Abstract
Pit1 is the human receptor for gibbon ape leukemia virus (GALV) and feline leukemia virus subgroup B (FeLV-B), while the related human protein Pit2 is a receptor for amphotropic murine leukemia virus (A-MuLV). The A-MuLV-related isolate 10A1 can utilize both Pit1 and Pit2 as receptors. A stretch of amino acids named region A was identified in Pit1 (residues 550 to 558 in loop 4) as critical for GALV and FeLV-B receptor function. We have here investigated the role of region A in A-MuLV and 10A1 entry. Insertion of a single amino acid in region A of mouse Pit1 resulted in a functional A-MuLV receptor, showing that region A plays a role in A-MuLV infection. Moreover, the downregulation of 10A1 receptor function by changes in region A of human Pit1 indicates that this region is also involved in 10A1 entry. Therefore, region A seems to play a role in infection by all viruses utilizing Pit1 and/or Pit2 as receptors.
Retroviruses are dependent on specific cell surface receptors for infection. Recently, two related proteins were identified as receptors for different C-type viruses. Pit1 (formerly GLVR1) was cloned as the human receptor for gibbon ape leukemia virus (GALV) (16) and is also a receptor for feline leukemia virus subgroup B (FeLV-B) (24). In addition, a number of Pit1 homologs from other species have been cloned (9, 10, 28, 29); however, not all of these support GALV and FeLV-B infection, e.g., that from mice (Mus musculus musculus) (MusPit1, formerly Glvr1) (9). A related protein, Pit2, was cloned as receptor for the amphotropic murine leukemia virus (A-MuLV) from rats (RatPit2, formerly Ram-1), humans (Pit2, formerly GLVR2), and hamsters (HaPit2, formerly EAR) (15, 26, 29). Miller and Miller demonstrated that Pit1, MusPit1, Pit2, and RatPit2 proteins are receptors for the A-MuLV-related isolate 10A1 (14). Furthermore, by interference studies, 10A1 was shown also to utilize Pit1 and Pit2 from hamsters for entry (29).
The cellular function of both Pit1 and Pit2 is sodium-dependent phosphate transport (11, 17, 30), and the human forms of these proteins have about 62% amino acid identity (26). Both proteins are predicted to have 10 transmembrane domains, five extracellular loops, and a large intracellular hydrophilic domain between the sixth and seventh transmembrane domains (9, 26). This topological model is supported by recent results obtained on Pit2 topology by Chien and colleagues (5).
MusPit1 and human Pit1 differ in their amino acid sequences at only 64 positions, and in the putative extracellular loops, divergent residues are found only in loops 2 and 4 (9). As mentioned above, unlike Pit1, MusPit1 does not allow infection by GALV or FeLV-B and chimeras between human and mouse Pit1 revealed a stretch of nine residues named region A (Pit1 positions 550 to 558 in loop 4) as critical for GALV and FeLV-B infection (10, 23). Moreover, only chimeras between Pit1 and the less related Pit2 and RatPit2 proteins which harbored Pit1 region A were permissive for GALV and FeLV-B entry, confirming the critical role of region A in receptor function for these viruses (14, 21). FeLV-B was, however, found also to be dependent on other Pit1 sequences in addition to region A for infection (21). Recently, Chaudry and Eiden have obtained results indicating that receptor regions in addition to region A are also important for GALV entry (4).
Little is known about what specifies A-MuLV receptor function. Pit1 is not an efficient A-MuLV receptor (6, 14, 21), and both the N-terminal two-thirds (comprising loops 1, 2, and 3 and the intracellular domain) and the C-terminal third (comprising loops 4 and 5) of the human and rat Pit2 proteins conferred A-MuLV receptor function on Pit1-Pit2 hybrids (6, 14, 21). However, substitution of the human and rat Pit2 region A sequence alone for the corresponding Pit1 sequence also conferred A-MuLV receptor function on Pit1 (14, 21). At present, the receptor requirements of the A-MuLV-related isolate 10A1 have not been studied.
To further examine the role of region A in A-MuLV receptor function, we tested whether region A mutants generated from Pit1 or MusPit1 would allow infection by A-MuLV. The same mutants were also assayed for 10A1 receptor function.
Expression plasmids encoding the MusPit1 and Pit1 region A mutants shown in Table 1 were tested for their ability to support A-MuLV and 10A1 infection in CHO K1 cells. The construction of the plasmids has been described elsewhere (10). Expression plasmids encoding wild-type MusPit1 (pOJ19) (10), Pit1 (pOJ75), and Pit2 (pOJ74) (21) were included as controls. The constructs were tested for A-MuLV and 10A1 receptor function by a transient transfection-infection assay essentially as described previously (21). Briefly, CHO K1 cells (ATCC CCL-61) were seeded at 4 × 104 cells per 60-mm-diameter dish and transfected the following day by the calcium phosphate-DNA precipitation method (8). Each precipitate contained 10 μg of a CsCl-purified expression plasmid and 5 μg of CsCl-purified pUC19 plasmid as carrier in 1 ml. From each precipitate, aliquots of 200 μl, corresponding to 2 μg of expression plasmid, were added to two 60-mm-diameter dishes. Forty-eight hours after transfection, dishes transfected with the same construct were selected at random and challenged with A-MuLV or 10A1 pseudotypes in the presence of Polybrene (8 μg/ml). A-MuLV pseudotypes of the β-galactosidase-encoding vector G1BgSvN (12) were obtained from the producer cell line PA317GBN (12, 13). 10A1 pseudotypes of the β-galactosidase-encoding vector LNPOZ (1) were obtained by infecting an NIH 3T3 cell clone harboring the LNPOZ construct with viruses derived from plasmid pRR151, which encodes Moloney Gag-Pol and 10A1 Env proteins (19). The titers of A-MuLV and 10A1 pseudotypes, determined as previously described (21), were 105 and 104 CFU/ml on D17 cells (ATCC CCL-183), respectively. Challenging of transfected cells with 10A1 pseudotypes was performed in the presence of medium conditioned by CHO K1 cells to block the endogenous 10A1 receptors (14). Forty-eight hours after challenge, the cells were fixed in 0.05% glutaraldehyde and assayed for β-galactosidase activity with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as the substrate (25). The plates were examined for stained (blue) cells with a light microscope, and the number of blue cells per dish was counted. The results obtained are shown in Table 1.
TABLE 1.
Permissiveness for infection in cells expressing Pit1, MusPit1, Pit2, or mutantsa
| Plasmid | Region A sequenceb | Permissiveness (%)c for infection with:
|
|
|---|---|---|---|
| A-MuLV | 10A1 | ||
| pOJ74 (Pit2) | KQGGVTQEA | 100 ± 26 | 100 ± 49 |
| pOJ75 (Pit1) | DTGDVSSKV | 0.3 ± 0.1 | 59 ± 27 |
| pOJ34 | –KQEASTKA | 0.02 ± 0.02 | 13 ± 3 |
| pOJ36 | SKVTSGVDD | 0.03 ± 0.02 | 8 ± 2 |
| pOJ71 | –TGDVSSKV | 0.006 ± 0.008 | 7 ± 2 |
| pOJ19 (MusPit1) | –KQEASTKA | 0.6 ± 0.1 | 109 ± 21 |
| pOJ64 | KTQEASTKA | 33 ± 20 | 45 ± 13 |
| pcDNA1ARtkpA | NAd | <0.002e | 7 ± 2 |
CHO K1 cells were transfected with DNA from the indicated plasmids and challenged with A-MuLV or 10A1 as described in the text. Plasmids pOJ34, pOJ36, and pOJ71 were derived from Pit1 and plasmid pOJ64 was derived from MusPit1 by site-directed mutagenesis of region A.
–, gap introduced for alignment.
Data are averages of three independent transfections ± the standard deviations of the actual means. The two different virus pseudotypes were tested with the same three precipitates of a given construct. The number of blue cells per 60-mm-diameter dish transfected with Pit2 was assigned a value of 100% (about 40,000 and 1,400 blue cells for A-MuLV and 10A1, respectively).
NA, not applicable (empty vector).
Based on one blue cell in three 60-mm-diameter dishes.
A role of region A in A-MuLV receptor function.
As previously observed for Pit1 (6, 14, 21), Pit1 (pOJ75) and MusPit1 (pOJ19) did not efficiently support A-MuLV infection. Three expression plasmids encoding Pit1-derived chimeras harboring either mouse Pit1 region A sequence (pOJ34), a scrambled Pit1 region A (pOJ36), or Pit1 region A with a deletion of aspartic acid at position 550 (pOJ71) also did not promote A-MuLV infection. It is, however, not known whether the proteins encoded by pOJ36 and pOJ71 are correctly processed, in that they did not support infection by any of the tested viruses (Table 1) (10). Interestingly, a MusPit1-derived protein with a threonine inserted between lysine at position 553 and glutamine at position 554 (pOJ64) afforded A-MuLV infection at a level about 60-fold greater than that afforded by MusPit1 (pOJ19).
The observation that insertion of a single threonine residue in MusPit1 region A resulted in an efficient A-MuLV receptor shows that region A is involved in A-MuLV receptor function. This result is in agreement with the previous observation that Pit1 harboring Pit2/RatPit2 region A sequence affords A-MuLV infection (14, 21).
Observations by us (21) and others (6, 14) suggest, however, that region A alone does not specify A-MuLV receptor function. As mentioned above, both chimeras harboring the N-terminal two-thirds of Pit1 and the C-terminal third of Pit2 or RatPit2 and the reciprocal chimeras allowed efficient A-MuLV entry (6, 14, 21). Thus, identical region A sequences are present both in Pit1 with no A-MuLV receptor function and in a chimeric A-MuLV receptor. Moreover, comparable chimeras in which the N- or C-terminal parts were derived from hamster Pit2 afforded only very low or no A-MuLV infection, respectively, although HaPit2 is as efficient an A-MuLV receptor as is RatPit2 (6). In summary, these results suggest that A-MuLV receptor function is defined by a combination of both N- and C-terminal receptor domains. Therefore, although region A is a C-terminal determinant of A-MuLV receptor function (14, 21; also this study), the region A sequence requirements for A-MuLV infection might be predicted to differ depending on the protein backbone. Accordingly, no consensus region A sequence can be expected to be revealed by comparing region A sequences from all wild-type and chimeric proteins known to support A-MuLV entry. However, comparison of A regions permissive for A-MuLV infection when present in identical or homologous receptor backbones might be informative.
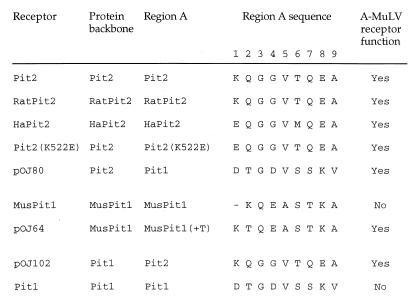
In Fig. 1 is shown a comparison of region A sequences from wild-type Pit1 or Pit2 proteins tested for A-MuLV receptor function and of region A mutant proteins permissive for A-MuLV infection (6, 9, 15, 16, 21, 26, 29; also this study). Alignment of region A sequences permissive for A-MuLV infection in either Pit1 or Pit2 backbones [receptors include Pit2, RatPit2, HaPit2, Pit2(K522E), pOJ64, pOJ80, and pOJ102] reveals that all nine residues can vary without affecting A-MuLV entry; only the differences in positions 5 and 9 are conservative (Fig. 1). However, the region A sequences from different Pit2 homologs and Pit2(K522E), which all support A-MuLV infection in Pit2 backbones, differ only in positions 1 and 6. Moreover, a comparison of the sequences of Pit1 region A and Pit2 region A, which allow similar levels of infection by A-MuLV in a human Pit2 context (pOJ80 and Pit2) (21), shows two conserved amino acids (positions 3 and 5) and four conservative differences (positions 2, 6, 7, and 9). Furthermore, the Pit2/RatPit2 region A and that of pOJ64, which allow A-MuLV infection in Pit1 (pOJ102) and MusPit1 (pOJ64) backbones, respectively, exhibit amino acid identity in positions 1 and 9 and show four conservative differences (positions 2, 5, 6, and 7). Moreover, interestingly, in region A of pOJ64, the presence of the threonine residue, resulting in A-MuLV receptor function, created a TQEA sequence (region A, positions 2 through 5). A similar motif is found in the C terminus of Pit2/RatPit2 region A (region A, positions 6 through 9) (Fig. 1). Whether the TQEA motif or the conserved residues actually play a role in the A-MuLV receptor function dependent on the receptor backbone, however, remains to be investigated.
FIG. 1.
Region A sequence comparison between receptor constructs tested for A-MuLV receptor function. Pit2, the human A-MuLV receptor (26); RatPit2, the rat Pit2 homolog (15); HaPit2, the hamster Pit2 homolog (29); Pit2(K522E), Pit2 with lysine at position 522 replaced with glutamic acid (6); pOJ80, Pit2 with region A replaced by the Pit1 region A (21); MusPit1, the M. musculus musculus Pit1 homolog (9; also this study); pOJ64, MusPit1 with a threonine inserted between lysine (position 553) and glutamine (position 554) (10; also this study); pOJ102, Pit1 with region A replaced by the Pit2 region A (21); Pit1, the human GALV receptor (16). Positions 1 to 9 of region A correspond to positions 522 to 530 in Pit2 homologs, pOJ80, and Pit2(K522E); positions 550 to 558 in Pit1 and pOJ102; and positions 553 to 561 in pOJ64. The dash at position 1 indicates a gap, and positions 2 to 9 correspond to positions 553 to 560 in MusPit1.
Roles of loops 2 and 4 in 10A1 receptor function.
Pit1 (pOJ75), MusPit1 (pOJ19), and Pit2 (pOJ74) were found to support 10A1 infection efficiently (Table 1) in agreement with previous observations (14). The construct pOJ64 derived from MusPit1 by insertion of a threonine in region A also supported 10A1 infection. However, the introduction of MusPit1 region A in Pit1 (pOJ34) reduced 10A1 infection to a level just above background, indicating that region A also can influence 10A1 receptor function. In a wild-type MusPit1 backbone, MusPit1 region A is compatible with 10A1 receptor function. Between MusPit1 and pOJ34, the only differences in the extracellular loops besides those in region A are found in loop 2 [MusPit1/pOJ34 positions 149(N)/145(K) and 152(K)/148(E)] (9, 10). These results indicate that both loops 2 and 4 are involved in 10A1 receptor function and that whether a certain region A sequence is permissive for 10A1 infection is dependent on the sequence present in loop 2 of the protein.
The human Pit1-derived constructs pOJ36, harboring a scrambled region A sequence, and pOJ71, with the aspartic acid in position 550 deleted, did not promote 10A1 entry. These observations are in agreement with a possible role of region A in 10A1 infection; it is, however, not known whether the proteins encoded by pOJ36 and pOJ71 are present on the cell surface, in that they do not support infection by any of the tested viruses (Table 1) (10, 23).
The observation that substitution of Pit1 region A for the corresponding MusPit1 sequence (pOJ34) reduced 10A1 receptor function is interesting given the broad receptor usage of 10A1. On the other hand, 10A1 and A-MuLV show only six amino acid differences in the N-terminal part of the SU protein (18), three of which are found in the variable regions A and B which have been suggested to be directly involved in receptor recognition by C-type viruses (2, 3, 7). It is therefore not unlikely that A-MuLV and 10A1 depend on the same receptor domain(s) for infection. In addition to the C-terminal region A sequences, our results also indicated a role of N-terminal loop 2 sequences in 10A1 entry. Interestingly, as discussed above, A-MuLV infection also seems to depend on N-terminal sequences, and we are currently undertaking studies to assess the possible role of loop 2 in both A-MuLV and 10A1 entry.
Region A sequences are critical for both GALV and FeLV-B infection (6, 10, 14, 21–23); e.g., none of the region A mutants in Table 1 tested for GALV or FeLV-B receptor function supported entry by these viruses (10, 23). However, results obtained with Pit1-Pit2 chimeras revealed that FeLV-B is dependent on other N- or C-terminal Pit1 sequences in addition to region A for entry (21). The presence of Pit1 region A was sufficient to confer GALV receptor function on Pit2 (21). Indeed, a single amino acid change in Pit2 region A resulted in a functional GALV receptor (6, 22). Moreover, the presence of 12 Pit1-specific amino acids, the C-terminal 9 of which comprise region A, were sufficient to confer receptor function for GALV on Pho-4 (20), a sodium-dependent phosphate transporter from Neurospora crassa distantly related to Pit1 and Pit2 (27). However, Chaudry and Eiden recently identified two mutant Pit1 A regions [positions 550(D) and 553(D) mutated to 550(G) and 553(G) and to 550(G) and 553(Q)] which were both permissive for GALV infection when present in a Pit1 backbone; in contrast, when present in a Pit2 backbone, they were not compatible with efficient GALV infection (4). These results indicate that GALV is also dependent on sequences in addition to those in region A for infection. In summary, the requirements for FeLV-B, GALV, A-MuLV, and 10A1 receptor function might be quite similar, all involving region A in addition to at least one other receptor domain. Moreover, we suggest that at least for GALV, A-MuLV, and 10A1, the sequence requirements for A regions compatible with receptor function are dependent on the sequence present in the other not-yet-identified domain(s) in the receptor.
Acknowledgments
We thank Maribeth V. Eiden for the PA317GBN cell line, Alan Rein for the 10A1 virus clone pRR151, and A. Dusty Miller for the pLNPOZ plasmid.
This work was supported by the Karen Elise Jensen Foundation and the Danish Biotechnology Programme.
REFERENCES
- 1.Adam M A, Ramesh N, Miller A D, Osborne W R A. Internal initiation of translation in retroviral vectors carrying picornavirus 5′ nontranslated regions. J Virol. 1991;65:4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudry G J, Eiden M V. Mutational analysis of the proposed gibbon ape leukemia virus binding site in Pit1 suggests that other regions are important for infection. J Virol. 1997;71:8078–8081. doi: 10.1128/jvi.71.10.8078-8081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien M-L, Foster J L, Douglas J L, Garcia J V. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J Virol. 1997;71:4564–4570. doi: 10.1128/jvi.71.6.4564-4570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eiden M V, Farrell K B, Wilson C A. Substitution of a single amino acid residue is sufficient to allow the human amphotropic murine leukemia virus receptor to also function as a gibbon ape leukemia virus receptor. J Virol. 1996;70:1080–1085. doi: 10.1128/jvi.70.2.1080-1085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 8.Gorman C. High efficiency gene transfer into mammalian cells. In: Glover D M, editor. DNA cloning. II. A practical approach. Oxford, United Kingdom: IRL Press; 1985. pp. 143–190. [Google Scholar]
- 9.Johann S V, Gibbons J J, O’Hara B. GLVR1, a receptor for gibbon ape leukemia virus, is homologous to a phosphate permease of Neurospora crassa and is expressed at high levels in the brain and thymus. J Virol. 1992;66:1635–1640. doi: 10.1128/jvi.66.3.1635-1640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johann S V, van Zeijl M, Cekleniak J, O’Hara B. Definition of a domain of GLVR1 which is necessary for infection by gibbon ape leukemia virus and which is highly polymorphic between species. J Virol. 1993;67:6733–6736. doi: 10.1128/jvi.67.11.6733-6736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell surface receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are inducible sodium-dependent phosphate transporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLachlin J R, Mittereder N, Daucher M B, Kadan M, Eglitis M A. Factors affecting retroviral vector function and structural integrity. Virology. 1993;195:1–5. doi: 10.1006/viro.1993.1340. [DOI] [PubMed] [Google Scholar]
- 13.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 14.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hara B, Johann S V, Klinger H P, Blair D G, Rubinson H, Dunn K J, Sass P, Vitek S M, Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990;1:119–127. [PubMed] [Google Scholar]
- 17.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 18.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott D, Keller J, Sill K, Rein A. Phenotypes of murine leukemia virus-induced tumors: influence of 3′ viral coding sequences. J Virol. 1992;66:6107–6116. doi: 10.1128/jvi.66.10.6107-6116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen L, van Zeijl M, Johann S V, O’Hara B. Fungal phosphate transporter serves as a receptor backbone for gibbon ape leukemia virus. J Virol. 1997;71:7619–7622. doi: 10.1128/jvi.71.10.7619-7622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen L, Johann S V, van Zeijl M, Pedersen F S, O’Hara B. Chimeras of receptors for gibbon ape leukemia virus/feline leukemia virus B and amphotropic murine leukemia virus reveal different modes of receptor recognition by retrovirus. J Virol. 1995;69:2401–2405. doi: 10.1128/jvi.69.4.2401-2405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneiderman R D, Farrell K B, Wilson C A, Eiden M V. The Japanese feral mouse Pit1 and Pit2 homologs lack an acidic residue at position 550 but still function as gibbon ape leukemia virus receptors: implications for virus binding motif. J Virol. 1996;70:6982–6986. doi: 10.1128/jvi.70.10.6982-6986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tailor C S, Takeuchi Y, O’Hara B, Johann S V, Weiss R A, Collins M K. Mutation of amino acids within the gibbon ape leukemia virus (GALV) receptor differentially affects feline leukemia virus subgroup B, simian sarcoma-associated virus, and GALV infections. J Virol. 1993;67:6737–6741. doi: 10.1128/jvi.67.11.6737-6741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi Y, Vile R G, Simpson G, O’Hara B, Collins M K, Weiss R A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner D L, Cepko C L. A common progenitor for neurons and glia cells persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 26.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O’Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Versaw W K, Metzenberg R L. Repressible cation-phosphate symporters in Neurospora crassa. Proc Natl Acad Sci USA. 1995;92:3883–3887. doi: 10.1073/pnas.92.9.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson C A, Farrell K B, Eiden M V. Comparison of cDNAs encoding the gibbon ape leukaemia virus receptor from susceptible and non-susceptible murine cells. J Gen Virol. 1994;75:1901–1908. doi: 10.1099/0022-1317-75-8-1901. [DOI] [PubMed] [Google Scholar]
- 29.Wilson C A, Farrell K B, Eiden M V. Properties of a unique form of the murine amphotropic leukemia virus receptor expressed on hamster cells. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson C A, Eiden M V, Anderson W B, Lehel C, Olah Z. The dual-function hamster receptor for amphotropic murine leukemia virus (MuLV), 10A1 MuLV, and gibbon ape leukemia virus is a phosphate symporter. J Virol. 1995;69:534–537. doi: 10.1128/jvi.69.1.534-537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]