Abstract
The thalamus is a small, bilateral structure in the diencephalon that integrates signals from many areas of the CNS. This critical anatomical position allows the thalamus to influence whole-brain activity and adaptive behaviour. However, traditional research paradigms have struggled to attribute specific functions to the thalamus and it has remained under-studied in the human neuroimaging literature. Recent advances in analytical techniques and increased accessibility to large, high-quality datasets have brought forth a series of studies and findings that (re)establish the thalamus as a core region of interest in human cognitive neuroscience, a field which otherwise remains cortico-centric. In this Perspective, we argue that using whole-brain neuroimaging approaches to investigate the thalamus and its interaction with the rest of the brain is key for understanding systems-level control of information processing. To this end, we highlight the role of the thalamus in shaping a range of functional signatures, including evoked activity, inter-regional connectivity, network topology and neuronal variability, both at rest and during the performance of cognitive tasks.
Introduction
The brain expresses rich, dynamic patterns of neural activity across multiple spatial and temporal scales. At the local level, the amplitude and tuning properties of neural responses exhibit task-dependent sensitivity1. Local neural responses are in turn transmitted across brain networks via white matter connections2, wherein inter-regional communication can be adjusted in response to cognitive and behavioural demands3–6. When considering the structural organization of brain networks (that is, topology), both micro-scale and macro-scale white-matter connections in the brain can be described as encompassing a modular architecture7, with tight interconnections between regions forming communities, which are then weakly connected to one another. This particular anatomical organization strikes a balance between segregated processes within local circuits and brain-wide integration2,8, and can be well-described by biophysical models9–11. In short, dynamic neural activities are richly expressed over seconds to minutes, and in circuits and systems, to facilitate a wide range of complex behaviours.
How does the brain instantiate this wide variety of spatiotemporal dynamics? An implicit assumption inherent within much of the human neuroimaging community is that these features arise due primarily to the organization of the cerebral cortex. As discussed in other reviews12–14, in this article, we argue that many features of neural activity, connectivity and topology measured by standard whole-brain neuroimaging approaches are inextricably linked to the organization of the subcortex — in particular, we focus our attention on the thalamus. A small, bilateral structure in the diencephalon (Box 1), the thalamus is highly interconnected with various structures in the CNS. It is sensitive to neuromodulatory input15,16, receives excitatory projections from both the superficial and deep superior colliculus17–19, integrates inputs from the cerebellum and the basal ganglia14,20, and projects to the cerebral cortex with a diverse range of different anatomical connectivity motifs21,22 (see REFS22–25 for excellent reviews of the complex anatomy of the thalamus and its connections with the rest of the nervous system; and Box 1 for more details). This dense interconnectivity places the thalamus in an ideal position to shape multiple aspects of brain dynamics and to contribute to diverse cognitive and behavioural functions.
Box 1 ∣. Thalamic neuroanatomy.
The thalamus is anatomically perched between the tectum and midbrain at one end, and the hypothalamus and telencephalon (that is, the cerebral cortex, basal ganglia and hippocampus, etc.) at the other. Like many other regions in the CNS, the thalamus comprises both excitatory and inhibitory neurons; however, their projection patterns are quite distinct. Excitatory thalamic neurons project primarily outside of the thalamus, albeit with fewer local synapses onto inhibitory neurons, such as those in the reticular nucleus. By contrast, inhibitory neurons have predominantly local effects. For instance, the GABAergic reticular nucleus engulfs the excitatory cells of the thalamus164, providing crucial inhibitory dampening of ongoing thalamic activity123,165 that is thought to play a crucial computational role in shaping feedback projections to the cerebral cortex166. By blanketing the thalamus with inhibition, the preferential lack of inhibition to a specific sub-set of thalamic neurons acts to boost the signal-to-noise properties of precisely the sub-set of regions that escape the reticular inhibition, particularly when compared to the surrounding, inhibited regions164,165,167. Optogenetic studies in rodents have provided empirical support for this hypothesis123,168. Specifically, focal stimulation of the reticular nucleus leads to sleep-like dynamics in the cortex that are spatially restricted to the area of inhibited thalamic projections123, suggesting that the reticular nucleus contains a spatially organized circuit that can enable focal enhancement or suppression of cortical processing (that is, what some might call the attentional searchlight166). The thalamus also receives inhibitory inputs from the pretectal nuclei and the zona incerta24,169, which in turn provide other circuits through which thalamic activity can be modulated.
Based on the patterns of afferent connections, excitatory thalamic nuclei are generally divided into two classes40: first-order thalamic nuclei (for example, the lateral geniculate nucleus), which are predominantly composed of neurons that receive ‘driver’ inputs from ascending sensory pathways (that is, large glutamatergic synapses that directly propagate action potentials in target cells40) or other subcortical brain regions and ‘modulatory’ input from the cerebral cortex (that is, change the excitability and receptivity of target cells; see REF.40 for further elaboration), and higher-order thalamic nuclei (for example, mediodorsal and pulvinar nuclei), which are composed of thalamic neurons that receive both driver and modulatory inputs from the cerebral cortex. Importantly, this two-class schema does not cover all glutamatergic thalamic populations — for instance, the intralaminar nuclei are a class of ‘nonspecific’ nuclei whose projections innervate multiple cortical regions throughout the cerebral cortex22 as well as the corpus striatum of the basal ganglia170. It is also possible to characterize thalamic regions according to their efferent projections to the cortex22. First, parvalbumin-staining ‘core’ cells (which are prevalent in sensory nuclei, but also exist within some higher-order thalamic nuclei) typically act as drivers of activity in relatively granular cortices, sending projections to layer IV of the cerebral cortex. Second, calbindin-staining ‘matrix’ cells (which are more common in higher-order nuclei22) fulfil a more integrative function by sending their projections to supragranular layers of cortex across multiple distinct neural regions, where they make contact with both cross-columnar layer II/III pyramidal cells and with apical dendrites of large, sub-cortically projecting thick-tufted, layer V pyramidal cells that recruit the involvement of diverse populations of subcortical regions171,172. It is important to note that the microarchitecture of the thalamus is highly diverse, with numerous individual neurons that are not easily classified into one of these groupings21, suggesting that is best to conceptualize these extreme classes as different ends of a complex spectrum.
There are several important subcortical inputs to the thalamus. For instance, there are strong excitatory projections from the retina and cochlea into the lateral geniculate nucleus and medial geniculate nucleus, respectively23, and the superficial superior colliculus acts as a way-station between different thalamic nuclei, in that it receives inputs from the lateral geniculate nucleus, but projects back to the pulvinar18. In the ventral tier of the thalamus, the core cells of the thalamus predominantly receive glutamatergic driver inputs, either from sensory nuclei or from the deep nuclei of the cerebellum14,62, whereas the calbindin-staining matrix cells are instead under the GABAergic control of the globus pallidus internus, which represents the main inhibitory output from the basal ganglia14,62. There are also other structures that send inhibitory projections into the thalamus, such as the zona incerta, pretectal nuclei and pontine reticular formation (for a review in rodents, see REF.24). Finally, there are many neuromodulatory structures in the brainstem, hypothalamus and basal forebrain that also strongly innervate the thalamus15,16,, including major histaminergic, noradrenergic, dopaminergic and cholinergic projections36,113,173, wherein they alter the gain and timescales of ongoing neural processing113. With all this said, it bears mention that the majority of information we have about thalamic circuitry arises from non-human model organisms, and there may be features of thalamic anatomy that are distinct across species.
Decades of important work in animal models — particularly in rodents, cats and non-human primates — has confirmed that the thalamus has a critical role in a wide range of cognitive processes. In more recent years, rodent models have enabled precise measurements and causal manipulations demonstrating key circuit properties of the thalamus. Many of these properties probably generalize to the human brain, as there is widespread homology in the morphology, physiology and gene expression of the thalamus across species26,27. There are however some differences between the rodent and human thalamus. For example, in humans, there is a greater proportion of GABAergic neurons in thalamic sensory nuclei28. Moreover, different mammalian species, including rodents, non-human primates and humans, have distinct neuromodulatory innervation pathways29,30. For these reasons, a deeper understanding of the human thalamus is needed, both to understand its shared and distinct functions relative to the thalamus in other species, and to understand its role in complex higher-order cognition.
While the physiology of the thalamus has been less studied in humans than in other species, the human thalamus is similarly implicated as a key node for coordinating cognitive function. Indeed, human lesion studies strongly suggest that the thalamus is involved in a wide range of cognitive functions. For instance, thalamic lesions are associated with aphasia31, amnesia32, executive dysfunction33, neglect and attention deficits34. Despite these compelling case reports and other tantalizing clues from early studies using electrophysiology35,36 and thalamic functional MRI (fMRI)37,38, there are few whole-brain neuroimaging approaches that directly study the thalamus. This is partly because MRI head coils are typically designed to augment signal quality in superficial structures (that is, the cortex). In addition, there have long been (perhaps overemphasized) concerns that the proximity of subcortical regions to ventricular structures can make it difficult to disentangle putative neural signals from ventricular noise39. However, as the resolution and sensitivity of fMRI have advanced in recent years, so too has our ability to detect and measure thalamic dynamics, leading to a rich set of new investigations of thalamocortical function in the human brain (Box 2). In addition, computational modelling approaches have further enhanced our understanding of thalamic computational properties in humans.
Box 2 ∣. Neuroimaging the thalamus: past, present, and future.
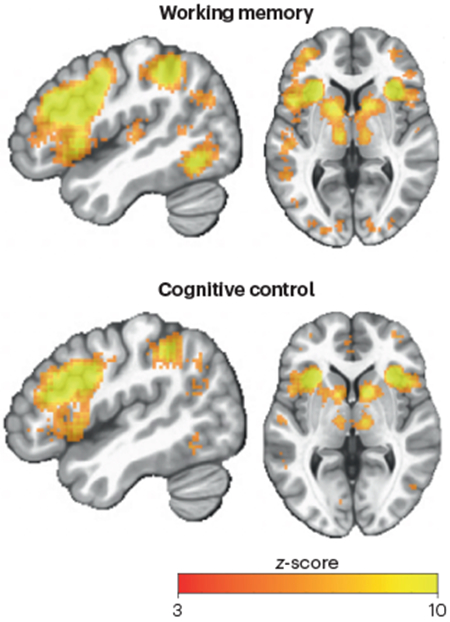
Cognitive neuroimaging has the potential to reveal how mental functions are supported by distributed brain regions and systems, owing to the whole-brain coverage of imaging methods such as functional MRI (fMRI). However, while fMRI can image functional signals from both cortical and subcortical structures, the field has remained largely cortico-centric. For example, neuroimaging studies on cognitive control have largely focused on determining the functional specialization and interactions of the frontoparietal systems, with relatively fewer studies focused on thalamic nuclei that are known to project to frontal parietal cortices. Nevertheless, the few studies that investigated thalamic contributions have reported notable task-linked activity in thalamus. This relative negligence of the thalamus may be due to several unfounded biases: the thalamus is small (relative to the resolution of typical functional neuroimaging scans), close to noise sources (for example, the ventricles) and not traditionally associated with higher-level mental functions, such as cognitive control. As a result, findings in the thalamus from human neuroimaging studies are often either omitted, reported with no interpretation, or discussed without consideration of its anatomical organization (that is, not describing the subregions involved, despite the major functional and anatomical differences between thalamic nuclei). Contrary to these pervasive biases, neuroimaging studies indeed obtain reliable task-related signals from the human thalamus with anatomical specificity that directly reflects cognitive capacities typically ascribed primarily to the cerebral cortex. Indeed, an automated meta-analyses performed by the Neurosynth database174 found that the mediodorsal, anterior, ventral lateral and intralaminar nuclei were frequently associated with terms “working memory” and “cognitive control” in the literature (meta analyses performed on June 2022; see the figure). In the figure, maps were created by entering the terms 'working memory' and 'cognitive control' individually into the Neurosynth database. These maps depict results from the 'uniformity test', which reports z-scores (thresholded at z > 3) summarizing the likelihood of a voxel being reported in studies that included the selected terms. Specifically, the Neurosynth query tool performs a one-way ANOVA test to determine whether the proportion of studies reporting activation at a given voxel differs from the rate that would be expected if activations were uniformly distributed throughout grey matter. Importantly, these results showed that in the fMRI literature corpus, reliable and robust task-related signals have already been consistently reported in specific thalamic regions. With the results of the aforementioned human fMRI studies in mind, we foresee excellent opportunities for the field to leverage reliable neuroimaging signals to improve our understanding of thalamic function. Future studies would benefit from combining the inferential power of computational modelling, model-based analysis and cutting-edge neuroimaging techniques with improved spatial and temporal resolution (for example 7T MRI and fast blood oxygen level-dependent (BOLD) imaging), greatly increasing sensitivity to thalamic activity. In addition, hemodynamic responses in subcortex are often faster than those in cortex175, which should be leveraged to take full advantage of the most advanced neuroimaging methods176. Researchers also must improve anatomical specificity when reporting of thalamic results. To that end, there are now thalamic atlases widely available, parcellating the thalamus based on variety of structural, histological, and functional information177,178.
In this Perspective, we review recent human neuroimaging studies of the thalamus that highlight a variety of unique functional signatures through which the thalamus impacts the dynamics in the rest of the brain. Specifically, we highlight the highly diverse functional repertoire of the thalamus: it modulates neuronal activity, supports inter-regional connectivity, facilitates shifts in network topology, mediates heightened neuronal variability, and is involved in modulating both subtle and overt shifts in systems-level arousal (Figure 1). Through this approach, we argue that multimodal human neuroimaging approaches have helped the field to move away from the notion that the thalamus is a simple ‘relay’25 to the cerebral cortex and, instead, have provided evidence to support a nuanced perspective of the thalamus as a structure that facilitates flexible, multi-scale, coordinated dynamics that characterize arousal, cognition and conscious awareness.
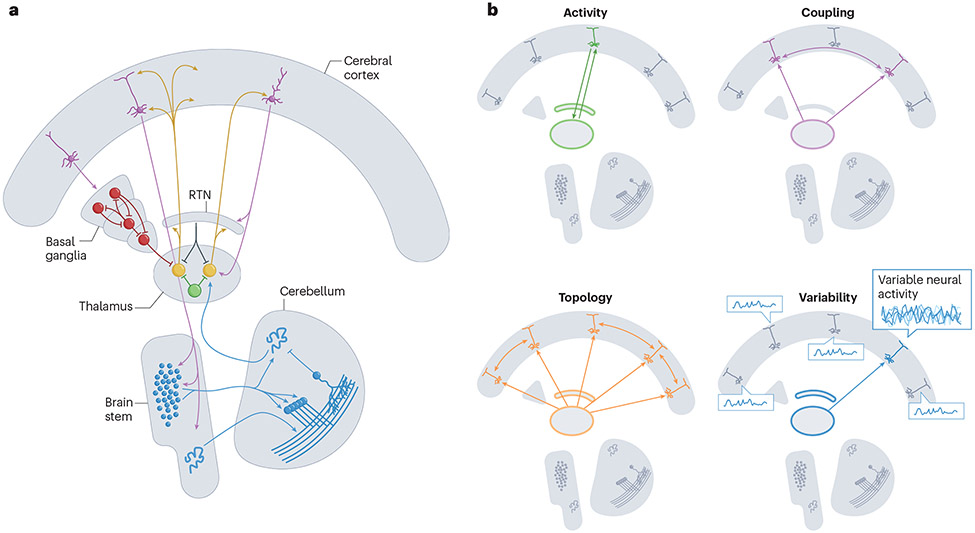
Figure 1. The functional neuroanatomy of the thalamus.
A) The thalamus is embedded in a distributed neural architecture: different populations of neurons in the thalamus (orange and brown) are interconnected with pyramidal neurons in the cerebral cortex (purple), inhibitory input from the basal ganglia (red), excitatory input from the cerebellum (blue) and local inhibition, both from the reticular nucleus (RTN; grey) and GABAergic interneurons (INs; green). B) The thalamus has a crucial role in at least four partially overlapping canonical functions: promoting focused cortical activity (green), facilitating inter-regional coupling (purple), supporting changes in network topology (orange) and dimensionality (that is, the higher-order structure of the interactions between neurons), and enabling and modulating temporal neuronal variability (blue).
The functional repertoire of the thalamus
Modulating local cortical evoked activity
There is a large-body of neuroimaging literature linking task-related activity in the human thalamus with a wide array of different behaviours, including attention, motor coordination, working memory and sleep–wake cycles6,35,40–44 (Box 2). Insights from animal studies can help interpret thalamic activity reported in human neuroimaging studies. For instance, findings from mice and non-human primate studies indicate that across each of these behavioural domains, the thalamus is capable of enhancing (or inhibiting) local activity patterns in the cerebral cortex45–47, potentially through the recruitment of the inhibitory reticular nucleus (Fig. 1A). For example, pharmacological or optogenetic inhibition of pulvinar or the reticular nucleus during a visual selective attention task reduced attentional modulation of evoked responses recorded in the visual cortex47,48. Specifically, the attentional enhancement in evoked response amplitude for the attended target was greatly reduced, suggesting that thalamocortical interactions are necessary for attentional amplification of task-relevant evoked responses, a process that has long been hypothesized to be mediated by top-down biasing signals.
The above studies suggest that thalamocortical projections can modulate cortical evoked activity (Fig. 1B) associated with cognitive processes that depend on top-down attentional biasing, such as working memory. For working memory, neural activity occurring after the presentation of a sensory input and before the contingent response (known as ‘delay activity’49) is thought to support the active maintenance of working memory content50. Persistent delay activity during working memory-related tasks has been found in the mediodorsal thalamus in both non-human primates and human fMRI studies51–56. In rodents, inhibiting activity in the ventrolateral or mediodorsal nucleus diminished delay activity in premotor45 and prefrontal cortex46 respectively, whereas stimulated mediodorsal thalamic activity enhanced information coding in rodents’ medial frontal cortex48. In humans, analogous findings have been reported, in which evoked responses in the thalamus can successfully predict evoked activity patterns in the cerebral cortex during working memory57, language, motor, attention and perceptual tasks58. These findings highlight the potential role of thalamic activity in influencing the cortical signatures of working memory and other task contents via thalamocortical interactions.
Beyond working memory maintenance, thalamocortical modulation of local cortical activity can be extended to inputting and updating task-relevant information into working memory. Computational models suggest that the process of inputting and updating working memory content can be implemented via a ‘gating’ mechanism59: when the gate is ‘open’, new information can enter the working memory system, whereas when the gate is ‘closed’, no new information can be entered into working memory. Crucially, this process is hypothesized to be mediated by the striatal-thalamic circuit60,61, wherein the striatum disinhibits a thalamocortical loop to facilitate new information to enter cortical circuits through targeted thalamocortical projections. While these models typically model the thalamic nodes according to known features of core-type thalamocortical projections (Fig. 1A; Box 1), it is unclear whether similar capacities will be associated with the gating of matrix-type projections, which are typically more diffuse and far more prominent targets of basal ganglia-mediated inhibition than core-type neurons14,62. Given that different cortical regions may be encoding different features, categories, or modalities of working memory content63, having a specific thalamocortical projection pathway that is selective for specific information may be advantageous, as different core cells can be selectively activated to allow precise control over distributed cortical activity.
While these observations mostly came from electrophysiology findings in model systems, various techniques can be used to study the effects of thalamic modulation on cortical activity in humans. In rare cases when electrophysiological activity can be simultaneously recorded from the human thalamus and the cortex, thalamic activity has been found to influence the level of high-frequency cortical activity64. Deep brain stimulation-mediated manipulation of mediodorsal thalamic activity in humans affected working memory performance43, and anterior thalamic activity recorded with an intracranial electrode showed increased synchrony with neocortical activity measured by electroencephalography (EEG) during successful memory encoding65. fMRI studies have also successfully imaged thalamic activity during visual attention tasks and have shown that such activity is correlated with local sensory responses and attentional modulation66,67. Technical developments in high-field fMRI have improved its spatial resolution, positioning it as an effective tool for detecting task-related thalamic activity for different cognitive functions. Thalamocortical interactions have also been found to be altered in disease states, suggesting that disrupted thalamocortical modulation of cortical evoked activity may be a core deficit in some psychiatric and neurological disorders68. Altogether, evidence from animal and human studies highlights the critical importance of thalamocortical function for modulating cortical evoked responses, and a major challenge is to determine how this mechanism is involved in diverse range of cognitive functions, beyond memory and attention.
Mediating inter-regional coupling
Cognition and behaviour are not only correlated with localized evoked activity in individual brain regions but also influenced by the interactions among circuits, regions and systems. In recent studies, inter-regional communication between brain regions has been proposed to be facilitated via coherence of local neural oscillations69,70, in which modulating the consistency in phase relationship between neural oscillations regulates patterns of information processing. Different classes of oscillations (that is, α-, β- and γ-band oscillations) have been demonstrated to be controlled by different cortical GABAergic inhibitory interneurons23,71,72, which in turn may be crucial targets for thalamocortical projections to control and facilitate inter-regional communication. Indeed, distinct families of GABAergic interneurons are known to be innervated by different classes of thalamocortical projections73,74. In addition, the inactivation of the thalamus diminishes cortico-cortical communication75, suggesting that the thalamus is directly or indirectly involved in facilitating information transfer between cortical regions.
There is evidence that the thalamic control of inter-regional communication supports a wide range of cognitive and behavioural processes (Fig. 1B). For instance, while monkeys performed a visual attention task, the level of alpha-band neural synchrony between V4 and the inferior temporal area of the cerebral cortex was modulated by the level of attention directed to the specific spatial receptive field42. A subsequent Granger causality analysis suggested that the thalamic pulvinar nucleus modulated alpha-band activity in both V4 and TEO6. Importantly, the regions that were functionally coupled also belong to similar thalamocortical projection zones determined via diffusion tensor imaging76, suggesting that thalamic regions are involved in coordinating the functional interactions between regions. These findings were further corroborated by a study demonstrating the pharmacologically inactivating the macaque pulvinar reduced attention-driven neural synchrony between V4 and the inferior temporal cortex47. These findings are consistent with studies that have mapped the resting state correlations between the pulvinar and cerebral cortex (using fMRI77), as well as those that found pulvinar lesions in human patients are associated with various attention deficits34. Importantly, there is detailed computational modelling work that reinforces the plausibility of these mechanistic explanations78. It remains an open question as to whether context-dependent shifts in inter-regional coupling observed in fMRI79 are also associated with similar thalamocortical interactions80,81, although there is emerging evidence that supports this hypothesis82.
Thalamic control of inter-regional communication may also be particularly important for top-down cognitive control, during which a hypothetical control signal (also referred to as a ‘top-down biasing’ signal) is communicated to regulate perceptual and motor functions to facilitate goal-directed behaviours83,84. This top-down control mechanism requires selectivity and flexibility to allow control signals to reach targeted brain regions accurately and selectively. As described in the previous section, the thalamus is well-placed to mediate the communication of top-down biasing signals, such as those that occur when selected cortical activity needs to be enhanced or inhibited via attentional modulation85. Many fMRI studies typically link attention modulation as observed in changes in blood oxygen level-dependent (BOLD) signal amplitude to biasing effects from the frontoparietal and dorsal attention networks86, but evidence discussed in the above section strongly implicate that thalamocortical interactions contribute heavily to this biasing mechanism. Because the thalamic regions supporting this frontoparietal network span both the mediodorsal nucleus and pulvinar87, it is possible that effective attentional performance may require heretofore underexplored coordination between different thalamic subnuclei.
Topology and dimensionality
Functional connections in the brain are not limited to pairwise interactions — indeed, the human brain can be conceptualized as a complex network consisting of interconnected circuits, brain regions and neural systems2. The architecture of brain networks can be effectively studied by graph theory-based methods, in which brain networks are represented as graphs, brain regions are modeled as nodes and connectivity among regions is modeled as edges88. Studies of brain network organizations have consistently reported they have a modular structure7, in which brain regions within the same module have many connections with other brain regions in the same module, and fewer connections with brain regions outside the module. For example, distributed regions within the sensory and motor cortices form visual, auditory, and somatomotor networks, whereas regions within the fronto-parietal association cortices form several distributed networks including the dorsal-attention, ventral-attention, cingulo-opercular, and frontal-parietal networks89. In addition to revealing the modular structure of functional brain networks, graph theoretic approaches can also be used to study network properties of individual brain regions in a functional network. For example, a brain region with many between-network connections has a strong ‘connector hub’ property, presumably to mediate interactions between functional networks90. Studies have found that in the cerebral cortex, such hubs are primarily located in frontal and parietal association areas that have been implicated in higher-order, integrative functions5,91,92.
Within the context of large-scale brain network organization, it has been shown that all cortical functional networks are strongly connected to distinct but also overlapping thalamic regions87,93,94 (Fig. 2A). Critically, multiple thalamic nuclei have also been found to exhibit strong connector hub properties (Fig. 2B). The anterior, mediodorsal, intralaminar, ventrolateral, ventroposterior and pulvinar nuclei all exhibit strong connector hub properties, which were found to be stronger than cortical connector hubs87 (Fig. 2B). This finding suggests that, similar to cortical regions such as the prefrontal and parietal cortices, thalamic nuclei are in a privileged position for supporting integrative functions across multiple functional networks87,95.
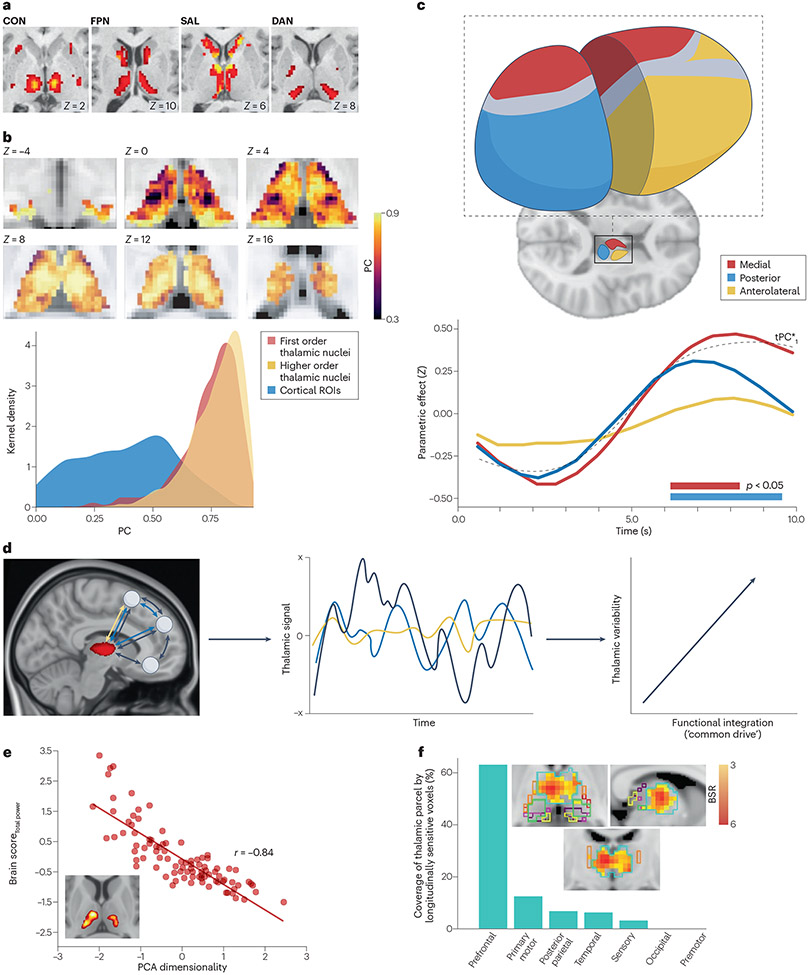
Figure 2. Functional parcellation, connector hub properties and variability profile of the thalamus.
A) Thalamic regions associate with cortical functional networks that are putatively involved in cognitive control related functions , namely the cingulo-opercular network (CON), the frontoparietal network (FPN), the saliency network (SAL) and the dorsal attention network (DAN). B) Top: The medial, mediodorsal, and posterior portions of the thalamus exhibit particularly strong connector hub properties, which are indicated by their higher scores on the participation coefficient (PC) axis. The colour bar depicts the PC scale, which is a measure of connector hubness and ranges from 0 to 1 (low to high). Bottom: Most of the thalamus exhibits connector hub properties that are stronger than those of cortical regions. C) The mean blood oxygen level-dependent (BOLD) signal in three different thalamic groups — the medial (pink), posterior (blue) and anterolateral (orange) — is strongly aligned with the parametric effect of cognitive load on the low-dimensional distributed activity — coloured bars designate significant parametric effects (p < 0.05 from non-parametric permutation testing) and the dotted black (tPC*1) line depicts mean low-dimensional trajectories. This indicates a low-dimensional relationship between activity in the thalamus and activity in the cerebral cortex. D) Toy example of three functional interaction scenarios between the prefrontal cortex and the thalamus. Here, the expectation is that with increasing functional connection (that is, going from 1 (green) to 2 (blue) to 3 (black) connected nodes; left panel), moment-to-moment brain signal variability expressed by the thalamus should increase in kind (middle panel). Accordingly, higher temporal variability in the thalamus may reflect higher functional integration among connected regions. E) Heightened BOLD variability in thalamus was the strongest signature of lower principal component analysis (PCA) dimensionality (that is, higher moment-to-moment functional integration between brain regions). F) Thalamic regions projecting to the prefrontal cortex were particularly sensitive to joint longitudinal changes between BOLD signal variability, functional integration and cognition; individuals who maintained moment-to-moment thalamic variability also maintained functional integration and cognition over 2.5 years. Part a adapted with permission from REF.94. Part b adapted with permission from REF.87. Part c adapted with permission from REF.82. Parts d and e adapted with permission from REF.102. Part f adapted with permission from REF.108.
This central topological position of the thalamus also makes specific predictions regarding its behavioural relevance. Given converging connectivity with multiple processing systems and functional networks, thalamic hubs should be involved in cognitive functions across multiple domains. This prediction can be best tested by studying human patients with focal thalamic lesions. As described in previous sections, lesions to different thalamic regions have been reported to be associated with behavioural impairments across many domains, including language96, memory32, executive function33 and attention34. Furthermore, a recent study provides causal support of the behavioural significance of thalamic hubs, reporting that a single, circumscribed lesion to an anterior dorsal thalamic subregion in humans can produce widespread impairment in executive, memory, and language-related functions97. Critically, this anterior-to-medial-dorsal thalamic pattern also exhibits a strong connector hub property, and lesions to thalamic regions with weaker hub properties has a more limited impact on behaviour.
Given these findings, thalamic hubs are probably not (completely) functionally specialized for specific cognitive and behavioural functions, but rather provide more domain-general contributions to brain functions. One hypothesis is that thalamic hubs coordinate the topological organization of cortical networks, and a focal lesion in a critical thalamic nucleus could have widespread and distal effects on cortical functions (‘diaschisis’). In support of this, focal thalamic lesions disrupt the modular organization of cortical functional networks in humans87. Furthermore, thalamic regions whose lesions are associated with behavioural deficits across multiple-domains also contain higher densities of the diffusely-projecting matrix thalamocortical cells. These findings thus provide causal support of both the network and behavioural significance of thalamic hubs.
A natural question to ask is whether there are inherent relationships between thalamic architecture and the well-established low-dimensional patterns (such as resting state networks89 and gradients in spatiotemporal organisation98) observed in cortico-cortical functional connectivity98. Low-dimensional organization indicates that the number of variables required to explain a large amount of variance in distributed neural activity is far lower than the total number of variables in the data99. Indeed, these simple descriptions of the basic patterns of covariance observed in whole-brain neuroimaging data spatially coincide with regions that strongly correlate with either core or matrix thalamic populations during the resting state in humans100. In this study, the authors used high-resolution 7T resting-state fMRI data and the relative amount of two calcium-binding proteins, parvalbumin and calbindin, to infer the relative distribution of core and matrix (respectively) nuclei within thalamic voxels. Tracking the time series within these voxels and comparing them to time series extracted from the cerebral cortex, it was shown that differences in thalamocortical functional connectivity recapitulated functional connectivity patterns within the cerebral cortex100. These same patterns have previously been shown to explain substantial amounts of variance across resting state functional connectivity studies in humans, and also to relate to distinct cognitive capacities, as estimated using large-scale, automated meta-analyses of the cognitive neuroscience literature98. The striking correspondence between the thalamocortical and corticocortical functional connectivity patterns provides further evidence for the importance of thalamic organization in shaping whole-brain functional connectivity patterns.
The patterns identified in the previous analysis100 are themselves relatively low-dimensional. There is also evidence to suggest a link between thalamic activity and the low-dimensional network patterns engaged during cognitive task performance82 (Fig. 2C). One potential explanation for these low-dimensional patterns is that thalamic activity broadcasts gamma-frequency ‘up’ states in the cerebral cortex36, but then uses the inhibitory blanket of the reticular nucleus to protect the cortical state from otherwise distracting thalamic input101. These results suggest that the thalamus facilitates integration by providing low-dimensional constraints over network-level dynamics in the cerebral cortex, but also simultaneously ensures that the resultant patterns are relatively segregated as well82. This interpretation is consistent with previous work investigating network dimensionality and temporal variability using resting state fMRI102.
Variability and integration
Brain activity exhibits remarkable variability from moment to moment, fluctuating across multiple spatial (neurons to networks) and temporal (milliseconds to days) scales103,104. While the source of this variability is still unknown, it is thought that dynamic neural activity at the regional level may reflect summed synaptic inputs105, directly linking local dynamics to communication between regions. This suggests that a greater number of functional inputs to any local brain region may increase the variability of its output (Fig. 2D). Consistent with this notion, computational modelling of visual cortex has shown that the majority of ‘noise variation’ is shared across neurons that share tuning properties106, suggesting a plausible general phenomenon that more temporal variability at the regional level may be characterized by a more integrated (that is, lower dimensional) network structure. As the thalamus is thought to dynamically relay and modulate information flow throughout the entire brain25,95, thalamic variability may provide a key temporal signature of how functional network integration emerges overall.
Evidence in humans is now building that this is indeed the case. For example, in resting state fMRI data, elevated moment-to-moment variability in thalamic activity was the strongest marker of heightened functional integration (that is, lower dimensionality) across the brain102 (Fig. 2E). In addition, individual differences in the temporal variability from thalamus to its structurally connected cortical targets predicted lower network dimensionality (higher functional integration) over and above local variability in thalamus alone102, a result that echoes similar findings in macaque106 and cat visual cortices107. These findings thus suggest that greater local temporal variability in thalamus and heightened upregulation of variability from the thalamus to the cortex provide strong and unique signatures of how the whole-brain systems functionally integrate.
Beyond individual differences, it has been unclear whether thalamic variability is also associated with functional integration at the within-person level, and if so, whether such changes are cognitively relevant. In a recent longitudinal resting-state fMRI study, individuals who exhibited loss of moment-to-moment variability in thalamic activity also expressed a loss of functional network integration and a concomitant loss of cognitive function (fluid and crystallized intelligence, and perceptual speed) over the course of 2.5 years108. Crucially, the observed changes in thalamic variability co-occurred with changes in variability across much of the prefrontal cortex and striatum, implicating the larger fronto-striato-thalamic system in behaviourally relevant longitudinal changes in neuronal variability. Thalamic effects were most expressed within subregions with structural connections to the prefrontal cortex (Fig. 2F), as well as thalamic nuclei known to primarily project to the prefrontal cortex and receive striatal input (that is, mediodorsal and ventral anterior nuclei). These results highlight thalamic variability as a primary target in future longitudinal work in ageing populations.
What mechanisms may allow an individual to exhibit higher temporal variability in thalamic activity? Although understudied to date, higher variability — which often correlates with optimal cognitive performance104,109–112 — may be a direct reflection of pooling over more differentiated inputs106. Given that the fMRI signal in an individual voxel is thought to reflect activity distributed across millions of ‘local’ neurons, the more these neurons differ in their level and form of temporal variability, the higher that voxel-level temporal variation should be. Greater thalamic variability (perhaps from the diffuse projections of higher-order, matrix-type thalamic nuclei14,22) may then mediate both an increase in functional integration over differing sets of axonal inputs, as well as patterns of cellular differentiation that contribute to diversity in the observed local (ensemble) dynamics within more integrated brains102.
Another plausible mechanism underlying elevated variability in thalamus is fluctuations in neural gain — that is, variability in the transfer of input to output due to neuromodulatory influences113. For example, noradrenaline-mediated elevations in neural gain can lead to a more integrated whole-brain network structure5,11,114, a topological feature that may relate to the effects of noradrenaline on oscillatory bursting and single spike firing modes in thalamus115. Furthermore, previous studies on the relationship between dopamine, brain signal variability and cognitive performance111,116 support theories of how dopaminergic neural gain117 influences thalamic variability for modulating functional integration (but see REF 118). Indeed, dopaminergic neurons naturally exhibit dominant low-frequency tonic firing patterns along with intermittent phasic bursts119. Mouse data highlight that dopamine-deficient animals display a complete lack of phasic bursting activity in the thalamus, but that dynamic bursting can be restored via dopaminergic agonism119. In addition, trial-to-trial variability in dopamine release appears to increase with increasing task proficiency120. Crucially, although the nature and direction of relations between dopaminergic and noradrenergic neurons can be highly complex, these systems are almost certainly coupled121 and should serve as key targets for future research on the association between thalamic dynamics and functional integration.
Arousal influences
Based on the functional repertoire reviewed above, the thalamus is probably not associated with one simple functional signature, but rather is well-positioned to modulate ongoing cortical activity, connectivity, topology, and variability through a variety of mechanisms. For example, the glutamatergic driver outputs from the thalamic nuclei can strongly drive cortical responses and thalamocortical inputs have been shown to elicit stronger cortical-evoked activity than cortico-cortical inputs122. Thalamocortical inputs to cortical regions are adaptive, as they can be modulated by inhibitory influences from the basal ganglia and the reticular nucleus24. Thus, the combination of excitatory and inhibitory thalamocortical interactions can dynamically shape cortical activities across spatial scales, giving rise to a wide range of activity profiles that are related to a multitude of behavioural phenomena. Another key question then becomes: what other factors modulate thalamic dynamics to shape the expression of the functional repertoire?
A major modulator of thalamic dynamics is arousal state. Multiple studies have shown that altering excitation or inhibition in the thalamus from the ascending arousal system leads to altered spontaneous dynamics and arousal states within the cortex123–125, suggesting that the thalamus acts as a key convergent pathway shaping arousal in cortical networks. Intriguingly, while inhibiting thalamic activity can drive slow wave (that is, low-frequency) activity123, lesioning or ablating the thalamus does not have the same effect126,127, suggesting an active role for inhibitory thalamic circuits in generating the slow waves, rather than simply reflecting the absence of or reduction in thalamic input to the cortex.
In humans, the question of how large-scale thalamocortical networks shift their dynamics across arousal states has been studied via fMRI. These studies have demonstrated distinct thalamocortical dynamics across a spectrum of arousal states, with maintained cortical connectivity128 but sharp suppression of thalamocortical connectivity at the onset of sleep129. More recent studies have distinguished distinct patterns of thalamocortical functional connectivity across arousal states130: specifically, thalamocortical connectivity selectively increased to sensorimotor networks during light sleep, and intra-thalamic connectivity increased broadly during the transition into sleep. While changes in systemic physiology could also modulate functional connectivity across arousal states due to their direct effects on hemodynamics131,132, the distinctive spatial patterning of these observations during sleep nonetheless suggests distinct neural activity changes across nuclei and networks.
Within low arousal states such as sleep, ongoing cognitive processes take place spontaneously, such as the memory consolidation that occurs during non-rapid eye movement (NREM) sleep. These memory processes are associated with distinct EEG signatures with clear links to function — for instance, the slow waves characteristic of deep sleep have been linked to stabilization and consolidation of memory133. Electrophysiological studies have clearly established a key role for intrinsic thalamic oscillations in sleep slow waves, originally in foundational studies in animal models35,134. For instance, there is evidence that sleep spindles demonstrate coupled activity in the thalamus135. Invasive electrophysiological recording of a subset of thalamic nuclei in human patients136 has now elucidated the temporal links between cortical and thalamic oscillations during sleep. These invasive recordings suggest cortical excitation drives a thalamic up-state, leading to thalamic coordination of sleep spindles and synchrony across the distributed network. Building on this already-identified critical role for thalamo-cortical networks in synchronizing cortical oscillations linked to memory, further dissection of the thalamic correlates of sleep oscillations remains a key area for future work, especially with regards to how distinct thalamic nuclei may drive this phenomenon.
In addition to expressing distinct dynamics during different arousal states, the thalamus also appears to play a distinctive role in effecting the transition between arousal states (Fig. 3A). Rare direct recordings from the human thalamus demonstrated that changes in spontaneous thalamic activity preceded changes in cortical arousal by several minutes137. The precise nature and origin of these thalamic changes is not yet fully understood. Studies using simultaneous EEG and fMRI have identified transient increases in thalamic activity occurring at the transition between sleep and wakefulness138, as well as at co-fluctuating with low- and high-arousal within the awake state (Fig. 3B)139. Recently, a study using accelerated fMRI to detect sub-second thalamic activity identified a temporal sequence of activity across thalamic nuclei that precedes transitions in arousal states140, with the intralaminar centromedian thalamus activating earlier than other thalamic nuclei, and preceding the behavioural signatures of arousal (Fig. 3A). These temporal sequencing results point to a key role for the thalamus in regulating transitions between states in humans, consistent with causal evidence from the animal literature, and moreover illustrate how specific nuclei play distinct roles relative to cortical network structure. In particular, a switch in oscillatory state in a single nucleus could potentially trigger a cascade throughout the cortical feedback loops leading to a widespread switch in thalamic and cortical activity states. Another possibility is that slow changes in release of neuromodulatory substances within the thalamus can produce such temporal sequences, owing to local differences in receptor densities across individual nuclei.
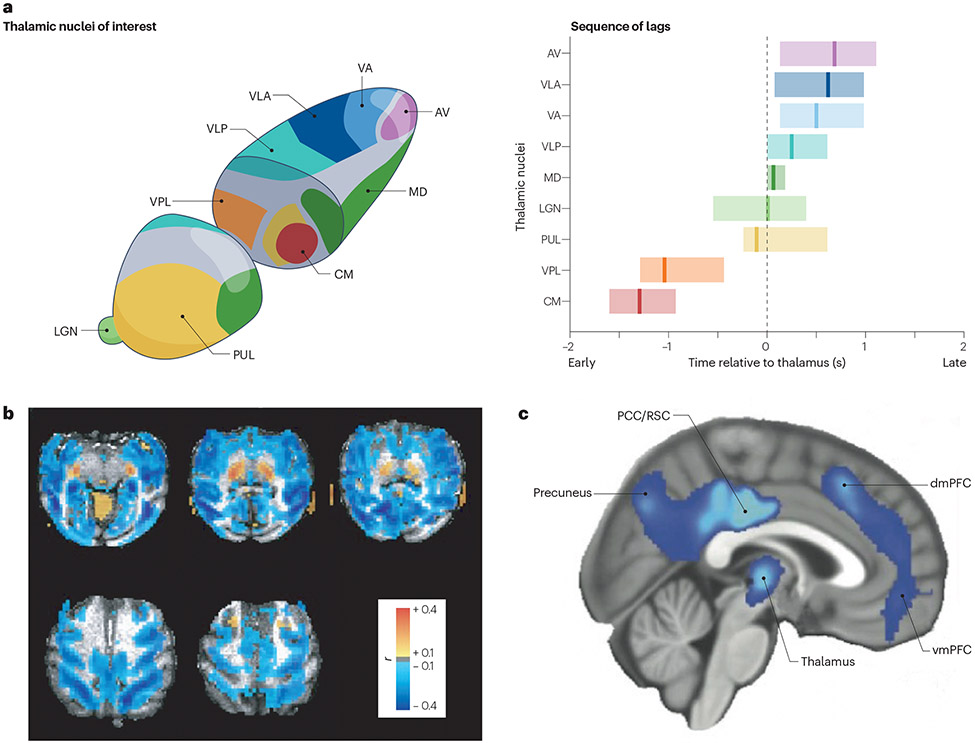
Figure 3. Arousal shapes thalamic activity and thalamocortical dynamics.
A) Schematic of individual nuclei of the thalamus imaged with 7T functional MRI (fMRI), locked to arousal state transitions. A temporal sequence of activity emerges across thalamic nuclei, preceding the moment of transition to higher arousal states. Shading shows 95% confidence interval for activity timing in each thalamic nucleus, locked to arousal. B) A spatial template of key fMRI predictors of arousal shows thalamic correlations with arousal state. The colour intensity shows the strength of each region’s correlation with behavioural arousal measured via eye closures; thalamus shows strong positive correlations. C) Inducing sedation with dexmedetomidine, a drug that induces decreased noradrenergic neuromodulatory tone, causes decreased thalamic glucose metabolism and connectivity. AV, anteroventral nucleus; CM, centromedian nucleus; dmPFC, dorsomedial prefrontal cortex; LGN, lateral geniculate nucleus; MD, mediodorsal nucleus; PCC, posterior cingulate cortex; PUL, pulvinar nucleus; RSC, retrosplenial cortex; VA, ventral anterior nucleus; VLA, ventral lateral anterior nucleus; VLP, ventral lateral posterior nucleus; vmPFC, ventromedial prefrontal cortex; VPL, ventral posterolateral nucleus. Part a adapted with permission from REF.140. Part b adapted with permission from REF.139. Part c adapted with permission from REF.144.
Neuromodulatory circuits are known to be a primary controller of these arousal states in the thalamus, not just within sleep, but also as arousal is dynamically modulated during wakefulness. Arousal is regulated by distributed circuits across the brainstem, hypothalamus and basal forebrain141, and the thalamus is a primary target on which these neuromodulatory circuits converge16,141,142. Dopaminergic, noradrenergic and cholinergic tone each influence the properties of spontaneous thalamic activity and thalamocortical dynamics143, allowing the thalamus to differentially engage the functional repertoire described above (Fig. 1B). Neuromodulatory pathways exhibit distinct projections across different thalamic nuclei15,16 and could form a control mechanism for dynamically shaping which aspects off the thalamic functional repertoire are engaged. These neuromodulatory effects on thalamus are beginning to be studied through causal manipulations in humans as well. A unique study using a combined positron emission tomography–magnetic resonance approach to image the neural effects of a noradrenergic sedative (the α2-adrenergic agonist, dexmedetomidine) showed that decreased noradrenergic signalling causes disruption of thalamocortical connectivity and decreased thalamic activity144 (Fig. 3C). Interestingly, recent work has shown that noradrenergic receptors are heterogeneously expressed in the non-human primate thalamus, with a greater expression in intralaminar and midline structures145, suggesting that neuromodulatory ligands16 may afford precise control over the thalamocortical functional repertoire.
Finally, thalamic activity is not only a critical component of spontaneous shifts in arousal but also a key mechanism of general anesthesia, consistent with the powerful role for the thalamus in shaping large-scale cortical dynamics. While distinct anesthetic agents act through a diversity of unique mechanisms, disruption of thalamocortical dynamics is generally observed across many of these medications. Propofol, one of the most widely used anesthetics, is a GABAergic agonist that binds widely throughout the brain and causes a shift in EEG dynamics towards high-amplitude slow oscillations and frontal alpha oscillations146–148. Computational modelling has demonstrated a key role for thalamus in generating these frontal alpha oscillations, specifically with tonic inhibition slowing oscillations within the corticothalamic feedback loop, and synchronized inhibitory input from the reticular nucleus to multiple thalamic nuclei, producing high synchrony throughout the frontal cortex149. A recent study demonstrated that stimulation of the central lateral thalamus can drive awakening in anesthetized animals150, and suggested that this effect is mediated by restoring wake-like intracolumnar and intracortical functional connectivity. Similar effects have been observed in mice151 and relate to clinical observations linking abnormal conscious arousal states to thalamic lesions152. Finally, it is notable that general anesthetics typically induce qualitatively distinct brain states as compared with sleep153, suggesting that a diverse range of thalamocortical state dynamics can be induced by non-naturalistic manipulations of neuromodulatory tone throughout the brain.
The thalamus shapes and constrains adaptive brain dynamics
In the preceding sections, we outlined the central role played by the thalamus in shaping evoked responses, functional connectivity, network topology and neuronal variability, often through its tight connections with the ascending arousal system (Fig. 1B). Note that each of these features is also specific to the empirical lens (for example, our emphasis is on human functional neuroimaging) through which they were investigated, and thus under different contexts these features could reflect different or similar underlying neural capacities. Henceforth, how might this functional repertoire work together to support cognitive functions? While the role of the thalamus in sensory and motor processes is relatively easy to link to relatively concrete measures — such as the perception of a particular stimulus, or the movement a particular effector — the same cannot be said for cognitive processes, which are often far more abstract in nature. However, in the cases where we can break down cognitive processes into smaller components, there are clear predictions that can be made regarding the role of the thalamus in cognition. For instance, if an item needs to be held over a delay in working memory, then thalamocortical normalization is of great importance, as evidenced in rodents45. Alternatively, if interactions in the cerebral cortex are required to support flexible decision making, the diffuse projections of higher-order or matrix thalamus could help to ensure coordinated synchrony between relatively specialized pyramidal neurons in the cerebral cortex6. Similar integration is required for large-scale circuits of the brain, such as the cerebellum and basal ganglia, and there is ample evidence that the thalamus plays a crucial role in amalgamating these signals to maximize adaptive behaviour14. Finally, the evidence that diffusely projecting intralaminar nuclei of the thalamus are involved in the transition between sleep and wake140,150 further highlights the importance of the thalamus for coordinating the global brain dynamic modes required for cognitive function10.
To provide a concrete example of how these different computations may play out at the systems level, we highlight new work showing how the functional repertoire of the thalamus (that is, relating to activity, coupling/topology, variability and arousal) can aid decision making under uncertainty154. When faced with a complex perceptual scene, the deliberate processing of specific, cognitively relevant perceptual features (likely requiring selective gain control) can cause certain features to ‘pop out’ of the noisy background. Such selective processing may be akin to sitting deep in a single attractor within a landscape of potential processing modes, a phenomenon linked to cortical alpha rhythms155 that likely emerge from relatively selective thalamocortical loop interactions156. Conversely, when we are less certain about what to attend to in our environment, the brain may instead need to track multiple stimulus features at the same time157,158. Doing so may require a ‘flatter’ attractor landscape, within which the brain can more easily switch processing modes, a process likely requiring higher neural ‘excitability’ (that is, variability) and neuromodulatory arousal159 via more distributed interactions between the thalamus and cerebral cortex14,62. Due to a variety of design-level challenges in past human work, testing whether and how the functional repertoire of the thalamus is engaged in these distinct cognitive challenges had been inherently difficult.
A multi-modal EEG–fMRI experiment was devised to capture these various phenomena using both fast cortical dynamics (EEG), slower cortical and subcortical activity (fMRI), and pupillometry (arousal) while participants performed the multi-attribute attention task154 (Fig. 4). Briefly, participants were presented with stimuli comprising four unique dimensions present on every trial, while ‘uncertainty’ was manipulated by varying the number of dimensions that were task-relevant. The results revealed that the brain indeed shifted into a more excitable (for example, more variable or entropic) and aroused cortical state with increasing uncertainty, effects dominantly associated with elevated activity in thalamic regions with fronto-parietal projections (for example, the mediodorsal thalamus (MD) and the anterior pulvinar; Fig. 4). Elevated mediodorsal activity is further consistent with recent findings reporting distinct MD neuronal populations in mice that resolve decision uncertainty from conflicting or low signal task inputs160. Crucially, the effects in the EEG-fMRI experiment were especially pronounced in those adults better able to perform the task (Fig. 4). It may thus be that processing uncertainty drives a topologically broad-scale ‘thalamo-fronto-parietal’ system to permit dynamic target selection within high-dimensional contexts161–163 in higher performing adults. Indeed, findings from this study154 highlight all core features of our proposed functional repertoire of the thalamus; the thalamus appears to influence local cortical activity at fast and slow temporal scales, reflect coupling or topology within the broader thalamo-fronto-parietal system, drive moment-to-moment variability in cortical activity, as well as reflect neuromodulatory arousal. In short, the capacity for deploying flexible, multi-demand attentional resources is critically dependent on the architecture of the thalamus and its interactions with the rest of the brain.
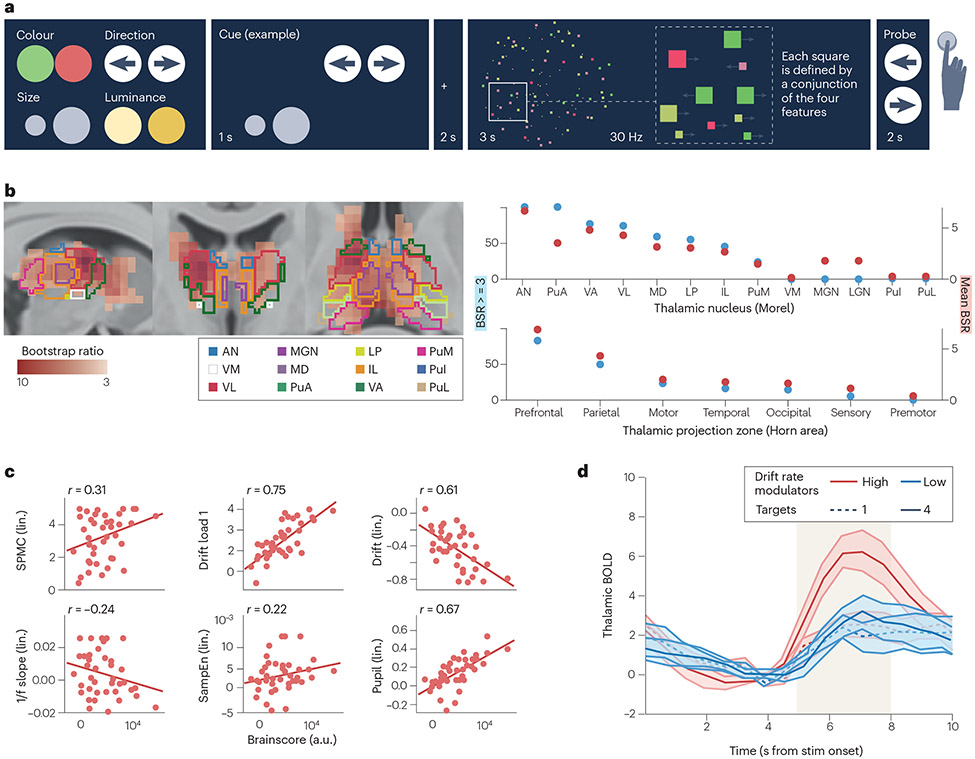
Figure 4. The functional repertoire of the thalamus serves decision-making under parametric uncertainty.
A) In the multi-attribute attention task, participants were first cued with a set of 1-4 potentially task relevant stimulus features (any set of color, direction, size, or luminance). They were then shown a stimulus that contains all four features, and were subsequently asked to make a decision about one feature. Participants underwent fMRI and EEG during the task, in separate sessions. Results indicated that B) participants with larger parametric increases in thalamic BOLD (especially in antero-medial nuclei that project to frontoparietal cortical targets) were more likely to C) upregulate EEG-based ‘excitability’ (reduced alpha and increased gamma, expressed by a spectral power modulation component (SPMC); flatter 1/f spectral slopes; higher sample entropy (SampEn), exhibit a higher drift rate and drift rate modulation, and show heightened arousal (first derivative of pupil responses). D) Those who expressed higher uncertainty-related modulation of behavioural drift rate expressed higher thalamic modulation particularly during stimulus presentation (yellow shading). Figure adapted with permission from REF.154.
Future outlook and conclusions
While previous generations of cognitive neuroscientists may have been dissuaded from incorporating the thalamus into their imaging protocols and experimental hypotheses (Box 2), we hope that the ideas conveyed in this perspective will encourage others to further investigate this crucial structure nestled deep within the subcortex. We have argued that many key features of brain function, including local activity, connectivity, network topology, variability, and systems-level coordination are crucially dependent on the integrity and influence of thalamic organization. Although the role of the thalamus in systems-level organization is becoming increasingly appreciated, there are a number of open questions that remain to be clarified. First, while the thalamus appears to act as a connector hub in the network organization of the brain87,95, it is unclear whether this topological role is maintained across arousal states. Indeed, the precise relationship between different arms of the neuromodulatory arousal system and the distributed thalamocortical system remains to be effectively clarified in vivo. Second, more careful characterization of the involvement of different thalamic subregions across diverse cognitive contexts will also vastly improve our understanding of how distributed cortico-subcortical architectures dynamically reconfigure to facilitate complex, adaptive behaviour. We also foresee a major role for studies designed to integrate across species, delineating the similarities and differences inherent within thalamic organization across phylogeny, and how these might underpin crucial cognitive idiosyncrasies across evolutionary time. Last, it will be important to determine precisely how the macroscopic features that cognitive neuroscientists measure (for example, the BOLD signal or event-related potentials) relate to (dys)function within the thalamus. Although this is undoubtedly a complex issue, we envisage that advances in neuronal recordings and the integration of neuronal signals with generative computational modelling approaches will further enhance the conclusions that can be made.
Footnotes
Competing interest statement:
The authors declare no competing interests.
References
- 1.Luo TZ & Maunsell JHR Attention can be subdivided into neurobiological components corresponding to distinct behavioral effects. Proc Natl Acad Sci USA 116, 26187–26194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullmore E & Sporns O The economy of brain network organization. Nature Reviews Neuroscience 13, 336–349 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Buschman TJ & Miller EK Top-Down Versus Bottom-Up Control of Attention in the Prefrontal and Posterior Parietal Cortices. Science 315, 1860–1862 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Krienen FM, Yeo BTT & Buckner RL Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Phil. Trans. R. Soc. B 369, 20130526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shine JM et al. The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron 92, 544–554 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saalmann YB, Pinsk MA, Wang L, Li X & Kastner S The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science 337, 753–756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporns O & Betzel RF Modular Brain Networks. Annu. Rev. Psychol 67, 613–640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H-J & Friston K Structural and Functional Brain Networks: From Connections to Cognition. Science 342, 1238411–1238411 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Breakspear M. Dynamic models of large-scale brain activity. Nature Neuroscience 20, 340–352 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Müller EJ, Munn BR & Shine JM Diffuse neural coupling mediates complex network dynamics through the formation of quasi-critical brain states. Nat Commun 11, 6337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shine JM, Aburn MJ, Breakspear M & Poldrack RA The modulation of neural gain facilitates a transition between functional segregation and integration in the brain. Elife 7, e31130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janacsek K. et al. Subcortical Cognition: The Fruit Below the Rind. Annu. Rev. Neurosci 45, 361–386 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J. Corticocentric myopia: old bias in new cognitive sciences. Trends in Cognitive Sciences 13, 354–359 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Shine JM The thalamus integrates the macrosystems of the brain to facilitate complex, adaptive brain network dynamics. Progress in Neurobiology 199, 101951 (2021). [DOI] [PubMed] [Google Scholar]
- 15.McCormick DA Cholinergic and noradrenergic modulation of thalamocortical processing. Trends in Neurosciences 12, 215–221 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Varela C. Thalamic neuromodulation and its implications for executive networks. Frontiers in neural circuits 8, 69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merker B. Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behav Brain Sci 30, 63–81 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Basso MA & May PJ Circuits for Action and Cognition: A View from the Superior Colliculus. Annu. Rev. Vis. Sci 3, 197–226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shine JM Adaptively navigating affordance landscapes: How interactions between the superior colliculus and thalamus coordinate complex, adaptive behaviour. Neuroscience & Biobehavioral Reviews 143, 104921 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Houk JC & Wise SP Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral Cortex 5, 95–110 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Clascá F, Rubio-Garrido P & Jabaudon D Unveiling the diversity of thalamocortical neuron subtypes. The European journal of neuroscience 35, 1524–1532 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Jones EG The thalamic matrix and thalamocortical synchrony. Trends in Neurosciences 24, 595–601 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Halassa MM & Sherman SM Thalamocortical Circuit Motifs: A General Framework. Neuron 103, 762–770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halassa MM & Acsády L Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends in Neurosciences 39, 680–693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman SM The thalamus is more than just a relay. Current Opinion in Neurobiology 17, 417–422 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt LI & Halassa MM Interrogating the mouse thalamus to correct human neurodevelopmental disorders. Mol Psychiatry 22, 183–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips JW et al. A repeated molecular architecture across thalamic pathways. Nature Neuroscience 22, 1925–1935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Cabezas MA, Rico B, Sánchez-González MA & Cavada C Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 34, 965–984 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Arcelli P, Frassoni C, Regondi MC, Biasi SD & Spreafico R GABAergic Neurons in Mammalian Thalamus: A Marker of Thalamic Complexity? Brain Research Bulletin 42, 27–37 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M & Cavada C Dopamine Innervation in the Thalamus: Monkey versus Rat. Cerebral Cortex 19, 424–434 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graff-Radford NR, Eslinger PJ, Damasio AR & Yamada T Nonhemorrhagic infarction of the thalamus: Behavioral, anatomic, and physiologic correlates. Neurology 34, 14–14 (1984). [DOI] [PubMed] [Google Scholar]
- 32.Von Cramon DY, Hebel N & Schuri U A Contribution to the Anatomical Basis of Thalamic Amnesia. Brain 108, 993–1008 (1985). [DOI] [PubMed] [Google Scholar]
- 33.Hwang K, Bruss J, Tranel D & Boes AD Network Localization of Executive Function Deficits in Patients with Focal Thalamic Lesions. Journal of Cognitive Neuroscience 32, 2303–2319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snow JC, Allen HA, Rafal RD & Humphreys GW Impaired attentional selection following lesions to human pulvinar: Evidence for homology between human and monkey. Proc. Natl. Acad. Sci. U.S.A 106, 4054–4059 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steriade M, McCormick D & Sejnowski T Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993). [DOI] [PubMed] [Google Scholar]
- 36.McCormick DA, McGinley MJ & Salkoff DB Brain state dependent activity in the cortex and thalamus. Current Opinion in Neurobiology 31, 133–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastner S. et al. Functional Imaging of the Human Lateral Geniculate Nucleus and Pulvinar. Journal of Neurophysiology 91, 438–448 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Zhu X-H, Thulborn KR & Ugurbil K Retinotopic mapping of lateral geniculate nucleus in humans using functional magnetic resonance imaging. Proceedings of the National Academy of Sciences 96, 2430–2434 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi EY, Yeo BTT & Buckner RL The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108, 2242–2263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman SM & Guillery RW The role of the thalamus in the flow of information to the cortex. Phil. Trans. R. Soc. Lond. B 357, 1695–1708 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kastner S, Fiebelkorn IC & Eradath MK Dynamic pulvino-cortical interactions in the primate attention network. Current Opinion in Neurobiology 65, 10–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usrey W & Kastner S Functions of the visual thalamus in selective attention. in The Cognitive Neurosciences vol. 6th Edition (2020). [Google Scholar]
- 43.Peräkylä J. et al. Causal Evidence from Humans for the Role of Mediodorsal Nucleus of the Thalamus in Working Memory. Journal of Cognitive Neuroscience 29, 2090–2102 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Dacre J. et al. A cerebellar-thalamocortical pathway drives behavioral context-dependent movement initiation. Neuron 109, 2326–2338.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo ZV et al. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolkan SS et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 20, 987–996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Schafer RJ & Desimone R Pulvinar-Cortex Interactions in Vision and Attention. Neuron 89, 209–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt LI et al. Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sreenivasan KK & D’Esposito M The what, where and how of delay activity. Nat Rev Neurosci 20, 466–481 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nobre AC & Stokes AM. Memory and attention: the back and forth. (2020). The Cognitive Neurosciences, 6th Edition (pp. 291–300). Cambridge: MIT Press. [Google Scholar]
- 51.Watanabe Y & Funahashi S Neuronal Activity Throughout the Primate Mediodorsal Nucleus of the Thalamus During Oculomotor Delayed-Responses. I. Cue-, Delay-, and Response-Period Activity. Journal of Neurophysiology 92, 1738–1755 (2004). [DOI] [PubMed] [Google Scholar]
- 52.DeNicola AL, Park M-Y, Crowe DA, MacDonald AW & Chafee MV Differential Roles of Mediodorsal Nucleus of the Thalamus and Prefrontal Cortex in Decision-Making and State Representation in a Cognitive Control Task Measuring Deficits in Schizophrenia. J. Neurosci 40, 1650–1667 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell AS The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neuroscience & Biobehavioral Reviews 54, 76–88 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Pergola G. et al. The Regulatory Role of the Human Mediodorsal Thalamus. Trends in Cognitive Sciences 22, 1011–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Bourbon-Teles J. et al. Thalamic Control of Human Attention Driven by Memory and Learning. Current Biology 24, 993–999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manoach DS, Greve DN, Lindgren KA & Dale AM Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. NeuroImage 20, 1670–1684 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Sorenson E & Hwang K Thalamocortical contributions to working memory processes during the n-back task. Neurobiology of Learning and Memory 197, 107701 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang K, Shine JM, Cole MW & Sorenson E Thalamocortical contributions to cognitive task activity. eLife 11, e81282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatham CH, Frank MJ & Badre D Corticostriatal Output Gating during Selection from Working Memory. Neuron 81, 930–942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frank MJ, Loughry B & O’Reilly RC Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci 1, 137–160 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Hazy TE, Frank MJ & O’Reilly RC Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Phil. Trans. R. Soc. B 362, 1601–1613 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuramoto E. et al. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cerebral cortex (New York, N.Y. : 1991) 19, 2065–2077 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Christophel TB, Klink PC, Spitzer B, Roelfsema PR & Haynes J-D The Distributed Nature of Working Memory. Trends in Cognitive Sciences 21, 111–124 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Malekmohammadi M, Elias WJ & Pouratian N Human Thalamus Regulates Cortical Activity via Spatially Specific and Structurally Constrained Phase-Amplitude Coupling. Cerebral Cortex 25, 1618–1628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweeney-Reed CM et al. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. eLife 3, e05352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connor DH, Fukui MM, Pinsk MA & Kastner S Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5, 1203–1209 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Ling S, Pratte MS & Tong F Attention alters orientation processing in the human lateral geniculate nucleus. Nat Neurosci 18, 496–498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang AS, Rogers BP & Woodward ND Disrupted modulation of thalamus activation and thalamocortical connectivity during dual task performance in schizophrenia. Schizophrenia Research 210, 270–277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bastos AM et al. Canonical microcircuits for predictive coding. Neuron 76, 695–711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fries P. Rhythms for Cognition: Communication through Coherence. Neuron 88, 220–235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cardin JA et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schofield CM, Kleiman-Weiner M, Rudolph U & Huguenard JR A gain in GABAA receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proceedings of the National Academy of Sciences 106, 7630–7635 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ährlund-Richter S et al. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat Neurosci 22, 657–668 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Cruikshank SJ, Lewis TJ & Connors BW Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10, 462–468 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Theyel BB, Llano DA & Sherman SM The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci 13, 84–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behrens TEJ et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience 6, 750–757 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Guedj C & Vuilleumier P Functional connectivity fingerprints of the human pulvinar: Decoding its role in cognition. NeuroImage 221, 117162 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Jaramillo J, Mejias JF & Wang X-J Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 101, 321–336.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lurie DJ et al. Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Network Neuroscience 4, 30–69 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen X. et al. Exploring communication between the thalamus and cognitive control-related functional networks in the cerebral cortex. Cogn Affect Behav Neurosci 21, 656–677 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Geier KT, Buchsbaum BR, Parimoo S & Olsen RK The role of anterior and medial dorsal thalamus in associative memory encoding and retrieval. Neuropsychologia 148, 107623 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Shine JM et al. The Low-Dimensional Neural Architecture of Cognitive Complexity Is Related to Activity in Medial Thalamic Nuclei. Neuron 104, 849–855.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 83.D’Esposito M. From cognitive to neural models of working memory. Phil. Trans. R. Soc. B 362, 761–772 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL & Petersen SE A dual-networks architecture of top-down control. Trends in Cognitive Sciences 12, 99–105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McAlonan K, Cavanaugh J & Wurtz RH Guarding the gateway to cortex with attention in visual thalamus. Nature 456, 391–394 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbetta M & Shulman GL Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Hwang K, Bertolero MA, Liu WB & D’Esposito M The Human Thalamus Is an Integrative Hub for Functional Brain Networks. J. Neurosci 37, 5594–5607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van den Heuvel MP & Sporns O Network hubs in the human brain. Trends in Cognitive Sciences 17, 683–696 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Thomas Yeo BT et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology 106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guimerà R & Nunes Amaral LA Functional cartography of complex metabolic networks. Nature 433, 895–900 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bertolero MA, Yeo BTT & D’Esposito M The modular and integrative functional architecture of the human brain. Proc. Natl. Acad. Sci. U.S.A 112, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Power JD, Schlaggar BL, Lessov-Schlaggar CN & Petersen SE Evidence for hubs in human functional brain networks. Neuron 79, 798–813 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawabata K et al. Bridging large-scale cortical networks: Integrative and function-specific hubs in the thalamus. iScience 24, 103106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greene DJ et al. Integrative and Network-Specific Connectivity of the Basal Ganglia and Thalamus Defined in Individuals. Neuron 105, 742–758.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bell PT & Shine JM Subcortical contributions to large-scale network communication. Neuroscience & Biobehavioral Reviews 71, 313–322 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Crosson B. Thalamic mechanisms in language: A reconsideration based on recent findings and concepts. Brain and Language 126, 73–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang K, Shine JM, Bruss J, Tranel D & Boes A Neuropsychological evidence of multi-domain network hubs in the human thalamus. eLife 10, e69480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Margulies DS et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences of the United States of America 113, 12574–12579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunningham JP & Yu BM Dimensionality reduction for large-scale neural recordings. Nature Neuroscience 17, 1500–1509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Müller EJ et al. Core and matrix thalamic sub-populations relate to spatio-temporal cortical connectivity gradients. NeuroImage 222, 117224 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Watson BO, MacLean JN & Yuste R UP states protect ongoing cortical activity from thalamic inputs. PloS one 3, e3971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garrett DD, Epp SM, Perry A & Lindenberger U Local temporal variability reflects functional integration in the human brain. NeuroImage 183, 776–787 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Faisal AA, Selen LPJ & Wolpert DM Noise in the nervous system. Nat Rev Neurosci 9, 292–303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waschke L, Kloosterman NA, Obleser J & Garrett DD Behavior needs neural variability. Neuron 109, 751–766 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Britten KH, Shadlen MN, Newsome WT & Movshon JA Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci 10, 1157–1169 (1993). [DOI] [PubMed] [Google Scholar]
- 106.Goris RLT, Movshon JA & Simoncelli EP Partitioning neuronal variability. Nat Neurosci 17, 858–865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scholvinck ML, Saleem AB, Benucci A, Harris KD & Carandini M Cortical State Determines Global Variability and Correlations in Visual Cortex. Journal of Neuroscience 35, 170–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garrett DD et al. Lost Dynamics and the Dynamics of Loss: Longitudinal Compression of Brain Signal Variability is Coupled with Declines in Functional Integration and Cognitive Performance. Cerebral Cortex 31, 5239–5252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grady CL & Garrett DD Brain signal variability is modulated as a function of internal and external demand in younger and older adults. NeuroImage 169, 510–523 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Garrett DD et al. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neuroscience & Biobehavioral Reviews 37, 610–624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garrett DD et al. Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc Natl Acad Sci USA 112, 7593–7598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garrett DD, Kovacevic N, McIntosh AR & Grady CL The Importance of Being Variable. Journal of Neuroscience 31, 4496–4503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shine JM et al. Computational models link cellular mechanisms of neuromodulation to large-scale neural dynamics. Nat Neurosci 24, 765–776 (2021). [DOI] [PubMed] [Google Scholar]
- 114.Shine JM, van den Brink RL, Hernaus D, Nieuwenhuis S & Poldrack RA Catecholaminergic manipulation alters dynamic network topology across cognitive states. Network Neuroscience 2, 381–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCormick DA, Pape HC & Williamson A Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Progress in brain research 88, 293–305 (1991). [DOI] [PubMed] [Google Scholar]
- 116.Alavash M. et al. Dopaminergic modulation of hemodynamic signal variability and the functional connectome during cognitive performance. NeuroImage 172, 341–356 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Li S-C, Lindenberger U & Sikström S Aging cognition: from neuromodulation to representation. Trends in Cognitive Sciences 5, 479–486 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Shafiei G. et al. Dopamine Signaling Modulates the Stability and Integration of Intrinsic Brain Networks. Cerebral Cortex 29, 397–409 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Venton BJ et al. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing: Decoding dopamine neurotransmission. Journal of Neurochemistry 87, 1284–1295 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Owesson-White CA, Cheer JF, Beyene M, Carelli RM & Wightman RM Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proceedings of the National Academy of Sciences 105, 11957–11962 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guiard BP, El Mansari M, Merali Z & Blier P Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. International Journal of Neuropsychopharmacology 11, 625–639 (2008). [DOI] [PubMed] [Google Scholar]
- 122.Zhang W & Bruno RM High-order thalamic inputs to primary somatosensory cortex are stronger and longer lasting than cortical inputs. eLife 8, e44158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis LD et al. Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife 4, e08760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gent TC, Bandarabadi M, Herrera CG & Adamantidis AR Thalamic dual control of sleep and wakefulness. Nat Neurosci 21, 974–984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poulet JFA, Fernandez LMJ, Crochet S & Petersen CCH Thalamic control of cortical states. Nature Neuroscience 15, 370–372 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Constantinople CM & Bruno RM Effects and Mechanisms of Wakefulness on Local Cortical Networks. Neuron 69, 1061–1068 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.David F. et al. Essential Thalamic Contribution to Slow Waves of Natural Sleep. Journal of Neuroscience 33, 19599–19610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Larson-Prior LJ et al. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences 106, 4489–4494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spoormaker VI et al. Development of a Large-Scale Functional Brain Network during Human Non-Rapid Eye Movement Sleep. Journal of Neuroscience 30, 11379–11387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hale JR et al. Altered thalamocortical and intra-thalamic functional connectivity during light sleep compared with wake. NeuroImage 125, 657–667 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Birn RM, Murphy K, Handwerker DA & Bandettini PA fMRI in the presence of task-correlated breathing variations. NeuroImage 47, 1092–1104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang C, Cunningham JP & Glover GH Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 44, 857–869 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diekelmann S & Born J The memory function of sleep. Nat Rev Neurosci 11, 114–126 (2010). [DOI] [PubMed] [Google Scholar]
- 134.McCormick DA & Bal T Sleep and Arousal: Thalamocortical Mechanisms. Annu. Rev. Neurosci 20, 185–215 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Schabus M et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences 104, 13164–13169 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mak-McCully RA et al. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat Commun 8, 15499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Magnin M. et al. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci USA 107, 3829–3833 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zou G. et al. Functional MRI of arousals in nonrapid eye movement sleep. Sleep 43, zsz218 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Chang C. et al. Tracking brain arousal fluctuations with fMRI. Proc Natl Acad Sci U S A 113, 4518–4523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Setzer B. et al. A temporal sequence of thalamic activity unfolds at transitions in behavioral arousal state. Nat Commun 13, 5442 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jones BE Arousal and sleep circuits. Neuropsychopharmacol. 45, 6–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchez-Gonzalez MA The Primate Thalamus Is a Key Target for Brain Dopamine. Journal of Neuroscience 25, 6076–6083 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lőrincz ML & Adamantidis AR Monoaminergic control of brain states and sensory processing: Existing knowledge and recent insights obtained with optogenetics. Progress in Neurobiology 151, 237–253 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Akeju O. et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife 3, e04499 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pérez-Santos I, Palomero-Gallagher N, Zilles K & Cavada C Distribution of the Noradrenaline Innervation and Adrenoceptors in the Macaque Monkey Thalamus. Cerebral Cortex 31, 4115–4139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gugino LD et al. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. British Journal of Anaesthesia 87, 421–428 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Purdon PL et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proceedings of the National Academy of Sciences 110, E1142–E1151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Waschke L. et al. Modality-specific tracking of attention and sensory statistics in the human electrophysiological spectral exponent. eLife 10, e70068 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ching S, Cimenser A, Purdon PL, Brown EN & Kopell NJ Thalamocortical model for a propofol-induced -rhythm associated with loss of consciousness. Proceedings of the National Academy of Sciences 107, 22665–22670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Redinbaugh MJ et al. Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron 106, 66–75.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Honjoh S. et al. Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nat Commun 9, 2100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schiff ND Central Thalamic Contributions to Arousal Regulation and Neurological Disorders of Consciousness. Annals of the New York Academy of Sciences 1129, 105–118 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Akeju O & Brown EN Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Current Opinion in Neurobiology 44, 178–185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kosciessa JQ, Lindenberger U & Garrett DD Thalamocortical excitability modulation guides human perception under uncertainty. Nat Commun 12, 2430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haegens S, Nacher V, Luna R, Romo R & Jensen O Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences 108, 19377–19382 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones SR et al. Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: rhythmogenesis and modulation of sensory-evoked responses. Journal of Neurophysiology 102, 3554–3572 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pettine WW, Louie K, Murray JD & Wang X-J Excitatory-inhibitory tone shapes decision strategies in a hierarchical neural network model of multi-attribute choice. PLoS Comput Biol 17, e1008791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mo C. et al. Competing rhythmic neural representations of orientations during concurrent attention to multiple orientation features. Nat Commun 10, 5264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Munn BR, Müller EJ, Wainstein G & Shine JM The ascending arousal system shapes neural dynamics to mediate awareness of cognitive states. Nat Commun 12, 6016 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mukherjee A, Lam NH, Wimmer RD & Halassa MM Thalamic circuits for independent control of prefrontal signal and noise. Nature 600, 100–104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rikhye RV, Gilra A & Halassa MM Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nature Neuroscience 21, 1753–1763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mack ML, Preston AR & Love BC Ventromedial prefrontal cortex compression during concept learning. Nat Commun 11, 46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rigotti M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Research Reviews 46, 1–31 (2004). [DOI] [PubMed] [Google Scholar]
- 165.Crabtree JW Functional Diversity of Thalamic Reticular Subnetworks. Front. Syst. Neurosci 12, 41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proceedings of the National Academy of Sciences 81, 4586–4590 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakajima M, Schmitt LI & Halassa MM Prefrontal Cortex Regulates Sensory Filtering through a Basal Ganglia-to-Thalamus Pathway. Neuron 103, 445–458.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Higashikubo B & Moore CI Systematic Examination of the Impact of Depolarization Duration on Thalamic Reticular Nucleus Firing in vivo. Neuroscience 368, 187–198 (2018). [DOI] [PubMed] [Google Scholar]
- 169.Jager P. et al. Dual midbrain and forebrain origins of thalamic inhibitory interneurons. eLife 10, e59272 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith Y, Raju DV, Pare J-F & Sidibe M The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in Neurosciences 27, 520–527 (2004). [DOI] [PubMed] [Google Scholar]
- 171.Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ & Clascá F Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cerebral cortex (New York, N.Y. : 1991) 19, 2380–2395 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Solari SVH & Stoner R Cognitive consilience: primate non-primary neuroanatomical circuits underlying cognition. Frontiers in Neuroanatomy 5, 65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee S-H & Dan Y Neuromodulation of Brain States. Neuron 76, 209–222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC & Wager TD Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665–670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lewis LD, Setsompop K, Rosen BR & Polimeni JR Stimulus-dependent hemodynamic response timing across the human subcortical-cortical visual pathway identified through high spatiotemporal resolution 7T fMRI. NeuroImage 181, 279–291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Polimeni JR & Lewis LD Imaging faster neural dynamics with fast fMRI: a need for updated models of the hemodynamic response. Progress in Neurobiology 102174 (2021) doi: 10.1016/j.pneurobio.2021.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Iglesias JE et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 183, 314–326 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alkemade A. et al. A unified 3D map of microscopic architecture and MRI of the human brain. Sci. Adv 8, eabj7892 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]