Abstract
Background:
Formaldehyde (FA) and oxytetracycline (OTC) are the chemicals commonly used in aquaculture to prevent or treat fish diseases due to protozoa, parasites, and bacteria.
Aim:
The goal of the present study is to assess the liver injury and oxidative stress induced by exposure of sea bass (Dicentrarchuslabrax L) to therapeutic doses of FA (200 ml.m-3) and OTC (40 g.m-3) under the same conditions being applied in intensive aquaculture systems in Tunisia.
Methods:
The liver histopathological survey was achieved after 5 and 10 days of exposure to FA, OTC separately or mixed. In parallel, liver catalase activity and malondialdehyde (MDA) were measured to assess oxidative stress.
Results:
Results showed that treatment with FA and OTC used alone or in combinations induced liver damage as measured by sinusoid dilatation, intensive vacuolization, blood congestion, and focal necrosis. Significant elevation in catalyze activity and MDA levels were also observed in liver homogenates by the treatment (p ≤ 005).
Conclusion:
Combined treatment induced higher effects suggesting the critical hazards associated with FA and OTC when released to the environment.
Keywords: Liver histopathology, Oxidative damage, Sea bass, Formaldehyde, Oxytetracycline
Introduction
The decrease in fisheries worldwide has resulted in an increased commercial interest in aquaculture (Naylor et al., 2000) for the demand for fishery products, which is about 80 million tons by 2016 (FAO, 2018). However, aquaculture expansion is known to cause many environmental problems, such as (a) organic matter in water, (b) large use of chemicals (antibiotics, antifoulants), (c) presence of pathogens, and (d) introduction of new strains of cultured fish with a clear reduction of biodiversity (Katranitsas et al., 2003; Akeem et al., 2019). Among the panoply of these impacts, special attention should be attributed to chemical use regarding raising awareness of the need to detect and assess their adverse effects on aquatic organisms. Particularly in intensive aquaculture, the intake and the release of these chemicals are continuous (Douet et al., 2009). Among the most used substancesall over the world, we found: formaldehyde (FA) (Pedersen et al., 2010; Lalonde et al. 2015) and oxytetracycline (OTC) (Austin, 1984; Leal et al., 2016; Rodrigues et al., 2018).
FA, named also formalin was reclassified by the International Agency for Research on Cancer (IARC) in 2006 as a human carcinogen (Group 1) with sufficient evidence in humans for the carcinogenicity of FA (IARC, 2006). In aquaculture, it is commonly applied as an anti-parasitic agent (Andrade-Porto et al., 2017). FA is known to be resistant in an environment with an estimated half-life of about 24–168 hours in surface water under aerobic conditions and 48–336 hours in groundwater under anaerobic conditions (Kamata, 1966; Howard et al., 1991, Leal et al., 2018). Diverse acute and chronic effects of formalin on many species of fish were described in the literature. In fact, acute toxicities were conducted in different species of fish and the most sensitive freshwater fish was the striped bass, Roccus saxatilis (Walbum) with 96 hours LC50 of 1084 mgL−1 in water (Reardon and Harrell, 1990). In the same context, a study made in Ghana in response to the consumption of several species of fish showed the presence of FA in all analysed species with a value ranging from 0.174 to 3.710 μgg−1(4.233 × 10−4 and 3.661 × 10−3 mg/kg BW/day) (Asare et al., 2018). Indeed, it was reported that the acceptable daily intake concentrations of FA suggested by the World Health Organization and the United States Environmental Protection Agency for FA intake were 0.15 and 0.2 mg/kg BW/day suggested, respectively (Asare et al., 2018).
On rainbow trout, Oncorhynchus mykiss (Walbaum) epithelial lesions have been described, as changes in mucous cell densities and production of high levels of stress hormone cortisol (Lumsden et al., 1994; Buchmann et al., 2004; Jørgensen and Buchmann, 2007; Bulut et al., 2015).
On the molecular level, FA damages the oxidative status and alters cell functions. Indeed, FA causes protein denaturation and formscross linkages to avoid protein unfolding (Aitcheson et al., 2000). However, in spite of all these side effects, at present many fish farms use high concentrations of FA to control parasitic outbreaks.
Otherwise, OTC is used in aquaculture systems as an antibiotic worldwide (Miranda and Zemelmen, 2002; Delépée et al., 2004; Reed et al., 2004; Ueno et al., 2004). OTC is administrated through two modes; via a diet of 50 to 60 mg kg-1 (Mayor et al., 2008) or via a bath of 25 to 40 gm-3 (Rigos et al., 2006). OTC presents a broad spectrum with activity against Gram-positive and Gram-negative germs. OTC is approved by the US Food and Drug Administration for use in food fish and for use in invertebrates (Nolan et al. 2007). It acts against vibriosis and necrotizing hepatopancreatitis infections in fish and other bacterial diseases (Reed et al., 2004). Despite the fact that OTC is effective against a large spectrum of infections, OTC was found to be partially absorbed by the digestive tract (Cravedi et al., 1987; Jacobsen, 1989; Bjorklund and Bylund, 1990). Besides that, Rigos et al. (2004) found that Greek farms release annually 1,900 kg of OTC.
The half-lives of OTC in marine sediments were relatively high. In fact, it was about 151 days for the surface layer until 1 cm deep and about 300 days to be 5–8 cm deep (Hektoen et al., 1995). In addition, it persists in fish tissues (Bjorklund et al., 1990; Rodrigues et al., 2019), may cause hepatotoxicity (Bruno 1989; Rodrigues 2019), immunotoxicity in fish and freshwater mussels (Lundén and Bylund, 2000; Gust et al., 2012), and alter the thyroid function in zebrafish (Yu et al., 2020).
In most cases, FA and OTC are used together in aquaculture practices. The goal of this study was the assessment of the adverse effects induced by combined treatment of FA and OTC. The experiment was carried out with juvenile sea bass, Dicentrarchuslabrax L, and exposure to these chemicals was applied under the same conditions being used in aquaculture farms. A histopathological examination and a survey of the oxidative stress status of specimens’ livers were performed to investigate the occurrence of any adverse effects.
Materials and Methods
Chemicals
NOÉ Laboratories (FRANCE) was the supplier of OTC in COMPOMIX TERRASOL in powder form (purity > 98%). Whereas FA 37% was commercially purchased from BASF Company (GERMANY).
Animals acclimatation
150 specimens of sea bass with an average weight of 80 ± 10 g, were supplied by Dr. Raouf Besbes from the National Institute of Science and Technology of the Sea, Monastir, where the experiment was performed. Before the experiments, animals were acclimated under laboratory conditions (25°C room temperature, 12 hours light, 12 hours dark) for 1 week. Animal handling and all experimental designs were approved according to the National Institute of Health Guidelines for Animal Care and approved by the local Ethics Committee.
Fish treatments
Specimens were randomly distributed in 12 tanks of 60 l at a population density of 12 fish in each tank. Each set of three tanks underwent the same treatment while three tanks were kept as a control. The dosage was 40 gm-3 for OTC and 200 ppm for FA. The combined treatment was administrated respecting the same doses of OTC and FA. The experiment lasted for 10 days during which specimens were exposed by bath to tested chemicals for one hour every day. Sampling was achieved after five days of exposure and by the end of the experiment. Fishes undergoing the same treatment were, then, killed by decapitation. Livers were separated and divided into two portions; the first portion, which is intended for the histological analysis, was fixed in aqueous BOUIN’s solution, whereas the second was conserved at -80°C for oxidative stress assessment.
Histological analysis
The tissues thus fixed were routinely dehydrated in a graded series of alcohols, washed in toluene, and enveloped in paraffin. Sections of 5 μm using a wax microtome (LEICA) were prepared. These sections were placed onto microscope slides and stretched using albumin and distilled water solution. After overnight drying, the slides were stained with hematoxylin-eosin before light microscopy observation.
All tissue samples were examined under an optical microscope (ZEISS) for different abnormalities (lipid infiltration, necrosis, and degeneration) in hepatocytes.
Oxidative stress assessment
Preparation of liver extracts
The frozen livers were homogenized (ULTRA TURRAX T25, GERMANY) 1/4, weight per volume) in an ice-cold phosphate buffer (01M, pH 74) and centrifuged (4,000 rpm for 15 minutes, 4°C); supernatants were frozen at -80°C until enzymatic assays were used. Protein concentrations were determined according to the Protein BioRad assay where bovine serum albumin was taken as a standard (Bradford, 1979).
Evaluation of lipid peroxidation status
Our method was based on the formation of a fluorescent complex between the MDA molecule and thiobarbituric acid (TBA) which will be quantified spectrophotometrically. To this end, 250 μl of each liver and kidney extract was mixed with 750 μl of both acetic acid (20%) and TBA (0.8%). The mixture was heated at 90°C for 2 hours and then chilled on the ice for 10 minutes. Finally, 2.5 ml n-butanol pyridine (15:1/v/v) was added before reading the optical density at 540 nm and the MDA level was reported as μmol mg–1 of proteins (Ohkawa et al., 1979).
Determination of catalase activity
To determine the catalase activity in fish livers, we placed in a quartz spectrophotometer cuvette, 780 μl of phosphate buffer solution (pH 7.0), 20 μl of each liver extract, and 200 μl of H2O2 (1 M) (the substrate of the enzyme). Then, we measured the optical density at 240 nm for an interval of time of 1 minute. For controls, we introduced the same compounds except the organ extract supernatants which were replaced by phosphate buffer. Catalase activity results were expressed in millimole of decomposed H2O2/min/mg of proteins.
Statistical analysis
Oxidative stress assessment results were expressed as mean ± standard deviation statistical differences between the treated and control sgroups were assessed by the Tuckey (HSD) test with p ≤ 005.
Ethical approval
Animal handling and all experimental designs were approved according to the National Institute of Health Guidelines for Animal Care and approved by the local Ethics Committee.
Results
Histopathological survey
The control group has largely presented a normal hepatic architecture (Fig. 1), showing normal cells with a transparent cytoplasm without inclusions; this shows good storage of glycogen (Simpson, 1992). Nuclei are constant in size and shape. Many sinusoids are observed lining the hepatic cords and branch from large vessels.
Fig. 1. A and B Liver tissue of the control specimens: (H) hepatocytes, (CLV) centro lobular vein; (S) sinusoids; (N) nuclei within hepatocytes. A (HE × 20) and B (HE × 100).
In a global overview, histopathological changes in the liver were observed for all treatments though, the intensity of cell damage was more severe in the case of combined treatment followed by the FA and the OTC. After five days of exposure, no alterations were observed in the treated group in reference to the control.
First, in the histological liver investigation of the OTC-treated fish, the following was observed. Vacuolated hepatocytes and slight sinusoid dilatation (SD) were observed in areas of the liver of most specimens. However, individual hepatocytes were clearly identifiable. No congestion of major blood vessels was evident. Notably, the liver histological structure of all specimens appeared relatively intact and the hepatic cord structure associated with normal fish liver histology was clearly visible in all specimens (Fig. 2).
Fig. 2. Liver tissue of the OTC treated specimens: (V) Vacuolisation, (N) Nucleus within hepatocytes; SD (HE × 100).

Second, the liver of the FA-treated specimens showed a relative loss of liver integrity through the appearance of significant vacuolization and SD in comparison with OTC samples (Fig. 3). Several cases of nucleus reduction were also obvious in many analyzed areas. Moderate congestion was recorded in major blood vessels. As an extreme impact of the application of FA, a restrained focal necrosis of the hepatic tissue was registered.
Fig. 3. A–C: Liver tissue of the formaldehyde 37% treated specimens: (V) vacuolation; (BC) blood congestion, (Ne) Necrosis; (H) hepatocyte; SD; (N) Nucleus within hepatocyte (HE × 100).
Finally, in the histological assessment of specimens’ livers exposed to the combination of FA and OTC, the following alterations were observed. The histological integrity was lost throughout the liver sample (Fig. 4). Individual hepatocytes were not always clearly visible. The histological structure did not appear relatively intact. The hepatic cord structure associated with normal fish liver histology was not clearly visible. Highly vacuolated hepatocytes were observed in the majority of investigated areas. As was the case with specimens exposed to FA, congestion was observed in most blood vessels with a prevalent SD. By the end, and in a more severe level of alteration, nuclear degeneration and necrosis of the hepatic tissue were also evident in several investigated areas.
Fig. 4. A–D: Liver tissue of the formaldehyde 37% and OTC treated specimens: (Ne) Necrosis; (V) vacuolation; (SC) sinusoid congestion; (CV) central vein with melanomacrophage centers (MMCs); SD; (CLVC) centrolobular vein congestion; (RBCs) red blood cells. A and B (HE × 10) and C and D (HE × 100).
Oxidative stress assessment
Catalase activities determination
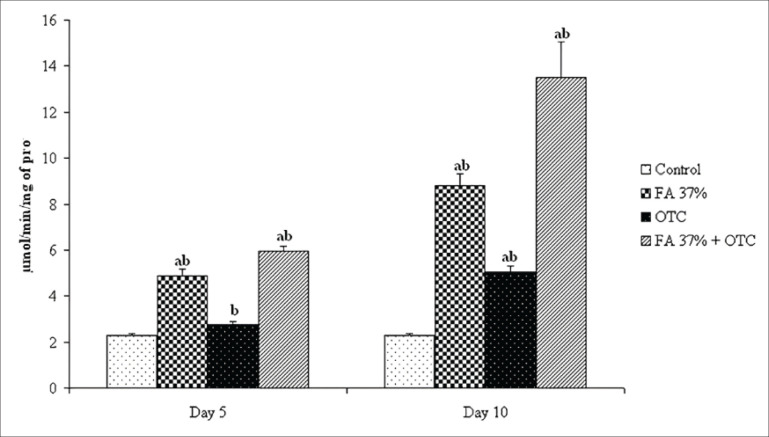
Figure 5 showed significant changes in catalase activity (p < 005) for all tested substances except OTC on day 5 where the induced catalase activity remains not significantly different from the control group. However, it seems evident that ten days of exposure is enough to provoke catalase induction in sea bass liver exposed to the latter component. Our analysis also showed a time-dependent increase in catalase activity. The highest value was reached for the combined treatment where the measured activity was about 14 μmol min-1 mg-1 reflecting a cumulative effect of FA37% and OTC when applied at the same time. Finally, as can be seen in Figure 5, the cumulative effect of FA37% + OTC was observed only after 10 days of treatment.
Fig. 5. Catalyse activity in the liver of sea bass control group, sea bass exposed to FA 37% (Formaldehyde), OTC (oxytetracycline), and FA 37% + OTC, respectively. (a) Significantly different from the control at P ≤ 0.05. (b) Significantly different from other treatments at P ≤ 0.05.
MDA measurement
The potential oxidative stress of FA 37%, OTC, and their combination was also assessed by MDAtest and the results are reported in Figure 6. We found significantly higher MDA levels in the exposure groups compared with the control group on day 5 and day 10, respectively (p < 005). The MDA concentration showed an increase in a time-dependent manner.
Fig. 6. Malondialdehyde (MDA) assessment in the liver of the sea bass control group, sea bass exposed to FA 37% (Formaldehyde), OTC (oxytetracycline), and FA 37% + OTC, respectively. (a) Significantly different from the control at P ≤ 0.05. (b) Significantly different from other treatments at P ≤ 0.05.
Obviously, there are no significant differences between the MDA levels after the exposure to FA37% and to OTC. As was the case with catalase, the combined exposure induced a cumulative effect particularly well pronounced on the tenth day where the MDA level in the assessed liver reached 059 μM (Fig. 6).
Discussion
Cancer, cardiovascular, and neurodegenerative diseases were related to the cellular imbalance in the antioxidant defense system and the overproduction of free radicals (Pizzino et al., 2017). This phenomenon is called oxidative stress and can alter proteins, lipids, and DNA which results in their oxidations (Lopez-Pedrera et al., 2016).
Therefore, oxidative stress is one of the most implicated processes in many chemicals’ toxicity (Satpute et al., 2017). In the present study, we assessed histopathological alterations and oxidative stress induction in sea bass liver after exposition to two chemicals commonly used in aquaculture; FA and OTC. The namely compounds are known as treatments to prevent fish diseases caused by protozoa, parasites, and bacteria.
To investigate the probable interaction between these chemicals, their combined effects were evaluated. The exposure lasted for 10 days during which two samples were achieved; the first at day 5 and the second by the end of the experiment.
Results indicate that the exposure to FA and OTC induced significant histological alterations in the sea bass liver. In the case of FA, obtained results are expected since it was previously reported that fish species are particularly sensitive to formalin, and even lower doses could adversely affect many fish species if used repeatedly (Omoregie et al., 1994; Omoregie et al., 1998; Yacoob et al., 2002; Santos et al., 2012).
From histopathological viewing, FA induces many changes in the Amazon ornamental fish, blue-spotted coridora (Corydoras melanistius). Indeed, when exposed to ten concentrations of formalin (40%) (0, 3, 6, 12, 25, 50, 100, 150, 200, and 250 mgL-1for 96-hours, Rainbow trout showed severe histopathological changes such as gill hyperplasia, with a filling of interlamellar spaces, disorganization of liver arrangement (Santos et al., 2012).
Moreover, liver tissues of Nile tilapia, Oreochromis niloticus L. fingerlings showed vacuolated areas (Perera and Pathiratne, 2005). Moreover, FA induces toxic effects in both liver and kidney silver pomfret (Pampus argenteus).
More recently, Bulut et al. (2015) showed that 250 mg L-1 (1 hour) and 500 mg L-1 (45 minutes) concentrations of FA in aquaculture of Rainbow Trout caused interlamellar necrosis and degeneration of the muscle tissue, dilatation in the liver, congestion in veins, and degeneration in hepatocytes and damage in the blood vessels. It is mentioned that formalin causes tissue damage and shows genotoxic effects even at 15 mg L-1 in Nile tilapia.
All these observations are in agreement with our findings, as a matter of fact, we found that the therapeutic exposure to FA has mainly induced SD and intensive vacuolization. Hepatocytes from FA-treated groups were distinguished from the reference by a greater degree of vacuolation.
In parallel, OTC-exposed specimens did not differ significantly from the reference group, and the hepatic damage was limited to SD and slight vacuolization. Accordingly, Elsayed et al. (2013), revealed that a 40 mg kg-1 dose of OTC basal diet showed non-significant increases in chromosomal aberrations compared with control in Nile Tilapia fish.
In a retrospective view, it is clear that recorded alterations depend on the treatment at both quantitative and qualitative levels. Hence, FA-treated groups have shown more severe damage than the OTC ones. It is also noteworthy to mention that the observed alterations were particularly well pronounced at the end of the experiment and that five days of exposure was not enough to induce relevant liver changes in comparison with the control.
In the present study, the absence of necrosis and fibrosis in specimens’ livers exposed to OTC and to FA separately suggests that the accumulation of lipids was relatively mild and that consequently may not be pathological. However, as was mentioned in our results, the combined exposure to FA and OTC has induced more severe histopathological alterations characterized by necrosis and intensive blood vessel congestion. Thus, it is obvious that the combined use of both tested chemicals presented a considerable additive effect and precautions should be paid during their parallel use and particularly during their release to the receiving environment especially because of the persistent proprieties of OTC (Kerry et al., 1996; Coyne et al., 1997). Observed alterations were particularly well pronounced at the end of the experiment and those five days of exposure were not enough to induce relevant liver changes in comparison with control.
The administration of FA and OTC disrupted the hepatic architecture of the treated fish. Thus, histopathological changes covered inflammatory disorder, which might be due to the generation of reactive oxygen species (ROS) that provoked damage to different membrane constituents in hepatocytes (Rjeibi et al., 2016).
Besides that, variations of CAT and MDA between treated and control groups are an indication of oxidative stress induction and consequently a general metabolism perturbation Correspondingly, in our study it was obvious that FA has induced significant oxidative stress seen as CAT activity induction and increase in the MDA tissue content as shown by the generation of ROSs of animal exposed to FA, inflammation, neurodegenerative disorders and carcinogenesis (Lambert and Shank, 1988; Kilburn, 1994; Gurel et al., 2005; Saito et al., 2005; Jung et al., 2007; Vosoughi et al., 2013).
As was the case with FA, the present study clearly shows that OTC induced oxidative stress in the liver tissue. However, the magnitude of oxidative damage was significantly less important than FA treated group. It is demonstrated that OTC acts through the inhibition of mitochondrial β-oxidation disturbing the respiratory chain and generating superoxide anion which are important in the production of both ROSs and nitrogen species (Gibson, 2005).
In the same context, showed that OTC increased lipogenesis, induced oxidative stress, damaged the mitochondrial structure and functions, and inhibited the lipolysis in the liver tissues and hepatocytes of grass carp Ctenopharyngodon Idella (Xu et al., 2021).
These reactive species could damage the cell membrane as a result of lipid peroxidation (Skakun and Vysotski, 1982; Lopez-Pedrera et al., 2016). In accordance with our study, Reda et al. (2013), reported that after 12 weeks of the feeding of cultured O. niloticus with a basal diet supplemented with OTC at 100 mg kg-1 diet, pathological alterations in the liver and kidney of the treated fish were observed. Other studies have also demonstrated that OTC produces steatosis of the liver in men (Pessayre et al., 2001) and hepatic damage (Rodrigues et al., 2018).
Thus, both assessed chemicals have induced oxidative stress in the liver tissue with a significant dominance of the signals induced by the FA treatment In accordance with the histopathological findings, the latter result may clearly indicate that FA presents a more toxic potential than OTC. In conclusion, the mixture of FA and OTC has induced the most important effect reflecting thus the already enounced postulate about the cumulative effect of tested chemicals when administrated by bath at the same time.
It was reported that the FA and OTC could be accumulated in fish mussels. Indeed, the study of Saha and Sahoo (2021) detected the presence of FA in some storage fish samples with a concentration of 3.90 μM. In addition, Abraham et al. (2021) showed that OTC caused mortality reduced feed intake, and biomass reduction in Nile tilapia (O. niloticus). The authors indicated the presence of OTC residues in this fish, at the concentration of 161.40 ± 11.10 ng/g.
The present study reveals serious threats to the combined use of FA-OTC in intensive aquaculture to prevent adverse effects, these recommendations should be taken into account:
– Optimize administered doses.
– Do not use FA and OTC at the same time in large-scale fish aquaculture.
– Develop less toxic chemical compounds to prevent or treat diseases.
Acknowledgments
The authors would like to express their gratitude to Dr. Omar Khabour for his fruitful comments on the primary version of the manuscript. They especially thank Professor Hassan Bencheikh for his permission to conduct the histological experiments in his laboratory.
Authors contributions
Zouhour Ouanes-Ben Othmen, Raouf Besbes, Lotfi Achour, and Adnen Kacem designed the experiments. Mohamed Ali JERBI, Raouf Besbes, and Zouhour Ouanes-Ben Othmen performed the experiment, Zouhour Ouanes-Ben Othmen, Zohra Haous, Lazhar Zorgui, Abdurraouf Zaet, and Mohamed Ali Jerbi analyzed the data, Mohamed Ali and Rim Timoumi wrote the manuscript. Zouhour Ouanes-Ben Othmen, Altayeb Elazomi, and Rim Timoumi, Lazhar Zourgui revised the manuscript and all authors read and approved the final manuscript.
Funding
The submitted work was funded by “The Tunisian Ministry of Higher Education and Scientific Research.”
Conflict of interest
The authors declare that there is no conflict of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
- Abraham T.J, Julinta R.B, Roy A, Singha J, Patil P.K, Kumar K.A, Paria P, Behera B.K. Dietary therapeutic dose of oxytetracycline negatively influences the antioxidant capacity and immune-related genes expression in Nile tilapia Oreochromis niloticus (L.) Environmen. Toxicol. Pharmacol. 2021;87:103685. doi: 10.1016/j.etap.2021.103685. [DOI] [PubMed] [Google Scholar]
- Aitcheson S.J, Arnett J, Murray K.R, Zhang J. Removal of aquaculture therapeutants by carbon adsorption: 1. Equilibrium adsorption behaviour of single components. Aquacult. 2000;183:269–284. [Google Scholar]
- Akeem B.D, Abdullateef A, Adenike S.T.F, Ayoola O.A. Waste production in aquaculture: sources, components and managements in different culture systems. Aquacult. Fisheries. 2019;4:81–88. [Google Scholar]
- Andrade-Porto S.M, Gusmão A.E, Daiani K.D, Oliveira M.J.C, Tavares-Dias M. Antiparasitic efficacy and blood effects of formalin on Arapaima gigas (Pisces: Arapaimidae) Aquacult. 2017;479:38–44. [Google Scholar]
- Asare D, Noah K, Raymond Akanwi A, Ray B, Voegborlo A, Apeke A. Risk assessment of kumasi metropolis population in ghana through consumption of fish contaminated with formaldehyde. J. Toxicol. 2018;1:4785031. doi: 10.1155/2018/4785031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B. The control of bacterial fish diseases by antimicrobial compounds. In: Woodbine M, editor. In Antimicrobials in agriculture. London: Butterworths; 1984. pp. 255–268. [Google Scholar]
- Bjorklund H, Bondestam J, Bylund G. Residues of oxytetracycline in wild fish and sediments from fish farms. Aquaculture. 1990;86:359–368. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruno D.W. An investigation into oxytetracycline residues in Atlantic salmon. Salmo salar L. J. Fish Dis. 1989;12:77–86. [Google Scholar]
- Buchmann K, Bresciani J, Jappe C. Effects of formalin treatment on epithelial structure and mucous cell densities in rainbow trout, Oncorhynchus mykiss (Walbaum) skin. J. Fish Dis. 2004;27:99–104. doi: 10.1111/j.1365-2761.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- Bulut C, Kubilay A, Hanol B.Z, Birden B. Histopathological effects of formaldehyde (CH2O) on rainbow trout (Oncorhynchus mykiss Walbaum, 1792) J. Limnol. Freshwater Fisheries Res. 2015;1:43–48. [Google Scholar]
- Claiborne A. Catalase activity. In Handbook of methods for oxygen radical research. In: Greenwald R.A, editor. Boca Raton, FL: CRC Press; 1985. pp. 283–284. [Google Scholar]
- Coyne R, Hiney M, Smith P. Transient presence of oxytetracycline in blue mussels (Mytilus edulis) following its therapeutic use at a marine Atlantic salmon farm. Aquacult. 1997;149:175–181. [Google Scholar]
- Cravedi J.P, Choubert G, Delous G. Digestibility of cloramphenicol, oxolinic acid and oxytetracycline in rainbow trout and influence of these antibiotics on lipid digestibility. Aquacult. 1987;60:133–141. [Google Scholar]
- Delépée R, Pouliquen H, Bris H.L. The bryophyte Fontinalis antipyreticaHedw. bioaccumulates oxytetracycline, flumequine and oxolinic acid in the freshwater environment. Sci. Total Environ. 2004;322:243–253. doi: 10.1016/j.scitotenv.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Douet D.G, Le Bris H, Giraud E. Environmental aspects of drug and chemical use in aquaculture: an overview. In: Rogers C, Basurco B, editors. The use of veterinary drugs and vaccines in Mediterranean aquaculture. 86. Zaragoza: CIHEAM. (Options Méditerranéennes: Série A. Séminaires Méditerranéens; 2009. pp. 105–126. [Google Scholar]
- Elsayed A, Soltan M.A, Radwan H.A, Mohamed M.G. Effect of oxytetracycline and florfenicol on the cytogenetic picture of Nile Tilapia (Oreochromis niloticus) Fish. JABS. 2013;7(3):102–106. [Google Scholar]
- FAO. The state of the world fisheries and aquaculture, opportunities and challenges. Food and Agricultural Organization of the United Nations; 2018. [Google Scholar]
- Gibson B.W. The human mitochondrial proteome: oxidative stress, protein modifications and oxidative phosphorylation. Int. J. Biochem. Cell Biol. 2005;37:927–934. doi: 10.1016/j.biocel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Gurel A, Coskun O, Armutcu F, Kanter M, Ozen O.A. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J. Chem. Neuroanat. 2005;29:173–178. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Gust M, Gélinas M, Fortier M, Fournier M, Gagné F. In vitro immunotoxicity of environmentally representative antibiotics to the freshwater mussel Elliptiocomplanata Environ. Pollut. 2012;169:50–58. doi: 10.1016/j.envpol.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Hektoen H, Berge J.A, Hormazabal V, Yndestad M. Persistente of antibacterial agents in marine sediments. Aquacult. 1995;133:175–184. [Google Scholar]
- Howard P.H, Boethling R.S, Jarvis W.F, Meylan W.M, Michalenko E.M. Handbook of environmental degradation rates. Chelsea, MI: Lewis Publishers; 1991. [Google Scholar]
- IARC. Formaldehyde, 2-butoxyethanol and 1-tertbutoxypropan-2-ol. Vol. 88. IARC Monogr. Eval. Carcinog. Risks Hum; 2006. pp. 1–478. [PMC free article] [PubMed] [Google Scholar]
- Jacobsen M.D. Withdrawal times of freshwater rainbow trout, Salmo gairdneri Richardson, after treatment with oxolinic acid, oxytetracycline and trimethoprim. J. Fish Dis. 1989;12:29–36. [Google Scholar]
- Jørgensen T.R, Buchmann K. Stress response in rainbow trout during infection with Ichthyophthiriusmultifiliis and formalin bath treatment. Acta Ichthyol. Pisc. 2007;37:25–28. [Google Scholar]
- Jung W.W, Kim E.M, Lee E.H, Yun H.J, Ju H.R, Jeong M.J, Hwang K.W, Sul D, Kang H.S. Formaldehyde exposure induces airway inflammation by increasing eosinophil infiltrations through the regulation of reactive oxygen species production. Environ. Toxicol. Pharmacol. 2007;24:174–182. doi: 10.1016/j.etap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kamata E. Aldehyde in lake and seawater. Bull. Chem. Soc. Jpn. 1966;36:1227. [Google Scholar]
- Katranitsas A, Castritsi-Catharios J, Persoone G. The effects of a copper-based antifouling paint on mortality and enzymatic activity of a non-target marine organism. Mar. Pollut. Bull. 2003;46:1491–1494. doi: 10.1016/S0025-326X(03)00253-4. [DOI] [PubMed] [Google Scholar]
- Kerry J.R, Coyne D, Gilroy M, Hiney P.S. Spatial distribution of oxytetracycline and elevated frequencies of oxytetracycline resistance in sediments beneath a marine salmon farm following oxytetracycline therapy. Aquaculture. 1996;145:31–39. [Google Scholar]
- Kilburn K.H. Neurobehavioural impairment and seizures from formaldehyde. Arch. Environ. Health. 1994;49:37–44. doi: 10.1080/00039896.1994.9934412. [DOI] [PubMed] [Google Scholar]
- Lalonde B.A, Ernst W, Garron C. Formaldehyde concentration in discharge from land based aquaculture facilities in Atlantic. Can. Bull. Environ. Contam Toxicol. 2015;94(4):444–447. doi: 10.1007/s00128-015-1493-9. [DOI] [PubMed] [Google Scholar]
- Lambert C.E, Shank R.C. Role of formaldehyde hydrazone and catalase in hydrazine induced methylation of DNA guanine. Carcinogenesis. 1988;9:65–70. doi: 10.1093/carcin/9.1.65. [DOI] [PubMed] [Google Scholar]
- Leal J.F, Esteves V.I, Santos E.B.H. Use of sunlight to degrade oxytetracycline in marine aquaculture's waters. Environ. Pollut. 2016;213:932–939. doi: 10.1016/j.envpol.2016.03.040. [DOI] [PubMed] [Google Scholar]
- Leal J.F, Maria G.P.M.S, Neves E.B.H.S, Valdemar I.E. Use of formalin in intensive aquaculture: properties, application and effects on fish and water quality. Rev. Aquacult. 2018;10(2):281–295. [Google Scholar]
- Lopez-Pedrera C, Barbarroja N, Jimenez-Gomez Y, Collantes-Estevez E, Aguirre M.A, Cuadrado M.J. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology (Oxford) 2016;55:2096–2108. doi: 10.1093/rheumatology/kew054. [DOI] [PubMed] [Google Scholar]
- Lumsden J.S, Ferguson H.W, Ostland V.E, Bryne P.J. The mucous coat on gill lamellae of rainbow trout (Oncorhynchus mykiss) Cell Tissue Res. 1994;275:187–193. [Google Scholar]
- Lundén T, Bylund G. The influence of in vitroin vivo exposure to antibiotics on mitogen-induced proliferation of lymphoid cells in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2000;10:395–404. doi: 10.1006/fsim.1999.0247. [DOI] [PubMed] [Google Scholar]
- Mayor D.J, Solan M, Martinez I, Murray L, McMillan H, Paton G.I, Killham K. Acute toxicity of some treatments commonly used by the salmonid aquaculture industry to Corophiumvolutatorand Hedistediversicolor: whole sediment bioassay tests. Aquaculture. 2008;285:102–108. [Google Scholar]
- Miranda C.D, Zemelmen R. Bacterial resistence to oxytetracycline in Chilean salmon farming. Aquaculture. 2002;212:31–47. [Google Scholar]
- Naylor R.L, Goldburg R.J, Primavera J.H, Kautsky N, Beveridge M.C.M, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- Nolan M.W, Smith S.A, Jones D. Pharmacokinetics of oxytetracycline in the American horseshoe crab, Limulus polyphemus. J. Vet. Pharmacol. Ther. 2007;30:451–455. doi: 10.1111/j.1365-2885.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omoregie E, Eseyin T.G, Ofojekwu P.C. Chronic effects of formalin on erythrocyte counts ans plasma glucose of the Nile tilapia, Orechromisniloticus. Asian Fish Sci. 1994;7:1–6. [Google Scholar]
- Omoregie E, Ofojekwu P.C, Amali E.I. Effects of sub-lethal concentrations of formalin on weight gain in the Nile tilapia, Orechromisniloticus (Trewavas) Asian Fish Sci. 1998;10:323–327. [Google Scholar]
- Pedersen L.F, Pedersen P.B, Nielsen J.L, Nielsen P.H. Long term/low dose formalin exposure to small-scale recirculation aquaculture systems. Aquacult. Eng. 2010;42:1–7. [Google Scholar]
- Perera H.A.C.C, Pathiratne A. J. Natl. Sci. Found. Vol. 33. Sri Lanka: 2005. Effects of short-term exposure to therapeutic levels of formalin on health status of Nile tilapia, Oreochromis niloticus; pp. 239–245. [Google Scholar]
- Pessayre D, Berson A, Formenty B, Mansouri A. Mitochondria in steatohepatitis. Semin. Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxidative Med. Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon I.S, Harrell R.M. Acute toxicity of formalin and copper sulfate to striped bass fingerlings held in varying salinities. Aquaculture. 1990;87:255–270. [Google Scholar]
- Reda R.M, Ibrahim R.E, Ahmed El-Nobi. G, El-Bouhy Z.M. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egyptian J. Aquatic Res. 2013;39:241–248. [Google Scholar]
- Reed L.A, Siewicki T.C, Shah J.C. Pharmacokinetics of oxytetracycline in the white shrimp, Litopenaeussetiferus. Aquaculture. 2004;232:11–28. [Google Scholar]
- Rigos G, Nengas I, Alexis M. Oxytetracycline (OTC) uptake following bath treatment in gilthead sea bream (Sparus aurata) Aquaculture. 2006;261:1151–1155. [Google Scholar]
- Rigos G, Nengas I, Alexis M, Troisi G.M. Potential drug (oxytetracycline and oxolinic acid) pollution from Mediterranean sparid fish farms. Aquat. Toxicol. 2004;69:281–288. doi: 10.1016/j.aquatox.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rjeibi I, Ben Saad A, Hfaiedh N. Oxidative damage and hepatotoxicity associated with deltamethrin in rats: the protective effects of Amaranthus spinosus seed extract. Biomed. Pharmacother. 2016;84:853–860. doi: 10.1016/j.biopha.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes S.C, Nunes B, Correia A.T. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ. Sci. Pollut. Res. Int. 2019;26(15):15481–15495. doi: 10.1007/s11356-019-04954-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes S.C, Correia A.T, Nunes B. Oxytetracycline effects in specific biochemical pathways of detoxification, neurotransmission and energy production in Oncorhynchus mykiss. Ecotoxicol. Environ. Saf. 2018;30:100–108. doi: 10.1016/j.ecoenv.2018.07.124. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nishio K, Yoshida Y, Niki E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology. 2005;210:235–245. doi: 10.1016/j.tox.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Santos R.F.B, Dias H.M, Fujimoto R.Y. Acute toxicity and histopathology in ornamental fish amazon bluespottedcorydora (Corydoras melanistius) exposed to formalin. An. Acad. Bras. Cienc. 2012;84(4):1001–1007. doi: 10.1590/s0001-37652012000400014. [DOI] [PubMed] [Google Scholar]
- Satpute R.M, Pawar P.P, Puttewar S, Sawale S.D, Ambhore P.D. Effect of resveratrol and tetracycline on the subacute paraquat toxicity in mice. Hum. Exp. Toxicol. 2017;36:1303–1314. doi: 10.1177/0960327116688070. [DOI] [PubMed] [Google Scholar]
- Simpson M.G. Histopathological changes in the liver of dab (Limandalimanda) from a contamination gradient in the North Sea. Mar. Environ. Res. 1992;34:39–43. [Google Scholar]
- Skakun N.P, Vysotski I.I.U. Effect of tetracycline antibiotics on lipid peroxidation. Antibiotiki. 1982;27:684–687. [PubMed] [Google Scholar]
- Ueno R, Kinoshita A, Wakabayashi J. Comparative pharmacokinetics of oxytetracycline in eel and its fate in a closed aquatic environment. Aquaculture. 2004;235:53–63. [Google Scholar]
- Vosoughi S.H, Khavanin A, Salehnia M, Asilian M.H, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int. J. Fertil. Steril. 2013;6(4):250–255. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Hogstrand C, Xu Y.C, Zhao T, Zheng H, Luo Z. Environmentally relevant concentrations of oxytetracycline and copper increased liver lipid deposition through inducing oxidative stress and mitochondria dysfunction in grass carp Ctenopharyngodonidella. Environ. Pollut. 2021;283:117079. doi: 10.1016/j.envpol.2021.117079. [DOI] [PubMed] [Google Scholar]
- Yacoob S.Y, Anraku K, Archdale M.V, Matsuoka T, Kiyohara S. Exposure of taste buds to potassium permanganate and formalin suppresses the gustatory neural response in the Nile tilapia Oreochromis niloticus (Linnaeus) Aquacult. Res. 2002;33:445–453. [Google Scholar]
- Yu K, Li X, Qiu Y, Zeng X, Yu X, Wang W, Yi X, Huang L. Low-dose effects on thyroid disruption in zebrafish by long-term exposure to oxytetracycline. Aquat. Toxicol. 2020;227:105608. doi: 10.1016/j.aquatox.2020.105608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.