Abstract
Heterologous priming and boosting with antigens expressed by DNA, viral vectors, or as proteins, are experimental strategies to induce strong immune responses against infectious diseases and cancer. In a preclinical study we compared the ability of recombinant modified vaccinia Ankara encoding HIV antigens (MVA-CMDR), and/or recombinant gp140C (rgp140C), to boost responses induced by a multigene/multisubtype HIV DNA vaccine delivered by electroporation (EP). Homologous DNA immunizations augmented by EP stimulated strong cellular immune responses. Still stronger cellular immune responses were observed after DNA priming and MVA-CMDR boosting, which was superior to all other immunization schedules tested in terms of antigen-specific IFN-γ, IL-2, and bifunctional IFN-γ and IL-2 responses. For HIV Env-specific antibody responses, mice receiving repeated rgp140C immunizations, and mice boosted with rgp140C, elicited the highest binding titers and the highest numbers of antibody-secreting B cells. When considering both cellular and humoral immune responses, a combination of DNA, MVA-CMDR, and rgp140C immunizations induced the overall most potent immune responses and the highest avidity of HIV Env-specific antibodies. These data emphasize the importance of including multiple vaccine modalities that can stimulate both T and B cells, and thus elicit strong and balanced immune responses. The present HIV vaccine combination holds promise for further evaluation in clinical trials.
Introduction
Several means of enhancing the potency of plasmid DNA vaccines have been evaluated. One of the most common approaches is to combine DNA priming immunizations with boosting immunizations using another vaccine modality such as a recombinant viral vector or a protein vaccine. This so-called heterologous prime-boost immunization strategy takes advantage of the unique immune profiles elicited by the different vaccine modalities (1,2). For both DNA and viral vectors the antigen is being expressed endogenously, which allows for post-translational modifications of the antigen, and the induction of humoral as well as cell-mediated immune responses. Protein vaccines, on the other hand, generally lack the ability to induce cytotoxic T-cell responses. Instead such vaccines are preferred when strong antibody responses are desired. Regardless if viral vectors or proteins are used for boosting, heterologous prime-boost immunization typically induces a more balanced immune response in terms of cellular and humoral immune responses, and has also proven to enhance the magnitude and quality of immune responses compared to homologous vaccination using either vaccine modality alone (3–5). Additionally, by using similar but not identical vaccine antigens during boosting, the breadth and the cross-reactivity to several variants of an antigen can be further increased (6–8).
For HIV, sterile protection against infection would require the induction of antibodies able to recognize a large number of HIV subtypes and strains. However, owing to the rather disappointing results from early HIV vaccine trials applying repeated protein immunizations to induce neutralizing antibodies (9,10), many HIV vaccine studies have employed a prime-boost strategy using DNA and viral vectors, with the primary aim being to induce T-cell responses that suppress HIV replication (11–14). The immunogenicity and efficacy of this strategy have been demonstrated in several animal models, including rhesus monkeys, where DNA priming followed by boosting with recombinant modified vaccinia Ankara (MVA) (15) or adenoviral (Ad) (16) vaccine vectors have been shown to protect monkeys from subsequent challenge with pathogenic simian HIV (SHIV). More importantly, DNA priming followed by viral vector boost immunizations can induce strong immune responses in humans (1,4,7,17–19).
Although recombinant subunit vaccines do not induce cytotoxic T-cell responses or potent HIV-specific neutralizing antibodies when used as a single vaccine modality, combining DNA prime and protein boost immunizations has been shown to enhance both cellular and humoral immune responses (20,21). Protein boosting can also increase the avidity and neutralization capacity of antibodies in rabbit (5,22–24) and human vaccine studies (8). The advantage of using protein as a boosting modality was perhaps most obvious in the RV144 HIV efficacy trial, where two seemingly poorly immunogenic vaccine candidates, one recombinant viral vector and one recombinant protein, were combined and shown to confer some protection against HIV-1 infection (31%, p=0.04) (25). Recent reports have suggested a possible association between antibodies directed to a conserved part of the V2 loop of gp120 and protection against HIV acquisition (26).
As an attempt to combat the vast genetic variability of HIV-1, we have constructed a DNA vaccine consisting of plasmids encoding HIV-1 antigens of different subtypes (27). To further enhance the potency of this vaccine, termed HIVIS, DNA priming immunizations are boosted with MVA encoding HIV antigens corresponding to the DNA prime (MVA-CMDR), but of different subtypes (28). The addition of the MVA-CMDR boost has been shown to enhance the magnitude and breadth of immune responses in mice (6) and humans (1,7). Since the HIVIS and MVA-CMDR vaccine combination primarily stimulates cellular immune responses, we evaluated the impact of boosting HIVIS and/or MVA-CMDR with a recombinant HIV gp140C (rgp140C) protein, which is a potent inducer of antibody responses (29). Furthermore, we assessed if in vivo electroporation [EP; i.e., the use of short electrical pulses that increase transfection efficacy and subsequently the immunogenicity of DNA vaccines (30)] could enhance the potency of repeated DNA immunizations to match immune responses induced by heterologous prime-boost immunizations.
We observed that DNA priming followed by an MVA-CMDR boost induced superior cellular immune responses, while rgp140C immunizations, alone or for boosting, elicited the highest antibody titers to Env. By combining all three vaccine modalities we show that both strong cell-mediated and humoral immune responses can be induced.
Material and Methods
Vaccine components
The HIVIS vaccine is composed of vaccine plasmids encoding several HIV-1 antigens of different subtypes (Gag p37A and B; Env gp160 A, B, and C; and reverse transcriptase [RT] and Rev B), and have been described in detail elsewhere (27,31–33). MVA-CMDR was designed and produced by the Walter Reed Army Institute, and encodes clade A Gag p55, protease, and RT, and clade E Env gp150 (28). Trimeric rgp140C was produced by Polymun Scientific (Vienna, Austria). Amino acid sequence similarities between vaccine constructs were determined using ALIGN web-based software (xylian.igh.cnrs.fr/bin/align-guess.cgi).
Immunizations
Female BALB/c mice (Charles River Laboratories, Sulzfeld, Germany), 5–9 weeks old, were immunized weeks 0, 4, and 8 with different combinations of HIVIS plasmids, MVA-CMDR, and rgp140C. DNA was diluted in saline and delivered intradermally (ID) by needle at two separate injection sites on the flank of the mice. Plasmids encoding Gag and RT were mixed and injected on one side (40 μg in 20 μL saline, 13.5 μg/plasmid), and Env- and Rev-encoding plasmids were mixed and delivered on the other side (40 μg in 20 μL saline, 10 μg/plasmid) (34). ID injections were directly followed by EP using the DermaVax™ EP device (Cellectis, Romainville, France). Then 107 plaque-forming units (PFU) of MVA-CMDR was delivered intramuscularly (IM) in the hind leg in 50 μL phosphate-buffered saline (PBS). Then 20 μg rgp140C was formulated in TiterMax Gold adjuvant (Sigma-Aldrich, St. Louis, MO), according to the manufacturer's instructions, to a final volume of 50 μL/mouse and injected subcutaneously. The study was repeated once.
In a subsequent experiment female BALB/c mice (Charles River Laboratories), 5–9 weeks old, were immunized weeks 0, 4, and 8 with rgp140C alone, or with rgp140C, and either MVA-CMDR (28), alum (MicroGeneSys Inc., West Haven, CT), TiterMax Gold adjuvant (Sigma-Aldrich), or wild-type MVA. A total of 20 μg rpg140C and adjuvant, or 107 PFU MVA, was mixed in 2×50 μL saline and delivered IM in both hind legs. MVA-CMDR and rgp140C were also injected separately in the opposite legs or at the same injection sites but subsequent to each other.
FluoroSpot and ELISpot
The mice were sacrificed 2 wk after the last immunization and the spleens were collected. Splenocytes were purified by Ficoll-Paque separation (GE Healthcare, Solna, Sweden), and cellular responses were assessed by interferon-γ (IFN-γ)/interleukin-2 (IL-2) FluoroSpot, or IFN-γ enzyme-linked immunosorbent spot (ELISpot) assays (Mabtech, Nacka Strand, Sweden), according to the manufacturer's guidelines and as previously described (35). Briefly, the plates used for both assays were treated with ethanol prior to antibody coating. In FluoroSpot assays, low-fluorescence plates were simultaneously coated with monoclonal antibodies AN18 and 1A12 (1.5 μg/well/antibody). In ELISpot assays, the plates were coated with either AN18 for IFN-γ detection or 1A12 for IL-2 detection (1.5 μg/well). Then 1×105 viable cells were plated per well and stimulated in 5% CO2 at 37°C for 20 h with: peptide pools of 15mers with 10-amino acid overlap representing Gag p55 A and p24 B and Env gp160 B and gp150 E; MHC class I-restricted epitopes AMQMLKETI (H2-Kd, Gag p24) and RGPGRAFVTI (H2-Dd, Env gp120); and rgp140C. The peptides and rgp140C used for stimulation were added in a concentration of 5 μg/mL/peptide and 10 μg/mL, respectively. The remaining cells were frozen group-wise. In FluoroSpot assays, a co-stimulatory anti-CD28 monoclonal antibody (0.1 μg/mL) was added to the cells during incubation. ELISpot plates were developed with biotinylated detection monoclonal antibodies R4-6A2 (1 μg/mL) or 5H4 (1 μg/mL), followed by streptavidin-ALP and BCIP/NBT substrate. In the FluoroSpot assay, bound cytokines were detected using FITC-labeled R4-6A2 and biotinylated 5H4, followed by anti-FITC antibody conjugated to a green fluorochrome, and streptavidin conjugated to a red fluorochrome. The numbers of spot-forming cells (SFCs) in the ELISpot and FluoroSpot assays were determined using an iSpot reader (AID GmbH, Strassberg, Germany), with software enabling overlay analysis of cells secreting both cytokines.
ELISA
Serum from individual mice was collected before each immunization and 2 wk after the last immunization at the time of sacrifice. Antibody titers to Gag p17/p24B (ARP6010; Centre for AIDS Reagents, NIBSC), Env gp 160B (MicroGeneSys) (10), and rgp140C, were assessed by enzyme-linked immunosorbent assay (ELISA). Plates were coated with 1 μg/mL of each antigen and the assay was performed as previously described (36).
Avidity of Gag- and Env-specific antibodies was examined by ELISA in serum pooled group-wise. The ELISA was performed as described (36), with some minor changes (37). Briefly, serum dilutions were added to plates in duplicate, and one-half of the samples were incubated in 8 M urea (Sigma-Aldrich), and the other half were incubated in saline for 5 min following incubation of serum dilutions.
Levels of IgG1 and IgG2a antibody titers to the Gag and Env antigens in serum samples pooled group-wise were examined using the Mouse Monoclonal Antibody Isotyping Reagents (Sigma-Aldrich) according to the manufacturer's protocol.
B cell ELISpot
The numbers of gp140C-specific antibody-secreting cells (ASCs) were determined using a B-cell ELISpot assay (Mabtech) according to the manufacturer's guidelines. Briefly, plates were treated with ethanol prior to coating with either 10 μg/mL rgp140C or 15 μg/mL α-IgG in PBS and incubated overnight. Previously purified splenocytes were thawed and viable cells were counted. Then 5×105 and 1×105 cells were plated per well for the detection of gp140C-specific and total IgG ASCs, respectively.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA), and a two-tailed Mann-Whitney U test was used to analyze differences between groups. p Values<0.05 were taken as significant.
Results
DNA prime and MVA-CMDR boost induce the strongest cell-mediated immune responses
Mice were immunized with combinations of multigene HIVIS DNA, MVA-CMDR, and rgp140C, with the aim of evaluating different boosting regimens for DNA priming immunizations delivered ID with EP (Table 1). The Env- and Gag-encoding plasmids were delivered at separate injection sites to avoid interference between these immunogens (34). Cellular responses were assessed by IFN-γ/IL-2 FluoroSpot, which detects and quantifies cells secreting IFN-γ, IL-2, or both cytokines (35).
Table 1.
Immunization Schedule
| Group | Week 0 | Week 4 | Week 8 |
|---|---|---|---|
| DDD | DNA | DNA | DNA |
| DDP | DNA | DNA | rgp140C |
| DDM | DNA | DNA | MVA-CMDR |
| DMP | DNA | MVA-CMDR | rgp140C |
| _MP | _ | MVA-CMDR | rgp140C |
| PPP | rgp140C | rgp140C | rgp140C |
| Naive | _ | _ | _ |
DNA, multigene/multisubtype HIVIS vaccine (subtypes A, B, and C); M, modified vaccinia Ankara, MVA-CMDR (subtypes A and E); P, recombinant gp140C (subtype C).
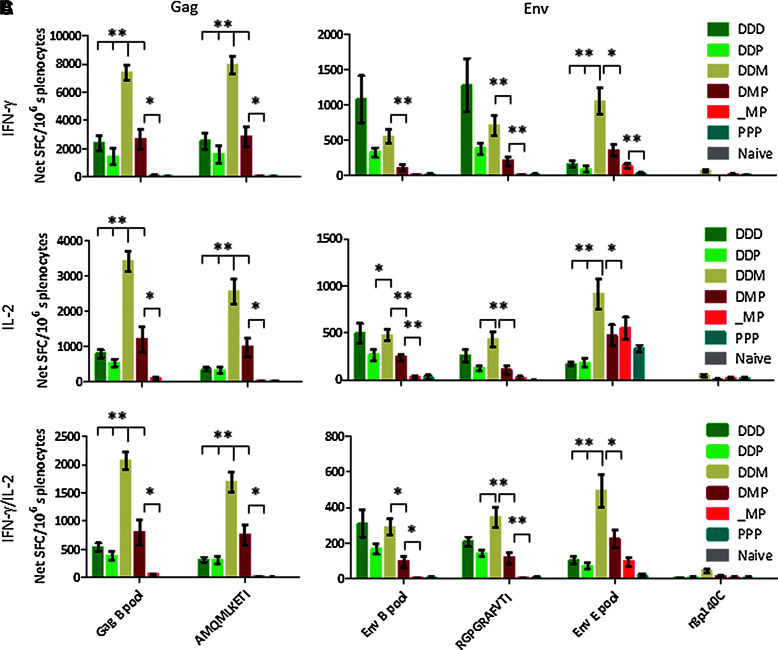
First we investigated whether the addition of EP could enhance the potency of repeated DNA immunizations to match the immune responses induced by heterologous prime-boost immunizations. Hence three repeated ID DNA immunizations with EP were compared to two ID DNA immunizations with EP, followed by immunization with either MVA-CMDR or rgp140C. Repeated DNA immunizations with EP induced strong Gag and Env clade B-specific IFN-γ and IL-2 responses (Fig. 1). When substituting the last DNA immunization with an rgp140C boost, the IFN-γ and IL-2 responses appeared somewhat weaker than after repeated DNA immunizations (p>0.05).
FIG. 1.
Cellular immune responses after prime-boost vaccination. Cellular immune responses were assessed by IFN-γ/IL-2 FluoroSpot on splenocytes collected 2 weeks after the last immunization. The FluoroSpot assay detects cells secreting IFN-γ (A), IL-2 (B), or both (C) cytokines in response to various stimuli. A Gag B peptide pool and the MHC class I-restricted AMQMLKETI peptide (present in DNA [Gag A and B] and MVA-CMDR [Gag A]) were used to assess Gag-specific responses. Env B and E peptide pools, MHC class I-restricted RGPGRAFVTI peptide (present in DNA [Env B]), and recombinant gp140C, were used to assess Env-specific responses. Bars represent mean values with standard error of the mean (n=6; *p<0.05, **p<0.01; D, DNA; M, MVA-CMDR; P, rgp140C).
When DNA priming immunizations were boosted with MVA-CMDR, the Gag B- and Env E-specific IFN-γ and IL-2 responses were significantly stronger compared to both repeated DNA immunizations, as well as DNA followed by rgp140C. However, DNA prime-MVA-CMDR boost and repeated DNA immunizations induced similar levels of Env B-specific IFN-γ and IL-2 responses. The discrepancy between Gag and Env B responses is most probably a consequence of a high amino acid sequence similarity between Gag B and A (87.5% sequence similarity), and less sequence similarity between Env B and E (71.7% sequence similarity), encoded by HIVIS DNA and MVA-CMDR, respectively. Moreover, the MHC class I-restricted AMQMLKETI (H2-Kd, Gag p24) peptide is present in both HIVIS DNA and MVA-CMDR, whereas the MHC class I-restricted RGPGRAFVTI (H2-Dd, Env gp120) peptide is only represented in the DNA.
Combining single immunizations with DNA, MVA-CMDR, and rgp140C, induced somewhat stronger Gag B and Env E, but not Env B, cellular responses than did both repeated DNA immunizations and DNA followed by rgp140C (p>0.05). Again, the lack of Env B responses is most likely explained by the rather low sequence similarity between Env B and Env E that are encoded by HIVIS DNA and MVA-CMDR, respectively. Still, the combination of three vaccine modalities did not induce as potent cellular immune responses as when employing DNA priming immunizations followed by MVA-CMDR boost, probably because one potent DNA immunization was exchanged for what was in terms of IFN-γ and IL-2 responses, a poorly immunogenic rgp140C immunization. Interestingly, when excluding the priming DNA immunization, leaving only the MVA-CMDR and rgp140C immunizations, almost no Gag B or Env B responses were detected. This clearly demonstrates the importance of including the multisequence DNA, representing subtypes A, B, and C, in the priming immunizations.
Stimulation with peptide pools representing the full Gag and Env B antigens induced similar magnitudes of IFN-γ and IL-2 responses as the single MHC class I-restricted AMQMLKETI (H2-Kd, Gag p24), and RGPGRAFVTI (H2-Dd, Env gp120) peptides (p>0.05 for all groups and antigens). This demonstrates that CD8+ T cells account for the majority of the cellular immune responses induced.
Boosting with rgp140C enhances the magnitude of Env-specific antibody responses
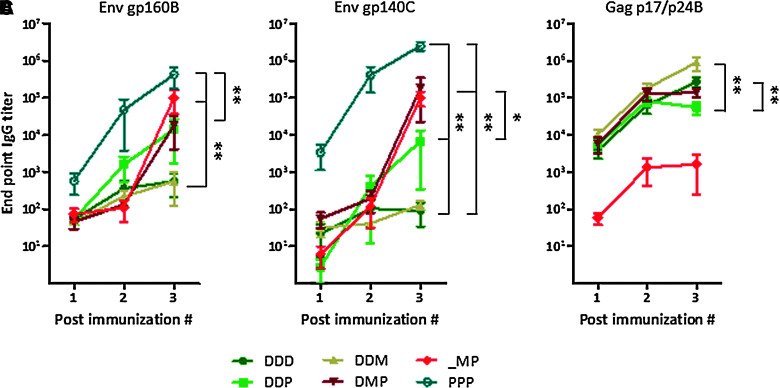
Binding antibody titers to recombinant gp160B, gp140C, and p17/p24B were assessed by ELISA with sera collected after each immunization. The different prime-boost immunization protocols elicited similar levels of gp160B- and gp140C-specific antibodies, demonstrating that these antibodies were able to cross-react between different subtypes of Env (Fig. 2A and B). The overall highest antibody titers to both gp160B and gp140C were induced after repeated rgp140C immunizations, confirming the ability of protein immunogens to induce strong humoral immune responses.
FIG. 2.
Humoral immune responses after prime-boost immunizations. Antibody titers to (A) gp160B, (B) gp140C, and (C) p17/p24B were measured by ELISA on serum collected before each immunization and 2 weeks after the last immunization. Results are shown as mean values and standard error of the mean (n=6; *p<0.05, **p<0.01; D, DNA; M, MVA-CMDR; P, rgp140C).
Repeated DNA immunizations and DNA priming immunizations followed by an MVA-CMDR boost induced similar and low levels of Env antibodies, whereas DNA priming followed by rgp140C boost appeared to induce somewhat improved Env antibody titers (p>0.05; Fig. 2A and B). For Gag-specific antibodies, repeated DNA immunizations and DNA priming immunizations followed by an MVA-CMDR boost induced strong antibody responses (Fig. 2C).
Combining DNA, MVA-CMDR, and rgp140C immunizations generated higher magnitudes of gp140C-specific antibodies than the DNA prime-rgp140C boost approach. For Gag-specific antibodies, this combined approach induced similar levels of antibodies as repeated DNA immunizations. Unlike what was observed for cellular responses, there was no difference in the magnitude of Env-specific antibodies when we excluded the DNA priming immunizations, leaving only the MVA-CMDR and rgp140C immunizations. However, antibody responses to Gag were significantly lower when we excluded the DNA prime (p=0.0043). Since the DNA was the only vaccine component containing Gag B, this emphasizes the importance of including several subtypes for the induction of antibodies.
DNA and MVA-CMDR induce different isotypes of IgG
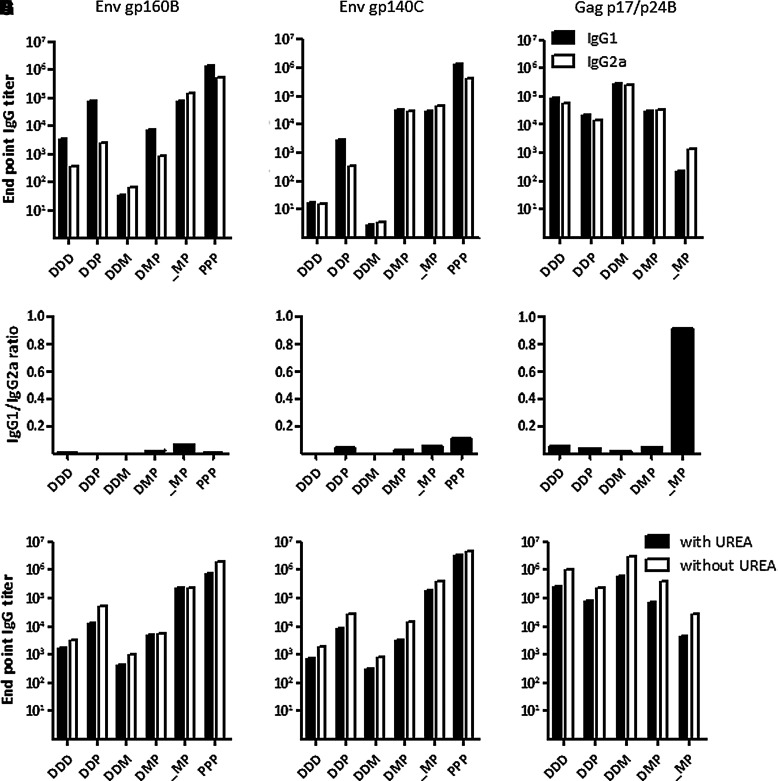
Levels of IgG1 and IgG2a antibodies were examined to determine whether the different prime-boost protocols skew the immune response towards a Th-2- or Th-1-type of immune response, respectively. Since the ratio of titers between IgG subtypes would not account for the sometimes vast difference in antibody titers between groups, we chose to present both the IgG1:IgG2a ratio, as well as the binding titers of each isotype. Only end-point titers above 102 were regarded as positive.
Mice receiving DNA priming immunizations and mice repeatedly injected with rgp140C had stronger IgG1 responses, indicating a Th-2-skewed antibody response (Fig. 3A–F). MVA-CMDR and rgp140C immunization without DNA prime, on the other hand, induced slightly more IgG2a, indicating a Th-1-skewed immune response that would suggest that the initial MVA-CMDR immunization directed the immune response towards a Th-1-type of immune response.
FIG. 3.
Isotype distribution and avidity of antibodies after prime-boost immunizations. Titers of IgG1 and IgG2a to (A) gp160B, (B) gp140C, and (C) p17/p24B, were assessed by ELISA on pooled serum (n=6) collected 2 weeks after the last immunization. Additionally, ratios of IgG1 to IgG2a titers were calculated for (D) gp160B, (E) gp140C, and (F) p17/p24B. Avidity of binding antibodies to (G) gp160B, (H) gp140C, and (I) p17/p24B were determined on pooled sera (n=6) collected 2 weeks after the last immunization. Duplicates of serum dilutions were incubated in an ELISA assay followed by incubation in either urea or PBS. The difference in titers with and without incubation with urea corresponds to the avidity of antibodies (D, DNA; M, MVA-CMDR; P, rgp140C).
MVA-CMDR prime enhances avidity of Env-specific antibodies
To further evaluate the quality of antibody responses, we examined the avidity of Env- and Gag-specific antibodies using urea treatment. Similarly to the IgG subtyping, the results are presented as titers with or without urea treatment.
As expected, incubation with urea reduced the binding titers to gp160B, gp140C, and p17/p24B in most of the groups (Fig. 3G–I). However, one exception was observed in serum from mice immunized with MVA-CMDR and rgp140C with or without a DNA priming immunization. These sera demonstrated almost identical levels of gp160B-specific antibodies both with and without incubation in urea, suggesting that the Env E encoding MVA-CMDR can efficiently prime the subsequent rgp140C boost to induce high-avidity antibodies. Additionally, repeated rgp140C immunizations induced high-avidity antibodies to the corresponding antigen.
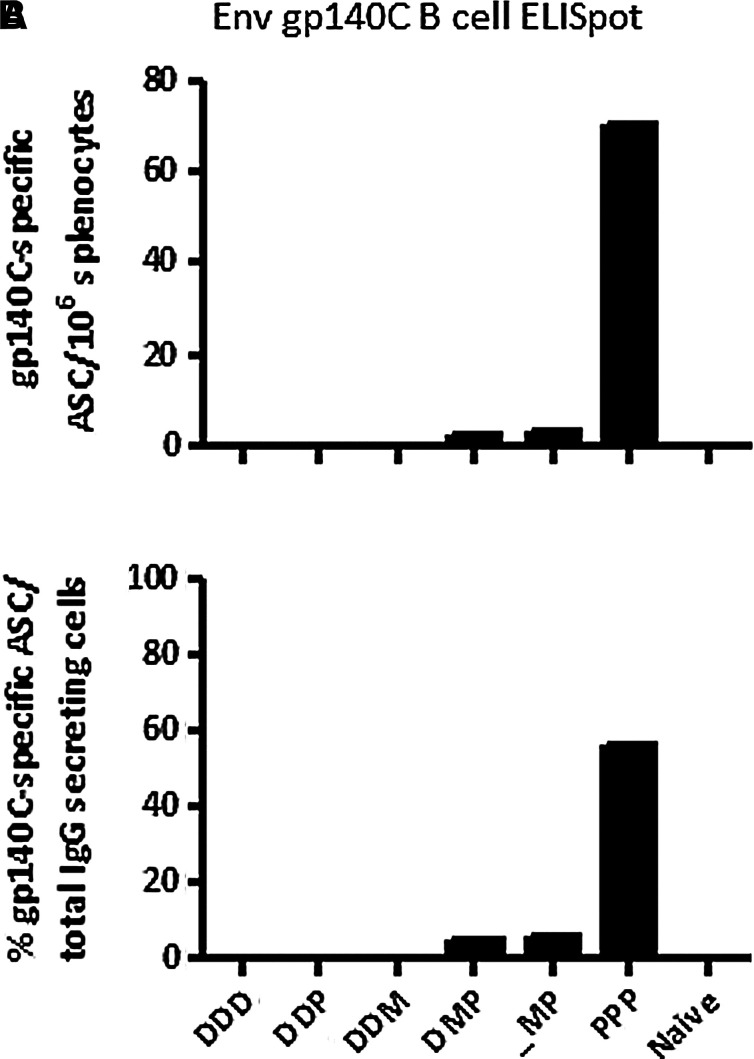
Repeated rgp140C immunizations induce the highest number of antibody-secreting cells
The number of gp140C-specific antibody-secreting cells (ASCs) was determined by B-cell ELISpot on thawed splenocytes pooled group-wise. Repeated immunizations with rgp140C induced the highest number of ASCs (Fig. 4). Also MVA-CMDR and rgp140C immunizations with or without a DNA priming immunization induced gp140-specific ASCs, but of considerably lower quantities. Hence, ASCs detected in the B-cell ELISpot assay mirrored the accumulated serum antibody titers to rgp140C as measured by ELISA.
FIG. 4.
Quantification of antibody-secreting cells (ASCs). The number of gp140C-specific IgG-secreting cells was determined by B-cell ELISpot on splenocytes pooled group-wise (n=6). (A) Number of gp140C-specific ASCs/million splenocytes. (B) Percent gp140C-specific ASCs of total IgG-secreting cells (D, DNA; M, MVA-CMDR; P, rgp140C).
Effect of MVA-CMDR on rgp140C-specific immune responses
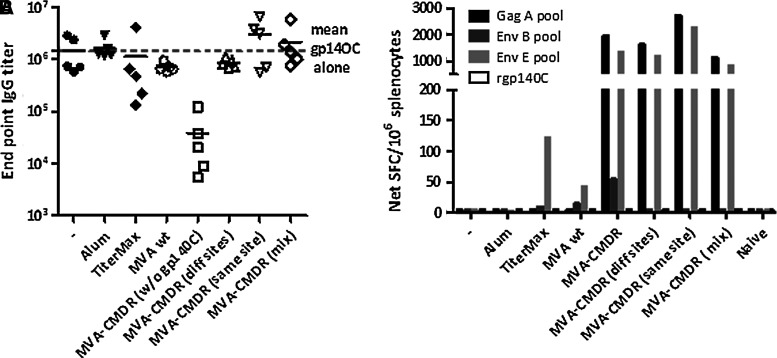
To evaluate if the inherent ability of MVA to stimulate innate immune responses could act to enhance immune responses to rgp140C, MVA-CMDR and rgp140C were injected as two separate entities into each hind leg of the mouse, at the same injection site one after the other, or as a mixture. Alum and TiterMax mixed with rgp140C were compared with MVA-CMDR for their ability to augment gp140C-specific immune responses. Immunizations with wild-type MVA mixed with rgp140C were included to determine the augmenting effect of the MVA vector alone.
After three immunizations, neither alum, Titermax, or wild-type MVA enhanced the magnitude of gp140C-specific antibody titers (Fig. 5A). MVA-CMDR tended to enhance antibody responses when the two vaccine modalities were delivered at the same injection site or as a mixture, but the increase was not significant (p=0.5476 and p=0.4206, respectively). Similarly to what was observed in the previous experiment, MVA-CMDR, but not rgp140C, elicited IFN-γ responses (Fig. 5B).
FIG. 5.
Effect of MVA-CMDR on rgp140C-specific immune responses. BALB/c mice were immunized with rgp140C alone or mixed with the adjuvants wild-type (wt) MVA or MVA-CMDR. rgp140C and MVA-CMDR were also delivered to different injection sites and subsequent to each other at the same injection site. (A) Binding gp140C-specific antibody titers were assessed by ELISA on sera collected 2 weeks after the third and last immunization. (B) Cell-mediated immune responses were measured by IFN-γ ELISpot on splenocytes pooled group-wise (n=5). A Gag A peptide pool was used to assess Gag-specific immune responses. Env B and E peptide pools and rgp140C were used to assess Env-specific responses.
Discussion
In this study we analyzed different prime-boost immunization protocols with multigene HIVIS DNA (subtypes A, B, and C) priming followed by boosting with MVA-CMDR and/or an rgp140C protein vaccine (Table 1). The MVA-CMDR (subtype A_E) and rgp140C (subtype C) vaccine candidates used for boosting represent the antigens present in the HIVIS vaccine, but of different subtypes. Hence the prime-boost immunizations can be regarded as heterologous in terms of vaccine modalities as well as antigen subtypes.
The study showed that the combination of DNA, MVA-CMDR, and rgp140C induced high antibody titers directed to both Env and Gag. Strong IFN-γ, IL-2, and IFN-γ/IL-2 responses were also elicited, particularly after stimulation with peptides representing or resembling the antigens encoded by MVA-CMDR. Interestingly, when excluding the DNA prime, leaving only MVA-CMDR and rgp140C immunizations, the cellular immune responses as well as Gag-specific antibodies were drastically reduced, stressing the importance of a DNA prime prior to MVA-CMDR and rgp140C boost. For Env-specific antibodies, however, excluding the DNA prime did not influence the magnitude of antibody responses, indicating that a single immunization with the gp160 DNA constructs does not efficiently prime a B-cell response to Env. Only a few publications have studied a similar set up with three different vaccine modalities. These include studies in mice in which DNA, MVA, and protein vaccines representing Plasmodium falciparum or hepatitis B antigens enhanced antibody responses compared to immunizations with DNA and recombinant MVA (38,39). A study in macaques examined the effect of boosting multigene HIV DNA and MVA immunizations by co-delivering rgp120 with the second DNA prime and the MVA booster immunization. The addition of rgp120 induced only a transient increase in Env-specific antibody titers, which failed to protect from subsequent SHIV challenge (40).
We also examined how immune responses induced by repeated ID DNA immunizations augmented by EP compared to immune responses induced by DNA priming with EP followed by boosting with MVA-CMDR, rgp140C, or both (Table 1). EP-augmented ID DNA delivery has been shown to efficiently boost DNA priming immunizations without EP, and induces stronger cell-mediated and humoral immune responses than when using a protein boost (41). Based on this finding, and the fact that EP considerably enhances transfection efficacy of plasmids and thus immune responses (30,42), we hypothesized that repeated DNA delivery by EP would induce immune responses similarly to the DNA prime-MVA-CMDR boost, which in our hands has been the optimal immunization strategy for the induction of strong cellular immune responses. However, cell-mediated immune responses induced by repeated DNA immunizations with EP could not match the magnitude of those elicited by DNA prime with EP and MVA-CMDR boost, which was superior to all other immunization protocols tested in its cellular immune responses to antigens represented by the different vaccine modalities. We thus confirm our findings, as well as those of others, that a combination of DNA and viral vectors, both having the potential to induce potent T-cell responses, is superior for the induction of optimal cell-mediated immune responses (3,4,6). Repeated DNA immunizations with EP as well as DNA prime-MVA-CMDR boost induced modest Env-specific antibody responses, but high binding titers to Gag, similarly to our previous observations (6).
Although protein immunogens are regarded as poor inducers of cell-mediated immune responses, studies in animal models and humans have shown that adding a protein-boosting immunization to DNA-priming immunizations can enhance the magnitude and quality of cellular immune responses (8,21). Still, our study showed that replacing the last of three DNA immunizations with a rgp140C boost did not elicit stronger IFN-γ/IL-2 responses than three homologous DNA immunizations, or two DNA-priming immunizations followed by an MVA-CMDR boost. Moreover, except for IL-2 responses, repeated rgp140C immunizations failed to induce detectable cell-mediated immune responses, indicating the limited use of these immunogens alone for the induction of cellular immune responses. For humoral responses, on the other hand, repeated protein immunizations elicited exceptional levels of antibody titers to Env of matched and unmatched subtypes. Mice boosted with rgp140C induced higher titers to Env antigens than mice immunized with repeated DNA or DNA followed by MVA-CMDR. These results were confirmed by the gp140C B-cell ELISpot.
One of the attractive features of heterologous prime-boost immunization is that a more balanced immune response can be induced compared to immunization with either vaccine alone (43,44). Here we show that priming immunizations with DNA or repeated immunizations with rgp140C induced a Th-2-skewed immune response. In contrast, excluding the DNA prime, leaving only MVA-CMDR followed by rgp140C immunization, slightly skewed the immune response towards a Th-1-type of response to all antigens, demonstrating the capacity of MVA to induce a strong Th-1-response. In addition, the avidity of Env B-specific antibodies was also strongest after MVA-CMDR and rgp140C immunizations. This was surprising, as DNA priming has been reported to enhance the avidity of antibodies induced after boosting with protein immunogens (5,22). Thus in these settings, priming with recombinant MVA was superior to DNA for the induction of high-avidity antibodies.
The inherent ability of viral vectors to stimulate innate immune responses (45) can contribute to the induction of strong antigen-specific immune responses. For example, Hutchings et al. demonstrated that both recombinant and non-recombinant MVA increased antibody responses to a recombinant hepatitis B surface antigen (38), and a recombinant poxvirus added to a protein immunogen was successful in the RV144 trial (25). We thus evaluated the adjuvant effect of MVA-CMDR for rgp140C. The only groups showing signs of improved antibody responses to gp140C were mice immunized with a mixture of MVA-CMDR and rgp140C, or MVA-CMDR and rgp140C delivered subsequent to each other at the same injection site. This was not seen when MVA-CMDR and protein were injected in separate legs, and the modest cross-reactive antibodies to Env E elicited by MVA-CMDR alone does not seem to account for the somewhat higher antibody titers seen when MVA-CMDR and rgp140C are delivered at the same injection site. Still, in our hands the delivery of rgp140C with wild-type MVA or MVA-CMDR did not enhance gp140C-specific binding titers significantly.
Taken together, our results support the use of heterologous prime-boost immunizations to enhance both the magnitude and quality of vaccine-induced immune responses. By combining three vaccine modalities of different subtypes, a balanced immune response including both cell-mediated immune responses and high-magnitude binding antibodies was induced. The combined vaccine approach also demonstrates increased breadth as immune responses to several immunogens were induced. Thus, using several vaccine modalities representing full-length natural sequences could constitute a powerful means of inducing broad immune responses on various human leukocyte antigen backgrounds. Additionally, as opposed to, for example mosaic sequences (46), these antigens retain the ability to stimulate potent antibody responses to linear and conformational epitopes. Even though the immunization protocols were limited to three immunization events to maintain a feasible protocol for the clinic, this is a rather advanced immunization schedule. Still, the benefits of improved immune responses may outweigh the complexity, especially for hard-to-target diseases like HIV. The combination of multigene HIV DNA, MVA-CMDR, and rgp140C, will be evaluated in a clinical trial, and the results presented herein are supportive of this concept.
Acknowledgments
We thank the EU programs EUROPRISE (LSHP-CT-2006-037611) and NGIN (Health-F3-2008-201433) for funding, and Polymun Scientific for supplying the gp140C protein vaccine candidate.
Author Disclosure Statement
A.K.M. was employed at Cyto Pulse Sciences Inc., and K.N. is employed at Mabtech AB. None of the other authors have any competing financial interests.
References
- 1.Bakari M. Aboud S. Nilsson C, et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine. 2011;29:8417–8428. doi: 10.1016/j.vaccine.2011.08.001. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill AV. Reyes-Sandoval A. O'Hara G, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. . [DOI] [PubMed] [Google Scholar]
- 3.Kent SJ. Zhao A. Best SJ, et al. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koup RA. Roederer M. Lamoreaux L, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One. 2010;5:e9015. doi: 10.1371/journal.pone.0009015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaine M. Wang S. Hackett A, et al. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine. 2010;28:2999–3007. doi: 10.1016/j.vaccine.2010.02.006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brave A. Boberg A. Gudmundsdotter L, et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther. 2007;15:1724–1733. doi: 10.1038/sj.mt.6300235. . [DOI] [PubMed] [Google Scholar]
- 7.Sandstrom E. Nilsson C. Hejdeman B, et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198:1482–1490. doi: 10.1086/592507. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S. Kennedy JS. West K, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:3947–3957. doi: 10.1016/j.vaccine.2007.12.060. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitisuttithum P. Gilbert P. Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. . [DOI] [PubMed] [Google Scholar]
- 10.Sandstrom E. Wahren B. Therapeutic immunisation with recombinant gp160 in HIV-1 infection: a randomised double-blind placebo-controlled trial. Nordic VAC-04 Study Group. Lancet. 1999;353:1735–1742. doi: 10.1016/s0140-6736(98)06493-9. . [DOI] [PubMed] [Google Scholar]
- 11.Deeks SG. Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. . [DOI] [PubMed] [Google Scholar]
- 12.Kiepiela P. Ngumbela K. Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. . [DOI] [PubMed] [Google Scholar]
- 13.Koup RA. Safrit JT. Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland-Jones SL. Dong T. Fowke KR, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–1765. doi: 10.1172/JCI4314. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amara RR. Villinger F. Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. . [DOI] [PubMed] [Google Scholar]
- 16.Shiver JW. Fu TM. Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. . [DOI] [PubMed] [Google Scholar]
- 17.De Rosa SC. Thomas EP. Bui J, et al. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol. 2011;187:3391–3401. doi: 10.4049/jimmunol.1101421. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari A. Bart PA. Stohr W, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaoko W. Karita E. Kayitenkore K, et al. Safety and immunogenicity study of multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010;5:e12873. doi: 10.1371/journal.pone.0012873. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett SW. Rajasekar S. Legg H, et al. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. doi: 10.1016/s0264-410x(96)00264-2. . [DOI] [PubMed] [Google Scholar]
- 21.Cristillo AD. Wang S. Caskey MS, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006;346:151–168. doi: 10.1016/j.virol.2005.10.038. . [DOI] [PubMed] [Google Scholar]
- 22.Richmond JF. Lu S. Santoro JC, et al. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72:9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S. Arthos J. Lawrence JM, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79:7933–7937. doi: 10.1128/JVI.79.12.7933-7937.2005. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S. Pal R. Mascola JR, et al. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350:34–47. doi: 10.1016/j.virol.2006.02.032. . [DOI] [PubMed] [Google Scholar]
- 25.Rerks-Ngarm S. Pitisuttithum P. Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. . [DOI] [PubMed] [Google Scholar]
- 26.Nakamura GR. Fonseca DP. O'Rourke SM, et al. Monoclonal antibodies to the V2 domain of MN-rgp120: Fine mapping of epitopes and inhibition of alpha4beta7 binding. PLoS One. 2012;7:e39045. doi: 10.1371/journal.pone.0039045. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brave A. Ljungberg K. Boberg A, et al. Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol Ther. 2005;12:1197–1205. doi: 10.1016/j.ymthe.2005.06.473. . [DOI] [PubMed] [Google Scholar]
- 28.Earl PL. Cotter C. Moss B, et al. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine. 2009;27:5885–5895. doi: 10.1016/j.vaccine.2009.07.039. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cranage MP. Fraser CA. Stevens Z, et al. Repeated vaginal administration of trimeric HIV-1 clade C gp140 induces serum and mucosal antibody responses. Mucosal Immunol. 2010;3:57–68. doi: 10.1038/mi.2009.110. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos AK. Eriksson F. Timmons JA, et al. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaguliants MG. Petrakova NN. Zuber B, et al. DNA-encoding enzymatically active HIV-1 reverse transcriptase, but not the inactive mutant, confers resistance to experimental HIV-1 challenge. Intervirology. 2000;43:288–293. doi: 10.1159/000053996. . [DOI] [PubMed] [Google Scholar]
- 32.Kjerrstrom A. Hinkula J. Engstrom G, et al. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology. 2001;284:46–61. doi: 10.1006/viro.2001.0905. . [DOI] [PubMed] [Google Scholar]
- 33.Ljungberg K. Rollman E. Eriksson L, et al. Enhanced immune responses after DNA vaccination with combined envelope genes from different HIV-1 subtypes. Virology. 2002;302:44–57. doi: 10.1006/viro.2002.1547. . [DOI] [PubMed] [Google Scholar]
- 34.Brave A. Ljungberg K. Boberg A, et al. Reduced cellular immune responses following immunization with a multi-gene HIV-1 vaccine. Vaccine. 2006;24:4524–4526. doi: 10.1016/j.vaccine.2005.08.018. . [DOI] [PubMed] [Google Scholar]
- 35.Hallengard D. Haller BK. Maltais AK, et al. Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation. Clin Vaccine Immunol. 2011;18:1577–1581. doi: 10.1128/CVI.05045-11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallengard D. Haller BK. Petersson S, et al. Increased expression and immunogenicity of HIV-1 protease following inactivation of the enzymatic activity. Vaccine. 2011;29:839–848. doi: 10.1016/j.vaccine.2010.10.083. . [DOI] [PubMed] [Google Scholar]
- 37.Ohlin M. Hinkula J. Broliden PA, et al. Human MoAbs produced from normal, HIV-1-negative donors and specific for glycoprotein gp120 of the HIV-1 envelope. Clin Exp Immunol. 1992;89:290–295. doi: 10.1111/j.1365-2249.1992.tb06947.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchings CL. Gilbert SC. Hill AV. Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175:599–606. doi: 10.4049/jimmunol.175.1.599. . [DOI] [PubMed] [Google Scholar]
- 39.Miao J. Li X. Liu Z, et al. Immune responses in mice induced by prime-boost schemes of the Plasmodium falciparum apical membrane antigen 1 (PfAMA1)-based DNA, protein and recombinant modified vaccinia Ankara vaccines. Vaccine. 2006;24:6187–6198. doi: 10.1016/j.vaccine.2006.05.099. . [DOI] [PubMed] [Google Scholar]
- 40.Buge SL. Ma HL. Amara RR, et al. Gp120-alum boosting of a Gag-Pol-Env DNA/MVA AIDS vaccine: poorer control of a pathogenic viral challenge. AIDS Res Hum Retroviruses. 2003;19:891–900. doi: 10.1089/088922203322493067. . [DOI] [PubMed] [Google Scholar]
- 41.Brave A. Hallengard D. Gudmundsdotter L, et al. Late administration of plasmid DNA by intradermal electroporation efficiently boosts DNA-primed T and B cell responses to carcinoembryonic antigen. Vaccine. 2009;27:3692–3696. doi: 10.1016/j.vaccine.2009.04.013. . [DOI] [PubMed] [Google Scholar]
- 42.Vasan S. Hurley A. Schlesinger SJ, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazumder S. Maji M. Das A. Ali N. Potency, efficacy and durability of DNA/DNA, DNA/protein and protein/protein based vaccination using gp63 against Leishmania donovani in BALB/c mice. PLoS One. 2011;6:e14644. doi: 10.1371/journal.pone.0014644. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang QM. Sun SH. Hu ZL, et al. Improved immunogenicity of a tuberculosis DNA vaccine encoding ESAT6 by DNA priming and protein boosting. Vaccine. 2004;22:3622–3627. doi: 10.1016/j.vaccine.2004.03.029. . [DOI] [PubMed] [Google Scholar]
- 45.Delaloye J. Roger T. Steiner-Tardivel QG, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Barouch DH. O'Brien KL. Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–323. doi: 10.1038/nm.2089. . [DOI] [PMC free article] [PubMed] [Google Scholar]