± Deviation | ||
|---|---|---|---|---|
| Mann-Whitney | Significance | High | HC | |
| miR-7-5p | 0.2475 | ns | 1.6 ± 1.7 | 0.6 ± 0.3 |
| miR-19b-3p | 0.9118 | ns | 10.1 ± 18.2 | 3.3 ± 3.1 |
| miR-20a-5p | 0.8534 | ns | 2.9 ± 5.0 | 1.2 ± 0.9 |
| miR-21-5p | 0.6842 | ns | 7.9 ± 13.2 | 2.6 ± 2.3 |
| miR-25-3p | 0.9118 | ns | 1.9 ± 3.7 | 0.8 ± 0.7 |
| miR-27b-3p | 0.8534 | ns | 13.7 ± 24.4 | 4.3 ± 3.8 |
| miR-29a-3p | 0.9705 | ns | 7.6 ± 12.5 | 2.2 ± 1.7 |
| miR-29b-3p | >0.9999 | ns | 6.4 ± 10.8 | 1.8 ± 1.3 |
| miR-92a-1-5p | 0.0220 | s | 3.2 ± 2.5 | 0.7 ± 0.5 |
| miR-103a-3p | 0.7394 | ns | 4.2 ± 7.4 | 1.2 ± 1.4 |
| miR-106b-5p | 0.9705 | ns | 3.3 ± 5.4 | 1.5 ± 1.2 |
| miR-122-5p | 0.4813 | ns | 1.4 ± 2.2 | 2.4 ± 3.6 |
| miR-125b-5p | 0.6842 | ns | 2.0 ± 3.5 | 1.2 ± 1.2 |
| miR-130a-5p | No amplification | ns | - | - |
| miR-150-5p | 0.6305 | ns | 3.7 ± 9.6 | 0.8 ± 0.5 |
| miR-155-5p | 0.4173 | ns | 42.3 ± 99.2 | 30.2 ± 45.9 |
| miR-186-5p | 0.5787 | ns | 4.3 ± 7.7 | 1.9 ± 1.8 |
| miR-192-5p | 0.5787 | ns | 3.2 ± 3.9 | 1.5 ± 1.0 |
| miR-193b-3p | 0.6048 | ns | 1.3 ± 1.6 | 0.9 ± 1.0 |
| miR-205-5p | 0.2176 | ns | 5.5 ± 6.2 | 2.1 ± 1.1 |
| miR-214-5p | No amplification | ns | - | - |
| miR-221-3p | 0.7394 | ns | 6.7 ± 10.7 | 1.8 ± 1.5 |
| miR-221-5p | 0.8125 | ns | 24.2 ± 51.1 | 5.3 ± 6.6 |
| miR-361-5p | 0.4359 | ns | 6.8 ± 10.3 | 2.6 ± 3.2 |
| miR-451 | >0.9999 | ns | 1.9 ± 3.2 | 0.7 ± 0.4 |
| miR-486-5p | 0.6305 | ns | 2.1 ± 3.7 | 0.8 ± 0.6 |
| miR-494-3p | 0.8286 | ns | 36.6 ± 72.0 | 16.1 ± 17.4 |
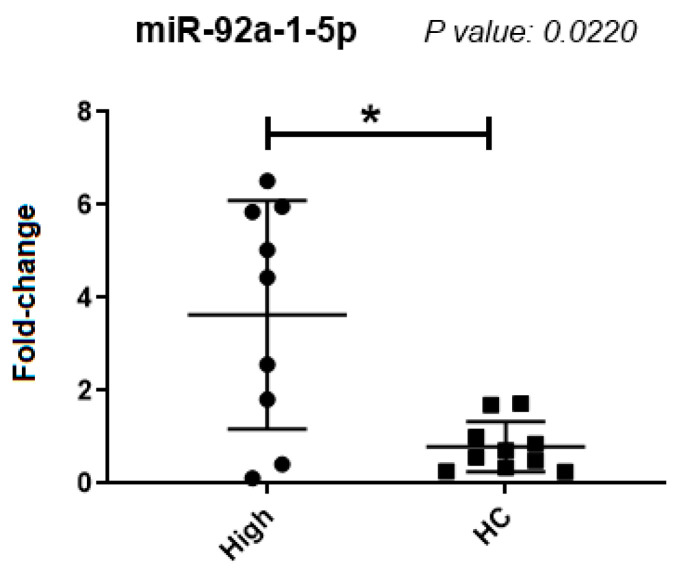
There was a significantly higher expression of miR-92a-1-5p in samples from patients with elevated percentages of the BCR::ABL1 transcripts, compared to the healthy control group (p = 0.0220) (Figure 1), in spite of great variation among CML samples. The other microRNAs tested did not show significant differential expressions between the sample groups.
Figure 1.
miR-92a-1-5p expression. Comparative results in a group of samples from patients with CML and healthy donors. High: samples with a high percentage of leukemic cells; HC (Healthy Controls): Samples from healthy donors. *: Significant difference (p < 0.05).
Among the 27 microRNAs tested, miR-92a-1-5p and three other miRNAs were analyzed in a greater number of samples in the groups (n = 20): miR-92a-1-5p, miR-7-5p, miR-486-5p, and miR-361-5p (Table 4). These three other miRNAs were those with a tendency for significant results between groups in the first 10 samples.
Table 4.
p-values found in Mann–Whitney analysis showing the general rank p value and significance of statistical difference between samples with High percentages of BCR::ABL1 transcript (0.1% or higher percentage of BCR::ABL1 transcript) and Healthy controls. s: significant; ns: non- significant.
| miRNA | General Rank | Mean ± Deviation | ||
|---|---|---|---|---|
| Mann-Whitney | Significance | High | HC | |
| miR-92a-1-5p | 0.057 | ns | 909.1 ± 4049.7 | 1.7 ± 5.1 |
| miR-7-5p | 0.0353 | s | 2.6 ± 3.1 | 9.5 ± 39.2 |
| miR-486-5p | 0.0829 | ns | 3.6 ± 5.8 | 6.2 ± 23.8 |
| miR-361-5p | 0.1494 | ns | 16.4 ± 36.4 | 7.4 ± 19.7 |
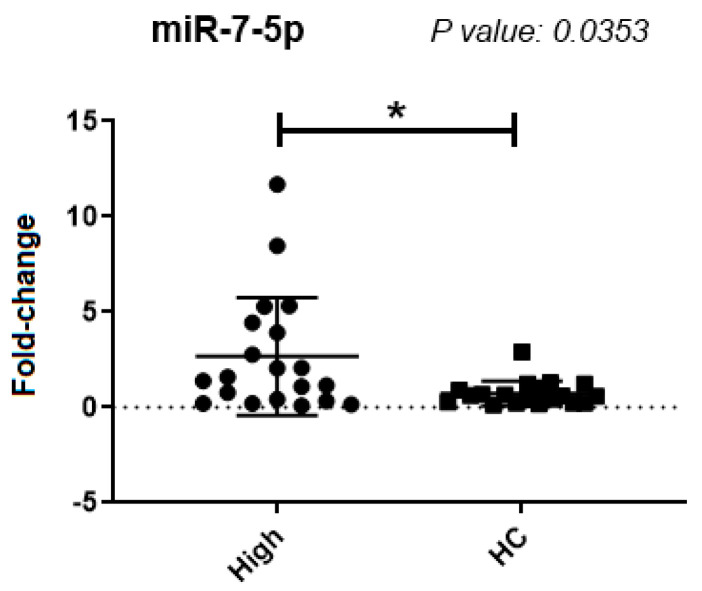
There was a significantly higher expression of miR-7-5p in samples from patients with a higher BCR::ABL1 proportion, when compared to the control group (p = 0.0353) (Figure 2). The microRNAs miR-486-5p and miR-361-5p did not show a significant difference in expression between the tested groups. When we increased the number of samples, miR-92a-1-5p had no significant difference in expression.
Figure 2.
miR-7-5p expression analysis with additional samples. High: samples with a high percentage of leukemic cells; HC (Healthy Controls): Samples from healthy donors. *: Significant difference (p < 0.05).
For the remaining analyses, only miR-7-5p was used for the evaluation of all samples from patients with expressions of the BCR::ABL1 transcript (30 samples) and in the group of samples from CML patients with undetectable levels of BCR::ABL1 transcripts (20 samples), as well as samples from healthy controls (Figure 3).
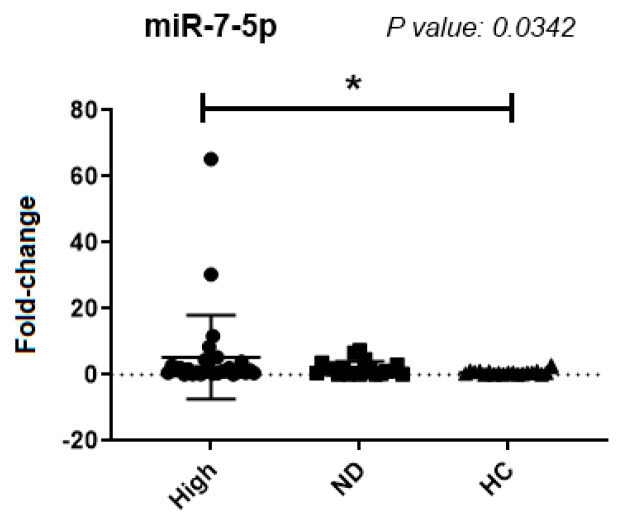
Figure 3.
miR-7-5p expression analysis between the group with a high percentage of BCR::ABL1, group with undetectable BCR::ABL1, and samples from healthy donors. High: samples with a high percentage of leukemic cells; ND (Non-Detected): Samples with an undetectable percentage of leukemic cells; HC (Healthy Controls): Samples from healthy donors. Mean ± deviation: High: 2.6 ± 3.1; ND: 1.8 ± 2.2 and HC: 9.5 ± 2.2. *: significant difference (p < 0.05).
There was a significant difference in expression between groups with a high percentage of BCR::ABL1 and healthy controls (p = 0.0342) (Figure 3). There was no significant difference between the groups with a high percentage of BCR::ABL1 and the group with undetected BCR::ABL1 (Figure 3).
2.3. Buffy Coat Single-Point Analysis
For expression analysis with RNA extracted from the buffy coat, the endogenous miUSB U6 was used as a normalizer [27,28]. The analyses were carried out mostly with the same patients that were used for the plasma analyses. However, some of the buffy coat samples were not available, so the final number of samples is not the same.
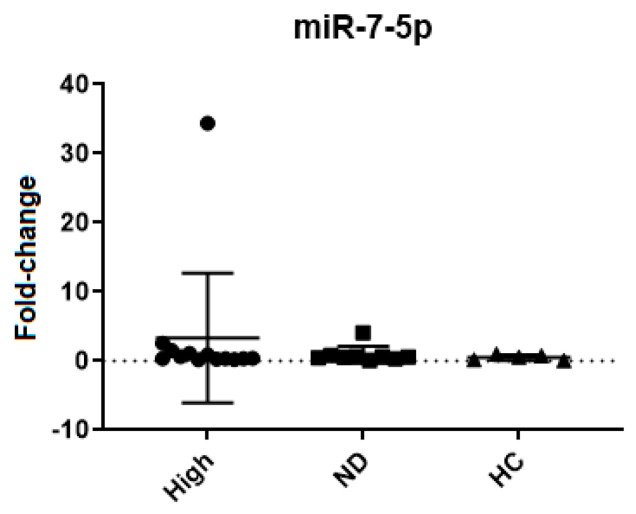
Thirteen samples from patients with a high percentage of BCR::ABL1, nine samples from patients with undetected BCR::ABL1, and five samples from the healthy control group were analyzed. miR-7-5p was evaluated in RNA from buffy coats with the aim of corroborating the results seen in plasma RNA. However, as opposed to plasma, no significant differences were seen in the expression of miR-7-5p in buffy coat RNA (Table 5 and Figure 4).
Table 5.
p value related to Kruskall–Wallis analysis showing the general rank p value and the p value for multiple comparisons analyzing samples of buffy coat. High: 0.1% or higher percentage of BCR::ABL1 transcript; ND: Non-Detected BCR::ABL1 transcript; HC: Healthy Control group.
| miRNA | General Rank | Multiple Comparisons | Mean ± Deviation | ||||
|---|---|---|---|---|---|---|---|
| High vs. ND | High vs. HC | ND vs. HC | High | ND | HC | ||
| miR-7-5p | 0.7897 | >0.9999 | >0.9999 | >0.9999 | 3.3 ± 9.3 | 0.9 ± 1.2 | 0.5 ± 0.4 |
Figure 4.
miR-7-5p expression analysis in RNA from buffy coat, the results shown non-significant (ns) statistics evaluating this group (data shown in Table 5). Groups: BCR::ABL1 high; undetectable BCR::ABL1, and healthy donors; High: samples with a high percentage of leukemic cells; ND (Non-Detected): Samples with an undetectable percentage of leukemic cells; HC (Healthy Controls): Samples from healthy donors.
2.4. Follow-Up Analysis
This analysis was carried out with miR-7-5p, and for this purpose, we selected patients that had two or more sample points collected, and subsequently divided them into two groups: those under treatment and those in treatment-free remission (TFR). The objective of this analysis was to evaluate a possible relationship between the expression of miR-7-5p and treatment interruption or failure, considering mainly the quantification of the BCR::ABL1 transcript as a reference parameter.
Patients undergoing follow-up were divided into two groups: patients undergoing treatment (initially with Imatinib) and patients who underwent treatment interruption (treatment-free remission); six patients were selected for the first group and four patients for the second group. The patients selected for these analyses may also be within one of the single-point analysis groups, and for this analysis, these patients were selected individually to carry out follow-up monitoring.
2.4.1. Group 1: Patients Undergoing Treatment
Regarding patients undergoing treatment, patients who started their treatment with Imatinib and had distinct progressions based on BCR::ABL1 levels were selected. A correlation was analyzed using Sperman’s rank correlation coefficient and was not found for any of the patients evaluated, despite some patients who showed a concordant relationship between BCR::ABL1 and miR-7 levels. However, in data from other patients, this relationship was not observed, and this may have happened due to other factors which we do not know, and may be interfering with the expression of this microRNA and not in the expression of the BCR::ABL1 transcript (Figure 5 and Table 6).
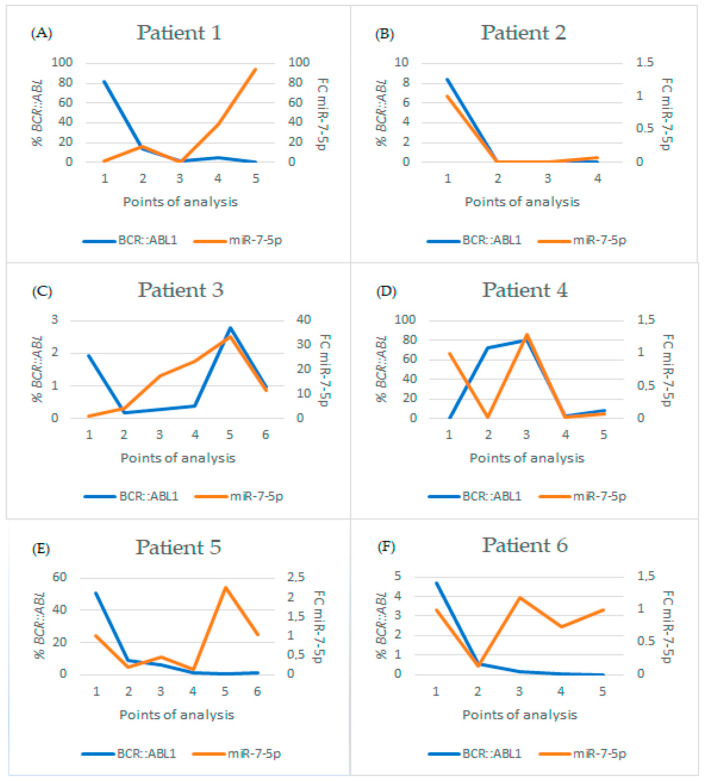
Figure 5.
Graphs showing the analysis of patients undergoing continuous follow-up to evaluate the progression of the pathology by evaluating the expression of microRNA-7-5p (fold-change, left scale, and orange line), expression of the BCR::ABL1 transcript (percentage, right scale, and blue line), treatment and treatment time, evaluating the date that is shown in Table 6. (A) Patient 1; (B) Patient 2; (C) Patient 3; (D) Patient 4; (E) Patient 5; and (F) Patient 6. BCR::ABL1: Percentage of BCR::ABL1 transcript in the sample; FC: Fold Change.
Table 6.
Analysis of patients undergoing continuous follow-up to evaluate the progression of the pathology by evaluating the expression of microRNA-7-5p, expression of the BCR::ABL1 transcript, treatment, and treatment time. BCR::ABL1: Percentage of BCR::ABL1 transcript in the sample; FC: Fold Change.
| Months of Treatment | BCR::ABL1 % | FC miR-7-5p | Treatment | ||
|---|---|---|---|---|---|
| Patient 1 | 0 | Point 1 | 81.74 | 1 | Imatinib |
| 4 | Point 2 | 13.68 | 15.637 | Imatinib | |
| 12 | Point 3 | 1.854 | 0.282 | Imatinib | |
| 14 | Point 4 | 5.291 | 38.241 | Imatinib | |
| 5 | Point 5 | 0 | 93.641 | Dasatinib | |
| Patient 2 | 3 | Point 1 | 8.39 | 1 | Imatinib |
| 6 | Point 2 | 0.000 | 0.003 | Imatinib | |
| 11 | Point 3 | 0.003 | 0.013 | Imatinib | |
| 15 | Point 4 | 0 | 0.068 | Imatinib | |
| Patient 3 | 8 | Point 1 | 1.943 | 1 | Imatinib |
| 18 | Point 2 | 0.179 | 3.985 | Imatinib | |
| 21 | Point 3 | 0.268 | 1.624 | Imatinib | |
| 23 | Point 4 | 0.364 | 23.589 | Imatinib | |
| 26 | Point 5 | 2.774 | 33.697 | Imatinib | |
| 27 | Point 6 | 0.96 | 11.446 | Imatinib | |
| Patient 4 | 0 | Point 1 | 0.143 | 1 | Imatinib |
| 6 | Point 2 | 72.738 | 0.020 | Imatinib | |
| 9 | Point 3 | 80.616 | 1.290 | Imatinib | |
| 3 | Point 4 | 2.239 | 0.025 | Dasatinib | |
| 5 | Point 5 | 8.192 | 0.071 | Dasatinib | |
| Patient 5 | 0 | Point 1 | 50.64 | 1 | Imatinib |
| 3 | Point 2 | 9.138 | 0.208 | Imatinib | |
| 6 | Point 3 | 6.288 | 0.447 | Imatinib | |
| 15 | Point 4 | 1.202 | 0.133 | Imatinib | |
| 22 | Point 5 | 0.902 | 2.251 | Imatinib | |
| 24 | Point 6 | 1.159 | 1.028 | Imatinib | |
| Patient 6 | 6 | Point 1 | 4.67 | 1 | Imatinib |
| 13 | Point 2 | 0.578 | 0.140 | Imatinib | |
| 16 | Point 3 | 0.141 | 1.188 | Imatinib | |
| 20 | Point 4 | 0.033 | 0.733 | Imatinib | |
| 29 | Point 5 | 0.000 | 1.002 | Imatinib |
In this context, the results of patient 1 (Table 6) at the fourth point show there was a new increase in the expression of both microRNA and the percentage of leukemic cells. However, at the fifth point, BCR::ABL1 levels decreased and microRNA expression increased. This patient had a failure in their treatment with Imatinib, and as we noted in the last point, the treatment was changed to Dasatinib. Therefore, the microRNA followed the expression levels of the BCR::ABL1 transcript and the last point may be related to the predisposition to instability or treatment failure. However, this hypothesis could only be deepened by analyzing subsequent samples from the same patient.
Patient 2 (Table 6) had a good treatment progression with Imatinib, since this patient’s results expressed a decreased percentage of BCR::ABL1, the expression of miR-7-5p continued showing low levels. Their data were consistent.
Regarding patient 3 (Table 6), we observed instability in the treatment with Imatinib, which may have been generated by a lack of correct adherence to the treatment. There is a fluctuation in the value of BCR::ABL1, which is accompanied by an increase in the expression of miR-7-5p, indicating that the greater the expression of miR-7-5p, the greater the possibility of worsening or treatment failure. At the last point for this patient, there was a decrease in the expression of miR-7-5p and in the percentage of the BCR::ABL1 transcript.
According to the results of patient 4 (Table 6), this also showed instability in the treatment, which is demonstrated by the increase in miR-7-5p expression levels at the third point, where BCR::ABL1 was already high. However, at the fourth point, there was a change in treatment to Dasatinib, and microRNA expression levels decreased, along with BCR::ABL1 levels. At point 5, both increased again, which could be a warning sign that the treatment may not be working. This hypothesis could only be supported when related to subsequent analyses of the same patient.
The results of patient 5 (Table 6) showed a reduction in BCR::ABL1 levels from points 1 to 5, where miR-7-5p levels were also reduced compared to the baseline. However, the levels of BCR::ABL1 at point 6 increased in relation to the previous point, while the level of miR-7-5p was already increased at the previous point, indicating that this miRNA may have had an increase prior to the elevation of BCR::ABL1, and, consequently, suggesting a relationship between micrRNA-7-5p and the increase in BCR::ABL1.
The sixth patient (Table 6) showed a good progression in treatment when we evaluated the expression of BCR::ABL1, but the expression of microRNA 7-5p remained unstable throughout the period, which could be a warning sign of possible future treatment failure.
2.4.2. Patients in Treatment-Free Remission
The patients evaluated in these analyses were selected by professionals at the Erasto Gaertner Hospital to begin treatment-free remission. The criteria for selecting these patients were evaluated according to the NCCN guidelines (NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [29], Chronic Myeloid Leukemia, Version 2.2024—5 December 2023). Regarding the results obtained in the analyses of the five selected patients in free remission (Table 7), it was observed that in patient 1 there were two follow-up points where the expression levels of miR-7-5p were increased, while the levels of BCR::ABL1 remained undetectable. In patient 2, the expression of miR-7-5p remained low at all points. The third patient had stable and low microRNA expression at all points in their free remission. Finally, the fourth patient had a long follow-up, and a point of instability was observed, with an increase in the expression of miR-7-5p (point 8), returning to stability shortly thereafter.
Table 7.
Results of follow-up analyses of patients undergoing free remission treatment, comparing TFR (Treatment Free-Remission) Time and Fold-Change of microRNA-7 (FC miR-7-5p).
| Time | FC miR-7-5p | ||
|---|---|---|---|
| Patient 1 | Point 1 | Pause | 1 |
| Point 2 | 2/3 months | 0.849 | |
| Point 3 | 5/6 months | 2.350 | |
| Point 4 | 14 months | 1.810 | |
| Point 5 | 20 months | 0.682 | |
| Patient 2 | Point 1 | In treatment | 1 |
| Point 2 | 5/6 months | 0.253 | |
| Point 3 | 11/12 months | 0.351 | |
| Point 4 | 14 months | 0.410 | |
| Patient 3 | Point 1 | In treatment | 1 |
| Point 2 | 1 month | 0.150 | |
| Point 3 | 2/3 months | 0.200 | |
| Point 4 | 5/6 months | 0.505 | |
| Point 5 | 7–10 months | 0.299 | |
| Point 6 | 11/12 months | 0.458 | |
| Point 7 | 14 months | 0.504 | |
| Patient 4 | Point 1 | In treatment | 1 |
| Point 2 | 1 months | 0.196 | |
| Point 3 | 2/3 months | 0.282 | |
| Point 4 | 5/6 months | 0.462 | |
| Point 5 | 5/6 months | 0.248 | |
| Point 6 | 5/6 months | 0.209 | |
| Point 7 | 7–10 months | 0.222 | |
| Point 8 | 7–10 months | 1.472 | |
| Point 9 | 7–10 months | 0.027 | |
| Point 10 | 11/12 months | 0.100 |
3. Discussion
Several studies have shown that MicroRNAs are associated with different biological pathways and have a great influence on the transduction of cell signaling [30]. The involvement of microRNAs in the development, progression, and resistance to chemotherapy in CML [31] shows that they can be important targets for studies related to chronic myeloid leukemia and other types of cancer. The present study addressed different clinical cohorts, forming different points of analysis aiming to evaluate possible biomarkers.
To achieve this, a screening of 35 microRNAs was first carried out in plasma samples from a cohort of patients with CML. These microRNAs were selected according to previous data in the literature, with some being correlated to leukemias in different contexts, such as diagnosis, prognostic assessment, and Treatment-Free Remission protocol, while some were selected based on their scientific relevance in other types of cancer. The results obtained showed that miR-7-5p had a significant differential expression among groups of patients separated according to their percentage of BCR::ABL1 expression.
The expression of miR-7-5p was significantly increased in samples from patients with a high percentage of BCR::ABL1 transcript compared to healthy controls. Surprisingly, as observed in the results, this microRNA had the highest expression in some CML patients treated with Imatinib and with a high percentage of BCR::ABL1, while other patients in this same group presented values similar to the average of controls. This result suggested that microRNA 7-5p may be related to the progression of the disease, considering that the increase in its expression could be related to the increase in the percentage of BCR::ABL1 transcript. These results led us to perform two additional analyses: single point analysis with RNA samples obtained from leukocytes (buffy coat) from the same patients, and analysis at various follow-up points of patients with CML.
To identify the origin of miR-7-5p in plasma, we evaluated its expression in leukocytes from the same patients. Unlike what was observed in plasma, miR-7-5p did not have a differential expression in the patients’ leukocytes. The increase of miR-7-5p in plasma could be related to the release of extracellular vesicles containing microRNA-7-5p or other factors, such as the hypothesis that this microRNA is being secreted by cells of the bone marrow and not directly from leukocytes in the bloodstream.
As another approach to investigate the role of miR-7-5p in CML, we evaluated a follow-up cohort of samples from the same patients collected at consecutive points throughout their progression with Imatinib treatment. Similar to what was found in the previous stage, miR-7-5p showed different behaviors in relation to the course of the disease in each patient, indicating a heterogeneous behavior of this target, suggesting that it can be modulated and altered by several factors.
These results indicate that microRNA-7-5p may be related to the expression of the BCR::ABL1 transcript. Some points of the analysis showed sudden increases in the expression of miR-7-5p, indicating that it can be a monitoring biomarker. Furthermore, it was also observed that microRNA-7 had a coincident progression relationship with the BCR::ABL1 transcript, where both increased or decreased at the same time, which may be related only to the BCR::ABL1 transcript and not with the prediction of prognosis. However, monitoring more patients for longer periods of time is necessary to confirm this hypothesis.
Another approach of the present study evaluated patients who started treatment with Imatinib, and, as they had excellent progression, were enrolled for treatment-free remission. All of these patients had undetectable BCR::ABL1 t at all follow-up points. In this context, the molecular monitoring of BCR::ABL1 expression in TFR patients together with the plasma quantification of miR-7-5p aimed to observe whether miR-7-5p could serve as an anticipatory marker of increased expression of BCR::ABL1, indicating the need to resume discontinuation for these patients.
Of the 4 TFR patients, 3 maintained stable miR-7-5p expression at all points of their follow-up, indicating that there may be some type of relationship with the worsening prognosis and the increase in the percentage of leukemic cells. Two patients had unstable results, such as patient 1 who had an instability where they had an increase in the expression of miR-7-5p in the third and fourth points. Considering the instability in patient 5, it can be hypothesized that there are other factors interfering in miR-7-5p expression. More follow-up points would be necessary so that we can draw any conclusions.
MicroRNA-7 has been studied in several types of cancer [32], such as lung, hepatocellular, breast, gliomas, colorectal, hematological neoplasms, among others [33]. This microRNA has been characterized as a tumor suppressor in several types of cancer [34] and has also been related to the modulation of signaling pathways [35,36]. MicroRNA-7 may have a functional performance related to the inhibition of DNA repair mechanisms, carried out by PARP-1 and BRCA [37]. A study indicated that there is a relationship between ANRIL/miR-7 in which microRNA-7 may function as a tumor suppressor in T-cell acute lymphoblastic leukemia [32]. A possible miRNA-TET2 pathway was also identified, where microRNAs, including miR-29b, miR-101, miR-125b, miR-29c, and miR-7 are overexpressed and thus may be involved in the pathogenesis of AML [38].
In the context of chronic myeloid leukemia, the roles for miR-7 are not yet well understood. Jiang et al. (2017) carried out studies demonstrating that miR-7 inhibited cell proliferation and promoted apoptosis in K562 cell lines focusing on the BCR::ABL1/PI3K/AKT signaling pathway [39]. The same study demonstrated that microRNA-7 may also be related to the sensitization of the K562 cell to Imatinib [39]. Considering the studies already carried out and the results obtained in this work, we can hypothesize that these pathways may be related to changes in the expression of microRNA-7/BCR::ABL1.
There is little evidence about the relationship between microRNA-7-5p and chronic myeloid leukemia patients. However, the present work found evidence that miR-7-5p may be related to chronic myeloid leukemia in a non-specific way, mainly considering its relationship with the BCR::ABL1 transcript. It is also important to highlight that the modern study of oncology, mainly focused on diagnosis, prognosis, and therapeutic evolution, is linked to individual differences and patient profiles. Therefore, the identification of a single sensitive and specific biomarker for a type of neoplasm is unlikely, requiring studies with a panel of biomarkers and the stratification of patients with a given neoplasm to elucidate more sensitive and specific biomarkers. This reiterates the importance of microRNAs as excellent accessories and mainly individualized biomarkers, being important in precision medicine, which is the main objective of clinical oncology, as it directs efforts, resources, time, and patient survival, which increases their quality of life.
4. Materials and Methods
4.1. Casuistry
Peripheral blood samples from patients with CML were collected at Erasto Gaertner Hospital (Curitiba, PR, Brazil), for research projects approved according to CAAE 08809419.0.0000.0098 and 53207021.5.0000.0098. The microRNAs addressed in this project were previously selected based on studies related to microRNAs in CML, consisting of 35 miRNAs: miR-7-5p, miR-17-5p, miR-19b-3p, miR-20a-5p, miR-21-5p, miR-23a-3p, miR-25-3p, miR-27b-3p, miR-29a-3p, miR-29b-3p, miR-92a-1-5p, miR-93-5p, miR-103a-3p, miR-106b-5p, miR-122-5p, miR-125b-5p, miR-130a-3p, miR-130a-5p, miR-142-5p, miR-148b-3p, miR-150-5p, miR-155-5p, miR-186-5p, miR-192-5p, miR-193b-3p, miR-199a-3p, miR-205-5p, miR-214-5p, miR-221-3p, miR-221-5p, miR-331-5p, miR-361-5p, miR-451, miR-486-5p, and miR-494-3p. The miRNAs were evaluated in cohorts of CML patients, who were divided into groups and selected considering the following variables: age, sex, type of leukemia, time of diagnosis, and type and duration of treatment. Thus, the cohorts analyzed at single-point samples were: (1) High, samples from Imatinib-treated patients, with BCR::ABL1 > 0.1% (n = 30); (2) ND, samples from Imatinib-treated patients, with BCR::ABL1 < 0.1% or undetectable (n = 20); (3) Healthy Control (HC) samples from healthy blood donors (n = 20); and a fourth group of serial samples; and (4) Follow-up cohort: samples from 11 patients that contained several collection points, at different points in the treatment, to evaluate the evolution of the disease through the quantification of BCR::ABL1 by RT-qPCR.
4.2. Buffy Coat RNA Extraction
After plasma separation by centrifugation, the buffy coat was subjected to total RNA extraction using the QIAamp® RNA Blood Mini Kit (Qiagen, Hilden, Germany). The extracted RNA was quantified in NanoDrop™ One (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −80 °C until use.
4.3. Quantification of BCR::ABL1
Total RNA extracted from the buffy coat was analyzed by RT-qPCR according to the protocol described by Marin et al., 2023 [40].
4.4. Synthesis of Endogenous cDNA from Buffy Coat Samples
After extracting the RNA from the buffy coat, this RNA was used to quantify the BCR::ABL1 transcript, as described in Marin et al., 2023, and for the quantification of microRNAs. The endogenous miUSB-U6 was used to quantify microRNAs, and cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), following the manufacturer’s instructions.
4.5. Extraction of Plasma RNA
Before starting the extraction, 1 µL of cel-miR-39-3p template at 1 nM (Spike-in-control) was added to each 100 µL of plasma. Then the total RNA was extracted using the MagmaxTM mirVanaTM Total RNA Isolation Kit (Thermo Fisher Scientific), following the instructions for use for RNA isolation from serum and plasma samples.
4.6. cDNA Synthesis of Free MicroRNAs in Plasma
For reverse transcription, the TaqManTM Advanced miRNA cDNA Synthesis kit (Thermo Fisher Scientific) was used, following the manufacturer’s instructions.
4.7. Real-Time PCR of microRNAs
For the relative quantification of miRNAs, assays were used with TaqMan probes specific for each microRNA (Thermo Fisher Scientific) and the TaqMan™ Fast Advanced Master Mix (Thermo Fisher Scientific). The PCR reaction was carried out on a QuantStudio 5TM real-time PCR platform (Thermo Fisher Scientific) using the exogenous microRNA cel-miR-39-3p as a normalizer. The qPCRs were performed following the manufacturer’s protocol, in duplicate for each sample and always using a negative control (the no template control, NTC).
4.8. Statistical Analyses
The data were calculated using the 2−ΔΔCT methodology [41] where the results obtained for single-point analyses were performed using the Graphpad Prism 7 software. Expression data was normalized with the median of the Ct values of endogenous control miUSB-U6 and the exogenous control cell- miR-39-3p.
5. Conclusions
35 microRNAs were evaluated, namely: miR-7-5p, miR-17-5p, miR-19b-3p, miR-20a-5p, miR-21-5p, miR-23a-3p, miR-25-3p, miR-27b-3p, miR-29a-3p, miR-29b-3p, miR-92a-1-5p, miR-93-5p, miR-103a-3p, miR-106b-5p, miR-122-5p, miR-125b-5p, miR-130a-3p, miR-130a-5p, miR-142-5p, miR-148b-3p, miR-150-5p, miR-155-5p, miR-186-5p, miR-192-5p, miR-193b-3p, miR-199a-3p, miR-205-5p, miR-214-5p, miR-221-3p, miR-221-5p, miR-331-5p, miR-361-5p, miR-451, miR-486-5p, and miR-494-3p. This work showed that the majority of microRNAS analyzed do not have a relationship with chronic myeloid leukemia. However, it was also observed that microRNA 7-5p may have a relationship with chronic myeloid leukemia and the BCR::ABL1 transcript in different ways.
Acknowledgments
We would like to thank Ana Claudia Martins Braga Gomes Torres, and Paula Barroso Litaiff.
Author Contributions
Conceptualization, M.N.A. and D.L.Z.; methodology, A.M.M. and D.K.W.; validation, D.K.W., A.M.M., G.M.K., R.N.O. and M.N.A.; data curation, E.C.M., J.S.d.H.F. and M.P.B.; writing—original draft preparation, D.K.W., A.M.M. and D.L.Z.; writing-review and editing, M.N.A. and D.L.Z.; project administration, M.N.A., D.K.W. and A.M.M.; funding acquisition, M.N.A. and D.L.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Erasto Gaertner Hospital Ethics Committee (CAAE 08809419.0.0000.0098 e 53207021.5.0000.0098).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors would like to thank to Fiocruz, Fiotec and Plataforma de Pesquisa Clínica—VPPCB by Inova Produtos (Grant VPPIS-004-FIO-18-58) and Geração de Conhecimento (Grant VPPCB-007-FIO-18-2-113), as well as the Brazilian National Council for Scientific and Technological Development (CNPq) (grant number 445940/2020-4).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang X.T., Dong S.H., Zhang J.Y., Shan B. MicroRNA-577 Promotes the Sensitivity of Chronic Myeloid Leukemia Cells to Imatinib by Targeting NUP160. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7008–7015. doi: 10.26355/eurrev_201908_18741. [DOI] [PubMed] [Google Scholar]
- 2.Luo J., Gao Y., Lin X., Guan X. Systematic Analysis Reveals a Lncrna-Mirna-Mrna Network Associated with Dasatinib Resistance in Chronic Myeloid Leukemia. Ann. Palliat. Med. 2021;10:1727–1738. doi: 10.21037/apm-20-343. [DOI] [PubMed] [Google Scholar]
- 3.Cumbo C., Anelli L., Specchia G., Albano F. Monitoring of Minimal Residual Disease (Mrd) in Chronic Myeloid Leukemia: Recent Advances. Cancer Manag. Res. 2020;12:3175–3189. doi: 10.2147/CMAR.S232752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou T., Medeiros L.J., Hu S. Chronic Myeloid Leukemia: Beyond BCR-ABL1. Curr. Hematol. Malig. Rep. 2018;13:435–445. doi: 10.1007/s11899-018-0474-6. [DOI] [PubMed] [Google Scholar]
- 5.Osman A.E.G., Deininger M.W. Chronic Myeloid Leukemia: Modern Therapies, Current Challenges and Future Directions. Blood Rev. 2021;49:100825. doi: 10.1016/j.blre.2021.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rencelj A., Gvozdenovic N., Cemazar M. MitomiRs: Their Roles in Mitochondria and Importance in Cancer Cell Metabolism. Radiol. Oncol. 2021;55:379–392. doi: 10.2478/raon-2021-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anelli L., Zagaria A., Specchia G., Musto P., Albano F. Dysregulation of Mirna in Leukemia: Exploiting Mirna Expression Profiles as Biomarkers. Int. J. Mol. Sci. 2021;22:7156. doi: 10.3390/ijms22137156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatnagar B., Garzon R. Clinical Applications of MicroRNAs in Acute Myeloid Leukemia: A Mini-Review. Front. Oncol. 2021;11:679022. doi: 10.3389/fonc.2021.679022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace J.A., O’Connell R.M. MicroRNAs and Acute Myeloid Leukemia: Therapeutic Implications and Emerging Concepts. Blood. 2017;130:1290–1301. doi: 10.1182/blood-2016-10-697698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherr M., Elder A., Battmer K., Barzan D., Bomken S., Ricke-Hoch M., Schröder A., Venturini L., Blair H.J., Vormoor J., et al. Differential Expression of MiR-17~92 Identifies BCL2 as a Therapeutic Target in BCR-ABL-Positive B-Lineage Acute Lymphoblastic Leukemia. Leukemia. 2014;28:554–565. doi: 10.1038/leu.2013.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mian Y.A., Zeleznik-Le N.J. The MiR-17~92 Cluster Contributes to MLL Leukemia through the Repression of MEIS1 Competitor PKNOX1. Leuk. Res. 2016;46:51–60. doi: 10.1016/j.leukres.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi S., Li Z., Chen P., He C., Cao D., Elkahloun A., Lu J., Pelloso L.A., Wunderlich M., Huang H., et al. Aberrant Overexpression and Function of the MiR-17-92 Cluster in MLL-Rearranged Acute Leukemia. Proc. Natl. Acad. Sci. USA. 2010;107:3710–3715. doi: 10.1073/pnas.0914900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chocholska S., Zarobkiewicz M., Szymańska A., Lehman N., Woś J., Bojarska-Junak A. Prognostic Value of the MiR-17~92 Cluster in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2023;24:1705. doi: 10.3390/ijms24021705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Gao X., Tian J., Zhang R., Qiao Y., Hua X., Shi G. LINC00261 Inhibits Progression of Pancreatic Cancer by Down-Regulating MiR-23a-3p. Arch. Biochem. Biophys. 2020;689:108469. doi: 10.1016/j.abb.2020.108469. [DOI] [PubMed] [Google Scholar]
- 15.Quan J., Pan X., Li Y., Hu Y., Tao L., Li Z., Zhao L., Wang J., Li H., Lai Y., et al. MiR-23a-3p Acts as an Oncogene and Potential Prognostic Biomarker by Targeting PNRC2 in RCC. Biomed. Pharmacother. 2019;110:656–666. doi: 10.1016/j.biopha.2018.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y., Kim S.J., Choo J., Heo G., Yoo J.-W., Jung Y., Rhee S.H., Im E. MiR-23a-3p Is a Key Regulator of IL-17C-Induced Tumor Angiogenesis in Colorectal Cancer. Cells. 2020;9:1363. doi: 10.3390/cells9061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang M., Xiao R., Wang X., Xiong Y., Duan Z., Li D., Kan Q. MiR-93-5p Regulates Tumorigenesis and Tumor Immunity by Targeting PD-L1/CCND1 in Breast Cancer. Ann. Transl. Med. 2022;10:203. doi: 10.21037/atm-22-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang Y., Liao X.-H., Yu C.-X., Yao A., Qin H., Li J.-P., Hu P., Li H., Guo W., Gu C.-J., et al. MiR-93-5p Inhibits the EMT of Breast Cancer Cells via Targeting MKL-1 and STAT3. Exp. Cell Res. 2017;357:135–144. doi: 10.1016/j.yexcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Jia J., Zhang X., Zhan D., Li J., Li Z., Li H., Qian J. LncRNA H19 Interacted with MiR-130a-3p and MiR-17-5p to Modify Radio-Resistance and Chemo-Sensitivity of Cardiac Carcinoma Cells. Cancer Med. 2019;8:1604–1618. doi: 10.1002/cam4.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klümper T., Bruckmueller H., Diewock T., Kaehler M., Haenisch S., Pott C., Bruhn O., Cascorbi I. Expression Differences of MiR-142-5p between Treatment-Naïve Chronic Myeloid Leukemia Patients Responding and Non-Responding to Imatinib Therapy Suggest a Link to Oncogenic ABL2, SRI, CKIT and MCL1 Signaling Pathways Critical for Development of Therapy Resistance. Exp. Hematol. Oncol. 2020;9:26. doi: 10.1186/s40164-020-00183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galka-Marciniak P., Kanduła Z., Tire A., Wegorek W., Gwozdz-Bak K., Handschuh L., Giefing M., Lewandowski K., Kozlowski P. Mutations in the MiR-142 Gene Are Not Common in Myeloproliferative Neoplasms. Sci. Rep. 2022;12:10924. doi: 10.1038/s41598-022-15162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Jing Y., Lei L., Peng M., Xiao Q., Ren J., Tao Y., Huang J., Zhang L. [MiR-148b-3p Inhibits the Proliferation and Autophagy of Acute Myeloid Leukemia Cells by Targeting ATG14] Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi. 2021;37:881–890. [PubMed] [Google Scholar]
- 23.Yuan L., Liu Y., Qu Y., Liu L., Li H. Exosomes Derived From MicroRNA-148b-3p-Overexpressing Human Umbilical Cord Mesenchymal Stem Cells Restrain Breast Cancer Progression. Front. Oncol. 2019;9:1076. doi: 10.3389/fonc.2019.01076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Yin J., Hou P., Wu Z., Wang T., Nie Y. Circulating MiR-375 and MiR-199a-3p as Potential Biomarkers for the Diagnosis of Hepatocellular Carcinoma. Tumor Biol. 2015;36:4501–4507. doi: 10.1007/s13277-015-3092-0. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Cui M.-M., Zhu H.-Z., Fu P.-Y., Wang G.-C., Huang L. MiR-199a-3p Overexpression Suppressed Cell Proliferation and Sensitized Chronic Myeloid Leukaemia Cells to Imatinib by Inhibiting MTOR Signalling. Acta Haematol. 2022;145:484–498. doi: 10.1159/000524158. [DOI] [PubMed] [Google Scholar]
- 26.Feng D.D., Zhang H., Zhang P., Zheng Y.S., Zhang X.J., Han B.W., Luo X.Q., Xu L., Zhou H., Qu L.H., et al. Down-Regulated MiR-331-5p and MiR-27a Are Associated with Chemotherapy Resistance and Relapse in Leukaemia. J. Cell Mol. Med. 2011;15:2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Tang S., Le S.-Y., Lu R., Rader J.S., Meyers C., Zheng Z.-M. Aberrant Expression of Oncogenic and Tumor-Suppressive MicroRNAs in Cervical Cancer Is Required for Cancer Cell Growth. PLoS ONE. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei R., Huang G.-L., Zhang M.-Y., Li B.-K., Zhang H.-Z., Shi M., Chen X.-Q., Huang L., Zhou Q.-M., Jia W.-H., et al. Clinical Significance and Prognostic Value of MicroRNA Expression Signatures in Hepatocellular Carcinoma. Clin. Cancer Res. 2013;19:4780–4791. doi: 10.1158/1078-0432.CCR-12-2728. [DOI] [PubMed] [Google Scholar]
- 29.Kristina Gregory N., Hema Sundar M., Drazer M., Maness L., Fred and Pamela Buffett Cancer Center Leland Metheny. Mohan S., Moore J.O., Oehler V., Pratz K., Pusic I., et al. NCCN Guidelines Version 2.2024 Chronic Myeloid Leukemia Continue NCCN Guidelines Panel Disclosures. National Comprehensive Cancer Network; Plymouth Meeting, PA, USA: 2023. [Google Scholar]
- 30.Chakraborty C., Ranjan Sharma A., Patra B.C., Bhattacharya M., Sharma G., Lee S.-S. MicroRNAs Mediated Regulation of MAPK Signaling Pathways in Chronic Myeloid Leukemia. Oncotarget. 2016;7:42683. doi: 10.18632/oncotarget.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X., Lin Z., Du J., Zhou X., Yang L., Liu G. Studies on MicroRNAs That Are Correlated with the Cancer Stem Cells in Chronic Myeloid Leukemia. Mol. Cell Biochem. 2014;390:75–84. doi: 10.1007/s11010-013-1958-2. [DOI] [PubMed] [Google Scholar]
- 32.Li G., Gao L., Zhao J., Liu D., Li H., Hu M. LncRNA ANRIL/MiR-7-5p/TCF4 Axis Contributes to the Progression of T Cell Acute Lymphoblastic Leukemia. Cancer Cell Int. 2020;20:335. doi: 10.1186/s12935-020-01376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales-Martinez M., Vega G.G., Neri N., Nambo M.J., Alvarado I., Cuadra I., Duran-Padilla M.A., Huerta-Yepez S., Vega M.I. MicroRNA-7 Regulates Migration and Chemoresistance in Non-Hodgkin Lymphoma Cells Through Regulation of KLF4 and YY1. Front. Oncol. 2020;10:588893. doi: 10.3389/fonc.2020.588893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye F. MicroRNA Expression and Activity in T-Cell Acute Lymphoblastic Leukemia. Oncotarget. 2018;9:5445–5458. doi: 10.18632/oncotarget.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Huang J., Peng J., Wu X., Zhang Y., Zhu W., Guo L. Upregulation of the Inwardly Rectifying Potassium Channel Kir2.1 (KCNJ2) Modulates Multidrug Resistance of Small-Cell Lung Cancer under the Regulation of MiR-7 and the Ras/MAPK Pathway. Mol. Cancer. 2015;14:59. doi: 10.1186/s12943-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X., Li J., Sun Y., Zhang Y., Dong L., Shen C., Yang L., Yang M., Li Y., Shen G., et al. MiR-7 Reverses the Resistance to BRAFi in Melanoma by Targeting EGFR/IGF-1R/CRAF and Inhibiting the MAPK and PI3K/AKT Signaling Pathways. Oncotarget. 2016;7:53558–53570. doi: 10.18632/oncotarget.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo H., Liang H., Chen Y., Chen S., Xu Y., Xu L., Liu J., Zhou K., Peng J., Guo G., et al. MiR-7-5p Overexpression Suppresses Cell Proliferation and Promotes Apoptosis through Inhibiting the Ability of DNA Damage Repair of PARP-1 and BRCA1 in TK6 Cells Exposed to Hydroquinone. Chem. Biol. Interact. 2018;283:84–90. doi: 10.1016/j.cbi.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Cheng J., Guo S., Chen S., Mastriano S.J., Liu C., D’Alessio A.C., Hysolli E., Guo Y., Yao H., Megyola C.M., et al. An Extensive Network of TET2-Targeting MicroRNAs Regulates Malignant Hematopoiesis. Cell Rep. 2013;5:471–481. doi: 10.1016/j.celrep.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang M., Dai J., Gu D., Huang Q., Tian L. MicroRNA-7 Inhibits Cell Proliferation of Chronic Myeloid Leukemia and Sensitizes It to Imatinib In Vitro. Biochem. Biophys. Res. Commun. 2017;494:372–378. doi: 10.1016/j.bbrc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Marin A.M., Wosniaki D.K., Sanchuki H.B.S., Munhoz E.C., Nardin J.M., Soares G.S., Espinace D.C., Farias J.S.d.H., Veroneze B., Becker L.F., et al. Molecular BCR::ABL1 Quantification and ABL1 Mutation Detection as Essential Tools for the Clinical Management of Chronic Myeloid Leukemia Patients: Results from a Brazilian Single-Center Study. Int. J. Mol. Sci. 2023;24:10118. doi: 10.3390/ijms241210118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.