Abstract
In order to elucidate the structure and morphology of hepatitis G virus (HGV), a recently isolated flavivirus, we generated a panel of eight monoclonal antibodies (MAbs) against the putative second envelope protein (E2) following DNA immunization. The MAbs were shown to be specific for four different epitopes on recombinant E2. MAb Mc6 was the only antibody able to detect the linear epitope LTGGFYEPL. In addition, Mc6 was able to immunoprecipitate viral particles in human blood samples as detected by reverse transcription-PCR amplification of HGV RNA. This precipitation could be competed by addition of saturating amounts of the linear peptide or abolished by addition of Nonidet P-40. We conclude that, albeit lacking the N-terminal sequence of a functional core protein, HGV builds classical viral particles displaying E2 envelope protein on their outer surfaces.
Introduction.
Recently, two groups reported independently on the isolation of new positive-strand RNA viruses, designated hepatitis G virus (HGV) (14) and GB virus C (GBV-C) (12). Sequence analysis revealed that both genomes are different isolates of the same virus and show ∼85% nucleotide sequence identity, including a single, continuous open reading frame encoding 2,873 amino acids with a number of motifs characteristic for members of the Flaviviridae family (2). The genetic organization of HGV resembles that of HCV, but the lack of a sequence coding for a functional core-like protein raises important questions with regard to the morphology of the virus (22). HGV is transmitted parenterally and is therefore commonly distributed among risk groups, such as intravenous drug users, hemophiliacs, and patients who receive multiple transfusions (14, 15, 23). Among apparently healthy blood donors, an HGV RNA prevalence of 0.9 to 3% has been reported (14, 15, 23). HGV can cause acute and persistent infection, but the clinical significance is still unclear. Based on the cloning sources, HGV was initially discussed as another potential causative agent of acute and chronic hepatitis, but studies so far have been unable to prove the link between HGV and liver disease (1).
Two different tools for HGV diagnosis in human blood specimens were available until now, reverse transcription-PCR (RT-PCR) detection of HGV RNA (20) and immunoassays for the detection of specific antibodies against the putative envelope protein E2 (anti-E2) (5, 17, 24, 25). The glycoprotein E2 features a C-terminal transmembrane anchor domain, three potential glycosylation sites, and 18 cysteine residues which might be involved in disulfide bonds. In analogy to other flaviviruses, E2 is presumed to play an important role in binding of the virus to target cells. In contrast to HCV, sequence variability of E2 is very low among isolates collected worldwide and the appearance of antibodies to E2 is normally associated with recovery from HGV viremia (5, 13, 24). Obviously, a high proportion of immunocompetent individuals infected with HGV are able to clear the virus, although viremia has been shown to persist in some patients (25). The present work describes the generation of monoclonal antibodies (MAbs) to E2 which share epitopes with antibodies present in sera of HGV-infected individuals. They provide tools for the characterization of HGV particles and the establishment of immunoassays for the detection of viral antigen in human sera.
DNA immunization and generation of E2-specific MAbs.
Immunization by intramuscular injection of plasmid DNA encoding the antigen seems to be advantageous over classic immunization with purified antigen, especially if the antigen is difficult to synthesize and/or to purify (28). In addition, the method allows host processing of newly synthesized proteins, correct glycosylation, and proteolytic processing. This method has recently been shown to induce both humoral and cellular immune responses against a number of infectious agents, including HBV surface antigen (3), influenza virus nucleoprotein (16), and HCV E2 (26).
The expression construct CHO-E2-TM8 used for plasmid DNA immunization was proven to correctly express glycosylated FLAG-E2 fusion protein in Chinese hamster ovary (CHO) cells (25). Viral E2 is expressed as part of a polyprotein, and therefore the construct features a heterologous signal sequence besides an N-terminal FLAG epitope (9) and the E2-coding sequence containing its C-terminal membrane anchor (25). Earlier reports claim higher efficiency of DNA uptake in regenerating muscle cells (3). Therefore, 80 μl of 10 μM cardiotoxin (Latoxan; Rosans) was injected into tibialis anterior muscles of five female 15-week-old BALB/c mice. Five days later, 50 μl of phosphate-buffered saline (PBS) containing plasmid DNA (1 μg/μl) was injected into each muscle. This was repeated after another 5, 10, 11, and 12 weeks. Serum samples collected after the second and the fifth immunizations were tested for E2-specific antibodies in a whole-cell enzyme-linked immunosorbent assay (ELISA): CHO cells displaying membrane-bound FLAG-E2 (25) were seeded overnight in 96-well tissue culture plates (4 × 104 cells/well). The next day, cells were first incubated for 2 h with medium containing 1% Byco C to block unspecific binding sites. Serum samples were added and incubated for another hour. After being washed with PBS–0.02% Tween 20, cells were incubated with horseradish peroxidase–anti-mouse immunoglobulin G (IgG)–Fab conjugate (50 mU/ml) for 1 h. The wells were washed, and an enzymatic color reaction was developed by adding the substrate solution, 1.9 mM 2,2′-azino-di(3-ethylbenzthiazolinesulfonate) diammonium salt (ABTS) in 100 mM phosphate citrate buffer (pH 4.4)–3.2 mM hydrogen peroxide (as sodium perborate). Adsorbance at 422 nm was read after 1 h. To check for unspecific binding, all supernatants were also tested on CHO cells expressing the human urokinase receptor with an N-terminal FLAG peptide.
After the second immunization, no significant titer was detectable. However, after the fifth immunization, three of five mice had developed an E2-specific titer over 1:1,000. One mouse was selected and given one booster by intravenous injection of 107 CHO cells expressing FLAG-E2 prior to fusion of spleen cells with the nonproducer cell line P3X63-Ag8.653 (ATCC CRL 1580) using polyethylene glycol (molecular weight, 4,000) (7). Supernatants of hybridomas obtained after selection with hypoxanthine aminopterine thymidine-containing medium were tested for E2-specific antibodies, and finally, eight cell lines producing IgG antibodies to HGV E2 were recloned by fluorescence-activated cell sorting. MAbs designated Mc3, Mc5, Mc6, Mc11, Mc13, Mc17, Mc19, and Mc30 were further purified and conjugated with biotin and digoxigenin (Table 1).
TABLE 1.
Epitope mapping of murine E2-specific MAbsa
| Capture conjugate | Detection conjugate isotype
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mc3 dig IgG2b(κ) | Mc5 dig IgG2b(λ) | Mc6 dig IgG2a(κ) | Mc11 dig IgG1(κ) | Mc13 dig IgG2b(κ) | Mc17 dig IgG1(λ) | Mc19 dig IgG2b(κ) | Mc30 dig IgG2b(κ) | |
| Mc3 bi | ++ | + | ++ | ++ | ++ | + | ++ | + |
| Mc5 bi | + | ++ | − | − | − | ++ | − | − |
| Mc6 bi | ++ | − | ++ | ++ | + | − | + | − |
| Mc11 bi | ++ | − | ++ | ++ | + | − | − | − |
| Mc13 bi | ++ | − | + | + | ++ | − | ++ | − |
| Mc17 bi | ++ | ++ | − | − | − | ++ | − | − |
| Mc19 bi | ++ | − | ++ | + | ++ | + | ++ | − |
| Mc30 bi | − | − | − | − | − | − | − | ++ |
Antibody isotypes were determined with the IsoStrip Mouse Monoclonal Antibody Isotyping Kit (BM). MAbs were used as biotin conjugates (bi) for capturing and digoxigenin conjugates (dig) for detection in pairwise combination in a checkerboard competition ELISA. ++, strong competition; +, slight competition; −, no competition.
Antibody isotyping revealed that two of eight cell lines produced IgG1, while one of eight and five of eight produced IgG2a and IgG2b, respectively (Table 1). This is in contrast to former studies reporting a preponderance of IgG2a antibodies after intramuscular inoculation (6, 16, 18). These reports used polyclonal sera for subtyping, whereas the present report describes the generation of MAbs after multiple injections of plasmid DNA followed by a final boost with cells expressing antigen. Cross-boosting experiments imply that the type of immune response is determined by the initial immunization; subsequent booster immunizations by an alternative method do not alter the established IgG isotype profile (6, 16). Therefore, the final boost with cells expressing antigen is unlikely to be the reason for the preponderance of subtype IgG2b. A selection for the subtype IgG2b antibodies during the screening of the hybridomas is also unlikely, since the polyclonal antibody which was used for the first screening was generated against mouse Fcγ. It recognizes the different mouse IgG subclasses with relative affinities in the following order (from greatest to least): IgG1, IgG2a, IgG2b, IgG3. Differences in the type or form of the antigen probably account for the different subtypes found, as membrane-bound, cytosolic, and secreted proteins may elicit different types of antibody isotype responses.
Epitope mapping by competition ELISA.
In order to analyze the binding sites of the MAbs on recombinant E2, a competitive binding inhibition ELISA was developed using the automated ES 300 serum analyzer system (Boehringer Mannheim [BM]): pairs of MAb-biotin conjugates for capturing by streptavidin and MAb-digoxigenin conjugates for detection (3 μg/ml each) were incubated with crude lysates of CHO cells expressing FLAG-E2 (25) for 3 h in streptavidin-coated test tubes. Some MAbs showed slight differences depending on their use as capture or detection antibody, probably due to differences in their binding affinities. Detection of digoxigenin-conjugated antibody complexes was done by incubation with horseradish peroxidase-antidigoxigenin conjugate (50 mU/ml) for 3 h. After the cells were washed, enzymatic color reactions were developed by adding ABTS substrate solution.
Simultaneous use of each of the E2-specific MAbs for capturing and detection resulted in strong inhibition in all eight cases (diagonal of Table 1), indicating that E2 is present as monovalent antigen in CHO E2 lysates. Different MAb combinations for capturing and detection indicated the presence of several MAb groups specific for epitope clusters (Table 1): Mc5 and Mc17 completely inhibited each other. They obviously bind to the same or correlated epitopes and thus constitute MAb group I. The same holds true for Mc6 and Mc11 (group II) and Mc13 and Mc19 (group III). MAbs of groups II and III slightly inhibited each other. Although the overall reactivity of Mc3 was relatively weak compared to that of the other MAb, Mc3 interfered with MAbs of groups II and III. Mc30 could be competed only by itself and is therefore specific for another epitope.
Pepscan.
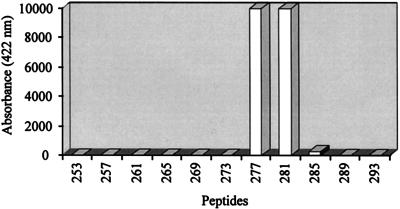
All E2-specific MAbs were tested for their reactivity against synthetic 13-mer peptides covering the 385-amino-acid putative primary sequence of mature HGV-E2 from APASVL to PAVEAA. Each peptide overlapped the adjacent peptide by nine residues and carried an N-terminal biotin tag for capturing followed by ɛ-lysyl-β-alanyl-ɛ-aminocaproyl-β-alanine as a spacer module. Peptides (200 ng/ml) were incubated with MAb-digoxigenin conjugates (1 μg/ml) and bound to streptavidin-coated test tubes for 3 h. Detection of digoxigenin-conjugated antibody complexes was done as described above. Mc6 strongly reacted with two peptides, 277 (biotin-spacer-GGAGLTGGFYEPL) and 281 (biotin-spacer-LTGGFYEPLVRRC), sharing residues 281 to 289 (LTGGFYEPL) (Fig. 1). This linear epitope is located near the C terminus of the extracellular domain of E2, assuming that the membrane anchor region starts around residue 340. Neither Mc11, which can be competed by Mc6, nor any other MAb showed reactivity with any of the peptides. These data imply that seven of eight MAbs are specific for conformational epitopes. This correlates with previous observations that no antigenic epitopes could be determined by screening the HGV genome for linear epitopes (20a).
FIG. 1.
Reaction profile of MAb Mc6 with synthetic overlapping peptides. Eleven peptides, spanning residues 253 to 305 of HGV E2, are shown. The amino acid residue numbers indicate the position of the first residue from each 13-mer; numbering starts with the first residue of the putative mature E2 protein. Only peptides 277 and 281, which share amino acids 281 to 289 (LTGGFYEPL), were recognized by Mc6.
Immunoprecipitation of viral particles.
An immunoprecipitation assay was developed in order to test whether MAbs generated against recombinant FLAG-E2 are able to bind native E2 present on viral particles. Serum samples were obtained from volunteer blood donors of the on-site medical center (sample designations starting with BM) and the blood bank of Salzburg (sample designations starting with SB). All sera tested negative for HBV, HCV, and human immunodeficiency virus markers and were preabsorbed with protein G-agarose to reduce unspecific IgG binding.
In a pilot experiment, 100 μl of a 10−2 dilution (in Dulbecco’s PBS) of serum BM7822 (HGV RNA positive, anti-E2 negative), corresponding to a final concentration of 105 GE/ml, was incubated for 2 h at 4°C under continuous shaking with 100 μl of a 10−2 dilution (in PBS) of serum SB9700575 (anti-E2 positive, HGV RNA negative) or SB315 (negative for both markers). Protein G-agarose was added, and antibody binding was allowed for 2 h. Immunoprecipitates were collected and extensively washed three times with PBS–1% bovine serum albumin, by using spin modules (Bio 101, Vista, Calif.). RNA was isolated using the High Pure RNA Isolation Kit (BM). For qualitative RT-PCR amplification of HGV RNA, we used primers derived from the 5′ noncoding region, 5′-CGGCCAAAAGGTGGTGGATG-3′ (forward) and 5′-biotin-CGACGAGCCTGACGTCGGG-3′ (reverse), in combination with the Titan One Tube RT-PCR Kit (BM). Automated detection of amplicons on a modified ELECSYS 1010 analyzer (BM) via electrochemiluminescence was performed as follows. After denaturation and addition of a ruthenium-labeled hybridization probe, 5′-CCACTATAGGTGGGTCTT-Ru(bpy)32+-3′, amplicons were captured onto streptavidin-coated microparticles via the biotinylated reverse primer and detected in an electrochemical reaction on the surface of an electrode. The luminescence at a wavelength of 620 nm was measured as arbitrary electrochemiluminescence counts (11).
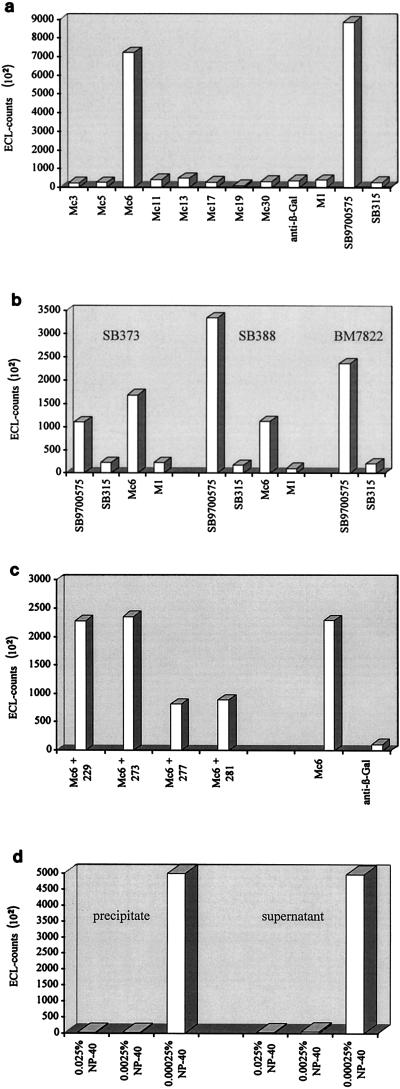
A significant amount of HGV RNA could be detected following precipitation with anti-E2-positive serum SB9700575, but not with anti-E2-negative serum SB315 (Fig. 2a). Precipitation of viral particles was not quantitative, since HGV RNA could also be detected in the supernatant (unbound fraction [data not shown]). Subsequently, all eight recombinant E2-specific MAbs were used instead of anti-E2-positive serum. As negative controls, MAbs specific for the FLAG epitope (M1, isotype IgG2b) and β-galactosidase (anti-β-Gal, isotype IgG2b) were included. Out of 10 MAbs used, only Mc6 was able to precipitate a considerable amount of viral particles from HGV RNA-positive serum BM7822 (Fig. 2a). To verify that immunoprecipitation was not only confined to serum BM7822, we examined two additional HGV RNA-positive human sera, SB373 (final concentration, 1 × 104 GE/ml) and BM388 (final concentration, 5 × 104 GE/ml), in combination with anti-E2-positive SB9700575, anti-E2-negative SB315, E2-specific Mc6, and FLAG-specific M1 (Fig. 2b). Again, only SB9700575 as well as Mc6 was able to precipitate significant amounts of viral particles (Fig. 2b). The results indicate that Mc6 can be used for immunoprecipitation of HGV RNA-containing particles present in different HGV PCR-positive human sera.
FIG. 2.
Immunoprecipitation of viral particles. HGV RNA-positive sera were incubated with either MAbs or human sera. Following precipitation by protein G-agarose, RNA was isolated and detected by RT-PCR. Shown are representative examples of three independent experiments. (a) HGV RNA-positive serum BM7822 was incubated with eight murine E2-specific MAbs, anti-β-Gal, FLAG-specific M1 (final concentration, 5 μg/ml), anti-E2-positive serum SB9700575, and anti-E2-negative serum SB315. Only Mc6 and SB9700575 were able to immunoprecipitate HGV. (b) HGV RNA-positive sera SB373, SB388, and BM7822 were incubated with anti-E2-positive serum SB9700575, anti-E2-negative serum SB315, E2-specific Mc6, and FLAG-specific M1. SB9700575 and Mc6 were able to immunoprecipitate HGV from all sera. (c) BM7822 was incubated with Mc6 in the presence of peptides 229, 273, 277, and 281 (final concentration, 1 μg/ml). For comparison, immunoprecipitation was performed with Mc6 without addition of a peptide and with anti-β-Gal. Peptides 277 and 281 competed for binding sites on viral E2. (d) BM7822 was incubated with Mc6 in the presence of different concentrations of NP-40. No viral RNA could be detected in the precipitate (bound) and supernatant (unbound fraction) at 0.025 and 0.0025% NP-40. ECL, electrochemiluminescence.
As described before, Mc6 was shown to be specific for a linear epitope. Therefore, the specificity of Mc6 could be proved by competition: HGV RNA-positive serum BM7822 was incubated with Mc6 in the presence of saturating amounts of peptides 277 and 281, which share the sequence LTGGFYEPL, and two peptides with nonrelated sequences, 229 (biotin- spacer-MTRIRDTLHLVEC) and 273 (biotin-spacer-SEALGGAGLTGGF), followed by immunoprecipitation and RT-PCR (Fig. 2c). As expected, only peptides 277 and 281 were able to compete with the binding of Mc6 to viral E2 (Fig. 2c).
All immunoprecipitation experiments described in this work so far were performed under nonstringent conditions to minimize disruption of viral particles. All steps were carried out at 4°C, serum was thawed only once, and denaturating reagents were avoided. Taken together, these experiments indicate the presence of viral particles displaying E2 on their surfaces. In another experiment, immunoprecipitation of HGV RNA-positive serum BM7822 in combination with anti-E2-positive serum SB9700575 was performed in the presence of different concentrations of lipid solvent. Nonidet P-40 (NP-40) concentrations below the critical micelle concentration (CMC) (CMC = 0.00025%) had no influence on the experiments, and HGV RNA could be isolated from the precipitate (bound fraction) and supernatant (unbound fraction) (Fig. 2d). However, NP-40 concentrations close to the CMC (0.0025%) or clearly above the CMC (0.025%) completely abolished immunoprecipitation. Importantly, HGV RNA could no longer be detected in either the precipitate or the supernatant in contrast to all previous experiments (Fig. 2d). Obviously, the viral particles were solubilized and the RNA was degraded by RNases present in the serum.
Conclusions.
Reports on HCV imply that detergents like NP-40 used at concentrations far above the CMC remove the viral envelope while leaving the capsid structure intact (10, 21). In these studies (10, 21), HCV RNA could be detected in detergent-treated virus preparations. This is in contrast to our observation that detection of HGV RNA in the supernatant (unbound fraction) was impaired at NP-40 concentrations around or above the CMC. Obviously, HGV RNA is not as well protected as HCV RNA, as already discussed in another study (19). The lack of core-like coding sequences in all HGV isolates analyzed so far might be related to the reduced stability of the viral RNA after disruption of the envelope.
Immunoprecipitation from human sera failed when conformation-dependent E2-specific MAbs were used. Their epitopes might be masked on the virus surface for several reasons, e.g., by complexation with envelope protein E1 or E2 oligomerization. Studies of HCV have shown that E1 and E2 glycoproteins interact to form a heterodimeric complex, which has been proposed to be a functional subunit of the HCV virion (4). Such oligomerization processes might induce conformational changes, altering the epitopes recognized by our MAbs generated against recombinant E2. As described for HCV, viral particles could also be associated with either Ig (8) or lipoproteins (27), making some epitopes inaccessible. Although the conformation-dependent MAbs were not able to precipitate viral particles, they share epitopes with antibodies present in sera of HGV-infected individuals. This was demonstrated by competition studies; binding of MAbs specific for the epitope cluster (groups II and III) could be competed by polyclonal antibodies present in sera of HGV-infected individuals, whereas the others (group I and Mc30) could hardly be competed by human antibodies. Future studies utilizing both Mc6 and conformation-dependent E2-specific MAbs will hopefully help to address open questions on HGV, such as the site of viral replication and clinical manifestations of HGV.
Acknowledgments
We thank the animal facilities of BM, Christa Huebner-Parajsz for helping us to generate MAbs, Volker Schlueter for support on RT-PCR methods, and Christoph Seidel for peptide synthesis.
REFERENCES
- 1.Alter H J, Nakatsuji Y, Melpolder J, Wages J, Wesley R, Shih J W-K, Kim J P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- 2.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 3.Davis H L, Michel M-L, Whalen R G. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum Mol Genet. 1993;2:1847–1851. doi: 10.1093/hmg/2.11.1847. [DOI] [PubMed] [Google Scholar]
- 4.Deleersnyder V, Pillez A, Wychowski C, Blight K, Xu J, Hahn Y S, Rice C M, Dubuisson J. Formation of native hepatitis C virus glycoprotein complexes. J Virol. 1997;71:697–704. doi: 10.1128/jvi.71.1.697-704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dille B J, Surowy T K, Gutierrez R A, Coleman P F, Knigge M F, Carrick R J, Aach R D, Hollinger F B, Stevens C E, Barbosa L H, Nemo G J, Mosley J W, Dawson G J, Mushahwar I K. An ELISA for detection of antibodies to the E2 protein of GB virus C. J Infect Dis. 1997;175:458–461. doi: 10.1093/infdis/175.2.458. [DOI] [PubMed] [Google Scholar]
- 6.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 7.Galfre G, Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73:3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- 8.Hijikata M, Shimizu Y K, Kato H, Iwamoto A, Shih J W, Alter H J, Purcell R H, Yoshikura H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993;67:1953–1958. doi: 10.1128/jvi.67.4.1953-1958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopp T P, Prickett K S, Price V L, Libby R T, March C J, Cerretti D P, Urdal D L, Conlon P J. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology. 1988;6:1204–1210. [Google Scholar]
- 10.Kanto T, Hayashi N, Takehara T, Hagiwara H, Mita E, Naito M, Kasahara A, Fusamoto H, Kamada T. Buoyant density of hepatitis C virus recovered from infected hosts: two different features in sucrose equilibrium density-gradient centrifugation related to degree of liver inflammation. Hepatology. 1994;19:296–302. [PubMed] [Google Scholar]
- 11.Kenten J H, Casadei J, Link J, Lupold S, Willey J, Powell M, Rees A, Massey R. Rapid electrochemiluminescence assays of polymerase chain reaction products. Clin Chem. 1991;37:1626–1632. [PubMed] [Google Scholar]
- 12.Leary T P, Muerhoff A S, Simons J N, Pilot-Matias T J, Erker J C, Chalmers M L, Schlauder G G, Dawson G J, Desai S M, Mushahwar I K. Sequence and genomic organization of GBV-C: a novel member of the Flaviviridae associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Lim M Y, Fry K, Yun A, Chong S, Linnen J, Fung K, Kim J P. Sequence variation and phylogenetic analysis of envelope glycoprotein of hepatitis G virus. J Gen Virol. 1997;78:2771–2777. doi: 10.1099/0022-1317-78-11-2771. [DOI] [PubMed] [Google Scholar]
- 14.Linnen J, Wages J, Zhang-Keck Z-Y, Fry K E, Krawczynski K Z, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J W-K, Young L, Piatak M, Hoover C, Fernandez J, Chen S, Zou J-C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 15.Masuko K, Mitsui T, Iwano K, Yamazaki C, Okuda K, Meguro T, Murayama N, Inoue T, Tsuda F, Okamoto H, Miyakawa Y, Mayumi M. Infection with hepatitis GB virus C in patients on maintenance hemodialysis. N Engl J Med. 1996;334:1485–1490. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- 16.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilot-Matias T J, Carrick R J, Coleman R F, Leary T P, Surowy T K, Simons J N, Muerhoff S, Buijk S L, Chalmers M L, Dawson G J, Desai S M, Mushahwar I K. Expression of the GB virus C E2 glycoprotein using the Semliki Forest virus vector system and its utility as a serologic marker. Virology. 1996;225:282–292. doi: 10.1006/viro.1996.0602. [DOI] [PubMed] [Google Scholar]
- 18.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K, Tanaka T, Okamoto H, Miyakawa Y, Mayumi M. Association of circulating hepatitis G virus with lipoproteins for a lack of binding with antibodies. Biochem Biophys Res Commun. 1996;229:719–725. doi: 10.1006/bbrc.1996.1871. [DOI] [PubMed] [Google Scholar]
- 20.Schlueter V, Schmolke S, Stark K, Hess G, Ofenloch-Haehnle B, Engel A M. Reverse transcription-PCR detection of hepatitis G virus. J Clin Microbiol. 1996;34:2660–2664. doi: 10.1128/jcm.34.11.2660-2664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Schmitt, U. Unpublished data.
- 21.Shindo M, Di Bisceglie A M, Akatsuka T, Fong T-L, Scaglione L, Donets M, Hoofnagle J H, Feinstone S M. The physical state of the negative strand of hepatitis C virus RNA in serum of patients with chronic hepatitis C. Proc Natl Acad Sci USA. 1994;91:8719–8723. doi: 10.1073/pnas.91.18.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons J N, Desai S M, Schultz D E, Lemon S M, Mushahwar I K. Translation initiation in GB viruses A and C: evidence for internal ribosome entry and implications for genome organization. J Virol. 1996;70:6126–6135. doi: 10.1128/jvi.70.9.6126-6135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark K, Bienzle U, Hess G, Engel A M, Hegenscheid B, Schlueter V. Detection of the hepatitis G virus genome among injecting drug users, homosexual and bisexual men, and blood donors. J Infect Dis. 1996;174:1320–1323. doi: 10.1093/infdis/174.6.1320. [DOI] [PubMed] [Google Scholar]
- 24.Tacke M, Kiyosawa K, Stark K, Schlueter V, Ofenloch-Haehnle B, Hess G, Engel A M. Detection of antibodies to a putative hepatitis G virus envelope protein. Lancet. 1997;349:318–320. doi: 10.1016/S0140-6736(96)06461-6. , 736. [DOI] [PubMed] [Google Scholar]
- 25.Tacke M, Schmolke S, Schlueter V, Sauleda S, Esteban J I, Tanaka E, Kiyosawa K, Alter H J, Schmitt U, Hess G, Ofenloch-Haehnle B, Engel A M. Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of HGV exposure among healthy blood donors. Hepatology. 1997;26:1626–1633. doi: 10.1002/hep.510260635. [DOI] [PubMed] [Google Scholar]
- 26.Tedeschi V, Akatsuka T, Shih J W-K, Battegay M, Feinstone S M. A specific antibody response to HCV E2 elicited in mice by intramuscular inoculation of plasmid DNA containing coding sequences for E2. Hepatology. 1997;25:459–462. doi: 10.1002/hep.510250234. [DOI] [PubMed] [Google Scholar]
- 27.Thomssen R, Bonk S, Propfe C, Heermann K-H, Koechel H G, Uy A. Association of hepatitis C virus in human sera with β-lipoprotein. Med Microbiol Immunol. 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 28.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]