Abstract
Background
Bile acid malabsorption (BAM) is characterized by chronic watery diarrhea resulting from excessive bile acids in the feces. BAM is often an overlooked cause of chronic diarrhea, with its prevalence not being sufficiently researched. This review aimed to assess existing literature that explores diverse treatment strategies, to review the published studies that examine the various therapies for BAM patients, emphasizing their influence on clinical results.
Methods
We conducted a comprehensive review of various databases, including PubMed, Scopus, Web of Science, Cochrane Database, and EMBASE. Our criteria for inclusion focused on randomized controlled studies (RCTs) that evaluated the effectiveness of different treatment options for patients with BAM. To rank the treatments, we adopted the frequentist approach through the “netrank” function of the network meta-analysis (NMA). Moreover, we utilized the “netsplit” function in the NMA to separate direct and indirect evidence. Our analysis was carried out using RStudio version 1.4.1717 (2009 - 2021 RStudio, Inc.), and we used the “netmeta” and “meta” packages for NMA.
Results
We found seven relevant articles involving 213 participants, the average age being approximately 50 years, including 53 males and 92 females. Of the drugs examined, tropifexor was proved to be the most effective in raising the fibroblast growth factor 19 (FGF19) levels and reducing the 7 alpha-hydroxy-4-cholesten-3-one (C4) levels, compared to the placebo (mean difference (MD) = 335.30, 95% confidence interval (CI) (334.86, 335.74), MD = -24.60, 95% CI (-25.37, -23.83); respectively). Compared to colesevelam and the placebo, liraglutide was more efficient in decreasing fecal bile acid concentration (liraglutide; MD = -19, 95% CI (-37.61, -0.39)).
Conclusions
Tropifexor has been identified as the most successful medication in mitigating BAM symptoms. To ensure more accurate results, there is a need for randomized controlled clinical trials that involve a larger participant pool.
Keywords: Bile acid malabsorption, Bile acid diarrhea, Colestyramine, Colesevelam, Tropifexor, Loperamide, Liraglutide, Network meta-analysis
Introduction
Bile acid malabsorption (BAM), also known as bile acid diarrhea (BAD), is a disorder in the enterohepatic circulation. This condition leads to an excess of bile acids making their way to the colon, resulting in increased fluid secretion, mucus production, and gut motility [1]. These effects present themselves as symptoms such as diarrhea, bloating, fecal urgency, and loss of bowel control [1]. BAM affects around 1% of the general population and is prevalent in 25% to 50% of patients diagnosed with irritable bowel syndrome with diarrhea (IBS-D) [2].
There are three recognized types of BAM. The first type, initially described by Hofmann et al [3] and Poley et al [4], is caused by the resection of the ileum and results in symptoms like diarrhea and mild steatorrhea. The second type is idiopathic, meaning it lacks an identifiable cause, and there are no discernible histological abnormalities in the ileum. However, recent research has indicated a link between this type and decreased plasma levels of fibroblast growth factor 19 (FGF19), which is known to inhibit bile acid production in the liver. The third type is associated with chronic pancreatitis, celiac disease, and bacterial overgrowth in the small intestine.
The selenium-75-homocholic acid taurine (75SeHCAT) test is one of the most dependable diagnostic methods for identifying BAM [1]. This test has high sensitivity and specificity, ranging between 80-90% and 70-100%, respectively. An alternative diagnostic tool is the 7 alpha-hydroxy-4-cholesten-3-one (C4) test, which indicates BAM when high levels of plasma C4 are detected. For type 1 BAM, this test has a sensitivity of 90% and a specificity of 77%. Type 2 BAM offers a sensitivity of 97% and a specificity of 74%. Furthermore, the evaluation of blood levels of FGF19 is also diagnostically relevant in BAM. This factor has an inverse relationship with C4 and a positive one with 75SeHCAT [4-9].
When the cause of BAM can be identified, therapy should be specifically targeted toward treating the underlying condition. In cases where the cause remains unknown, interventions may include a low-fat diet (less than 30 g per day), conventional anti-diarrheal medications, or bile acid sequestrants (BAS) [3]. BAS class of drugs contains three primary components: colestyramine, colestipol, and colesevelam, which are commercially available [10, 11].
While there is a lack of extensive studies on the efficacy of colesevelam, research by Odunsi-Shiyanbade et al in 2010 [10] and Wedlake et al in 2009 [11] suggested it could be effective. It has been observed that patients who failed to respond to colestyramine responded well to colesevelam, with many of them continuing long-term treatment. Given its palatable taste, colestipol may be a more practical and better-tolerated option than colestyramine [12].
Additional research is essential to determine the most effective medication for treating BAM. As such, we conducted a systematic review and network meta-analysis (NMA) to evaluate the efficacy of several drugs, including colestyramine, colesevelam, tropifexor, loperamide, and liraglutide, in managing BAM.
Materials and Methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, as well as the Cochrane Handbook for Systematic Reviews, in conducting our research [13, 14]. The study protocol was registered on the Open Science Framework (OSF) (registration digital object identifier (DOI): 10.17605/OSF.IO/4FRAC).
Institutional Review Board (IRB) approval
The NMA research involves synthesizing and comparing data from multiple previously published studies rather than collecting primary, new data from human participants. Given that it is a secondary analysis of existing public data, and no further data is being collected directly from participants, there is no direct interaction or intervention with human subjects. As a result, IRB approval, which primarily ensures the protection of the rights and welfare of human research participants, is not usually required for NMA.
Human research ethics
Our meta-analysis research study solely involved collecting and analyzing data from previously published studies and did not entail any direct experimentation on humans. Consequently, there was no necessity for human research ethics approval.
Search term
PubMed, Scopus, Web of Science, Cochrane, and EMBASE were searched through March, 2023, using the following search strategy without any restrictions: (colestyramine OR resin OR colestyramin OR MK-135 OR Questran OR cuemid OR quantalan OR Cholybar OR Olestyr OR Locholest OR Prevalite OR Novo-cholamine OR colestipol OR U-26597 OR Colestid OR Cholestabyl OR colesevelam OR CholestaGel OR Welchol OR GT-31104 OR Lodalis OR loperamide OR R-18553 OR Imodium OR Maalox OR Imotil OR Diamode OR codeine OR methylmorphine OR isocodeine OR Ardinex OR aluminium hydroxide OR Alumina OR Alugel OR Algeldrate OR Rocgel OR Brasivil OR Dialume OR Nephrox OR Pepsamer OR Alhydrogel OR Amphojel OR Alumanetriol OR Basalgel OR Aldrox OR Amphojel OR Alu-Cap OR Dialume OR Alu OR Alternagel OR Aloh OR liraglutide OR Victoza OR Saxenda OR NN-2211) AND (bile acid-induced diarrhea OR cholerheic OR choleretic OR bile salt diarrhea OR bile salt malabsorption OR bile acid malabsorption OR BAM).
Eligibility criteria
We exclusively focused on English-written randomized controlled trials (RCTs) that assessed the effectiveness of colestyramine, colesevelam, tropifexor, loperamide, liraglutide, or their combinations for treating BAM or BAD. We did not consider animal studies, cohort and observational studies, literature reviews, meta-analyses, book chapters, theses, editorials, letters, or publications in other languages. Two separate reviewers conducted the selection process, evaluating the studies’ appropriateness across three screening phases using an Excel spreadsheet. A third reviewer intervened in cases of discrepancies between the first two.
Risk of bias assessment
We utilized the Cochrane tool version 2 for both parallel and cross-over RCTs. This tool covers six domains: randomization process, divergence from planned interventions, missing outcome data, outcome measurement, selection of reported results, and overall bias. Notably, the risk of bias due to period and carryover effects is a domain unique to the cross-over tool. Each domain was classified as yes, probably yes, no, or no information. Any disagreements were subsequently discussed and resolved. Due to the limited number of studies included, we were unable to conduct an Egger’s test for publication bias [15].
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) RCT reporting the evaluation of the efficacy of several drugs, including colestyramine, colesevelam, tropifexor, loperamide, and liraglutide, in managing BAM; 2) publications reporting sufficient data to establish statistical analysis; and 3) studies published as original articles. The exclusion criteria were as follows: 1) Full text not electronically accessible; 2) Publication in a language other than English; 3) Observational studies, comments, letters, editorials, protocols, guidelines, and review papers; and 4) Studies with insufficient outcome data.
Extraction of data
We used an Excel spreadsheet to compile the following data. Initially, we collected fundamental and summary details such as the number of participants in the study, the study’s design, inclusion criteria, the treatment plan prescribed, any pre-existing gastrointestinal conditions, the participants’ gender, age, and body mass index (BMI). Subsequently, we documented effectiveness outcomes, which included daily bowel movement frequency, stool classification according to the Bristol Stool Form Scale (BSFS), FGF19 levels in pg/mL, C4 levels in ng/mL, and the total amount of fecal bile acids in mol/g. Two authors independently performed this data extraction process. Any discrepancies encountered were settled through a collective discussion among the reviewers.
Data synthesis and analysis
To synthesize the data, we calculated the post-treatment means. When the standard deviation was not presented directly, we computed it using algebraic recalculation or other approximation methods. If necessary, data for analysis were not provided, we inferred it from published median values, ranges, or confidence intervals. To assess the heterogeneity among the trials, we used I2 statistics. Where heterogeneity was observed, we utilized a random-effects model; otherwise, a fixed-effect model was applied. We employed the frequentist approach for ranking the various treatment options, using the “netrank” function within the network. The NMA “netsplit” function was used to separate direct and indirect evidence. All our analyses were conducted using RStudio version 1.4.1717 (2009- 2 021 RStudio, Inc.), applying the NMA packages “netmeta” and “meta”.
Results
Results of literature search and data collection
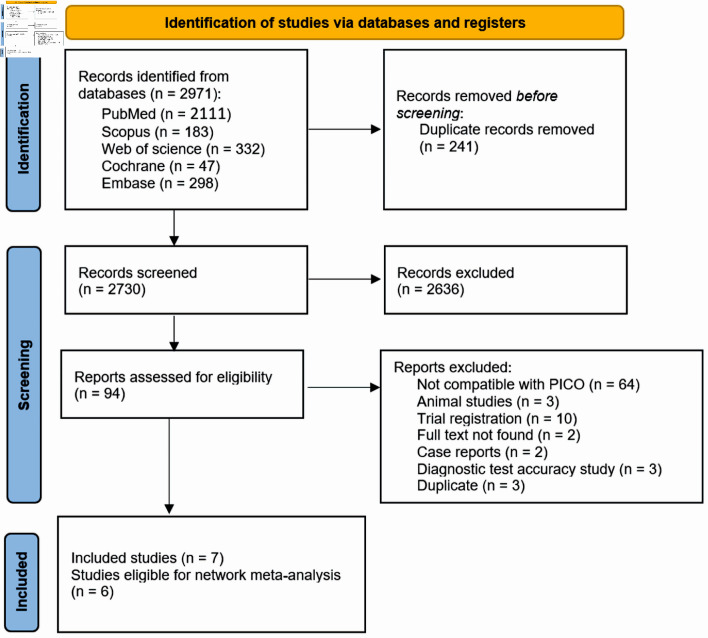
Our search across five databases resulted in 2,730 unique articles. Upon review of titles and abstracts, we discarded 2,636 papers that did not meet our eligibility criteria. After conducting a full-text screening, we further excluded 87 articles. We finally selected seven articles (with six suitable for analysis) that satisfied our inclusion criteria [10, 16-21]. Out of these, five were parallel RCTs, one was a cross-over study with full text available, and one was an abstract [10, 16-21]. Figure 1 presents a detailed breakdown of our search results and the reasons for article exclusion. Table 1 [10, 16-21] presents a summary of the included RCTs. Our research encompassed 213 participants, averaging around 50 years of age, consisting of 53 males and 92 females. The summary and foundational characteristics of the studies are illustrated here (Supplementary Material 1, 2, www.jocmr.org).
Figure 1.
PRISMA flow diagram for literature search. PRISMA: the Preferred Reporting Items for Systematic Reviews and Meta-Analysis; PICO: patient, intervention, comparison, and outcome.
Table 1. Summary of the Included Studies.
| References | Groups | Sample size | Design | Inclusion criteria | Regimen | Underlying GI problem | Findings | Country |
|---|---|---|---|---|---|---|---|---|
| Appleby et al, 2017 [16] | Cholestyramine (1 g/day) | 7 | RCT | Men and women aged 18 - 80 years with a BMI between 18.5 and 35: 1) three stools of BSFS 5 per day on average, calculated from 7 days off therapy; 2) SeHCAT 7-day retention of < 10% (moderate or severe impairment), within the last 5 years with no change in symptoms since; 3) 21 bowel motions in the last 7 days before randomization, of which > 50% are BSFS ≥ 5; 4) macroscopically normal colonoscopy with no histological evidence of microscopic colitis within the last 5 years. | A3384 250 mg bid. or A3384 1 g bid., or placebo for 2 weeks | - | Serum FGF19 was suppressed, and bile acid production upregulated on conventional bile acid sequestrants, but not with A3384. This colonic-release formulation of colestyramine produced symptomatic benefit in patients with BAD. | Sweden |
| Cholestyramine (250 mg/day) | 6 | |||||||
| Placebo | 6 | |||||||
| Beigel et al, 2014 [17] | Colesevelam | 15 | RCT | CD patients with ≥ 3 and ≤ 15 liquid stools/day in clinical remission, defined as CD activity index (CDAI) ≤ 150 points and CRP value ≤ 1 mg/dL were eligible for the study, age ≥ 18 and ≤ 65 years, contraception in women, medication with either oral aminosalicylates, azathioprine, mercaptopurine, methotrexate, oral steroids (10 mg/day), or anti-TNF alpha antibody and cholestenone serum level of ≥ 50 ng/mL, which was centrally assessed at the screening visit. | Patients received either colesevelam (3 × 2 tablets a 625 mg/day) or identically shaped placebo (3 × 2 tablets/ day). | CD | We found significant differences in favor for colesevelam treatment compared to placebo treatment for CD patients with BAM regarding the reduction of the number of liquid stools/day and stool consistency. | Germany |
| Placebo | 11 | |||||||
| Camilleri et al, 2020 [18] | Tropifexor (60 µg/day) - placebo | 10 | Cross over RCT | Patients with primary bile acid diarrhea, who had not used bile acid sequestrants, aged ≥ 18 years weighing ≥ 50 kg, with a history of diarrheal symptoms for at least 3 months prior to dosing, as defined by average stool frequency of ≥ 3 per day when off therapy and average stool form of > 5 on the Bristol Stool Chart; laboratory or radiological confirmation of bile acid malabsorption within the last 5 years as defined by evidence of fecal bile acid loss of ≥ 2,000 µmol/48 h with or 7-day 75SeHCAT retention of < 10%. | Tropifexor 60 µg/placebo or placebo/tropifexor 60 µg. In treatment period 1, patients received either tropifexor 60 µg or placebo orally once daily for 14 days. | - | Tropifexor 60 µg once daily had acceptable safety and tolerability. Changes in FGF19 and C4 showed effective target engagement; however, higher doses may be required to observe stool frequency changes. Slowing of ascending colon emptying suggests therapeutic potential of tropifexor in patients with primary bile acid diarrhea. | USA |
| Placebo - tropifexor (60 µg/day) | 10 | |||||||
| Devarakonda et al, 2019 [19] | Colesevelam | 17 | RCT | - | CD | We have demonstrated that colesevelam is an effective treatment for postoperative BAM in CD. Both colesevelam and cholestyramine were associated with a reduction in stool frequency but only colesevelam was associated with a reduction in CDAI and an improvement in quality of life. | UK | |
| Cholestyramine | 10 | |||||||
| Loperamide | 12 | |||||||
| Colesevelam and loperamide | 5 | |||||||
| Karhus et al, 2022 [20] | Liraglutide | 25 | RCT | White individuals aged 18 - 75 years with SeHCAT verified moderate-to-severe primary bile acid diarrhea (≤ 10% 7-day bile acid retention), with no diabetes, other GI diseases including CD, ileal resection, previous history of radiation to the abdomen or the pelvis, or recent or active malignancy. | Liraglutide (one daily subcutaneous injection up-titrated from 0.6 mg to 1.8 mg over 3 weeks) or colesevelam (three capsules of 625 mg twice daily) and the opposite dummy for 6 weeks. | IBS-D | The superiority of liraglutide compared with colesevelam in reducing stool frequency suggests consideration of liraglutide as a potential new treatment modality for bile acid diarrhea, although larger confirmatory trials powered for superiority are warranted | Denmark |
| Colesevelam | 25 | |||||||
| Odunsi-Shiyanbade et al, 2010 [10] | Colesevelam | 12 | RCT | All participants had fasting plasma 7alpha-C4 (C4) levels measured to assess for underlying BAM and had serum FGF19 levels measured. Participants also completed the following questionnaires: Hospital Anxiety and Depression Scale,16 Symptom Checklist 90 (SCL-90) (somatization), and bowel function by validated daily diaries including BSFS scores. | Colesevelam HCl (625-mg tablets) and the placebo (312.5-mg capsules) | IBS-D | Sodium chenodeoxycholate in health and colesevelam in IBS-D patients have opposite effects on colonic transit and fecal parameters. | USA |
| Placebo | 12 | |||||||
| Vijayvargiya et al, 2020 [21] | Colesevelam | 15 | RCT | Females and males aged 18 - 75. An IBS diagnosis based on the Rome III criteria for at least 3 months, with onset at least 6 months previously, of recurrent abdominal pain or discomfort. Biomarkers serum alpha C4 ≥ 40 ng/mL or FGF19 ≤ 80 pg/mL or fecal bile acid > 2,000 µmol/48 h | Colesevelam, 1,875mg (three tablets, 625 mg each), or matching placebo (ratio 1:1), taken orally, twice daily. | IBS-D | In a randomized trial, we found that colesevelam increases delivery of total and secondary BAs to stool, hepatic BA synthesis, and colonic mucosal expression of genes that regulate BA, farnesoid X, and GPBAR1 receptors. Larger studies are needed to determine the effects on clinical responses. | USA |
| Placebo | 15 |
RCT: randomized controlled study; BMI: body mass index; GI: gastrointestinal; CD: Crohn’s disease; BSFS: Bristol Stool Form Scale; 75SeHCAT: selenium-75-homocholic acid taurine; bid: twice daily; BAD: bile acid diarrhea; CDAI: Crohn’s disease activity index; BAM: bile acid malabsorption; IBS-D: irritable bowel syndrome with diarrhea; FGF19: fibroblast growth factor 19; BA: bile acid.
Results of risk of bias assessment
All studies, except those by Odunsi-Shiyanbade et al [10] (2010) and Camilleri et al [18] (2020), were evaluated as low risk in the randomization process due to the absence of adequate information about allocation concealment, randomization, and baseline balance. As for intended interventions, all the included studies apart from the study of Camilleri et al [18] (2020) were deemed as having a minimal risk of bias in terms of deviation from planned treatments. All incorporated articles were assessed as low risk concerning missing outcome data, outcome measurement, and selection of reported results. In terms of overall domains, all studies were judged to have a low risk, except for the study of Odunsi-Shiyanbade et al [10] (2010), which was deemed to have some concerns, and Camilleri et al [18] (2020), which was evaluated as high risk (Fig. 2).
Figure 2.
Risk of bias assessment for included studies.
Frequency of stool per day
This analysis revealed no significant difference in the ability of the investigated drugs, when compared to a placebo, to reduce daily stool frequency in patients with BAM (colestyramine; mean difference (MD) = -11.85, 95% confidence internal (CI) (-25.94, 2.25), liraglutide; MD = -1.01, 95% CI (-3.11, 1.09), colesevelam and loperamide; MD = -2.71, 95% CI (-37.95, 32.52), loperamide; MD = -0.01, 95% CI (-21.88, 21.85) and colesevelam; MD = -0.21, 95% CI (-1.17, 0.74)) (Fig. 3).
Figure 3.
The forest network plot for the analysis of the frequency of stool per day outcome. The study demonstrated no substantial difference between the examined drugs and placebo in terms of their efficacy in decreasing daily stool frequency. MD: mean difference; CI: confidence interval.
FGF19 (pg/mL)
As illustrated in Figure 4, tropifexor emerged as the most effective medication in increasing FGF19 levels compared to a placebo, among the drugs included in our study (MD = 335.30, 95% CI (334.86, 335.74)). Conversely, colesevelam (MD = -78.07, 95% CI (-119.13, -37.01)) and colestyramine (1 g/day) (MD = -51.70, 95% CI (-86.14, -17.26)) decreased FGF19 levels. The other medicines, liraglutide (MD = -21.41, 95% CI (-86.37, 43.55)) and colestyramine (250 mg/day) (MD = 85.60, 95% CI (-58.36, 229.56)), demonstrated inconclusive results.
Figure 4.
The forest network plot for the analysis of the FGF19 (pg/mL) outcome. The chart illustrates that tropifexor was the most potent drug in elevating FGF19 levels, outperforming the placebo and other drugs in our study. MD: mean difference; CI: confidence interval; FGF19: fibroblast growth factor 19.
C4 (ng/mL)
As shown in Figure 5, tropifexor emerged as the most potent medication in decreasing the C4 levels compared to the placebo among the drugs included in our study (MD = -24.60, 95% CI (-25.37, -23.83)). In contrast, colesevelam (MD = 102.84, 95% CI (52.52, -153.16)) and colestyramine (1 g/day) (MD = 39.45, 95% CI (8.95, 69.95)) caused an increase in C4 levels. Other medications like liraglutide (MD = -4.83, 95% CI (-92, 82.34)) and colestyramine (250 mg/day) (MD = 11.65, 95% CI (-22.27, 45.57)) did not present significant results.
Figure 5.
The network forest plot that analyses the C4 (ng/mL) outcome. This visualization demonstrates that tropifexor stands out as the most effective drug in reducing C4 levels when compared to placebo among the assessed medications. MD: mean difference; CI: confidence interval.
BSFS
There was not a significant difference between the drugs included and the placebo in decreasing the BSFS in patients with BAM. Specifically, colesevelam had a MD of -0.61, with a 95% CI ranging from -1.22 to 0.00. Similarly, liraglutide had an MD of -0.35, with a 95% CI between -1.65 and 0.95 (Fig. 6).
Figure 6.
The network forest plot that analyses the Bristol Stool Form Scale (BSFS) outcome. The plot reveals no substantial difference between the tested drugs and the placebo when it comes to reducing the BSFS score in patients diagnosed with BAM. MD: mean difference; CI: confidence interval; BAM: bile acid malabsorption.
Fecal total bile acids (µmol/g)
The findings from our study indicate that liraglutide was more effective than colesevelam in reducing the concentration of fecal bile acids when compared to the placebo. Specifically, liraglutide had a MD of -19, with a 95% CI ranging from -37.61 to -0.39. Conversely, colesevelam had an MD of -9.53, with a 95% CI between -26.11 and 7.05 (Fig. 7).
Figure 7.
The forest network plot for the analysis of total fecal bile acids (µmol/g) outcome. The plot demonstrates that liraglutide was more effective than colesevelam in diminishing fecal bile acid concentrations when compared with a placebo. MD: mean difference; CI: confidence interval.
Table 1 [10, 16-21] presents the characteristics of the studies included in our network meta-analysis, while Table 2 details the mechanisms of action for different treatments of BAM, including colestyramine, colesevelam, tropifexor, loperamide, and liraglutide.
Table 2. Mechanism of Action of Colestyramine, Colesevelam, Tropifexor, Loperamide, and Liraglutide for Treatment of Bile Acid Malabsorption.
| Drug | Mechanism of action |
|---|---|
| Colestyramine | Cholestyramine resin adsorbs and combines with the bile acids in the intestine to form an insoluble complex which is excreted in the feces. This results in a partial removal of bile acids from the enterohepatic circulation by preventing their absorption. |
| Colesevelam | Colesevelam hydrochloride is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. |
| Tropifexor | Activation of tropifexor inhibits bile acid synthesis and increases bile acid conjugation, transport, and excretion, thereby protecting the liver from the harmful effects of bile accumulation, leading to considerable interest in tropifexor as a therapeutic target for the treatment of cholestasis and nonalcoholic steatohepatitis. |
| Loperamide | Loperamide, a synthetic opiate agonist, decreases peristaltic activity and inhibits secretion, resulting in the reduction of fluid and electrolyte loss and an increase in stool consistency. |
| Liraglutide | Through liraglutide-induced prolongation of small intestinal transit time allowing a greater degree of passive and active bile acid reabsorption through the small intestine and thereby reducing spillover of bile acids. |
Discussion
This study examines the effectiveness of different treatment options for BAM. Our goal was to investigate and contrast the success of various therapeutic strategies for treating BAM in patients afflicted with inflammatory bowel disease. We specifically assessed their impact on clinical outcomes, focusing on FGF19 and C4 levels, along with fecal bile acid concentration.
Our NMA indicated that tropifexor outperformed other drugs in terms of increasing FGF19 levels and reducing C4 levels. Liraglutide emerged as the most effective drug in reducing bile concentration in stools. On the other hand, colesevelam and colestyramine, which bind to and isolate bile acid in the feces, were observed to increase C4 levels while simultaneously reducing FGF19 levels, thus elevating the total fecal bile acid.
Liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, was more effective in decreasing fecal bile acid concentration than colesevelam and placebo. This finding further establishes the role of liraglutide in gut motility and the secretion of gastrointestinal hormones, which can influence bile acid reabsorption, facilitating passive bile acid reabsorption and consequently decreasing the volume delivered to the colon [21, 22]. Bile acid triggers the activation of the Farnesoid X receptor (FXR) during reabsorption, which escalates the release of FGF19 and curbs bile acid secretion [22]. This sequence of events contributes to the reduction of fecal bile acid levels by diminishing motility and enhancing absorption. FXR agonists, both directly and indirectly, are crucial during bile acid biosynthesis via FGF19 [23]. Bile acid stimulates the FXR receptor, which is found in various organs including the liver, intestines, and kidneys [24]. FXR agonists include tropifexor, obeticholic acid, and chenodeoxycholic acid (CDCA).
Tropifexor, a non-bile acid FXR agonist, demonstrated the most substantial impact on raising FGF19 levels and reducing C4 levels compared to placebo, making it a promising option for managing BAM. Our results echo existing literature supporting the role of FGF19 in regulating bile acid synthesis and suggesting that increasing FGF19 levels could be beneficial in the management of BAM. Furthermore, our findings align with other studies reporting that an elevated C4 level, a marker of bile acid synthesis, is related to BAM, therefore, its reduction is a desired outcome. Research by Pellicciari et al [25] in 2004 suggested that obeticholic acid is more potent than chenodeoxycholic acid (CDCA) [25]. Moreover, two studies confirmed the effectiveness of obeticholic acid in BAM treatment [26, 27].
In the study by Appleby et al [16] in 2017, colestyramine was found to decrease FGF19 levels; however, it showed no impact on the release of colonic pellets in BAM patients. The reduction in FGF19 levels was 28% in BAM patients, compared to 58% in healthy participants. Nonetheless, due to the premature cessation of enrollment, the study suffered from reduced statistical power [16]. Another study conducted by Odunsi-Shiyanbade et al [10] in 2010 found no significant difference between colesevelam and placebo. Beigel et al [17] in 2014 observed that colesevelam significantly reduced stool frequency, improved stool consistency, and served as an alternative for patients with Crohn’s disease who did not respond to cholestyramine. However, the study fell short in terms of sample size, requiring 46 patients but analyzing only 26.
The 2020 study by Camilleri et al [18] also had similar statistical power limitations due to the early halt in recruitment. Nevertheless, they concluded that tropifexor demonstrated satisfactory safety and tolerability in primary BAM patients. While in a 2022 study by Karhus et al [20], colesevelam was found to reduce bowel frequency by 25% in patients with moderate-to-severe BAM, while liraglutide reduced stool frequency by 27% and absorbed bile acids and C4 more efficiently than colesevelam.
Existing research has demonstrated the effectiveness of colestyramine and colestipol in treating patients with BAM [12, 28]. Patients tend to tolerate colestipol better due to its more appealing taste. Colesevelam presents a viable alternative for those unresponsive to colestyramine [10-12]. However, it should be noted that it can limit the bioavailability of several drugs, including warfarin, digoxin, diuretics, beta-blockers, and fat-soluble vitamins. Therefore, these drugs should be consumed 1 h before ingesting BAS [29-30].
Additionally, regular monitoring for deficiencies in fat-soluble vitamins is recommended, especially for pregnant or breastfeeding women. Vitamin supplementation may be necessary, with careful scheduling to ensure appropriate intervals between doses. Dietary modifications may also significantly contribute to the alleviation of BAM symptoms [19, 31, 32].
Given the results of our NMA, clinicians should be aware of the potential therapeutic advantages of tropifexor in managing BAM, especially in patients where conventional treatments may have been suboptimal. The noted efficacy of tropifexor in elevating FGF19 levels and reducing C4 levels suggests its potential as a front-line therapy for BAM, particularly in patients with a more pronounced imbalance of these markers. Furthermore, the superior efficiency of liraglutide in decreasing fecal bile acid concentration compared to colesevelam and placebo sheds light on an alternative therapeutic avenue for clinicians to explore [19].
In terms of future research, it would be valuable to delve into the mechanism of action of these drugs at a molecular level to understand their effects more comprehensively. Additionally, observational studies could be beneficial in assessing long-term outcomes and potential side effects of these treatments in real-world settings. Lastly, investigating patient subgroups based on the severity of BAM or associated comorbidities might provide more tailored therapeutic recommendations for individualized patient care.
While our findings contribute meaningful insights into the management of BAM, they should be viewed tentatively due to several limitations. Firstly, the relatively small sample size, resulting from the limited availability of eligible studies, hinders the robustness of our results. The varied patient profiles included in our study may also affect the generalizability of the findings. In addition, our study primarily concentrated on the immediate impact of the interventions on clinical outcomes. However, future research should consider patients’ quality of life, given that symptoms like chronic diarrhea can considerably affect daily activities. It is also crucial to investigate these treatments’ potential long-term side effects, a perspective our study did not explore. In meta-analysis studies, subgroup analysis is a valuable method to explore potential sources of heterogeneity and to gain insights into specific subpopulations. However, when fewer than 10 RCTs are available, conducting a subgroup analysis can be challenging and potentially misleading. With a limited number of studies, there is a heightened risk of type I errors, where false-positive results can be identified. Moreover, with such a small sample size, the power to detect genuine subgroup effects is diminished, leading to potential type II errors. Additionally, the sparseness of data might lead to imprecise and unstable estimates, undermining the reliability and validity of the subgroup findings. Therefore, in the context of meta-analyses with fewer than 10 RCTs, it is generally advisable to approach or forgo subgroup analyses with caution.
Above all, the necessity for larger, high-quality RCTs is emphasized by our study. To secure a more solid evidence base for BAM management, it is essential that future research engage more participants in RCTs.
In sum, while our study illuminates possible treatment strategies for BAM, it also highlights the urgent need for further, more extensive research in this field. Considering the impact of BAM on the quality of life of patients with inflammatory bowel disease, the implementation of these interventions should be guided by more robust evidence to ensure the best patient outcomes.
Conclusions
Our study found tropifexor to be the most effective medication in boosting FGF19 levels and reducing C4 levels. Liraglutide emerged as the most potent treatment for reducing fecal bile acid concentrations.
Supplementary Material
Baseline characteristics of included studies.
Continued: baseline characteristics of included studies.
Acknowledgments
None to declare.
Funding Statement
This study required no funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
In our research project, which encompassed a meta-analysis and network meta-analysis, obtaining informed consent was deemed unnecessary. This decision is grounded in the nature of our study, which exclusively involved the analysis of existing published data, rather than direct interaction with human participants or the use of personal, identifiable data. Our methodologies relied solely on publicly available data from previous studies, ensuring that there was no breach of privacy or confidentiality for any individual. Since the data used were aggregated and anonymized, and our research did not entail any intervention or interaction with human subjects, it fell outside the scope of ethical considerations that necessitate informed consent. This approach is consistent with standard practices for secondary data analysis in research, where the primary data collection process has already addressed ethical considerations, including consent.
Author Contributions
Manuscript writing and overview of published works: Nooraldin Merza, Omar Saab, Yusuf Nawras, Roua Abdulhussein, Ahmed Elmoursi, Lena Daddoo, Zinah Yaqoob, Hiba Al Ani, Tamarah Al Hamdany, Ahmed Gadelmawla, Mohamed Khalil, Mona Hassan, and Abdallah Kobeissy. Manuscript preparation and editing: Mona Hassa, Nooraldin Merza, Ahmed Elmoursi, Ahmed Gadelmawla, Mohamed Khalil, Yusuf Nawras, and Abdallah Kobeissy. Final drafting, review, and approval: Nooraldin Merza, Abdallah Kobeissy, and Mona Hassan contributed to drafting the final version of the manuscript, undertaking critical revisions for important intellectual content, and endorsing the final approval of the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
- BAM
bile acid malabsorption
- BAD
bile acid diarrhea
- BAS
bile acid sequestrants
- NMA
network meta-analysis
- IBS-D
irritable bowel syndrome with diarrhea
- 75SeHCAT
selenium-75-homocholic acid taurine
- C4 test
7-alpha-C4 level test
- FGF19
fibroblast growth factor 19
- RCTs
randomized controlled trials
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- OSF
Open Science Framework
- BSFS
Bristol Stool Form Scale
References
- 1.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39(9):923–939. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9(3):332–339. doi: 10.5009/gnl14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281(8):397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62(5):918–934. [PubMed] [Google Scholar]
- 5.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7(11):1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8(2):117–123. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Eusufzai S, Axelson M, Angelin B, Einarsson K. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut. 1993;34(5):698–701. doi: 10.1136/gut.34.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauter GH, Munzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44(1):14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 9.Pattni SS, Brydon WG, Dew T, Walters JR. Fibroblast Growth Factor 19 and 7alpha-Hydroxy-4-Cholesten-3-one in the Diagnosis of Patients With Possible Bile Acid Diarrhea. Clin Transl Gastroenterol. 2012;3(7):e18. doi: 10.1038/ctg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J. et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8(2):159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJ. Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire. Clin Ther. 2009;31(11):2549–2558. doi: 10.1016/j.clinthera.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Smith MJ, Cherian P, Raju GS, Dawson BF, Mahon S, Bardhan KD. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond. 2000;34(5):448–451. [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane handbook for systematic reviews of interventions. 2019. [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appleby RN, Bajor A, Gillberg PG, Graffner H, Simren M, Ung KA, Walters J. Effects of conventional and a novel colonic-release bile acid sequestrant, A3384, on fibroblast growth factor 19 and bile acid metabolism in healthy volunteers and patients with bile acid diarrhoea. United European Gastroenterol J. 2017;5(3):380–388. doi: 10.1177/2050640616662432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, Rust C. et al. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study. J Crohns Colitis. 2014;8(11):1471–1479. doi: 10.1016/j.crohns.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Nord SL, Burton D, Oduyebo I, Zhang Y, Chen J, Im K. et al. Randomised clinical trial: significant biochemical and colonic transit effects of the farnesoid X receptor agonist tropifexor in patients with primary bile acid diarrhoea. Aliment Pharmacol Ther. 2020;52(5):808–820. doi: 10.1111/apt.15967. [DOI] [PubMed] [Google Scholar]
- 19.Devarakonda A, Arnott I, Satsangi J. The efficacy of colesevelam to treat bile acid malabsorption in Crohn's disease: data from TOPPIC trial. J Crohns Colitis. 2019;13(Supplement_1):S470–S471. doi: 10.1093/ecco-jcc/jjy222.824. [DOI] [Google Scholar]
- 20.Karhus ML, Bronden A, Forman JL, Haaber A, Knudsen E, Langholz E, Dragsted LO. et al. Safety and efficacy of liraglutide versus colesevelam for the treatment of bile acid diarrhoea: a randomised, double-blind, active-comparator, non-inferiority clinical trial. Lancet Gastroenterol Hepatol. 2022;7(10):922–931. doi: 10.1016/S2468-1253(22)00198-4. [DOI] [PubMed] [Google Scholar]
- 21.Vijayvargiya P, Camilleri M, Carlson P, Nair A, Nord SL, Ryks M, Rhoten D. et al. Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Clin Gastroenterol Hepatol. 2020;18(13):2962–2970.e2966. doi: 10.1016/j.cgh.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karhus ML, Bronden A, Roder ME, Leotta S, Sonne DP, Knop FK. Remission of Bile Acid Malabsorption Symptoms Following Treatment With the Glucagon-Like Peptide 1 Receptor Agonist Liraglutide. Gastroenterology. 2019;157(2):569–571. doi: 10.1053/j.gastro.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278(1):104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 25.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S. et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47(18):4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 26.Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, Donowitz M. et al. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63(5):808–817. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]
- 27.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41(1):54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 28.Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 29.Scaldaferri F, Pizzoferrato M, Ponziani FR, Gasbarrini G, Gasbarrini A. Use and indications of cholestyramine and bile acid sequestrants. Intern Emerg Med. 2013;8(3):205–210. doi: 10.1007/s11739-011-0653-0. [DOI] [PubMed] [Google Scholar]
- 30.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol. 2010;3(6):349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayvargiya P, Camilleri M. Update on bile acid malabsorption: finally ready for prime time? Curr Gastroenterol Rep. 2018;20(3):10. doi: 10.1007/s11894-018-0615-z. [DOI] [PubMed] [Google Scholar]
- 32.Jackson A, Lalji A, Kabir M, Muls A, Gee C, Vyoral S, Shaw C. et al. The efficacy of a low-fat diet to manage the symptoms of bile acid malabsorption - outcomes in patients previously treated for cancer. Clin Med (Lond) 2017;17(5):412–418. doi: 10.7861/clinmedicine.17-5-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of included studies.
Continued: baseline characteristics of included studies.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.